Abstract
The Bcl-2 family small molecule inhibitor navitoclax is being clinically evaluated to treat multiple cancers including lymphoid malignancies and small cell lung cancer. A sensitive and reliable method was developed to quantitate navitoclax in human plasma using liquid chromatography with tandem mass spectrometry to perform detailed pharmacokinetic studies. Sample preparation involved protein precipitation using acetonitrile. Separation of navitoclax and the internal standard, navitoclax-d8, was achieved with a Waters Acquity UPLC BEH C18 column using isocratic flow over a 3 minute total analytical run time. A SCIEX 4500 triple quadrupole mass spectrometer operated in positive electrospray ionization mode was used for the detection of navitoclax. The assay range was 5-5000 ng/mL and proved to be accurate (89.5-104.9%) and precise (CV ≤11%). Long-term frozen plasma stability for navitoclax at −70°C has been determined for at least 34 months. The method was applied for the measurement of total plasma concentrations of navitoclax in a patient with receiving a 250 mg daily oral dose.
Keywords: navitoclax, quantitative analysis, tandem mass spectrometry, validation
1. Introduction
The B-cell lymphoma-2 (BCL-2) family is comprised of anti-apoptotic and pro-apoptotic proteins that serve as key regulators of the intrinsic apoptotic pathway.(Kale, Osterlund, & Andrews, 2018; Korsmeyer, 1999) Overabundance of anti-apoptotic proteins, including BCL-2, BCL-XL or MCL-1, can contribute to pathogenesis and therapeutic resistance in multiple malignancies including chronic lymphocytic lymphoma (CLL), acute myelogenous leukemia (AML) and small cell lung cancer (SCLC).(Adams & Cory, 2018; Zhang, Ming, & Yu, 2007) BH3 mimetics, a class of small molecules designed to antagonize antiapoptotic BCL-2 proteins, are predicted to have therapeutic efficacy in cancers reliant on overexpression of anti-apoptotic proteins.(Merino et al., 2018) Venetoclax, a BCL-2 selective BH3-mimetic, is currently FDA-approved for the treatment of CLL and AML.
Navitoclax is an orally-bioavailable BH3 mimetic with high affinity for the anti-apopototic proteins BCL-2, BCL-XL and BCL-W.(Merino et al., 2012; Tse et al., 2008) In preclinical studies of acute lymphoblastic leukemia, SCLC, and oral cancers navitoclax demonstrated antitumor efficacy in vitro and in vivo.(Shoemaker et al., 2008; Tse et al., 2008; I. H. Yang et al., 2019) In early phase studies navitoclax was safe and tolerable at the recommended phase 2 dose of 150 mg/day 7 day lead-in followed by 325 mg daily.(Wilson et al., 2010) Navitoclax is substantially metabolized in humans as was demonstrated in a mass balance study with nearly 90% of radioactivity being recovered in the feces of which ~50% was metabolites.(J. Yang et al., 2014) The most prominent dose-limiting toxicity of navitoclax is thrombocytopenia, driven by on-target inhibition of BCL-XL, which controls platelet turnover.(Mason et al., 2007; Wilson et al., 2010) The thrombocytopenia could be mitigated by at least a 7 day lead-in the majority of patients. Modest anti-tumor activity has been observed in phase I/II trials including patients with SCLC.(Gandhi et al., 2011; Roberts et al., 2012; Wilson et al., 2010)
Previously reported clinical trials using navitoclax have not reported detailed analytic methods for pharmacokinetic analysis. However, a preclinical method has been reported over the range of ~5-10000 ng/mL but lacks sample preparation and reproducibility and reliability details.(Choo et al., 2014) To support the continued development of this therapeutic agent and ongoing clinical trials in oncology, we developed and validated method for the determination of navitoclax in plasma using LC-MS/MS. This method is applicable over clinically relevant concentration ranges with short run times to allow for reliable, high throughput analysis. We further demonstrate the application of this pharmacokinetic analysis in patients receiving oral navitoclax as part of a clinical trial.
