Abstract
The COVID-19 pandemic has potentially increased the risk for adolescent depression. Even pre-pandemic, <50% of youth with depression accessed care, highlighting needs for accessible interventions. Accordingly, this randomized-controlled trial (ClinicalTrials.gov: NCT04634903) tested online single-session interventions (SSIs) during COVID-19 in adolescents with elevated depression symptoms (N=2,452, ages 13-16). Adolescents from all 50 U.S. states, recruited via social media, were randomized to 1 of 3 SSIs: a behavioural activation SSI, an SSI teaching that traits are malleable, or a supportive control. We tested each SSI’s effects on post-intervention (hopelessness, agency) and 3-month outcomes (depression, hopelessness, agency, generalized anxiety, COVID-related trauma, restrictive eating). Versus control, both active SSIs reduced 3-month depressive symptoms (ds=0.18); decreased post-intervention and 3-month hopelessness (ds=0.16-0.28); increased post-intervention agency (ds=0.15-0.31); and reduced 3-month restrictive eating (ds=0.12-17). Several differences between active SSIs emerged. Results confirm the utility of free-of-charge, online SSIs for high-symptom adolescents, even in the high-stress COVID-19 context.
Keywords: COVID-19, single-session interventions, adolescence, depression, anxiety, intervention
In the early months of 2020, the COVID-19 pandemic swiftly and profoundly transformed the lives of youths nationwide. School closures affecting >50 million students led to isolation and disruption of educational and social-emotional supports; simultaneously, families grappled with collective trauma and economic recession.1 Together, these stressors might increase risk for adolescent depression—already the world’s leading cause of disability in young people.2-4 Even before the pandemic, fewer than 50% of adolescents with depression accessed services;9-10 among those who did, 40-65% have failed to respond.5-6 Newfound financial strain may further preclude families’ capacity to afford treatment for their children. Thus, a generation of youth exposed to unprecedented psychosocial adversity is poised to fall through the cracks of the mental healthcare system. It is critical to identify effective, scalable strategies to reduce adolescent depression, both during and beyond COVID-19. Accordingly, in a nationwide randomized-controlled trial, we tested whether two self-guided, online, single-session interventions for adolescent depression—one teaching growth mindset of personality, the belief that personal traits and symptoms can change, and another teaching behavioral activation, the practice of managing one’s mood via engagement in valued, enjoyable activities—could reduce hopelessness, strengthen perceived agency, and mitigate symptoms of depression, anxiety, and COVID-19-related trauma in high-symptom youth, versus a supportive control.
Well-powered trials of brief, focused, and rapidly-scalable interventions may overcome longstanding challenges to reducing adolescent depression—namely, the challenges of limited potency of existing treatments, and of low accessibility in predominant modes of care. Difficulties underlying limited treatment potency are thought to reflect depression’s heterogeneity.7-8 Diagnostic criteria for depression place youths with 5 of 9 diverse symptoms (such as activity withdrawal, fatigue, and hopelessness) into a single category including >1,400 possible symptom combinations.7 This heterogeneity has spurred the creation of interventions that target widely-ranging difficulties, some of which may be unrelated to an individual’s needs—suggesting the utility of highly-focused, targeted interventions, rather than those characterized by “extreme comprehensiveness” (e.g., cognitive behavioral therapy).19-20 Large-scale trials can rigorously and definitively gauge the promise of treatments that are designed for brevity, containing just one or two treatment elements rather than 10+ separate modules.
Separately, large trials of brief interventions may reveal solutions to the low accessibility of many depression interventions. This low accessibility stems from the formats of predominant treatments, which span many weeks and are intended for delivery in brick-and-mortar clinics by highly-trained clinicians, creating major dissemination barriers.21 Further, up to 59% of youths who do access mental health treatment drop out prematurely, compounding challenges posed by provider scarcity.22-23 Testing brief treatments deliverable by flexible means is a key component of solving this access-to-care crisis.
The need for accessible, brief, and targeted adolescent depression interventions has grown during the pandemic. Prior to the pandemic, research demonstrated that environmental stress, family instability, and social isolation can compound risk for youth depression—and each of these stressors has intensified since the pandemic’s emergence.2-5 Thus, it is possible that risk for youth depression may increase in upcoming years, yet access to effective services remains scarce. It is as critical as ever to rigorously evaluate rapidly-scalable, low-cost strategies to reduce youth depression.
Single-session interventions (SSIs) for youth depression may offer a sustainable path toward this goal. SSIs include core elements of evidence-based treatments, but their brevity makes them easier to disseminate to diverse settings.18 In a meta-analysis of 50 randomized trials, SSIs reduced diverse youth mental health difficulties (mean g=0.32), including self-administered SSIs (e.g., web-based SSIs; mean g=0.32).11 To date, systematic and narrative reviews have identified two types of SSIs have reduced adolescent depression symptoms.11,18 The first is a growth mindset (GM-)SSI, an online program teaching that personal traits are malleable, which has prevented and reduced adolescent depression in multiple trials.12-13,17,24-25 As examples, a GM-SSI led to 9-month depression symptom reductions in high-symptom adolescents, versus a supportive control (ds=0.60, 0.32 per parent and youth reports).13 In two school-based randomized trials, GM-SSIs reduced adolescent depressive symptoms versus active controls from 4-to-9-month follow-ups.12,24-25 The second, a behavioral activation (BA)-SSI, promotes values-based activity engagement to elicit pleasure and accomplishment.14-16 In randomized trials, therapist-guided BA-SSIs have reduced depressive symptoms in moderately-depressed adolescents across 2 weeks (d=1.61) and 1 month (d=0.57).14-15 Further, in an open trial, high-symptom adolescents reported increases in perceived problem-solving ability and agency after completing an online, self-guided BA-SSI; they also endorsed the BA-SSI’s overall acceptability and helpfulness.17 Notably, the GM- and BA-SSIs are the only two fully self-guided, single-session, digital interventions that have shown acceptability and (at minimum) short-term utility among U.S. adolescents with elevated depressive symptoms. However, their relative effectiveness has yet to be rigorously assessed, creating a need to understand their potentially-differential promise as widely-scaled supports for youth depression and related difficulties.
During the pandemic, large trials of multiple online SSIs can elucidate their relative utility for depression, as well as for other mental health difficulties, such as COVID-19-related trauma or anxiety symptoms. Indeed, proximal targets addressed by both the BA and GM SSIs, such as hope,17 relate to depression and anxiety alike. Likewise, interventions designed to increase perceived agency25 and active problem-solving26 have reduced both anxiety and depression in youth. Thus, SSIs targeting agency and hope, like the BA-SSI and GM-SSI, may help reduce depression symptom severity, anxiety symptoms, and trauma symptoms linked to COVID-19—and potentially other forms of psychopathology not directedly targeted by the SSIs (e.g., restrictive eating). SSIs circumvent many common treatment access barriers: they require no therapist, are completable from any location, and are < 30 minutes in length, eliminating premature drop-out concerns. Moreover, online SSIs hold advantages even over other digital interventions, which tend to require sustained effort and repeated use, leading to low engagement and rapid dropout.27 Thus, online SSIs offer a unique opportunity for rapid-large scale tests of accessible depression interventions while the pandemic remains underway.
We therefore conducted a nationwide randomized-controlled trial comparing the utility of online SSIs for adolescent depression during the COVID-19 pandemic (N=2,452 youth with elevated depressive symptoms, ages 13-16). The trial had three primary aims. Aim 1 was to test whether online, self-guided SSIs improved proximal, clinically-relevant targets (e.g., hopelessness and perceived agency, which have predicted longer-term SSI response25) and 3-month depressive symptoms (primary outcome), relative to an active control. Aim 2 was to test whether the GM-SSI versus the BA-SSI—currently, the only two self-guided, digital SSIs that have shown high acceptability in youths experiencing depressive symptoms—proved more impactful in this context. Aim 3 was to test whether either SSI improved secondary, clinically-relevant outcomes (COVID-related trauma, hopelessness, agency, generalized anxiety) versus the control across 3 months. We also conducted post-hoc, exploratory analyses to test intervention impacts on difficulties not directly targeted by the SSIs (restrictive eating). Adolescents recruited from across the U.S. via social media were randomized to one of three self-guided SSIs: a BA-SSI, designed to address activity withdrawal and low agency; a GM-SSI, designed to target hopelessness; or a supportive control. We tested each SSI’s relative benefits, versus the control, across 3 months of the COVID-19 pandemic (November 2020-March 2021). Notably, the trial took place approximately 8 months after school closures and social distancing mandates were first imposed in the United States, but before the COVID-19 vaccine was publicly available. Thus, the trial took place at a time when pandemic-related conditions were still evolving and unpredictable in many U.S. regions, and also when some adolescents might have begun to adjust to lifestyle changes and norms.
