Abstract
Objective
A higher proportion of circulating memory CD4+ T cells is associated with prevalent diabetes mellitus in persons with HIV (PWH) and HIV-negative persons. We assessed whether circulating T cell subsets could also identify individuals who will subsequently develop diabetes.
Design
This is a longitudinal follow-up study of PWH and similar HIV-negative individuals from the Veterans Aging Cohort Study who provided peripheral mononuclear blood cells between 2005 and 2007.
Methods
We quantified T cell subsets using flow cytometry and functional assays to identify CD4+ and CD8+ naïve, activated, senescent, memory (central, effector, and effector RA+), and TH1, TH2, and TH17-phenotype cells. The occurrence of an incident diabetes diagnosis (i.e., after baseline blood draw) was adjudicated by a two-physician chart review. Cox proportional hazards models adjusted for traditional risk factors, cytomegalovirus serostatus, and plasma inflammatory biomarkers assessed the relationship between T cell subsets and incident diabetes.
Results
1837 participants (1259 PWH) without diabetes at baseline were included; 69% were black, 95% were male, and median follow-up was 8.6 years. Higher baseline frequencies of CD4+ T effector memory RA+ (TEMRA) cells defined as CD45RA+ CD27− (p=0.04) and senescent T cells defined as CD4+ CD28− (p=0.04) were associated with incident diabetes in PWH only.
Conclusions
Higher frequencies of CD4+ TEMRA and CD4+ CD28− T cells were associated with incident diabetes in PWH only after adjustment for other factors. Additional studies are necessary to assess whether these cells act in blood via inflammatory mediators or reflect T cell populations in metabolically active tissues.
Keywords: Diabetes Mellitus, HIV, T-Lymphocytes, Adaptive Immunity, Metabolic Diseases
Introduction
Type 2 diabetes mellitus is increasingly common among persons with HIV (PWH) on long-term combination antiretroviral therapy (ART) [1, 2], and contributes to an increased risk of chronic kidney disease, cardiovascular disease, and overall mortality [3–5]. The development of diabetes in PWH has been previously linked to higher concentrations of circulating inflammatory markers including interleukin (IL)-6 and high-sensitivity C-reactive protein (CRP) [6, 7], which become elevated after HIV infection and do not completely normalize with viral suppression [8]. While the relationship of innate immune activation and diabetes risk has been demonstrated in PWH and HIV-negative persons, there are fewer data on the relationship between adaptive immunity and diabetes.
In the general population, a reduced proportion of naïve and regulatory (Treg) CD4+ T cells [9–11], an increased proportion of circulating memory CD4+ T cells [9, 12], and a shift towards pro-inflammatory type 1 (TH1) and type 17 (TH17) helper cells [11], have been associated with prevalent diabetes. An important question is whether T cell profiles change in advance of diabetes onset, which could provide insight into pathogenesis and serve as prognostic or risk stratification markers. The limited available studies provide inconclusive findings. A recent study prospectively followed 932 individuals from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort who were at risk for diabetes over a median 9.1 years and did not find any association between baseline circulating T cell subset frequencies and incident diabetes after adjusting for age, sex, race/ethnicity, educational status, and body mass index (BMI) [13]. In contrast, a prospective study of participants at high risk for cardiovascular disease followed 149 individuals for a median of 2.3 years and found that increased frequency of circulating CD8+CD57+ T cells, a marker of senescence, was associated with a greater rise in hemoglobin A1c and fasting serum blood glucose during follow up [14].
HIV infection induces detrimental changes to the immune system with depletion of naïve CD4+ T cells, persistent activation of CD4+ and CD8+ T cells, and higher frequencies of T effector memory RA+ (TEMRA), CD28− senescent, and exhausted T cell phenotypes [15–17]. These immunologic changes have been linked to increased cardiovascular, liver, and renal diseases [18–20]. In a small, cross-sectional study, higher proportions of CD4+ T effector memory (TEM) and TEMRA cells co-expressing CX3CR1, GPR56, and CD57 were associated with prevalent diabetes in PWH [21]. We previously assessed the relationship between circulating T cell subsets and prevalent diabetes in the Veterans Aging Cohort Study Biomarker Cohort (VACS-BC) and found that increased frequency of memory CD4+ T cells was associated with prevalent diabetes among both PWH and HIV-negative persons [22]. The objective of this study is to determine the relationships between circulating T cell subsets and the subsequent development of diabetes in Veterans with and without HIV.
Methods
Study participants
The Veterans Aging Cohort Study (VACS) is a longitudinal, prospective, multicenter observational study enrolling PWH and HIV-negative individuals of similar age, race/ethnicity, and geographic location from the Veterans Affairs healthcare system to study the role of comorbid diseases and behavior on HIV outcomes [23]. A subset of VACS participants had peripheral blood mononuclear cells (PBMCs) cryopreserved between 2005 and 2007 that comprised the VACS-BC and were included in this study [22, 24]. Information regarding demographic and clinical data was extracted from the Veterans Affairs electronic health record, the VA corporate data warehouse, the VA vital status file, and from self-reported surveys administered at annual VACS visits.
Incident diabetes definition
We employed a validated EMR algorithm (Document, Supplemental Digital Content 1, detailed methods) incorporating pharmacy data, laboratory values, and ICD-9 codes to identify potential diabetes cases present at participant specimen archiving in 2005–2007 or occurring after baseline blood draws through September 30, 2015 [25]. All potential cases were adjudicated by two physicians with expertise in endocrinology and metabolism (if there was a discrepancy, a third physician served as the final arbitrator of case classification). Given that all incident diabetes cases occurred in adulthood and after discharge from active military service, these were presumed to be type 2 in nature, though insulin measurements were frequently not available. Diabetes diagnoses were classified as prevalent (adjudicated onset date preceded baseline blood collection) or incident (occurred after blood collection). We excluded participants who had prevalent diabetes for this analysis. We have previously reported the sensitivity, specificity, and other agreement characteristics of this EMR algorithm with physician adjudication for this cohort [25].
Immunophenotyping
Cryopreserved PBMCs from the baseline blood draws were processed and stained as previously published [22, 26]. Briefly, PBMCs were thawed at 37°C for 15 minutes and suspended in fully supplemented RPMI at 10-fold dilution. The samples underwent repeat centrifugation and resuspension before filtering through a 70 μm filter. Antibodies and isotype-matched control antibodies used for surface staining and intracellular staining are listed in supplemental materials (Table, Supplemental Digital Content 2, surface and intracellular markers). For CD4+ functional assays, samples were first stimulated with phorbol myristate acetate in the presence of brefeldin A for three hours, then resuspended in PBS, pH 7.4, and stained for viability before staining with PE-conjugated anti-IL-4, PEVio-conjugated anti-IFN-γ and APCVio-conjugated anti-IL-17, or isotype controls.