2. Experimental
2.1. Chemical and reagents
Navitoclax and the internal standard, navitoclax-d8, were purchased from Toronto Research Chemicals (Toronto, ON). HPLC grade acetonitrile and formic acid were purchased from EMD Chemical Inc. (Billerica, MA). Deionized water was obtained from the Millipore Milli-Q-UF filtration system (Milford, MA). All other chemicals were of molecular biological grade or higher and were obtained from Sigma–Aldrich (St. Louis, MO). Drug-free sodium heparin human plasma was purchased from Biological Specialty Company (Colmar, PA, USA).
2.2. Chromatography
The LC system was a Shimadzu Nexera X2 UHPLC system (Columbia, MD) with the autosampler operating at 5°C. Analyte separation was achieved using Waters Acquity UPLC BEH C18 column (50 mm × 2.1 mm, 1.7 μm, Milford, MA) at 40°C. The mobile phase consisted of acetonitrile-water-formic acid (70:30:0.1, v/v/v) and was delivered using isocratic elution at a flow rate of 0.15 mL/min for a total runtime of 3 minutes. After each injection, the autosampler needle was washed with 0.5 mL of acetonitrile:water (50:50, v/v).
2.3. Mass spectrometry
An AB SCIEX 4500 triple quadrupole mass spectrometer operated in positive electrospray ionization utilizing multiple reaction monitoring (MRM) mode. The LC and the mass spectrometer were controlled by the Analyst software (version 1.6.2 and greater).
The settings for the mass spectrometer were as follows: curtain gas 10 psi, collision gas 8 psi, ion spray voltage 5500 V, probe temperature 450°C, ion source gas 1 16 psi, ion source gas 2 16 psi, and exit potential 10. The collision cell exit potentials were 12 for navitoclax and 10 for navitoclax-d8. The declustering potentials were 86 and 181 for navitoclax and IS, respectively. The collision energies were 19 and 43 for navitoclax and IS, respectively. MRM m/z transitions were the following: 487.76 → 742.06 for navitoclax and 983.12 → 751.20 for navitoclax-d8.
2.4. Preparation of calibration standards and quality control samples
Stock solutions for navitoclax and navitoclax-d8 were prepared independently at a concentration of 1 mg/mL in dimethyl sulfoxide (DMSO). All stock solutions were stored at −20 °C. All working solutions, standards, and quality control (QC) samples were prepared fresh daily unless otherwise stated. Working solutions were made in acetonitrile:water (1:1, v/v) at the following concentrations: 125, 250, 1,250, 2,500, 5,000, 12,500, 25,000, and 125,000 ng/mL. Working solutions (4:100, v/v) were spiked into blank sodium heparin human plasma to make the calibration curve at the following concentrations: 5, 10, 50, 100, 200, 500, 1000 and 5000 ng/mL. The QC samples were made at five different concentration levels for validation: a lower limit of quantitation (LLOQ) 5 ng/mL, low QC 15 ng/mL, medium QC 400 ng/mL, and high QC 4000 ng/mL, An above upper limits of quantitation (AULQ) QC 40000 ng/mL was prepared and diluted 1:10 (v/v) in pooled plasma for quantitation. Blank and zero calibrators were also part of the standard curve and made from blank human plasma. QC samples were prepared as a batch and stored at −70°C for long-term and freeze–thaw stability.
2.5. Sample preparation
A 50 μL aliquot of plasma sample was added to a borosilicate glass test tube and mixed with 200 μL of acetonitrile containing the internal standard (500 ng/mL of navitoclax-d8). For blank samples, 200 μL of acetonitrile was added in lieu of internal standard. Samples were vortex-mixed, centrifuged (1200 ×g for 10 minutes at ambient temperature) and transferred to an autosampler vial. A volume of 5 μL was injected onto the LC-MS/MS system using a temperature-controlled autosampling device operating at approximately 5 °C.
