Abstract
Purpose
To describe the unusual case of inflammatory CRB1-associated retinal dystrophy that initially presented with self-resolving opsoclonus.
Observations
We report the case of a now 2-year-old female who developed opsoclonus without myoclonus at the age of 4 months. An extensive workup for neuroblastoma and other systemic diseases was unremarkable, and all unusual eye movements self-resolved at age 10 months. Twenty-one months after initial presentation, she began having reduced visual behaviors, and comprehensive ophthalmic exam at that time revealed recurrent saccadic intrusions as well as severe, chronic retinal inflammation and dystrophic changes. An extensive infectious and inflammatory workup was negative. Genetic sequencing revealed two variants in CRB1: a heterozygous missense mutation and a heterozygous novel deletion involving exon 12. The patient was treated with monthly infliximab and methylprednisolone infusions with improvement in her optic disc and macular capillary leakage. The patient's 8-month-old sister also harbored the same variants in CRB1 and had early signs of retinal dystrophy and peripheral vascular leakage on exam.
Conclusion
Saccadic intrusions may be the first sign of a retinal dystrophy, and infants and children with this presentation should undergo a complete eye exam. We further highlight the link between CRB1-associated retinal dystrophy and inflammation, and how systemic steroids and tumor necrosis factor alpha (TNF-α) inhibitors may be effective therapies. Finally, we report a novel deletion in CRB1 that is likely highly penetrant.
Keywords: Opsoclonus-myoclonus, Retinal vasculitis, CRB1-Associated retinal dystrophy
1. Introduction
Opsoclonus is characterized by rapid, involuntary, multidirectional conjugate eye movements with horizontal, vertical, and torsional directions.1 Opsoclonus, which can sometimes present as part of opsoclonus-myoclonus (OM), is associated with a variety of diseases including paraneoplastic syndromes, infections, or toxic-metabolic derangements.1 In children, opsoclonus alone or OM is most frequently linked to neuroblastoma. In fact, over 50% of children with OM have underlying paraneoplastic neuroblastic tumors (neuroblastoma, ganglioneuroblastoma, and ganglioneuroma).2 Other etiologies include viral infections (HIV, CMV, VZV, and hepatitis C among others) and systemic inflammatory conditions like sarcoidosis or multiple sclerosis.2, 3, 4 There have been no prior reports in the literature of opsoclonus being associated with an inherited retinal dystrophy. Here we describe the case of a 4 month old who presented initially with opsoclonus without myoclonus and was later diagnosed with CRB1-associated retinal dystrophy.
2. Case report
The patient is a 4 month old female with an unremarkable birth and family history who presented with acute onset opsoclonus. She developed episodes of abnormal eye movements occurring every few minutes two weeks after a viral upper respiratory infection and on the same day that she received routine 4 month old vaccines. These vaccines consisted of the second of three doses of diphtheria/tetanus/pertussis (DTaP), H. influenzae type b (Hib), polio (IPV), pneumococcal disease (PCV13), and rotavirus (RV). The eye movements were episodic, conjugate, and rapid in all directions, consistent with opsoclonus (Video 1).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ajoc.2022.101444
The following is/are the supplementary data related to this article:
A full workup including labs (VMA/HVA, CMP, CRP, and CBC)1 and extensive imaging (abdominal ultrasound, full body magnetic resonance imaging (MRI), and MIBG2) was unremarkable. Over the next six months, the abnormal eye movements continued, but the episodes were less frequent and there was a transition to more purely vertical nystagmoid movement of the eyes with less frequent multidirectional chaotic movement as seen on review of parent videos comparing the eye movements at presentation and on video electroencephalogram (EEG) at 10 months of age. She was followed closely by oncology and neurology, undergoing regular screenings for neuroblastoma and seizures. No formal eye exam by an ophthalmologist occurred at this time due to normal visual behavior between these episodes and the temporal association of the viral illness and start of the eye movements. The opsoclonus and vertical nystagmoid movements resolved completely at ten months of age. The self-resolution of symptoms appeared most consistent with a viral-associated opsoclonus syndrome.
The patient continued to develop normally without any other health issues. At 25 months of age, she exhibited diminished visual behavior and recurrence of abnormal eye movements, most noticeably when she fixated on a particular object. There were no abnormal movements elsewhere in the body and no other systemic symptoms. A repeat neuroblastoma workup was negative and brain and spinal MRI was unremarkable. At this time, the patient was seen for her first eye examination by a neuro-ophthalmologist, who noted that her new eye movements were consistent with saccadic intrusions rather than opsoclonus or nystagmus. Her visual acuity (as measured by Teller Acuity) was 20/190 bilaterally and there was no relative afferent pupillary defect. Funduscopic exam revealed bilateral elevated optic nerves that were waxy and diffusely pale, parafoveal yellow discoloration and pigmented deposits, and peppered retinal pigment epithelium (RPE) changes in the peripheral retina concerning for a retinal dystrophy.
