Abstract
Objectives:
Tobacco smoking is linked to cognitive deficits and greater white matter (WM) abnormalities in people with HIV disease (PWH). Whether tobacco smoking additionally contributes to brain atrophy in PWH is unknown and was evaluated in this study.
Design:
We used a 2 × 2 design that included 83 PWH (43 nonsmokers, 40 smokers) and 171 HIV-seronegative (SN, 106 nonsmokers, 65 smokers) participants and assessed their brain structure and cognitive function.
Methods:
Selected subcortical volumes, voxel-wise cortical volumes and thickness, and total WM volume were analyzed using FreeSurfer. Independent and interactive effects of HIV and smoking were evaluated with two-way analysis of covariance on cognitive domain Z-scores and morphometric measures on T1-weighted MRI.
Results:
Regardless of smoking status, relative to SN, PWH had smaller brain volumes [basal ganglia, thalami, hippocampi, subcortical gray matter (GM) and cerebral WM volumes (p=0.002–0.042)], steeper age-related declines in the right superior-parietal (interaction-p<0.001) volumes, and poorer attention/working memory and learning (p=0.016–0.027). Regardless of HIV serostatus, smokers tended to have smaller hippocampi than nonsmokers (−0.6%, p=0.055). PWH smokers had the smallest total and regional subcortical GM and cortical WM volume and poorest cognitive performance.
Conclusions:
Tobacco smoking additionally contributed to brain atrophy and cognitive deficits in PWH. The greater brain atrophy in PWH smokers may be due to greater neuronal damage or myelin loss in various brain regions, leading to their poor cognitive performance. Therefore, tobacco smoking may exacerbate or increase the risk for HIV-associated neurocognitive disorders.
Keywords: HIV, tobacco, brain volume, cognitive function, MRI
1. INTRODUCTION
Tobacco smoking is more prevalent (36% vs. 16–21%) and associated with greater adverse consequences in people with HIV disease (PWH) [1–3] than in the general population. For instance, tobacco smoking may attributed to 62% of deaths among PWH compared to 34% in the general population [4]. In addition, tobacco smoking is associated with worse viral suppression [5, 6] and lower CD4 cell counts [7] in PWH. Conversely, HIV infection accelerates nicotine metabolism [8, 9], which leads to greater tobacco usage [10], more severe craving and withdrawal symptoms [11], and difficulty with smoking cessation in HIV-seropositive (PWH) smokers compared to HIV-seronegative (SN) smokers [12].
Both HIV infection and tobacco smoking are associated with pathological changes in the brain. Persistent HIV-mediated neuroinflammation and neurotoxicity may result in neuronal and glial loss or white matter (WM) damage, even in treated individuals, contributing to HIV-associated neurocognitive disorders (HAND) observed in 30–50% of PWH [13–15]. Tobacco smoke induces oxidative stress, particularly in the cerebrovascular endothelial cells, and causes brain-blood barrier (BBB) leakage [16, 17]. Tobacco smoking was also associated with reduced brain volumes and WM abnormalities [18–22], cognitive impairment [23], and increased risk of dementia [24]. Despite the popularity of and vulnerability to tobacco smoking in PWH, surprisingly little is known regarding whether tobacco smoking exacerbates the neuropathology in PWH.
Preclinical studies have provided indirect evidence that the combination of HIV infection and tobacco smoke may lead to greater neuroinflammation. For example, cigarette smoke exposure induced greater neuroinflammatory gene expression and more impaired locomotor activity in HIV transgenic rodents than wild-type [25]. In addition, nicotine, the major addictive component of tobacco, suppressed neuroinflammation in wild-type [26], but promoted neuroinflammation and induced neuronal apoptosis in HIV transgenic rodents [27]. Clinical studies showed tobacco smoking and HIV infection appear to have additive adverse effects on cognitive function [28–31], psychopathology [31], WM structural integrity [22], as well as cortical gray matter (GM) volume [28]. However, most clinical studies typically compared smokers and nonsmokers among PWH and did not delineate the independent or possible interactive effects of tobacco smoking and HIV infection on the brain.
Therefore, we aim to expand upon our prior findings on the additive effects of tobacco smoking and HIV infection on cognitive deficits, to evaluate for the possible additive or interactive effects on brain atrophy. We hypothesized that 1) PWH tobacco smokers would have the smallest cortical and subcortical volumes, and the poorest cognitive performance compared to SN smokers and nonsmokers with or without HIV disease; and 2) those with smaller brain volumes would have poorer cognitive performance regardless of HIV serostatus or smoking status.
2. MATERIAL AND METHODS
2.1. Participants
Participants were recruited from the local community through website advertisements, flyers, word of mouth, and referrals from health care providers. In total, 720 participants each completed a telephone screening, and 447 (62%) completed an in-person screening evaluation and provided written informed consent for several prior studies [32–35]. The clinical and MRI data from these prior studies were pooled for this current study and anonymized before data analyses. Ultimately, 304 (68%) subjects fulfilled the current study inclusion criteria, and 254/304 (84%) had usable imaging and cognitive datasets. This included 106 SN nonsmokers, 65 SN smokers, 43 PWH nonsmokers, and 40 PWH smokers. Inclusion criteria for PWH participants were: 1) 18–80 years old men or women; 2) HIV seropositivity confirmed by medical records; and 3) stable on a cART regiment ≥6 months and plasma viral load was undetectable. All SN participants fulfilled study criterion 1 and their HIV-negative serostatus was verified using Clearview® COMPLETE HIV 1/2 test kits (Alere, Waltham, MA). All tobacco smokers were current smokers (within the past 6 months) and smoked more than 10 cigarettes a day for more than two years, which is the typical pattern among PWH [36–39]. Nonsmokers had a lifetime use of tobacco less than 2 pack-years and had not smoked in the past 2 years. Participants were excluded if they: 1) had any confounding medical conditions (including active hepatitis C, verified by medical records, or with QraQuick® HCV test on-site); 2) had a confounding neurological or psychiatric disorder; 3) had a severe substance use disorder (according to the DSM-5 criteria, except for tobacco use disorders) within the past two years; 4) had an education level lower than 8th grade or intelligence quotient (IQ) less than 70 based on the Wechsler Test of Adult Reading (WTAR); or 5) any contraindications for MR studies. The participants in this study were allowed to have recreational marijuana and social alcohol use (up to 1 drink per day for women and up to 2 drinks per day for men), but a negative urine toxicology screening test was required on the day of the brain scan and neuropsychological testing. The original study protocols were approved by the Committee on Human Studies at the University of Hawaii at Manoa and the Queen’s Medical Center in Honolulu, HI, and a Certificate of Confidentiality was obtained from the National Institute on Drug Abuse.
2.2. Clinical and Cognitive Assessments
All participants were evaluated by a study physician, including the review of medical history and HIV disease-related information, such as the nadir and most recent CD4 cell counts and plasma HIV viral loads. The study physician also performed a neuropsychiatric examination, including screening for depressive symptoms with the Centers for Epidemiologic Studies-Depression Scale (CES-D) [40], daily functioning using the Karnofsky Performance Status Scale [41] and the Functional Activities Questionnaire [42] or Instrumental Activities of Daily Living [43], as well as clinical cognitive assessment from the neurological history and the HIV Dementia Scale [44]. Estimated verbal IQ was assessed using the WTAR [45]. The Hollingshead Four Factor Index of Socioeconomic Status was used to assess the participants’ socioeconomic status, which provided the Index of Social Position (ISP) [46].
We used a neuropsychological test battery that contained the 7 required cognitive domains to assess HAND [47], in addition to the clinical assessments described above, to determine the HAND status in PWH and HAND-equivalent status in SN participants. The seven domains included Fluency, Executive function, Learning, Attention/Working Memory, Memory, Information Processing Speed and Motor skill. The subtests included in each domain are listed in the Figure 1 legend. For each subtest, an age and education adjusted Z-score was calculated based on our existing normative database (which included 538 healthy SN controls tested using the same protocol). A domain Z-score was generated using the average value of subtest Z-scores.
