Abstract
Background/Objectives:
Lipopolysaccharide-binding protein (LBP), a biomarker of gut barrier permeability to lipopolysaccharides, is higher in adults with obesity and type 2 diabetes. Behavioral weight loss and metformin have distinct effects on the gut microbiome, but their impact on gut permeability to lipopolysaccharides is unknown. This study’s objective was to determine the effects of a behavioral weight loss intervention or metformin treatment on plasma LBP.
Subjects/Methods:
SPIRIT was a randomized trial of adults with overweight or obesity. Participants were randomized to one of three arms: metformin treatment, coach-directed behavioral weight loss on a DASH diet, or self-directed care (control). Of 121 participants, a random subset (n=88) was selected to have LBP measured at baseline, 6-months, and 12-months post intervention. Intervention effects on LBP over time were assessed using generalized estimating equations (GEE). We also examined whether the intervention effects were modified by change in diet and weight.
Results:
Arms were balanced by sex (83% female), race (51% white), and age (mean 60 years), with no differences in baseline LBP (median 4.23 μg/mL). At 1 year, mean weight change was −3.00% in the metformin arm, −3.02% in the coach-directed behavioral weight-loss arm, and +0.33% in the self-directed (control) arm. The corresponding change in LBP was +1.03, −0.98, +1.03 μg/mL. The behavioral weight-loss reduced LBP compared to self-directed care (β=−0.17, 95% CI: −0.33 to −0.01); no other between-arm comparisons were significant. Participants who reduced dietary fat showed the greatest reductions in 6-month LBP (β=−2.84, 95% CI: −5.17 to −0.50).
Conclusions:
Despite similar weight loss in the behavioral weight loss and metformin arms, only the behavioral weight loss intervention reduced LBP compared to control. Lifestyle weight loss interventions that promote a DASH diet may be effective at reducing gut barrier permeability to lipopolysaccharides.
Keywords: Metformin, Lifestyle, Intervention, Gut Permeability, Obesity
INTRODUCTION
Lipopolysaccharides (LPS) are a component of gram-negative bacterial cell walls that, when released from the bacteria, initiate a robust immune response.(1) Low levels of LPS originating from intestinal bacteria can translocate into the circulation in healthy individuals,(1) and it is thought that situations which increase circulating LPS levels may contribute to the chronic low-grade inflammation seen in obesity and cardiometabolic disorders.(2) LPS-binding protein (LBP), an acute-phase protein constitutively produced by the liver,(3) plays a pivotal role in the initial binding of LPS and the transfer to and from its innate immune receptor, toll-like receptor 4.(4) Difficulties exist in the accurate measurement of LPS in blood;(5) thus, LBP is used as a surrogate marker of circulating LPS activity.(6, 7)
Individuals with obesity have impaired intestinal barriers (8–12), and weight loss in individuals with obesity can improve markers of intestinal barrier permeability.(8, 13–17) Additionally, metformin, which enhances insulin sensitivity and also leads to weight loss,(18) was shown to reduce both LPS and LBP in mice fed a normal-chow diet.(8) Together, these findings suggest that different methods of weight loss may similarly lead to reduced LBP. However, weight lost through behavioral intervention or metformin treatment—current first-line treatments for obesity and diabetes, respectively—operate through different physiological mechanisms.(19) While a behavioral weight loss intervention has been shown to reduce LBP in adolescents with abdominal obesity,(20) there have been no direct comparison between the effects of behavioral weight loss interventions and metformin on LBP in humans.
In this trial, we compared the effects of a behavioral weight loss intervention, a metformin treatment intervention, and a self-directed control group on one-year changes in LBP. We hypothesized that either intervention arm reduces LBP when compared to the self-directed control arm. We further hypothesized that changes in diet and weight modify these associations.
METHODS
Study population
The Survivorship Promotion In Reducing IGF-1 Trial (SPIRIT) was a three-arm randomized trial that enrolled adult cancer survivors with overweight or obesity, with a primary endpoint of 6-month reduction in insulin-like growth factor. The full study protocol with more detailed information can be found at ClinicalTrials.gov (identifier: NCT02431676), and primary study results have been published (21). Study recruitment occurred between August 2015 and December 2016 at the Johns Hopkins ProHealth clinical research unit, Baltimore, MD. Eligibility criteria for the SPIRIT trial included: age ≥ 18 years; previous diagnosis of a solid malignant tumor, and an anticipated treatment-free lifespan of at least 1 year; a BMI ≥ 25 and weight ≤ 400 pounds; and a willingness to change diet, physical activity, and weight. Exclusion criteria included: medication-treated diabetes, fasting blood glucose ≥ 200 mg/dL, or both a fasting blood glucose ≥ 126 mg/dL and HbA1c ≥ 7%; current or prior use of metformin in the past 3 months; chemotherapy or radiation 3 months prior to the intervention start date; use of weight loss medications in the past 6 months; prior or planned bariatric surgery; and an estimated glomerular filtration rate (eGFR) < 45 mL/min. The study protocol was approved by the Institutional Review Board at Johns Hopkins University. All participants provided written informed consent.