A full eye examination under anesthesia (EUA) by a pediatric retinal specialist found 1+ non-pigmented cell in the anterior vitreous bilaterally with no other anterior segment findings. There was mild pallid edema of both optic nerves. In both eyes, there was macular atrophy, punctate RPE changes peripherally, and attenuation of the retinal vasculature with vascular sheathing (Fig. 1). Optical coherence tomography (OCT) in both eyes showed elevation of the optic disc without drusen as well as diffuse outer retina and RPE loss, with prominent central atrophy resulting in full-thickness macular holes (Fig. 2). Fluorescein angiography (FA) showed good retinal perfusion but leakage of the optic disc and retinal vasculature diffusely in both eyes (Fig. 3A–D). Taken together, the exam demonstrated features of both dystrophic and inflammatory processes.
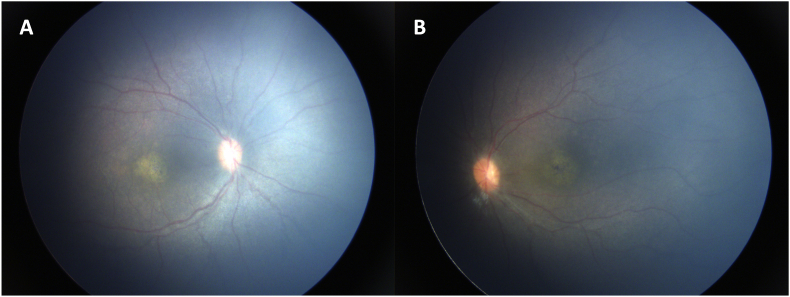
Fig. 1.
Fundus photography of the right eye (A) and left eye (B) showed evidence of a retinal dystrophy. In both eyes, macular atrophy, punctate RPE changes peripherally, and attenuation of the retinal vasculature with vascular sheathing are noted. Mild pallid edema of both optic nerves also present.
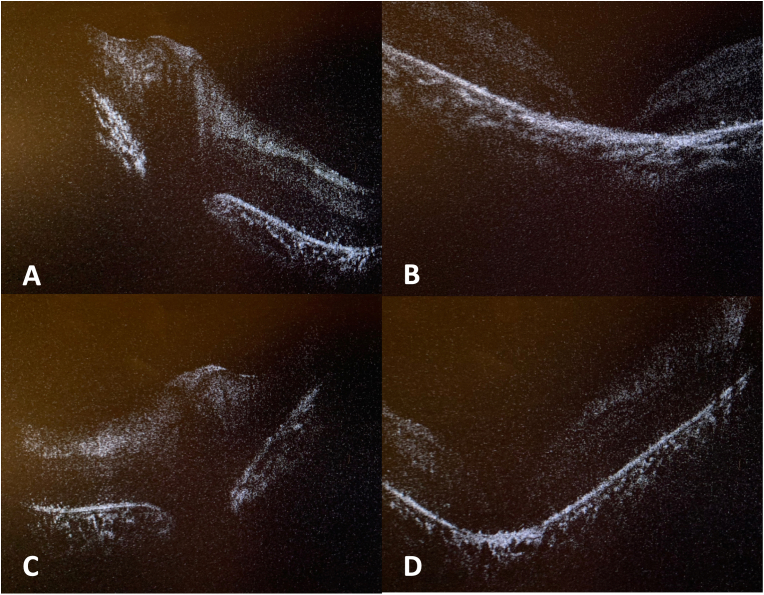
Fig. 2.
Optical coherence tomography (OCT) shows bilateral signs of inflammation and retinal dystrophy. In the right eye, there is elevation of the optic disc without drusen (A) as well as diffuse outer retina and RPE loss with prominent central atrophy resulting in full-thickness macular holes (B). Similar findings were present in the left eye (C and D).