Results
Sample Characteristics.
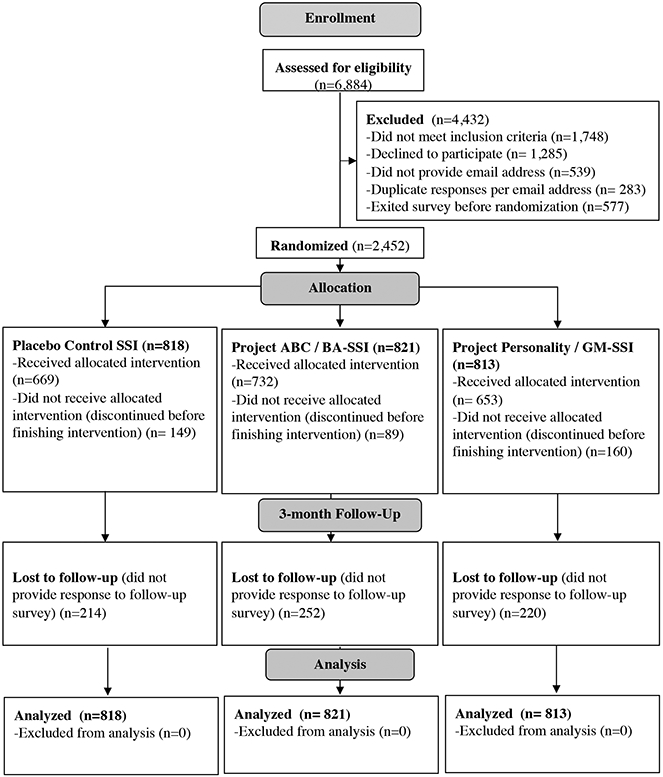
Table 1 presents characteristics of the 2,452 adolescents randomized to an SSI, and Figure 1 (CONSORT diagram) presents participant retention throughout the study. Per a cut-off score of ≥10 for the CDI-SF,31 86.17% of youths reported clinically-elevated depressive symptoms at baseline. Thus, this sample was comparable to those in treatment studies, wherein a majority of youths’ symptom severity scores exceed standard cutoffs. No group differences emerged on demographic factors or baseline depressive symptoms, indicating successful randomization. Participants were 88.09% female (sex assigned at birth) and 3.75% American Indian, 12.64% Asian, 10.48% Black, 1.59% Native Hawaiian, 19.21% Hispanic, and 66.56% White (categories were not mutually-exclusive). Approximately 80% of adolescents identified with a sexual minority identity, and participating youths lived in all 50 U.S. states (Supplementary Figure 2 illustrates participants’ locations throughout the United States).
Table 1.
Sample characteristics
| Treatment Received | |||
|---|---|---|---|
| Demographics | Placebo Control, N=8181 |
Project ABC, N=8211 |
Project Personality, N=8131 |
| Race/Ethnicity | |||
| American Indian/Alaska Native | 36 (4.4%) | 27 (3.3%) | 29 (3.6%) |
| Asian Including Asian Desi | 101 (12%) | 109 (13%) | 100 (12%) |
| Hispanic/Latinx | 159 (19%) | 164 (20%) | 148 (18%) |
| Native Hawaiian/Other Pacific Islander | 14 (1.7%) | 11 (1.3%) | 14 (1.7%) |
| White | 546 (67%) | 536 (65%) | 550 (68%) |
| Black/African-American | 85 (10%) | 84 (10%) | 88 (11%) |
| Other | 19 (2.3%) | 17 (2.1%) | 11 (1.4%) |
| Prefer Not to Answer | 9 (1.1%) | 5 (0.6%) | 10 (1.2%) |
| Gender Identity | |||
| Agender | 19 (2.3%) | 12 (1.5%) | 23 (2.8%) |
| Not sure/Questioning | 71 (8.7%) | 60 (7.3%) | 65 (8.0%) |
| Unspecified Gender | 32 (3.9%) | 27 (3.3%) | 28 (3.4%) |
| Androgynous | 50 (6.1%) | 52 (6.3%) | 55 (6.8%) |
| Non-binary | 128 (16%) | 127 (15%) | 129 (16%) |
| Two-spirited | 5 (0.6%) | 5 (0.6%) | 6 (0.7%) |
| Transgender - Female to Male | 59 (7.2%) | 59 (7.2%) | 62 (7.6%) |
| Trans Female/Trans-Feminine | 7 (0.9%) | 9 (1.1%) | 9 (1.1%) |
| Trans Male/Trans-Masculine | 67 (8.2%) | 65 (7.9%) | 64 (7.9%) |
| Gender Expansive | 7 (0.9%) | 11 (1.3%) | 9 (1.1%) |
| Third Gender | 2 (0.2%) | 1 (0.1%) | 3 (0.4%) |
| Genderqueer | 46 (5.6%) | 45 (5.5%) | 49 (6.0%) |
| Transgender - Male to Female | 6 (0.7%) | 3 (0.4%) | 6 (0.7%) |
| Man/Boy | 122 (15%) | 115 (14%) | 126 (15%) |
| Transgender | 70 (8.6%) | 58 (7.1%) | 58 (7.1%) |
| Woman/Girl | 514 (63%) | 514 (63%) | 516 (63%) |
| Biological Sex | |||
| Female | 723 (88%) | 718 (87%) | 719 (88%) |
| Male | 87 (11%) | 86 (10%) | 78 (9.6%) |
| Other | 2 (0.2%) | 12 (1.5%) | 7 (0.9%) |
| Prefer not to say | 6 (0.7%) | 5 (0.6%) | 9 (1.1%) |
| Sexual Orientation | |||
| Asexual | 39 (4.8%) | 46 (5.6%) | 38 (4.7%) |
| Bisexual | 228 (28%) | 230 (28%) | 220 (27%) |
| Gay/Lesbian/Homosexual | 86 (11%) | 76 (9.3%) | 91 (11%) |
| Heterosexual/Straight | 172 (21%) | 161 (20%) | 165 (20%) |
| Do not use a label | 52 (6.4%) | 41 (5.0%) | 47 (5.8%) |
| Do not want to respond | 3 (0.4%) | 5 (0.6%) | 2 (0.2%) |
| Other/Not listed | 32 (3.9%) | 23 (2.8%) | 38 (4.7%) |
| Pansexual | 87 (11%) | 98 (12%) | 79 (9.7%) |
| Queer | 49 (6.0%) | 47 (5.7%) | 51 (6.3%) |
| Unsure/Questioning | 70 (8.6%) | 94 (11%) | 82 (10%) |
| Baseline CDI Sum Score (0-24) | 14.31 (4.12) | 14.15 (4.06) | 14.22 (4.13) |
n (%); Mean (SD)
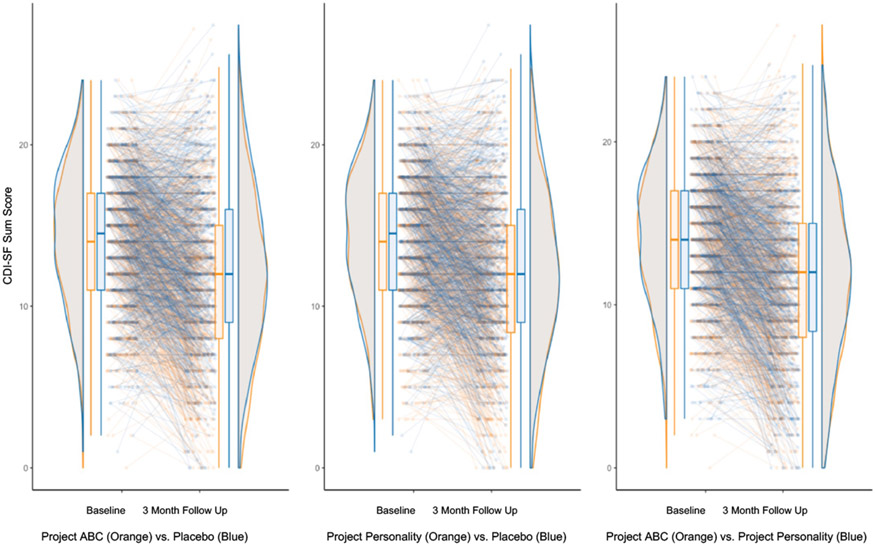
Figure 1.
