Abstract
Background & Aims:
Randomized clinical trials have proven the efficacy and safety of Food and Drug Administration (FDA) approved anti-obesity medications (AOMs) for long-term use. It is unclear whether these outcomes can be replicated in real-world clinical practice where clinical complexities arise. The aim of this study was to evaluate the effectiveness and side effects of these medications in real-world multidisciplinary clinical practice settings.
Methods:
We reviewed the electronic medical records (EMR) of patients with obesity who were prescribed an FDA approved AOM for long-term use in academic and community multi-disciplinary weight loss programs between January 2016 and January 2020.
Intervention:
We assessed percentage total body weight loss (%TBWL), metabolic outcomes, and side effect profile up to 24 months after AOM initiation.
Results:
The full cohort consisted of 304 patients (76% women, 95.2% White, median age of 50 years old [IQR, 39–58]). The median follow-up time was 9.1 months [IQR, 4.2–14.1] with a median number of 3 visits [IQR, 2–4]. The most prescribed medication was phentermine/topiramate extended-release (ER) (51 %), followed by liraglutide (26.3 %), bupropion/naltrexone sustained-release (SR) (16.5 %), and lorcaserin (6.2 %). %TBWL was 5.0%, 6.8%, 9.3%, 10.3%, and 10.5% at 3, 6, 12, 18, and 24 months. 60.2% of the entire cohort achieved at least 5% TBWL. Overall, phentermine/topiramate-ER had the most robust weight loss response during follow up, with the highest %TBWL at 12 months of 12.0%. Adverse events were reported in 22.4% of patients. Only 9% of patients discontinued the medication due to side effects.
Conclusions:
AOMs resulted in significant long-term weight loss, that was comparable to outcomes previously reported in clinical trials.
Keywords: Obesity, pharmacotherapy, phentermine-topiramate-ER, lorcaserin, liraglutide, naltrexone-bupropion-SR
INTRODUCTION
Obesity has become a major public health problem. More than one-third of adults in the U.S. meet criteria for obesity, defined as a body mass index (BMI) ≥30 kg/m2.(1, 2) Obesity is a major risk factor for developing metabolic, mechanical, and mental health diseases, in addition to a myriad of cancers. Furthermore, obesity represents a socio-economic burden with an estimated annual medical cost of $480 billion.(2) A recent meta-analysis also demonstrated that the annual medical cost for people with obesity was 42% or $3,429 higher than the annual medical cost for people with a normal BMI.(3, 4)
While behavioral modification based on increased exercise and caloric restriction remains the cornerstone of weight management, the success rates and long-term durability of this intervention are inconsistent.(5, 6) Anti-obesity medications (AOMs) can improve adherence to a low-calorie diet by decreasing appetite, increasing satiation, enhancing satiety, and modulating hedonic regulation of food intake. Several obesity management guidelines recommend the use of AOMs as an adjunct to behavior modification in patients with a BMI ≥30 kg/m2 and in those with a BMI ≥27 kg/m2 with weight-related comorbidities.(6–8)
The Food and Drug Administration (FDA) has approved the following AOMs for long-term use: phentermine/topiramate-ER, bupropion/naltrexone-SR, liraglutide, and orlistat. Lorcaserin, a medication initially approved for long-term use, was withdrawn from the market in 2020 after its use was associated with an increased cancer occurrence.(9) Recently, the FDA approved the use of semaglutide for chronic weight management(10). Phentermine and other noradrenergic sympathomimetic drugs have been approved for short-term use only (<12 weeks). Among these, only phentermine has safety data, although retrospective, supporting its use for longer than 12 weeks.(11)
Multiple clinical trials have shown that the use of AOMs approved for long-term use leads to greater total body weight loss (TBWL) in the short- and long-term when compared to placebo, with TBWL ranging from 3.6 to 11%.(12–19) Furthermore, a systematic review and network meta-analysis showed that the use of orlistat, lorcaserin, bupropion/naltrexone-SR, phentermine/topiramate-ER, and liraglutide led to a greater proportion of patients achieving at least 5% and 10% TBWL compared with placebo.(20) The same study showed that of all FDA-approved AOMs, phentermine/topiramate-ER had the highest odds ratio (OR) of achieving clinically significant weight loss compared to placebo. As with most medications, all AOMs have been associated with adverse events.(20)
To date, most of the information on the efficacy of AOMs derives from FDA-guided randomized clinical trials (RCTs). Although, RCTs are considered the gold standard for testing the efficacy and the safety of medical interventions, their outcomes are not consistently replicated in the clinical practice as a result of their strict eligibility criteria, specific study regulations and relatively short duration.(21) For instance, many clinical trials exclude individuals with complex medical histories including psychiatric diseases, conditions that are highly prevalent in patients with obesity.(22, 23) Furthermore, it is challenging to replicate the interventions of RCTs in a pragmatic way, mostly because of the costly resources that are usually involved.
Real-world data offers the opportunity to evaluate the efficacy of AOMs in the general population. There have been a few studies to date reporting weight loss outcomes of AOMs approved for long-term use in a real-world clinical setting, most reporting no more than 12-month weight loss outcomes, some focusing on medication adherence.(24–32) No study to date has assessed the effect of all the currently AOMs approved for long-term use in a less controlled clinical setting. Therefore, the aim of this study was to assess weight loss outcomes and side effects in patients with obesity receiving any of the AOMs approved by the FDA for long-term use, as an adjunct to lifestyle recommendations in a multidisciplinary clinical practice setting.
METHODS
Design and Eligibility Criteria
This was a retrospective multi-site study. Three multidisciplinary weight management clinics were included. The Institutional Review Board of each site approved the study and waived the need for informed consent due to its minimal-risk nature. We included patients evaluated between January 1, 2016, and January 31, 2020. Patients were selected through integrated medical record query tools (ACE-Advanced Cohort Explorer- and i2b2-Informatics for Integrating Biology and the Bedside) based on the following inclusion criteria: 1) patients with a BMI ≥27 kg/m2 with weight-related comorbidities or patients with BMI ≥30 kg/m2 with or without weight-related comorbidities; 2) patients prescribed AOMs approved for long-term use by the FDA; 3) follow-up of at least 3 months; and, 4) two or more face-to-face visits with a weight management provider. We excluded all patients who: 1) had prior major gastrointestinal surgery; 2) had prior endoscopic weight loss intervention; 3) did not fill the medication prescription due to health insurance coverage denial and/or high drug cost that could not be afforded out-of-pocket, and 4) were taking FDA-approved AOMs prior to their first visit to the weight management program. All the information was collected from providers’ medical documentation and vital signs collected during their in-person clinic visits. All this information is part of the patients’ electronic medical records (EMR). Further information on the systematic approach to the EMR review process is provided in the supplementary document.