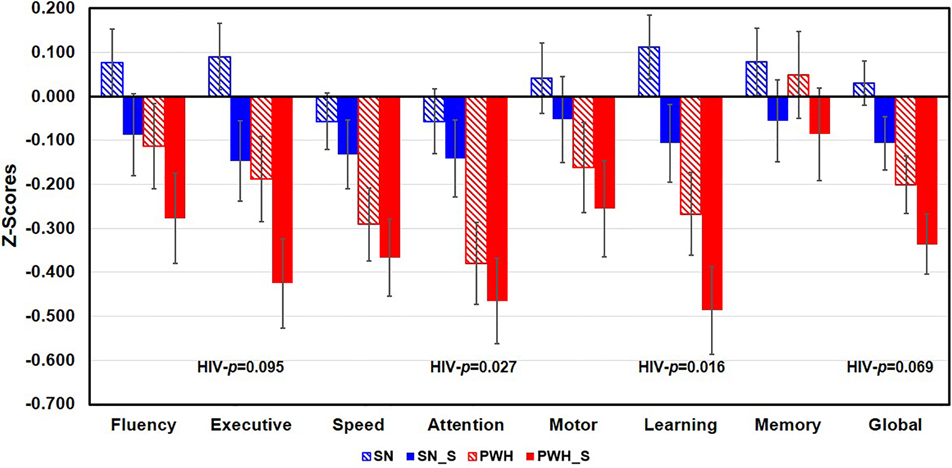
Figure 1. Cognitive Domain Z-Scores in the Four Participant Groups.
Regardless of smoking status, PWH participants had lower Z-scores in the Attention (p=0.027) and Learning (p=0.016) than SN participants. PWH participants also tended to have lower Z-scores on Executive function (p=0.095) and Global function (p=0.069) than SN participants. PWH=people with HIV disease and are non-smokers, SN= HIV seronegative non-smokers, SN_S=HIV seronegative tobacco smokers, PWH_S=PWH tobacco smokers. Subtests in each domain were listed below. Fluency: DKEFS Design Fluency and Verbal Fluency (with letters FAS); Executive Functions: DKEFS- Color-Word Interference Test Inhibition & Inhibition/Switching, DKEFS-Trail making Number-Letter Switching; Speed of Information Processing: Symbol Digit, DKEFS Trail-making Number Sequencing, DKEFS Color naming, and California Computerized Assessment Package (CalCAP) Simple Reaction Time; Attention/Working Memory: Arithmetic from Wechsler Adult Intelligence Scale-VI, Digit Span Backward, Letter-Number Sequencing, Arithmetic and Paced Auditory Serial Addition Test 1; Learning: Rey Auditory Verbal Learning Test Trial 5 and Rey-Osterreith Complex Figure Test-Immediate Recall; Memory: Rey Auditory Verbal Learning Test Delayed Recall (Trial 7) and Rey Complex Figure-Delayed Recall; Motor Skills, Grooved Pegboard Dominant and Non-dominant hands.
2.3. Image Acquisition
All participants were scanned on a 3 Tesla TIM Trio scanner (Siemens Medical Solutions, Erlangen, Germany) with a 12-channel head coil. Structural imaging was performed for morphometry and to assess for possible neuroanatomical abnormalities. The protocol included a T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence (TR/TE/TI=2200/4.47/1000 ms, flip angle=12°, FOV=256mm, 256*256 matrix, thickness=1 mm) and an axial fluid-attenuated inversion recovery scan (FLAIR, TR/TE/TI=9100/84/2500 ms, flip angle=150°, FOV 230mm, 256×256 matrix, 3 mm slices). All images were reviewed by a Neurologist (LC). Scans from two participants were excluded due to encephalomalacia or multiple lesions in the brain from unreported prior brain disorders. Regional brain volume data from the putamen were also excluded for 3 participants due to silent lacunar infarcts.
2.4. Image Processing
T1-weighted images were first processed with a FMRIB Software Library (FSL)-based tool [48] for skull stripping and brain extraction, and then processed with FreeSurfer 6.0 (http://surfer.nmr.mgh.harvard.edu/) for automated segmentation and cortical surface reconstruction overlaid on the Desikan-Killiany atlas [49–51]. All processed images were visually inspected to ensure accurate segmentations. Selected subcortical brain volumes included the following 7 regions of interest (ROIs): amygdala, hippocampi, thalami, caudates, globus pallidus, putamen, and nucleus accumbens. These subcortical ROIs were selected because prior studies of either PWH [52, 53] or tobacco smokers [54–58] showed volume abnormalities in those regions. Since neither HIV infection nor tobacco smoking showed significant volume laterality in these subcortical regions, the left and right volumes were averaged. The volumes of total cortical GM, total cerebral WM, total subcortical GM, and estimated intracranial volume (eICV) were also evaluated. Lastly, vertex-wise cortical volume and thickness were determined, using a 10-mm smoothing at the full-width at half-maximum.
2.5. Statistical Analyses
Data were analyzed using R (version 3.6.3). Demographical data and clinical variables between groups were compared using analyses of variance (ANOVA), t-tests, Chi-square, Fisher’s exact, Mann-U or Kruskal-Wallis tests, depending on the type and distribution of variables. For cognitive domain z-scores, the main effects and interactions of HIV and tobacco smoking status were analyzed using 2-way ANCOVA, co-varying for sex, estimated verbal IQ and depressive symptom scores. These covariates were evaluated individually in the model, and summarized in Supplemental Table 1. Atlas-based ROI volumes were analyzed by 2-way ANCOVA, and vertex-wise cortical measures were analyzed using the vertex-based general linear model (GLM) analysis in FreeSurfer (https://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/GroupAnalysisV6.0); both analyses covaried for age, sex, and other potential confounds, including ISP [59], estimated verbal IQ [60], and depressive symptom scores [61, 62]. Volume measures were additionally covaried for eICV. Vertex-wise measures were corrected for multiple comparisons using a Monte Carlo simulation with a corrected cluster-value of p < 0.05 [63]. Average values of the significant clusters from the vertex-based analysis were extracted for association analyses. Associations between brain morphometric measures (age, sex and eICV adjusted) and cognitive domain Z-scores, HIV disease severity and tobacco smoking were explored using GLM. These GLM analyses were limited to the morphometric measures and cognitive domain scores that showed significant group differences. Since these correlations were performed for exploratory purposes, the significance level was set at p≤0.05. Group comparisons on cognitive performance and volume measures were repeated with marijuana use duration as an additional covariate in order to explicate the possible confounding effect of marijuana. Methamphetamine use was not covaried due to the small number of users.
