Abstract
Background
In the current COVID-19 pandemic, Multiple Sclerosis (MS) patients represent a population of particular interest as they might be at higher risk of COVID-19 infection and it's complications. The present study aimed to investigate a one year follow up of patients with MS during the COVID-19 pandemic, in Qom province, Iran.
Methods
This study was performed at the MS Clinic of Beheshti Hospital from June 1, 2020 to November 1, 2021. 202 patients with a diagnosis of MS and negative self-reported history of COVID-19 at the beginning of the pandemic, were enrolled. First, the demographic characteristics of patients were collected. Second, the patients underwent serological testing for anti-SARS-CoV-2 IgG antibodies. Then, a year later, they were revalauted and asked about the occurrence of clinical relapse leading to hospitalization, disease progression, DMT profile, COVID-19 vaccination, and history of COVID-19 infection. We considered six weeks after COVID-19 regarding relapse occurrence. Eventually, statistical analysis was carried out by using SPSS 26.0
Results
Of 202 patients, 26 patients (12.87%) had initially a positive index antibody result. During the follow-up periods, 25 patients (12.37%) were infected with COVID-19 which was mainly mild (74.8%), and significantly lower than general population. 118 patients (58.41%) were vaccinated for COVID-19 which reduced the risk of COVID-19 development (P<001). Except a case of myelitis associated with vaccination, no serious adverse event was reported. Additionally, only one patient developed MS relapse following COVID-19 infection. Except clinical relapse (P = 0.001), other demographic and MS characteristics, and DMT type were not associated with COVID-19. In terms of MS course, 12 patients (5.94%) discontinued their DMTs regardless of the DMT adverse events or treatment failure. 41 patients (20.3%) experienced a clinical relapse, of whom 12 were escalated to a second line DMT. Further, 27 patients (13.4%) noted a history of worsening disability which mainly occurred after COIVD-19 infection.
Conclusion
The present study showed a significant lower incidence of COVID-19 infection in MS patients. Except for clinical relapse, other demographic and MS characteristics, and DMT type were not associated with COVID-19 infection. In addition, COVID-19 vaccination reduced the risk of COVID-19 development, and the prognosis was favorable in the majority of MS patients.
Keywords: Multiple sclerosis, COVID-19, Vaccination, EDSS, Relapse
1. Introduction
In December 2019, a cluster of unexplained pneumonia in Wuhan, China, penetrated borders and became a global pandemic. The current pandemic and the numerous unexplained issues regarding the post-pandemic phase, make it more difficult to manage individuals who require immunosuppressive drugs. A large number of studies have shown that a high level of innate immunity, along with a deficiency in adaptive immunity, may worsen the COVID-19 infection, and the production of a significant variety of inflammatory cytokines may result in an adverse prognosis (Fauci et al., 2020; Ge et al., 14).
Multiple sclerosis (MS) is an immune-mediated central nervous system (CNS) disease that necessitates immunosuppressive or immunomodulatory disease-modifying therapy (DMTs). Up to 70% of MS patients are managed with DMTs, which affect the immune response and may enhance the risk of infection (Koch-Henriksen and Magyari, 2021). Previous studies have shown a higher rate of respiratory tract infections in MS patients compared to general population, which rises with age, and degree of disability, especially in male sex. Notably, immunosuppressive DMTs as B cell therapies are more likely to be associated with severe infection as SARS CoV-2 infection and lower response to COVID-19 vaccination (Wijnands et al., 2017; Möhn et al., 2020; Grebenciucova and Pruitt, 2017). Moreover, while MS is often thought to be a disease hampering young people, ageing patients with MS are increasing worldwide, who might be at higher risk of severe morbidity and death from COVID-19 (Minden et al., 2004).
Considering the chronicity of MS, the importance of rehabilitation therapy which might be limited during the current pandemic, the long-term consequences of DMTs, and the challenges in DMTs initiation and continuation on one hand, and psychological burden of COVID-19 on the other, along with the possible complications of COVID-19 vaccination which all might increase the risk of MS relapse, in this cross-sectional study, we aimed to evaluate a one-year follow-up of patients with MS, in Qom province, Iran to investigate the change of MS course in COVID-19 pandemic, and determine the COVID-19 occurrence according to the clinical profile of patients and different DMTs usage.