Depression symptom severity at baseline and three-month follow-up for adolescents in each intervention condition (Project ABC/BA-SSI, N=818; Project Personality/GM-SSI, N=821; and the Sharing Feelings Project/Placebo Control, N=818). Adolescents randomized to the BA-SSI reported significantly decreases in depression symptom severity from pre-intervention to 3-month follow-up than did adolescents in the Placebo Control, padj<0.001, as did adolescents randomized to the GM-SSI relative to control-group adolescents, padj<0.001. Adolescents assigned to the BA- and GM-SSIs did not significantly differ in their depression symptom reductions, padj=0.845.
Intervention Acceptability.
Adolescents completed the baseline survey in Median=43.31 minutes, including pre-SSI questionnaires, one randomly-assigned SSI, and post-SSI surveys. Among adolescents randomized to an SSI, 83.76% completed it in full (range across conditions: 80.32%-89.16%). Youths found both active SSIs and the Placebo Control SSI acceptable across all Program Feedback Scale items (item-level means were all >3.50/5; see Table 2 for item-level ratings by condition).
Table 2.
Program Feedback Scale item-level means and standard deviations by intervention condition, among adolescents who completed each SSI in full.
| Single-Session Intervention Received | |||
|---|---|---|---|
| Program Feedback Scale Items (range across items: 1-5) |
Placebo Control, N = 6301 |
Project ABC (BA- SSI), N = 7291 |
Project Personality (GM- SSI) N = 6531 |
| Enjoyed | 3.80 (0.79) | 3.93 (0.73) | 3.83 (0.77) |
| Understood | 4.54 (0.56) | 4.46 (0.59) | 4.48 (0.64) |
| Easy to Use | 4.46 (0.64) | 4.42 (0.64) | 4.39 (0.68) |
| Tried My Hardest | 4.45 (0.65) | 4.42 (0.69) | 4.45 (0.67) |
| Helpful to Other Kids | 4.24 (0.81) | 4.30 (0.76) | 4.20 (0.83) |
| Would Recommend to a Friend | 3.97 (0.95) | 4.12 (0.89) | 3.93 (1.00) |
| Agree with Message | 4.50 (0.62) | 4.54 (0.57) | 4.47 (0.70) |
Mean (SD).
Note. Most item-level acceptability ratings did not differ by condition; exceptions were that youths reported (1) enjoying the BA- and Placebo more than the GM-SSI, (2) understanding the Placebo more than the BA-SSI, and (3) being more likely to recommend the BA-SSI to a friend, versus both other conditions.
Differential SSI completion and follow-up survey completion.
Assumptions were met for interpretation of two-tailed tests regarding differential SSI and follow-up survey completion. Participants in Project ABC dropped out significantly less (χ2(2) = 41.47, p<.001, 11.08%) during the intervention than participants from Project Personality (19.68%) or the Sharing Feelings Placebo (22.86%). There were no significant differences in who initiated the 3-month follow up across conditions (χ2(2) = 2.14, p=0.34, Project ABC: 37.39%; Project Personality: 38.02%; Sharing Feelings Placebo: 40.71%). Logistic regressions using baseline depression symptom and demographic data were unable to predict whether participants would drop out during the intervention (AUC: 0.51) or at 3-month follow up (AUC: 0.56) better than chance (see analyses on the Open Science Framework for further detail: https://osf.io/8mk6x/).
Depression severity outcomes
(pre-registered primary outcome; see Table 3 for descriptives for primary and secondary outcome variables at baseline, post-intervention, and follow-up; Figure 2 for visualization of results for depression; and Supplementary Table 1 for full results of regression analysis, for which all assumptions for interpretation were met). Compared to adolescents randomized to the control SSI, adolescents randomized to the BA-SSI reported significant decreases in depression symptom severity from pre-intervention to 3-month follow-up (t(1673)=−3.62, padj<0.001, d=0.18, 95% CI 0.08, 0.28). Likewise, adolescents randomized to the GM-SSI reported significant decreases in depression symptom severity from pre-intervention to 3-month follow-up, relative to control-group adolescents. (t(1629)=−3.53 padj<0.001, d=0.18, 95% CI 0.08, 0.27). Adolescents randomly assigned to the BA- and GM-SSIs did not significantly differ in their depression symptom reductions from pre-intervention to 3-month follow-up (t(1632)=−0.20, padj=0.845, d=−0.01, 95% CI −0.11, 0.09).
Table 3.
Means and standard deviations for primary and secondary study outcomes at baseline, post-intervention (where applicable), and 3-month follow-up
| Outcome by Treatment Assignment | Baseline M(SD) | Post Intervention M(SD) | 3M Follow Up M(SD) |
|---|---|---|---|
| Placebo Control | |||
| Beck Hopelessness Scale - 4 item version | 1.71 (0.76) | 1.36 (0.75) | 1.51 (0.82) |
| State Hope Scale - Agency Subscale | 3.88 (1.39) | 5.26 (1.52) | 4.89 (1.64) |
| Children's Depression Inventory-SF | 14.31 (4.12) | -- | 12.57 (4.97) |
| COVID-19 Trauma Symptoms | 2.80 (0.65) | -- | 2.83 (0.63) |
| Generalized Anxiety Disorder-7 | 3.01 (0.73) | -- | 2.78 (0.79) |
| Dietary Restriction Screener | 0.66 (0.43) | 0.63 (0.43) | |
| Project ABC (BA-SSI) | |||
| Beck Hopelessness Scale - 4 item version | 1.72 (0.77) | 1.22 (0.73) | 1.37 (0.80) |
| State Hope Scale - Agency Subscale | 3.93 (1.40) | 5.68 (1.43) | 5.06 (1.63) |
| Children's Depression Inventory-SF | 14.15 (4.06) | -- | 11.47 (5.04) |
| COVID-19 Trauma Symptoms | 2.75 (0.66) | -- | 2.73 (0.64) |
| Generalized Anxiety Disorder-7 | 2.96 (0.76) | -- | 2.73 (0.82) |
| Dietary Restriction Screener | 0.69 (0.41) | 0.57 (0.43) | |
| Project Personality (GM-SSI) | |||
| Beck Hopelessness Scale - 4 item version | 1.72 (0.76) | 1.21 (0.72) | 1.39 (0.83) |
| State Hope Scale - Agency Subscale | 3.96 (1.44) | 5.50 (1.48) | 5.17 (1.65) |
| Children's Depression Inventory-SF | 14.22 (4.13) | -- | 11.58 (5.08) |
| COVID-19 Trauma Symptoms | 2.82 (0.64) | -- | 2.76 (0.66) |
| Generalized Anxiety Disorder-7 | 3.00 (0.74) | -- | 2.68 (0.81) |
| Dietary Restriction Screener | 0.68 (0.42) | 0.60 (0.43) | |
Figure 2.
CONSORT Diagram.