2.6. Method validation
The validation of this method includes precision and accuracy, sensitivity and selectivity, carryover, stock and plasma stability, and freeze/thaw stability. The FDA guidelines were followed for all acceptance criteria.(Food and Drug Administration Center for Drug Evaluation and Research, May, 2018.)
2.6.1. Sensitivity and selectivity
The sensitivity of the assay was determined by the signal to noise ratio of the LLOQ QC from the three precision and accuracy runs. The selectivity of the method was evaluated using blank human heparin plasma assessing for the presence of endogenous or exogenous interfering peaks. The interfering peak area needed to be less than 20% of the peak area of the analytes at the LLOQ in blank plasma. In the same six lots, the plasma was spiked at the low QC concentration level. Each plasma lot was analyzed in triplicate and the results were acceptable if at least 50% of the QCs in each lot were within 85-115% of the nominal concentration.
2.6.3. Precision and accuracy
The precision and accuracy validation runs were performed on three consecutive days. This included a calibration curve processed in duplicate, QC samples at five different concentrations (LLOQ, low, medium, high, and AULQ QC) in quintuplicate, a single blank, and zero-level standard (blank with internal standard). Estimates of the inter-day precision, intra-day precision and accuracy were obtained.
2.6.4. Carryover
In order to determine if carryover is present after an injection of a sample with high navitoclax concentration, a zero-level standard (blank with internal standard) was injected following a 5,000 ng/mL (upper limit of quantitation (ULOQ)) standard in replicates of six. Next, a lower limit of quantitation (LLOQ, 5 ng/mL) standard was injected following a ULOQ standard in replicates of six. Carryover was deemed not significant if the zero-level standards are below the limit of quantitation and LLOQ samples are within 20% of 5 ng/mL.
2.6.4. Stability
The long-term stability of navitoclax stock solution stored at −20°C was determined by injecting the test stock solution compared to freshly prepared stock solution. Each stock was injected five times, and long-term stock stability was evaluated up to 13 months. Long term stability in human heparin plasma at −70°C was evaluated in triplicate for up to 13 months. The stability was evaluated after three freeze/thaw cycles by assaying QC samples that had been frozen (−70°C) and thawed on three separate days and comparing the results to samples that had not been thawed. The processed sample stability was assessed by injecting freshly prepared low and high QCs and storing at 5°C (autosampler temperature) for ~26 hours and reinjecting the sample.
2.8. Assay application
To assess the applicability of the assay, we applied the method to a patient who received navitoclax in an ongoing Phase I clinical trial (ETCTN10070 ClinicalTrials.gov Identifier NCT03366103). The protocol was approved by the National Cancer Institute’s Central Institutional Review Board. The patient provided written informed consent.
Navitoclax was administered orally at a dose of 150 mg daily for 7 days followed by a dose of 250 mg daily. Navitoclax in patient plasma samples were determined using the validated method described above. The pharmacokinetic parameters for the navitoclax were estimated using noncompartmental analysis as implemented in Phoenix WinNonlin version 8.3 (Pharsight A Certara Company, Cary, NC).
3. Results
3.1. Chromatographic separation and detection
The LC/MS/MS method was developed and validated to determine navitoclax in human heparin plasma. The assay was validated with a Waters Acquity UPLC BEH C18 analytical column. The total run time was 3.0 minutes, and the retention times for navitoclax and the internal standard (navitoclax-d8) were approximately 1.0 minute. The column eluent was diverted to waste for the first 0.6 minutes and last 0.8 minutes of each injection. Mass spectrometer detection was using a Sciex 4500 triple quadrupole mass spectrometer with a Tubro V ion source in MRM in positive electrospray mode. The mass transitions, the double charged molecular ion was selected as the precursor ion for navitoclax since it had better sensitivity in the extracted plasma samples. The internal standard was optimized for the single charged molecular ion.