2. Material and methods
2.1. Study designs
This cross-sectional study was conducted at the MS Clinic of Qom province, Iran, from June 1, 2020 to November 1, 2021. The initial assessment was performed in a two-month period from June 1, 2020 to August 1, 2020. The second evaluation was performed in a similar period from August 1, 2021 to September 1, 2021.
The study received approval from the ethics committee of Qom University of Medical Sciences and Health (ethic code: IR.MUQ.REC.1400.102). Additionally, all patients fulfilled the informed consents prior to their participation in the study.
2.2. Study population
Patients were recruited from the MS clinic of Beheshti hospital, Qom, Iran. Eligible participants were selected according to inclusion criteria: patients with a diagnosis of MS based on McDonald Criteria 2017 and age over 18 years.
Patients with a self-reported history of COVID-19 prior to enrollment, patients who attempted to conceive, and those who were pregnant or in the postpartum period were excluded. Eventually, 202 patients met the eligibility criteria.
2.3. Interventions and data collection
First, in a face-to-face interviews with patients, a questionnaire of demographic characteristics consisted of age, gender, type of MS, DMTs use, Expanded Disability Severity Scale score (EDSS),and comorbidities was fulfilled. EDSS was assessed by the neurology resident, who was participated in the study.
Second, all patients underwent serological testing for anti-SARS-CoV-2 IgG antibodies. Then, a year later, the patients were reassessed. In the next visit, the clinical data including of history of clinical relapse leading to hospitalization, disease progression, DMT profile, COVID-19 vaccination, and history of COVID-19 infection were collected.
COVID-19 infection was confirmed based on a positive nasopharyngeal Polymerase chain reaction (PCR) test for SARS-CoV-2. Additionally, given the China's National Health Commission, the severity of COVID-19 was classified as mild, moderate, and severe.
MS relapse was defined as a monophasic clinical episode with objective findings typical of MS, which was developed acutely or subacutely, with a duration of at least 24 h (Repovic, 2019). We considered six weeks as the period attributed to MS relapse in association with COVID-19 infection or vaccination.
2.4. Statistical analysis
Statistical analysis was carried out by using SPSS 26.0 (SPSS Inc, Chicago, IL) software. Data were expressed as mean ± SD for quantitative variables and counts (%) for categorical variables. One-sample Kolmogorov-Smirnov test, Chi-Square test, and Whitney-Mann statistical tests were used for data analysis, and multi nominal regression model was used to evaluate the relation between DMT type and COVID-19 severity. The significance difference was considered P < 0.05.
3. Results
As June 1, 2020 to November 1, 2021, a total of 202 MS patients were included. One hundred and sixty-four patients (81.2%) were female, with a mean (SD) age of 37.52 ± 8.95 years. Approximately, 25.61% patients had one or more comorbidity, of that hypothyroidism was most frequently reported.
The results revealed that, the majority of patients (77.7%) had EDSS score ≤ 2 with a mean score of 1.76 on a scale from 0 to 8. Relapse remitting MS (RRMS) was found as the most frequent MS type in all patients (71.78%). After a year, 27 patients (13.4%) noted a history of worsening disability, particularly after COIVD-19 infection. However, this association was not considered to be significant (P = 0.0.719).
At the beginning of the study, 94.55% of the patients received DMTs, with IFN-β1a (n = 66, 34.55%) and anti-CD20 (n = 47, 24.60%) being the most frequently prescribed medications. However, in the following, 12 patients (5.94%) discontinued their DMTs regardless of the DMT adverse events or treatment failure, of whom 10 were receiving interferons, one was receiving glatiramer acetate, and one was taking dimethyl fumarate (DMF). Moreover, 41 patients (20.3%) experienced a clinical relapse which was mainly treated with methylprednisolone 1 gr/d for 3–5 consecutive days. only one rituximab-treated RRMS patient experienced myelitis in the course of COVID-19 infection which was managed with therapeutic plasma exchange (TPE) for 5 sessions (alternate days). One standard TPE session was 2.5 plasma volume exchange using 5% albumin as a replacement fluid. The relapses were not significantly associated with COVID-19 infection (P = 0.604). Contrariwise, a statistical significance was demonstrated between relapse and COVID-19 vaccination (P = 0.02).