Regarding within-group effects (presented here to contextualize within-group symptom changes across conditions, not as indicators of efficacy), adolescents randomized to each SSI condition reported significant reductions in depressive symptom severity from baseline to 3-month follow-up; within-group reductions were numerically larger for BA-SSI (t(820)=−9.62, p<0.001, d=−0.47, 95% CI −0.54, −0.39) and GM-SSI (t(812)=−12.29, p<0.001, d=−0.43, 95% CI −0.50, −0.36) than for the control (t(817)=−12.29, p<0.001, d=−0.34, 95% CI −0.41, −0.27)
Hopelessness and agency outcomes
(pre-registered secondary outcomes at post-intervention; exploratory secondary outcomes at follow-up; see Supplementary Tables 4, 5, 6, and 7 for full results of regression analyses, for which all assumptions for interpretation were met). Compared to adolescents randomized to the supportive control, adolescents randomized to the BA-SSI reported significant reductions in hopelessness at post-intervention (t(1637)=−5.22, padj<0.001, d=0.26, 95% CI 0.16, 0.36) and 3-month follow-up (t(1637)=−3.49, p<0.001, d=0.17, 95% CI 0.08, 0.27). The BA-SSI also led to increases in perceived agency at post-intervention (t(1637)=6.20, padj<0.001, d=−0.31, 95% CI 0.21, 0.40) but not follow-up (t(1637)=1.57, p=0.12, d=0.08, 95% CI −0.02, 0.17) versus the control. Likewise, adolescents randomized to the GM-SSI reported significant reductions in hopelessness at post-intervention ( t(1629)=−5.80, padj<0.001, d=0.28, 95% CI 0.18, 0.38) and follow-up (t(1629)=−3.00, p=0.002, d=0.15, 95% CI 0.05, 0.25), and increases in perceived agency at post-intervention (t(1629)=−3.22, padj<0.001, d=−0.15, 95% CI −0.24, −0.05) and follow-up (t(1629)=2.46, p= 0.01, d=−0.12, 95% CI 0.02, 0.22), versus control-group adolescents. Improvements in hopelessness at post-intervention and follow-up did not significantly differ for adolescents assigned to the BA-SSI, relative to adolescents assigned to the GM-SSI (at post-intervention, t(1632)=0.50, p=0.497, d=−0.03, 95% CI −0.06, 0.13; at follow-up, t(1632)=−0.55, p=0.585, d=0.03, 95% CI −0.12, 0.07). However, adolescents assigned to the BA-SSI reported larger increases in perceived agency, relative to adolescents assigned to the GM-SSI, at post-intervention (t(1632)=3.22, padj=0.001, d=0.16, 95% CI 0.06, 0.26). This difference did not persist at follow-up (t(1632)=−1.04, p=0.30, d=0.05, 95% CI −0.15, 0.05).
COVID-19-related trauma and anxiety outcomes
(pre-registered secondary outcomes; see Supplementary Tables 2 and 3 for full results of regression analyses, for which all assumptions for interpretation were met). Compared to adolescents randomized to the control SSI, adolescents randomized to the BA-SSI did not report decreases in generalized anxiety (t(1637)=−0.37, p=0.72, d=0.02, 95% CI −0.08, 0.12) or COVID-19-related trauma symptoms (t(1637)=−1.94, p=0.05, d=0.10, 95% CI −0.001, 0.19) from baseline to 3-month follow-up. However, adolescents randomized to the GM-SSI did report significant decreases in generalized anxiety symptom severity (t(1629)=−2.08, p=0.038, d=0.10, 95% CI 0.006, 0.20) and COVID-19-related trauma symptoms (t(1629)=−2.08, p=0.037, d=0.10, 95% CI 0.006, 0.20) at 3-month follow-up. Adolescents assigned to the BA- and GM-SSIs did not differ in their COVID-19-related trauma symptom reductions from pre-intervention to 3-month follow-up (t(1632)=0.28, p=0.78, d=−0.01, 95% CI −0.008, 0.11); however, GM-SSI led to greater reductions in generalized anxiety symptom severity compared to BA-SSI (t(1632)=2.01, p=0.044, d=−0.10, 95% CI 0.002, 0.20).
Restrictive eating outcomes
(post-hoc, exploratory secondary outcome; see Supplementary Table 8 for full results of regression analysis, for which all assumptions for interpretation were met). Compared to control group adolescents, those who received the BA-SSI reported significant reductions in past-month restrictive eating from pre- to 3-month follow-up (t(1637)=3.07, p=0.002, d=0.15, 95% CI 0.06, 0.25). Likewise, adolescents randomized to the GM-SSI reported significant reductions in restrictive eating from pre-intervention to 3-month follow-up (t(1629)=1.99, p=0.047, d=0.10, 95% CI 0.001, 0.20). Adolescents randomly assigned to the BA- and GM-SSIs did not significantly differ in restrictive eating changes from pre-intervention to 3-month follow-up (t(1632)=−1.31, p=0.153, d=−0.07, 95% CI −0.16, 0.03).
Sensitivity analyses.
To test the robustness of our results, we ran two sets of additional analyses using alternative approaches to handling missing data for all pre-registered analyses. Both of these were “completers-only” analyses; one used listwise deletion for participants lacking follow-up data, and the other used multiple imputation to estimate follow-up outcomes for all participants, but excluded participants who did not complete their assigned SSI. Code and results from these analyses are available on Open Science Framework (https://osf.io/8mk6x/). Compared to the control SSI, overall effects of the GM-SSI and BA-SSI on depressive symptoms, generalized anxiety symptoms, post-intervention and 3-month hopelessness, post-intervention and 3-month perceived agency; 3-month generalized anxiety symptoms) were generally unchanged using alternative missing data approaches. Only a few minor differences emerged from pre-registered imputation analyses, exclusively with respect to secondary outcomes. In our completers-only analyses with listwise deletion, the effect of the GM-SSI on COVID-related trauma symptoms at 3-month follow-up was non-significant, versus the control (t(987)=1.81, p=0.07, d=0.12, 95% CI −0.01, 0.12); second, the GM-SSI showed a significantly stronger (more positive) effect on generalized anxiety symptoms at 3-month follow-up than did the BA-SSI (t(994)=2.00, p=.045, d=0.13, 95% CI 0.002, 0.25). In our completers-only analyses with imputation for follow-up non-completers, the BA-SSI significantly reduced COVID-related trauma symptoms at 3-month follow-up, versus the control (t(1637)=2.51, p=0.01, d=0.12, 95% CI 0.03, 0.22), and the GM-SSI had a significantly greater, positive effect on generalized anxiety symptoms, versus the BA-SSI (t(1632)=2.20, p=0.02, d=0.11, 95% CI 0.01, 0.21). Thus, overall results patterns were similar—showing only minor differences with respect to secondary outcomes—regardless of our approach to handling missing data.
Discussion
In a nationwide randomized trial, we tested whether two half-hour, self-administered single-session interventions (SSIs) teaching behavioral activation (BA-SSI) and growth mindsets of personality (GM-SSI), respectively, reduced hopelessness, improved perceived agency, and lessened symptoms of depression, anxiety, and COVID-19-related trauma in high-symptom adolescents. Compared a supportive control SSI, both active interventions led to significantly greater improvements in depressive symptoms from baseline to 3-month follow-up (BA-SSI d=0.18; GM-SSI d=0.18). Additionally, both active interventions led to greater decreases in hopelessness at post-intervention (BA-SSI d=0.26; GM-SSI d=0.28) and follow-up (BA-SSI d=0.17; GM-SSI d=0.16), and increases in perceived agency at post-intervention (BA-SSI d=−0.31; GM-SSI d=−0.15) and follow-up (GM-SSI d=0.17), relative to the control. Regarding differential intervention effects, the GM-SSI (but not the BA-SSI) reduced three-month generalized anxiety symptoms (d=0.10) and COVID-19-related trauma symptoms (d=0.10) relative to the control. The BA-SSI led to greater post-SSI increases in perceived agency than did the GM-SSI (d=.16), whereas the GM-SSI led to greater 3-month reductions in generalized anxiety symptoms than did the BA-SSI (d=.10). Furthermore, exploratory post-hoc analyses revealed that both the BA-SSI and the GM-SSI significantly reduced restrictive eating from pre-intervention to 3-month follow-up, compared to the control. Sensitivity analyses using two alternative approaches to handling missing data yielded similar patterns of results, lending confidence to observed outcomes for pre-registered tests. Indeed, effect sizes observed for the BA-SSI and the GM-SSI’s effects on depressive symptoms were identical to meta-analytic estimates of single-session interventions’ effects on youth depressive symptoms (e.g., Schleider and colleagues (2017) identified a meta-analytic effect on depressive symptoms of d=0.1811).
