Abstract
Nowadays, the importance of detection of myositis-specific antibodies (MSA) and myositis-associated antibodies (MAA) in diagnosis and in delineating disease subsets of idiopathic inflammatory myopathy (IIM) is highly acknowledged by IIM experts. Consequently, MSA/MAA are increasingly integrated in expert-based myositis (sub)classification criteria as well as in routine diagnostics. In contrast, MSA/MAA are under-represented in data-based (sub)classification criteria, mostly related to the lack of sufficient data on the wide spectrum of MSA/MAA in large multicenter cohorts. Unfortunately, the current commercially available assays to detect MSA/MAA show variable analytical and clinical performance characteristics. This challenges the design of prospective multicenter studies on MSA/MAA as well as the optimization of their routine clinical use. Additional validation studies and continuous harmonization initiatives on MSA/MAA detection from the pre-analytical to the post-analytical phase (e.g. from defining request criteria to guidelines for reporting), will be needed to overcome these hurdles. To speed up this process, we encourage close collaborations between IIM clinical experts, laboratory professionals and diagnostic companies.
Keywords: Idiopathic inflammatory myopathy, Classification, Autoantibodies, Validation, Harmonization
1. Introduction
The idiopathic inflammatory myopathies (IIM) are a heterogeneous group of rare acquired immune-mediated muscle diseases, with distinct clinical, serological and histological features [1]. In addition to muscle inflammation, patients may also present with extra-muscular manifestations involving the skin, lungs, heart and joints [2].
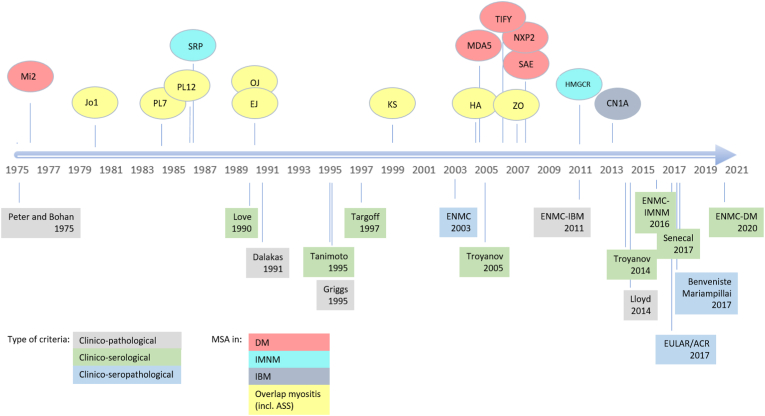
For many years, the IIM have been subdivided in three main subgroups, namely polymyositis (PM), dermatomyositis (DM), including juvenile dermatomyositis (jDM), and inclusion body myositis (IBM) [[3], [4], [5]]. The last decade, the landscape of IIM underwent a total make over, largely due to the discovery of several new myositis-specific and myositis-associated antibodies (respectively MSA and MAA). MSA are a well-defined group of autoantibodies present in >50% of IIM, which are generally considered highly disease-specific and mutually exclusive (reviewed in Ref. [1]). An overview of the antibodies denominated as MSA in relation to their discovery and proposed disease criteria is provided in Fig. 1. MSA are associated with specific clinical features in the IIM spectrum and enable identification of subsets of patients with specific phenotypes of skin, muscle and lung disease and malignancy [1,8]. In contrast, MAA are less disease-specific, as they are also found in other systemic autoimmune rheumatic diseases (SARD) and often associated with overlap disease [9,10]. The MAA group includes anti-PM/Scl (75 and 100), anti-U1RNP, anti-Ro52/TRIM21 and anti-Ku. Some authors also categorize anti-La/SSB and anti-Ro60/SSA as MAA [1,11]. Controversies exist on categorizing anti-CN1A as MSA or MAA, as anti-CN1A are also found in systemic lupus erythematosus and Sjögren's syndrome, we considered anti-CN1A as MAA. Frequencies of MAA in (overlap) IIM ranges from <1% for anti-Ku to >25% for anti-Ro52/TRIM21 (reviewed in Ref. [1]). Expansion of the characterized MSA/MAA together with the development of easy accessible detection methods for these antibodies, has led to better understanding, diagnosis, classification and treatment of IIM [12].
Fig. 1.
Proposed criteria for IIM in relation to MSA discovery (adapted from Ref. [13]). MSA were abbreviated by their target antigen. Subclassification of the MSA in relation to clinical subtype according to Ref. [14]. There is no consensus on whether anti-CN1A should be considered an MSA or an MSA. DM: dermatomyositis; IMNM: immune mediated necrotizing myopathy; IBM: inclusion body myositis; ASS: anti-synthetase syndrome; EULAR/ACR: European League Against Rheumatism and American College of Rheumatology; ENMC: European Neuromuscular Centre.
In this review we first provide a historical overview of the diagnostic/(sub)classification criteria of IIM with focus on the entry of MSA/MAA in these criteria, including a summary on the scientific background supporting their inclusion. Next, we elaborate on the reliability of the different methods for MSA/MAA detection and the challenges related to this in the context of designing studies/defining future disease criteria and daily routine application. Finally, we discuss the integration of these tests in current laboratory practice as well as opportunities for further improvement of the clinical utility of these tests.
2. Autoantibodies in disease criteria: an historical overview until 2016
In general, classification criteria are meant to define homogeneous disease groups to allow for optimal research possibilities and in the long term for better understanding of the underlying pathogenesis, improved management and outcome of the disease. Table 1 gives a historical overview of the proposed criteria for IIM with MSA included, some focussing on ‘defining’ the disease subset, while other serving subclassification.
Table 1.