In terms of DMT adjustment, 12 patients with clinical relapse were escalated to a second line DMT, four of them to rituximab. Further, the rituximab was infused in an extended interval of 9–12 months in four patients.
Based on the results, a total of 26 patients (12.87%) showed seropositive for SARS CoV-2 IgG at the beginning of the study. Within one year, 25 patients (12.37%) developed COVID-19 which was considered to be mild in 74.8%. No one experienced recurrence of COVID-19 or persistent viral shedding. We also did not demonstrate a significant association between prior seropositive SARS CoV-2 status and future COVID-19 (P = 0.24).
As shown in Table 1 , there was no significant association between demographic characteristics and COVID-19 (P>0.05). In terms of MS characteristics, neither MS type (P = 0.306) nor EDSS score (P = 0.319) was associated with COVID-19. Conversely, a significant relationship was shown between clinical relapse and COVID-19 infection (P<0.001). In parallel, there was a significant correlation between the change of DMTs (escalation therapy) and the incidence of COVID-19 (P<001). However, escalation to a particular type of DMT as rituximab was not associated with COVID-19 (P = 0.712).
Table 1.
The baseline clinical characteristics of MS patients.
| Variable | COVID-19 infection | P-value | ||
|---|---|---|---|---|
| Negative | Positive | |||
| Gender | Female (%) | 144 (71.28%) | 20 (9.9%) | 0.698 |
| Male (%) | 32 (15.84%) | 5 (2.47%) | ||
| Age (mean ± SD) | 38.09 ± 10.440 | 37.45 ± 8.768 | 0.095 | |
| MS course | CIS (%) | 4 (1.98%) | 1 (0.49%) | 0.306 |
| Primary-progressive (%) | 8 (3.96%) | 1 (0.49%) | ||
| Secondary progressive (%) | 39 (19.30%) | 4 (1.98%) | ||
| Relapsing-remitting (%) | 126 (62.37%) | 19 (0.49) | ||
| EDSS (mean) | 1.76 ± 1.65 | 1.77 ± 1.52 | 0.319 | |
| Comorbidity | Negative | 132 (65.34%) | 19 (9.40%) | 0.652 |
| Hypothyroidism | 19 (19.40%) | 6 (2.97%) | ||
| Hyperlipidemia | 10 (4.95%) | 0 (0.00%) | ||
| Hypertension | 8 (3.96%) | 0 (0.00%) | ||
| Diabetes mellitus | 6 (2.97%) | 0 (0.00%) | ||
| Others | 2 (0.99%) | 0 (0.00%) | ||
| SARS-CoV 2 IgG level | Positive | 22 (10.89%) | 4 (1.98%) | 0.24 |
| Negative | 155 (76.73%) | 21 (10.39%) | ||
MS: multiple sclerosis; CIS: clinically isolated syndrome; RRMS: relapse remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis; PPMS: primary progressive multiple sclerosis; EDSS: Expanded Disability Status Scale.