Both active SSIs yielded modest reductions, on average, in adolescent depressive symptoms. Effect sizes were small, reflecting mean 3-month reductions of 2.64 points on the CDI-Short Form. Nonetheless, for several reasons, results hold clinical and practical importance. First, before and especially during the COVID-19 pandemic, adolescents experiencing depression have remained unlikely to access any mental health treatment—let alone evidence-based support—due to structural and logistical barriers. Many of these barriers, such as poverty, proximity to providers, and insurance status, are impossible to modify absent large-scale policy intervention. Thus, even modest overall clinical improvements produced by cost-free, 20-to-30-minute, self-guided interventions suggest their promise to enable efficient clinical benefits, which could be multiplied at the population level. Moving forward, research might focus less on how to strengthen the average magnitude of these SSIs’ impacts and more on identifying subsets of “best-responder” adolescents, guiding tailored dissemination based on individual odds of benefit.
Second, this trial represents a confirmatory extension of prior work suggesting the promise of SSIs for adolescent mental health. The use of an active control (which itself produced significant within-group decreases in depression symptoms), three-month follow-up, large sample, and high reproducibility (due to pre-registration and open-access intervention materials, data, and analytic code) strengthen confidence in results. Indeed, effects of many SSIs fall to near-zero after multi-month follow-up periods, and the effects of most adolescent mental health treatments reduce substantially when tested against active (versus no-treatment) controls.11, 21 Further, the average number of participants in randomized trials of youth psychotherapies to date has been 68.69 participants, rendering many trials underpowered for robust effectiveness tests.21 This study is among the largest U.S.-based randomized-controlled trials of any psychosocial treatment for adolescent depression to date.
Third, this study supports acceptability and effectiveness for brief, self-guided depression interventions among highly diverse adolescents. The sample included population-congruent representation of Black, Hispanic, Asian, and Native American youth in the United States; participants included youths living in all 50 U.S. states; and 80% of participants identified as sexual minorities. Although sexual minority youths were arguably over-represented in this study, most youth psychotherapy trials routinely include samples that are >90% White and seldom assess sexual orientation.21 Thus, this sample’s diversity extends the youth mental health knowledge-base, filling gaps in our knowledge of the youth intervention literature that have long remained unaddressed.
Fourth, positive effects on depressive symptoms are notable because (1) both active interventions yielded significant benefits, even in the high-stress context of a global pandemic; and (2) results address the need for more potent adolescent depression interventions. In fact, overall effect sizes across all trials of youth depression treatments have decreased from 1960 to the present.28 The SSIs in this trial might help improve population-level youth depression symptoms and outcomes, if disseminated broadly.
Both active SSIs yielded improvements in post-intervention hopelessness and perceived agency, although the BA-SSI increased agency more than the GM-SSI. These results align with evidence that many psychotherapy change-mechanisms may be more similar than different across programs, even when those programs are thought to target distinct mechanisms.29 Further, both SSIs are designed per a common set of components17 including neuroscience-based psychoeducation, narratives from peers, and opportunities to provide advice to others. These shared elements may promote a common set of adaptive thinking styles—perhaps reflected by the SSIs’ equivalent impacts on hopelessness. By contrast, the BA-SSI’s larger impacts on short-term perceived agency may reflect its unique elements, such as creating a personalized action plan for engaging in valued activities. Creating this plan may strengthen adolescents’ confidence in their ability to solve future mood-related problems, at least in the short-term. Indeed, items included in this study’s assessment of agency (e.g., “There are lots of ways around problems I am facing right now”) map more directly onto the BA-SSI than the GM-SSI.
Notably, per pre-registered analyses, the GM-SSI reduced 3-month generalized anxiety and COVID-19-related trauma versus the control, but the BA-SSI did not. This pattern fits with intended content specificity (or lack thereof) of each SSI. The BA-SSI provides psychoeducation specific to depression and low mood, whereas the GM-SSI is intentionally not diagnosis-specific; its message is that everyone has potential for change across multiple domains, including abilities to cope with anxiety and depression. Prior studies testing the GM-SSI in high-symptom adolescents have also found program benefits for both depression and anxiety symptoms.13 Future work may test the prospect of matching youth to the BA- or GM-SSI based on individual clinical needs and the strengths of each intervention.
Post-hoc exploratory analyses revealed that both the GM-SSI and the BA-SSI significantly reduced restrictive eating behaviors at 3-month follow-up, relative to the control. This result was unanticipated but aligned with research suggesting close ties between mood problems and disordered eating,30 with interventions targeting restrictive eating often yielding improvements in mood.31 However, few youth depression-focused intervention trials have evaluated restrictive eating as a secondary outcome. Present results support the utility of including such outcomes in depression treatment studies, including trials of SSIs. Given the dearth of treatment resources available to youths experiencing eating disorder symptoms32 and the inherent accessibility of the interventions in this trial, the potential for SSIs to mitigate restrictive eating directly merits future study.
How might these SSIs fit into broader youth mental healthcare ecosystems? In considering possibilities, it is crucial to note that SSIs are not panaceas. Overall effects were modest, some adolescents will still require longer-term services, and policy changes remain needed to improve youth access to higher-intensity treatments in addition to brief, immediately-accessible resources. Nonetheless, the SSIs in this trial may offer rapidly-scalable, evidence-based support for all youths with depressive symptoms—many of whom would otherwise access no support at all. Given the brevity and flexibility of these programs, they may be offered within diverse youth care systems (e.g., primary care, emergency rooms, and schools), complementing existing support structures while retaining potential to benefit youths unable to access further care.
A notable feature of this study is adolescents’ ability to “self-refer” into SSIs. In many U.S. states, adolescents aged ≥12 may consent, without a parent/guardian, to mental and physical healthcare. These laws were enacted to increase adolescents’ likelihood of seeking and obtaining care. For myriad reasons, allowing youth participation in minimal-risk online SSIs without requiring parental consent may be a critical path toward increasing youth treatment access and use. This study suggests that adolescents can safely participate in online SSIs, and that SSIs may reduce both short- and longer-term mental health difficulties. Requiring parent/guardian approval for adolescents to try these online activities could prevent thousands of youth from receiving minimal-risk, free, evidence-based support.33-34 Unfortunately, requiring parent permission may pose disproportionate barriers for sexual minority, gender minority, and racial/ethnic minority youth, for whom concerns about caregiver stigma or dismissal (linked to rejection of one’s identity, beliefs about mental health treatment-seeking, or both) often prevent youth from disclosing treatment needs to family.35 Although not all states have yet adopted these guidelines, SSIs tested here pose minimal risk to safety and may offer a common-sense path to increasing access to mental health support among adolescents.
Some study limitations warrant consideration and suggest directions for future work. First, eligibility screening relied on adolescents’ interest in completing an online study and required internet access. Some adolescents may require support from adults to access mental health treatment, some may lack Internet access, others may not have the time for this type of study, and other still might speak languages other than English (the SSIs were not available in other languages at the time of this trial). Thus, findings may not apply uniformly across adolescents. However, most prior trials of psychosocial interventions for adolescents have required parent referral and consent; by eliminating this barrier, this study may have included adolescents under-represented in prior clinical trials, such as adolescents who are not open with caregivers about mental health difficulties.
Second, attrition at follow-up was ~40%. Missing data were addressed via a rigorous approach that has shown utility with higher missing data rates than observed here, and sensitivity analyses using alternative data approaches yielded similar patterns of results.36 Although results may have been influenced by these missing data, this rate is comparable to those observed in previous randomized trials of youth depression interventions (e.g. The Treatment for Adolescents with Depression Study).5, 37 Future investigations might formally compare methods for increasing participant retention across longer-term study periods.
Third, some groups of youth were potentially over-represented in our sample (e.g., sexual minority youth), whereas others were underrepresented (e.g., boys). These sample characteristics are unlikely to reflect our Instagram-based recruitment approach, as 72% of teens ages 13-17 actively use Instagram, nearly half of whom are boys.38 Thus, our sample may reflect youths most readily drawn to taking part in online self-help activities. Focus group-based and mixed-methods research, geared toward gathering youth feedback and guidance, may forward efforts to engage adolescent boys in digital, mental health-focused SSIs.
Fourth, although SSI did not predict attrition at 3-month follow-up, youths randomized to the BA-SSI were more likely than those in the GM-SSI and control conditions to complete their assigned intervention (although completion rates were very high across conditions, >80%, compared to the majority of self-guided digital mental health programs27). It is possible that the greater interactivity of the BA-SSI (e.g., creation of an action plan) contributed to this higher completion rate, but tests of engagement-enhancing components of online SSIs remains a key direction for future work.
