Abstract
Objective:
This study compared the mutation profile and tumor mutational burden (TMB) in women living with HIV (WLWH) diagnosed with lung adenocarcinoma (n=8) or breast ductal neoplasm (n=13) that were enrolled into the Women’s Interagency and HIV Study (WIHS).
Design:
Previous studies tend to focus on single-institutions based on sample availability, while this study is based on a representative, multi-center cohort that represents the racial and ethnic composition of women with HIV in the United States
Methods:
The study sequenced the complete human exome of n = 26 cancer samples from HIV+ women, using Ion torrent next generation sequencing. The study cohort was compared to a HIV- cohort obtained from the Genomic Data Commons Data Portal of the NCI.
HIV+ and HIV- cohorts were compared using ion torrent next-generation sequencing.
Results:
There were no differences in known cancer mutations between breast cancer and lung cancer that developed in WLWH and those that developed in HIV seronegative (HIV-) women; however, WLWH presented a significantly higher tumor mutational burden (TMB) in comparison to HIV- patients. 75% of lung cancers and 61% of breast cancers were defined as TMB-high (more than 10 mutation/mb of DNA).
Conclusions:
This study affirms the recommendation that WLWH be included in clinical trials of novel treatments for these cancers. While these data are preliminary, the high TMB in WLHV suggests, paradoxically, that this immune challenged population may benefit greatly from immune checkpoint inhibitor therapies.
Keywords: High-Throughput Nucleotide Sequencing, Women, Exome, Mutation, Breast, Lung
Introduction
Infection with human immunodeficiency virus (HIV) increases the risk of cancer in people living with HIV (PLWH). The oncogenic properties of HIV go beyond systemic immunodeficiency due to CD4 depletion during the end stage of HIV disease. They may be attributable to viral proteins directly, or to events over the long pre-clinical course of infection. Little is known how cancers that develops in PLWH differ from cancer that develops in persons who were not exposed to HIV-related immune suppression and persistent low-level inflammation. Consequently, HIV was classified an oncogenic virus [1]. Using whole exome sequencing, this study ascertained the mutational status of lung and breast cancers that developed in a well-characterized cohort of women living with HIV (WLWH) and compared it to the general population. We hypothesized that incident cancers in HIV infected persons would exhibit genomic patterns that could inform cancer therapy in this population.
The introduction, early and lifelong application of effective combination antiviral therapy (cART) reduced the risk of severe immune deficiency and progression to Acquired Immune Deficiency Syndrome (AIDS), which was defined as a combination of opportunistic infections and viral associated cancers, such as Kaposi Sarcoma and Lymphoma. In low- and middle-income countries (LMIC) with less than optimal access to cART and a less than ideal public health structure, most cancers that develop in PLWH are still those associated with viral infections and loss of immune function [2]. In countries with ready access to cART non-infection associated cancers, such as breast cancer and lung cancer are on the rise and are predicted to become the most prevalent cancer types in PLWH [3]. This rise in breast and lung cancer is primarily attributable to the increased median age of PLWH [4, 5]. Lung cancer is emerging as one the leading causes of deaths among PLWH, as it already is in the general population. The increased incidence of lung cancer has been attributed to the more frequent smoking in PLWH [6–10]. However, some studies found that lung cancer incidence remained significantly elevated in PLWH, even after adjustment for smoking habits [11]. Adenocarcinoma, the most common type of lung cancer in PLWH, are not strongly linked to smoking. These epidemiological observations raise the possibility that HIV-infection may be an additional risk factor for lung cancer [12, 13].
Another common cancer in PLWH is breast cancer. Here, the epidemiological data are less clear. Breast cancer is the most common cancer in women world-wide. WLWH do not have a higher risk of developing breast cancer than HIV seronegative (HIV-) women [14]; however, this may be explained by competing comorbidities in HIV+ women prior to the introduction of cART. The proportion of HIV+ women with breast cancer is increasing over time. Importantly, HIV+ women with breast cancer have a 2-fold lower survival relative to cases in the general population, despite -- presumably -- equal access to cancer treatment and cART. This survival difference motivated this study of common genomic alterations that define breast cancer in HIV+ women as compared to HIV- women.