Weight Management Programs
The weight management programs of each institution practice obesity management based on the principles outlined in the multi-societal, multi-disciplinary Practice Guide on Obesity and Weight Management, Education, and Resources (POWER) program(6). The POWER program provides physicians with a comprehensive, multidisciplinary process to guide and personalize innovative obesity care for safe and effective weight management. This program incorporated previously published societies-approved guidelines for best care of patients with obesity.
-
Mayo Clinic Rochester and La Crosse Weight Management Programs
The Mayo Clinic Weight Management programs in Rochester and La Crosse involve a multi-disciplinary team that includes obesity medicine physicians, registered dietitians, advanced practice providers (Physician Assistants and Nurse Practitioners), and behavioral psychologists. Upon initial evaluation, patients are encouraged but not obligated to meet with a dietitian and the behavioral psychology team. All patients are encouraged but not obligated to participate in a standardized behavioral program. The general recommendations are to: 1) reduce dietary intake to 1200–1500 calories per day for women and 1500–1800 calories per day for men, 2) achieve a goal of 10,000 steps or more per day and 150 minutes or more of moderate intensity activity per week, and 3) limit the consumption of liquid calories (e.g., sodas, juices, alcohol). Calorie restriction and counseling on activity might vary widely based on weight-related comorbidities and functional capacity. Some patients are prescribed AOMs. All AOMs were included in the analysis if they were prescribed for weight loss, regardless of the dose achieved. Patients were encouraged to return for follow-up visits 4–6 weeks after starting the medication and every 3 months thereafter. During each visit, providers recorded information on body weight and weight-related comorbidities, gathered information on medication adherence based on patients’ report and pharmacy data on prescriptions filled, and side effects.
-
University of Iowa Hospitals and Clinics Weight Management Program
The University of Iowa weight management program consists of a multi-disciplinary team that assesses patients on a screening visit to ensure they are proper candidates for this kind of intervention. Patients are encouraged to meet with the dietitian team and are screened and referred to psychology/psychiatry services if they have a history suggestive of eating disorders or other warranted indications. The general recommendations are to: 1) reduce dietary caloric intake to 500 kcal less than their basal metabolic rate as measured by the Mifflin St. Jeor equation or indirect calorimetry, 2) achieve a physical activity goal of at least 150 minutes per week of moderate to high intensity exercise, and 3) limit the consumption of high calorie liquids. After patients are prescribed AOMs, at each follow-up visit, information on body weight, adherence to medications and recommendations, and side effects was collected.
Study Endpoints
The primary endpoint was the percentage total body weight loss %TBWL during the first 2 years of follow-up for patients prescribed AOMs approved for long-term use. Secondary endpoints included: 1) the proportion of patients who had a reduction from the baseline body weight of ≥5%, ≥10%, ≥15%, and ≥20%; 2) health status changes from baseline including: lipids [total cholesterol, low density lipoprotein (LDL) cholesterol, high- density lipoprotein (HDL) cholesterol and triglycerides], glycemic variables [fasting glucose and glycated hemoglobin (HbA1c)], and blood pressure (systolic and diastolic blood pressures); 3) weight regain after medication discontinuation at 3, 6 and 12 months from stop date.
Statistical analysis
All continuous data are summarized as medians and interquartile ranges (IQR). Categorical data are presented as frequencies and percentages. We used Pearson χ2 and Wilcoxon test for between-group comparisons for baseline categorical and continuous variables, respectively. We used paired t-test to compare %TBWL and all secondary endpoints at follow-up with their respective baseline values. Wilcoxon test was used to compare %TBWL between medications at each time-point. To estimate the contribution of biological and clinical variables on the %TBWL at each time point, we conducted a multiple regression analysis and summarized the results based on parameter estimates (PE) with 95% confidence intervals (95% CI) and significance values. We used logistic regression and summarized the results as OR and 95% CI to assess the impact of the same variables on >5%, >10%, >15%, and >20% TBWL. All P-values <0.05 were considered statistically significant.
RESULTS
Baseline Characteristics and Study Disposition
Between January 1, 2016, and January 31, 2020, a total of 433 patients were prescribed long-term FDA-approved AOMs. One-hundred and twenty-nine patients were excluded because they did not meet the inclusion criteria (prescription not filled by the patient, prescription coverage denied and patients were not able to afford the cost of the medication [n=107], and previous or concomitant endoscopic/surgical bariatric procedure [n=22]).
The evaluated study cohort consisted of 304 patients: 76% women, 99% White, median age 50 years [IQR, 39–58], median BMI 41.5 kg/m2 [IQR, 36.5–47.7]) (Table 1A). Most patients had obesity class III (61.2%). During the first 12 months, 99% of patients had at least 1 follow-up visit, and between months 13th and 24th, 45.4% of patients had at least 1 follow-up visit with an obesity medicine provider after the initial evaluation. The median time of follow-up for the entire cohort was 9.1 months (IQR, 4.2–14.1), with a median number of visits of 3 (IQR, 2–4). Fifty four percent of patients had at least one dietitian visit and 33.5% had at least one psychology visit. There was no significant difference in the length of follow-up, number of provider visits, and percentage of patients having at least one visit with a dietitian among the different medications. Patients taking naltrexone/bupropion combination were more likely to have a psychology visit compared to the rest of the cohort (Table 1C).
Table 1.
