Abstract
Furosemide is a diuretic drug used to increase urine flow in order to reduce the amount of salt and water in the body. It is commonly utilized to treat preterm infants with chronic lung disease of prematurity. There is a need for a simple and reliable quantitation of furosemide in human urine. We have developed and validated an ultra-high performance liquid chromatographic-tandem mass spectrometry method for furosemide quantitation in human urine with an assay range of 0.100 – 50.0 μg/mL. Sample preparation involved solid-phase extraction with 10 μL of urine. Intra-day accuracies and precisions for the quality control samples ranged from 94.5 – 106% and 1.86 – 10.2%, respectively, while inter-day accuracies and precision ranged from 99.2 – 102% and 3.38 – 7.41%, respectively. Recovery for furosemide had an average of 23.8%, with an average matrix effect of 101%. Furosemide was stable in human urine under the assay conditions. Stability for furosemide was shown at 1 week (room temperature, 4 °C, −20 °C, and −78 °C), 6 months (−78 °C), and through three freeze-thaw cycles. This robust assay demonstrates accurate and precise quantitation of furosemide in a small volume (10 μL) of human urine. It is currently implemented in an ongoing pediatric clinical study.
Keywords: furosemide, solid phase extraction, LC-MS/MS, pediatrics
1. INTRODUCTION
The loop diuretic furosemide is common in both adult and pediatric clinical settings. Use is particularly frequent in intensive care units, where furosemide is used to manage edema resulting from or complicating hepatic, renal, cardiac and respiratory failure (Feudtner, Dai, Hexem, Luan and Metjian 2012, Hsieh, Hornik, Clark, Laughon, Benjamin, Smith and Best Pharmaceuticals for Children Act-Pediatric Trials 2014, McCoy, Chertow and Chang 2019). Among preterm infants with severe bronchopulmonary dysplasia, furosemide is the most commonly used pharmacotherapy in United States children’s hospitals (Bamat, Kirpalani, Feudtner, Jensen, Laughon, Zhang, Monk, Passarella and Lorch 2019).
Furosemide urine concentrations are most relevant to its therapeutic effect. A diuretic response requires drug delivery to the Na+/2Cl−/K+ (NCCK) co-transporter on the luminal aspect of the renal tubule, expressed predominantly in the ascending loop of Henle (Cotton, Suarez and Reese 2012, Orlov, Koltsova, Kapilevich, Gusakova and Dulin 2015). Inhibition of this electrolyte carrier increases urinary sodium and water excretion, thereby decreasing extracellular water (O’Donovan and Bell 1989, Segar, Chemtob and Bell 1997). Because the mechanism of action is luminal rather than peritubular, furosemide must reach the urine to exert its therapeutic effect. Urinary excretion rates are accepted as the most accurate effective drug measurement, and follow a sigmoid-shaped drug-response relationship (Brater 2011). Blood concentrations correlate poorly with diuretic action and are more relevant to evaluations of pharmacokinetic parameters like bioavailability, volume of distribution, clearance and pharmacodynamic effects related to off-target harms (Brater 2011, Mirochnick, Miceli, Kramer, Chapron and Raye 1990, Ponto and Schoenwald 1990).
Despite its common use, important knowledge gaps in the clinical pharmacology of furosemide remain. This is particularly true in neonates and infants, in whom the identification of optimal therapeutic practices is challenged by dynamic developmental changes in furosemide pharmacology (NCT02527798). Furosemide is highly protein bound. Glomerular filtration of free furosemide is limited, and drug delivery to the renal tubular lumen relies on active secretion by high-affinity organic acid transporters in the proximal peritubular capillaries (Brater 2011, Odlind and Beermann 1980). Tubular secretion drives both drug delivery to the therapeutic target and drug elimination. This process matures dynamically in extremely preterm infants, increasing variably after 32 weeks postmenstrual age (Mirochnick, Miceli, Kramer, Chapron and Raye 1988). These developmental changes and the therapeutic strategies to account for them remain inadequately characterized.
Reliable quantitation of urinary furosemide is also needed to better describe pharmacodynamic tolerance to furosemide diuresis. Tolerance is characterized by an initial robust diuretic response that sharply diminishes over time, and has been demonstrated in adults, children and preterm infants (Kim, Capparelli, Romanowski, Proudfoot and Tremoulet 2017, Mirochnick, Miceli, Kramer, Chapron and Raye 1990, Rao, Planavsky, Hanberg, Ahmad, Brisco-Bacik, Wilson, Jacoby, Chen, Tang, Cherney, Ellison and Testani 2017, Segar, Robillard, Johnson, Bell and Chemtob 1992). Tolerance is likely mediated by redundant homeostatic mechanisms that include upregulation of receptors distal to the loop of Henle to help reabsorb the sodium and water blocked by furosemide. These likely lead to dynamic changes in the furosemide dose-response profile during sustained exposure. In one study in preterm infants, a five-fold increase in furosemide urinary excretion rate was required to exert an equivalent diuretic effect after a week of sustained therapy (Mirochnick, Miceli, Kramer, Chapron and Raye 1990). Accurate quantitation of urinary drug concentrations is needed to further characterize this phenomenon in clinical populations.
Furosemide concentrations are typically quantified in human plasma or serum by validated liquid chromatography – tandem mass spectrometry (LC-MS/MS) methods (Heffron, Taddei, Benoit and Negrusz 2013, Ritscher, Georges, Wunder, Wallemacq, Persu and Toennes 2020, Ritscher, Hoyer, Wunder, Obermuller and Toennes 2019). Sample analysis with urine is a useful approach for quantitative analysis of drugs, especially in pediatrics. The quantitation of furosemide concentrations in children will be useful to clinicians to make dose adjustments that will improve safety and efficacy. To our knowledge, this is the first study to report a LC–MS/MS furosemide assay in human urine utilizing only 10 μL of sample, which has been evaluated for the analysis of representative pediatric samples from an ongoing clinical study.
2. EXPERIMENTAL
2.1. Materials
Analytical powders of furosemide and furosemide-d5 (Figure 1) were purchased from Toronto Research Chemicals (Toronto, ON, Canada). LCMS-grade acetonitrile and methanol, HPLC-grade isopropanol (IPA), and ACS grade formic acid (98%) were obtained from EMD Millipore Corporation (Burlington, MA). ACS grade phosphoric acid (85%) was purchased from Fisher Scientific (Waltham, MA) and ACS grade ammonium carbonate was purchased from Avantor (Radnor Township, PA). Ultrapure water from a Synergy®UV-R purification system (Millipore Sigma, Burlington, MA) was used for sample preparation, mobile phase preparation and in all experiments where required. Oasis HLB plates (30 μm) from Waters Corporation (Milford, MA) were used for solid phase extraction (SPE).
Figure 1.