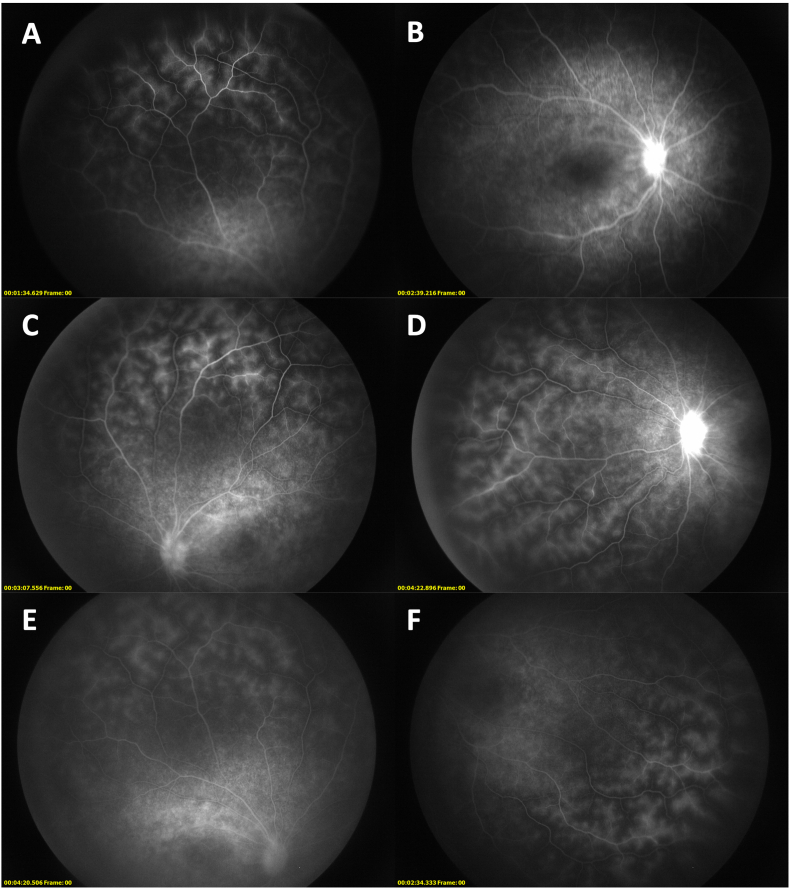
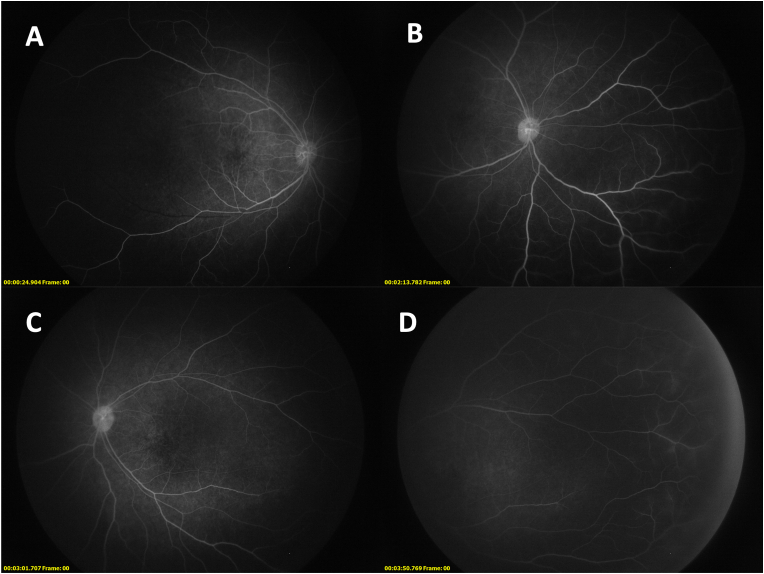
Fig. 3.
Fluorescein angiography shows bilateral disc and vascular leakage that is improved after months of treatment. Early phase (A) and late phase (B) images of the right eye show prominent vascular leakage both centrally and peripherally and hyperfluorescence indicative of disc leakage. Similar findings were present in the left eye (C and D). After three months of oral prednisone and monthly methylprednisolone and infliximab infusions, repeat FA shows marked improvement in the degree of optic disc and macular capillary leakage, with peripheral vascular leakage mostly unchanged, in both the right eye (E) and left eye (F). It should be noted that the peripheral hyperfluorescence may be an artifact relative to the hypofluorescence of the degenerated retina.