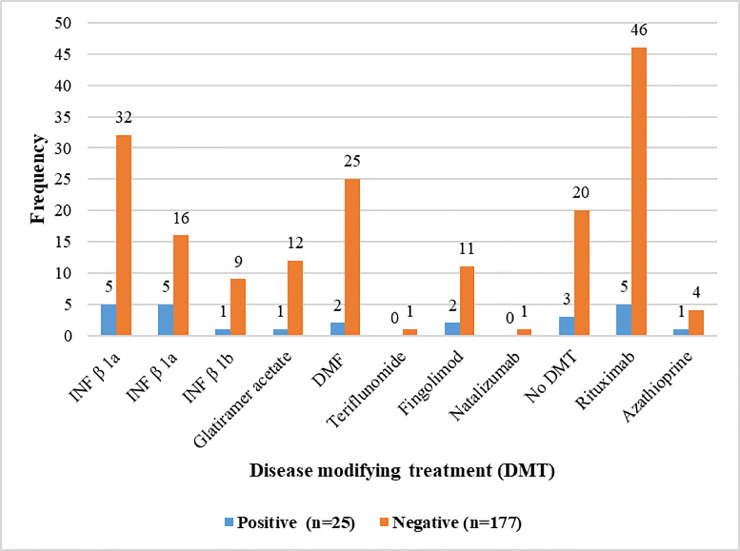
Table 2 shows the medication history of patients after 12 months in both groups. The data did not reveal a statistical association between DMT type and COVID-19 (P = 0. 0.190), as patients with rituximab and immune cell trafficking inhibitors were not more likely to develop COVID-19 compared to other DMTs (Fig. 1 ).
Table 2.
The DMT medication of MS patients after 12 months and association between COVID-19 infection and DMT category.
| COVID-19 infection | Disease modifying treatment (DMT) | P-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INF β 1a IM injection |
INF β 1a SC injection |
INF β 1b SC injection |
Glatiramer acetate | DMF | Teriflunomide | Fingolimod | Natalizumab | No DMT | Rituximab | Azathioprine | ||
| Positive (n = 25) | 5 (2.79%) | 5 (2.79%) | 1 (0.55%) | 1 (0.55%) | 2(1.11%) | 0 (0%) | 2 (1.11%) | 0 (0.00%) | 3 (1.67) | 5 (2.79%) | 1 (0.55%) | 0.190 |
| Negative (n = 177) | 32 (17.87%) | 16 (8.93%) | 9 (5.02%) | 12 (6.70%) | 25 (13.96) | 1 (0.55%) | 11 (6.14%) | 1 (0.55%) | 20 (11.17%) | 46 (25.69%) | 4 (2.23%) | |
Fig. 1.
The DMT medication of MS patients after 12 months and association between COVID-19 infection and DMT category IFN-β 1a: interferon β 1a, INF-β 1b: interferon β 1b, DMF: dimethyl fumarate, DMT: disease modifying treatment,.
The results also revealed that, 118 patients (58.41%) were vaccinated for COVID-19, which reduced the risk of severe COIVD-19 development (P<0.001).Except the one with PPMS and rituximab treatment who experienced an attack of transverse myelitis following vaccination, no one reported significant adverse event after vaccination.
DMT: disease modifying treatment; MS: multiple sclerosis; IFN-β 1α IM: interferon-β 1α intramuscular; IFN-β 1α SC: subcutaneous; DMF: dimethyl fumarate
4. Discussion
4.1. COVID-19 infection
In the present study, an 11.8% seroprevalence of COVID-19 was demonstrated in MS population at the beginning, which was much higher than the officially confirmed cases. The results were lower than a report in Iran on 8902 participants from the general population across 17 provinces, which revealed a total 17.1% seroprevalence of COVID-19 with a greater prevalence in Qom province (58.5%) (Poustchi et al., 2021).However, amid the COVID-19 pandemic, regardless of the nationwide increasing trend of COIVD-19 prevalence and mortality in Iran (Hazbavi et al., 2021), only 12.3% of MS patients developed COVID-19 which was significantly lower than general population. Moreover, the majority of MS patients (74.8%) developed mild COVID-19, and no death was reported. The results also did not reveal a significant association between prior seropositivity of SARS CoV-2 and risk of later COVID-19.