Treatment groups
Participants were randomized 1:1:1 to a coach-directed behavioral weight loss arm, a metformin treatment arm, or a self-directed control arm, using a race and BMI-stratified randomization with variable block sizes. Randomization was performed by a biostatistician, and treatment group assignment was performed by a staff member uninvolved with follow-up data collection. Study staff and lab staff involved in follow-up data collection were blinded to randomization assignment.
The coach-directed intervention arm was a remotely delivered, behavioral intervention based on the previously published POWER trial;(22) this intervention promoted increased physical activity, reduced caloric intake, and a Dietary Approaches to Stopping Hypertension (DASH)-style diet. Participants were coached remotely via telephone calls which decreased in frequency from weekly (first three months) to monthly (remainder of intervention), where they were encouraged to set goals and self-monitor progress in achieving and maintaining weight loss. Participants were also provided access to a study website for self-monitoring and learning modules. The target weight loss for coach-directed intervention participants was 5% of baseline body weight at 6 months, with maintenance of this weight loss at 12 months. Individuals in the metformin arm were provided oral metformin which was titrated up to 2000 mg/day, depending on medication tolerance. Individuals in the self-directed arm met with study staff once at the beginning of the trial at which time they received written information on weight loss.
Of the 121 participants initially randomized in SPIRIT, complete baseline, 6-month, and 12-month laboratory measures were available for 115 participants (N=40/40 for self-directed control arm, N=38/39 for coach-directed behavioral weight loss arm, and N=37/42 for metformin arm). Of those with available measures at all three timepoints, a random sample of 88 individuals had LBP measured in plasma samples collected at baseline, 6-months, and 12-months post baseline. A participant flow diagram can be found in the supplementary materials (Supplementary Figure 1). There were no baseline differences between participants with and without LBP measures (Supplementary Table 1).
Primary outcome: lipopolysaccharide-binding protein (LBP)
We measured concentrations of plasma LBP in duplicate in samples diluted 1:100, using an ELISA kit (Biomatik, Cambridge, Ontario, Canada) per the manufacturer’s instructions. Assays were performed at the Integrated Physiology Ligand Assay and Biomarker Subcore of the Johns Hopkins University-University of Maryland Diabetes Research Center. Final concentrations were determined with reference to a standard curve.
Diet and other variables
Participants filled out a fruit and vegetable screening questionnaire (National Cancer Institute Eating at America’s Table Study Quick Food Scan) and a dietary fat screening questionnaire (National Cancer Institute Percentage Energy from Fat Screener). Fiber (in grams) was estimated using a regression model based on self-reported fruit/vegetable/legumes intake, age, and sex. Percentage energy intake from total dietary fat was estimated using the publicly available National Cancer Institute’s Percentage Energy from Fat Screener, a 16-item questionnaire which asks about the past-year usual consumption of foods that are associated with variability in the percentage energy from fat. Individual predicted percentage energy from fat was estimated using a regression equation with gender-specific coefficients. A log2 transformation was applied to the ratio of percentage energy from fat sources to the percentage energy from non-fat sources.
Age, sex, and race/ethnicity were self-reported. Weight was measured each visit using a high-quality, calibrated digital scale, without shoes. Height was measured at the study entry, and BMI was calculated from body weight and standing height (kg/m2). Interleukin 6 (IL-6) was measured using an ELISA (Quantikine; R&D Systems, Minneapolis, Minnesota). High-sensitivity C-reactive protein (hsCRP) was measured using Dimension Vista® System (Siemens Healthcare Diagnostics Inc. Newark, DE).
Statistical analyses
All analyses were performed by assigned treatment groups, and using all N=88 participants with measured LBP (unless otherwise stated). Baseline between-group differences were compared using a one-way ANOVA test or Kruskal-Wallis test for continuous variables or a chi squared test for categorical variables. We assessed baseline correlations using Spearman correlations. We compared changes in LBP, dietary variables, and weight between groups using a one-way ANOVA and Tukey post-hoc test or Kruskal-Wallis test as appropriate. The effects of interventions on LBP over 12 months were assessed using generalized estimating equations (GEE) with an exchangeable correlation structure and model-based standard errors. We tested unstructured, auto-regressive, and exchangeable correlation structures and found exchangeable to be preferred based on the lowest quasi-likelihood information criteria (QIC). Terms for time (continuous), for both intervention arms (with self-directed as the reference group), and time-by-intervention arm interactions were included. The time-by-intervention arm interaction slopes were used to determine the between-group differences in LBP over 12 months.