An extensive infectious workup was negative for syphilis, tuberculosis, herpesviruses (HSV-1, HSV-2, VZV, EBV),3 toxoplasmosis, Bartonella, Lyme, measles, and HIV. She had positive IgG titers (1:20) for CMV but a negative serum polymerase chain reaction. An inflammatory workup consisting of CBC, CMP, ESR, CRP, ANA, ACE,4 lysozyme and antiphospholipid antibodies was also unremarkable. Given the degree of bilateral ocular inflammation and risk of significant visual impairment, she was admitted to the hospital for 3 days of 10 mg/kg IV methylprednisolone and a single dose of 7.5 mg/kg infliximab. Upon discharge she was initiated on an oral steroid taper along with monthly infliximab and methylprednisolone infusions at the above doses. An EUA done three months after starting treatment showed slight improvement in vitreous cell and haze bilaterally and marked improvement in the degree of optic disc and macular capillary leakage, with peripheral vascular leakage mostly unchanged (Fig. 3E and F). Other findings, including the diffuse outer retinal/RPE loss and prominent central atrophy resulting in complete retinal thinning in the foveal center, were similarly unchanged.
A full-field electroretinogram (ffERG) after the initial exam under anesthesia was consistent with an inherited retinal dystrophy. After dark adaptation, the rod response (to a dim flash) was undetectable. The combined rod-cone response to a strong flash had minimal amplitudes less than 10% of minimum. Oscillatory potentials were absent. After light adaptation, the single-flash cone responses had an amplitude 20–30% of normal minimum values. The 30 Hz flicker responses showed implicit times that were significantly delayed. Overall, the study demonstrated severe, diffuse retinal dysfunction that affected rods slightly more than cones, though both were significantly affected.
Genetic testing performed using the retinal dystrophy panel from Blueprint Genetics found the patient had two variants in CRB1, a gene associated with Leber's congenital amaurosis (LCA) type 8 (Mendelian Inheritance in Man (MIM) #613835), autosomal recessive retinitis pigmentosa (RP) type 12 (MIM #600105), and pigmented paravenous chorioretinal atrophy (MIM #172870).5, 6, 7 One of the two variants was a heterozygous missense mutation (c.2300T > C, p.Leu767Pro) known to be pathogenic, and the other was a heterozygous novel deletion involving exon 12 (c.(4005 + 1_4006–1)_(*1_?)del) categorized as likely pathogenic.17, 18, 19 Two variants in CDH3 were also detected -- one heterozygous point mutation leading to early truncation (likely pathogenic) and one heterozygous point mutation in an intronic splice region (variant of uncertain significance). However, the patient's phenotype was thought to be less consistent with known CDH3-associated disease. Mutations in CDH3 are associated with two autosomal recessive diseases: congenital hypotrichosis and juvenile-onset macular dystrophy (MIM #601553) and ectodermal dysplasia, ectrodactyly, and macular dystrophy syndrome (MIM #225280). The patient did not have any abnormalities involving the hair or limbs and had an earlier onset of retinal dystrophy compared to patients with these syndromes.8
Of note, although both parents are asymptomatic, the patient's 8-month-old sister also tested positive for both CRB1 variants and one of the CDH3 variants. No changes in visual behavior were noted by the parents, and no abnormal eye movements were found on exam. An EUA done at 8 months of age showed no abnormalities in the anterior chamber and normal optic discs, but diffuse granularity of the RPE (worse in the macula than periphery) and blunted foveal reflex bilaterally (Fig. 4A and B). OCT showed macular outer retinal and retinal pigment epithelial loss with early pigment migration and abnormal foveal contour secondary to a foveal lamellar hole (Fig. 4C and D). FA showed no leakage of the optic nerve or macula and very minimal faint late leakage of far peripheral vessels in both eyes (Fig. 5). Given the milder findings, she was not immediately started on treatment but will be closely monitored with plans for a repeat EUA in 3 months.
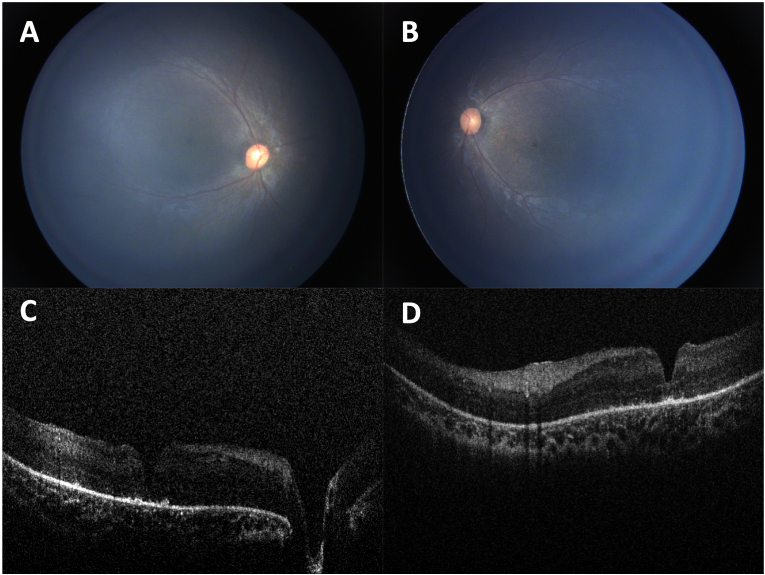
Fig. 4.