Given the autoimmune nature of MS and DMT treatment, which in most part, target the adaptive rather than the innate immune system, MS patients are theoretically more likely to succumb to serious infection, which raises concerns about the outcome of COVID-19 (Vishnevetsky and Levy, 2020).However,COVID-19 incidence was lower in our patients. This might be related to different factors. The results showed that 58.41% of patients received two doses of Sinopharm vaccine. Further, a substantial number of our patients were female and young with no or few comorbidities. They were also more likely to adhere with strict quarantine and social distance. Also, potentially the reduced mobility in those with greater disability may make them less likely to be out in public areas where exposure might occur, even despite there being no difference in mean EDSS between COVID groups. Finally, the majorly of patients continued their conventional treatment (93.71%), of which interferon-βs were the most common (37.98%). Previous studies have shown the down regulatory effects of interferon-βs on both pro- and anti-inflammatory cytokines which might inhibit SARS-CoV2 and attenuate the symptoms of COVID-19 (Zheng et al., 2020; Berger et al., 2020; McNab et al., 2015).
A large number of studies have investigated the risk factors of COVID-19, of which age, gender, and comorbidities have been considered as the main factors. However, there are conflicting results regarding the role of MS type and EDSS in the occurrence of COVID-19 (Saeed et al., 2021). Our data indicated that except for recent clinical relapse (P = 0.001), other demographic and MS characteristics (P > 0.05) did not remain independent factors for COVID-19. These results might be related to the relatively younger age of our patients with low frequency of individual comorbidities. Furthermore, patients with more benign course of MS and COVID-19 were more likely to participate in the study, which all resulted in a confounding bias. On the other hand, a negative association of clinical relapse and COVID-19 might be related to the corticosteroids therapy, as some guidelines have recommended to avoid or prescribe corticosteroids at the lowest possible dose, especially in cases of mild relapses (Dhamija et al., 2021). However, a report in our center did not reveal the increased risk of COVID-19 following pulse steroid therapy in MS patients (Naser Moghadasi et al., 2021). Moreover, the variable interval of pulse therapy and onset of COVID-19 makes this relationship unlikely.
In terms of DMT type, it is noteworthy that, a particular attention has been given to the DMTs in the COVID-19 era. While, prior studies revealed no apparent increase in the risk of COVID-19 with DMT type, later reports highlighted the possibly increased risk of infection and decreased immune response of COVID-19 vaccination for B cell–depleting agents (Zheng et al., 2020; Berger et al., 2020; Baker et al., 2020; Yamout et al., 2021; Zrzavy et al., 2021). The present study, did not demonstrate a significant association between DMT type and risk of COVID-19 or the severity of COVID-19. We assume, our results might be related to the small sample size of the study, and specifically the small number of patients with severe COVID-19 leading to a selection bias. Further, a relatively low prevalence of rituximab administration, extended intervals of rituximab infusion, and lack of ocrelizumab administration might have played a role in improper interpretation.
4.2. MS course
In the current study, only one of the RRMS patients (4%) experienced a clinical relapse following COVID-19 infection, and except for a report of MS relapse (0.84%) following the first dose of Sinopharm, the post-vaccination adverse events were mild and comparable to previous reports (Achiron et al., 2021). However, an increased risk of relapse associated with COVID-19 vaccination was contrary to other reports, ranging from 0.9% in an Iranian report to 2.1% following the second dose of BNT162b2 COVID-19 vaccine in Israel (Ali Sahraian et al., 2021; Barzegar et al., 2021a).
Notably, while other types of coronaviruses have been reported to be related with demyelinating diseases, little association has been shown between COVID-19 infection and MS exacerbation or even MS manifestation. Similarly, the same is true of COVID-19 vaccination (Montalvan et al., 2020). The recently reports of a case of clinically isolated syndrome (CIS) and two cases of MS initiation with prior COVID-19 infection, and five cases of initial manifestation of MS associated with COVID-19 vaccine exposure, have alarmed such considerations (Domingues et al., 2020; Palao et al., 2020; Moore et al., 2021; Fujimori et al., 2021). In addition, reports of COVID-19 infection as a trigger of relapse in MS patients, have highlighted this association (Barzegar et al., 2021b; Michelena et al., 2021).