3. RESULTS
3.1. Participants’ Characteristics (Table 1)
Table 1:
Participant Demographics and Clinical Characteristics (Mean ± S.D., median or range)
| SN Nonsmoker (N=106) |
SN-Smoker (N=65) |
PWH Nonsmoker (N=43) |
PWH Smoker (N=40) |
p-value | |
|---|---|---|---|---|---|
| Age (years) Age range |
47.2 ± 13.6 (18 – 77) |
43.7 ± 12.4 (20 – 73) |
51.0 ± 9.5 (24 – 73) |
48 ± 10.1 (22 – 64) |
0.280a |
| # Men (%) | 89 (84.0%) | 51 (78.5%) | 41 (93.2%) | 37 (94.9%) | 0.051b |
| Education (years) | 15 ± 2.6 | 14 ± 2.1 | 15 ± 2.2 | 14 ± 2.7 | 0.080a |
| WTAR Predicted Verbal IQ# | 110 ± 7.4 | 104 ± 9.8 | 106 ± 8.7 | 104 ± 10 | <0.001 a |
| Race (W/As/B/NH/NA/Mixed) | 54/27/3/7/0/15 | 27/14/0/10/0/14 | 24/5/2/3/2/8 | 22/7/3/3/0/7 | 0.066b |
| Index of Social Position (8–66) | 37.3 ± 16.0 | 43.5 ± 13.6 | 35.2 ± 15.1 | 45.9 ± 15.4 | 0.074 a |
| CES-D scale score (0–60) | 6.44 ± 5.1 | 10.8 ± 9.3 | 12.7 ± 10.2 | 12.8 ± 9.2 | <0.001 a |
| HIV disease-related | |||||
| Duration (years) | - | - | 16.6 (1.8–36.6) | 14.1 (0.9–30.9) | 0.318c |
| CD4 count (#/mm3) | - | - | 546.7 (105–1331) | 550 (107–1275) | 0.963c |
| Nadir CD4 count (#/mm3) | - | - | 204 (0–1021) | 206 (3–1018) | 0.562c |
| HIV dementia Scale (0–16) | - | - | 13.9 ± 2.6 | 12.7 ± 3.4 | 0.088d |
| Karnofsky score (0–100) | - | - | 90.4 ± 9.4 | 94.2 ± 6.8 | 0.040 d |
| # (%) with HAND or “HAND-equivalent** | <0.001 b | ||||
| Normal (no HAND) | 82 (83.7%) | 47 (72.3%) | 31 (70.5%) | 23 (60.0%) | |
| ANI | 12 (12.2%) | 11 (16.9%) | 4 (9.1%) | 4 (10.3%) | |
| MND | 4 (4.1%) | 3 (4.6%) | 3 (6.8%) | 9 (23.1%) | |
| HAD | 0 | 0 | 3 (6.8%) | 3 (7.7%) | |
| Vascular disease risk factors *** | |||||
| With vascular risks, n (%) | 17 (39.5%) | 13 (32.5%) | 30 (28.3%) | 14 (21.5%) | 0.228 b |
| History of hypertension | 10 (23.3%) | 9 (22.5%) | 13 (12.3%) | 9 (13.8%) | 0.237 b |
| History of Hyperlipidemia | 12 (27.9%) | 7 (17.5%) | 17 (16%) | 4 (6.2%) | 0.024 b |
| Systolic blood pressure | 120.4±11.6 | 122.3±11.9 | 121.3±12.1 | 121.6±13.7 | 0.926 a |
| Diastolic blood pressure | 78.9±12.0 | 80.5±8.9 | 79.2±11.0 | 81.3±10.8 | 0.584 a |
| Body Mass Index | 25.3±3.3 | 24.9±3.8 | 27.1±4.7 | 27.1±6.1 | 0.024 a |
| White matter hyperintensities on FLAIR images, n (%) | 20 (46.5%) | 22 (55%) | 56 (54.9%) | 28 (43.8%) | 0.466 b |
| Nicotine usage, Median (range) | |||||
| Age at first use (years) | 18.5 ± 15.2 | 18.9 ± 6.7 | 21.3 ± 9.5 | 19.2 ± 7.8 | 0.824a |
| Total lifetime used (pack-years) | - | 16.0 (0.2–120.9) | - | 20.6 (0.9–68.2) | 0.319c |
| Duration of use (months) | - | 320 (27.3–723.6) | - | 340 (47.2–669.3) | 0.186c |
| Duration of abstinence (months) | 204 (9–1341) | - | 253 (49–713) | - | 0.830c |
| Marijuana usage, Median (range) | |||||
| #Lift time user (%) | 46 (43.4) | 40 (61.5) | 25 (58.1) | 33 (82.5) | <0.001 b |
| Daily average use (gram) ¶ | 0.02 (0.00–7.09) | 0.06 (0.00–7.90) | 0.01 (0.00–6.75) | 0.09 (0.00–3.00) | 0.076e |
| Total lifetime use (gram) ¶ | 57.9 (0–51774) | 355.5 (2.1–37180) | 15.7 (0–55054) | 772.3 (0.1–56250) | 0.074e |
| Duration of MJ use (years) ¶ | 8.48 (0.00–38.4) | 16.4 (1.00–43.9) | 16.1 (0.01–43.9) | 21.0 (1.00–51.2) | 0.027 e |
| Month since last use¶ | 63.5 (0.00–478) | 24 (0.00–425) | 5.00 (0.00–339) | 0.00 (0.00–488) | 0.246e |
| Methamphetamine usage, Median (range) | |||||
| #Lift time user (%) | 9 (8.5) | 18 (27.7) | 12 (27.3) | 21 (53.8) | <0.001 b |
| Daily average use (gram) ¶ | 0.02 (0.00–1.00) | 0.00 (0.00–0.71) | 0.00 (0.00–0.07) | 0.01 (0.00–0.62) | 0.279e |
| Total lifetime use (gram) ¶ | 11.4 (0.11–5350) | 2.8 (0.01–783) | 10.4 (0.02–218.3) | 6.8 (011–1370) | 0.441e |
| Duration of use (years) ¶ | 5.0 (0.00–17.6) | 2.5 (0–15.0) | 8.4 (0.00–33.4) | 5.0 (1.60–29.1) | 0.182e |
| Duration of abstinence (months) ¶ | 106 (0.00–344) | 131 (0.00–431) | 123 (0.00–308) | 62 (0.00–445) | 0.878e |
| Alcohol usage, Median (range) | |||||
| #Lift time user (%) | 92 (86.8) | 53 (81.5) | 38 (86.4%) | 38 (97.4%) | 0.139b |
| Daily average use (mL) ¶ | 6.1 (0.04–85.2) | 9.11 (0.05–317.8) | 11.4 (0.10–659.6) | 6.6 (0.04–295.7) | 0.104e |
| Total lifetime use (Liter) ¶ | 36.9 (0. – 714.5) | 47.6 (0.6 – 4362.6) | 97 (1.3 – 8805.2) | 51.7 (0.1 – 3870.11) | 0.140e |
| Duration of use (years) ¶ | 21.5 (0.00–61.1) | 23.1 (0.52–54.3) | 22.5 (0.00–46.5) | 28.2 (8.9–46.5) | 0.443e |
| Duration of abstinence (months) ¶ | 0.00 (0.00–432) | 0.00 (0.00–341) | 0.00 (0.00–303) | 0.00 (0.00–358) | 0.489e |
Description of variables were presented as mean ± S.D, median (range) or count (%). Significant p values were bolded. WTAR = Wechsler Test of Adult Reading; CES-D = Center for Epidemiological Studies – Depression Scale; Index of Social Position: assessed using the Hollingshead Four Factor Index of Social Position. Race = White/ Asian/ Black/Native Hawaiian/Native American/More than one race. PWH=people living with HIV disease, SN=HIV seronegative.
: ANOVA
: χ2;
: Mann-Whitney U
: T test
: Kruskal-Wallis Test.
Plasma HIV RNA was calculated from 7 PWH participants and 5 PWH+Smokers with detectable viruses.
HAND = HIV-associated Neurocognitive Disorder or HAND-equivalent, 15 subjects missing this information
6 participants did not have blood pressure data, 4 did not have Fluid attenuated inversion recovery imaging (FLAIR) data (2 due to excessive motion, 2 did not complete FLAIR scans). Hyperlipidemia was defined as having an abnormally high total cholesterol or triglyceride level or taking lipid-lowering medications.
These variables were calculated only among the users.
The significance was driven by the smoking main effect (p<0.01)
Regardless of HIV serostatus, smokers had a lower verbal IQ (p<0.001) than nonsmokers. Furthermore, PWH had more depressive symptoms than SN individuals (p<0.001), especially in smokers (HIV x smoking interaction-p=0.052). The four groups had similar age, sex distribution, socioeconomic status, racial distributions and years of educations. The two PWH groups had similar HIV disease severity, including duration of HIV infection and current and nadir CD4 cell counts. As expected, compared to SN, more PWH participants had HAND (or HAND-equivalent cognitive deficits for SN) (p<0.001), but the percentage with HAND was similar between the two PWH groups. However, PWH nonsmokers had lower daily function than PWH smokers (p=0.040). In terms of vascular disease risk factors, regardless of smoking status, PWH participants had a higher prevalence of hyperlipidemia (p=0.024) but lower body mass index (p=0.024) than SN controls. The participant groups had similar but low prevalence of vascular risk factors such as hypertension or diabetes. Furthermore, the percentages of participants who had white matter lesions on the FLAIR images were similar across participant groups, which further suggests that they had similar cerebrovascular disease burden. The two tobacco smoking groups were similar in their tobacco usage patterns. PWH had a higher percentage of marijuana (p<0.001) or methamphatamine (p<0.001) use, and used marijuana for a longer duration than SN participants (p=0.027). Lastly, the four groups had similar recreational alcohol use.