Fundus photography and OCT of the patient's 8-month-old sister shows early stage retinal dystrophy. Fundus photographs of the right eye (A) and left eye (B) show diffuse granularity of the RPE which is more pronounced in the macula than periphery. OCT of the right eye (C) and left eye (D) showed macular outer retinal and retinal pigment epithelial loss with early pigment migration and abnormal foveal contour secondary to a foveal lamellar hole.
Fig. 5.
Fluorescein angiography of the patient's 8-month-old sister shows mild signs of peripheral vascular leakage. Early phase (A) and late phase (B) images of the right eye showed no leakage of the optic nerve or macula and very minimal faint late leakage of far peripheral vessels. Similar findings were present in the left eye (C and D).
3. Discussion
This is an unusual case of a now 2 year old patient with CRB1-associated retinal dystrophy who initially presented with a 6 month period of self-resolving episodic opsoclonus at 4 months old, followed 21 months later with recurrent saccadic intrusions and severe, chronic retinal inflammation and dystrophic changes. It is suspected that the opsoclonus was the first initial presentation of her inherited retinopathy, especially given that her eye examination at 25 months old revealed severe and chronic signs of inflammation (retinal vascular sheathing and optic disc leakage) and dystrophy (RPE changes and significant central macular atrophy). It is likely that the eye movements never fully abated and were not noticed by family during the 15 months of reported quiescence given that the eye movements at 25 months appear roughly similar to those seen on video EEG at 10 months old and less multidirectional than at presentation at 4 months old.
Although there are no reported cases of any retinal disorder presenting with opsoclonus, other types of saccadic intrusions and nystagmus -- particularly in infancy -- have been associated with retinal dystrophies.9,10 A recent Italian study of 50 children with ERG-confirmed retinal dystrophy showed that 76% of these patients initially presented with nystagmus as noted by their parents. In fact, nystagmus was the first symptom reported by parents if the disease onset was before six months of age.11 Another Chinese study of 136 patients with a specific type of nystagmus (pendular low amplitude, high frequency) showed that 52% had abnormal fundi and 34% had genetic mutations causing various inherited retinal dystrophies.12 Nystagmus has been reported in several specific cone and rod dystrophies, including congenital stationary night blindness, achromatopsia, LCA, Bardet-Biedl syndrome, Joubert's syndrome, and Alstrom's syndrome.9,13 This case highlights the importance of considering a full ophthalmic examination in a pediatric patient with opsoclonus or any other abnormal eye movements like saccadic intrusions or nystagmus, as it could be an initial sign of retinal dystrophy, especially in young patients who are not able to verbalize a decrease in vision.
The patient's dystrophic retinal findings, as well as those of her younger sister, are best explained by variants in CRB1. CRB1 is a human homolog of the Drosophila melanogaster protein crumbs (crb) -- expressed in the retina and the brain -- and encodes a transmembrane protein that is crucial for establishing polarity within the developing photoreceptor.14,15 Mutations in CRB1 in humans were shown to have abnormal retinal architecture, lacking the distinct layers in the adult retina and demonstrating thickness of the nerve fiber layer around the optic nerve head.16 The pattern of inheritance for CRB1-associated retinal dystrophy tends to be autosomal recessive. However, our patient had significant disease expression despite heterozygous mutations. It is likely that her two mutations were located on separate alleles, thus leading to the disease phenotype as a compound heterozygote.