In terms of MS course, 12 patients (5.94%) discontinued their DMTs regardless of the DMT side effects or escalation therapy. 41 patients (20.3%) experienced a clinical relapse, of whom 12 were escalated to a second line DMT. Further, 27 patients (13.4%) had a history of slowly disability worsening, which mainly occurred after COIVD-19 infection. However, this association was not considered to be significant (P = 0.719). To the best of our knowledge, there are no reports of MS progression in relation to COVID-19. Our preliminary results did not suggest the negative impact of COVID-19 on MS course. Still, more studies are needed to confirm our data.
4.3. Limitations and recommendations
To the best of knowledge, this is the first report in Iran evaluating a one year follow up of MS patients with a baseline COVID-19 serostatus. However, our work has some limitations. First, patients with significant disability and progressive form of MS were less likely to participate in the study leading to a healthy bias. Second, the small sample size of COVID-19 cases and especially those with a severe course, along with small sample size of patients on immunosuppressive therapy as B cell depleting agents might have led to a high degree of uncertainty. Third, as we did not check the serostatus of COVID-19 at the end of the study, there was a possibility to miss asymptomatic, mild or atypical COVID-19 cases. Eventually, the present study is restricted to MS patients who could be on DMTs, excluding women who were pregnant or attempting to conceive. This is potentially a limitation in terms of general disability, as it is possible that among all persons, including pregnant women or those attempting to conceive, the epidemiology of COVID-19 could differ.
Taking all considerations, as the COVID-19 pandemic is still ongoing, the demand for data on the impact of COVID-19 infection and COVID-19 vaccination on MS patients is crucial. Large multicenter studies are required to better understand the association of COVID-19 infection and vaccination with the demyelinating disorders to optimize the best practice in MS management.
5. Conclusion
The present study shows that the incidence of COVID-19 in MS patients was similar to the general population, early in the pandemic. However, in the following, it significantly decreased, and COVID-19 vaccination decreased the risk of severe COVID-19 development. Except for clinical relapse, other demographic and MS characteristics, and DMT type were not associated with COVID-19. In addition, the majority of patients continued their conventional treatment, and the prognosis was generally favorable. Except for one case of MS relapse after vaccination and one case following COVID-19 infection, MS disease activity was not affected by COVID-19. Our results highlights the importance of comprehensive studies to elucidate the possible association of COVID-19 and MS, and provide a therapeutic guideline in MS population in COVID-19 era.
CRediT authorship contribution statement
Sepideh Paybast: Visualization, Funding acquisition, Methodology, Writing – original draft. Seyed Amir Hejazi: Visualization, Funding acquisition. Payam Molavi: . Mohammad Amin Habibi: Formal analysis, Methodology, Writing – original draft. Abdorreza Naser Moghadasi: Writing – review & editing, Methodology, Writing – original draft, Formal analysis.
Acknowledgments
Acknowledgments
We thank the staff of the Department of Neurology for their kind help to manage the patients.
Funding
The authors have not declared a specific grant for this research from any funding agency.
References
- Achiron A., Dolev M., Menascu S., Zohar D.-.N., Dreyer-Alster S., Miron S. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. J. 2021;27(6):864–870. doi: 10.1177/13524585211003476. http://journals.sagepub.com/doi/10.1177/13524585211003476 [Internet]May 15Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Sahraian M., Ghadiri F., Azimi A. Naser Moghadasi A. Adverse events reported by Iranian patients with multiple sclerosis after the first dose of Sinopharm BBIBP-CorV. Vaccine. 2021;39(43):6347–6350. doi: 10.1016/j.vaccine.2021.09.030. https://linkinghub.elsevier.com/retrieve/pii/S0264410×21012214 [Internet]OctAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Roberts C.A.K., Pryce G., Kang A.S., Marta M., Reyes S. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin. Exp. Immunol. 2020;202(2):149–161. doi: 10.1111/cei.13495. https://academic.oup.com/cei/article/202/2/149/6402965 [Internet]Oct 30Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar M., Mirmosayyeb O., Gajarzadeh M., Afshari-Safavi A., Nehzat N., Vaheb S. COVID-19 among patients with multiple sclerosis. Neurol Neuroimmunol. Neuroinflammation. 2021;8(4):e1001. doi: 10.1212/NXI.0000000000001001. http://nn.neurology.org/lookup/doi/10.1212/NXI.0000000000001001 [Internet]Jul 20Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar M., Vaheb S., Mirmosayyeb O., Afshari-Safavi A., Nehzat N., Shaygannejad V. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult. Scler. Relat. Disord. 2021;52 doi: 10.1016/j.msard.2021.102947. https://linkinghub.elsevier.com/retrieve/pii/S2211034821002145 [Internet]JulAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J.R., Brandstadter R., Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol. Neuroimmunol. Neuroinflammation. 2020;7(4) doi: 10.1212/NXI.0000000000000761. http://nn.neurology.org/lookup/doi/10.1212/NXI.0000000000000761 [Internet]Jul 15e761. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamija R., Srivastava A., Chauhan S., Shah U., Nagda T., Palande D. Consensus statement on neurorehabilitation during COVID-19 times: expert group on behalf of the Indian federation of neurorehabilitation (IFNR) Ann. Indian Acad. Neurol. 2021;24(2):138–141. doi: 10.4103/aian.AIAN_997_20. http://www.annalsofian.org/preprintarticle.asp?id=311621;type=0 [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues R.B., Mendes-Correa M.C., de Moura Leite F.B.V., Sabino E.C., Salarini D.Z., Claro I. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J. Neurol. 2020;267(11):3154–3156. doi: 10.1007/s00415-020-09996-w. https://link.springer.com/10.1007/s00415-020-09996-w [Internet]Nov 20Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A.S., Lane H.C., Redfield R.R. COVID-19 – Navigating the Uncharted. N. Engl. J. Med. 2020;382(13):1268–1269. doi: 10.1056/NEJMe2002387. [Internet]Mar 26Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori J., Miyazawa K., Nakashima I. Initial clinical manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J. Neuroimmunol. 2021;361 doi: 10.1016/j.jneuroim.2021.577755. https://linkinghub.elsevier.com/retrieve/pii/S0165572821002824 [Internet]DecAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H., Wang X., Yuan X., Xiao G., Wang C., Deng T. The epidemiology and clinical information about COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(6):1011–1019. doi: 10.1007/s10096-020-03874-z. https://link.springer.com/10.1007/s10096-020-03874-z [Internet]Jun 14Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenciucova E., Pruitt A. Infections in patients receiving multiple sclerosis disease-modifying therapies. Curr. Neurol. Neurosci. Rep. 2017;17(11):88. doi: 10.1007/s11910-017-0800-8. http://link.springer.com/10.1007/s11910-017-0800-8 [Internet]Nov 22Available from: [DOI] [PubMed] [Google Scholar]
- Hazbavi Z., Mostfazadeh R., Alaei N., Azizi E. Spatial and temporal analysis of the COVID-19 incidence pattern in Iran. Environ. Sci. Pollut. Res. 2021;28(11):13605–13615. doi: 10.1007/s11356-020-11499-0. http://link.springer.com/10.1007/s11356-020-11499-0 [Internet]Mar 14Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch-Henriksen N., Magyari M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat. Rev. Neurol. 2021;17(11):676–688. doi: 10.1038/s41582-021-00556-y. https://www.nature.com/articles/s41582-021-00556-y [Internet]Nov 28Available from: [DOI] [PubMed] [Google Scholar]
- McNab F., Mayer-Barber K., Sher A., Wack A., O'Garra A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015;15(2):87–103. doi: 10.1038/nri3787. http://www.nature.com/articles/nri3787 [Internet]Feb 23Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelena G., Casas M., Eizaguirre M.B., Pita M.C., Cohen L., Alonso R., ¿ Can COVID-19 exacerbate multiple sclerosis symptoms? A case series analysis. Mult. Scler. Relat. Disord. [Internet]. 2021 Nov;103368. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2211034821006350. [DOI] [PubMed]