Lastly, we did not formally assess youths’ use of other mental health supports during the study period. Notably, SSIs may function best as complements to (rather than replacements for) other forms of care. Indeed, a prior randomized trial demonstrated no effect of external mental health treatment (e.g., psychotherapy and/or medication) on response to the GM-SSI, versus a placebo control, across a 9-month follow-up.13 Future trials might collect data to capture the role of SSIs in the context of youths’ full range of formal and informal mental health supports.
In sum, results suggest that free-of-charge, 20-to-30 minute online interventions can reduce depressive symptoms, hopelessness, and anxiety and strengthen perceived agency across three-months in high-symptom adolescents, even in the context of a global pandemic. Although differences across the active interventions emerged, these brief supports could be made freely available for adolescents to complete anytime, from any location, regardless of traditional barriers to mental health care.
Method
Ethics Information.
Procedures were approved by the University of Denver institutional review board, and adolescents provided online assent prior to participating. To maintain adolescents’ confidentiality and minimize access barriers (e.g., discomfort disclosing psychological distress, as parents are often unaware of their adolescents’ depressive symptoms, including suicidal ideation, in up to 80% of cases),39 parent permission was not required to participate in this study (waived by the University IRB). All procedures were pre-registered prospectively, before enrollment of the first participant (ClinicalTrials.gov: NCT04634903). As a minimal-risk study (per the IRB’s determination), we did not expect any adverse events to occur during the study period; as such, we included no explicit assessments of adverse events. No incidental adverse events of any kind were reported by participants or identified by the researchers during the study period.
Recruitment and Resulting Sample.
Participants learned about the study through Instagram advertisements following established ethics guidelines for passive, social media-based recruitment.40 Posts included invitations to determine eligibility for a confidential, online psychology study, for which participants could earn up to $20 USD in gift cards; Supplemental Figure 1 depicts an example advertisement from our study. Instagram posts linked to a Qualtrics survey; the first page included study details and an invitation to complete an eligibility screener. Inclusion criteria were (1) being between 13-16 years old (inclusive); (2) comfort reading and writing in English; (3) internet and computer, laptop, or smartphone access; and (4) endorsement of elevated depressive symptoms, per a score of >=2 on the Patient Health Questionnaire-2 (this cut-off prioritizes sensitivity over specificity in identifying youth with elevated depressive symptoms).41 Eligible adolescents then reviewed an online assent form inviting them to participate. Recruitment took place from November 19-December 6, 2020 (on which date >2,400 youth had been randomized to an intervention condition). Three-month follow-up surveys were completed by March 15, 2021 (youth received 3-week windows to finish their follow-up survey). Of 6,884 youths who completed the screener, 3,851 were eligible and agreed to participate; 2,452 completed the baseline survey and were randomized to an SSI; and 1,766 of those randomized were retained at follow-up. Rates of follow-up survey completion did not differ by intervention condition. Participants could complete the study from any U.S. location (Supplementary Figure 1 illustrates the geographic distribution of study participants), using any internet-equipped device.
Procedures.
Per the CONSORT Diagram (Figure 1), participants were invited to complete pre-SSI questionnaires; one of three 20-30 minute SSIs (youths were randomly assigned to one of three SSIs in a 1:1:1 ratio per a Qualtrics-embedded randomizer; youth and investigators were masked to condition assignment until after all data collection was complete); and post-SSI questionnaires, within one 50-to-60-minute session. Youths then received an email invitation three months later to complete a 10-minute follow-up questionnaire, also via Qualtrics, including a subset of measures from the baseline survey, to assess the SSIs’ effects on primary and secondary outcomes. After all data collection was complete, the researchers learned each youth’s condition assignment, and both active interventions were offered to all participants. Additionally, participants received a resource list of hotlines, textlines, and online psycho-educational resources to facilitate engagement with additional mental health supports, if desired. Adolescents were also invited to contact the research team at any time during the study with questions or for further support in accessing mental health support beyond the study’s scope.
Interventions.
Behavioral Activation SSI (BA-SSI) — ABC Project.17 The BA-SSI includes 5 elements: (1) An introduction to the program’s rationale: that engaging in values-based activities that build pleasure and accomplishment can combat sad mood and low self-esteem; (2) Psychoeducation about depression, including how behavior shapes feelings and thoughts; (3) A life values assessment, where youth identify key areas (family relationships, friendships, school, or hobbies) from which they draw (or once drew) enjoyment and meaning; (4) Creation of an activity “action plan,” where youth identify (from pre-generated lists) and personalize (in guided exercises) 3 activities to target for change; and (5) An exercise in which youths write about benefits that might result from engaging in each activity; an obstacle that might keep them from doing the activities; and a strategy for overcoming identified obstacles. Intervention materials are available here: https://osf.io/ch2tg/.
Growth Mindset SSI (GM-SSI) — Project Personality.12-13 This self-administered youth program includes: (1) An introduction to the brain and a lesson on neuroplasticity; (2) Testimonials from older youths who describe their views that traits are malleable, due to the brain's plasticity; (3) Further stories by older youths, describing times when they used “growth mindsets” to persevere during social/emotional setbacks; (4) Study summaries noting how/why personality can change; and (5) An exercise in which youths write notes to younger students, using scientific information to explain people's capacity for change. Intervention materials are available here: https://osf.io/a9uv2/
Supportive Therapy SSI (Placebo).12-13 This self-administered supportive therapy (placebo) is structurally similar to the other SSIs (e.g., it is length-matched; includes peer narratives; and contains the same number of writing activities), but is designed to control for nonspecific aspects of completing a generally-supportive online activity. The program encourages participants to express emotions to close others; it does not teach specific skills. In a previous trial, this program predicted smaller reductions in youth internalizing problems versus a GM-SSI.13 Intervention materials are available here: https://osf.io/u4axs/.
Per prior open and randomized trials including the ABC Project, Project Personality, and the Supportive Therapy SSI, each of these interventions take approximately 20-30 minutes for adolescents experiencing depressive symptoms to complete.13,17
Outcomes.
Listed below are the key primary and secondary trial outcomes for which we pre-registered confirmatory (3-month depressive symptoms; post-intervention hopelessness; post-intervention perceived agency) and exploratory analyses (3-month hopelessness; 3-month perceived agency; 3-month generalized anxiety; 3-month COVID-19-related trauma). We also included a handful of additional, exploratory secondary outcomes, as noted in our clinicaltrials.gov registration. These were: 3-month restrictive eating; presence of lifetime suicidal ideation; 3-month approach-based coping; and post-intervention implicit theory of personality. Because no analyses were pre-registered regarding these outcomes, results of post-hoc analyses testing intervention effects on these variables are detailed in the Supplemental Tables 8-11. Additionally, we include results for one of these variables (restrictive eating) in the main text due to unanticipated, significant intervention effects revealed in post-hoc analyses. Lastly, also in the supplemental materials (Supplementary Table 12), we list all measures collected as part of the trial, defined as primary, secondary, or ‘other’, as well as the time-points at which they were administered to participants (including baseline-only, exploratory “other” measures for which no analyses were pre-registered and no descriptive statistics are reported in this manuscript).
Adolescent depressive symptoms (primary outcome). Depressive symptom severity was assessed using the Children’s Depression Inventory (CDI) 2 - short form (CDI-SF), a reliable, valid measure of youth depression severity.42 Twelve items are scored from 0-2, with higher summed scores reflecting greater overall depression symptom severity. Changes in CDI-SF scores from pre-SSI to three-month follow-up was the primary study outcome. Alphas were 0.77 and 0.85 at baseline and follow-up.
Adolescent anxiety symptoms. Generalized anxiety symptom severity was measured using the Generalized Anxiety Disorder 7 (GAD-7), which includes 7 items asking respondents how often, during the last 2 weeks, they were bothered by various anxiety symptoms.43 Items are rated on a 0-3 scale and averaged to yield an overall score. Changes in GAD-7 scores from pre-SSI to follow-up was a secondary outcome. Alphas were 0.89 and 0.90 at baseline and follow-up.