3.2. Cognitive Measurements (Figure 1)
Independent of smoking status, PWH had lower Z-scores compared to SN participants for Attention (p=0.027) and Learning (p=0.016) domains, and a trend for lower Z-scores on Executive function (p=0.095) and Global cognition (p=0.069). However, regardless of HIV serostatus, smokers performed similarly on the cognitive tasks as nonsmokers. PWH smokers consistently showed the lowest Z-scores across the four groups, indicating an additive adverse impact of HIV infection and tobacco smoking on these cognitive domains. Marijuana use duration was not associated with any of the cognitive domain scores.
Models with or without the covariates sex, IQ, or CES-D are listed in Supplemental Table 1. Without covariates, the PWH groups performed worse in 5 cognitive domains compared to the SN participants, while smokers performed poorer in 2 domains than the nonsmokers. When sex was included as a covariate, smoking main effects remained but HIV main effect on Motor function became non-significant. With CES-D score as a covariate, the HIV main effect became non-significant for Motor function and the smoking main effects became a trend. With IQ as a covariate, only HIV (except for Motor function), but none of the smoking main effects, remained significant. For the final model that covaried for all three variables (sex, CES-D, and IQ ), only the HIV main effects on Attention/Working Memory and Learning remained significant.
3.3. Subcortical Brain Volume Measures (Figure 2)
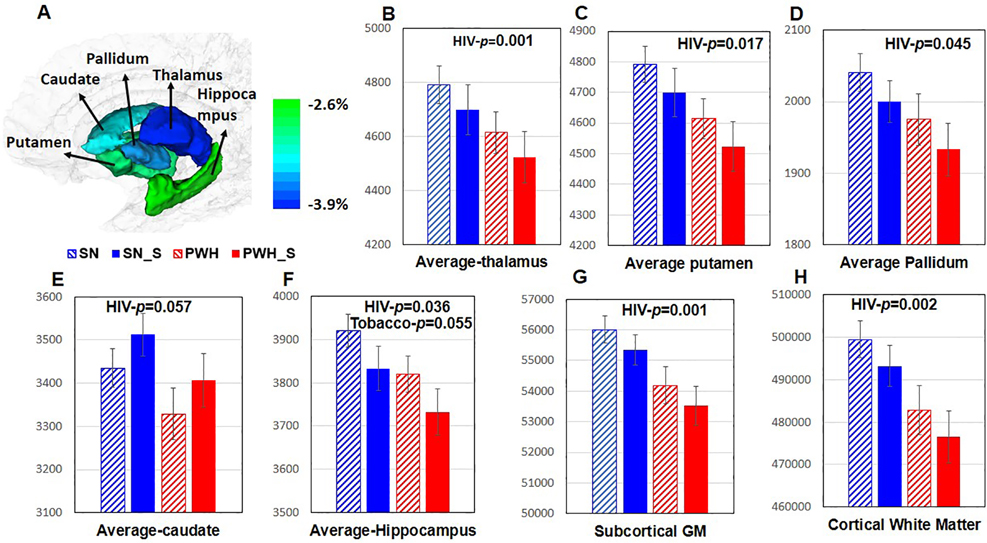
Figure 2. Volume of Whole Brain and Subcortical Regions in the Four Participant Groups.
A ROIs on a standard brain template. Color bar indicates the % of volume difference between PWH and SN participants. B-D Regardless of smoking status, PWH participants had smaller thalamus (−3.9%, p=0.001), putamen (−3.7% p=0.017) and pallidum (−3.3%, p=0.045) than SN participants. E Caudate tended to be smaller in PWH compared to SN participants (−3.1%, p=0.057). F Regardless of smoking status, PWH participants had smaller hippocampus (−2.5%, p=0.036) than SN participants. Regardless of HIV serostatus, tobacco smokers tended to have smaller hippocampi than nonsmokers (−2.87%, p=0.055). G-H Regardless of tobacco smoking status, PWH participants had smaller total subcortical gray matter (−3.0%, p=0.001) and cortical white matter (−2.7%, p=0.002) than SN individuals. PWH=people with HIV disease and are non-smokers, SN= HIV seronegative non-smokers, SN_S=HIV seronegative tobacco smokers, PWH_S=PWH tobacco smokers, GM=gray matter.
Regardless of smoking status, PWH had smaller thalami (−3.9%, p=0.001), putamen (−3.7%, p=0.017), pallidum (−3.3%, p=0.045), a trend of smaller caudates (−3.1%, p=0.057), and smaller hippocampi (−2.6%, p=0.036) than SN participants (Figure 2B–F). Regardless of HIV serostatus, tobacco smokers tended to have smaller hippocampi (−0.6%, p=0.055, Figure 2F) than nonsmokers. The PWH groups also had smaller total subcortical GM (−3.0%, p=0.001) and cortical WM (−2.7%, p=0.002) volumes than SN individuals, irrespective of tobacco smoking status (Figure 2G–H). Notably, PWH smokers had the smallest volumes in the thalami, putamen, pallidum, hippocampi, total subcortical GM, and cerebral WM across the four groups, indicating the additive effects of HIV infection and tobacco smoking on brain atrophy in these regions. The four groups were similar in the total cortical GM volume or age-related volume declines in all ROIs. Likewise, marijuana use duration was not associated with any of these ROI volumes.
3.4. Vertex-wise Cortical Volume and Thickness Measures (Figure 3)
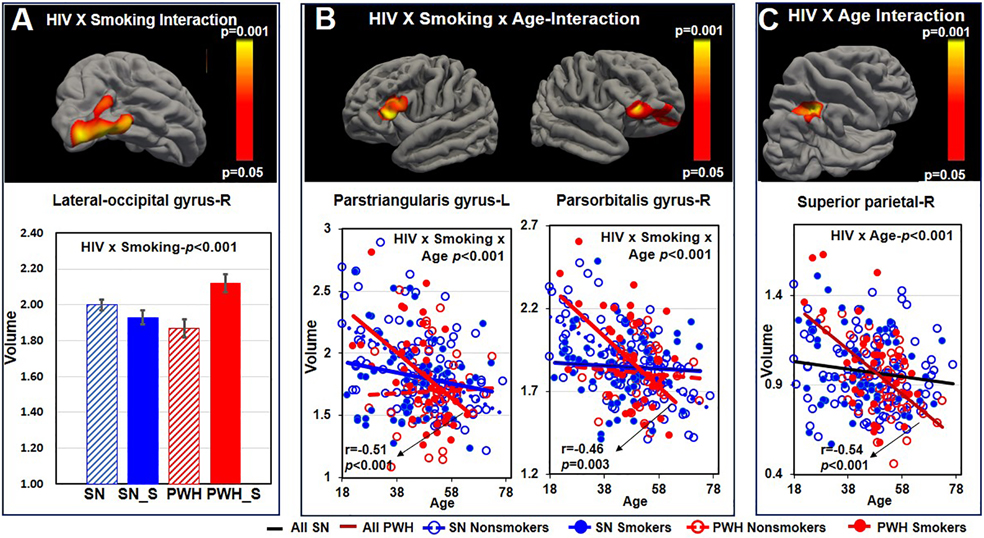
Figure 3. Vertex-wised Comparisons of Cortical Volumes across the Four Participant Groups.
A HIV-by-smoking interaction was observed in the right lateral-occipital gyrus. PWH smokers had larger volume than PWH nonsmokers whereas there was no such difference between the two SN groups (interaction p<0.001). B HIV-by-smoking interaction on the age-volume association was observed in the left parstriangularis gyrus (HIV-by-age interaction p<0.001; r=−0.507, p<0.001 in PWH smokers), and the right parsorbitalis gyrus (HIV-by-Smoke-by-age interaction<0.001; r=−0.460, p=0.003 among PWH smokers). C HIV main effect on the age-volume association was observed in the right superior parietal gyrus. Steeper age-related volume decline was observed in the PWH participants but not in the SN groups (HIV-by-age interaction p<0.001; r=−0.544, p<0.001 in PWH). PWH=people with HIV disease, SN=HIV seronegative, SN_S=HIV seronegative tobacco smokers, PWH_S=people with HIV disease and tobacco smoking, L=left, R=right. Color bar indicates p value significant levels for all clusters from A-C.