Baseline Demographic and Clinical Characteristics, and Follow Up Information of the Entire Cohort and by Anti-Obesity Medication
| All Patients | Phentermine/ Topiramate | Liraglutide | Lorcaserin | Bupropion/ Naltrexone | ANOVA P | |
|---|---|---|---|---|---|---|
| A. Baseline Demographic information | ||||||
| N, % | 304 | n=155 (51.0%) | n=80 (26.3%) | n=19 (6.2%) | n=50 (16.5%) | |
| Age, Years | 50(39–57.8) | 47 (39–56) | 54 (42–63) | 54 (49–63) | 47.5 (38–53) | 0.0009 |
| Sex, Female | 230 (75.7%) | 126 (81.2%) | 48 (60%) | 9 (47.4%) | 47 (94%) | <0.0001 |
| Race, White | 290 (99.0%) | 148 (99.3%) | 76 (97.4%) | 19 (100%) | 47 (100%) | 0.7 |
| B. Baseline Clinical Information | ||||||
| Weight, Kg | 118 (98.2–139.5) | 114.3 (97.5–139.3) | 119.5 (104.0–139.5) | 142.3 (104.4–156.8) | 113.6 (5.7–133.6) | 0.1 |
| BMI, kg/m2 | 41.5 (36.5–47.7) | 41.2 (34.8–47.7) | 42.2 (37.2–47.5) | 44.0 (37.5–50.7) | 41.5 (37.1–48.2) | 0.7 |
| BMI (Class I) | 62 (20.4%) | 39 (25.2%) | 12 (15.0%) | 1 (10.5%) | 9 (18.0%) | 0.5 |
| BMI (Class II) | 56 (18.4%) | 25 (16.1%) | 16 (20.0%) | 5 (26.3%) | 10 (20.0%) | |
| BMI (Class III) | 186 (61.2%) | 91 (58.7%) | 52 (65.0%) | 12 (63.1%) | 31 (62.0%) | |
| SBP, mmHg | 128 (121–137) | 128 (121–136) | 129 (122.8–138.5) | 131 (114–143) | 130 (121–136) | 0.9 |
| DBP, mmHg | 79 (70–86) | 81.5 (73.8–87.2) | 78 (69–85.5) | 89 (69–92) | 77 (73–83) | 0.27 |
| Glucose, mg/dL (n=149) | 105 (93–129) | 100 (92.3–113) | 131 (105–170) | 105 (90–135) | 97 (91–113) | <0.0001 |
| HbA1c, % (n=147) | 6 (5.4–7.2) | 5.5 (5.2–6) | 7.2 (6.2–8.4) | 5.5 (5.1–7.1) | 5.6 (5.1–6.1) | <0.0001 |
| LDL, mg/dL (n=155) | 97 (74–115) | 109 (90.8–123.3) | 81 (60–102) | 82.5 (73.8–95.8) | 97 (80–130) | <0.0001 |
| HDL, mg/dL (n=161) | 46 (39–54.5) | 47 (40.5–62) | 41 (35.5–49) | 34 (30–49) | 52 (44–60) | 0.001 |
| Total Cholesterol, mg/dL (n=161) | 170 (150.5–200.5) | 189 (169–208.8) | 160 (130–192) | 149 (132–160.5) | 175 (155.8–222) | 0.0001 |
| Triglycerides, mg/dL (n=163) | 142 (105–207) | 124 (95–177) | 168 (119.3–229) | 98 (81–257) | 157 (89–218) | 0.1 |
| Dyslipidemia | 161 (53.0%) | 78 (50.3%) | 57 (71.3%) | 6 (31.6%) | 20 (40.0%) | 0.004 |
| Obstructive sleep apnea | 108 (35.5%) | 52 (33.5%) | 36 (45.0%) | 6 (31.6%) | 14 (28.0%) | 0.2 |
| Hypertension | 153 (50.3%) | 63 (40.6%) | 55 (68.7%) | 12 (63.2%) | 21 (42.0%) | 0.0002 |
| Degenerative joint disease | 91 (29.9%) | 51 (32.9%) | 24 (30.0%) | 4 (21.0%) | 12 (24.0%) | 0.5 |
| Diabetes Mellitus | 100 (32.9%) | 22 (14.2%) | 62 (77.5%) | 5 (26.3%) | 11 (22.0%) | <0.0001 |
| GERD | 76 (25.0) | 39 (25.2%) | 20 (25.0%) | 5 (26.3%) | 12 (24.0%) | 0.9 |
| NAFLD | 39 (12.8%) | 17 (11.0%) | 14 (17.5%) | 4 (21%) | 4 (8.0%) | 0.2 |
| # Diabetes meds (n=100) | 1 (0–2) | 0 (0–0.1.5) | 1 (0–2) | 0 | 0 (0–1) | 0.06 |
| # Blood pressure meds (n=153) | 1 (0–1) | 0 (0–1) | 1 (0–2) | 0 (0–1.5) | 0 (0–1.5) | 0.004 |
| # Dyslipidemia meds (n=161) | 0 (0–1) | 0 (0) | 1 (0–1) | 0 | 0 (0–1) | 0.0004 |
| C. Follow up Information | ||||||
| Follow-up, Months | 9.1 (4.2–14.7) | 9.9 (4.2–14.1) | 10.4 (4.9–16.3) | 4.4 (3.3–14.2) | 7.6 (3.2–13.5) | 0.3 |
| Number of follow-up Visits | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.2 |
| Patients with >1 follow-up visit during year 1 with an obesity specialist | 301 (99.0%) | 153 (98.7%) | 80 (100%) | 19 (100%) | 49 (98.0%) | 0.6 |
| Patients with > 1 follow-up visit during year 2 with an obesity specialist | 138 (45.4%) | 70 (45.1%) | 40 (50.0%) | 7 (36.8%) | 21 (42.0%) | 0.6 |
| Patients with ≥ 1 dietitian visit | 163 (53.6%) | 73 (47.1%) | 47 (58.7%) | 12 (63.2%) | 31 (62.0%) | 0.1 |
| Patients with ≥ 1 psychology visit | 68 (33.5%) | 25 (23.1%) | 23 (42.6%) | 3 (33.3%) | 17 (53.1%) | 0.005 |
Continuous data are summarized as median and interquartile ranges. Categorical data are presented as frequencies and percentages.
All P-values <0.05 were considered significant.
Abbreviations used: BMI, body mass index; DBP, diastolic blood pressure; GERD, gastroesophageal reflux disease; HbA1c; glycated hemoglobin A1c; HDL, high density lipoprotein; LDL, low density lipoprotein; NAFLD, non-alcoholic fatty liver disease; SBP, systolic blood pressure.
Figure 1 summarizes the cohort’s follow-up. From our 304 patients included, 194 patients had medical information relevant to this study in their EMR for at least 24 months after AOMs were prescribed. From those, only 52, were on AOMs for the duration of the 24 months. The remaining were lost to follow-up (n= 76),discontinued the medication due to switching to another AOMs (n=15), side effects (n=26), achieving the desired weight loss (n=3), insurance renewal denial (n=12), having a bariatric endoscopic procedure (n=2), and having bariatric surgery (n=8).
Figure 1.