Cells were analyzed on an 8-color MQ10 (Miltenyi Biotec) flow cytometer. Bead calibration was used to standardize runs and single-color compensation and isotype controls were used to set compensation and negative gates, respectively. Flow cytometry data were analyzed using the MACSQuantify software (Miltenyi Biotec). We evaluated CD4+ and CD8+ central memory (TCM; CD45RO+CCR7+CD27+), effector memory (TEM; CD45RO+CCR7−CD27−), effector memory re-expressing CD45RA (TEMRA; CD45RA+ CD57+CD28− or CD45RA+CD27−), and senescent T cells (CD57+CD28−; CD28−). We used two definitions for TEMRA cells to reflect differing definitions in the literature. We characterized type 1 (TH1; IFN-γ producing), type 2 (TH2; IL-4 producing), and type 17 (TH17; IL-17 producing) helper CD4+ T cells and regulatory CD4+ T cells (Treg; CD4+CD25+FoxP3+). The missingness for functional assays and surface marker phenotypes was 11% and 8% respectively due to poor cell viability.
Interleukin-6, D-dimer, soluble CD14, and Cytomegalovirus Assays
Serum IL-6, d-dimer, and soluble CD14 concentration were measured as described in the Supplementary Materials. The cytomegalovirus (CMV) IgG concentration was determined using an ELISA from Diamedix (Miami Lakes, FL) and CMV seropositivity was defined as CMV IgG concentration > 8 EU/ml.
Statistical Analyses
Covariate data were obtained closest to the date of blood sample collection except for BMI, which was time-updated. HIV-1 RNA was obtained closest to the blood collection date (up to 180 days after). We compared demographic and clinic characteristics between PWH and HIV-negative individuals stratified by incident diabetes. We used chi-square and Wilcoxon rank sum tests to compare categorical and continuous variables, respectively. To evaluate the association of T cell subset with risk of incident diabetes, we used a Cox proportional hazards model with the T cell subset as the main exposure and incident diabetes as the outcome. We estimated the hazard ratio (HR) and 95% confidence intervals (CI) for 1-standard deviation increment in each immune cell. The proportional hazards assumption was tested by the Schoenfeld residuals and included the interaction term of the covariates by time as evaluated by the “cox.zph” function in R. We modeled PWH and HIV-negative individuals separately because the heterogeneity of viral load, which may affect the outcome of interest, could not be accounted for in a combined model.
We first performed unadjusted analysis of baseline T cell subsets and incident diabetes. We then created three sequential Cox proportional hazards models. Model 1 adjusted for age, race, CMV serostatus, and serum HIV-1 RNA quantification (PWH only). Model 2 further adjusted for hepatitis C serostatus, high-density lipoprotein, low-density lipoprotein, total cholesterol, history of alcohol abuse, and time-updated BMI. Model 3 further adjusted for serum concentrations of IL-6, d-dimer, and soluble CD14. As a sensitivity analysis, we incorporated time-updated HIV-1 RNA quantification (PWH only). To assess whether the relationship between baseline T cell subsets and incident diabetes was non-linear, we used restricted cubic splines to model the subsets with 4 knots. Missing data were imputed using multiple imputation by chained equations with five complete datasets based on predictive mean matching using the Hmisc library of R programming language. Cox survival models were fitted in each imputed dataset and then combined to obtain pooled HRs and standard errors. All analyses were performed using R software (version 4.0.2; www.r-project.org).
This study was reviewed and approved by the Veterans Affairs and the Vanderbilt University Medical Center Institutional Review Boards. All participants provided written informed consent.
Results
Cohort Characteristics
Of 2,386 participants who provided PBMCs, 549 participants were excluded from the analysis; this included those with no further clinical encounters after baseline blood draw (n = 7), a discrepancy between HIV status and HIV specific variables (i.e., no record of HIV diagnosis or positive screening test but measured HIV viral load or CD4 T cell count, or ART prescription [n = 14]), last VA utilization date listed before date of blood draw (n = 5), and adjudicated prevalent diabetes (n = 523) leaving 1259 PWH and 578 HIV-negative participants (Figure 1). Incident diabetes occurred in 238 participants (133 in PWH and 105 in HIV-negative individuals) over a median follow-up time of 8.6 years. Table 1 describes demographic and clinical characteristics of the cohort, stratified by HIV status and incident diabetes during follow-up. The cohort was 69% black and 95% male. PWH and HIV-negative individuals who developed diabetes had a higher BMI compared to those who did not developed diabetes (p < 0.01 for both groups). The majority of PWH had viral suppression (65%) and were on ART at the time of enrollment (84%). Circulating inflammatory biomarker concentration and T cell subset percentage were also compared by HIV serostatus and incident diabetes (Table, Supplemental Digital Content 3, Inflammatory biomarkers and T cell subsets).
Figure 1.