PWH smokers had larger volumes than PWH nonsmokers while the two SN groups had similar volumes in the right lateral occipital gyrus (interaction-p<0.001, Figure 3A). An HIV-by-smoking interaction on age-volume association was observed in the left parstriangularis and right parsorbitalis gyrus (both 3-way interaction p-values<0.001), with steeper age-related declines in SN nonsmokers and PWH smokers than in the other two groups (Figure 3B). Furthermore, regardless of smoking status, PWH showed steeper age-dependent volume declines than SN participants in the right superior parietal cortex (HIV-by-age interaction-p<0.001; r=−0.544, p<0.001 for PWH ) (Figure 3C). Vertex-based measures were similar between smokers and nonsmokers, regardless of HIV serostatus. Marijuana use duration was not associated with any of these vertex-wise measures.
3.5. Association between Abnormal Brain Volumes and Cognitive Function or Clinical Features (Figure 4 and Supplemental Figures 1–2)
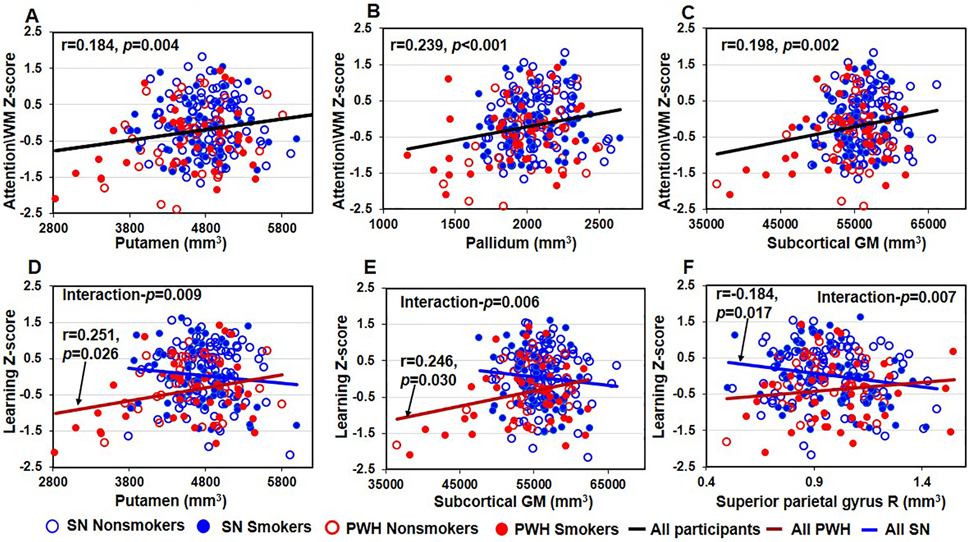
Figure 4. Smaller Brain Volume Predicted Poorer Cognitive Function.
A-C Regardless of smoking status, smaller putamen, pallidum, and subcortical total GM predicted poorer attention/working memory function in all participants (putamen: r=0.148, p=0.004, Figure 4A; pallidum: r=0.239, p<0.001, Figure 4B; subcortical total GM : r=0.198, p=0.002, Figure 4C). D-E Among PWH participants, smaller putamen and subcortical total GM predicted poorer Learning function (putamen: r=0.251, p=0.026 in PWH participants, interaction p=0.009, Figure 4D; subcortical total GM : r=0.246, p=0.030 in PWH participants, interaction p=0.006, Figure 4E). F Larger right superior parietal gyrus predicted poorer learning function in SN participants, but not in PWH participants (r=−0.184, p=0.017 in SN participants, interaction p=0.007). GM=gray matter, PWH=people with HIV disease, SN=HIV seronegative.
Smaller putamen, pallidum, and subcortical GM predicted poorer attention/working memory performance in all participants, independent of smoking or HIV serostatus (r=184, p=0.004, Figure 4A; pallidum: r=0.239, p<0.001, Figure 4B; subcortical GM: r=0.198, p=0.002, Figure 4C). Across all PWH, but not the SN participants, smaller putamen (r=0.251, p=0.026, interactionp=0.009, Figure 4D), and subcortical total GM (r=0.246, p=0.030, interaction-p=0.006, Figure 4E) predicted poorer learning function. In the right superior parietal gyri, larger volume predicted poorer learning function in SN but not in PWH (r=−0.184, p=0.017, interaction-p=0.007, Figure 4F).
In PWH smokers, longer duration of smoking predicted smaller left superior praieral (r=−0.387, p=0.018, HIV x smoking duration-p=0.009) and left parstriangularis gyrus (r=−0.379, p=0.021, HIV x smoking duration-p=0.036). Greater pack-years also predicted smaller caudate (r=−0.364, p=0.027, HIV x smoking duration-p=0.024) and subcortical GM in PWH smokers (r=−0.350, p=0.033, HIV x smoking duration-p=0.032) (Supplemental Figures 1A–D). Regardless of HIV serostatus, greater tobacco smoking pack-years predicted smaller thalamus (r=−0.341, p<0.001) and pallidum (r=−0.248, p=0.012) among all smokers (Supplemental Figure 1E–F).
Among all PWH, lower nadir CD4 counts also predicted smaller thalami (r=0.233, p=0.022) and subcortical GM (r=0.251, p=0.027) (Supplemental Figure 2A–B). In addition, lower nadir CD4 cell counts predicted smaller caudate volumes in PWH smokers only (r=0.385, p=0.020, smoking by-nadir CD4 interaction-p=0.033, Supplemental Figure 2C). In addition, PWH with lower current CD4 cell counts had smaller volumes in thalamus (r=0.236, p=0.032, Supplemental Figure 2D) and putamen (r=0.246, p=0.026, Supplemental Figure 2E). Lower current CD4 cell counts also predicted smaller hippocampi (r=0.330, p=0.042, smoking-by-current CD4 interaction-p=0.010, Supplemental Figure 2F) in PWH smokers only. The estimated duration of HIV infection was not associated with any of the volume measures.
4. DISCUSSION
The main findings of this study are: 1) tobacco smoking and chronic HIV infection have additive negative effects on cognition and regional brain atrophy; 2) smaller regional brain volumes typically predicted poorer cognition; 3) greater pack-years smoked or the duration of smoking predicted smaller brain volumes, particularly in PWH smokers; and 4) greater immune suppression, with lower nadir and current CD4 counts, predicted smaller brain volumes in specific brain regions in PWH.
4.1. HIV Infection and Tobacco Smoking on Cognitive Performance
Consistent with prior studies in the post cART era [64], we found PWH had poorer cognitive performance than SN participants even after covarying for depressive symptoms and verbal IQ. The poorest cognitive performance in PWH smokers validated our earlier findings [31] and again indicates an additive effect of tobacco smoking on cognitive deficits in PWH.
Independent of HIV serostatus and different from prior studies [28–30, 65], we found that tobacco smokers performed similarly on cognitive assessments to nonsmokers. Potential confounds in priors studies might be the typically lower socioeconomic status and the greater depressive symptoms of smokers. Earlier studies typically did not covary for depressive symptoms [30, 31, 65, 66]. Since acute mood disorders were exclusionary in the current study, the relatively high CES-D scores in PWH participants or smokers indicate sub-clinical depression in these individuals. Sub-clinical or mild depressive symptoms in PWH might be associated with elevated levels of a wide range of plasma inflammatory markers (e.g., interferon γ-induced protein 10, interleukin-6, and tumor necrosis factor-α) [67]. However, whether such elevated peripheral inflammatory markers also reflected greater neuroinflammation and contributed to the cognitive deficits in PWH is unclear.