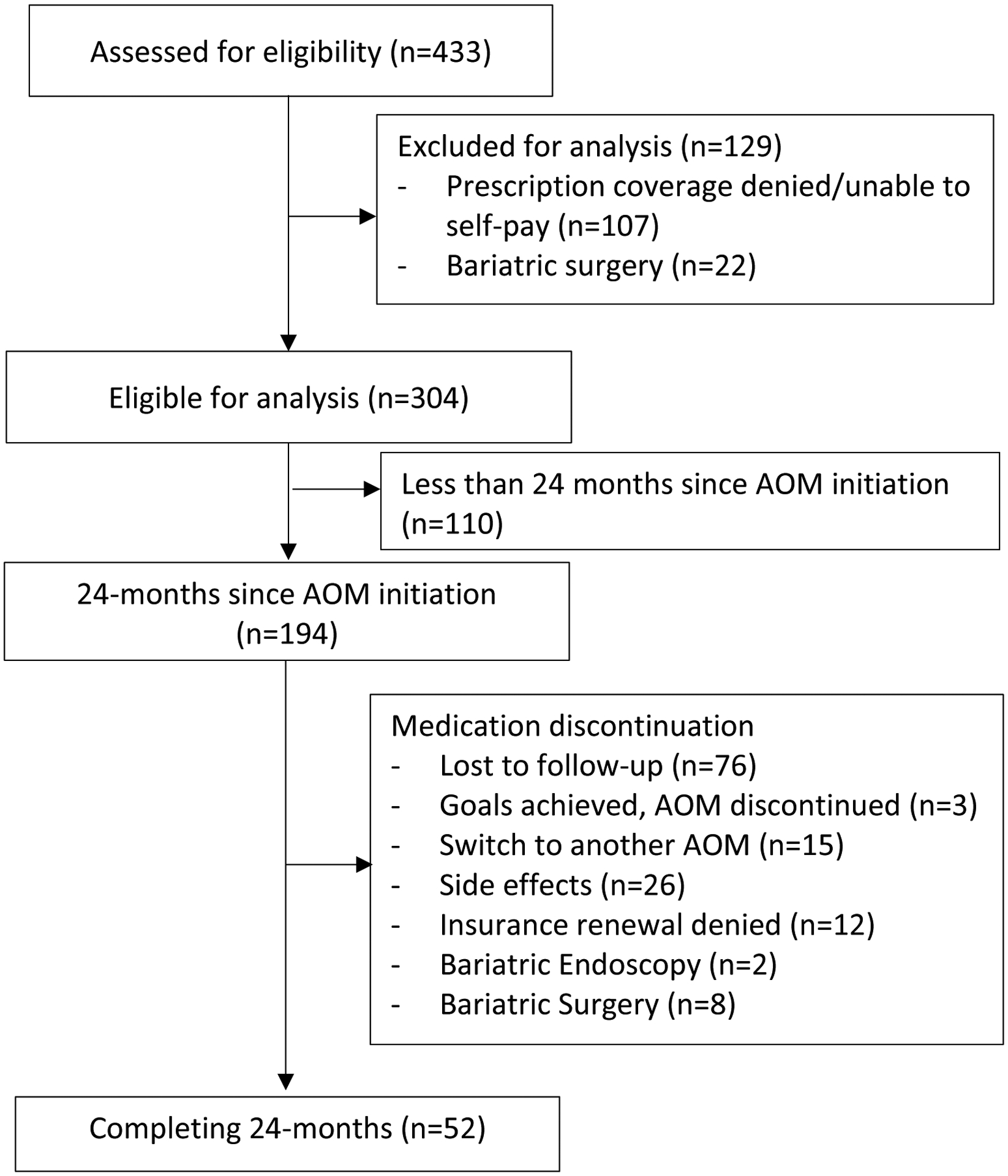
Follow-up flowchart.
Weight-related Comorbidities
The most common diagnosed weight-related comorbidities present at the initiation of pharmacotherapy were dyslipidemia (53.0%) and hypertension (50.3%), followed by obstructive sleep apnea (35.5%), type 2 diabetes mellitus (32.9%), degenerative joint disease (29.9%), gastroesophageal reflux disease (25.0%) and nonalcoholic fatty liver (12.8%). The median number of medications per patient for diabetes, hypertension, and dyslipidemia were 1 (IQR, 0–2), 1 (IQR, 0–1), and 0 (IQR, 0–1), respectively (Table 1B). Mean values of glucose, HbA1c, LDL, HDL, total cholesterol, and triglycerides at baseline are presented in Table 1B. Among the treatment groups, there was a significant difference in age, sex, and prevalence of hypertension, dyslipidemia, and diabetes. The prevalence of diabetes, dyslipidemia, and hypertension were higher in the liraglutide group compared to the other medication groups; diabetes: 77.5% vs. 15.5% (p<0.0001); dyslipidemia 71.3% vs 43.7%(p=0.003); hypertension 68.7% vs 42.8% (p=0.0002).
Prescribed Medications
Phentermine/topiramate-ER was the most prescribed AOM (51%), followed by liraglutide (26.3%), bupropion/naltrexone-SR (16.5%), and lorcaserin (6.2%). Phentermine/topiramate-ER was prescribed at 7.5–46 mg daily in 54% of patients, while 43% received 15–92 mg and 3% received 3.75–23 mg. A total of 80 patients were prescribed and approved liraglutide as a weight loss therapy. Full 3 mg daily dose, weight loss dose of liraglutide, was only achieved in 15% of patients with liraglutide. Lorcaserin was prescribed at 10 mg twice daily in 63.2% of patients. All patients taking bupropion/naltrexone received 16–180 mg twice daily.
Post-treatment Total Body Weight Loss
Among those who participated in follow-up visits at 3, 6, 12, 18, and 24 months, percentage total body weight losses were 5.0%, (95% CI [−4.2 to −5.7], n=138), 6.8% (95% CI [−6.1 to −7.5], n=213), 9.3% (95% CI [−8.2 to −10.5], n=146), 10.3% (95% CI [−8.8 to −11.7], n=121), and 10.5% (95% CI [−8.2 to −12.8], n=52), respectively (Figure 2A). Figure 2B–E shows %TBWL of each medication individually at 3, 6, 12, 18, and 24 months. Overall, phentermine/topiramate-ER had the most robust weight loss response during follow up, with the highest %TBWL at 12 months of 12.0% (Figure 2C). At 24 months, the highest %TBWL was achieved with naltrexone/bupropion-SW, with 12% TBWL (Figure 2D).
Figure 2.