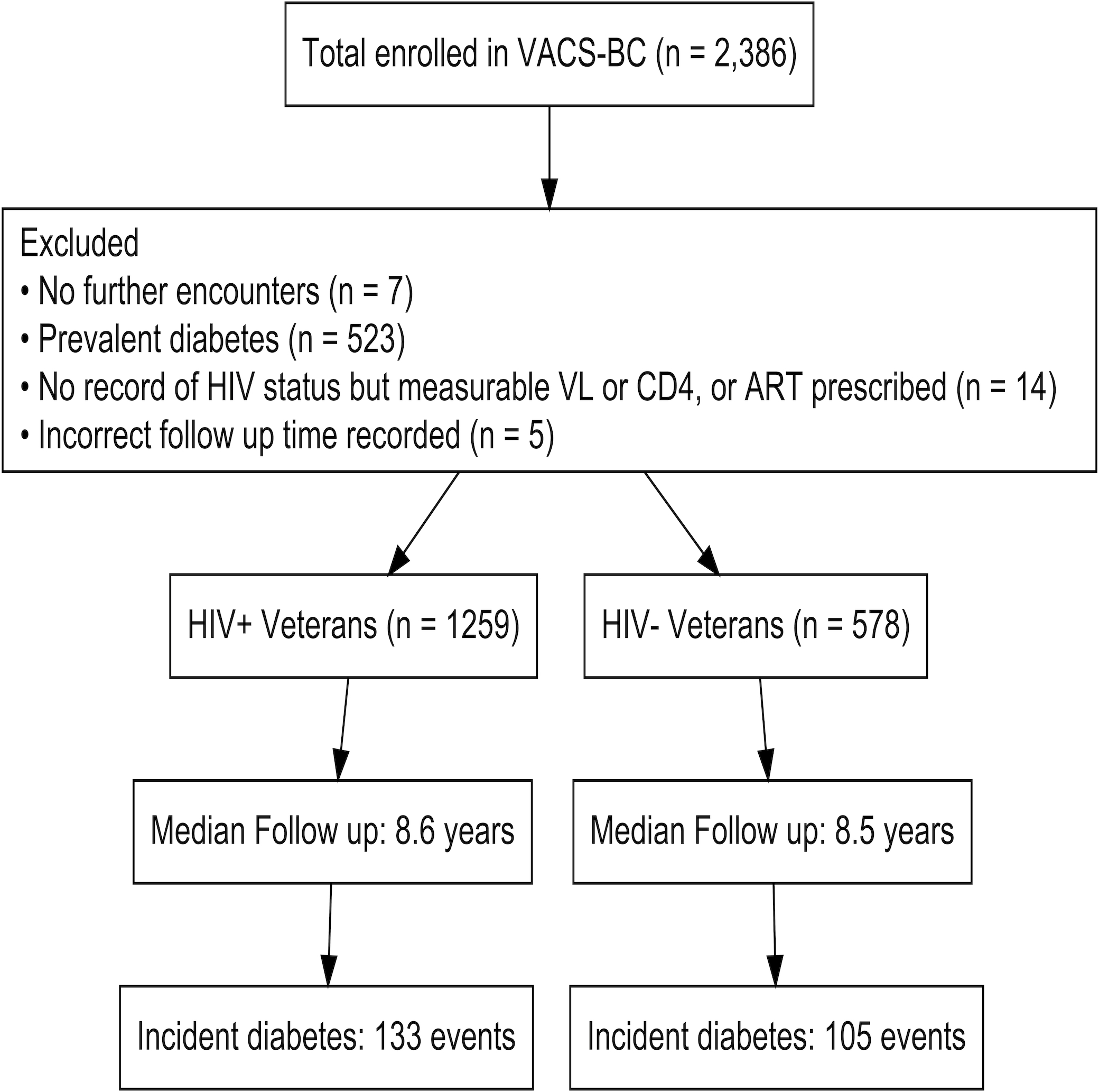
Overview of study design and participant inclusion. Abbreviations: ART, antiretroviral therapy; HIV+, persons with HIV; HIV-, HIV-negative; VACS-BC, Veterans Aging Cohort Study Biomarker Cohort; VL, viral load.
Table 1.
Baseline characteristics stratified by HIV serostatus and incident diabetes
| Persons with HIV | HIV-negative persons | |||||
|---|---|---|---|---|---|---|
| Incident diabetes (n = 133) |
Non-diabetic (n = 1126) |
P value | Incident diabetes (n = 105) |
Non-diabetic (n = 473) |
P value | |
| Demographics | ||||||
| Age, years | 52.1 (47.1, 57.9) | 51.4 (46.1, 57.0) | 0.13 | 51.8 (47.1, 56.9) | 52.5 (47.2, 58.2) | 0.41 |
| Race/ethnicity | ||||||
| White | 19 (14.3) | 232 (20.6) | 0.25 | 19 (18.1) | 99 (20.9) | 0.45 |
| Black | 101 (75.9) | 766 (68.0) | 72 (68.6) | 323 (68.3) | ||
| Hispanic | 10 (7.5) | 87 (7.7) | 12 (11.4) | 35 (7.4) | ||
| Other | 3 (2.3) | 41 (3.6) | 2 (1.9) | 16 (3.4) | ||
| Body mass index, kg/m2 | 27.1 (24.0, 30.6) | 24.8 (22.4 27.4) | <0.01 | 31.6 (28.9, 35.7) | 27.9 (24.6, 32.0) | <0.01 |
| Sex (male) | 129 (97.0) | 1091 (96.9) | 0.99 | 98 (93.3) | 422 (89.2) | 0.28 |
| Comorbid diseases | ||||||
| Alcohol abuse history | 62 (46.6) | 446 (39.6) | 0.14 | 54 (51.4) | 237 (50.1) | 0.89 |
| eGFR ml/min/1.73 m2 < 60 | 8 (6.0) | 77 (6.8) | 0.86 | 4 (3.8) | 38 (8.0) | 0.18 |
| Total cholesterol ≥ 200 mg/dL | 41 (30.8) | 287 (25.5) | 0.23 | 30 (28.6) | 133 (28.1) | 0.26 |
| LDL cholesterol ≥ 160 mg/dL | 6 (4.5) | 51 (4.5) | 0.84 | 10 (9.5) | 35 (7.4) | 0.76 |
| HDL cholesterol < 40 mg/dL | 63 (47.4) | 447 (39.7) | 0.22 | 44 (41.9) | 123 (26.0) | <0.01 |
| Triglyceride ≥ 200 mg/dL | 44 (33.1) | 264 (23.4) | 0.05 | 20 (19.0) | 59 (12.5) | 0.08 |
| HCV antibody positive | 51 (38.3) | 382 (33.9) | 0.87 | 25 (23.8) | 116 (24.5) | 0.89 |
| CMV antibody positive | 117 (88.0) | 1029 (91.4) | 0.35 | 71 (67.6) | 337 (71.2) | 0.76 |
| HIV Characteristics | ||||||
| HIV-1 RNA copies/mL ≥ 500 | 41 (30.8) | 405 (36.0) | 0.28 | |||
| CD4+ T cell count, cells/mm3 | ||||||
| < 200 | 30 (22.6) | 216 (19.2) | 0.51 | |||
| 211–499 | 55 (41.4) | 519 (46.1) | ||||
| ≥ 500 | 48 (36.1) | 391 (34.7) | ||||
| ART at enrollment | 112 (84.2) | 947 (84.1) | 0.99 | |||
Continuous variables shown as median values with interquartile range (parentheses). Categorical variables are shown as total ‘n’ and column percentage.
Abbreviations: ART, antiretroviral therapy; CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate; HCV, hepatitis c virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein
Higher baseline frequencies of CD4+ CD28− and CD4+ TEMRA cells in PWH, but not HIV-negative individuals, are associated with a greater risk of incident diabetes
We first performed unadjusted Cox proportional hazards modeling to assess the relationship between baseline demographic and clinical variables and the risk of incident diabetes in PWH and HIV-negative individuals (Figure 2). Higher BMI was associated with an increased hazard of incident diabetes in both PWH (HR=1.46, p<0.01) and HIV-negative individuals (HR=1.56, p<0.01). Among T cell subsets, a higher proportion of CD4+ TEMRA cells defined as either CD45RA+CD57+CD28− or CD45RA+CD27− was associated with increased hazard of incident diabetes in PWH (p=0.04 and p=0.03, respectively). A similar relationship was observed for CD4+CD28− T cells (p=0.06) in PWH. One definition of CD4+ TEMRA cells, CD45RA+CD27−, was associated with a higher risk of incident diabetes in HIV-negative participants (p=0.05) (Table, Supplemental Digital Content 4, Cox proportional hazards model).