Overview of the proposed criteria for idiopathic inflammatory myopathy with myositis-specific antibodies (MSA) included.
| Criteria Year Type |
Role of the MSA in the critera | Number and identity of the MSA included |
Basis for MSA inclusion |
|---|---|---|---|
| Love et al. [20] 1990 Clinico-serological |
No ‘inclusion’ role, rol in subclassification Specific MSA were linked to clinical subtype, demographic characteristics, HLA type and prognosis as defined by survival |
Anti-SRP Anti-Mi2 Anti-ARS |
Data-driven (n = 212) Subgroups included: PM, DM, IBM, CAM and myositis overlap |
| Tanimoto et al. [21] 1995 Clinico-serological |
Diagnostic criterion (1 out of 9) |
Anti-Jo1 (Anti-Ku, anti-U1RNP, anti-SSA also analyzed but not retained) |
Data-driven (n = 341 PM/DM; n = 381 controls [SSc, SLE, neuromuscular diseases]) Retrospective questionnaire-based study amongst rheumatologist, dermatologists, neurologists |
| Targoff et al. [22] 1997 Clinico-serological |
Diagnostic criterion (1 out of 6) Net effect of the addition of MSA is that diagnosis of definite PM without muscle biopsie |
Anti-SRP Anti-Mi2 Anti-ARS |
Expert-based/literature-driven Modification of the Peter and Bohan (PM/DM) criteria with inclusion of the newer diagnostic modalities (MSA and MRI) |
| ENMC IIM [32] 2003 Clinico-(sero) pathological |
Inclusion criterion, not for subclassification Focus was on immunohistological patterns (required biopsy in all cases) |
MSA only limitedly represented and not specified |
Expert-based consensus guideline Subgroups included: IBM, PM, DM, ADM, possible DM sine dermatitis, non-specific myositis and IMNM |
| Troyanov et al. [18] 2005 Clinico-serological |
Core of criteria = clinical overlap features (no histology) Presence of clinical overlap features and/or ‘overlap Ab’ define overlap myositis (exclude PM/DM) Exception: anti-Mi2 defines DM Classification was shown to predict response to immunosuppression and IIM course |
‘Overlap Ab’ = MSA/MAA and SSc-Ab MSA: ARS, anti-Mi2, -SRP MAA: anti-PM/Scl, -U1-RNP, -Ku SSc-Ab: anti-CENP-B, -Scl70, -Th/To, -RNA polymerase III |
Data-supported (n = 100 IIM) Subgroups: Overlap myositis, DM, PM |
| Troyanov et al. [24] 2014 Clinico-serological |
MSA used for subdifferentiation of DM Presence of DM-specific antibodies/absence of ‘overlap Ab’ define pure DM |
DM-specific MSA (anti-Mi2, anti-TIF1γ, anti-NXP2) |
Data-supported (n = 100 IIM, including 44 DM) Subgroups included: pure DM and overlap myositis with DM features |
| Senecal et al. [25] 2017 Clinico-serological |
Classification/subdifferentiation in more subsets (IBM/IMNM) |
Antibodies of Troyanov (2014) expanded with: MSA: anti-MDA5, -HMGCR DM-specific MSA: anti-SAE SSc-Ab: anti-fibrillarin |
Expert-based expansion of the Troyanov 2014 criteria supplemented with more Ab/more subsets Subgroups: Overlap myositis, DM, PM, IBM, IMNM |
| Benveniste et al. [14] Mariampillai et al. [17] 2016 Clinico-seropathological |
MSA/MAA are used to create 4 subgroups of IIM |
MAA: ARS, anti-PM/Scl, -Ku, -U1RNP, - Ro52 DM-specific MSA: Anti-Mi2, -TIF1γ, -NXP2, - SAE, -NXP2, -MDA5 IMNM MSA: anti-SRP, -HMGCR IBM MSA: anti-CN1A |
Initially expert-based/literature-based but later confirmed on data Subgroups included: overlap myositis (incl. ASS), DM, IMNM, IBM |
| EULAR [26] 2017 Clinico-(sero)pathological |
MSA is an inclusion criterion in the score system (1 out of 16) MSA assigned the highest score points |
Anti-Jo-1 |
Data-driven (976 IIM and 624 non-IM cases in an international multicenter study (42 centers)) Subgroups included: PM, IBM, (A)DM, (J)DM |
| ENMC-IMNM [16] 2016 Clinico-serological |
MSA is a diagnostic and subclassification criterion 3 subgroups within IMNM based on MSA (incl. Ab negative IMNM) Role of muscle biopsy is optional in case of anti-SRP/HMGCR detection, but remains necessary in case Ab negativity |
Anti-HMGCR Anti-SRP |
Expert-based consensus guideline |
| ENMC-DM [33] 2020 Clinico-serological |
MSA is a diagnostic and subclassification criterion 6 subgroups of DM based on the MSA (incl. Ab negative DM) In case of ARS, anti-SRP, anti-HMGCR: Dx of anti-synthetase syndrome/IMNM ‘with DM-like rash’ |
Anti-Mi2 Anti-NXP2 Anti-MDA5 Anti-SAE Anti-TIF1γ |
Expert-based consensus guideline |
PM: polymyositis; (A/J)DM: (amyopathic/juvenile) dermatomyositis; IBM: inclusion body myositis; CAM: cander-associated myositis; IMNM: immune-mediated necrotizing myopathy; SSc(-Ab): systemic-sclerosis (associated antibodies), SLE: systemic lupus erytomathosus; ARS: anti-aminoacyl-tRNAs.
In 1975, Peter and Bohan introduced a first set of criteria for both diagnosis and classification of IIM in PM/DM [3,4], which were widely adopted and therefore enabled tremendous evolution within the field. These earliest criteria were based on clinical (symmetric proximal muscle weakness, usually progressive) and histopathological features (evidence of inflammation on muscle biopsy) [3,4]. Technical investigations included electromyography and determination of the muscle enzymes (e.g. creatine kinase [CK], lactate dehydrogenase [LDH], aldolase, transaminases). The presence of classical DM skin rashes differentiated between DM and PM [3,4]. IBM was later incorporated in the Dalakas diagnostic criteria of 1991, which were in fact an expert-opinion based fine-tuning of the Peter and Bohan criteria [5]. More widely used clinico-pathological expert-based criteria for IBM are the Griggs criteria as proposed in 1995 [6], which were later updated by the use of unbiased machine learning approaches on patient data [7]. Of note, Dalakas already anticipated on the potential role of autoantibody detection in diagnosis, clinical description and immunopathogenesis of IIM, but this idea was denominated as yet premature [5].