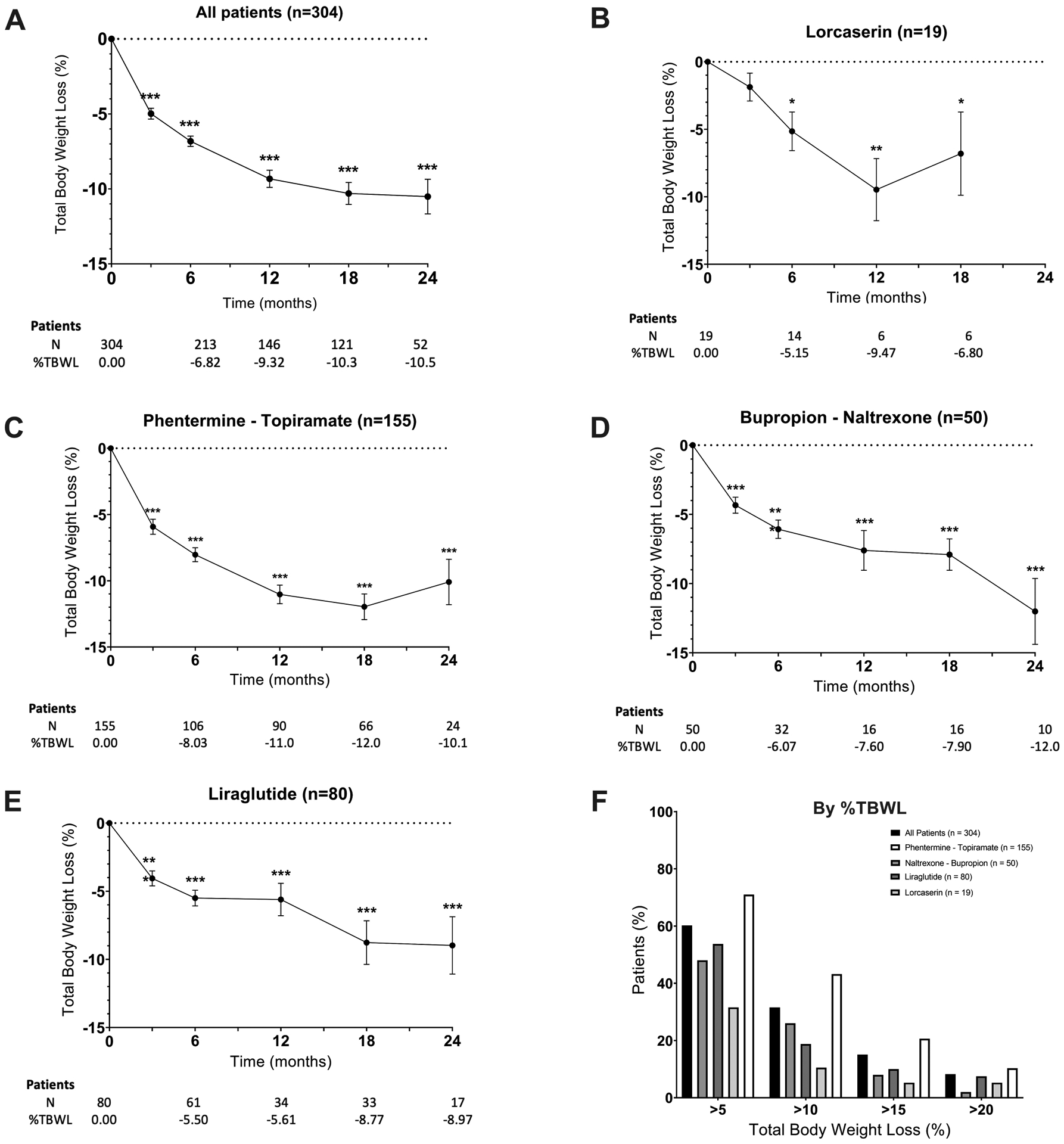
Weight loss outcomes after antiobesity medication initiation. Change in body weight from baseline (%TBWL) at each visit over the course of 24 months. A) All, b) Lorcaserin c) phentermine/topiramate-ER, d) Bupropion/naltrexone-SR, e) Liraglutide, and; f) Percentage of participants who at the last clinic visit had lost at least 5%, 10%, 15%, and 20% of their total body weight compared to baseline weight for all patients and by individual medication. Data presented in means+/−SE and as percentages. Paired-t test was used to make comparisons to baseline, *p<0.05, **p<0.005, ***p<0.0001
The percentage of participants who at the last clinic visit had a TBWL of at least 5%, 10%, 15%, and 20% was 60.2% (n=183/304), 31.6% (n=96/304), 15.1% (n=46/304) and 8.2% (n=25/304), respectively. The percentages of these responses for each drug are shown in Figure 2F. Patients on phentermine/topiramate-ER had higher rates of TBWL of at least 5%, 10%, 15%, and 20% compared to other medications.
Since most patients taking liraglutide received 1.8 mg daily, we performed a secondary analysis to compare 1.8 mg vs. 3 mg daily doses in the patients receiving this medication (n=80). The median time of follow-up was not different among both groups (10.5 vs. 11 months, p =0.1). There was no significant difference in weight loss with the 3 mg compared with the 1.8 mg dose at 3, 6, 12, or 18 months.
Percentage of Weight Regain after medication discontinuation
One-hundred and forty patients of our cohort had EMR weight information after discontinuing the AOM. The %TBWL in these patients was 4.6% [IQR (−9.8; −0.3)] at the time of medication discontinuation. The weight regain % from TBWL at 3, 6, and 12 months were 95.8% [IQR (91.7 – 100.5), n=83]; 97.2% [IQR (90.5 – 101.3), n=72]; and 96.3% [IQR (92.4 – 103.5), n=82]; respectively.
Multiple Regression and Logistic Regression Analyses
We used the following variables to perform multiple regression and logistic regression analyses: age, sex, BMI at baseline, and visits with a dietitian and a behavioral psychologist during follow-up. Multiple regression analysis showed that for %TBWL at 3 months, having at least one visit with a dietitian was the only variable associated with greater weight loss (PE [95% CI]: 1.6 [0.06–3.2], p=0.04). For %TBWL at 12 months, older age was the only variable associated with greater weight loss (PE [95% CI]: 0.18 [0.06–0.31]; p=0.001).
Logistic regression analysis showed that age was the only statistically significant predictor of attaining >15% and >20% TBWL at the last follow up (OR [95% CI]: 1.03 [1.01‐1.06], p=0.02 and OR 1.04 [1.01‐1.09], p=0.01). None of the variables studied was associated with attaining >5% or >10% TBWL at the last follow-up.
Secondary Endpoints
The results of secondary endpoints are summarized in Table 2. To study secondary endpoints, we used a paired t-test, as only a minority of patients had blood work at baseline and again while on treatment. Patients treated with AOMs approved for long-term use showed significant improvement in HbA1c compared to baseline (change HbA1C −1.1%, p=0.0003). This effect was mainly driven by patients with diabetes and treated with liraglutide (HbA1C decline from 7.2 to 6.3, p=0.004). There were clinically significant improvements in glucose, LDL, and total cholesterol, however, none of these observations were statistically significant.
Table 2.