COVID-19-related Trauma Symptoms. Child Trauma Screen—Reaction Scale (CTS-RS) was administered at pre-SSI and 3-month follow-up.44 The CTS-RS is a reliable, valid self-report measure of traumatic stress symptom severity, including event-related somatic symptoms, intrusive memories, avoidance, sleep problems, and mood and behavioral changes. Here, instructions read: “For many, the COVID-19 (or ‘coronavirus’) pandemic has been scary or very upsetting. Sometimes, events that are scary or upsetting can affect how people think, feel, and act. The next questions ask how you have been feeling and thinking recently.” Youth rated the frequency of 6 types of traumatic stress symptoms, per past-month frequency, based on a 0-3 scale; ratings were averaged to yield an overall severity score. Changes in CTS-RS scores from pre-SSI to follow-up was a secondary outcome. Alpha was 0.73 at both baseline and follow-up.
Perceived Agency. The “agency” subscale of the State Hope Scale (SHS) is a reliable, valid 3-item self-report measure of youth’ perceived ability to generate plans and work towards one’s goals.45 Pre- and post-intervention, youths rated their agreement with 3 statements reflecting how they felt about themselves “right now” on an 8-point scale. Alphas were 0.82, 0.83, and 0.80 at pre-SSI, post-SSI, and follow-up.
Hopelessness. The Beck Hopelessness Scale-4 (BHS-4) is a reliable, shortened version of the 20-item scale used to measure hopelessness in youth.46-47 Pre- and post-intervention and at follow-up, participants rated their agreement with 4 statements reflecting their sense of hopelessness “right now, in this moment” on a 0-3 Likert scale. Alphas were 0.84, 0.87, and 0.88 at pre-SSI, post-SSI, and follow-up.
Intervention Acceptability. The Program Feedback Scale (PFS) asks youth to rate agreement with 7 statements indicating perceived acceptability of an SSI (e.g. “I enjoyed the program”) on a 5-point Likert scale (1=“really disagree”; 5=“totally agree”).17, 48 We pre-registered that scores of ≥3.5/5 on any given PFS item would reflect an “acceptable” rating on that item.
Restrictive (disordered) Eating. Restrictive eating behaviors were measured at pre-intervention and 3-month follow-up using The Dietary Restriction Screener (DRS-2).49 The DRS-2 is a 2-item measure evaluating restrictive eating behaviors. The 2 items ask participants whether or not they have engaged in restrictive eating in the past year or in the past 3 months (0=no; 1=yes). The DRS-2 was administered at pre-SSI and 3-month follow-up. Given the timing of the assessments in this study, we examined intervention effects on the presence of past-month restrictive eating only. No analyses were pre-registered for this outcome, and all analyses were conducted post-hoc with respect to intervention effects on restrictive eating..
Power analysis.
The pwr R package was used to calculate the Power for all planned contrasts detailed below. An N=2,400 (800 per group) and 2 time-points (pre-intervention and 3-month follow-up) yields 98% Power to detect a small effect size of d=0.20 on all outcomes when comparing SSIs. Notably, our final sample (N=2,452) was 52 participants greater than our pre-registered sample size, as a group of youths completed our eligibility screener within hours of a study advertisement gaining traction on Instagram. We stopped recruitment as soon as we learned that our pre-registered threshold had been met.
Analysis Plan.
We ran a series of linear regressions to test whether the GM-SSI and BA-SSI predicted (1) improvements in proximal post-SSI targets (perceived agency; hopelessness), and (2) reductions in adolescent depressive symptom severity (primary study outcome) from pre-SSI to 3-month follow-up, relative to (1) each other, and (2) the control SSI, when considered independently. SSI condition was a categorical predictor in these models, and parallel linear regressions were conducted to assess intervention effects on secondary and other exploratory study outcomes. We imputed participant-level missing data using the expectation-maximization and bootstrapping algorithm implemented with Amelia II in R,50 allowing more conservative intent-to-treat analyses than other approaches (e.g., listwise deletion; last-observation carried-forward). We imputed as many datasets as there were percent of missing data for an outcome, rounding to the next-highest percentage (e.g., If 5.4% of data was missing on an outcome, we created 6 imputed datasets), allowing us to retain high power despite missing data. Cohen’s d and 95% confidence intervals for analyses were calculated using t-values for treatment effects obtained from analyses with the MOTE package in R.51 The false discovery rate (FDR) was applied to identify potential false-positive results.52 Results from pre-registered tests described above were considered significant if FDR-corrected p<0.05, and all tests were two-tailed. Lastly, for exploratory purposes (and to contextualize within-group changes in symptom levels over time in each condition), we computed effect sizes reflecting within-group intervention effects on depression (the primary outcome) for each SSI condition.
Differential SSI completion and attrition.
We ran post-hoc Z-tests of proportions analyses to assess whether rates of SSI completion and 3-month follow-up survey completion differed by condition assignment. We then ran 2 separate logistic regression models with baseline depression symptom severity, age, gender identity, sexual orientation, romantic/sexual attraction, and racial identity to determine if those characteristics could predict which participants would drop out either before completing the intervention or before initiating the 3-month follow-up.
Sensitivity analyses.
Given the high rate of missing data that characterizes many clinical trials, and to gauge whether results might have differed by our approach to handling missing data, we re-ran pre-registered analyses using two alternative approaches to handling missing data. First, we ran “completers-only” analysis, using listwise deletion for participants who did not complete the follow-up survey. Second, we ran a “completers-only” analyses, wherein participants who did not complete their assigned SSI were excluded, with multiple imputation (as described above) for 3-month follow-up outcomes.
Supplementary Material
Acknowledgements.
This study was supported by the Office of the Director, National Institutes of Health under an “Emergency COVID-19 Competitive Revision Award” linked with grant DP5OD028123 (PI: Schleider). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests.
JLS receives grant support from the National Institutes of Health (DP5OD28123), the Klingenstein Third Generation Foundation, the American Psychological Foundation, the Society for Clinical Child and Adolescent Psychology, and Limbix, Inc. JLS and KRF receive grant support from the Upswing Fund for Adolescent Mental Health. MLD receives grant support from a Stony Brook University Graduate Research Fellowship and the Psi Chi Honor Society. JLS, MLD, and MCM have co-authored and receive royalties from sales of a therapeutic workbook for adolescents, published by New Harbinger. JLS is under contract with Oxford University Press to co-edit a book on low-intensity mental health interventions for youth. The authors report no other financial conflicts.
Footnotes
ClinicalTrials.gov Identifier: NCT04634903; Open Science Framework pre-registration: osf.io/kumdv
Code availability. Analytic code is available on the Open Science Framework: https://doi.org/10.17605/OSF.IO/8MK6X
Data availability.