The heterozygous p.Leu767Pro variant that the patient and her sister harbor is a known pathogenic mutation that has been seen as a homozygous mutation in two patients with LCA.17,18 Blueprint genetics has also previously detected this variant in at least 4 other patients with CRB1-associated retinal dystrophy, including one compound heterozygote. The other heterozygous variant in our patient and her sister is a deletion of approximately 4900 base pairs, including the final exon - exon 12 - of the gene. This specific deletion has not been previously described in the literature or in databases such as ClinVar (NCBI, NIH, Bethesda, MD), but other deletions affecting specific exons have been described and noted to be pathogenic as the truncation of the CRB1 protein leads to loss of function.17,19
Retinal dystrophies such as LCA, Bardet-Biedl syndrome, RP, and others have previously been shown to present with uveitis or other signs of inflammation.20, 21, 22 In fact, elevated inflammatory markers have been noted in the aqueous and vitreous of patients with RP compared to controls.23 In addition, certain inherited retinal diseases, namely CAPN5 neovascular inflammatory vitreoretinopathy (MIM #193235), have combined clinical features of RP and uveitis.24 A growing number of studies have demonstrated that CRB1-associated retinal dystrophy can present with uveitis or other signs of ocular inflammation, often in pediatric patients who have already undergone an extensive but unremarkable workup for other causes of uveitis. This association was first reported in 2001, when CRB1 mutations were detected in five of nine RP patients with Coats-like exudative vasculopathy.25 A recent study of 40 Belgian patients with CRB1-associated retinal dystrophies showed that intermediate or posterior uveitis was found in 8% of these patients.7 In a separate case series about retinal dystrophy presenting as refractory intermediate uveitis, 3 out of the 6 patients (5, 12, and 13 years of age) had mutations in CRB1, with 2 being compound heterozygotes and the other having a homozygous missense mutation.26 Specific cases have also been described, including an 8-year-old male with intermediate uveitis, retinal capillaritis, and cystoid macular edema who was subsequently found to have compound heterozygous mutations in CRB1 and a 26-year-old male with a homozygous missense mutation in CRB1 who presented with diffuse retinal vascular leakage.27,28 The exact mechanism of localized inflammation is still unclear but appears to involve microglia. It is thought that photoreceptor cell death or other insults to the retina activate microglia to become migratory phagocytes, releasing a mixture of pro-inflammatory cytokines from the retina.29,30 Mouse models of RP have already shown that suppression of microglial activation was protective of photoreceptors and led to decreased pro-inflammatory markers in the retina.31
The inflammation found in CRB1-associated retinal dystrophy has been detected systemically. Verhagen et al. studied a 14-year-old female with compound heterozygous mutations in CRB1 who initially was found to have intermediate uveitis, cystoid macular edema, and lesions resembling multifocal choroiditis. She was treated with several local and systemic anti-inflammatory agents including systemic steroids, methotrexate, mycophenolate mofetil, TNF-alpha inhibitors, and tocilizumab. Interestingly, the patient's serum levels of various pro-inflammatory markers including CCL2-2, CXCL9, CXCL10, interferon-beta, and various interleukins (including IL-17 and IL-21, central drivers of autoimmune uveitis) were all initially elevated and subsequently decreased after tocilizumab. Ultimately, the various immunosuppressive/immunomodulatory agents resulted in slight improvements in her visual acuity, from 20/50 to 20/40 in the right eye and 20/66 to 20/33 in the left eye.30 The presence of this systemic inflammatory signature is suggestive of a secondary immune response against the damaged retina.
Our patient had multiple signs of ocular inflammation, including significant optic disc and retinal vasculature leakage FA, vascular sheathing on fundus exam, and vitreous haze. This inflammation is most likely a feature of her CRB1-retinal dystrophy. Although it is possible that the vaccines she received and her upper respiratory infection at the time of her initial presentation (with opsoclonus) were contributing to her ocular inflammation, this is highly unlikely given the duration and severity of the inflammation. Furthermore, none of the vaccines she received at 4 months (DTaP, Hib, polio, pneumococcal, or rotavirus) have been reported to cause vaccine-associated uveitis.
Ultimately, her exam improved after 3 months of steroids and TNF-alpha inhibitors, with a marked improvement in optic disc and posterior pole capillary leakage on an FA and some decrease in vitreous haze, although peripheral vascular leakage was unchanged. (It is plausible that the peripheral hyperfluorescence is partially due to artifact, with increased fluorescence relative to the hypofluorescence of the degenerated retina.) Taken together, these findings suggest that systemic immunosuppression may be an effective way of treating CRB1-associated retinal dystrophy. More time is needed to determine if this decrease in inflammation will lead to improvements in visual acuity. In addition, the fact that the patient's 8-month-old sister had early signs of peripheral vascular leakage on FA suggests that the inflammation and dystrophic retinal changes may proceed in parallel, and perhaps earlier intervention with immunosuppressive or immunomodulatory therapies can lead to greater preservation of visual acuity.