We examined whether the effects of the interventions on LBP were modified by change in weight or change in diet at 6 months, as this timepoint was prespecified for the primary analyses of the parent trial. We regressed the 6-month change in LBP on the coach-directed vs. self-directed indicator (N=60), or on the metformin vs. self-directed indicator (N=59); these models were adjusted for changes in fiber, percent energy from fat, or weight, and additionally adjusted for an interaction term between the intervention arm indicator and change variable. One individual in the metformin group had missing data on dietary fat intake at 6-month; for analyses using change in percent of energy from dietary fat, complete case analysis (N=58) was performed. As interactions were identified for changes in percent energy from dietary fat (p < 0.10), samples were stratified by decreased vs. increased % energy intake from fat, and we regressed the 6-month change in LBP on the intervention vs. self-directed arm within each fat change stratum.
To investigate associations of factors related to LBP in the coach-directed arm, we assessed longitudinal correlations using a mixed model approach from the SAS macro MMCorr_NormalApprox.(23) IL-6 and hsCRP were log-transformed prior to correlation analysis as distributions were not normal. To further investigate metformin-specific treatment effects, additional analyses restricted to the metformin arm looked at medication by dosage, and stratified by metformin compliance at 6 months. All 6-month change analyses were performed similarly for the corresponding 12-month changes, but as findings were consistent at 12 months, only the 6-month changes are presented.
Statistical significance was based on α = 0.05, and analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, North Carolina). Graphs were generated using ggplot2(24) in R version 4.0.2.(25)
RESULTS
Baseline characteristics and changes in characteristics over time
Table 1 displays baseline characteristics, which were similar across the three randomized arms. Baseline correlations with LBP are presented in Supplementary Table 2; fiber intake was inversely associated with LBP (r = −0.28, p = 0.0078), though no other baseline characteristics were.
Table 1:
Baseline Characteristics by Study Arm
| Characteristic | Overall (N=88) | Self-Directed (N=31) | Metformin (N=28) | Coach-Directed (N=29) |
|---|---|---|---|---|
| Age (y) | 60.1 (9.0) | 59.1 (9.3) | 60.0 (8.8) | 61.4 (9.0) |
| Sex (female) | 73 (83.0 %) | 26 (83.9%) | 24 (85.7%) | 23 (79.3%) |
| Race (White) | 45 (51.1%) | 17 (54.8%) | 14 (50.0%) | 14 (48.3%) |
| BMI (kg/m2) | 34.4 (5.3) | 34.3 (5.1) | 34.9 (6.5) | 34.2 (4.1) |
| Weight (kg) | 94.1 (16.7) | 94.7 (15.7) | 97.1 (21.4) | 90.6 (12.1) |
| Time from last cancer treatment (y) * | 6.0 (3.0, 13.0) | 5.5 (3.0, 10.0) | 5.0 (2.5, 15.5) | 6.0 (2.5, 11.5) |
| Diet | ||||
| Fiber (g/day) | 10.6 (4.8) | 11.5 (4.2) | 10.5 (5.1) | 9.8 (5.0) |
| Energy from Fat (%) | 33.1 (3.4) | 33.4 (3.1) | 32.8 (3.5) | 33.1 (3.7) |
| Biomarkers | ||||
| LBP (μg/mL) | 4.2 (3.1, 6.4) | 4.0 (2.8, 6.0) | 4.2 (3.1, 5.8) | 5.9 (3.6, 7.2) |
| hsCRP (mg/dL) | 3.7 (2.0, 6.4) | 4.2 (1.9, 7.0) | 3.4 (1.9, 7.1) | 3.8 (2.5, 5.4) |
| IL-6 (pg/mL) | 3.5 (2.3, 5.0) | 4.1 (2.5, 5.5) | 3.2 (1.9, 3.9) | 3.5 (2.4, 5.0) |
Data presented as mean (SD), median (IQR), or N(%)
N = 86 (missing 1 each in self-directed and coach-directed arms)
Within-group changes in weight, diet, and LBP between baseline and 6- or 12-month follow-ups are displayed in Supplementary Table 3. Both the metformin and coach-directed arms showed similar and significant 12-month reductions in weight (~3% from baseline) compared to no weight loss in the self-directed control arm. Overall changes in diet, inflammatory markers, and LBP were not statistically significantly different between treatment groups.