Figure 2.
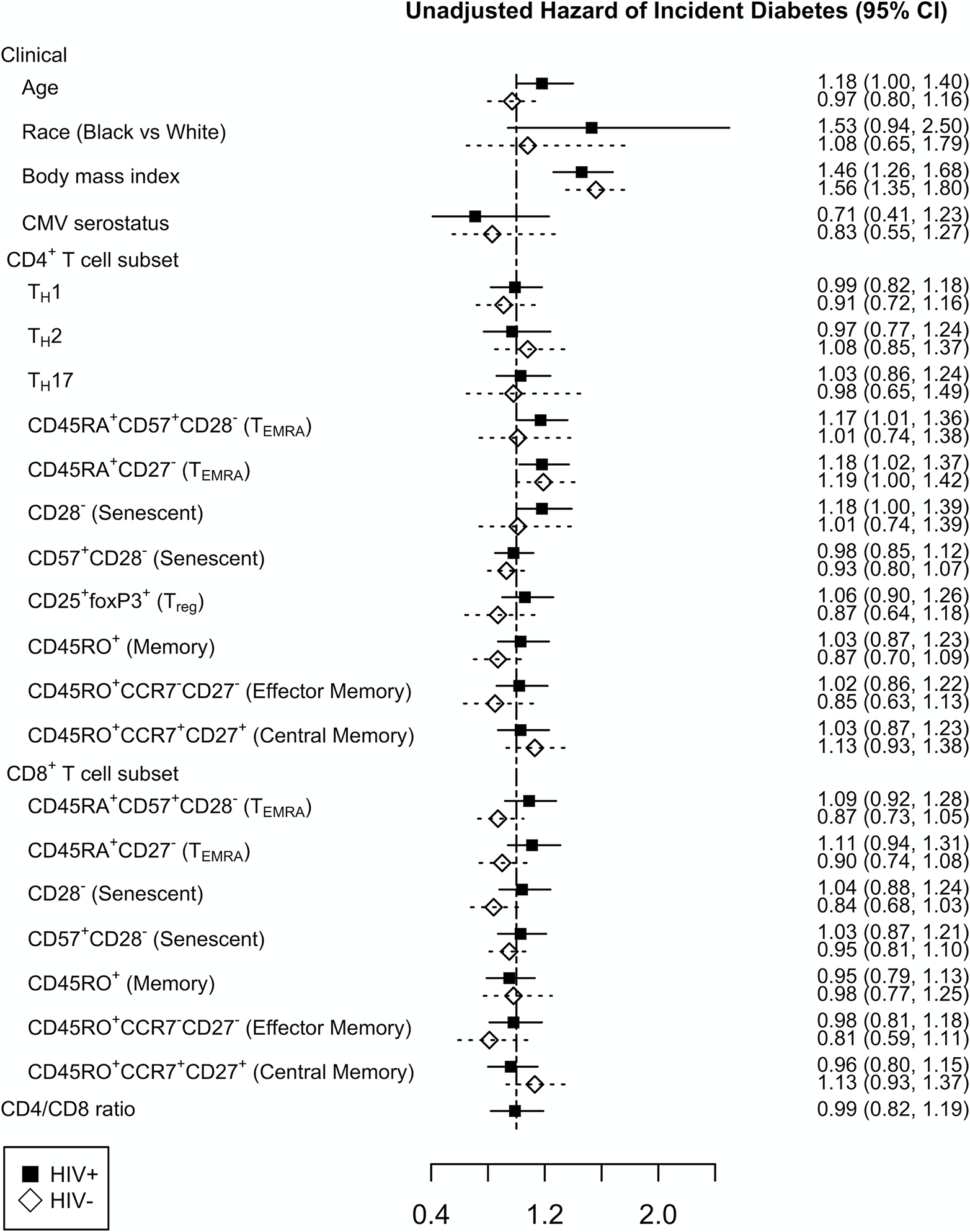
Unadjusted Cox proportional hazards model assessing the relationship between baseline clinical variables and incident diabetes in persons with HIV (black square) and HIV-negative individuals (open diamond). For linear variables, the hazard ratio is reported per kg/m2 for body mass index, per year for age, and per standard deviation (SD) increment for T cell subsets with 95% confidence interval (95% CI).
Figure 3 shows the results for PWH and HIV-negative individuals for the fully adjusted model. A higher baseline frequency of CD4+ TEMRA cells defined as CD45RA+CD27− was significantly associated with an increased hazard of incident diabetes in PWH (HR=1.17, p=0.04), and approached significance for CD4+ TEMRA cells defined as CD45RA+CD57+CD28− (HR=1.15, p=0.07). A higher frequency of senescent CD4+ T cells defined as CD28− (HR=1.18, p=0.04), but not CD57+CD28−, was also significantly associated with hazard of incident diabetes in the PWH (Table, Supplemental Digital Content 5, fully adjusted cox proportional hazards model in PWH). In contrast, no T cell subsets were significantly associated with incident diabetes in HIV-negative individuals (Table, Supplemental Digital Content 6, fully adjusted cox proportional hazards model in HIV-negative individuals). To test whether there is an interaction between HIV status and the CD4+ T cell subsets, we used the full model combining PWH and HIV-negative individuals and included an interaction term. There was no significant interaction between any CD4+ T cell subset and HIV status.
Figure 3.