4. Conclusions
In summary, we report the remarkable case of a now 2-year-old female with CRB1-associated retinal dystrophy who initially presented with self-limited opsoclonus. Her presentation suggests that opsoclonus or nystagmoid eye movements such as saccadic intrusions – even if self-limited – may be the first sign of a retinal dystrophy. Thus, it is important to consider performing a complete eye exam on children and particularly infants with this presentation as these patients may not be able to verbalize any decrease in vision. This patient's bilateral optic disc leakage and retinal vascular sheathing/leakage further highlights the link between CRB1-associated retinal dystrophy and inflammation, and her response to systemic steroids and TNF-alpha inhibitors show that these therapies may be an effective way to treat CRB1 retinal dystrophy. Finally, we report a new mutation in CRB1, a heterozygous deletion of exon 12 (c.(4005 + 1_4006–1)_(*1_?)del). Given the patient's early onset of symptoms and severe presentation, this new mutation -- along with the missense mutation p.Leu767Pro that the patient also harbors -- is likely highly penetrant.
Patient consent
The patient's legal guardians consented to publication of the case in writing.
Funding
No funding or grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship
Declaration of competing interest
The following authors have no financial disclosures – ASL, MVP, KM, QDN, SJB, EHW.
Acknowledgements
None
Footnotes
vanillylmandelic acid, homovanillic acid, comprehensive metabolic panel, C-reactive protein, complete blood count.
A nuclear scan using MIBG (metaiodobenzylguanidine), a radioactive tracer to detect the presence of neuroblastoma or pheochromocytoma.
Herpes simplex virus 1 and 2, varicella zoster virus, and Epstein-Barr virus..
complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, antinuclear antibody, angiotensin-converting enyzme
References
- 1.StatPearls. 2020 [Google Scholar]
- 2.Wong A. An update on opsoclonus. Curr Opin Neurol. Feb 2007;20(1):25–31. doi: 10.1097/WCO.0b013e3280126b51. [DOI] [PubMed] [Google Scholar]
- 3.Hero B., Schleiermacher G. Update on pediatric opsoclonus myoclonus syndrome. Neuropediatrics. Dec 2013;44(6):324–329. doi: 10.1055/s-0033-1358604. [DOI] [PubMed] [Google Scholar]
- 4.Oh S.Y., Kim J.S., Dieterich M. Update on opsoclonus-myoclonus syndrome in adults. J Neurol. Jun 2019;266(6):1541–1548. doi: 10.1007/s00415-018-9138-7. [DOI] [PubMed] [Google Scholar]
- 5.Abouzeid H., Li Y., Maumenee I.H., Dharmaraj S., Sundin O. A G1103R mutation in CRB1 is co-inherited with high hyperopia and Leber congenital amaurosis. Ophthalmic Genet. Mar 2006;27(1):15–20. doi: 10.1080/13816810500481840. [DOI] [PubMed] [Google Scholar]
- 6.Bujakowska K., Audo I., Mohand-Saïd S., et al. CRB1 mutations in inherited retinal dystrophies. Hum Mutat. Feb 2012;33(2):306–315. doi: 10.1002/humu.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talib M., Van Cauwenbergh C., De Zaeytijd J., et al. -associated retinal dystrophies in a Belgian cohort: genetic characteristics and long-term clinical follow-up. Br J Ophthalmol. Feb 2021 doi: 10.1136/bjophthalmol-2020-316781. [DOI] [PubMed] [Google Scholar]
- 8.Souied E., Amalric P., Chauvet M.L., et al. Unusual association of juvenile macular dystrophy with congenital hypotrichosis: occurrence in two siblings suggesting autosomal recessive inheritance. Ophthalmic Genet. Mar 1995;16(1):11–15. doi: 10.3109/13816819509057848. [DOI] [PubMed] [Google Scholar]
- 9.Papageorgiou E., McLean R.J., Gottlob I. Nystagmus in childhood. Pediatr Neonatol. Oct 2014;55(5):341–351. doi: 10.1016/j.pedneo.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Osborne D., Theodorou M., Lee H., et al. Supranuclear eye movements and nystagmus in children: a review of the literature and guide to clinical examination, interpretation of findings and age-appropriate norms. Eye. 2019;33(2):261–273. doi: 10.1038/s41433-018-0216-y. 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suppiej A., Marino S., Reffo M.E., et al. Early onset retinal dystrophies: clinical clues to diagnosis for pediatricians. Ital J Pediatr. Dec 2019;45(1):168. doi: 10.1186/s13052-019-0760-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P., Ya P., Li D., Lv S., Yang D. Nystagmus with pendular low amplitude, high frequency components (PLAHF) in association with retinal disease. Strabismus. 2020;28(1):3–6. doi: 10.1080/09273972.2019.1707237. 03. [DOI] [PubMed] [Google Scholar]