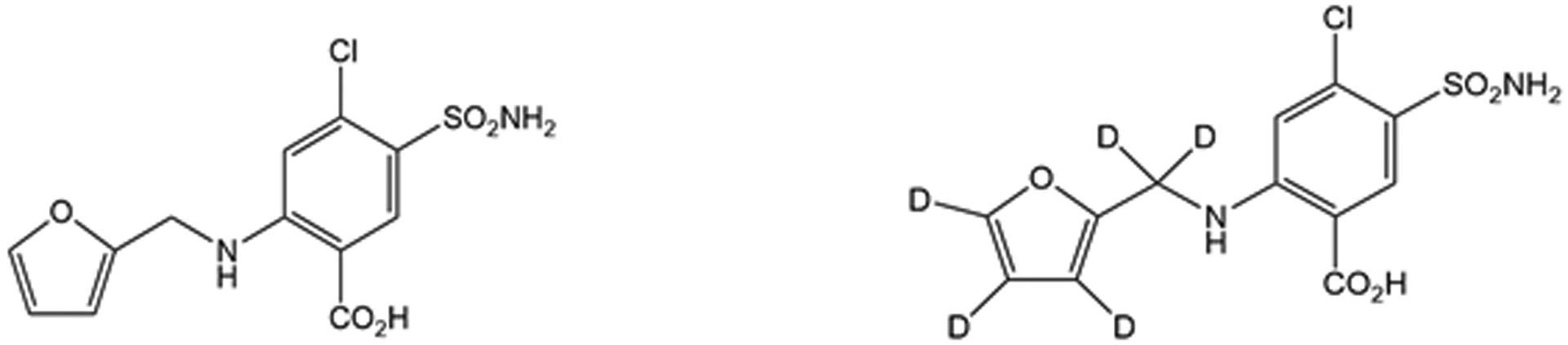
The chemical structure of furosemide (left) and the internal standard furosemide-d5 (right).
2.2. Preparation of stock solutions, standard, and quality control samples
Urine used for the preparation of standards and quality control samples were collected from healthy volunteers (employees) at the Children’s Hospital of Philadelphia. Stock solutions for the calibration standards and quality control (QCs) samples were prepared independently in dimethyl sulfoxide (DMSO) at a concentration of 1.0 mg/mL and stored at −20 °C in amber glass vials when not in use. From the stock solution, serial dilutions were prepared for the calibration standards in human urine at concentrations of 0.100, 0.250, 0.500, 1.00, 2.50, 5.00, 10.0, 25.0, and 50.0 μg/mL. Calibration standards 10.0, 25.0, and 50.0 were spiked and left at room temperature for 15 minutes for equilibration, mixing periodically, before continuing with further dilutions. QC samples were prepared in human urine at four concentrations: 0.100, 0.200, 20.0 and 40.0 μg/mL. QCs 20.0 and 40.0 μg/mL were spiked in urine and left at room temperature to equilibrate for 15 minutes and mixed every few minutes prior to continuing with further dilutions. Furosemide-d5 (internal standard, IS) stock solution was prepared at a concentration of 0.5 mg/mL in 50:50 (v/v) methanol: DMSO and stored in an amber glass vial at −20 °C when not in use. The working solution for the IS was prepared at a concentration of 5.0 μg/mL in acetonitrile, then diluted to a final concentration of 5 ng/mL in blank human plasma, which was stored at −78 °C when not in use.
2.3. Sample preparation
Figure 2 illustrates a simple representation for the SPE procedure for furosemide quantitation in human urine samples. Calibration standards, QCs, blanks and unknown samples (10 μL) were pre-mixed with 90 μL of the IS solution (5 ng/mL of furosemide-d5 in human plasma) and 260 μL of water containing 1% formic acid in 1.5 mL Eppendorf tubes before loading onto the SPE plate. The SPE plate was: 1) conditioned with 200 μL of methanol + 200 μL of water, 2) equilibrated with 200 μL of 1% formic acid, 3) loaded with 300 μL of pre-mixed sample (mentioned above), 4) washed with 200 μL of 10 mM ammonium carbonate and, 5) eluted with 100 μL of methanol (2x) and diluted with 300 μL (2x) of water through the SPE column. Eluent from Step 5 is collected into a clean 96-well plate, then vortexed for 10 minutes at 500 rpm, and finally injected (2 μL) for ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) analysis.
Figure 2.
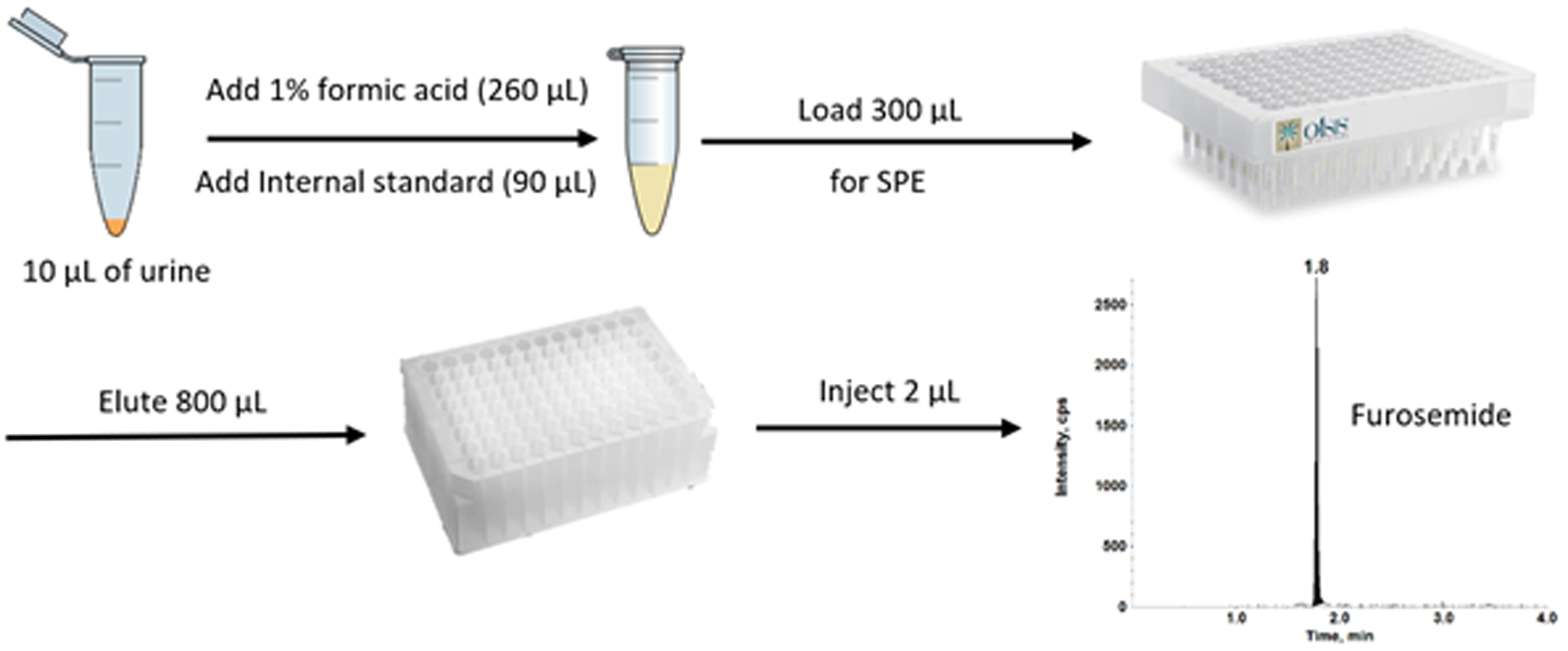
A schematic of sample preparation for furosemide analysis in human urine samples.