Next, the classification of IIM evolved from a clinico-pathological classification with limited subgroups, towards a clinico-seropathological classification, with additional distinct subgroups [[15], [16], [17], [18], [19]]. This evolution was mainly driven by the booming discovery of new autoantibodies associated with IIM and particular IIM subtypes (Fig. 1). The first MSA-based IIM classification was published by Love et al. in 1990 [20]. Their proposal focussed not on serving a diagnostic role but on an adjunct subclassification of IIM patients based on their MSA, and included the major antibodies characterized at that moment: anti-Mi2, anti-SRP, anti-synthetase antibodies (ASA, including anti-Jo1, -PL12, -PL7, -OJ). This group also described clinical features associated with each MSA subgroup, including the association of the anti-synthetase antibodies with interstitial lung disease (ILD), fever, arthritis, mechanic's hands and myositis (today referred to as the “anti-synthetase syndrome”[ASS]) and the association of anti-Mi2 with a classical DM with typical skin rash and good response to immunosuppressive therapy. In the nineties, two other groups proposed IIM classifications with MSA included [21,22]. In both proposals, MSA positivity was not intended for subclassification but rather served as one of the diagnostic criteria for use in epidemiological studies within a total set of nine and six criteria in the respective studies. Of note, these classifications included only a very limited set of the MSA because laboratories did not test for all specificities known at that time [21,22].
The next important steps towards a clinico-serological classification were the proposals of Troyanov and colleagues [[23], [24], [25]]. Their proposals did not take into account histopathological features but considered clinical overlap features as the core of their classification. Additionally, they used a large set of autoantibodies to distinguish pure DM, overlap myositis with DM features, overlap myositis, IMNM, IBM and PM. According to their definition, the presence of myositis in combination with ‘overlap antibodies’ (anti-CENP-B, -Scl70, -Th/To, -RNA polymerase III, -PM/Scl, -U1RNP, -Ku, ASA, -SRP and -HMGCR) and/or ‘overlap clinical features’ was sufficient to define ‘overlap myositis’ and exclude PM and DM. The presence of DM-specific antibodies (anti-Mi2, -NXP2, -SAE, -TIF1γ, and -MDA5) defined DM. Around the same period another clinico-seropathological approach was proposed by Benveniste and colleagues [14,17]. They retained 4 subgroups in IIM defined by autoantibody presence: overlap myositis was associated with ASA or systemic sclerosis-associated antibodies (anti-PM/Scl, anti-Ku, anti-U1RNP), DM was associated with anti-Mi2, anti-SAE, anti-NXP2, anti-TIF1γ and anti-MDA5, IMNM was associated with anti-HMGCR and anti-SRP and IBM was associated with anti-CN1A. In their view, which is in line with the idea of Troyanov and colleagues, the ‘classic’ PM group is an overdiagnosed entity and should be abandoned as most of these patients can be reclassified in ASS, IMNM or IBM if the autoantibodies are taken into account [14].
3. Scientific basis for inclusion of MSA in the disease criteria and their role in classification since 2017
Today, the clinical utility of MSA detection, and to a lesser extend also MAA detection, is highly acknowledged by IIM experts. Nevertheless, before 2017 the inclusion of the autoantibodies in the disease criteria were mostly derived empirically and were not supported by large international studies (Table 1). In 2017, the European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) jointly presented new EULAR/ACR classification criteria for adult and juvenile IIM in which they distinguish 4 groups: PM, (J)DM, IBM and amyopathic DM. The subgroup of PM patients includes patients with IMNM [26]. A set of 93 candidate variables for analysis in IIM cases and comparators were first identified from published criteria and further fine-tuned based on data availability and expert opinion using standard consensus methodology. In parallel, a glossary and definition list was developed for each of the variables. This candidate list also included an extensive list of autoantibodies including antinuclear antibodies (ANA), 5 MSA (anti-Jo1, -Mi2, -SRP, -PL7, -PL12), 4 MAA (anti-Ku, -PM/Scl, -Ro52/TRIM21, -U1RNP) and 6 other anti-extractable nuclear antigens (anti-ENA)(anti-Ro60/SSA, -La/SSB,-CENP-B, -Scl70, -Ribo-P and -Sm). Of note, the glossary and definition list did not specify details on the antibody detection method. The association of each candidate variable with the diagnosis (IIM, non-IIM) was assessed by odds ratios and tested with the Fisher's exact test on a set of 976 IIM and 624 comparator (non-IIM) cases. Final variables were selected by applying three statistical approaches independently of the other: (1) a probability score, (2) a sum of items and (3) a classification tree. The items that finally emerged as potentially relevant for the prospective criteria were closely examined for statistical performance, clinical relevance and practicability. Statistical performance was measured by classification accuracy and area under the receiver operating characteristic curve (AUC ROC). This finally resulted in a set of 16 variables from six categories which were assigned a weight (score) by the use of a probability score approach in which score values of the candidate items were estimated by multivariate logistic regression. These new criteria were then internally cross-validated on 200 bootstrap samples and validated in independent cohorts. In this final list of variables only 1 autoantibody (anti-Jo1) was retained, but compared to the other variables assigned the highest score points. Whether the reasons for not retaining the other MSA/MAA from the candidate variables list were attributed to the lack of sufficient data for these autoantibodies or to inferior discriminative performance was not clear in the initial publication [26], but was later partially clarified in subsequent communications mentioning that variables with less than 800 valid observations were not considered [27,28]. Anti-Jo1 (n = 1062) reached this criterion, but this was not the case for most of the other MSA/MAA (all less than 350 valid observations) reflecting the limited availability of the detection methods for MSA/MAA at the start of the study [28]. In contrast, acceptable numbers of valid observations were observed for ANA, anti-Ro60/SSA, anti-La/SSB, anti-U1RNP, anti-Sm, suggesting that these autoantibodies were not retained based on inferior discriminative performances [27]. However, this was not explicitly stated in the results. The only additional information about the autoantibodies in the EULAR/ACR classification criteria for IIM includes that anti-Jo1 should be performed with a standardized and validated test showing positive results.