- 13.Gottlob I. Eye movement abnormalities in carriers of blue-cone monochromatism. Invest Ophthalmol Vis Sci. Aug 1994;35(9):3556–3560. [PubMed] [Google Scholar]
- 14.den Hollander A.I., ten Brink J.B., de Kok Y.J., et al. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12) Nat Genet. Oct 1999;23(2):217–221. doi: 10.1038/13848. [DOI] [PubMed] [Google Scholar]
- 15.Izaddoost S., Nam S.C., Bhat M.A., Bellen H.J., Choi K.W. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. Mar 2002;416(6877):178–183. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson S.G., Cideciyan A.V., Aleman T.S., et al. Crumbs homolog 1 (CRB1) mutations result in a thick human retina with abnormal lamination. Hum Mol Genet. May 2003;12(9):1073–1078. doi: 10.1093/hmg/ddg117. [DOI] [PubMed] [Google Scholar]
- 17.Stone E.M. Leber congenital amaurosis - a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. Dec 2007;144(6):791–811. doi: 10.1016/j.ajo.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Bryant L., Lozynska O., Maguire A.M., Aleman T.S., Bennett J. Prescreening whole exome sequencing results from patients with retinal degeneration for variants in genes associated with retinal degeneration. Clin Ophthalmol. 2018;12:49–63. doi: 10.2147/OPTH.S147684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellingford J.M., Horn B., Campbell C., et al. Assessment of the incorporation of CNV surveillance into gene panel next-generation sequencing testing for inherited retinal diseases. J Med Genet. 2018;55(2):114–121. doi: 10.1136/jmedgenet-2017-104791. 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spalton D.J. Posterior uveitis and retinal dystrophy. A case report. Trans Ophthalmol Soc U K. Sep 1977;97(4):462–464. [PubMed] [Google Scholar]
- 21.Weller J.M., Michelson G., Juenemann A.G. Unilateral retinitis pigmentosa: 30 years follow-up. BMJ Case Rep. Feb 2014;2014 doi: 10.1136/bcr-2013-202236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stunkel M., Bhattarai S., Kemerley A., et al. Vitritis in pediatric genetic retinal disorders. Ophthalmology. Jan 2015;122(1):192–199. doi: 10.1016/j.ophtha.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida N., Ikeda Y., Notomi S., et al. Clinical evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. Jan 2013;120(1):100–105. doi: 10.1016/j.ophtha.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Tang P.H., Chemudupati T., Wert K.J., et al. Phenotypic variance in Calpain-5 retinal degeneration. Am J Ophthalmol Case Rep. Jun 2020;18:100627. doi: 10.1016/j.ajoc.2020.100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.den Hollander A.I., Heckenlively J.R., van den Born L.I., et al. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet. Jul 2001;69(1):198–203. doi: 10.1086/321263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hettinga Y.M., van Genderen M.M., Wieringa W., Ossewaarde-van Norel J., de Boer J.H. Retinal dystrophy in 6 young patients who presented with intermediate uveitis. Ophthalmology. 2016;123(9):2043–2046. doi: 10.1016/j.ophtha.2016.03.046. 09. [DOI] [PubMed] [Google Scholar]
- 27.Murro V., Mucciolo D.P., Sodi A., et al. Retinal capillaritis in a CRB1-associated retinal dystrophy. Ophthalmic Genet. 2017;38(6):555–558. doi: 10.1080/13816810.2017.1281966. 12. [DOI] [PubMed] [Google Scholar]
- 28.Alsulaiman H.M., Schatz P., Nowilaty S.R., Abdelkader E., Abu Safieh L. Diffuse retinal vascular leakage and cone-rod dystrophy in a family with the homozygous missense C.1429G>A (P.GLY477ARG) mutation IN CRB1. Retin Cases Brief Rep. 2020;14(2):203–210. doi: 10.1097/ICB.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 29.Alves C.H., Wijnholds J. Microglial cell dysfunction in CRB1-associated retinopathies. Adv Exp Med Biol. 2019;1185:159–163. doi: 10.1007/978-3-030-27378-1_26. [DOI] [PubMed] [Google Scholar]
- 30.Verhagen F., Kuiper J., Nierkens S., Imhof S.M., Radstake T., de Boer J. Systemic inflammatory immune signatures in a patient with CRB1 linked retinal dystrophy. Expet Rev Clin Immunol. Dec 2016;12(12):1359–1362. doi: 10.1080/1744666X.2016.1241709. [DOI] [PubMed] [Google Scholar]
- 31.Peng B., Xiao J., Wang K., So K.F., Tipoe G.L., Lin B. Suppression of microglial activation is neuroprotective in a mouse model of human retinitis pigmentosa. J Neurosci. Jun 2014;34(24):8139–8150. doi: 10.1523/JNEUROSCI.5200-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.