2.4. UHPLC-MS/MS analysis
An AB Sciex Exion UHPLC system interfaced with an AB Sciex 6500+ QTRAP mass spectrometer (Framingham, MA) equipped with a Turbo IonSpray source was used for the quantitation of furosemide in human urine with furosemide-d5 as the IS. Infusion solutions for furosemide and furosemide-d5 were prepared at concentrations of 10 ng/mL in 50:50 (v/v) 10 mM ammonium acetate/ 0.1% formic acid in acetonitrile to optimize UHPLC-MS/MS conditions. The product ion spectra of furosemide and furosemide-d5 are shown in Figure 3. Selected reaction monitoring (SRM) was employed for quantification. Transitions of m/z 329.0 → 77.9 (quantitation) and 329.0 → 125.8 (qualification) for furosemide, and m/z 334.0 → 206.0 for furosemide-d5 were determined utilizing electrospray ionization in the negative mode.
Figure 3:
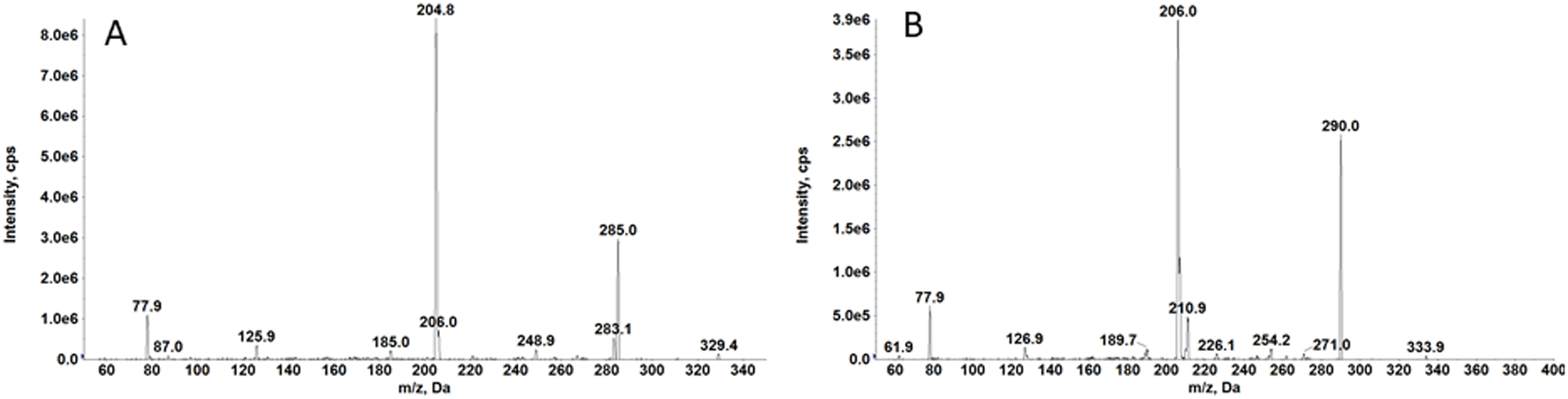
Production spectra of furosemide (A) and furosemide-d5 (B)
A nitrogen generator (PEAK Scientific; Scotland, UK) was used for the collision, nebulizer and curtain gases. Optimized gas parameters for curtain gas, collision gas, GS1, and GS2 were 20, 10, 65, and 65, respectively. IonSpray voltage was −4500 V with a source temperature of 450 °C. The declustering potential (DP) and entrance potential (EP) for furosemide and furosemide-d5 were −55 V and −10 V, respectively. Collision energies (CE) for furosemide were determined at −40 V (quantification) and −44 (qualification), and −31 V for the IS. Exit potentials (CXP) were −9 V (quantification), and −13 V (qualification) for furosemide, and −15 V for furosemide-d5. Analyst software (version 1.6.3) from AB Sciex was used for all data acquisition and processing.
All chromatographic separation was achieved with a Waters (Milford, MA) Acquity HSS C18 column (2.6 μm, 100 Å, 2.1 × 100 mm) with 0.1% formic acid in water as mobile phase A, and 0.1% formic acid in acetonitrile as mobile phase B. Supernatants were injected at 2 μL with a flow rate of 0.500 mL/min. Initial gradient conditions were maintained at 2% B from 0.00 – 0.50 min. At 0.70 minutes, B increased to 40%, then increased further to 98% at 1.50 minutes, and remained at 98% until 2.50 minutes. The gradient returned to the starting conditions of 2% B at 3.00 minutes until the completion of the 4.50-minute run. To reduce carryover, a strong wash solvent composed of 2: 2: 2: 2: 1 (v/v/v/v/v) water/ acetonitrile/ isopropanol/ phosphoric acid and a weak wash solvent of 1:1 (v/v) water/ acetonitrile with 0.1% formic acid were used.
2.6. Validation
A bioanalytical method for the quantitation of furosemide in a small volume (10 μL) of human urine was developed and validated by accuracy, precision, linearity, sensitivity, carryover, selectivity/specificity, recovery, matrix effect, stability based on the guidance from the FDA for bioanalytical validation (FDA 2018).
2.6.1. Accuracy and precision
Four QC concentrations at 0.100, 0.200, 20.0 and 40.0 μg/mL were assessed for inter- and intra-day accuracy and precision for the quantitation of furosemide in human urine. Based on a single day validation run (n=6), intra-day accuracy and precision were calculated. Inter-day accuracy and precision were calculated based on the 3-day analytical validation runs (n=18).
2.6.2. Linearity, sensitivity, and carryover
The 3-day validation run was assessed for linearity over the calibration range of 0.100 – 50.0 μg/mL in human urine. Concentrations from clinical samples are expected to be higher than 0.100 μg/mL, therefore this concentration was chosen as the lowest limit of quantitation (LLOQ). Sensitivity of the assay was determined by the signal-to-noise ratios. Observations for carryover were monitored by injecting a blank sample after the upper limit of quantitation and analyzing the peak area of the analyte in the blank sample.
2.6.3. Selectivity and specificity
Eight lots of human urine from eight individuals and one pooled lot (mixture of the eight individual lots) were analyzed for selectivity and specificity of furosemide and furosemide-d5. LLOQ samples (n=3) and double blank samples (n=1) were prepared in each lot and extracted following the validated method. This was to ensure there were no interfering peaks at the retention times of furosemide and furosemide-d5, as well as no cross-interference between the two.
2.6.4. Recovery, matrix effect and non-specific binding
Recovery and matrix effect for furosemide and furosemide-d5 were assessed at all four QC concentrations (n=6). Comparison of the peak areas between samples subjected through regular extraction (with furosemide and furosemide-d5) and double blank samples back-spiked post-extraction with furosemide and furosemide-d5 into the final matrix were observed to calculate the recovery for both analyte and IS. Evaluation for the matrix effect of furosemide and furosemide-d5 was performed by comparing the peak areas of post-extracted back-spiked double blank samples with the peak areas of neat samples spiked at the same QC concentrations.
An experiment was designed to evaluate non-specific binding of furosemide in urine samples following the published method (Chen, Bajpai, Mollova and Leung 2009). Furosemide QCs spiked in urine were transferred multiple times from one polypropylene tube to another to determine the loss, if any, of furosemide. We compared urine samples subjected to 0 (normal extraction), 1, 3 and 6 transfers in polypropylene tubes. The non-specific binding of furosemide to polypropylene tubes was evaluated (n=4) at 3 different QC concentrations (200, 20,000 and 40,000 ng/mL) following 0 (normal extraction), 1, 3 and 6 transfers.
2.6.5. Stability
Stability studies for the quantitation of furosemide in human urine were evaluated at the four QC concentration levels (n=6) for long-term storage for 1 week at room temperature, 4 °C, −20 °C and −78 °C, and 6 months (190 days) at −78 °C. Freeze-thaw stability was assessed by subjecting QC samples (n=6 at all four concentrations) to 3 freeze-thaw cycles. Samples analyzed and kept in the autosampler for 24 hours were reinjected to assess autosampler stability at 10 °C.