The assertion of a direct role for HIV in oncogenesis, beyond modulating the immune system, is controversial. No HIV-specific mechanisms driving increased lung and breast cancer risk in PLWH has been identified. Few genomic studies of lung and breast cancer in WLWH have been reported [15, 16]. They tended to be single institution-based and relied on convenience samples or case reports. By comparison, this study is based on a multi-center cohort of women with HIV in the United States. The Women’s Interagency and HIV Study (WIHS) is a large, prospective cohort study designed to investigate the consequences of HIV disease in women.
Within the sample size limitations of this study, no evidence for specific genomic differences between breast cancer and lung cancer were discovered; however, tumor mutational burden (TMB) was far greater in PLWH than matched controls. It remains unclear why both cancer risk and cancer survival is worse among PLWH than for the general population. This study supports the recent recommendation that PLWH should be included in all clinical trials of novel treatment approaches for these two cancers and receive the same consistent clinical treatment according to the same standards of care as HIV negative persons.
Methods
Samples.
The Women’s Interagency HIV Study (WIHS) is a cohort of women with and at risk for HIV infection [17]. Seven WIHS sites (New York, Chicago, Washington D.C., San Francisco, Chapel Hill, Atlanta and Miami) contributed 26 tissue blocks plus matching PBMC from breast and lung cancer cases between 2000–2017 after obtaining participant consent. Specimens were de-identified replacing the patient information with a WIHS ID number. The study was IRB approved at each participating WIHS site. Lung (n=219) and breast (n=719) cancer sequencing data from HIV negative individuals were obtained from Genomic Data Commons Data Portal.
DNA extraction.
Tumor DNA was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen Inc.) per manufacturer protocol with 3 hrs tissue digestion. PBMC DNA was extracted using a MagNA Pure Compact Instrument and Nucleic Acid Isolation Kit I -Large Volume (Roche Inc.).
Exome analysis.
Barcoded libraries were prepared from 100 ng DNA with an Ion AmpliSeq Exome RDY library preparation kit (Thermo Inc.) quantitated by Qubit dsDNA HS assay, sized with an Agilent Bioanalyzer 2100 high-sensitivity DNA assay (Agilent Technologies), and pooled to 80 pM final concentration before sequencing on the Ion Chef and S5 sequencer (Thermo Inc.). Base calling, quality filtering, and demultiplexing were performed on the instrument with default parameters. Reads >50 bp were mapped to the human genome (NCBI build hg38_2016) using CLC Genomics Workbench 9 (Qiagen Inc) and low frequency variant detection tool with the parameters: minimum frequency = 10%, and minimum coverage = 100x. Only non-synonymous variants with a minimum average quality score of ≥19 and a forward/ reverse balance of 0.5 were included. Tumor variants were filtered against germline variants obtained from PBMC for each patient.
Statistical analysis.
Results are reported as mean ± standard deviation (SD). The unpaired 2-tailed t-test with Welch correction was used to compare groups.
Results
The study sequenced the human exomes of initially n = 26 tumor biopsies from HIV+ women provided as formalin-fixed paraffin-embedded (FFPE) blocks or slides, as well as matched PBMC samples. Two samples were excluded due to the slides being stained, two samples were excluded due to low DNA yield and one additional sample was excluded due to low library quality. A total of 21 samples, 8 lung and 13 breast cancers, and matched normal PBMC, were included in the final analysis. All samples were primary tumors and confirmed by pathology. The clinical information for the study cohort is provided in Table 1. The participants were between 41–73 years old, with an average of 54.5 and 56.8 years for the lung and breast cohort respectively. Most patients were either white or African American. Only one person identified as Hispanic. For the lung cohort, 87.5% were adenocarcinomas and 12.5% were squamous cell carcinomas. For the breast cancer cohort 100% were ductal carcinomas, 38.5% were classified as in situ and 61.5% as invasive.