4.2. HIV Infection and Tobacco Smoking on Brain Morphometry
Our findings suggest that the basal ganglia and cortical WM regions are particularly vulnerable to HIV. Previous studies consistently found that PWH had abnormally smaller thalami [52, 68–71], basal ganglia [52, 68, 70–75], total subcortical GM [69] and cortical WM [68–70, 74]. The basal ganglia and WM are proximal to the ventricles; therefore, these structures are more easily infiltrated by HIV-infected monocytes, exposed to inflammatory factors, and have the highest levels of HIV burden (HIV RNA or antigen levels) in the brain [76–78]. The vulnerability of the basal ganglia to HIV is further supported by the finding that only caudate and putamen volumes correlated with plasma HIV-infected monocyte density in acutely infected individuals [71]. In addition, the basal ganglia also has the highest density of dopaminergic neurons that are sensitive to HIV protein-induced toxicity [78, 79]; as such, dopamine transmission in these regions is reduced proportionately by the severity of cognitive deficits in PWH [80, 81]. In our study, PWH and SN participants had minimally different cortical volume or thickness, which is consistent with some [70, 74, 82, 83] but not all previous studies [52, 84, 85]. These discrepancies may be due to varied clinical characteristics, including genotypes or host immune responses. For example, individuals with the APOEε4 allele, especially those with HIV, also demonstrate greater brain atrophy and cognitive deficits [34, 68]. In addition, a postmortem study of HAND showed those with HIV encephalitis had strong upregulation of inflammatory genes in both the striatum and cortical regions compared to those without HIV encephalitis[86].
Consistent with prior studies[19, 87], chronic smokers in the current study tended to have smaller hippocampi than nonsmokers, a region vulnerable to tobacco smoke-induced oxidative stress [88]. In this study, the additive effect of HIV and tobacco smoking on brain atrophy was also predominantly located in the cortical WM and subcortical GM, indicating greater injury to these brain regions. This additive atrophy in the GM and WM of PWH smokers may be mediated by the greater neuroinflammation, which is consistent with findings on diffusion tensor imaging that showed greatest diffusivity in various white matter tracts in PWH smokers than nonsmokers with or without HIV disease [22].
4.3. Association between Age, Cognitive Function, HIV Disease Severity, Smoking Severity, and Brain Volume
Consistent with prior reports, we found an apparent accelerated age-related decline in cortical volumes in PWH [69, 70]. In addition, consistent with previous findings [69, 85, 89, 90], we found that smaller brain volumes predicted poorer cognitive function, particularly in PWH participants. These volume-cognition associations suggest that HIV-associated brain atrophy may contribute to cognitive deficits in PWH.
Consistent with previous studies [18, 19], we found that greater tobacco usage predicted brain atrophy, especially among PWH smokers, which, again, suggests greater brain injury might result from tobacco smoking in PWH smokers. Lastly, Among PWH participants, we found that both lower nadir and current CD4 cell counts predicted subcortical atrophy, indicative of both the legacy effect of HIV infection [85] and ongoing immunosuppression [71, 75].
4.4. Limitations
Due to the cross-sectional design, we cannot attribute causation between the major variables (HIV and tobacco smoking) and the outcome measures of brain volumes and cognitive performance. Other limitations include the small sample size for the subgroups and the variable clinical features across subject groups (e.g., depressive symptoms and estimated verbal IQ), which might have diminished the statistical power to detect small effect sizes on brain morphometric and cognitive measures. In addition, the duration of viral suppression in PWH was not documented in this study which might have contributed to the cognitive performance or white matter abnormalities in PWH. Lastly, since we evaluated only individuals with viral suppression and excluded those with significant vascular risk factors (e.g., those with uncontrolled hypertension or diabetes), these findings may not be generalizable to other PWH.
4.5. Conclusions
This study demonstrated that chronic tobacco smoking is associated with greater brain atrophy and cognitive deficits in PWH. Therefore, tobacco smoking is likely a risk factor for HAND. Longitudinal studies are needed to confirm these findings by evaluating the combined effects of HIV infection and tobacco smoking on brain atrophy and cognitive deficits or impairment over time. In addition, depressive symptoms might moderate the effects of chronic tobacco smoking and HIV infection on brain atrophy and cognitive deficits.
Supplementary Material
Supplemental Figure 1. Smoking Severity Predicted Brain Volume A-B Longer duration of smoking predicted smaller left superior parietal (r=−0.387, p=0.018, HIV x smoking duration-p=0.009) and smaller left parstriangularis gyrus (r=−0.379, p=0.021, HIV x smoking duration-p=0.036) in PWH smokers. C-D Greater tobacco smoking pack-years predicted smaller caudate (r=−0.364, p=0.027, HIV x pack-years-p=0.024) and smaller subcortical GM in PWH smokers (r=−0.350, p=0.033, HIV x pack-years -p=0.032). E-F Regardless of HIV serostatus, greater tobacco smoking pack-years predicted smaller thalamus (r=−0.341, p<0.001) and pallidum (r=−0.248, p=0.012) among all smokers. GM=gray matter, L= left, PWH=people with HIV disease, TS=tobacco smoking.
Supplemental Figure 2. Nadir CD4 and CD4 Predicted Brain Volume A-B Lower nadir CD4 cell count predicted smaller thalamus (r=0.261, p=0.021) and subcortical GM (r=0.251, p=0.027) in all PWH participants. C Lower nadir CD4 cell count predicted smaller caudates in PWH smokers (r=0.385, p=0.020, Smoking-by-nadir interaction p=0.033). D-E Regardless of smoking status, lower current CD4 cell count predicted smaller thalamus (r=0.236, p=0.032) and putamen (r=0.246, p=0.026) among all PWH participants. F Lower current CD4 cell count predicted smaller hippocampal in PWH smokers (r=0.330, p=0.042, Smoking-by-CD4 interaction p=0.010). GM= gray matter, PWH=people with HIV disease
Acknowledgments
We are grateful to our research participants, the referral physicians from our community providers in Honolulu, Hawai’i, including Dr. Drew Kovach, Dr. Dominic Chow, Dr. Jennifer Frank, Dr. Cyril Goshima, and the personnel at the Life Foundation, the Gregory House and at Save the Food Basket. We also appreciate the meticulous and hard work from the multiple clinical and technical research staff members who assisted in the data collection of this study. Lastly, we thank Ms. Brigitte Pocta for her assistance in the editing of this manuscript.
Funding Resources
This work was supported by the National Institutes of Health (grants numbers 2K24-DA16170; U54-NS56883; R01-DA035659).
Footnotes
Conflicts
The authors have no conflicts of interest to disclose.