2.6.6. Clinical Application and Analysis
This validated method was implemented for the analysis of clinical samples from infants in an ongoing prospective clinical research study (IRB# 20–017936) at the Children’s Hospital of Philadelphia. The goal of the study is to determine how the diuretic response to furosemide changes over time following repeated dose administrations in infants with severe bronchopulmonary dysplasia. Urine samples were obtained from infants at baseline in the 12 hours preceding furosemide initiation and then longitudinally for the duration of furosemide exposure. The body weights, dose of furosemide, and sampling times for two subjects are shown in Table 4. Subject 1 received a furosemide dose every 12 hours for 5.5 days (11 doses) and Subject 2 received a dose every 24 hours for 3 days (3 doses). Urine samples were collected during diaper changes, which occurred approximately every 3 hours as part of routine clinical care. To obtain samples, each diaper was fitted with 10 cotton balls. Urine volumes were first calculated by measuring the difference in weight between soiled and clean cotton ball-containing diapers and estimating the proportion of output attributable to urine. Cotton balls were then removed from the diaper and placed in a clean 30 mL syringe with the plunger removed. The plunger was then replaced and depressed to express urine from the cotton balls into a urine collection vial. All urine from each consecutive 6-hour period was expressed into a collection vial. Representative 10 μL samples were obtained for furosemide quantitation. Furosemide urinary excretion rates (μg/kg/h) were calculated as the product of urine volume in (mL/kg/h) and furosemide concentration (μg/mL) as reported by the assay described in this report.
Table 4:
Measured concentrations of furosemide in urine samples from infant subjects with bronchopulmonary dysplasia.
| Subject 1 (q12h furosemide) | Subject 2 (q24h furosemide) | ||||
|---|---|---|---|---|---|
| Dosing Weight (kg): 3.0 | Dosing Weight (kg): 2.7 | ||||
| Dose 1: 3.0 mg (1 mg/kg, via nasogastric tube) Dose 2: 3.0 mg (1 mg/kg, via nasogastric tube) |
Dose: 5.4 mg (2 mg/kg, via nasogastric tube) | ||||
| Sample ID (Time) | Furosemide Concentration (μg/mL) | Furosemide Urinary Excretion Rate (μg/kg/h) | Sample ID (Time) | Furosemide Concentration (μg/mL) | Furosemide Urinary Excretion Rate (μg/kg/h) |
| B1 (12 – 6 h before 1st dose) | BLQ | - | B1 (12 – 6 h before 1st dose) | BLQ | - |
| B2 (6 – 0 h before 1st dose) | BLQ | - | B2 (6 – 0 h before 1st dose) | BLQ | - |
| F1 (0 – 6 h after 1st dose) | 5.35 | 36.0 | F1 (0 – 6 h after 1st dose) | 17.7 | 180.3 |
| F2 (6 – 12 h after 1st dose) | 1.06 | 4.7 | F2 (6 – 12 h after 1st dose) | 5.29 | 6.8 |
| F3 (0 – 6 h after 2nd dose) | 6.70 | 24.6 | F3 (12 – 18 h after 1st dose) | 6.87 | 3.5 |
| F4 (6 – 12 h after 2nd dose) | 1.64 | 11.8 | F4 (18 –24 h after 1st dose) | 0.440 | 1.0 |
3. RESULTS
3.1. Method Development
Initial method development studies involved resolving issues with carryover and different extraction methods to help keep the instrument clean. It was critical to use SPE plates since urine is known to be a dirty matrix due to its high salt content. This helped to keep the instrument as clean as possible during sample injection and analysis. Because the analyte response was extremely high, we increased the volume of the elution solvent in order to avoid signal saturation. With the additional solvent, carryover remained a noticeable issue after the ULOQ injection. Several wash solvents were used to address the carryover issue. We found that a strong wash solvent composed of 2: 2: 2: 2: 1 (v/v/v/v/v) water/ acetonitrile/ isopropanol/ phosphoric acid best reduced the carryover.
3.2. Validation
3.2.1. Accuracy and precision
Results for the intra- and inter-day accuracies and precisions for the QC samples are shown in Table 1. Intra-day accuracies (within 1 day) ranged from 94.5 – 106% with precisions (%CV) of 1.86 – 10.2. Inter-day accuracies (over the 3-day validation run) showed 99.2 – 102% with %CV of 3.38 – 7.41. Results were within the acceptable range (± 15%) noted in the FDA guidance for bioanalytical validation.
Table 1:
Validation results (intra- and inter-day accuracy and precision) for furosemide in human urine from a 3-day validation study.
| Furosemide Concentration (μg/mL) |
Quality Controls | |||||
|---|---|---|---|---|---|---|
| Day | n | LLOQ | LQC | MQC | HQC | |
| 0.100 | 0.200 | 20.0 | 40.0 | |||
| Mean ± SD | 1 | 6 | 0.103 ± 0.006 | 0.212 ± 0.006 | 20.9 ± 0.609 | 42.2 ± 1.91 |
| Accuracy (%) | 1 | 6 | 103 | 106 | 104 | 105 |
| %CV | 1 | 6 | 5.93 | 2.37 | 2.92 | 4.53 |
| Mean ± SD | 2 | 6 | 0.095 ± 0.010 | 0.195 ± 0.007 | 19.8 ± 0.547 | 39.2 ± 0.849 |
| Accuracy (%) | 2 | 6 | 95.0 | 97.6 | 98.8 | 98.0 |
| %CV | 2 | 6 | 10.2 | 3.78 | 2.77 | 2.16 |
| Mean ± SD | 3 | 6 | 0.100 ± 0.004 | 0.189 ± 0.008 | 20.1 ± 0.373 | 40.6 ± 1.91 |
| Accuracy (%) | 3 | 6 | 99.9 | 94.5 | 100 | 101 |
| %CV | 3 | 6 | 4.02 | 4.06 | 1.86 | 4.70 |
| Mean ± SD | 1, 2, 3 | 18 | 0.099 ± 0.007 | 0.199 ± 0.012 | 20.2 ± 0.683 | 40.6 ± 1.98 |
| Accuracy (%) | 1, 2, 3 | 18 | 99.2 | 99.3 | 101 | 102 |
| %CV | 1, 2, 3 | 18 | 7.41 | 5.94 | 3.38 | 4.87 |
3.2.2. Linearity, sensitivity, and carryover
For the 3-day validation study, urine was collected from a healthy volunteer to quantitate furosemide in the calibration range of 0.100 – 50 μg/mL (n=2 calibration curves per run), which showed acceptable linearity and reproducibility (Table 2). The correlation coefficient (r2) for each validation run was ≥ 0.999 employing linear regression, 1/x2 weighting. The slope, y-intercept, and r2 values (mean ± standard deviation) were 0.00452 ± 0.000145, −1.49e-3 ± 6.75e-3, and 0.9991 ± 0.0001, respectively. Representative chromatograms for the double blank, blank with IS and LLOQ samples are shown in Figure 4. Signal-to-noise ratio was determined at ≥1000, showing adequate assay sensitivity.