In the meanwhile, several independent validation studies on the EULAR/ACR criteria for IIM have been published, some of them also further elaborating on the potential of the MAA/MSA [[29], [30], [31]]. In the study of Luu et al. the impact of the addition of an extended myositis antibody panel (MAA/MSA), as detected by lineblot, to the criteria was evaluated [30]. They concluded that adding the non-anti-Jo1 MSA (no data on the level of individual MSA reported) as covariate could possibly improve the accuracy of determining the probability of IIM diagnoses [30].Casal-Dominguez and colleagues applied the EULAR/ACR criteria on a large set of 524 MSA-positive myositis patients, and observed that 91% of them were correctly classified [31]. However, certain MSA-defined subgroups, including those with autoantibodies against HMGCR, SRP, and PL7, were frequently misclassified as non-IIM (20% of anti-HMGCR and 50% of anti-PL7) or as IBM (∼10% of anti-SRP and anti-HMGCR patients). Moreover, they observed that in MSA-positive patients, autoantibodies outperform the EULAR/ACR-defined subgroups to predict clinical phenotypes, underscoring the need to include MSA in a revised myositis classification scheme [31].
The advancement in knowledge of MSA and their detection methods were also one of the major drivers for the European Neuromuscular Centre (ENMC) experts to revise their mainly clinico-pathological oriented expert-based classification of IIM as was published in 2004 [32]. A clinico-seropathological oriented update was published for IMNM and DM [16,33]. The clinico-pathological ENMC IBM criteria were published before characterization of anti-CN1A, and therefore do not elaborate on the role of these antibodies [34]. For IMNM, the ENMC agreed upon three subgroups (anti-SRP myopathy, anti-HMGCR myopathy and antibody negative IMNM) based on elevated serum CK levels, proximal muscle weakness, and the presence of anti-SRP and anti-HMGCR autoantibodies. Muscle biopsy was only needed in case of negative MSA findings; in the antibody positive subsets muscle biopsy, electromyography (EMG) and magnetic resonance imaging were considered optional for further characterization of the patients. Similarly for DM, the ENMC updated their criteria in 2020 [33]. According to their proposal, a diagnosis of DM can be made based on cutaneous DM features in combination with: (a) interface dermatitis on skin biopsy or (b) muscle features (proximal muscle weakness, elevated CK, DM-specific muscle biopsy features) or (c) presence of DM-specific MSA (anti-Mi2, anti-NXP2, anti-MDA5, anti-SAE, anti-TIF1γ). Thus, the presence of DM-specific antibodies excludes the need for a skin/muscle biopsy. DM patients with MSA are subclassified according to their antibody; DM without MSA are classified as autoantibody negative DM. When other MSA such as ASA, anti-SRP or anti-HMGCR are detected in patients with a DM-like rash, these antibodies overrule the classification of DM, and patients should be classified as ASS or IMNM with DM-like features, respectively.
4. Reliability of the different methods and/or assays
Next to CK levels, EMG and muscle biopsy, MSA/MAA detection plays already an important role in clinical practice in diagnosis as well as subclassification of IIM. Moreover, MSA/MAA analysis have the potential to overrule ‘classic’ criteria of IIM diagnosis such as biopsy. This expanding role obviously demands reliable and routinely applicable MSA/MAA assays. Conventional techniques, such as protein- and RNA immunoprecipitation (P-IP, RNA-IP), western blotting (WB), and double immunodiffusion (DID) have historically been used to identify and characterize the MSA/MAA. At present, however, these conventional techniques are confined to research laboratories as they require a high level of expertise, are labour-intensive and take weeks to complete. Today, there is an expanding list of commercially available options (mostly lineblots and dotblots) for multiparameter MSA/MAA detection, which represent easy accessible alternatives for the above-mentioned conventional techniques. Moreover, novel technologies are currently being validated (e.g. particle-based multi-analyte technology [PMAT]) [35]. These assays are, however, not standardized or even harmonized. They use various techniques and antigen sources, and represent important challenges for validation/verification, especially in a rare disease context with low and diverse autoantibodies. For an extensive review on these challenges related to validation/verification of MSA/MAA tests see Ref. [12].
A large number of studies evaluating commercial platforms for MSA/MAA detection are already published. Here we discuss a selection of them to illustrate the current challenges related to the application of these assays in clinical routine practice. Despite that MSA were originally characterized as highly disease-specific and specifically linked to certain clinical subsets of the disease, this does not seem to hold for all antibodies and for all platforms. This is especially the case if data were generated on more generalized cohorts (such as healthy controls or consecutive routine cohorts) instead of delineated control cohorts of SARD patients. At least three studies documented that line/dot blots suffer from limited specificity [[36], [37], [38]]. Tansley et al. reported that 16% and 9.7% of healthy controls tested positive for MSA on lineblot and dotblot, respectively [36]. For lineblot, false positive results were generally low titer and false positive samples on both line/dot blot more often showed multiple autoantibody positivity [36]. Vulsteke et al. evaluated 2 lineblots and 1 dotblot and found differences in specificity between manufacturers and between individual antibodies [37]. MSA in healthy controls were detected in 12.5% by lineblot and in 2.5% by dotblot, with individual frequencies of the antibodies ranging from 0 to 9.2% [37]. Bundell et al. reported MSA/MMA reactivity in 22% of healthy controls (9% MSA, 14% MAA), and based on their results they suggested the use of locally-established reference ranges based on the 99th percentile of healthy individuals [38]. In contrast, not all studies using lineblot seem to confirm these observations. Espinosa–Ortega et al. reported a specificity of 99.7%. However, detailed analysis of their report shows that MSA were found in 3 out of a set of 60 healthy controls, for which we calculate a corresponding specificity of 95% instead of 99,7% [39]. This clearly illustrated that specificity calculations differ over studies (some probably taking the number of MSA measurements as the denominator instead of the number of samples) [39]. Specificity issues were also documented in consecutive routine cohorts with diagnostic workup in the context of IIM suspicion, with the study of Piette et al. as an example [40]. MSA (as detected by a combination of two assays) were observed in 7.9% of patients that were finally categorized as not having the disease (combined specificity 92%, lineblot specificity 97%, dotblot specificity 95%) [40]. The frequency of MSA/MAA as detected by lineblot documented in SARD control groups ranges between 4% and 8% [41,42]. For PMAT, 6% positivity for at least one MSA (mostly low levels) was observed in the control group consisting of both diseased controls and healthy donors (n = 200) [35].