List of Supplemental Digital Content
References
- 1.Centers for Disease Control and Prevention. Behavioral and Clinical Characteristics of Persons with Diagnosed HIV Infection—Medical Monitoring Project, United States, 2010 Cycle to 2015 Cycle. HIV Surveillance Special Report 9, 12, 16, 17 and 20. In. May 2018 ed; 2018. [Google Scholar]
- 2.Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, et al. Current Cigarette Smoking Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 2018; 67(2):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162(5):335–344. [DOI] [PubMed] [Google Scholar]
- 4.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis 2013; 56(5):727–734. [DOI] [PubMed] [Google Scholar]
- 5.Pollack TM, Duong HT, Pham TT, Do CD, Colby D. Cigarette smoking is associated with high HIV viral load among adults presenting for antiretroviral therapy in Vietnam. PLoS One 2017; 12(3):e0173534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman JG, Minkoff H, Schneider MF, Gange SJ, Cohen M, Watts DH, et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the women’s interagency HIV study. Am J Public Health 2006; 96(6):1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winhusen T, Feaster DJ, Duan R, Brown JL, Daar ES, Mandler R, et al. Baseline Cigarette Smoking Status as a Predictor of Virologic Suppression and CD4 Cell Count During One-Year Follow-Up in Substance Users with Uncontrolled HIV Infection. AIDS Behav 2018; 22(6):2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashare RL, Thompson M, Leone F, Metzger D, Gross R, Mounzer K, et al. Differences in the rate of nicotine metabolism among smokers with and without HIV. AIDS 2019; 33(6):1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earla R, Ande A, McArthur C, Kumar A, Kumar S. Enhanced nicotine metabolism in HIV-1-positive smokers compared with HIV-negative smokers: simultaneous determination of nicotine and its four metabolites in their plasma using a simple and sensitive electrospray ionization liquid chromatography-tandem mass spectrometry technique. Drug Metab Dispos 2014; 42(2):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghura S, Gross R, Jordan-Sciutto K, Dubroff J, Schnoll R, Collman RG, et al. Bidirectional Associations among Nicotine and Tobacco Smoke, NeuroHIV, and Antiretroviral Therapy. J Neuroimmune Pharmacol 2019:Epub on 2019/2012/2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology 2012; 37(6):1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledgerwood DM, Yskes R. Smoking Cessation for People Living With HIV/AIDS: A Literature Review and Synthesis. Nicotine Tob Res 2016; 18(12):2177–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clifford DB. HIV-associated neurocognitive disorder. Curr Opin Infect Dis 2017; 30(1):117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol 2010; 67(6):699–714. [DOI] [PubMed] [Google Scholar]
- 15.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nat Rev Neurol 2016; 12(5):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzone P, Tierney W, Hossain M, Puvenna V, Janigro D, Cucullo L. Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: expanding the awareness of smoking toxicity in an underappreciated area. Int J Environ Res Public Health 2010; 7(12):4111–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durazzo TC, Mattsson N, Weiner MW, Korecka M, Trojanowski JQ, Shaw LM, et al. History of cigarette smoking in cognitively-normal elders is associated with elevated cerebrospinal fluid biomarkers of oxidative stress. Drug Alcohol Depend 2014; 142:262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karama S, Ducharme S, Corley J, Chouinard-Decorte F, Starr JM, Wardlaw JM, et al. Cigarette smoking and thinning of the brain’s cortex. Mol Psychiatry 2015; 20(6):778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duriez Q, Crivello F, Mazoyer B. Sex-related and tissue-specific effects of tobacco smoking on brain atrophy: assessment in a large longitudinal cohort of healthy elderly. Front Aging Neurosci 2014; 6:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health 2010; 7(10):3760–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang L, Liang H, Kandel SR, He JJ. Independent and Combined Effects of Nicotine or Chronic Tobacco Smoking and HIV on the Brain: A Review of Preclinical and Clinical Studies. J Neuroimmune Pharmacol 2020; 15(4):658–693. [DOI] [PubMed] [Google Scholar]
- 22.Liang H, Chang L, Chen R, Oishi K, Ernst T. Independent and Combined Effects of Chronic HIV-Infection and Tobacco Smoking on Brain Microstructure. J Neuroimmune Pharmacol 2018; 13(4):509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti AA, McLean L, Tolomeo S, Steele JD, Baldacchino A. Chronic tobacco smoking and neuropsychological impairments: A systematic review and meta-analysis. Neurosci Biobehav Rev 2019; 96:143–154. [DOI] [PubMed] [Google Scholar]
- 24.Ott A, Slooter AJ, Hofman A, van Harskamp F, Witteman JC, Van Broeckhoven C, et al. Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: the Rotterdam Study. Lancet 1998; 351(9119):1840–1843. [DOI] [PubMed] [Google Scholar]
- 25.Royal W 3rd, Can A, Gould TD, Guo M, Huse J, Jackson M, et al. Cigarette smoke and nicotine effects on brain proinflammatory responses and behavioral and motor function in HIV-1 transgenic rats. J Neurovirol 2018; 24(2):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han H, Yang Z, Chang SL, Li MD. Modulatory Effects of Nicotine on neuroHIV/neuroAIDS. J Neuroimmune Pharmacol 2018; 13(4):467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capo-Velez CM, Morales-Vargas B, Garcia-Gonzalez A, Grajales-Reyes JG, Delgado-Velez M, Madera B, et al. The alpha7-nicotinic receptor contributes to gp120-induced neurotoxicity: implications in HIV-associated neurocognitive disorders. Sci Rep 2018; 8(1):1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durazzo TC, Rothlind JC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Chronic cigarette smoking and heavy drinking in human immunodeficiency virus: consequences for neurocognition and brain morphology. Alcohol 2007; 41(7):489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monnig MA, Kahler CW, Lee H, Pantalone DW, Mayer KH, Cohen RA, et al. Effects of smoking and alcohol use on neurocognitive functioning in heavy drinking, HIV-positive men who have sex with men. AIDS Care 2016; 28(3):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryant VE, Kahler CW, Devlin KN, Monti PM, Cohen RA. The effects of cigarette smoking on learning and memory performance among people living with HIV/AIDS. AIDS Care 2013; 25(10):1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang L, Lim A, Lau E, Alicata D. Chronic Tobacco-Smoking on Psychopathological Symptoms, Impulsivity and Cognitive Deficits in HIV-Infected Individuals. J Neuroimmune Pharmacol 2017; 12(3):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging 2010; 32(5):1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang L, Holt JL, Yakupov R, Jiang CS, Ernst T. Lower cognitive reserve in the aging human immunodeficiency virus-infected brain. Neurobiol Aging 2013; 34(4):1240–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang L, Jiang C, Cunningham E, Buchthal S, Douet V, Andres M, et al. Effects of APOE epsilon4, age, and HIV on glial metabolites and cognitive deficits. Neurology 2014; 82(24):2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andres T, Ernst T, Oishi K, Greenstein D, Nakama H, Chang L. Brain Microstructure and Impulsivity Differ between Current and Past Methamphetamine Users. J Neuroimmune Pharmacol 2016; 11(3):531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akhtar-Khaleel WZ, Cook RL, Shoptaw S, Surkan PJ, Teplin LA, Stall R, et al. Long-Term Cigarette Smoking Trajectories Among HIV-Seropositive and Seronegative MSM in the Multicenter AIDS Cohort Study. AIDS Behav 2016; 20(8):1713–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bekele T, Rueda S, Gardner S, Raboud J, Smieja M, Kennedy R, et al. Trends and Correlates of Cigarette Smoking and Its Impacts on Health-Related Quality of Life Among People Living with HIV: Findings from the Ontario HIV Treatment Network Cohort Study, 2008–2014. AIDS Patient Care STDS 2017; 31(2):49–59. [DOI] [PubMed] [Google Scholar]
- 38.Chang JT, Anic GM, Rostron BL, Tanwar M, Chang CM. Cigarette Smoking Reduction and Health Risks: A Systematic Review and Meta-analysis. Nicotine & Tobacco Research 2020; 23(4):635–642. [DOI] [PubMed] [Google Scholar]
- 39.Akbari M, Hasani J, Seydavi M. Negative affect among daily smokers: A systematic review and meta-analysis. J Affect Disord 2020; 274:553–567. [DOI] [PubMed] [Google Scholar]
- 40.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 1997; 12(2):277. [DOI] [PubMed] [Google Scholar]
- 41.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984; 2(3):187–193. [DOI] [PubMed] [Google Scholar]
- 42.Pfeffer RI, Kurosaki TT, Harrah CH, Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982; 37(3):323–329. [DOI] [PubMed] [Google Scholar]
- 43.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9(3):179–186. [PubMed] [Google Scholar]
- 44.Power C, Selnes OA, Grim JA, McArthur JC. HIV Dementia Scale: a rapid screening test. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 8(3):273–278. [DOI] [PubMed] [Google Scholar]
- 45.Wechsler D Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001. [Google Scholar]
- 46.Hollingshead AA. Four-factor index of social status. New Haven, CT: 1975; (Yale University.). [Google Scholar]
- 47.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002; 17(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 50.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999; 9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 51.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004; 14(1):11–22. [DOI] [PubMed] [Google Scholar]