Table 1:
Patient characteristics
| Lung | Breast | |||
|---|---|---|---|---|
| Characteristics | HIV− (N=219) | HIV+ (N=8) | HIV− (N=719) | HIV+ (N=13) |
| Age mean (range) | 61.6 (41–73) | 54.5 (41–73) | 57.0 (41–73) | 56.8 (41–73) |
| Ethnicity | ||||
| White | 160 (73.1%) | 5 (38.5%) | 512 (71.2%) | 2 (25.0%) |
| African American | 24 (11.0%) | 5 (38.5%) | 115 (16.0%) | 6 (85.7%) |
| Other | 35 (16.0%) | 3 (23.1%) | 92 (12.8%) | 0 (0.0%) |
| Race | ||||
| Hispanic | 3 (1.4%) | 1 (7.69%) | 48 (6.7%) | 1 (12.5%) |
| Non-Hispanic | 716 (99.6%) | 12 (92.3%) | 671 (93.3%) | 7 (87.5%) |
| Subtype | ||||
| Adenocarcinoma | 219 (100%) | 7 (87.5 %) | NA | NA |
| Ductal Carcinoma | NA | NA | 719 (100%) | 13 (100%) |
| Other | NA | 1 (12.5%) | NA | NA |
All samples were subjected to targeted amplification and next generation sequencing. The reads were aligned to the human genome (NCBI build hg38_2016) and single nucleotide variants (SNVs) identified. A median of 42,106,610±13,447,761 reads were obtained for each tumor sample, translating to 88.07%±0.03% median coverage at 10x. A median of 33,157,434±11,861,795 reads was obtained for each PBMC sample, translating to 93.08%±0.04% median coverage at 10x. Any tumor SNVs that were also present in the matched PBMC samples were removed. To focus the data set further, all non-synonymous SNVs were removed as well.
The study cohort was compared to a HIV- cohort obtained from the Genomic Data Commons Data Portal of the NCI. This cohort contained n=719 breast and n = 219 lung cancer samples. The HIV seronegative cohort was filtered by the following parameters sex: female, age: 41–74 years, sample type: primary tumor, subtype: breast ductal and lobular neoplasms and lung adenocarcinomas, respectively. The HIV- group in lung cancer was further divided into smokers (>1 cigarette/day) and non-smokers (<1 cigarette/day).
The most mutated gene in lung and breast samples was for TP53. There were no significant differences between the HIV+ and HIV- cohorts, regarding the frequencies of key oncogenes and tumor suppressor genes.
Increased TMB in breast and lung cancer in PLWH
The term TMB describes the total number of mutations in DNA. It has emerged as a useful clinical predictor in certain cancer types and therapy types, such a checkpoint inhibitor therapy [18, 19]. For lung cancer the TMB for the HIV+ cohort (mean = 53.13/MB; range: 146.13–1.07/MB) was significantly higher compared to HIV- both non-smoker (mean = 14.09/MB; range: 77.97–0.03/MB) and smoker (mean =15.23/MB; range: 54.40–0.43/MB). (Figure 1). However, TMB did not differ significantly between of non-smokers and smokers in the HIV- cohort. For breast cancer the same phenomenon is observed, where the HIV+ cohort (mean = 82.46/MB; range: 673.7–0.40/MB) has a significantly higher TMB in comparison to the HIV- cohort (mean = 4.38/MB; range: 264.93–0.23/MB).
Figure 1: Tumor mutational burden of HIV+ vs. HIV- patients.