Another point of concern is the variability observed between commercial assays. Piette et al. documented that in a combined consecutive routine/diseased control cohort (n=214) with clinical data available 22 out of a total of 36 MSA positive results could not be confirmed with an alternative assay [40]. Discrepancies were seen concerning both the novel autoantibodies, as well as the established autoantibodies, with discrepancies most apparent for anti-TIF1γ (ĸ = −0.021), anti-SRP (ĸ = −0.006) and anti-SAE (ĸ = 0.395). Differences between assays were mostly observed in non-IIM patients, in whom also significantly lower blot signal intensities were found compared to IIM patients (p = 0.0013) [40]. Nevertheless, differences in reactivities between manufacturers/methods were also observed in IIM patients, with the most pronounced differences documented for anti-TIF1γ [37,40,43].
Concerns were also raised on the concordance of commercial platforms with the conventional techniques. Historically, IP was used to identify and characterize MSA/MAA in relation to clinical observations, and therefore often considered the reference method. Accordingly, it is important that commercial assays are compared with IP. In the study of Ghirardello and colleagues, a general concordance rate of 91% was reported between IP and lineblot [42]. Others documented that the agreement between IP and commercial assays is dependent on the commercial method as well as the antibody [36,44,45], but the results in these studies did not identify the same ‘problem’ antibodies. In the study of Cavazzana comparing IP with PMAT and lineblot, with focus on the antibodies to EJ, SRP, Jo1, NXP2, MDA5, TIF1γ, and Mi2, the PMAT assay showed a slightly better overall correlation with IP (ROC with IP used as reference: PMAT AUC = 0.83, 95% confidence interval [CI] 0.70–0.95 vs. lineblot AUC = 0.70, 95% CI 0.56–0.84), while kappa agreement was strongly dependent on the type of autoantibody [44]. For example, for anti-TIF1γ a good agreement between IP and lineblot, and between IP and PMAT was documented in the studies of Cavazzana and colleagues, while in the more recent study of Tansley, in which only IP-positive samples were included, both lineblot and dotblot were found unreliable for detecting anti-TIF1γ [36,44,45]. Low sensitivity for anti-TIF1γ on lineblot compared to IP was also reported by Espinosa-Ortega and colleagues [39]. For some antibodies, for example anti-OJ, the commercial assays are considered not suitable, a shortcoming probably linked to the presence of conformational epitopes [36,46]. It should be noted, however, that also IP may have shortcomings and these assays are not harmonized. Indeed, some workers used K562 cells while others used HeLa cells and it is not unlikely that autoantigen configurations in the extracts vary between cell lines as well as culture conditions [47]. Some IP studies even use cells that have been transfected with the target antigen in order to increase sensitivity [48]. Some autoantibodies are also indistinguishable on P-IP due to identical molecular weight, and IP should therefore be supplemented with other techniques such as ELISA or western blotting, e.g. anti-MDA5 and NXP2 antibodies both having a molecular weight of 140kD [39]. Moreover, although IP is often considered the gold standard, differences between IP and the commercial test do not necessarily reflect superiority of IP and definition of the ‘correct’ assay might be influenced by the patient population as well as the clinical association that was selected to validate the assay (e.g. diagnosis of DM, or link with malignancy). Within this context, it must also be mentioned that in most studies using IP (both in the historical studies as well the more recent comparative studies) no or only very few disease controls were included. Therefore, information on the trade-off between sensitivity and specificity for IP is scarce. Further studies in large numbers of patients with careful clinical characterization as well as diseased controls analyzed on both commercial test as well as IP are necessary to answer this question on eventual superiority. An advantage of IP compared to solid phase assays is that IP detects antibodies that react to conformational epitopes.
Finally, users of the commercial platforms must be aware that differences may occur in the antigens included in the assays, and that none of the current commercially available tests contain the whole spectrum of established MSA/MAA. For example, some assays contain anti-OJ, anti-CN1A and/or anti-HMGCR, while not being present on other platforms. Geographical differences in antigen selection within one commercial method may also occur, probably related to commercial patent limitations. Moreover, some assays contain variants on a particular antigen, either alone or in combination. This is for instance the case for anti-Mi2 (anti-Mi2α and anti-Mi2β), anti-SAE (anti-SAE1 and anti-SAE2), and anti-PM/Scl (anti-PM/Scl75 and anti-PM/Scl100), potentially impacting the results. The reasons for targeting variants on these particular antigens are in fact not very clear and published data on the added value are scarce. Contradictory results have been published on the added value of detecting both anti-Mi2α and anti-Mi2β [49,50] In a recent study comparing IP vs. lineblot detecting both anti-Mi2α and anti-Mi2β, it was shown that the diagnostic contribution of combined reactivity (positive predictive value with IP as a reference was 96%) was higher than the contribution of isolated reactivity. Isolated positivity for just anti-Mi2β was more frequently false positive (positive predictive value of 7%) compared to isolated positivity for anti-Mi2α (positive predictive value of 62%) [49]. In contrast, Richards and colleagues concluded that the detection of anti-Mi2β as detected by PMAT was sufficient, as all anti-Mi2α positive patients also reacted with Mi2β (while some of them were negative on lineblot) [50]. To our knowledge no studies on the importance of differentiating anti-SAE1/SAE2 are available. For PM/Scl antibodies, the diagnostic contribution of subdifferentiation was mainly studied in systemic sclerosis patients [[51], [52], [53]]. For IIM, a lower specificity for anti-PM/Scl75 compared to anti-PM/Scl100 (94.6%–95.4% vs. 96.7%–97%) was documented [37,54]. Larger studies are needed to evaluate whether subdifferentiation on these particular antibodies has added value in significantly improving diagnostic performance and/or allowing further relevant clinical subdifferentiation. Some authors also reported on multiple reactivities on lineblot despite MSA being principally mutually exclusive [45,55,56] and on temperature-dependent variability of the results [41].