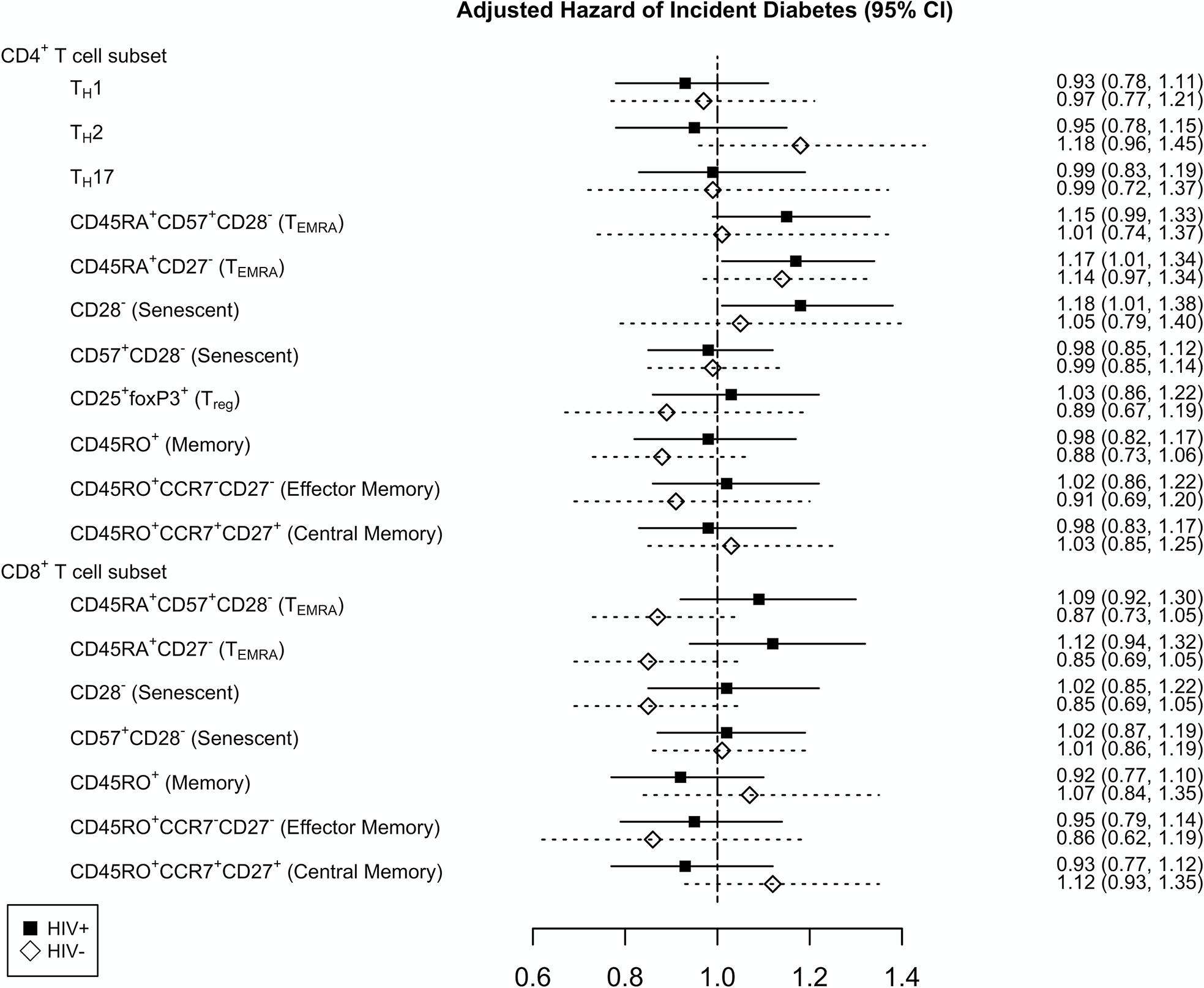
Cox proportional hazards model assessing the relationship of baseline T cell subsets and incident diabetes in persons with HIV (black square) and HIV-negative individuals (open diamond) adjusted for age, race, cytomegalovirus serostatus, plasma HIV-1 RNA copies/mL (persons with HIV only), high-density lipoprotein, low-density lipoprotein, total cholesterol, time-updated body mass index, hepatitis C virus serostatus, history of alcohol abuse, and circulating concentrations of interleukin-6, d-dimer, and soluble CD14. Hazard ratio is reported per standard deviation (SD) increment with 95% confidence interval (95% CI).
As a sensitivity analysis, we adjusted for time-updated plasma HIV-1 RNA level, which did not significantly change the hazard ratios (Table, Supplemental Digital Content 7, Cox proportional hazard sensitivity analysis). Using a restricted cubic spline regression model, we found the relationship between CD4+ and CD8+ TEMRA cells and incident diabetes was not significantly different from the linear model (Figure, Supplemental Digital Content 8, Restricted cubic spline regression).
Cytomegalovirus serostatus does not influence the relationship of T cell subsets with incident diabetes
In our cohort, CMV seropositivity was associated with the inflation of CD4+ and CD8+ TEMRA and senescent T cells (Table, Supplemental Digital Content 9, Demographic characteristics and T cell subsets by CMV serostatus). We therefore stratified PWH by CMV serostatus and repeated the analysis. Among CMV-positive PWH, the hazard for incident diabetes with baseline CD4+ TEMRA cells and CD28− senescent T cells was similar (Figure 4) (Table, Supplemental Digital Content 10, Cox proportional hazards model for CMV-positive PWH). We next evaluated CMV-negative PWH, which included only 14 incident diabetes events. Given the small number of events, we only adjusted for time-updated BMI and age since these variables had the strongest hazard for incident diabetes (Figure 2). Higher proportion of CD4+ TEMRA cells defined by CD45RA+CD27− was associated with a higher risk of incident diabetes (HR=1.61, p=0.001) (Table, Supplemental Digital Content 11, Cox proportional hazards model for CMV-negative PWH).
Figure 4.