Table 2:
Inter-day accuracy and precision for quantitation of furosemide in human urine in the calibration standards (n=6) from the 3-day validation study.
| Nominal Furosemide Concentration (μg/mL) | Mean ± SD (μg/mL, n=6) |
CV (%) | Accuracy (%) |
|---|---|---|---|
| 0.100 | 0.101 ± 0.003 | 3.27 | 101 |
| 0.250 | 0.245 ± 0.009 | 3.75 | 97.9 |
| 0.500 | 0.492 ± 0.020 | 4.03 | 98.5 |
| 1.00 | 1.00 ± 0.031 | 3.06 | 100 |
| 2.50 | 2.48 ± 0.069 | 2.79 | 99.1 |
| 5.00 | 4.98 ± 0.152 | 3.05 | 100 |
| 10.0 | 10.2 ± 0.438 | 4.32 | 102 |
| 25.0 | 24.8 ± 0.833 | 3.36 | 99.3 |
| 50.0 | 51.4 ± 2.02 | 3.92 | 103 |
Figure 4.
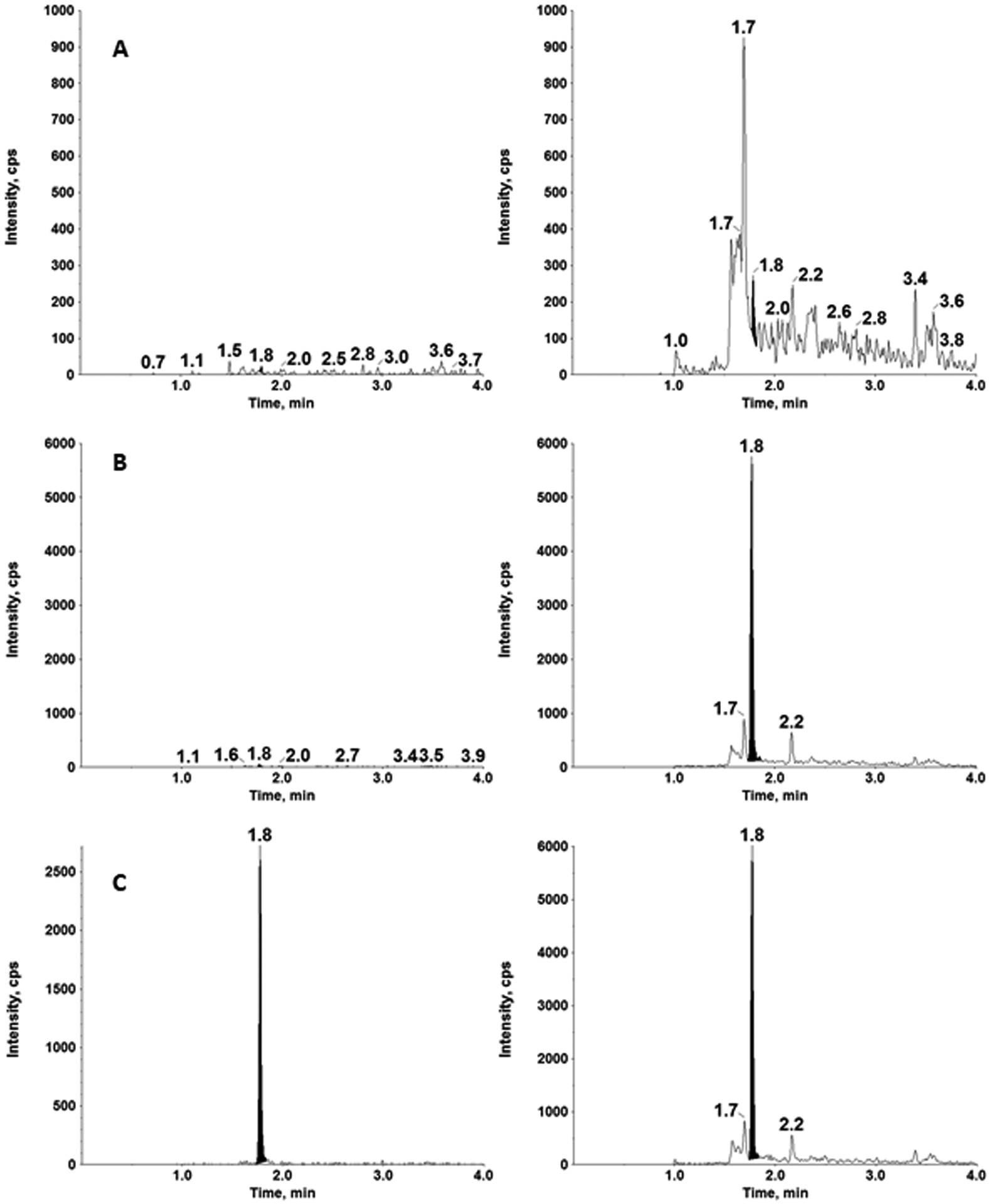
Representative chromatograms of furosemide (left) and furosemide-d5 (right) in a double blank (A), blank with IS (B) and the LLOQ of 0.100 μg/mL in human urine.
Initial method development involved improvement of carryover. With the optimized wash solvents, the signal for furosemide in the blank sample following the ULOQ was <20 % of the LLOQ, exhibiting minimal carryover. Our initial assay range was 0.0100 – 100 μg/mL with 50 ng/mL of IS solution. To eliminate the carryover of IS, the concentration was reduced to 5 ng/mL. Since the 100 μg/mL calibration standard had signal saturation and carryover, the assay range was narrowed to 0.100 – 50 μg/mL with an IS concentration of 5 ng/mL. We have utilized the less abundant fragment (m/z 77.9) for quantitation of furosemide, while utilizing the major product ion for internal standard (Figure 3). Therefore, we get higher response for the internal standard compared to the LLOQ as shown in Figure 4. We have compared the original IS concentration (5 ng/mL) with a higher IS concentration (50 ng/mL) to determine if the IS concentration influenced quantitation. We performed two sets of calibration curves (n=2) with internal standard concentration of 5 ng/mL (normal assay condition) and 50 ng/mL (10-times higher internal standard concentration). The results confirmed that calibration curves were superimposable (r2 = 0.9974) and the change in internal standard concentration did not affect the robustness of furosemide quantitation in urine samples.
3.2.3. Selectivity and specificity
Urine was collected from eight healthy volunteers to assess selectivity and specificity. The lots (n = 8 individual lots; n = 1 pooled lot) were extracted as double blank samples through SPE following the validated method. Chromatography showed that there were no interference peaks at the retention times of furosemide and furosemide-d5. Individual lots (n=8) and a pooled lot (n=1) of human urine were spiked at the LLOQ concentration of 0.100 μg/mL (n=3). LLOQ samples were quantified with STDs and QCs and extracted following the validated method. Accuracies in the individual lots and pooled lot were within acceptable range (± 20%) with accuracies ranging from 88.7 – 101% with %CV of 5.61 – 14.2.
3.2.4. Recovery, matrix effect and non-specific binding
Recovery and matrix effect were observed at the four QC concentrations (n=6) as well as the IS at 5 ng/mL. The average recovery across the four QC levels and the IS were 23.8%, and 25.9%, respectively. Results for the average matrix effect of furosemide (in the LLOQ, low, medium and high QCs), and furosemide-d5 were 101% and 93.7%, respectively. These results show that furosemide and furosemide-d5 have consistent recovery when extracted from urine samples with minimal matrix effect.