Between-group differences in LBP slope over 12-months
The effects of intervention arm on LBP were assessed using GEE (Figure 1). Compared to the self-directed control arm, the coach-directed behavioral weight loss intervention significantly reduced LBP over time (β = −0.17, 95%CI: −0.33 to −0.01). Metformin treatment did not affect LBP compared to the self-directed arm (β = 0.0003, 95%CI: −0.16 to 0.16).
Figure 1: Change in LBP distribution over time, by trial arm.
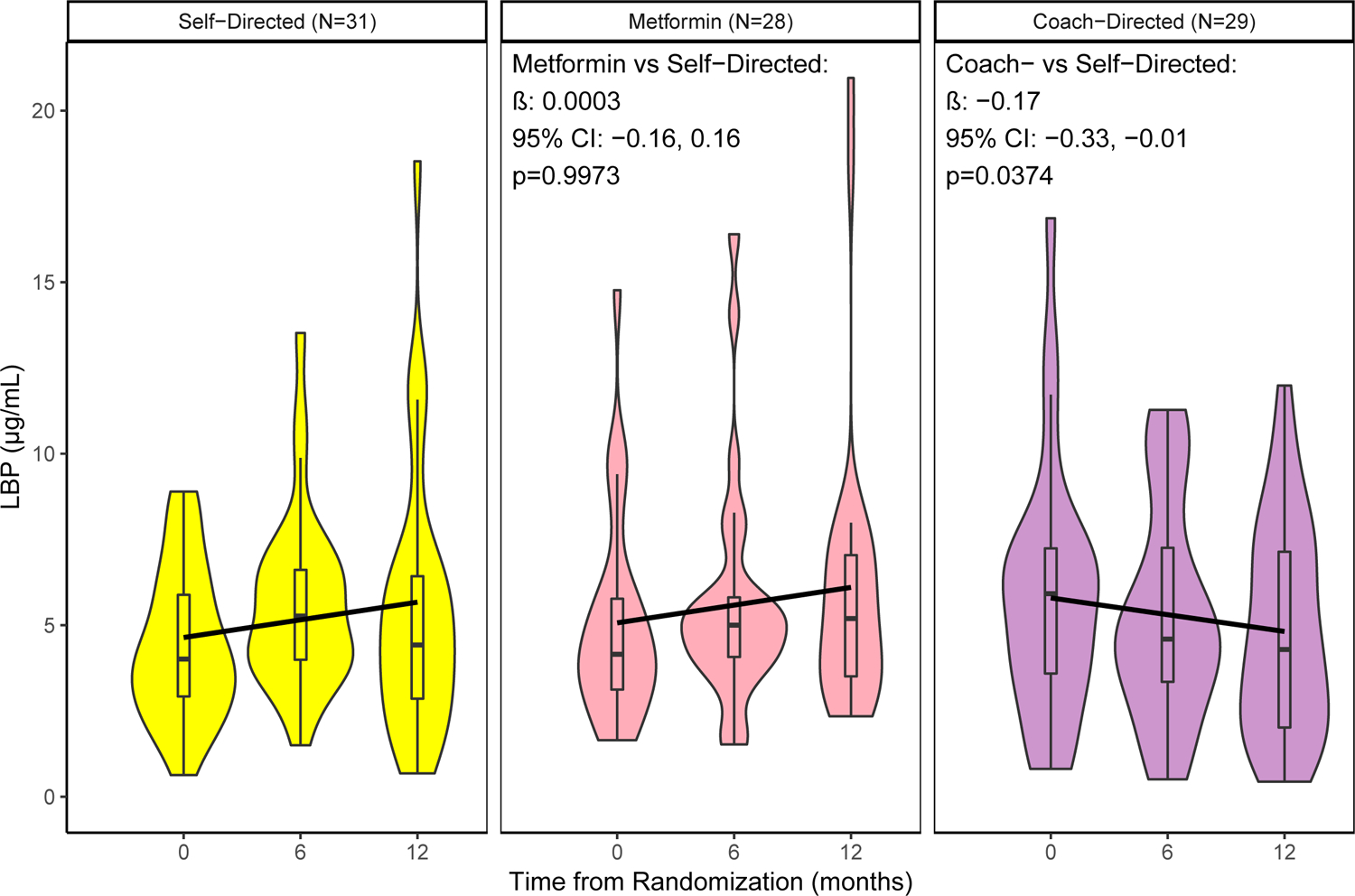
β (95%CI) and p-value from GEE model for test if intervention arm slope significantly differs from self-directed (control) arm.