Secondary endpoints at follow/up: Glucose Metabolism, Blood Pressure, and Cholesterol Parameters
| N | Baseline | Last Follow-up | Mean Difference (95% Confidence Interval) | P | |
|---|---|---|---|---|---|
| Liraglutide | (n=9) | 130.5 (105 – 188) | 135.5 (105.8 – 169) | −18.3 (−45.4 to −8.8) | 0.2 |
| Liraglutide | (n=31) | 7.2 (6.3 – 8.5) | 6.3(5.7 – 8.1) | −0.7 (−1. to −0.2) | 0.004 |
| SBP | (n=168) | 128 (120.7 – 137) | 125 (117 – 133.7) | −2.4 (−5.4 to 0.4) | 0.09 |
| DBP | (n=168) | 78 (71 – 85) | 78 (71 – 86) | −0.2 (−2.5 to 2.1) | 0.85 |
| LDL | (n=85) | 97 (74 – 115) | 90 (72 – 116.5) | 1.5 (−7.9 to 10.9) | 0.75 |
| HDL | (n=85) | 46 (39 – 54.5) | 47 (39 – 60) | 3.19 (−1.1 to 7.5) | 0.1 |
| Total Cholesterol | (n=85) | 170 (150.5 – 200.5) | 167 (146.5 – 198.5) | 1.6 (−9.3 to 12.4) | 0.7 |
| Triglycerides | (n=85) | 142.5 (105.3 – 207) | 140 (92.5 – 192.5) | −19.7 (−42.3 to 2.9) | 0.08 |
Continuous data are summarized as median and interquartile ranges.
All P-values <0.05 were considered significant.
Abbreviations used: DBP, diastolic blood pressure; HbA1c; glycated hemoglobin A1c; HDL, high density lipoprotein; LDL, low density lipoprotein; SBP, systolic blood pressure.
Adverse Events
Bupropion/naltrexone-SR was the medication with the highest number of documented adverse events (30.0%), followed closely by liraglutide (23.7%) and phentermine/topiramate ER (20.6%). Adverse events in all groups were most frequently gastrointestinal symptoms, with nausea being the most common. Treatment was suspended in 26 patients in the cohort (8.6%), at a median time of 2.6 months (IQR 1.9–3.5) after starting the medication, with the highest proportion coming from bupropion/naltrexone-SR users (9/50 or 18%). All documented adverse events are reported in Table 3.
Table 3.
Documented Adverse Events
| Incidence (n=304 per year) | Treatment Suspended | Adverse Events | |
|---|---|---|---|
| Total | 68 (22.4%) | 26 (8.6%) | Treatment was suspended in 9% of the subjects due to side effects. None were severe enough to prompt hospitalization. |
| Phentermine/ Topiramate-ER | 32/155 (20.6%) | 10 (6.4%) |
Insomnia (4.3%), paresthesia (3.6%). Dry mouth (2.9%), constipation (1.4%), Nausea (2.2%), Restlessness (1.4%), Anxiety (2.9), Others (3.6%) |
| Liraglutide | 19/80 (23.7%) | 4 (5.0%) |
Nausea (14.3%), Diarrhea (7.1%) Vomiting (4.3%), dyspepsia (2.9%) Constipation (1.4%), mood change (1.4%), others (7.1%) |
| Lorcaserin | 2/19 (10.5%) | 2 (10.5%) | Dizziness (10.5%) |
| Bupropion/Naltrexone-SR | 15/50 (30.0%) | 9 (18.0%) |
Nausea (18.8%) Mood changes (6.3%), Vomiting (2%), Constipation (4.1%). Other (10.4%) |
DISCUSSION
In this study, we showed that the use of AOMs approved for long-term use in a real-world multidisciplinary weight management practice promotes significant weight loss at 3, 6, 12, 18, and 24 months, with a maximum %TBWL of −10.5% at 24 months. When the medication was discontinued, we observe a weight regain of 96% of TBWL after 12 months. Phentermine/topiramate-ER was the most used medication and was associated with the highest %TBWL at 3, 6, 12, and 18 months (−6%, 8%, 11%, and 12%, respectively). There is a highly variable response to treatment: 60.2% of patients lost at least 5% of their total body weight, 31.6% lost at least 10%, 15.1% lost at least 15%, and 8.2% lost at least 20% at their last follow-up. Older age and visiting a dietitian were associated with greater weight loss and greater chances of achieving a higher %TBWL. However, the clinical significance of these findings requires further studies. No other demographic or clinical variables predicted weight loss outcomes. Adverse events were common with all medications, particularly with bupropion/naltrexone-SR. Lorcaserin had the lowest incidence of side effects. The rate of drug discontinuation due to adverse events was low, at 9%. About half of our patients had obesity with weight-related comorbidities, including type 2 diabetes, dyslipidemia, hypertension, and/or obstructive sleep apnea. We observed a significant improvement in diabetes and hypertension. Importantly, the rate of loss to follow-up is 25% in our cohort. Based on available information, the reasons for this high rate are unknown but some of the factors that may contribute include the elevated cost of AOMs and the lack of insurance coverage.
Weight loss outcomes in this study are comparable to those seen in large RCTs. The greatest weight loss with AOMs compared with placebo has been observed with once-weekly subcutaneous semaglutide 2.4mg where patients achieved 14.9% TBWL.(33) For other AOMs, TBWL varied from 6.5–8.1% with lorcaserin versus 9.5% in our study(13, 34); 6.7–9.2% with liraglutide versus 5.6% in our study(15, 16), 6.7–14.4% with phentermine/topiramate-ER versus 11% in our study(12, 19), and 6.7–11.5% with bupropion/naltrexone-SR versus 7.6% in our study(14, 35, 36). Real-world studies to date have assessed weight loss outcomes of liraglutide 3 mg daily and orlistat.(25–28) Compared to these studies, %TBWL and the percentage of patients achieving >5%, >10%, and >15% TBWL with liraglutide in our cohort were slightly lower. Although we included patients prescribed liraglutide for weight loss, not all these patients were able to get up to 3.0mg daily due to the lack of insurance coverage or development of side effects. This may explain the difference in weight loss outcome compared to these other studies. Our study did not include patients on Orlistat; however, it is the first study to compare weight loss outcomes among several weight loss medications approved for long-term use in a real-world setting multidisciplinary clinical practice. Similar to weight loss, the improvement in metabolic outcomes paralleled what has been reported in large RCTs and other real-world studies.(13–15, 19, 25–28, 34–37) It is important to note that our weight management programs follow standard dietetic and behavioral therapies recommended by current obesity management guidelines.(6, 7) In our practices, we aim to incorporate a multi-disciplinary team approach by integrating clinical dietetics and bariatric psychology to mimic what is done in RCTs. Although patients are encouraged to meet with these teams, only 50% of patients met with a dietitian and only a third had a psychologic evaluation. This suggests that AOMs can be effective in less structured weight loss programs as long as obesity medicine healthcare providers spend adequate time counseling patients about dietary and behavioral changes before prescribing these drugs.