Non-specific binding of furosemide in polypropylene tubes was evaluated at the low, medium and high QC concentrations (n=4). The ranges of accuracy and precision of furosemide under various conditions are: 97.1 ± 8.59 to 99.6 ± 8.96 (normal extraction), 94.4 ± 4.65 to 99.9 ± 8.14 (1 transfer), 93.8 ± 7.63 to 98.1 ± 5.65 (3 transfers), and 88.1 ± 2.64 to 95.3 ± 7.04 (6 transfers). The data suggests that furosemide has no or minimal non-specific binding after multiple transfers. Since the clinical study required a broad assay range and furosemide was very sensitive under the assay conditions, our focus was to get cleaner samples with consistent recovery across the assay range. Since, furosemide is an acidic compound, the recovery could be improved by utilizing Oasis WAX SPE plates. Since our challenge was signal saturation at high concentrations, we did not further optimize the conditions to improve the recovery. Our method demonstrates a consistent recovery across the assay range with minimal matrix effect.
3.2.5. Stability
Stability studies for the quantitation of furosemide in urine were assessed for 1 week at room temperature, 4 °C, −20 °C and −78 °C, and 6 months (190 days) at −78 °C at all four QC concentrations (n=6). Results showed that furosemide is stable at all these conditions (Table 3). Freeze-thaw cycles were performed to observe the stability of furosemide at four QC levels (n=6) after subjecting QC samples to three freeze-thaw cycles. Results (accuracy ± %CV) for the three freeze-thaw cycles at the LLOQ, low, medium and high QCs were 110 ± 7.40, 105 ± 5.93, 105 ± 4.09, and 102 ± 3.92, respectively, and were within acceptable range. Samples analyzed and kept in the autosampler for 24 hours at 10 °C were reinjected to observe extract stability for the four QC levels (n=6). Autosampler stability results showed acceptable accuracy and precision with accuracies ranging from 102 – 106% with %CV ranging from 1.64 – 3.35.
Table 3:
Accuracy and precision for furosemide (n=6) in human urine at various temperatures (room temperature, 4, −20 and −78 °C) for 1 week and 6 months, and 3 freeze-thaw cycles.
| Accuracy (%) of Furosemide Concentrations ± %CV | |||
|---|---|---|---|
| Furosemide (μg/mL) | 1 week at RT | 1 week at 4 °C | 1 week at −20 °C |
| 0.100 | 101 ± 6.62 | 98.6 ± 6.04 | 110 ± 3.11 |
| 0.200 | 97.0 ± 3.94 | 97.3 ± 4.67 | 103 ± 7.76 |
| 20.0 | 94.3 ± 4.49 | 91.8 ± 1.49 | 95.4 ± 3.25 |
| 40.0 | 92.4 ± 3.83 | 94.1 ± 3.83 | 98.7 ± 4.08 |
| Furosemide (μg/mL) | 1 week at −78 °C | 6 months at −78 °C | 3 Freeze Thaw Cycles |
| 0.100 | 104 ± 6.87 | 95.1 ± 11.0 | 110 ± 7.40 |
| 0.200 | 92.9 ± 6.03 | 98.1 ± 5.33 | 105 ± 5.93 |
| 20.0 | 96.1 ± 1.63 | 103 ± 3.25 | 105 ± 4.09 |
| 40.0 | 90.9 ± 3.01 | 103 ± 5.66 | 102 ± 3.92 |
3.2.6. Clinical Application and Analysis
This validated method for the quantitation of furosemide in human urine was applied to an ongoing prospective clinical research study. The results for furosemide concentrations in urine samples from two infant subjects can be found in Table 4 with representative chromatograms (Figures 5 and 6) of a baseline pre-dose sample and the first sample after the primary dose of furosemide. Results show that this validated method is suitable for the analysis of furosemide in human urine using a small volume of sample with extraction through SPE. Both subjects had plausible results, where the baseline pre-dose samples (B1 and B2) were below the limit of quantitation, and the samples following furosemide administration had a spike in furosemide concentration followed by decreasing levels in subsequent samples until the next dose was administered.
Figure 5.
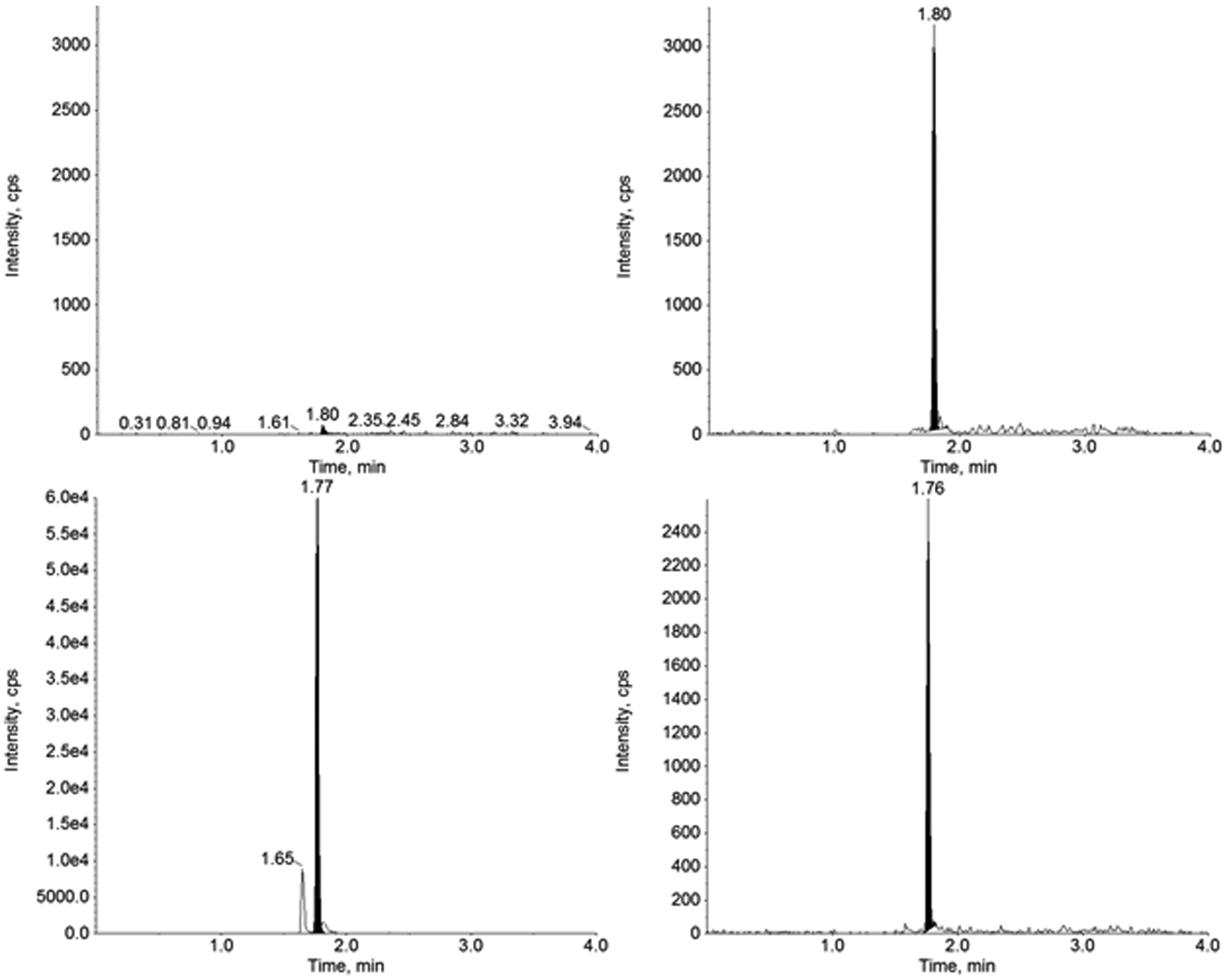
Representative chromatograms of furosemide (left) and furosemide-d5 (right) in baseline pre-dose (top) and after the first dose in Subject 1.