From the above overview of the limitations of the commercial assays, it must be clear that the application of these multiparameter assays in routine care as well as in multicenter studies is not without challenges. Obviously, assay variability impacts diagnostic and prognostic classification, and might even add an extra layer of complexity in the challenging diagnosis and classification of a heterogeneous disease such as IIM. In this context, the concerns raised tempt the design of retrospective studies on large datasets missing details on the MSA/MAA detection method (e.g. for defining future classification/disease criteria). Moreover, even if this information is readily available this may not overcome the hurdles as it will be provocative how to handle the variability. For future prospective studies variability of assays should be taken into account and anticipated upon already when designing the study protocol. Thereby, close collaborations between IIM clinical experts, laboratory professionals and diagnostic companies will be of utmost importance.
Some authors already suggested minimal actions to improve the quality of the assays such as the integration of quality control procedures (e.g. participation in external quality control programs and the inclusion of internal quality control material) [57,58], adapting the cut-off values [38,45,57,59] and correlating with the IFA pattern [40,59]. Automated reading of the line/dot blot is also of utmost importance to improve reproducibility. Moreover, it must be mentioned that differences depending on wet versus dry reading of the lineblot strips may occur [57]. Clearly, these suggestions may represent a relevant step toward harmonization of MSA/MAA detection. However, none of these suggestions have been proven all-encompassing to solve the issues raised. For instance, generally increasing the cut-offs to only regard samples with moderate and high reactivity as positive will also affect individual antibody specificities differently with the risk of missing clinically significant cases such as patients presenting with amyopathic dermatomyositis and rapidly progressive ILD with low level anti-MDA5 [57].
5. Position of MSA/MAA detection in reflex testing of the clinical laboratory
The last decades, autoantibody requests in general have increased tremendously in the clinical routine lab. From being tests requested only by SARD/IIM-experts, we are now faced with a wide spectrum of requesters. This clearly impacts overall pre-test probability of having SARD/IIM, which implies in a context of a rare disease with low antibody frequency a high chance of having false positive results (which is even more challenging for antibodies with specificity issues). In the clinical laboratory pre-test probability might be improved by limiting testing to patients fulfilling a set of predefined symptoms (e.g. DM-specific MSA on patients with a DM-like skin rash only) or alternatively by elaborating the autoantibodies by the use of testing algorithms. Of note, the appropriateness of requesting MSA/MAA testing (and potential solutions) was recently addressed in an ENMC workshop (8–10th October 2021), and an expert-based guideline on this topic amongst others is currently being prepared for publication. Regarding selective analysis based on symptoms, routine clinical laboratories are often blinded from this information and expert-based recommendations on this topic are currently lacking for IIM. In contrast, testing algorithms for autoantibody testing are widely applied. In general most laboratories screen for ANA by indirect immunofluorescence (IIF) on HEp-2 cells [60]. This technique offers a first clue to the presence of autoantibodies directed against various nuclear and/or cytoplasmic antigens [64]. Additionally, IIF can provide useful information on the immunofluorescence pattern and intensity [60]. Certain immunofluorescence patterns are predictive for the presence of disease specific autoantibodies (e.g. a centromere pattern for anti-centromere antibodies in systemic sclerosis, and a fine speckled cytoplasmic pattern for ASA in ASS) [[60], [61], [62], [63], [64], [65], [66]]. In case of positive HEp-2 IIF, it is recommended to perform specific solid phase immunoassay tests for anti-ENA [62,67,68], whether or not with an intermediate pooled anti-ENA screening test, creating a three step cascade testing algorithm [69]. Despite still being regarded as the best screening test for most SARD, HEp-2(000) IIF is, however, not suited for MSA screening as the technique lack sensitivity for some of the MSA (e.g. for ASA, anti-MDA5 and anti-HMGCR) [12,62,[70], [71], [72]]. Furthermore, since antibodies against cytoplasmic antigens are by definition not anti-nuclear, there is no strict consensus on reporting non-nuclear patterns as a negative or positive test result [60,73,74]. Of note, in case screening for ANA is done using a solid phase assay with pooled antigens one should be aware that most of the current tests contain only a very limited set of MSA/MAA (mostly only anti-Jo1, in some assays also anti-Mi2). Consequently, in case of suspicion of IIM, neither HEp-2 IIF nor solid phase ANA screenings assays with pooled antigens represent a good screening option, implying that even in case of negative HEp-2 IIF results MSA/MAA testing should be performed. However, the above arguments to overrule negative HEp-2 IIF with adequate reflex testing, does not imply that IIF is of no importance in IIM. In some studies finding a pattern on HEp-2 compatible with the MSA/MAA result on the specific assay improved specificity, suggesting that HEp-IIF can be used as a kind of confirmation test [40,66,75]. Moreover, IIM frequently overlaps with other SARD, most often but not limited to systemic sclerosis, where HEp-2 IIF remains the screening method of choice [70].