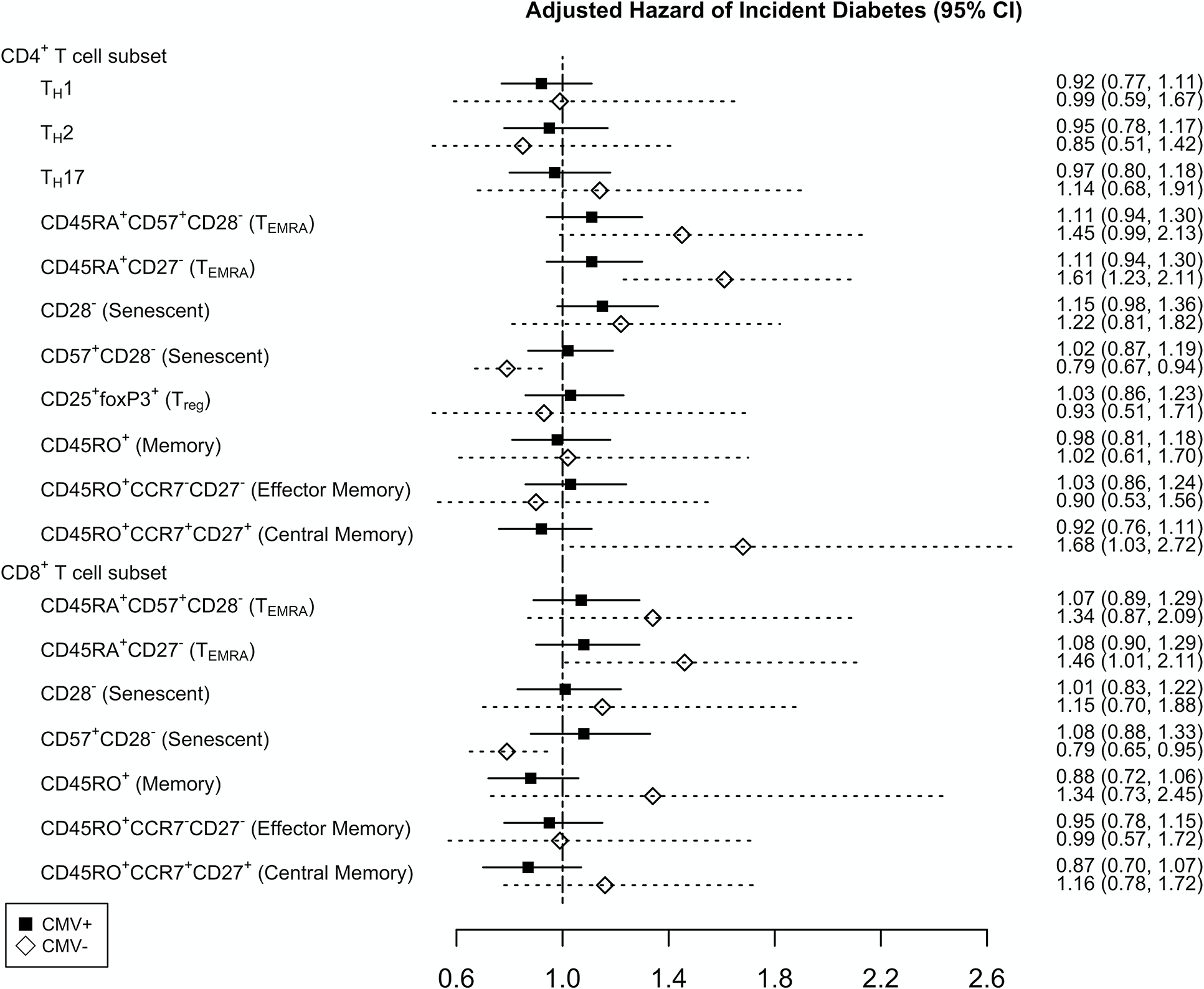
Cox proportional hazards model assessing the relationship of baseline T cell subsets and incident diabetes in cytomegalovirus (CMV) seropositive (black square) with HIV adjusted for age, race, plasma HIV-1 RNA copies/mL, high-density lipoprotein, low-density lipoprotein, total cholesterol, time-updated body mass index (BMI), hepatitis C virus serostatus, history of alcohol abuse, and circulating concentrations of interleukin-6, d-dimer, and soluble CD14. The model for CMV seronegative PWH (open diamond) was only adjusted for age and time-updated BMI. Hazard ratio is reported per standard deviation (SD) increment with 95% confidence interval (95% CI).
Discussion
The burden of diabetes is high in PWH and the complex immunologic changes induced by HIV infection may contribute to the development of metabolic diseases. We have previously shown that a greater proportion of memory CD4+ T cells was associated with prevalent diabetes in PWH [22]. Here, we show that higher baseline frequencies of CD4+ TEMRA and CD4+CD28− T cells are associated with an increased hazard of incident diabetes in PWH, but not in HIV-negative individuals, after adjusting for multiple covariates.
CD28 is a co-stimulatory receptor that is important for activation, proliferation, and maintenance of CD4+ T cells [27]. CD4+CD28− T cells are a heterogeneous population of cells that demonstrate reduced proliferative capacity in the setting of antigen stimulation, have higher TCR clonality than CD28+ T cells [28], have cytotoxic properties, and secrete both TNF-α and IFN-γ [29]. Furthermore, these cells are resistant to the anti-inflammatory stimuli of T regulatory cells [30] and have been associated with autoimmune disorders, cardiovascular disease, and diabetes in HIV-negative persons [31, 32]. While expansion of CD4+CD28− T cells has been associated with inflammatory disorders and chronic infections including CMV [33], the underlying cause of their expansion is unclear. CD4+ TEMRA cells are closely related to CD28− T cells as they frequently lack CD28 expression. Functionally, they are cytotoxic with high expression of perforin and granzyme B [34–36], and a subset of TEMRA expressing GPR56 and CX3CR1 are highly clonal and frequently have anti-viral activity [34]. CMV is a dominant antigenic target of CD4+ TEMRA cells, and they have been associated with atherosclerosis and cardiovascular disease in both PWH and HIV-negative individuals [37, 38]. The link with diabetes has been less clear, but a small study found CD4+ T cells co-expressing CD57, GPR56, and CX3CR1, a predominantly CD4+ TEMRA population, were higher in the blood and adipose tissue of PWH with greater glucose intolerance [21].
In this study, we did not find any association between CMV serostatus and risk of incident diabetes. In subgroup analysis, CMV-negative PWH had a similar increased risk of diabetes with a higher proportion of CD4+ TEMRA cells. This finding was unexpected given the role of CMV in TEMRA cell inflation, and we considered whether some participants were incorrectly classified as CMV-seronegative. However, the IgG antibody titers in those defined as CMV-seronegative and who subsequently developed diabetes were below our defined IgG titer for seropositivity (highest IgG titer was < 5.4 EU/mL). Taken together, our results suggest that a higher proportion of proinflammatory CD4+CD28− and CD4+ TEMRA cells may contribute to elevated systemic inflammation that predisposes to the development of diabetes. Importantly, these findings were specific to PWH, as we did not find any T cell subsets that were associated with risk of incident diabetes in HIV-negative individuals, similar to previous studies [13].
The lack of any observed associations between T cell subsets and diabetes in HIV-negative individuals may reflect three important factors. First, HIV-negative individuals had a higher incidence of diabetes compared with PWH, potentially driven by a higher prevalence of obesity. This likely reflects the fact that PWH and HIV-negative persons engage in care for differing reasons; while many PWH may establish care primarily to address their HIV diagnosis, HIV-negative individuals may seek care to address underlying cardiometabolic conditions (e.g., obesity, hypertension, hyperlipidemia, among others) associated with an increased risk of developing diabetes. When we analyzed only non-obese individuals, the incidence of diabetes was more similar between HIV-negative individuals and PWH (11.4% vs 8.7%, respectively, compared to 18.2% and 10.6% in the full cohort). Since obesity is a major risk factor for diabetes, the smaller effect size of T cell subsets for risk of diabetes may not be detected if obese HIV-negative individuals are included in the model. Second, the T cell subsets associated with incident diabetes in PWH are often expanded after HIV infection, which may predispose these individuals to metabolic disease. Finally, the sample size for HIV-negative individuals was smaller, so we cannot rule out that we failed to detect a difference in this group due to a type 2 error.