Figure 6.
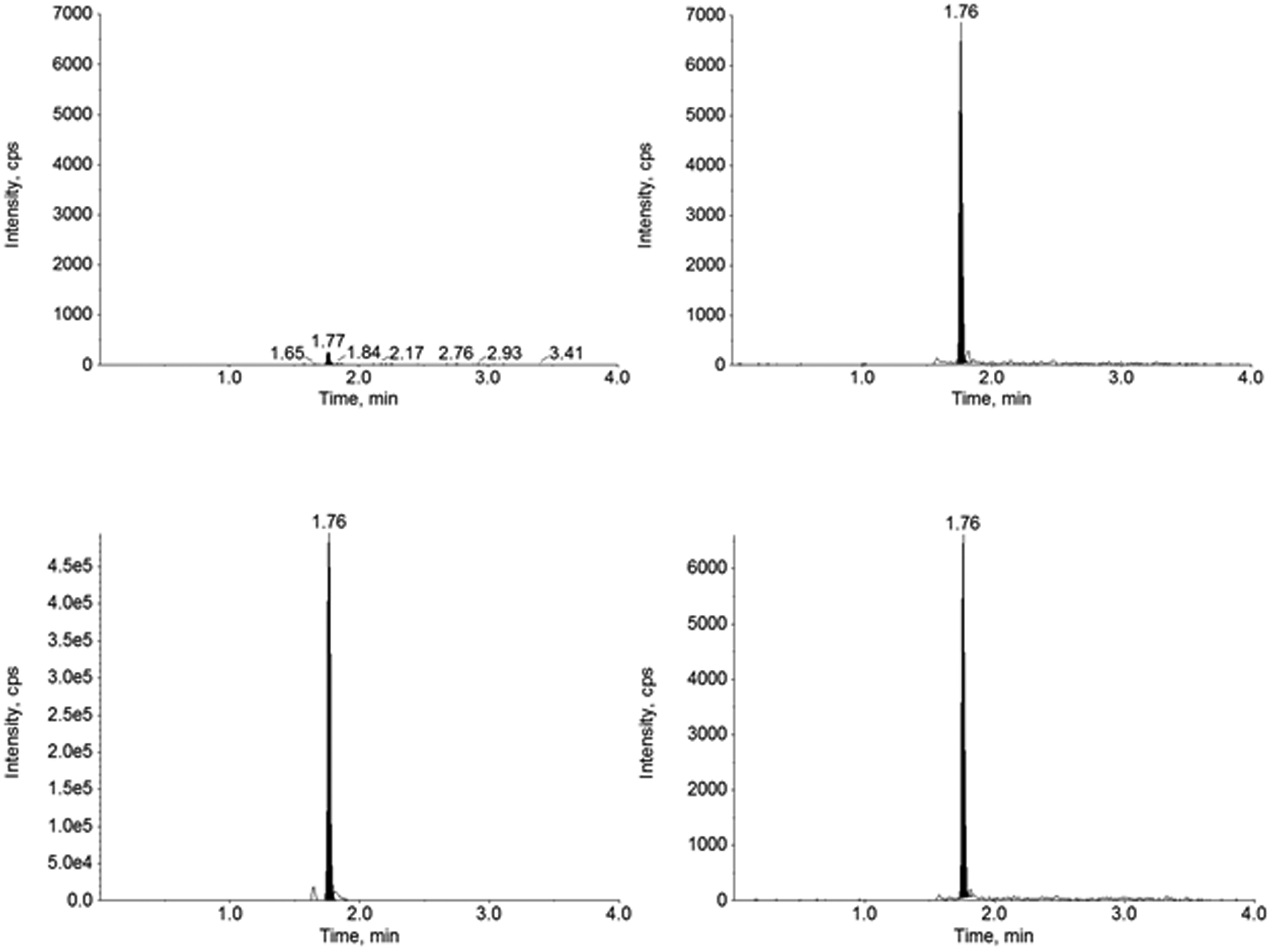
Representative chromatograms of furosemide (left) and furosemide-d5 (right) in baseline pre-dose (top) and after the first dose in Subject 2.
4. DISCUSSION
There were few methods that were reported for the analysis of furosemide in urine samples. An earlier study (Aranda, Lambert, Perez, Turmen and Sitar 1982, Aranda, Perez, Sitar, Collinage, Portuguez-Malavasi, Duffy and Dupont 1978) utilized a gas-liquid chromatographic method to analyze furosemide in urine samples. They employed calibration curves in heparinized blood samples with an assay range of 0.05 – 64 μg/mL with a run time of 20 minutes per sample. Another study (Ayalasomayajula, Schuehly, Pal, Chen, Zhou, Sunkara and Langenickel 2018) employed a LC–MS/MS method to quantify furosemide in urine samples. They pretreated urine samples with 1% H3PO4 and performed solid-phase extraction for the sample clean up. The LLOQ for furosemide in urine samples was 0.05 μg/mL. However, the details of chromatographic column, mobile phase solvents, run time, internal standard used, assay range were not reported. In a recent study (Ritscher, Georges, Wunder, Wallemacq, Persu and Toennes 2020, Schmieder, Ott, Schmid, Friedrich, Kistner, Ditting, Veelken, Uder and Toennes 2016) the analyses of urine extracts of furosemide and other antihypertensive drugs were performed with LC-MS/MS with methodone-d9 as an internal standard. The assay employed liquid-liquid extraction of 200 μL of urine samples with ethyl acetate followed by evaporation and reconstitution in mobile phase solvents. Furosemide was separated with Kinetex XB-C18 column with a runtime of 7.2 minutes to determine adherence to medication. Previously reported methods lack the details of a bioanalytical method and in the present study we report the details of a bioanalytical method validation.
We have developed a quick and efficient UHPLC-MS/MS assay for furosemide quantitation in a small volume (10 μL) of human urine with furosemide-d5 as the internal standard. This method utilizes a simple SPE sample preparation method for easy sample clean-up without the need for evaporation and reconstitution of samples. Our method was linear over the range of 0.100 – 50.0 μg/mL with an LLOQ of 0.100 μg/mL since clinically relevant concentrations for furosemide were expected to be greater. Samples were injected at 2 μL on the UHPLC-MS/MS system with chromatographic separation using a Waters Acquity HSS C18 column (2.6 μm, 100 Å, 2.1 × 100 mm) and a runtime of 4.5 minutes. This method is currently utilized for an ongoing prospective clinical research study.
5. CONCLUSIONS
Development and validation for an UHPLC-MS/MS assay for the quantitation of furosemide in 10 μL of human urine has been established and applied to the analysis of urine samples from a prospective clinical research study. The assay range was linear and reproducible over the calibration range of 0.100 – 50.0 μg/mL. Stability studies have shown that samples can be kept at room temperature for up to 1 week if a refrigerator or freezer is not readily available. Long-term stability was proven at −78 °C for 6 months, which allows for grouping large batches of samples for analysis. The validated method was implemented for clinical samples from infants receiving furosemide for bronchopulmonary dysplasia in an ongoing study.
ACKNOWLEDGMENT
This research is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD101651 (Bamat).
Footnotes
CONFLICTS OF INTEREST
The authors disclose no conflicts.