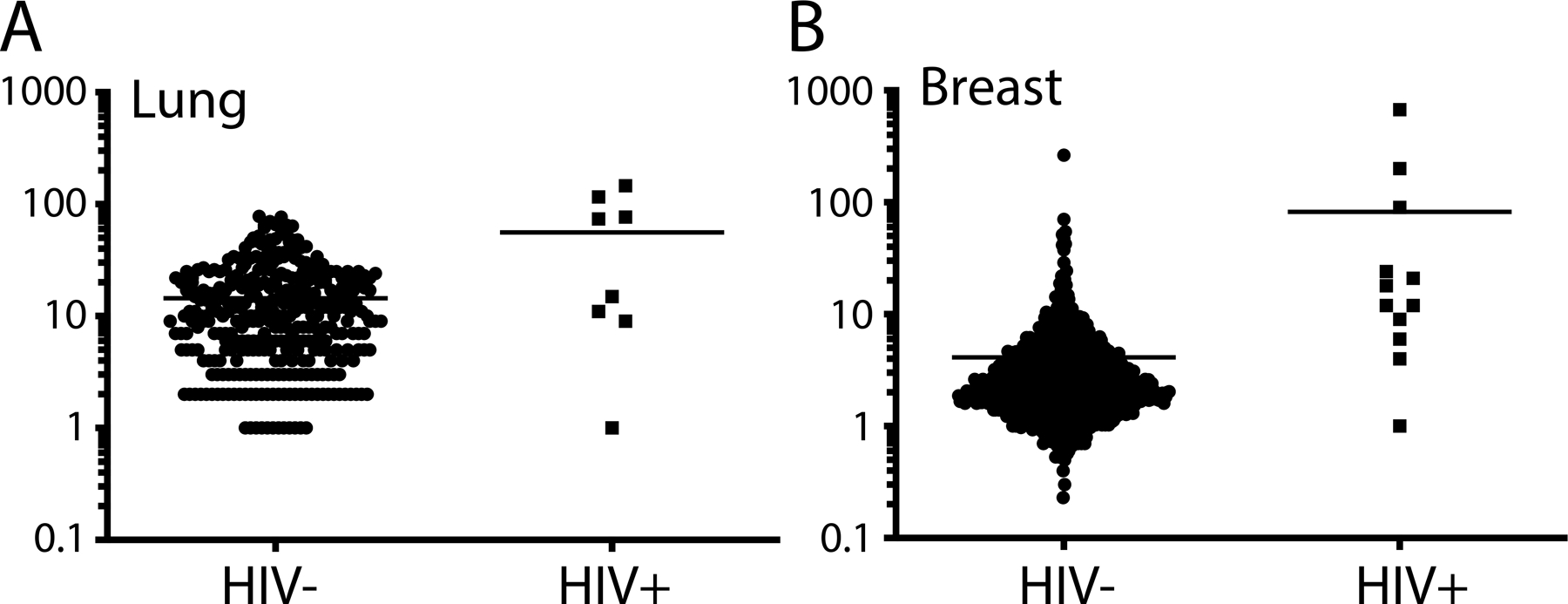
Dot plot showing the total number of mutations per Mb observed in whole-exome sequencing of HIV+ patients included in this study and HIV- patient obtained from the TCGA database for (A) breast and (B) lung malignancies. Each dot represents a tumor.
Discussion
Cancer has become the leading cause of mortality and morbidity in PLWH (reviewed in [20]). Recent improvements to cART, such as long-lasting injectables, promise further advancements in the lifelong suppression of HIV viral loads; however, HIV infection rates are no longer declining in many countries, and a HIV cure or an HIV vaccine remains elusive. Hence, one can project an increase in the number of patients living with HIV and cancer [21].
This study tried to address a fundamental question in the field. Do cancers that develop in PLWH differ from those that develop in the general population, and should we make cancer treatment recommendations specific to PLWH?
In our cohort of HIV+ women with lung adenocarcinoma or breast ductal cancer, we observed no significant differences in the frequency of the most common mutated oncogenes; however, this study observed a significantly higher TMB in the HIV+ vs. the matched HIV- cohort. 75% of lung cancer cases and 61.5% of breast cancer cases were TMB-high, while the matched HIV- cohort were 52.4% and 31% for lung and breast cancer, respectively. The main limitation of this study is the small sample size, as both HIV infection and cancer are relatively rare events in the US.
The incidences of breast and lung cancer have been rising in PLWH and are projected to become the leading cause of mortality for this population. As TMB-high cancers are more susceptible to immune checkpoint inhibitor therapies-, the results from this study reemphasize the notion that HIV+ cancer patients on successful cART should not a priori be excluded from immune therapies. This conjecture is supported by recent successful clinical trials with pembrolizumab and nivolumab in HIV+ KS, HIV+ lung cancer and in Merkel Cell Carcinoma[22, 23].
Acknowledgments
This work was supported by public health service grant CA163217 to DPD and U01-HL146194 and P30-AI-050410 to AAA. Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS), now the MACS/WIHS Combined Cohort Study (MWCCS)
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), and P30-MH-116867 (Miami CHARM).
Footnotes
Conflict of interest statement
The authors have declared that no conflict of interest exists.