Another important contribution of this study is that it assesses the incidence of side effects of the approved AOMs in clinical practice. We found that these drugs had a higher frequency of side effects compared to clinical trials. The most common adverse events were gastrointestinal. Phentermine/topiramate-ER use was the most likely to achieve ≥5% TBWL (76.3%) but also carried a high probability of adverse events (20.6%). Conversely, lorcaserin was associated with a lower rate of side effects but was the least effective for weight loss, with only 36.8% achieving ≥5% TBWL. These findings are comparable to the network meta-analysis by Khera et al.(20) where the combination of phentermine/topiramate-ER was the most effective for weight loss but was the third most likely to be associated with side effects (after liraglutide and bupropion/naltrexone-SR) and where lorcaserin had the least documented adverse events.
Finally, we found that 12 months after discontinuing the medication, patients had regained up to 95% of the TBWL. This emphasizes the need for longer-term treatment, which is often limited by changes in drug availability (e.g., lorcaserin no longer available), insurance coverage, and long-term safety data. Long-term safety has been evaluated in clinical trials for liraglutide, phentermine/topiramate, and naltrexone/bupropion, and in real-world observational studies for phentermine and phentermine/topiramate.(38, 39)
This study has several limitations. First, by definition, this study included only patients who were prescribed AOMs for at least 3 months. In the United States, the cost of weight loss medications is high, and if not covered by insurance companies, their cost can be prohibitive. In our practices, 24% of the prescriptions written for AOMs approved for long-term use were denied by insurance companies and not filled by patients. Furthermore, most insurance companies require that these prescriptions go through a preauthorization process, an additional bureaucratic barrier that leads to reluctance among physicians to prescribe these medications. For this reason, providers often opt to prescribe off-label AOMs such as metformin, topiramate or bupropion either alone or in combination with phentermine, currently approved for short-term use only. As a result, although we demonstrate that AOMs approved by the FDA for long-term use are effective and overall safe in clinical practice, access to these pharmacologic interventions may negatively impact the role of pharmacologic weight management interventions in the real-world setting.
Second, this was a retrospective study with no control group as in RCTs. However, our results mirror what has been reported in other studies around the world, suggesting that AOMs do indeed lead to clinically relevant weight loss in clinical practice. Importantly, obesity-directed pharmacotherapy should be evaluated in a prospective pragmatic controlled trial where control patients are exposed to the same overall management minus AOMs. Larger sample sizes with longer term follow-up are also needed to better understand the outcomes of AOMs in clinical practice.
Third, AOMs were prescribed based on the prescriber’s perception that a specific drug may prove more beneficial than others for each patient. In other words, the selection of a specific AOM was not random. This is demonstrated by the fact that patients with hyperglycemia or established diabetes were predominantly prescribed liraglutide. Therefore, the results do not represent an unbiased sample, but they presumably reflect the prescriber’s perception of the best suited medication for a specific patient.
Fourth, given the nature of the study, results are dependent on EMR documentation. Because EMR documentation is imperfect and varies from provider to provider, there is a risk of misreporting, particularly relevant when it comes to side effects.
Fifth, eating behaviors were not assessed. It is unknown if loss of control over eating, or binge eating episodes had an impact on outcomes.
Lastly, it is conceivable that the non-pharmacological components of our multidisciplinary weight loss programs (i.e. dietetic and behavioral modification counseling) could have impacted the results. However, as demonstrated in univariate analyses, these two factors in our study did not affect weight loss outcomes. This is particularly important as visits with a dietitian and behavioral psychologist are encouraged but not mandatory.
CONCLUSION
Our findings demonstrate that AOM approved by the FDA for long-term treatment of obesity can result in significant long-term weight loss over the course of two years in the clinical practice. These outcomes are comparable to the ones reported in randomized clinical trials. Providers prescribing AOMs should monitor for side effects carefully, given their high incidence. In the future, focused research efforts should be made to enhance efficacy and, possibly, tolerance of AOMs.
Supplementary Material
Table 4.
Weight Loss Outcomes by Anti-obesity Medication in the Current Study at 1 year Compared to RCTs Outcomes
| Anti-obesity Medication | Total Body Weight Loss | Reference |
|---|---|---|
| All Drugs | 9.3% | Current study |
| Lorcaserin | 9.5% | Current study |
| 6.5–7.9% | Fidler et al.18 | |
| 8.1% | Smith et al.11 | |
| Phentermine/Topiramate-ER | 11% | Current study |
| 6.7–14.4% | Allison et al.19 | |
| 9.6–12.4% | Gadde et al.10 | |
| Liraglutide | 5.6% | Current study |
| 6.7% | Wadden et al.21 | |
| 9.2% | Pi-Sunyer et al.13 | |
| Bupropion/Naltrexone-SR | 7.6% | Current study |
| 6.7–8.1% | Greenway et al.12 | |
| 11.5% | Wadden et al.20 | |
| 8.2% | Apovian et al 22 |
Acknowledgements:
We would like to thank Dr. Michael Jensen for his critical feedback on the reporting of the findings and the discussion.
Competing Interests
Dr. Mundi has research grants from Fresenius Kabi, Nestle, Realfood Blends, and VectivBio and is a consultant for Baxter.
Dr. Acosta reports other from Gila Therapeutics, other from Phenomix Sciences, personal fees from Rhythm Pharmaceuticals, personal fees from General Mills, outside the submitted work.
Dr. Abu Dayyeh has served as a consultant for Boston Scientific, Metamodix, BFKW, DyaMx, and USGI Medical, has received research support for Boston Scientific, Apollo Endosurgery, USGI, Spatz Medical, GI Dynamics, Caim Diagnostics, Aspire Bariatrics, and Medtronic, and has been a speaker for Johnson & Johnson, Endogastric Solutions, and Olympus.
Dr. Camilleri is a stockholder in Phenomix Sciences and Enterin and serves as a consultant to Takeda, Allergan, Rhythm, Kallyope, and Arena with compensation to his employer, Mayo Clinic.
Other authors declare no conflict of interest.
Grant Support:
No external funding for this manuscript.