References
- Aranda JV, Lambert C, Perez J, Turmen T and Sitar DS Metabolism and renal elimination of furosemide in the newborn infant. J Pediatr 1982; 101 (5): 777–81. DOI: 10.1016/s0022-3476(82)80319-3. [DOI] [PubMed] [Google Scholar]
- Aranda JV, Perez J, Sitar DS, Collinage J, Portuguez-Malavasi A, Duffy B and Dupont C Pharmacokinetic disposition and protein binding of furosemide in newborn infants. J Pediatr 1978; 93 (3): 507–11. DOI: 10.1016/s0022-3476(78)81181-0. [DOI] [PubMed] [Google Scholar]
- Ayalasomayajula S, Schuehly U, Pal P, Chen F, Zhou W, Sunkara Gand Langenickel TH Effect of the angiotensin receptor-neprilysin inhibitor sacubitril/valsartan on the pharmacokinetics and pharmacodynamics of a single dose of furosemide. Br J Clin Pharmacol 2018; 84 (5): 926–936. DOI: 10.1111/bcp.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamat NA, Kirpalani H, Feudtner C, Jensen EA, Laughon MM, Zhang H, Monk HM, Passarella M and Lorch SA Medication use in infants with severe bronchopulmonary dysplasia admitted to United States children’s hospitals. J Perinatol 2019; 39 (9): 1291–1299. DOI: 10.1038/s41372-019-0415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brater DC Update in diuretic therapy: clinical pharmacology. Semin Nephrol 2011; 31 (6): 483–94. DOI: 10.1016/j.semnephrol.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Chen C, Bajpai L, Mollova N and Leung K Sensitive and cost-effective LC-MS/MS method for quantitation of CVT-6883 in human urine using sodium dodecylbenzenesulfonate additive to eliminate adsorptive losses. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877 (10): 943–7. DOI: 10.1016/j.jchromb.2009.02.045. [DOI] [PubMed] [Google Scholar]
- Cotton R, Suarez S and Reese J Unexpected extra-renal effects of loop diuretics in the preterm neonate. Acta Paediatr 2012; 101 (8): 835–45. DOI: 10.1111/j.1651-2227.2012.02699.x.FDA Bioanalytical Method Validation: Guidance for Industry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feudtner C, Dai D, Hexem KR, Luan X and Metjian TA Prevalence of polypharmacy exposure among hospitalized children in the United States. Arch Pediatr Adolesc Med 2012; 166 (1): 9–16. DOI: 10.1001/archpediatrics.2011.161. [DOI] [PubMed] [Google Scholar]
- Heffron B, Taddei L, Benoit M and Negrusz A Quantification of several acidic drugs in equine serum using LC-MS-MS. J Anal Toxicol 2013; 37 (8): 600–4. DOI: 10.1093/jat/bkt069. [DOI] [PubMed] [Google Scholar]
- Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK Jr., Smith PB and Best Pharmaceuticals for Children Act-Pediatric Trials N Medication use in the neonatal intensive care unit. Am J Perinatol 2014; 31 (9): 811–21. DOI: 10.1055/s-0033-1361933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GJ, Capparelli E, Romanowski G, Proudfoot JA and Tremoulet AH Development of Tolerance to Chronic Intermittent Furosemide Therapy in Pediatric Patients. J Pediatr Pharmacol Ther 2017; 22 (6): 394–398. DOI: 10.5863/1551-6776-22.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy IE, Chertow GM and Chang TI Patterns of diuretic use in the intensive care unit. PLoS One 2019; 14 (5): e0217911. DOI: 10.1371/journal.pone.0217911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirochnick MH, Miceli JJ, Kramer PA, Chapron DJ and Raye JR Furosemide pharmacokinetics in very low birth weight infants. J Pediatr 1988; 112 (4): 653–7. DOI: 10.1016/s0022-3476(88)80192-6. [DOI] [PubMed] [Google Scholar]
- Mirochnick MH, Miceli JJ, Kramer PA, Chapron DJ and Raye JR Renal response to furosemide in very low birth weight infants during chronic administration. Dev Pharmacol Ther 1990; 15 (1): 1–7. DOI: 10.1159/000457612. [DOI] [PubMed] [Google Scholar]
- NCT02527798 National Library of Medicine (U.S.). Safety of Furosemide in Premature Infants at Risk of Bronchopulmonary Dysplasia (BPD) (2015, November - ). National Library of Medicine (U.S.); ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT02527798. [Google Scholar]
- O’Donovan BH and Bell EF Effects of furosemide on body water compartments in infants with bronchopulmonary dysplasia. Pediatr Res 1989; 26 (2): 121–4. DOI: 10.1203/00006450-198908000-00010. [DOI] [PubMed] [Google Scholar]
- Odlind B and Beermann B Renal tubular secretion and effects of furosemide. Clin Pharmacol Ther 1980; 27 (6): 784–90. DOI: 10.1038/clpt.1980.111. [DOI] [PubMed] [Google Scholar]
- Orlov SN, Koltsova SV, Kapilevich LV, Gusakova SV and Dulin NO NKCC1 and NKCC2: The pathogenetic role of cation-chloride cotransporters in hypertension. Genes Dis 2015; 2 (2): 186–196. DOI: 10.1016/j.gendis.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponto LL and Schoenwald RD Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part I). Clin Pharmacokinet 1990; 18 (5): 381–408. DOI: 10.2165/00003088-199018050-00004. [DOI] [PubMed] [Google Scholar]
- Rao VS, Planavsky N, Hanberg JS, Ahmad T, Brisco-Bacik MA, Wilson FP, Jacoby D, Chen M, Tang WHW, Cherney DZI, Ellison DH and Testani JM Compensatory Distal Reabsorption Drives Diuretic Resistance in Human Heart Failure. J Am Soc Nephrol 2017; 28 (11): 3414–3424. DOI: 10.1681/ASN.2016111178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritscher S, Georges C, Wunder C, Wallemacq P, Persu A and Toennes SW Assessment of adherence to diuretics and beta-blockers by serum drug monitoring in comparison to urine analysis. Blood Press 2020; 29 (5): 291–298. DOI: 10.1080/08037051.2020.1763775. [DOI] [PubMed] [Google Scholar]
- Ritscher S, Hoyer M, Wunder C, Obermuller N and Toennes SW Evaluation of the dose-related concentration approach in therapeutic drug monitoring of diuretics and beta-blockers - drug classes with low adherence in antihypertensive therapy. Sci Rep 2019; 9 (1): 15652. DOI: 10.1038/s41598-019-52164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder RE, Ott C, Schmid A, Friedrich S, Kistner I, Ditting T, Veelken R, Uder M and Toennes SW Adherence to Antihypertensive Medication in Treatment-Resistant Hypertension Undergoing Renal Denervation. J Am Heart Assoc 2016; 5 (2). DOI: 10.1161/JAHA.115.002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segar JL, Chemtob S and Bell EF Changes in body water compartments with diuretic therapy in infants with chronic lung disease. Early Hum Dev 1997; 48 (1–2): 99–107. DOI: 10.1016/s0378-3782(96)01841-5. [DOI] [PubMed] [Google Scholar]
- Segar JL, Robillard JE, Johnson KJ, Bell EF and Chemtob S Addition of metolazone to overcome tolerance to furosemide in infants with bronchopulmonary dysplasia. J Pediatr 1992; 120 (6): 966–73. DOI: 10.1016/s0022-3476(05)81972-9. [DOI] [PubMed] [Google Scholar]