Interactions of intervention arm effect on LBP by 6-month changes in diet and weight
We next explored whether dietary or weight changes modified the differences in LBP change between study arms. In the coach-directed vs. self-directed analysis, interaction with fiber change and weight change were not significant (p = 0.96 and p = 0.15, respectively). There was evidence of interaction with change in percent energy intake from dietary fat (p = 0.06). Similar results were seen in the metformin arm, with an interaction identified for change in percent energy intake from dietary fat (p = 0.04), while no interactions were noted for changes in dietary fiber (p = 0.19) or changes in weight (p = 0.53). The effect of coach-directed (vs. self-directed) on change in LBP was stronger in individuals who decreased their percentage of energy intake from fat (β = −2.84, 95%CI: −5.17, −0.50; Figure 2 Panel A). Similarly, metformin (vs. self-directed) reduced LBP more in individuals who decreased their percentage of energy intake from fat (β = −1.82, 95%CI: −3.69, 0.04; Figure 2 Panel B). Results were largely consistent when looking at 12-month changes.
Figure 2: Stratified 6-month change in LBP in the intervention arms vs. self-directed weight loss arm.
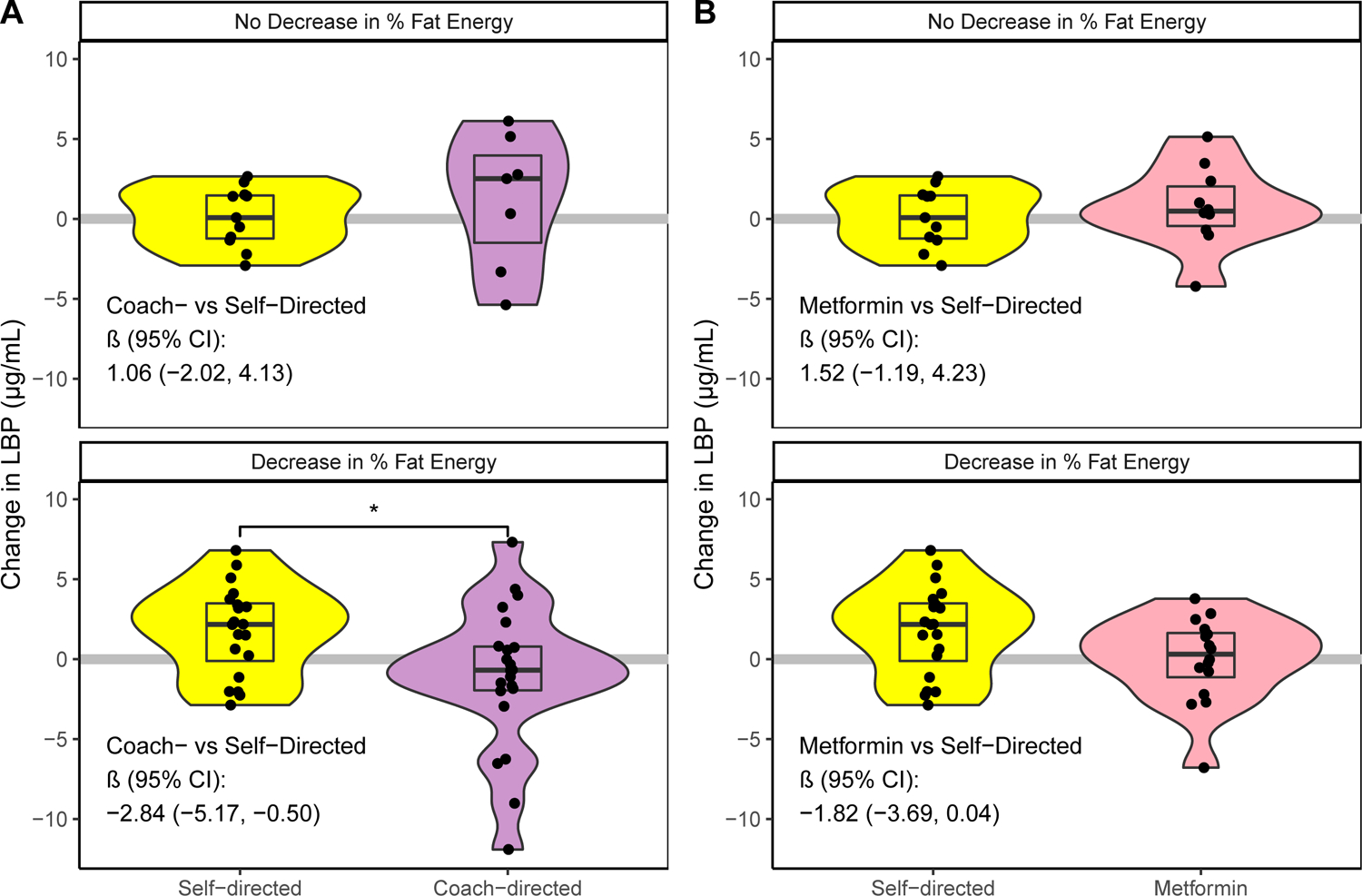
Study arms were stratified by changes in percent of energy intake from dietary fat (defined as decreased % fat intake or did not decrease % fat intake). (A) Coach-directed vs. self-directed weight loss; (B) metformin vs. self-directed weight loss. Regression coefficients for the treatment arm indicator within each stratified grouping are included.