References
- 1.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol 2009; 10(4):321–322. [DOI] [PubMed] [Google Scholar]
- 2.Dittmer DP, Krown SE, Mitsuyasu R. Exclusion of Kaposi Sarcoma From Analysis of Cancer Burden. JAMA Oncol 2017; 3(10):1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS 2006; 20(12):1645–1654. [DOI] [PubMed] [Google Scholar]
- 4.Mahale P, Engels EA, Coghill AE, Kahn AR, Shiels MS. Cancer risk in older people living with human immunodeficiency virus infection in the United States. Clin Infect Dis 2018. [DOI] [PMC free article] [PubMed]
- 5.Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Medicine 2013; 14(4):195–207. [DOI] [PubMed] [Google Scholar]
- 6.Herida M, Mary-Krause M, Kaphan R, Cadranel J, Poizot-Martin I, Rabaud C, et al. Incidence of Non–AIDS-Defining Cancers Before and During the Highly Active Antiretroviral Therapy Era in a Cohort of Human Immunodeficiency Virus–Infected Patients. Journal of Clinical Oncology 2003; 21(18):3447–3453. [DOI] [PubMed] [Google Scholar]
- 7.Frisch M Association of Cancer With AIDS-Related Immunosuppression in Adults. JAMA 2001; 285(13):1736. [DOI] [PubMed] [Google Scholar]
- 8.Dal Maso L, Franceschi S, Polesel J, Braga C, Piselli P, Crocetti E, et al. Risk of cancer in persons with AIDS in Italy, 1985–1998. British Journal of Cancer 2003; 89(1):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer Risk in the Swiss HIV Cohort Study: Associations With Immunodeficiency, Smoking, and Highly Active Antiretroviral Therapy. JNCI Journal of the National Cancer Institute 2005; 97(6):425–432. [DOI] [PubMed] [Google Scholar]
- 10.Hessol NA, Barrett BW, Margolick JB, Plankey M, Hussain SK, Seaberg EC, et al. Risk of smoking-related cancers among women and men living with and without HIV. AIDS 2021; 35(1):101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karp J, Profeta G, Marantz PR, Karpel JP. Lung Cancer in Patients With Immunodeficiency Syndrome. Chest 1993; 103(2):410–413. [DOI] [PubMed] [Google Scholar]
- 12.Cadranel J, Garfield D, Lavole A, Wislez M, Milleron B, Mayaud C. Lung cancer in HIV infected patients: facts, questions and challenges. Thorax 2006; 61(11):1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sigel K, Wisnivesky J, Gordon K, Dubrow R, Justice A, Brown ST, et al. HIV as an independent risk factor for incident lung cancer. AIDS 2012; 26(8):1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370(9581):59–67. [DOI] [PubMed] [Google Scholar]
- 15.Molto J, Moran T, Sirera G, Clotet B. Lung cancer in HIV-infected patients in the combination antiretroviral treatment era. Transl Lung Cancer Res 2015; 4(6):678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crequit P, Ruppert AM, Rozensztajn N, Gounant V, Vieira T, Poulot V, et al. EGFR and KRAS mutation status in non-small-cell lung cancer occurring in HIV-infected patients. Lung Cancer 2016; 96:74–77. [DOI] [PubMed] [Google Scholar]
- 17.Adimora AA, Ramirez C, Benning L, Greenblatt RM, Kempf MC, Tien PC, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol 2018; 47(2):393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rozeman EA, Hoefsmit EP, Reijers ILM, Saw RPM, Versluis JM, Krijgsman O, et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat Med 2021; 27(2):256–263. [DOI] [PubMed] [Google Scholar]
- 19.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020; 21(10):1353–1365. [DOI] [PubMed] [Google Scholar]
- 20.Gabuzda D, Jamieson BD, Collman RG, Lederman MM, Burdo TH, Deeks SG, et al. Pathogenesis of Aging and Age-related Comorbidities in People with HIV: Highlights from the HIV ACTION Workshop. Pathog Immun 2020; 5(1):143–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer Burden in the HIV-Infected Population in the United States. JNCI: Journal of the National Cancer Institute 2011; 103(9):753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med 2016; 374(26):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uldrick TS, Goncalves PH, Abdul-Hay M, Claeys AJ, Emu B, Ernstoff MS, et al. Assessment of the Safety of Pembrolizumab in Patients With HIV and Advanced Cancer-A Phase 1 Study. JAMA Oncol 2019; 5(9):1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]