3.2. Method validation
3.2.1. Sensitivity and selectivity
The LLOQ for navitoclax was determined to be 5 ng/mL in three precision and accuracy runs (Table 1). No significant interferences were seen at the retention times for navitoclax and the internal standard for six lots of plasma (Fig. 1). Additionally, the low QC spiked in the same six lots of plasma was within acceptance criteria. The average signal to noise ratio of the LLOQ was 152.8 (data not shown). Since the metabolites of navitoclax were unknown and not commercially available, we compared the ionization response of the stable label internal standard (navitoclax-d8) between the spiked calibration standards and QCs and the unknown patient samples. There was no statistically significant difference in the internal standards over analytical runs (p = 0.24; Fig. 2) suggesting that the unknown metabolites do not interfere with navitoclax ionization.
Table 1.
Validation characteristics of navitoclax
| LLOQ (5 ng/mL) |
Low (15 ng/mL) |
Medium (400 ng/mL) |
High (4000 ng/mL) |
AULQ (40000 ng/mL) |
|
|---|---|---|---|---|---|
| n | 15 | 15 | 15 | 15 | 15 |
| Average | 4.5 | 14.8 | 410 | 4197.8 | 40918 |
| Standard Deviation | 0.5 | 0.9 | 24.4 | 71.3 | 2894 |
| Accuracy (%) | 89.5 | 98.5 | 102.5 | 104.9 | 102.3 |
| Within-day Precision (%) | 7.3 | 6.8 | 5.9 | 1.0 | 7.4 |
| Between-day Precision (%) | 11.0 | 6.3 | 5.9 | 1.7 | 7.1 |
|
| |||||
| Plasma Stability (−70°C) | |||||
| 3.5 months (%) | 107.5 | 105.2 | 104.3 | ||
| 7 months (%) | 97.0 | 91.6 | 98.9 | ||
| 13 months (%) | 99.6 | 90.8 | 95.0 | ||
|
| |||||
| Freeze-thaw cycles | |||||
| 3 (%) | 109.4 | 99.7 | - | ||
Figure 1.
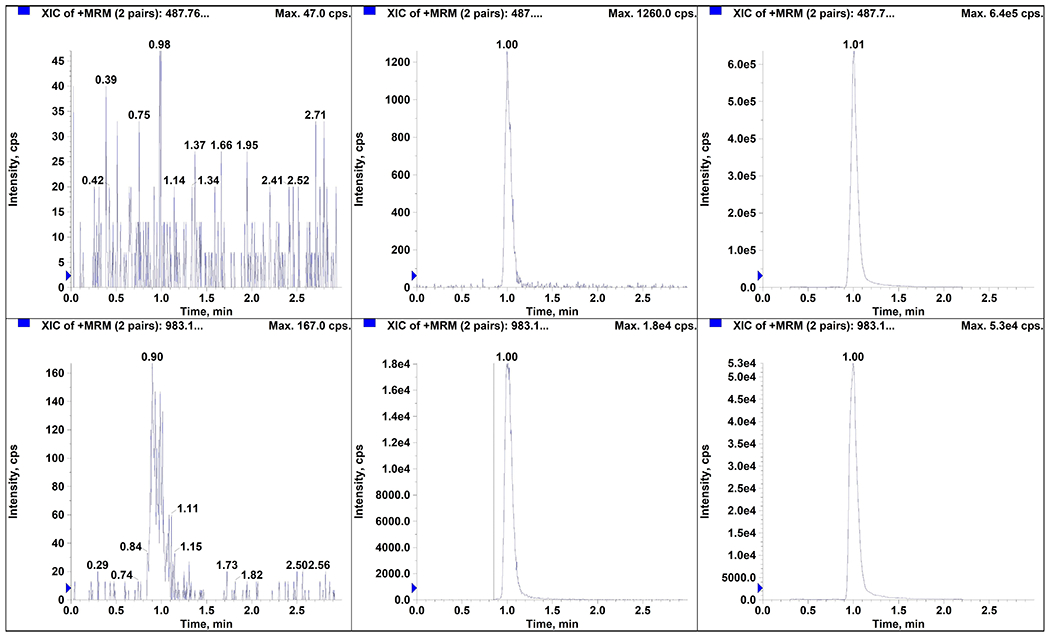
Chromatograms of plasma for navitoclax (A, B, C) and internal standard (navitoclax-d8; D, E, F): (A, D) blank human plasma (B, E) LLOQ (5 ng/mL), and (C, F) patient sample with 1990 ng/mL of navitoclax.