Anonymized participant-level data is available on the Open Science Framework, https://doi.org/10.17605/OSF.IO/8MK6X
References
- 1.Golberstein E, Wen H, Miller BF Coronavirus disease 2019 (COVID-19) and mental health for children and adolescents. JAMA Pediatrics 174(9), 819–820 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Whiteford HA et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. The Lancet 382, 1575–1586 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Hammen C, Brennan PA, & Keenan-Miller D Patterns of adolescent depression to age 20: The role of maternal depression and youth interpersonal dysfunction. Journal of Abnormal Child Psychology 36, 1189–1198 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Wilson S, Hicks BM, Foster KT, McGue M, & Iacono WG Age of onset and course of major depressive disorder: associations with psychosocial functioning outcomes in adulthood. Psychological Medicine 45, 505–514 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.March JS et al. The Treatment for Adolescents With Depression Study (TADS): long-term effectiveness and safety outcomes. Archives of General Psychiatry 64, 1132–1144 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Vitiello B et al. Long-term outcome of adolescent depression initially resistant to SSRI treatment. Journal of Clinical Psychiatry 72, 388–396 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried EI, & Nesse RM Depression sum-scores don’t add up: why analyzing specific depression symptoms is essential. BMC Medicine 13, 72 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried EI, & Nesse RM Depression is not a consistent syndrome: an investigation of unique symptom patterns in the STAR*D study. Journal of Affective Disorders 172, 96–102 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forman-Hoffman V et al. Screening for Major Depressive Disorder in Children and Adolescents: A Systematic Review for the U.S. Preventive Services Task Force. Evidence synthesis no. 116. AHRQ publication no. 13-05192-EF-1. Rockville, MD. Agency for Healthcare Research and Quality; (2016). [Google Scholar]
- 10.Avenevoli S, Swendsen J, He JP, Burstein M, & Merikangas KR Major depression in the National Comorbidity Survey–Adolescent Supplement: prevalence, correlates, and treatment. Journal of the American Academy of Child & Adolescent Psychiatry 54, 37–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schleider JL, & Weisz JR Little treatments, promising effects? Meta-analysis of single session interventions for youth psychiatric problems. Journal of the American Academy of Child & Adolescent Psychiatry 56, 107–115 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Schleider JL, Burnette JL, Widman L, Hoyt C, & Prinstein MJ Randomized trial of a single-session growth mindset intervention for rural adolescents’ internalizing and externalizing problems. Journal of Clinical Child & Adolescent Psychology 49(5), 660–672 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schleider J, & Weisz J A single-session growth mindset intervention for adolescent anxiety and depression: Nine-month outcomes of a randomized trial. Journal of Child Psychology and Psychiatry 59, 160–170 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Gawrysiak M, Nicholas CRN, & Hopko DR Behavioral activation for moderately depressed university students: Randomized controlled trial. Journal of Counseling Psychology 56, 468–475 (2009). [Google Scholar]
- 15.Armento MEA, McNulty JK, & Hopko DR Behavioral activation of religious behaviors (BARB): Randomized trial with depressed students. Psychology of Religion and Spirituality 4, 206–222 (2012). [Google Scholar]
- 16.Huguet A, Rao S, McGrath PJ, Wozney L, Wheaton M, Conrod J, & Rozario S A systematic review of cognitive behavioral therapy and behavioral activation apps for depression. PloS one, 11, e0154248 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schleider JL, Dobias ML, Sung JY, Mumper E, & Mullarkey MC Acceptability and utility of an open-access, online single-session intervention platform for adolescent mental health. JMIR Mental Health 7(6), e20513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schleider JL, Dobias ML, Sung JY, Mullarkey MC Future directions in single-session youth mental health interventions. Journal of Clinical Child and Adolescent Psychology 2, 264–278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollon S, Garber J, & Shelton R Treatment of depression in adolescents with cognitive behavior therapy and medications: A commentary on the TADS project. Cognitive and Behavioral Practice 12, 149–155 (2005). [Google Scholar]
- 20.Chu BC, Colognori D, Weissman AS, & Bannon K An initial description and pilot of group behavioral activation therapy for anxious and depressed youth. Cognitive and Behavioral Practice 16, 408–419 (2009). [Google Scholar]
- 21.Weisz JR et al. What five decades of research tells us about the effects of youth psychological therapy: A multilevel meta-analysis and implications for science and practice. American Psychologist 72, 79–117 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Kataoka SH, Zhang L, & Wells KB Unmet need for mental health care among U.S. children: variation by ethnicity and insurance status. American Journal of Psychiatry 159, 1548–1555 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Harpaz-Rotem I, Leslie D, & Rosenheck RA Treatment retention among children entering a new episode of mental health care. Psychiatric Services 55, 1022–1028 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Miu AS, & Yeager DS Preventing symptoms of depression by teaching adolescents that people can change: Effects of a brief incremental theory of personality intervention at 9-month follow-up. Clinical Psychological Science 3, 726–743 (2015). [Google Scholar]
- 25.Schleider JL, Abel M, & Weisz JR Do immediate gains predict long-term symptom change? Findings from a randomized trial of a single-session intervention for youth anxiety and depression. Child Psychiatry and Human Development 50, 868–881 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Makover HB et al. Mediators of Youth Anxiety Outcomes 3 to 12 Years After Treatment. Journal of Anxiety Disorders 70, 102188 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumel A, Muench F, Edan S, & Kane J Objective User Engagement With Mental Health Apps: Systematic Search and Panel-Based Usage Analysis. Journal of Medical Internet Research 21, e14567 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisz JR et al. Are psychotherapies for young people growing stronger? Tracking trends over time for youth anxiety, depression, attention-deficit/hyperactivity disorder, and conduct problems. Perspectives on Psychological Science 14(2), 216–237 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Cuijpers P, Reijnders M, & Huibers MJ The role of common factors in psychotherapy outcomes. Annual Review of Clinical Psychology 15, 207–231 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Stice E, & Van Ryzin MJ A prospective test of the temporal sequencing of risk factor emergence in the dual pathway model of eating disorders. Journal of Abnormal Psychology 128, 119–128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stice E, Marti CN, Shaw H, & Rohde P Meta-analytic review of dissonance-based eating disorder prevention programs: Intervention, participant, and facilitator features that predict larger effects. Clinical Psychology Review 70, 91–107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissman RS & Rosselli F Reducing the burden of suffering from eating disorders: Unmet treatment needs, cost of illness, and the quest for cost-effectiveness. Behaviour Research and Therapy 88, 49–64 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Wilson CJ, & Deane FP Brief report: Need for autonomy and other perceived barriers relating to adolescents’ intentions to seek professional mental health care. Journal of adolescence 35(1), 233–237 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Samargia LA, Saewyc EM, & Elliott BA Foregone mental health care and self-reported access barriers among adolescents. The Journal of School Nursing 22(1), 17–24 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Brown A, Rice SM, Rickwood DJ, & Parker AG Systematic review of barriers and facilitators to accessing and engaging with mental health care among at-risk young people. Asia-Pacific Psychiatry 8(1), 3–22 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Taylor L, & Zhou XH Multiple imputation methods for treatment noncompliance and nonresponse in randomized clinical trials. Biometrics 65(1), 88–95 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Treatment for Adolescents with Depression Study (TADS) Team et al. The Treatment for Adolescents with Depression Study (TADS): Outcomes over 1 year of naturalistic follow-up. American Journal of Psychiatry 166, 1141–1149 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Pew Research Center. Teens’ social media habits and experiences. Retrieved from: https://www.pewresearch.org/internet/wp-content/uploads/sites/9/2018/11/PI_2018.11.28_teens-social-media_FINAL4.pdf. (2018).
- 39.Smith DMY, Lipson S,M, Wang SB, & Fox KR Online methods in adolescent self-injury research: Challenges and Recommendations. Journal of Clinical Child and Adolescent Psychology (2021). [DOI] [PubMed] [Google Scholar]
- 40.Gelinas L et al. Using social media as a research recruitment tool: ethical issues and recommendations. The American Journal of Bioethics 17(3), 3–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson LP et al. Evaluation of the PHQ-2 as a brief screen for detecting major depression among adolescents. Pediatrics 125, e1097–e1103 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovacs M Children’s Depression Inventory - 2 (2nd Ed). North Tonawanda, New York: Multi-Health Systems Inc. (2011). [Google Scholar]
- 43.Spitzer RL, Kroenke K, Williams JB, & Löwe B A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of internal medicine 166(10), 1092–1097 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Lang JM, & Connell CM Development and validation of a brief trauma screening measure for children: The Child Trauma Screen. Psychological Trauma: Theory, Research, Practice, and Policy 9, 390 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Snyder CR et al. Development and validation of the State Hope Scale. Journal of personality and social psychology 70(2), 321–335 (1996). [DOI] [PubMed] [Google Scholar]
- 46.Rhoades H et al. Homelessness, Mental Health and Suicidality Among LGBTQ Youth Accessing Crisis Services. Child Psychiatry & Human Development 49(4), 643–651 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Forintos DP, Rózsa S, Pilling J, & Kopp M Proposal for a short version of the Beck Hopelessness Scale based on a national representative survey in Hungary. Community Mental Health Journal 49, 822–830 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Schleider JL, Mullarkey MC, & Weisz JR Virtual reality and web-based growth mindset interventions for adolescent depression: Protocol for a three-arm randomized trial. JMIR research protocols 8, e13368 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haynos AF, & Fruzzetti AE Initial evaluation of a single-item screener to assess problematic dietary restriction. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity 20, 405–413 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honaker J, King G, & Blackwell M Amelia II: A Program for Missing Data. Journal of Statistical Software, 45(7), 1–47 (2011). [Google Scholar]
- 51.Buchanan E, Gillenwaters A, Scofield J, Valentine K MOTE: Measure of the Effect: Package to assist in effect size calculations and their confidence intervals. R package version 1.0.2, http://github.com/doomlab/MOTE (2019). [Google Scholar]
- 52.Benjamini Y, & Hochberg Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 57(1), 289–300 (1995). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized participant-level data is available on the Open Science Framework, https://doi.org/10.17605/OSF.IO/8MK6X