Abbreviations:
- AOMs
anti-obesity medications
- BMI
body mass index
- FDA
Food and Drug Administration
- HDL
high- density lipoprotein
- IQR
interquartile ranges
- ITT
intention-to-treat
- LDL
low density lipoprotein
- POWER
Practice Guide on Obesity and Weight Management, Education and Resources
- RCTs
randomized clinical trials
- TBWL
total body weight loss
- LOCF
last observation carried forward
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS data brief. 2015(219):1–8. [PubMed] [Google Scholar]
- 2.Waters H, Graf M. America’s Obesity Crisis. Milken Institute; 2018. [Google Scholar]
- 3.Su W, Huang J, Chen F, Iacobucci W, Mocarski M, Dall TM, et al. Modeling the clinical and economic implications of obesity using microsimulation. Journal of Medical Economics. 2015;18(11):886–97. [DOI] [PubMed] [Google Scholar]
- 4.Kim DD, Basu A. Estimating the Medical Care Costs of Obesity in the United States: Systematic Review, Meta-Analysis, and Empirical Analysis. Value in Health. 2016;19(5):602–13. [DOI] [PubMed] [Google Scholar]
- 5.Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med. 2017;376(15):1492. [DOI] [PubMed] [Google Scholar]
- 6.Acosta A, Streett S, Kroh MD, Cheskin LJ, Saunders KH, Kurian M, et al. White Paper AGA: POWER - Practice Guide on Obesity and Weight Management, Education, and Resources. Clin Gastroenterol Hepatol. 2017;15(5):631–49 e10. [DOI] [PubMed] [Google Scholar]
- 7.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American College of Cardiology. 2014;63(25, Part B):2985–3023. [DOI] [PubMed] [Google Scholar]
- 8.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–62. [DOI] [PubMed] [Google Scholar]
- 9.Sharretts J, Galescu O, Gomatam S, Andraca-Carrera E, Hampp C, Yanoff L. Cancer Risk Associated with Lorcaserin - The FDA’s Review of the CAMELLIA-TIMI 61 Trial. N Engl J Med. 2020;383(11):1000–2. [DOI] [PubMed] [Google Scholar]
- 10.CllinicalTrials.gov. A Research Study of NNC0165-1562 and Semaglutide in People Who Are Overweight or Obese 2020. [cited 2020 12/22/2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT03574584.
- 11.Lewis KH, Fischer H, Ard J, Barton L, Bessesen DH, Daley MF, et al. Safety and Effectiveness of Longer-Term Phentermine Use: Clinical Outcomes from an Electronic Health Record Cohort. Obesity. 2019;27(4):591–602. [DOI] [PubMed] [Google Scholar]
- 12.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–52. [DOI] [PubMed] [Google Scholar]
- 13.Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245–56. [DOI] [PubMed] [Google Scholar]
- 14.Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. [DOI] [PubMed] [Google Scholar]
- 15.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373(1):11–22. [DOI] [PubMed] [Google Scholar]
- 16.Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37(11):1443–51. [DOI] [PubMed] [Google Scholar]
- 17.Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36(6):843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. Jama. 2016;315(22):2424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey TA, Stiles WB. Some Problems with Randomized Controlled Trials and Some Viable Alternatives. Clinical Psychology & Psychotherapy. 2016;23(1):87–95. [DOI] [PubMed] [Google Scholar]
- 22.Grudell AB, Sweetser S, Camilleri M, Eckert DJ, Vazquez-Roque MI, Carlson PJ, et al. A controlled pharmacogenetic trial of sibutramine on weight loss and body composition in obese or overweight adults. Gastroenterology. 2008;135(4):1142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holgerson AA, Clark MM, Ames GE, Collazo-Clavell ML, Kellogg TA, Graszer KM, et al. Association of Adverse Childhood Experiences and Food Addiction to Bariatric Surgery Completion and Weight Loss Outcome. Obes Surg. 2018;28(11):3386–92. [DOI] [PubMed] [Google Scholar]
- 24.Gorgojo-Martínez JJ, Basagoiti-Carreño B, Sanz-Velasco A, Serrano-Moreno C, Almodóvar-Ruiz F. Effectiveness and tolerability of orlistat and liraglutide in patients with obesity in a real-world setting: The XENSOR Study. Int J Clin Pract. 2019;73(11):e13399. [DOI] [PubMed] [Google Scholar]
- 25.Park JH, Kim JY, Choi JH, Park HS, Shin H-Y, Lee JM, et al. Effectiveness of liraglutide 3 mg for the treatment of obesity in a real-world setting without intensive lifestyle intervention. International Journal of Obesity. 2021. [DOI] [PubMed] [Google Scholar]
- 26.Piantanida E, Gallo D, Tanda ML. Liraglutide is an effective drug for the treatment of obesity also in real life. Journal of Endocrinological Investigation. 2020;43(12):1827–8. [DOI] [PubMed] [Google Scholar]
- 27.Wharton S, Liu A, Pakseresht A, Nørtoft E, Haase CL, Mancini J, et al. Real-World Clinical Effectiveness of Liraglutide 3.0 mg for Weight Management in Canada. Obesity. 2019;27(6):917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrari F, Fierabracci P, Salvetti G, Jaccheri R, Vitti J, Scartabelli G, et al. Weight loss effect of liraglutide in real-life: the experience of a single Italian obesity center. Journal of Endocrinological Investigation. 2020;43(12):1779–85. [DOI] [PubMed] [Google Scholar]
- 29.Fakhreddine AY, Bagsic S, Fujioka K, Frenette CT. Safety and efficacy of pharmacologic weight loss in patients with cirrhosis. Obesity Science & Practice. 2021;7(2):159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganguly R, Tian Y, Kong SX, Hersloev M, Hobbs T, Smolarz BG, et al. Persistence of newer anti-obesity medications in a real-world setting. Diabetes Research and Clinical Practice. 2018;143:348–56. [DOI] [PubMed] [Google Scholar]
- 31.Shibuya K, Ali KF, Ji X, Milinoivh A, Bauman J, Kattan MW, et al. The Benefit of Short-Term Weight Loss with Anti-Obesity Medications in Real-World Clinical Practice. Endocrine Practice. 2019;25(10):1022–8. [DOI] [PubMed] [Google Scholar]
- 32.Elangovan A, Shah R, Smith ZL. Pharmacotherapy for Obesity—Trends Using a Population Level National Database. Obesity Surgery. 2021;31(3):1105–12. [DOI] [PubMed] [Google Scholar]
- 33.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine. 2021;384(11):989–1002. [DOI] [PubMed] [Google Scholar]
- 34.Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96(10):3067–77. [DOI] [PubMed] [Google Scholar]
- 35.Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O’Neil PM, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19(1):110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21(5):935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37(11):1443–51. [DOI] [PubMed] [Google Scholar]
- 38.Lei XG, Ruan JQ, Lai C, Sun Z, Yang X. Efficacy and Safety of Phentermine/Topiramate in Adults with Overweight or Obesity: A Systematic Review and Meta-Analysis. Obesity (Silver Spring). 2021;29(6):985–94. [DOI] [PubMed] [Google Scholar]
- 39.Lewis KH, Fischer H, Ard J, Barton L, Bessesen DH, Daley MF, et al. Safety and Effectiveness of Longer-Term Phentermine Use: Clinical Outcomes from an Electronic Health Record Cohort. Obesity (Silver Spring). 2019;27(4):591–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