In addition to validation of the assays, standardization/harmonization of the MSA/MAA test has been suggested the ideal approach to improve clinical utility of the assays. Indeed, the EULAR/ACR criteria for IIM state that anti-Jo1 detection should be performed by a ‘standardized and validated’ method [26]. The challenge of validation is addressed in the above paragraphs. The aim of standardization and harmonization of MSA/MAA testing is to provide accurate and comparable test results for an individual patient at any time, any place [77]. The process of standardization and harmonization should cover all phases of a diagnostic test: pre-analytical (request and sample collection), analytical and post-analytical (e.g. reporting). Standardization in autoimmune diagnostic is challenging and one may debate whether standardization will ever be possible [74]. Indeed, the possibility to achieve standardization depends on the availability of a standard reagent that can be used for calibration of assays and eventually reveals uniform test results independent from the type of assay used (reviewed in Refs. [[77], [79]]). For anti-Jo1, a reference reagent is available from the CDC [80]. However, this reagent, developed in the nineties, was intended to verify qualitative performance of the techniques used at that time (immunodiffusion, western blotting) [80]. Certified standard material/calibrators with documented commutability for anti-Jo1 are currently lacking and, therefore, the use of a standardized test is an utopia. Harmonization, seems to be the best alternative.
6. Conclusion
In conclusion, today the importance of MSA detection, and to a lesser extend also MAA detection, is highly acknowledged by IIM experts. As a consequence, MSA/MAA play a key role in expert-based (sub)classification criteria. In addition, the integrated MSA even have the potential to overrule ‘classic’ criteria of IIM diagnosis such as biopsy in IMNM and DM. The role of biopsy in diagnosis/subclassification of IIM seems to hold only for categorization of autoantibody negative IIM and IBM, and in support of new discoveries on the pathophysiology. In contrast, under-representation of MSA/MAA in recent data-based (sub)classification criteria is related to the lack of sufficient data on the wide spectrum of MSA in a large multicenter cohort (as the advancement in MSA knowledge and detection was only very high the last decade). Continuous efforts of the different partners involved (e.g. clinical experts, laboratory medicine specialists and diagnostic companies) are needed to generate these data.
At first sight, the availability of easy-to-use multiparameter test platforms seems to have paved the way to organize these studies. However, both retrospective as well as prospective studies to define criteria with MSA/MAA included will be challenged by the variability in commercial platforms as well as by the discrepancy with the conventional techniques. To address these issues we encourage additional initiatives for harmonization of the commercial assays as well as further prospective validation studies of the commercial platforms on large cohorts. These cohorts include, ideally, both well-characterized disease cohorts (to cover disease heterogeneity as well as low MSA/MAA frequency for the individual antibodies) and consecutive control cohorts. The latter being important to reflect the daily clinical practice, where these assays are being used in patients with a low to moderate suspicion of IIM. In such studies variability between the participating labs might be controlled and minimized by quality control programs and the use of digitalized reading. Alternatively, it should be even considered to perform all MSA/MAA detection in these studies in a single laboratory, to be able to document the inherent test performance characteristics per method independently of between-lab variability. Some authors suggested antibody and method-dependent cut-off optimization as a step forward towards harmonization (see above). A better approach to take the antibody titer into account, would be the determination of antibody- and method-dependent test-results-interval specific likelihood ratios for IIM diagnosis as well as for some relevant clinical associations (e.g. malignancy, rapid progressive ILD) [76,78].
One of the aims of this manuscript is to increase awareness on the pitfalls related to the application of commercial MSA/MAA tests both in a routine clinical context as well as in studies. This need was recently documented by a questionnaire of the International IMACS group (International Myositis Assessment and Clinical Studies group) amongst international health professionals (n = 111 participants: 76% rheumatologists, 8% neurologists, 5% dermatologists, 11% other). Their report illustrated that many of the MSA/MAA assay users are not very familiar with the concerns raised in this manuscript. For example, >80% of respondents reported that MSA results influenced their diagnostic confidence, the information provided to the patient as well as their therapy. In addition, it became clear that support from the laboratory on this topic can be improved as only 41% of respondents received guidance from the lab on the interpretation of a positive test and <20% received guidance from the laboratory on the interpretation in case of discrepancies between techniques [81].
Credit author statement
Carolien Bonroy: Conceptualization, Writing – original draft. Jan Damoiseaux: Conceptualization Writing- Reviewing and Editing. Yves Piette, Xavier Bossuyt and Yves Allenbach: Writing- Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.McHugh N.J., Tansley S.L. Autoantibodies in myositis. Nat. Rev. Rheumatol. 2018;14:290–302. doi: 10.1038/nrrheum.2018.56. [DOI] [PubMed] [Google Scholar]
- 2.Barsotti S., Lundberg I.E. Myositis an evolving spectrum of disease. Immunol. Med. 2018;41:46–54. doi: 10.1080/13497413.2018.1481571. [DOI] [PubMed] [Google Scholar]
- 3.Bohan A., Peter J.B. Polymyositis and dermatomyositis (first of two parts) N. Engl. J. Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 4.Bohan A., Peter J.B. Polymyositis and dermatomyositis (second of two parts) N. Engl. J. Med. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 5.Dalakas M.C. Polymyositis, dermatomyositis and inclusion-body myositis. N. Engl. J. Med. 1991;325:1487–1498. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- 6.Griggs R., Askanas V., DiMauro S., Engel A., Karpati G., Mendell J.R., Rowland L.P. Inclusion Body myositis and myopathies. Ann. Neurol. 1995;38:705–713. doi: 10.1002/ana.410380504. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd T.E., Mammen A.L., Amato A.A., Weiss M.D., Needham M., Greenberg S.A. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology. 2014;83:426–433. doi: 10.1212/WNL.0000000000000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunawardena H., Betteridge Z.E., McHugh N.J. Newly identified autoantibodies: relationship to idiopathic inflammatory myopathy subsets and pathogenesis. Curr. Opin. Rheumatol. 2008;20:675–680. doi: 10.1097/BOR.0b013e328313bff4. [DOI] [PubMed] [Google Scholar]