- Minden S.L., Frankel D., Hadden L.S., Srinath K.P., Perloff J.N. Disability in elderly people with multiple sclerosis: an analysis of baseline data from the Sonya slifka longitudinal multiple sclerosis study. NeuroRehabilitation. 2004;19(1):55–67. http://www.ncbi.nlm.nih.gov/pubmed/14988588 [Internet]Available from: [PubMed] [Google Scholar]
- Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin. Neurol. Neurosurg. 2020;194 doi: 10.1016/j.clineuro.2020.105921. https://linkinghub.elsevier.com/retrieve/pii/S030384672030264X [Internet]JulAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L., Ghannam M., Manousakis G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci. 2021;22 doi: 10.1016/j.ensci.2020.100299. https://linkinghub.elsevier.com/retrieve/pii/S2405650220300782 [Internet]MarAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhn N., Konen F.F., Pul R., Kleinschnitz C., Prüss H., Witte T. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. doi: 10.3390/jcm9124067. https://www.mdpi.com/2077-0383/9/12/4067 [Internet]Dec 16Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naser Moghadasi A., Shabany M., Heidari H., Eskandarieh S. Can pulse steroid therapy increase the risk of infection by COVID-19 in patients with multiple sclerosis? Clin. Neurol. Neurosurg. 2021;203 doi: 10.1016/j.clineuro.2021.106563. https://linkinghub.elsevier.com/retrieve/pii/S0303846721000901 [Internet]AprAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palao M., Fernández-Díaz E., Gracia-Gil J., Romero-Sánchez C.M., Díaz-Maroto I., Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult. Scler Relat. Disord. 2020;45 doi: 10.1016/j.msard.2020.102377. https://linkinghub.elsevier.com/retrieve/pii/S2211034820304521 [Internet]OctAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poustchi H., Darvishian M., Mohammadi Z., Shayanrad A., Delavari A., Bahadorimonfared A. SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: a population-based cross-sectional study. Lancet Infect. Dis. 2021;21(4):473–481. doi: 10.1016/S1473-3099(20)30858-6. https://linkinghub.elsevier.com/retrieve/pii/S1473309920308586 [Internet]AprAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovic P. Management of multiple sclerosis relapses. Contin. Lifelong Learn Neurol. 2019;25(3):655–669. doi: 10.1212/CON.0000000000000739. http://journals.lww.com/00132979-201906000-00007 [Internet]JunAvailable from: [DOI] [PubMed] [Google Scholar]
- Saeed B.Q., Al-Shahrabi R., Alhaj S.S., Alkokhardi Z.M., Adrees A.O. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int. J. Infect. Dis. 2021;111:219–226. doi: 10.1016/j.ijid.2021.08.013. https://linkinghub.elsevier.com/retrieve/pii/S1201971221006469 [Internet]OctAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnevetsky A., Levy M. Rethinking high-risk groups in COVID-19. Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102139. https://linkinghub.elsevier.com/retrieve/pii/S2211034820302157 [Internet]JulAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnands J.M., Kingwell E., Zhu F., Zhao Y., Fisk J.D., Evans C. Infection-related health care utilization among people with and without multiple sclerosis. Mult. Scler. J. 2017;23(11):1506–1516. doi: 10.1177/1352458516681198. http://journals.sagepub.com/doi/10.1177/1352458516681198 [Internet]Oct 21Available from: [DOI] [PubMed] [Google Scholar]
- Yamout B.I., Zakaria M., Inshasi J., Al-Jumah M., Zeineddine M., Dahdaleh M. MENACTRIMS practice guideline for COVID-19 vaccination in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2021;56 doi: 10.1016/j.msard.2021.103225. https://linkinghub.elsevier.com/retrieve/pii/S2211034821004922 [Internet]NovAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J. Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. https://linkinghub.elsevier.com/retrieve/pii/S0163445320302346 [Internet]AugAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrzavy T., Wimmer I., Rommer P.S., Berger T. Immunology of COVID-19 and disease-modifying therapies: the good, the bad and the unknown. Eur. J. Neurol. 2021;28(10):3503–3516. doi: 10.1111/ene.14578. https://onlinelibrary.wiley.com/doi/10.1111/ene.14578 [Internet]Oct 8Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]