While T cells are important contributors to adaptive immune responses, B cells have also been implicated in the development of type 2 diabetes. In persons with type 2 diabetes, 4–14% have glutamic acid decarboxylase, tyrosine phosphatase IA-2, or islet-cell antibodies, suggesting an autoimmune-related reduction in insulin production may be present in addition to the more common insulin resistance phenotype [39]. Furthermore, a cross-sectional study of persons with type 2 diabetes showed that circulating islet auto-reactive antibodies were associated with greater IL-17 secreting CD4+CXCR5+ TH17 cells [40]. In the MESA study, higher proportion of CD19+CD27+ B cells was associated with a reduced risk of incident diabetes [13]. These and other studies suggest a joint role for B-cell and T cell-mediated immune responses in diabetes, potentially acting at multiple sites and affecting both insulin production and tissue insulin sensitivity. Our group has previously described a specific CD4+ TEMRA subset that accumulates in both blood and adipose tissue in PWH with progressive glucose intolerance [21, 41], and similar translational studies are needed to assess the role of B cells in metabolic disease in the context of HIV. To our knowledge, only small case series have examined auto-antibodies in PWH [42], and future studies will need to simultaneously assess T cells, B cells and auto-antibodies in large cohorts of PWH versus HIV-negative controls, as well as in specific tissue compartments.
Our study had several strengths. First, to our knowledge, this is the largest cohort of PWH and HIV-negative individuals from the same health system prospectively followed with detailed cellular phenotyping and concurrent chart adjudication of incident diabetes cases. Second, we used two different definitions to define TEMRA cells given the multiple definitions in the literature. Using two definitions of TEMRA cells we found very similar results, which also suggests this was not a spurious finding. Finally, we were able to adjust for traditional risk factors, CMV serostatus, and inflammatory biomarkers in the model to assess the independent association of T cell subsets with risk of incident diabetes. However, this study did have several limitations. The VA healthcare system is largely male and may not be generalizable to other populations. Second, cellular phenotypes were analyzed on cryopreserved cells rather than fresh whole blood, which may influence the results. However, data on healthy individuals suggests this difference is small [43]. Third, CMV-negative PWH had higher HCV seropositivity and alcohol use compared with CMV-positive PWH for unclear reasons. While the model adjusted for both HCV serostatus and alcohol use, we cannot rule out an unrecognized confounder may exist that could affect our findings. Fourth, PWH had varying duration and stage of HIV infection, which may not be captured by a single flow cytometry measurement of T cell subsets at initiation of the study. Fifth, we do not have family history of diabetes or auto-antibody status available for this study, though this is unlikely to influence our conclusions. Finally, VACS-BC was not designed to investigate the potential biological role CD4+ TEMRA and CD4+CD28− T cells have in increasing the risk for diabetes and future studies will be needed to establish how these cells could mediate the risk of metabolic disease.
In summary, in a large cohort of PWH and HIV-negative individuals in the VACS-BC, we found that higher frequencies of baseline CD4+ TEMRA and CD28− T cells in PWH, but not HIV-negative individuals, were associated with risk of incident diabetes. These T cell subsets are highly inflammatory and have been previously linked to immune dysregulation, cardiovascular disease, and autoimmune diseases and may be an important risk factor for the development of metabolic disease in PWH. A better understanding of the role of adaptive immune cells in imparting risk of diabetes may lead to new prognostic test or therapeutic interventions.
Supplementary Material
Acknowledgements
Source of Funding
This work was supported by the National Institute on Alcohol Abuse and Alcoholism by COMpAAAS/Veterans Aging Cohort Study [grant numbers U24 AA020794, U01 AA020790, U01 AA020795, U01 AA020799, U10 AA013566]; the National Institute of Diabetes and Digestive and Kidney Diseases [R56 DK108352]; the National Heart Lung and Blood Institute [grant numbers R01 HL125032, K12 HL143956]; and the National Institute of Allergy and Infectious Diseases [grant numbers P30 AI110527, T32 AI00747426 to S.S.B.]. The funders had no role in the study design, data collection, and analyses, decision to publish, or preparation of the manuscript. Views presented in the manuscript are those of the authors and do not reflect those of the Department of Veterans Affairs, or the United States government.
Footnotes
Conflicts of Interest
The authors have no conflicts to declare.