Figure 2.
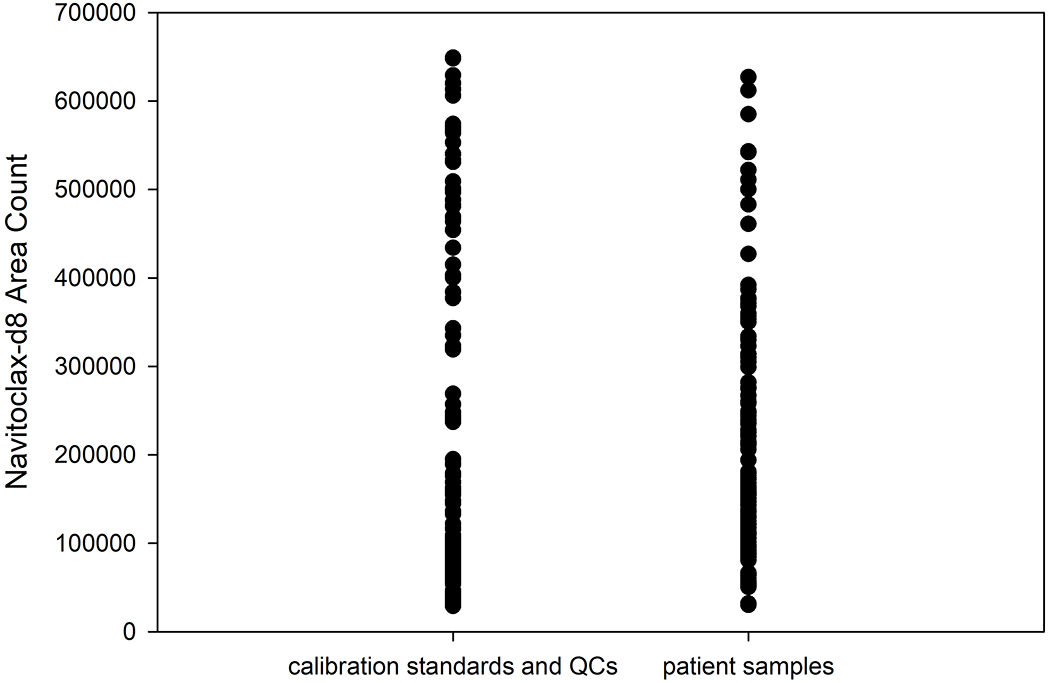
Comparison of the internal standard response in spiked calibration standards and QCs and the unknown patient samples. There was no statistically significant difference in the internal standards over analytical runs (p = 0.24).
3.2.2. Precision and accuracy
The calibration curve for navitoclax was constructed from the peak area ratio of the analyte to the peak area of its internal standard (navitoclax-d8) by using the least-squares quadratic regression analysis with 1/x2 weight for navitoclax. Validation experiments revealed excellent goodness of fit, with the r2 ≥ 0.99 with a calibration range of 5 ng/mL to 5,000 ng/mL. The average intra-day precision for low, medium and high quality controls ranged from 1.0% to 6.8% (Table 1). The inter-day precision for low, medium and high quality controls ranged from 1.7% to 6.3%. The percent accuracy ranged from 98.5% to 104.9%. The intra-day precision, inter-day precision and percent accuracy for the 1:10 dilution AULQ quality controls were 7.4%, 7.1%, and 102.3%, respectively (Table 1).
3.2.3. Stability
The master stock of navitoclax in DMSO determined to be stable up for approximately 13 months in DMSO at −20 °C. The stability of the analyte was evaluated in human heparin plasma at the low and high QC level stored at −70°C and was found to be stable up to 34 months (Table 1). The analyte was stable in plasma for three freeze-thaw cycles at −70°C (Table 1). Processed samples did not meet stability acceptance criteria after 26 hours at 5°C, with difference in mean concentration of 34.6% for low QC and 25.1% for high QC (threshold for acceptance ≤15%). Processed samples are considered stable for the length and of a precision and accuracy validation run (~2.3 hours, n=43 samples including calibrators and QCs).