- 52.Sanford R, Fernandez Cruz AL, Scott SC, Mayo NE, Fellows LK, Ances BM, et al. Regionally Specific Brain Volumetric and Cortical Thickness Changes in HIV-Infected Patients in the HAART Era. J Acquir Immune Defic Syndr 2017; 74(5):563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guha A, Brier MR, Ortega M, Westerhaus E, Nelson B, Ances BM. Topographies of Cortical and Subcortical Volume Loss in HIV and Aging in the cART Era. J Acquir Immune Defic Syndr 2016; 73(4):374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanlon CA, Owens MM, Joseph JE, Zhu X, George MS, Brady KT, et al. Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers. Addict Biol 2016; 21(1):185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durazzo TC, Meyerhoff DJ, Yoder KK, Murray DE. Cigarette smoking is associated with amplified age-related volume loss in subcortical brain regions. Drug Alcohol Depend 2017; 177:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elbejjani M, Auer R, Jacobs DR Jr., Haight T, Davatzikos C, Goff DC Jr., et al. Cigarette smoking and gray matter brain volumes in middle age adults: the CARDIA Brain MRI sub-study. Transl Psychiatry 2019; 9(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wetherill RR, Jagannathan K, Hager N, Childress AR, Rao H, Franklin TR. Cannabis, Cigarettes, and Their Co-Occurring Use: Disentangling Differences in Gray Matter Volume. Int J Neuropsychopharmacol 2015; 18(10):pyv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franklin TR, Wetherill RR, Jagannathan K, Johnson B, Mumma J, Hager N, et al. The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS One 2014; 9(8):e104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waldstein SR, Dore GA, Davatzikos C, Katzel LI, Gullapalli R, Seliger SL, et al. Differential Associations of Socioeconomic Status With Global Brain Volumes and White Matter Lesions in African American and White Adults: the HANDLS SCAN Study. Psychosom Med 2017; 79(3):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blackmon K, Barr WB, Kuzniecky R, Dubois J, Carlson C, Quinn BT, et al. Phonetically irregular word pronunciation and cortical thickness in the adult brain. Neuroimage 2010; 51(4):1453–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang H, Tang WK, Chu WCW, Ernst T, Chen R, Chang L. Striatal and white matter volumes in chronic ketamine users with or without recent regular stimulant use. Drug Alcohol Depend 2020; 213:108063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szymkowicz SM, Woods AJ, Dotson VM, Porges EC, Nissim NR, O’Shea A, et al. Associations between subclinical depressive symptoms and reduced brain volume in middle-aged to older adults. Aging Ment Health 2019; 23(7):819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagler DJ Jr., Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 2006; 33(4):1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison JD, Dochney JA, Blazekovic S, Leone F, Metzger D, Frank I, et al. The nature and consequences of cognitive deficits among tobacco smokers with HIV: a comparison to tobacco smokers without HIV. J Neurovirol 2017; 23(4):550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wojna V, Robles L, Skolasky RL, Mayo R, Selnes O, de la Torre T, et al. Associations of cigarette smoking with viral immune and cognitive function in human immunodeficiency virus-seropositive women. J Neurovirol 2007; 13(6):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu H, Surkan PJ, Irwin MR, Treisman GJ, Breen EC, Sacktor N, et al. Inflammation and Risk of Depression in HIV: Prospective Findings From the Multicenter AIDS Cohort Study. Am J Epidemiol 2019; 188(11):1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang L, Andres M, Sadino J, Jiang CS, Nakama H, Miller E, et al. Impact of apolipoprotein E epsilon4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. Neuroimage 2011; 58(4):1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nir TM, Jahanshad N, Ching CRK, Cohen RA, Harezlak J, Schifitto G, et al. Progressive brain atrophy in chronically infected and treated HIV+ individuals. J Neurovirol 2019; 25(3):342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cardenas VA, Meyerhoff DJ, Studholme C, Kornak J, Rothlind J, Lampiris H, et al. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol 2009; 15(4):324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kallianpur KJ, Jahanshad N, Sailasuta N, Benjapornpong K, Chan P, Pothisri M, et al. Regional brain volumetric changes despite 2 years of treatment initiated during acute HIV infection. AIDS 2020; 34(3):415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Connor EE, Zeffiro T, Lopez OL, Becker JT, Zeffiro T. HIV infection and age effects on striatal structure are additive. J Neurovirol 2019; 25(4):480–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, et al. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav 2011; 5(2):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr 2012; 59(5):469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nir TM, Fouche JP, Ananworanich J, Ances BM, Boban J, Brew BJ, et al. Association of Immunosuppression and Viral Load With Subcortical Brain Volume in an International Sample of People Living With HIV. JAMA Netw Open 2021; 4(1):e2031190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kure K, Weidenheim KM, Lyman WD, Dickson DW. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Pattern of involvement resembling a multisystem degeneration. Acta Neuropathol 1990; 80(4):393–400. [DOI] [PubMed] [Google Scholar]
- 77.McClernon DR, Lanier R, Gartner S, Feaser P, Pardo CA, St Clair M, et al. HIV in the brain: RNA levels and patterns of zidovudine resistance. Neurology 2001; 57(8):1396–1401. [DOI] [PubMed] [Google Scholar]
- 78.Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, et al. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol 2004; 61(3):369–376. [DOI] [PubMed] [Google Scholar]
- 79.Agrawal L, Louboutin JP, Marusich E, Reyes BA, Van Bockstaele EJ, Strayer DS. Dopaminergic neurotoxicity of HIV-1 gp120: reactive oxygen species as signaling intermediates. Brain Res 2010; 1306:116–130. [DOI] [PubMed] [Google Scholar]
- 80.Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain 2004; 127(Pt 11):2452–2458. [DOI] [PubMed] [Google Scholar]
- 81.Chang L, Wang G-J, Volkow ND, Ernst T, Telang F, Logan J, et al. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage 2008; 42(2):869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Janssen MA, Meulenbroek O, Steens SC, Góraj B, Bosch M, Koopmans PP, et al. Cognitive functioning, wellbeing and brain correlates in HIV-1 infected patients on long-term combination antiretroviral therapy. AIDS 2015; 29(16):2139–2148. [DOI] [PubMed] [Google Scholar]
- 83.Hines LJ, Miller EN, Hinkin CH, Alger JR, Barker P, Goodkin K, et al. Cortical brain atrophy and intra-individual variability in neuropsychological test performance in HIV disease. Brain Imaging Behav 2016; 10(3):640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ragin AB, Du H, Ochs R, Wu Y, Sammet CL, Shoukry A, et al. Structural brain alterations can be detected early in HIV infection. Neurology 2012; 79(24):2328–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang L, Shukla DK. Imaging studies of the HIV-infected brain. In: Handbook of Clinical Neurology, volume 152 (3rd series) The Neurology of HIV infection. Edited by Brew BJ. 3rd ed: Elsevier; 2018. pp. 229–264. [DOI] [PubMed] [Google Scholar]
- 86.Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One 2012; 7(9):e46178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Durazzo TC, Meyerhoff DJ, Nixon SJ. Interactive effects of chronic cigarette smoking and age on hippocampal volumes. Drug Alcohol Depend 2013; 133(2):704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2010; 2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Campbell LM, Fennema-Notestine C, Saloner R, Hussain M, Chen A, Franklin D, et al. Use of Neuroimaging to Inform Optimal Neurocognitive Criteria for Detecting HIV-Associated Brain Abnormalities. J Int Neuropsychol Soc 2020; 26(2):147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Küper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, et al. Structural gray and white matter changes in patients with HIV. J Neurol 2011; 258(6):1066–1075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Smoking Severity Predicted Brain Volume A-B Longer duration of smoking predicted smaller left superior parietal (r=−0.387, p=0.018, HIV x smoking duration-p=0.009) and smaller left parstriangularis gyrus (r=−0.379, p=0.021, HIV x smoking duration-p=0.036) in PWH smokers. C-D Greater tobacco smoking pack-years predicted smaller caudate (r=−0.364, p=0.027, HIV x pack-years-p=0.024) and smaller subcortical GM in PWH smokers (r=−0.350, p=0.033, HIV x pack-years -p=0.032). E-F Regardless of HIV serostatus, greater tobacco smoking pack-years predicted smaller thalamus (r=−0.341, p<0.001) and pallidum (r=−0.248, p=0.012) among all smokers. GM=gray matter, L= left, PWH=people with HIV disease, TS=tobacco smoking.
Supplemental Figure 2. Nadir CD4 and CD4 Predicted Brain Volume A-B Lower nadir CD4 cell count predicted smaller thalamus (r=0.261, p=0.021) and subcortical GM (r=0.251, p=0.027) in all PWH participants. C Lower nadir CD4 cell count predicted smaller caudates in PWH smokers (r=0.385, p=0.020, Smoking-by-nadir interaction p=0.033). D-E Regardless of smoking status, lower current CD4 cell count predicted smaller thalamus (r=0.236, p=0.032) and putamen (r=0.246, p=0.026) among all PWH participants. F Lower current CD4 cell count predicted smaller hippocampal in PWH smokers (r=0.330, p=0.042, Smoking-by-CD4 interaction p=0.010). GM= gray matter, PWH=people with HIV disease