References
- 1.Duncan AD, Goff LM, Peters BS. Type 2 diabetes prevalence and its risk factors in HIV: A cross-sectional study. PLoS One 2018; 13:e0194199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and Risk Factors for Prediabetes and Diabetes Mellitus Among HIV-infected Adults on Antiretroviral Therapy: A Systematic Review and Meta-analysis. Epidemiology 2018; 29:431–441. [DOI] [PubMed] [Google Scholar]
- 3.Medapalli RK, Parikh CR, Gordon K, Brown ST, Butt AA, Gibert CL, et al. Comorbid diabetes and the risk of progressive chronic kidney disease in HIV-infected adults: data from the Veterans Aging Cohort Study. J Acquir Immune Defic Syndr 2012; 60:393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friis-Moller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil 2010; 17:491–501. [DOI] [PubMed] [Google Scholar]
- 5.Putcharoen O, Wattanachanya L, Sophonphan J, Siwamogsatham S, Sapsirisavat V, Gatechompol S, et al. New-onset diabetes in HIV-treated adults: predictors, long-term renal and cardiovascular outcomes. AIDS 2017; 31:1535–1543. [DOI] [PubMed] [Google Scholar]
- 6.Betene ADC, De Wit S, Neuhaus J, Palfreeman A, Pepe R, Pankow JS, et al. Interleukin-6, high sensitivity C-reactive protein, and the development of type 2 diabetes among HIV-positive patients taking antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 67:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010; 33:2244–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuhaus J, Jacobs DR Jr., Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olson NC, Doyle MF, de Boer IH, Huber SA, Jenny NS, Kronmal RA, et al. Associations of Circulating Lymphocyte Subpopulations with Type 2 Diabetes: Cross-Sectional Results from the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One 2015; 10:e0139962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner NM, Brandhorst G, Czepluch F, Lankeit M, Eberle C, Herzberg S, et al. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity (Silver Spring) 2013; 21:461–468. [DOI] [PubMed] [Google Scholar]
- 11.Zeng C, Shi X, Zhang B, Liu H, Zhang L, Ding W, et al. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med (Berl) 2012; 90:175–186. [DOI] [PubMed] [Google Scholar]
- 12.Rattik S, Engelbertsen D, Wigren M, Ljungcrantz I, Ostling G, Persson M, et al. Elevated circulating effector memory T cells but similar levels of regulatory T cells in patients with type 2 diabetes mellitus and cardiovascular disease. Diab Vasc Dis Res 2019; 16:270–280. [DOI] [PubMed] [Google Scholar]
- 13.Olson NC, Doyle MF, Sitlani CM, de Boer IH, Rich SS, Huber SA, et al. Associations of Innate and Adaptive Immune Cell Subsets With Incident Type 2 Diabetes Risk: The MESA Study. J Clin Endocrinol Metab 2020; 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YH, Kim SR, Han DH, Yu HT, Han YD, Kim JH, et al. Senescent T Cells Predict the Development of Hyperglycemia in Humans. Diabetes 2019; 68:156–162. [DOI] [PubMed] [Google Scholar]
- 15.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol 2008; 214:231–241. [DOI] [PubMed] [Google Scholar]
- 16.Bofill M, Mocroft A, Lipman M, Medina E, Borthwick NJ, Sabin CA, et al. Increased numbers of primed activated CD8+CD38+CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1-infected patients. AIDS 1996; 10:827–834. [DOI] [PubMed] [Google Scholar]
- 17.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443:350–354. [DOI] [PubMed] [Google Scholar]
- 18.Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS 2008; 22:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS 2008; 22:2409–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis 2011; 203:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanjalla CN, McDonnell WJ, Barnett L, Simmons JD, Furch BD, Lima MC, et al. Adipose Tissue in Persons With HIV Is Enriched for CD4(+) T Effector Memory and T Effector Memory RA(+) Cells, Which Show Higher CD69 Expression and CD57, CX3CR1, GPR56 Co-expression With Increasing Glucose Intolerance. Front Immunol 2019; 10:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailin SS, McGinnis KA, McDonnell WJ, So-Armah K, Wellons M, Tracy RP, et al. T Lymphocyte Subsets Associated with Prevalent Diabetes in Veterans with and without HIV. J Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care 2006; 44:S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.So-Armah KA, Tate JP, Chang CH, Butt AA, Gerschenson M, Gibert CL, et al. Do Biomarkers of Inflammation, Monocyte Activation, and Altered Coagulation Explain Excess Mortality Between HIV Infected and Uninfected People? J Acquir Immune Defic Syndr 2016; 72:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGinnis KA, Justice AC, Bailin S, Wellons M, Freiberg M, Koethe JR. High concordance between chart review adjudication and electronic medical record data to identify prevalent and incident diabetes mellitus among persons with and without HIV. Pharmacoepidemiol Drug Saf 2020; 29:1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tracy RP, Doyle MF, Olson NC, Huber SA, Jenny NS, Sallam R, et al. T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2013; 2:e000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumitriu IE. The life (and death) of CD4+ CD28(null) T cells in inflammatory diseases. Immunology 2015; 146:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 2000; 101:2883–2888. [DOI] [PubMed] [Google Scholar]
- 29.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, et al. Characterization of CD4(+) CTLs ex vivo. J Immunol 2002; 168:5954–5958. [DOI] [PubMed] [Google Scholar]
- 30.Thewissen M, Somers V, Hellings N, Fraussen J, Damoiseaux J, Stinissen P. CD4+CD28null T cells in autoimmune disease: pathogenic features and decreased susceptibility to immunoregulation. J Immunol 2007; 179:6514–6523. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest 1996; 97:2027–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giubilato S, Liuzzo G, Brugaletta S, Pitocco D, Graziani F, Smaldone C, et al. Expansion of CD4+CD28null T-lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. Eur Heart J 2011; 32:1214–1226. [DOI] [PubMed] [Google Scholar]
- 33.Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol 2007; 81:7759–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Y, Babor M, Lane J, Schulten V, Patil VS, Seumois G, et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat Commun 2017; 8:1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truong KL, Schlickeiser S, Vogt K, Boes D, Stanko K, Appelt C, et al. Killer-like receptors and GPR56 progressive expression defines cytokine production of human CD4(+) memory T cells. Nat Commun 2019; 10:2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libri V, Azevedo RI, Jackson SE, Di Mitri D, Lachmann R, Fuhrmann S, et al. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+CD45RA+CD27+ T cells: the potential involvement of interleukin-7 in this process. Immunology 2011; 132:326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen B, Morris SR, Panigrahi S, Michaelson GM, Wyrick JM, Komissarov AA, et al. Cytomegalovirus Coinfection Is Associated with Increased Vascular-Homing CD57(+) CD4 T Cells in HIV Infection. J Immunol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pachnio A, Ciaurriz M, Begum J, Lal N, Zuo J, Beggs A, et al. Cytomegalovirus Infection Leads to Development of High Frequencies of Cytotoxic Virus-Specific CD4+ T Cells Targeted to Vascular Endothelium. PLoS Pathog 2016; 12:e1005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buzzetti R, Zampetti S, Maddaloni E. Adult-onset autoimmune diabetes: current knowledge and implications for management. Nat Rev Endocrinol 2017; 13:674–686. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Li W, Zhou J, Wan R, Hao W, Gao Y, et al. Autoantibody-positivity in lean type II diabetes patients was associated with elevated Th17-like CD4(+)CXCR5(+) T cell responses. Mol Immunol 2019; 112:305–311. [DOI] [PubMed] [Google Scholar]
- 41.Wanjalla CN, McDonnell WJ, Ram R, Chopra A, Gangula R, Leary S, et al. Single-cell analysis shows that adipose tissue of persons with both HIV and diabetes is enriched for clonal, cytotoxic, and CMV-specific CD4+ T cells. Cell Rep Med 2021; 2:100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takarabe D, Rokukawa Y, Takahashi Y, Goto A, Takaichi M, Okamoto M, et al. Autoimmune diabetes in HIV-infected patients on highly active antiretroviral therapy. J Clin Endocrinol Metab 2010; 95:4056–4060. [DOI] [PubMed] [Google Scholar]
- 43.Thyagarajan B, Barcelo H, Crimmins E, Weir D, Minnerath S, Vivek S, et al. Effect of delayed cell processing and cryopreservation on immunophenotyping in multicenter population studies. J Immunol Methods 2018; 463:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