3.2.4. Carryover
Carryover assessment was conducted to ensure accurate quantitation of patient samples. No peaks were detected in blank plasma following the highest calibration standard (ULOQ). Additionally, LLOQ QCs injected after the highest calibration standard all met acceptance criteria. Therefore the method results in no carryover for the analyte under the chromatographic conditions described in the methods.
3.3. Application of the method
The assay was capable of quantifying navitoclax in a single patient receiving a lead-in dose of 150 mg dose orally once daily for 7 days followed by 250 mg once daily (Fig. 3). The pre-treatment sample was undetectable. At steady state on Cycle 1 Day 15, the maximum total plasma concentration (Cmax) of 1990 ng/mL which occurred at 8.0 h with an AUCTau of 36.2 μg*h/mL.
Figure 3.
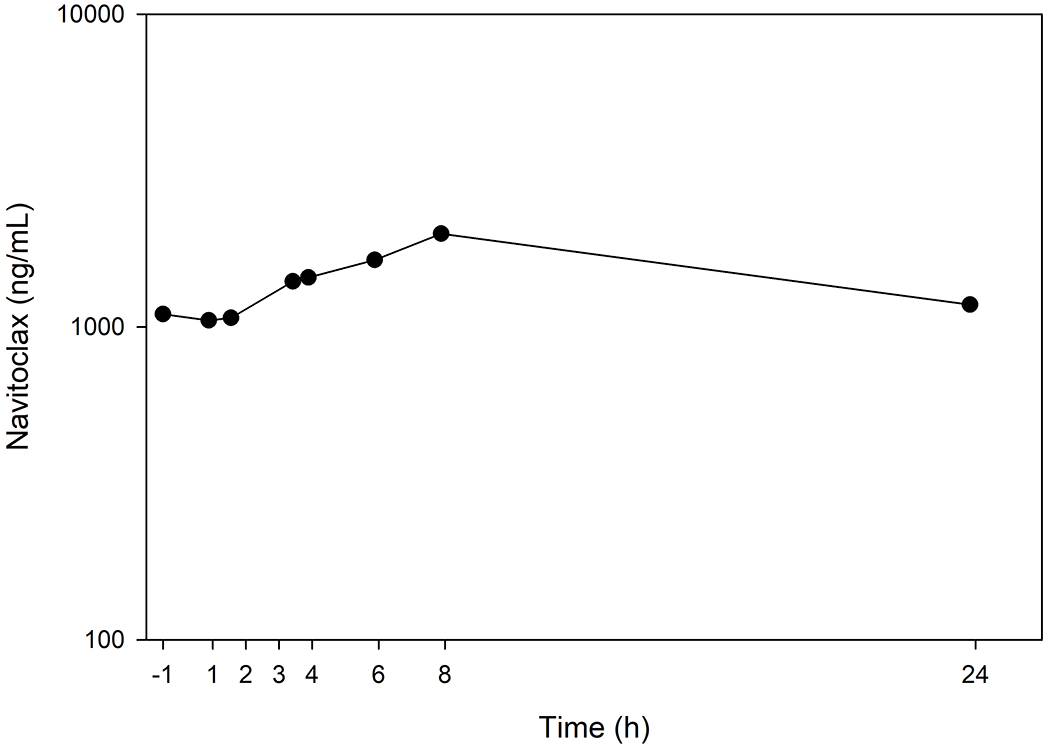
Navitoclax concentration-time profile from a single patient receiving a 7-day lead-in of 150 mg orally once daily followed by 15 days of 250 mg orally once daily.