*: p < 0.05
Associations of LBP with characteristics over time in the coach-directed behavioral weight loss intervention
As the coach-directed behavioral weight loss intervention demonstrated an effect on LBP, we next assessed longitudinal correlations of LBP with characteristics within this arm to determine what factor(s) may be associated with LBP change (Supplementary Table 4). LBP was significantly and positively associated with relative weight change (r = 0.29, p = 0.0480) and with hsCRP (r=0.28, p=0.0171).
Direct effects of metformin adherence on change in LBP
To explore a potential direct effect of metformin on change in LBP, we assessed the adherence to metformin treatment at 6 months as well as potential dose-response relationship (Supplementary Figure 2). Metformin adherence (Supplementary Figure 2 Panel A) was not associated with 6-month change in LBP, and there was no relationship between metformin dose and LBP (Supplementary Figure 2 Panel B). Results for 12-month changes were consistent with those from the 6-month changes.
DISCUSSION
In a biracial population of adult with overweight or obesity, a behavioral lifestyle intervention, but not metformin, lowered plasma levels of LBP despite both interventions having similar levels of weight loss. Furthermore, the coach-directed weight loss intervention was most effective at reducing LBP in participants who decreased their relative intake of dietary fat, a finding that may simply reflect better adherence to the DASH diet recommendations in the behavioral intervention. Collectively, our results suggest that a behavioral weight loss intervention focused on reduced calorie intake, improved diet quality, and physical activity can reduce gut permeability to lipopolysaccharides.
Our finding that behavioral weight loss reduces LBP is supported by several lines of evidence. Previous diet- or behavioral-induced weight loss interventions were also found to reduce LBP (8, 14, 20) or intestinal permeability assessed by lactulose:mannitol ratio.(13) In addition to behavioral weight loss, surgical weight loss has also been shown to reduce LBP, though LBP reduction may depend both on surgical method and timing of LBP measurement post-surgery.(15–17) Importantly, even a mean weight loss of 3% in our lifestyle intervention group was sufficient to reduce LBP, and there was a moderate correlation between lifestyle-induced weight loss and lower LBP, suggesting that greater behavioral weight loss could result in more marked improvements.
While a prior study in mice had suggested metformin reduces LBP,(8) here we report that metformin did not affect LBP over one year. Thus, while different methods of reducing weight (e.g. caloric restriction and surgical) may result in reduced LBP, our findings suggests that the weight loss effect on LBP does not generalize to weight lost via metformin. We postulate that metformin treatment may offset adipose-tissue related changes in LBP(26) through effects on the gut microbiome. Previously in SPIRIT we found that metformin increased gram-negative Escherichia,(27) similar to other studies,(28, 29) thus increasing overall LPS-producing potential. Alternatively, it is possible that metformin simply has a negligible direct effect on the gut barrier. Future research, including alternative measures of gut permeability (e.g., intestinal fatty acid binding protein), is needed to understand why metformin does not affect LBP.
In our trial, we found that changes in dietary fat intake modified the effects of the interventions on LBP. The changes in dietary fat may be a surrogate for better adherence to a healthful dietary pattern (e.g. DASH diet), suggesting that improvements in gut permeability to lipopolysaccharides are maximized by the combination of weight loss with adherence to a high quality diet. Additional work is needed to better understand the individual and joint effects of weight loss and other lifestyle factors on LPS translocation and other measures of gut permeability.
Within our lifestyle intervention arm, LBP was positively correlated with weight gain from baseline and with the inflammatory marker hsCRP. Similar to LBP, CRP is an acute-phase protein which is also elevated in obesity.(30) LBP was not, however, correlated with cytokine IL-6. Associations of IL-6 with inflammation and metabolism depend on several contexts including cellular source of production (31) and activated signaling pathways in targeted cells.(32) Future studies should include additional markers of inflammation to better understand the effects of lifestyle weight-loss interventions on systemic inflammation vis-à-vis gut permeability.
Our trial has limitations. First, our assessment of diet relied on self-report through a modified food frequency questionnaire. Our diet assessment approach prevented us from examining the contributions of specific types of fats, food groups, or dietary patterns as well as estimates of total energy intake. Second, we did not have measures of physical activity or body composition in our study, which could be important mediating or modifying factors to include in future studies investigating weight loss interventions on LBP. Additionally, we did not have other measures of intestinal permeability in our study, which could otherwise have provided a more comprehensive understanding of intervention effects on overall intestinal barrier permeability. Third, we may be underpowered to detect some associations, such as those involving metformin dose. Fourth, our study population consists of cancer survivors with overweight or obesity, and our results may not be generalizable to individuals with overweight or obesity with no history of cancer. Individuals receiving radiation or chemotherapy can experience acute intestinal barrier dysfunction; however, this is thought to resolve shortly after cessation of therapy.(33) In our study, participants had to be at least three months post-chemotherapy or radiation treatment (with the sample having a median of 6 years since last treatment), there were no baseline differences in time since last cancer treatment, and the time since last cancer treatment was not correlated with baseline levels of LBP; thus, it is unlikely that past cancer treatments influenced our findings.
Our study also has several strengths. First, this study is a randomized clinical trial and provides stronger inferences about causality of associations. Second, the study population is diverse in terms of race and sex. Third, this is the first study in humans to compare a behavioral lifestyle weight loss intervention to a metformin weight loss intervention on change in LBP, thus allowing us to investigate potential mechanisms beyond weight loss per se.
In conclusion, a behavioral lifestyle intervention, but not metformin, lowered plasma levels of LBP in the setting of similar levels of weight loss. Furthermore, the impact of either weight-loss intervention (behavioral or metformin) on LBP differed by the extent of change in dietary fat, which may reflect greater adherence to a healthful dietary pattern. Future studies are needed to replicate our findings, and to further identify the factors and mechanisms underlying the relationship between weight loss, diet composition and LPS translocation.
Supplementary Material
SOURCES OF SUPPORT:
The SPIRIT study was funded by the Maryland Cigarette Restitution Fund. N.T.M. was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (K01HL141589). H.C.Y. was supported in part by the National Cancer Institute’s Cancer Centers Support Grant to the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (5P30CA006973). C.T. was supported by the National Heart, Lung, and Blood Institute grant T32HL007024.
ABBREVIATIONS:
- DASH
Dietary Approaches to Stopping Hypertension
- eGFR
estimated glomerular filtration rate
- GEE
Generalized estimating equation
- hsCRP
high-sensitivity C-reactive protein
- IL-6
interleukin 6
- LBP
Lipopolysaccharide binding protein
- LPS
Lipopolysaccharide
- QIC
quasi-likelihood information criteria
- SPIRIT
Survivorship Promotion In Reducing IGF-1 Trial
Footnotes
CLINICAL TRIALS REGISTRATION NUMBER: NCT02431676, https://clinicaltrials.gov
COMPETING INTERESTS
The authors declared no conflict of interest.
DATA AVAILABILITY STATEMENT:
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.
REFERENCES
- 1.Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clinical microbiology reviews. 2003;16(3):379–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. [DOI] [PubMed] [Google Scholar]
- 3.Grube BJ, Cochane CG, Ye RD, Green CE, McPhail ME, Ulevitch RJ, et al. Lipopolysaccharide binding protein expression in primary human hepatocytes and HepG2 hepatoma cells. The Journal of biological chemistry. 1994;269(11):8477–82. [PubMed] [Google Scholar]
- 4.Wurfel MM, Monks BG, Ingalls RR, Dedrick RL, Delude R, Zhou D, et al. Targeted deletion of the lipopolysaccharide (LPS)-binding protein gene leads to profound suppression of LPS responses ex vivo, whereas in vivo responses remain intact. The Journal of experimental medicine. 1997;186(12):2051–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novitsky TJ. Limitations of the Limulus amebocyte lysate test in demonstrating circulating lipopolysaccharides. Annals of the New York Academy of Sciences. 1998;851:416–21. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz AG, Casafont F, Crespo J, Cayón A, Mayorga M, Estebanez A, et al. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obesity surgery. 2007;17(10):1374–80. [DOI] [PubMed] [Google Scholar]