4. Conclusion
An analytical assay method was developed and validated to quantify navitoclax concentrations in plasma. The analytical assay was shown to be robust and reproducible, with over 1 year stability of analyte in stock solution and in human heparin plasma. The method has been successfully applied to the study of plasma pharmacokinetics of navitoclax after its oral administration in a patient, and is being used to support the Phase I clinical trial.
Acknowledgments
We would like to thank Linping Xu for her quality assurance review of the data. The project described was supported by the NCI Experimental Therapeutics Clinical Trials Network (grants U01CA070095, UM1CA186691 and U24CA247648). The project described was also supported by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins [NIH grants P30CA006973 and UL1TR003098, and the Shared Instrument Grant S10OD020091]. The project described was also supported by grant number UL1TR003098 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCATS or NIH.
References
- Adams JM, & Cory S (2018). The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ, 25(1), 27–36. doi: 10.1038/cdd.2017.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo EF, Boggs J, Zhu C, Lubach JW, Catron ND, Jenkins G, Voorman R (2014). The role of lymphatic transport on the systemic bioavailability of the Bcl-2 protein family inhibitors navitoclax (ABT-263) and ABT-199. Drug Metab Dispos, 42(2), 207–212. doi: 10.1124/dmd.113.055053 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration Center for Drug Evaluation and Research, U.S. Department of Health and Human Services. (May, 2018.). Guidance for industry bioanalytical method validation. Retrieved from [Google Scholar]
- Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, Rudin CM (2011). Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol, 29(7), 909–916. doi: 10.1200/JCO.2010.31.6208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale J, Osterlund EJ, & Andrews DW (2018). BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ, 25(1), 65–80. doi: 10.1038/cdd.2017.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer SJ (1999). BCL-2 gene family and the regulation of programmed cell death. Cancer Res, 59(7 Suppl), 1693s–1700s. [PubMed] [Google Scholar]
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kile BT (2007). Programmed anuclear cell death delimits platelet life span. Cell, 128(6), 1173–1186. doi: 10.1016/j.cell.2007.01.037 [DOI] [PubMed] [Google Scholar]
- Merino D, Kelly GL, Lessene G, Wei AH, Roberts AW, & Strasser A (2018). BH3-Mimetic Drugs: Blazing the Trail for New Cancer Medicines. Cancer Cell, 34(6), 879–891. doi: 10.1016/j.ccell.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, Bouillet P (2012). Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood, 119(24), 5807–5816. doi: 10.1182/blood-2011-12-400929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Humerickhouse R (2012). Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol, 30(5), 488–496. doi: 10.1200/JCO.2011.34.7898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker AR, Mitten MJ, Adickes J, Ackler S, Refici M, Ferguson D, Elmore SW (2008). Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res, 14(11), 3268–3277. doi: 10.1158/1078-0432.CCR-07-4622 [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Elmore SW (2008). ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res, 68(9), 3421–3428. doi: 10.1158/0008-5472.CAN-07-5836 [DOI] [PubMed] [Google Scholar]
- Wilson WH, O’Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, Humerickhouse RA (2010). Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol, 11(12), 1149–1159. doi: 10.1016/S1470-2045(10)70261-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IH, Jung JY, Kim SH, Yoo ES, Cho NP, Lee H, Cho SD (2019). ABT-263 exhibits apoptosis-inducing potential in oral cancer cells by targeting C/EBP-homologous protein. Cell Oncol (Dordr), 42(3), 357–368. doi: 10.1007/s13402-019-00431-5 [DOI] [PubMed] [Google Scholar]
- Yang J, Pradhan RS, Rosen LS, Graham AM, Holen KD, & Xiong H (2014). Effect of rifampin on the pharmacokinetics, safety and tolerability of navitoclax (ABT-263), a dual inhibitor of Bcl-2 and Bcl-XL , in patients with cancer. J Clin Pharm Ther, 39(6), 680–684. doi: 10.1111/jcpt.12193 [DOI] [PubMed] [Google Scholar]
- Zhang L, Ming L, & Yu J (2007). BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat, 10(6), 207–217. doi: 10.1016/j.drup.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]