- 7.Lepper PM, Schumann C, Triantafilou K, Rasche FM, Schuster T, Frank H, et al. Association of lipopolysaccharide-binding protein and coronary artery disease in men. Journal of the American College of Cardiology. 2007;50(1):25–31. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. International Journal of Obesity. 2012;36(11):1442–9. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Lu L, Yao P, Ma Y, Wang F, Jin Q, et al. Lipopolysaccharide binding protein, obesity status and incidence of metabolic syndrome: a prospective study among middle-aged and older Chinese. Diabetologia. 2014;57(9):1834–41. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Quintela A, Alonso M, Campos J, Vizcaino L, Loidi L, Gude F. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PloS one. 2013;8(1):e54600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salden BN, Troost FJ, Wilms E, Truchado P, Vilchez-Vargas R, Pieper DH, et al. Reinforcement of intestinal epithelial barrier by arabinoxylans in overweight and obese subjects: A randomized controlled trial: Arabinoxylans in gut barrier. Clinical nutrition (Edinburgh, Scotland). 2018;37(2):471–80. [DOI] [PubMed] [Google Scholar]
- 12.González-Sarrías A, Romo-Vaquero M, García-Villalba R, Cortés-Martín A, Selma MV, Espín JC. The Endotoxemia Marker Lipopolysaccharide-Binding Protein is Reduced in Overweight-Obese Subjects Consuming Pomegranate Extract by Modulating the Gut Microbiota: A Randomized Clinical Trial. Molecular nutrition & food research. 2018;62(11):e1800160. [DOI] [PubMed] [Google Scholar]
- 13.Damms-Machado A, Louis S, Schnitzer A, Volynets V, Rings A, Basrai M, et al. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am J Clin Nutr. 2017;105(1):127–35. [DOI] [PubMed] [Google Scholar]
- 14.Ott B, Skurk T, Hastreiter L, Lagkouvardos I, Fischer S, Büttner J, et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Scientific reports. 2017;7(1):11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dielen FM, Buurman WA, Hadfoune M, Nijhuis J, Greve JW. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. The Journal of clinical endocrinology and metabolism. 2004;89(8):4062–8. [DOI] [PubMed] [Google Scholar]
- 16.Yang PJ, Lee WJ, Tseng PH, Lee PH, Lin MT, Yang WS. Bariatric surgery decreased the serum level of an endotoxin-associated marker: lipopolysaccharide-binding protein. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2014;10(6):1182–7. [DOI] [PubMed] [Google Scholar]
- 17.Clemente-Postigo M, Roca-Rodriguez Mdel M, Camargo A, Ocaña-Wilhelmi L, Cardona F, Tinahones FJ. Lipopolysaccharide and lipopolysaccharide-binding protein levels and their relationship to early metabolic improvement after bariatric surgery. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2015;11(4):933–9. [DOI] [PubMed] [Google Scholar]
- 18.Yerevanian A, Soukas AA. Metformin: Mechanisms in Human Obesity and Weight Loss. Current obesity reports. 2019;8(2):156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimoto WY, Jablonski KA, Bray GA, Kriska A, Barrett-Connor E, Haffner S, et al. Body size and shape changes and the risk of diabetes in the diabetes prevention program. Diabetes. 2007;56(6):1680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marti A, Martínez I, Ojeda-Rodríguez A, Azcona-Sanjulian MC. Higher Lipopolysaccharide Binding Protein and Chemerin Concentrations Were Associated with Metabolic Syndrome Features in Pediatric Subjects with Abdominal Obesity during a Lifestyle Intervention. Nutrients. 2021;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh HC, Maruthur NM, Wang NY, Jerome GJ, Dalcin AT, Tseng E, et al. Effects of Behavioral Weight Loss and Metformin on Insulin-like Growth Factors in Cancer Survivors: A Randomized Trial. The Journal of clinical endocrinology and metabolism. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appel LJ, Clark JM, Yeh H-C, Wang N-Y, Coughlin JW, Daumit G, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irimata K, Wakim P, Li X. Estimation of correlation coefficient in data with repeated measures. SAS Conference Proceedings: SAS Global Forum 2018: SAS Institute Inc; 2018. [Google Scholar]
- 24.Wickham H ggplot2: Elegant Graphics for Data Analysis: Springer-Verlag; New York; 2016. [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 26.Moreno-Navarrete JM, Escoté X, Ortega F, Serino M, Campbell M, Michalski M-C, et al. A role for adipocyte-derived lipopolysaccharide-binding protein in inflammation- and obesity-associated adipose tissue dysfunction. Diabetologia. 2013;56(11):2524–37. [DOI] [PubMed] [Google Scholar]
- 27.Mueller NT, Differding MK, Zhang M, Maruthur NM, Juraschek SP, Miller ER 3rd, et al. Metformin Affects Gut Microbiome Composition and Function and Circulating Short-Chain Fatty Acids: A Randomized Trial. Diabetes care. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nature medicine. 2017;23(7):850–8. [DOI] [PubMed] [Google Scholar]
- 29.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14(3):232–44. [DOI] [PubMed] [Google Scholar]
- 31.Han MS, White A, Perry RJ, Camporez J-P, Hidalgo J, Shulman GI, et al. Regulation of adipose tissue inflammation by interleukin 6. Proceedings of the National Academy of Sciences. 2020;117(6):2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2011;1813(5):878–88. [DOI] [PubMed] [Google Scholar]
- 33.Sonis ST. The pathobiology of mucositis. Nature Reviews Cancer. 2004;4(4):277–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.