- 9.Betteridge Z., McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J. Intern. Med. 2016;280:8–23. doi: 10.1111/joim.12451. [DOI] [PubMed] [Google Scholar]
- 10.Satoh M., Tanaka S., Ceribelli A., Calise S.J., Chan E.K. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin. Rev. Allergy Immunol. 2017;52:1–19. doi: 10.1007/s12016-015-8510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundberg I.E., Miller F.W., Tjarnlund A., Bottai M. Diagnosis and classification of idiopathic inflammatory myopathies. J. Intern. Med. 2016;280:39–51. doi: 10.1111/joim.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damoiseaux J., Vulsteke J.B., Tseng C.W., Platteel A.C.M., Piette Y., Shovman O., Bonroy C., Hamann D., De Langhe E., Musset L., Chen Y.H., Shoenfeld Y., Allenbach Y., Bossuyt X. Autoantibodies in idiopathic inflammatory myopathies: clinical associations and laboratory evaluation by mono- and multispecific immunoassays. Autoimmun. Rev. 2019;18:293–305. doi: 10.1016/j.autrev.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Tanboon J., Uruha A., Stenzel W., Nishino I. Where are we moving in the classification of idiopathic inflammatory myopathies? Curr. Opin. Neurol. 2020;33:590–603. doi: 10.1097/WCO.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 14.Benveniste O., Stenzel W., Allenbach Y. Advances in serological diagnostics of inflammatory myopathies. Curr. Opin. Neurol. 2016;29:662–673. doi: 10.1097/WCO.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg I.E., Tjarnlund A., Bottai M., Werth V.P., Pilkington C., Visser M., Alfredsson L., Amato A.A., Barohn R.J., Liang M.H., Singh J.A., Aggarwal R., Arnardottir S., Chinoy H., Cooper R.G., Danko K., Dimachkie M.M., Feldman B.M., Torre I.G., Gordon P., Hayashi T., Katz J.D., Kohsaka H., Lachenbruch P.A., Lang B.A., Li Y., Oddis C.V., Olesinska M., Reed A.M., Rutkowska-Sak L., Sanner H., Selva-O'Callaghan A., Song Y.W., Vencovsky J., Ytterberg S.R., Miller F.W., Rider L.G., International T.E.r. Myositis classification criteria project consortium, S. The juvenile dermatomyositis cohort biomarker, and repository, 2017 European League against rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017;76:1955–1964. doi: 10.1136/annrheumdis-2017-211468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allenbach Y., Mammen A.L., Benveniste O., Stenzel W., G. Immune-Mediated Necrotizing Myopathies Working 224th ENMC international workshop: clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14-16 october 2016. Neuromuscul. Disord. 2018;28:87–99. doi: 10.1016/j.nmd.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Mariampillai K., Granger B., Amelin D., Guiguet M., Hachulla E., Maurier F., Meyer A., Tohme A., Charuel J.L., Musset L., Allenbach Y., Benveniste O. Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis-specific autoantibodies. JAMA Neurol. 2018;75:1528–1537. doi: 10.1001/jamaneurol.2018.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troyanov Y., Targoff I.N., Tremblay J.L., Goulet J.R., Raymond Y., Senecal J.L. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine. 2005;84:231–249. doi: 10.1097/01.md.0000173991.74008.b0. [DOI] [PubMed] [Google Scholar]
- 19.Mariampillai K., Granger B., Amelin D., Guiguet M., Hachulla E., Maurier F., Meyer A., Tohme A., Charuel J.L., Musset L., Allenbach Y., Benveniste O. Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis-specific autoantibodies. JAMA Neurol. 2018;75:1528–1537. doi: 10.1001/jamaneurol.2018.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Love L.A., Leff R.L., Fraser D.D., Targoff I.N., Dalakas M., Plotz P.H., Miller F.W. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine. 1991;70:360–374. doi: 10.1097/00005792-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Tanimoto K., Nakano K., Kano S., Mori S., Ueki H., Nishitani H., Sato T., Kiuchi T., Ohashi Y. Classification criteria for polymyositis and dermatomyositis. J. Rheumatol. 1995;22:668–674. [PubMed] [Google Scholar]
- 22.Targoff I.N., Miller F.W., Medsger T.A., Jr., Oddis C.V. Classification criteria for the idiopathic inflammatory myopathies. Curr. Opin. Rheumatol. 1997;9:527–535. doi: 10.1097/00002281-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Troyanov Y., Targoff I.N., Tremblay J.L., Goulet J.R., Raymond Y., Senecal J.L. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine. 2005;84:231–249. doi: 10.1097/01.md.0000173991.74008.b0. [DOI] [PubMed] [Google Scholar]
- 24.Troyanov Y., Targoff I.N., Payette M.P., Raynauld J.P., Chartier S., Goulet J.R., Bourre-Tessier J., Rich E., Grodzicky T., Fritzler M.J., Joyal F., Koenig M., Senecal J.L. Redefining dermatomyositis: a description of new diagnostic criteria that differentiate pure dermatomyositis from overlap myositis with dermatomyositis features. Medicine. 2014;93:318–332. doi: 10.1097/MD.0000000000000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senecal J.L., Raynauld J.P., Troyanov Y. Editorial: a new classification of adult autoimmune myositis. Arthritis Rheumatol. 2017;69:878–884. doi: 10.1002/art.40063. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg I.E., Tjarnlund A., Bottai M., Werth V.P., Pilkington C., de Visser M., Alfredsson L., Amato A.A., Barohn R.J., Liang M.H., Singh J.A., Aggarwal R., Arnardottir S., Chinoy H., Cooper R.G., Danko K., Dimachkie M.M., Feldman B.M., Garcia-De La Torre I., Gordon P., Hayashi T., Katz J.D., Kohsaka H., Lachenbruch P.A., Lang B.A., Li Y., Oddis C.V., Olesinska M., Reed A.M., Rutkowska-Sak L., Sanner H., Selva-O'Callaghan A., Song Y.W., Vencovsky J., Ytterberg S.R., Miller F.W., Rider L.G., International t.E.R. Myositis classification criteria project consortium, S. The juvenile dermatomyositis cohort biomarker, and repository, 2017 European League against rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. 2017;69:2271–2282. doi: 10.1002/art.40320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bottai M., Tjarnlund A., Santoni G., Werth V.P., Pilkington C., de Visser M., Alfredsson L., Amato A.A., Barohn R.J., Liang M.H., Singh J.A., Aggarwal R., Arnardottir S., Chinoy H., Cooper R.G., Danko K., Dimachkie M.M., Feldman B.M., Garcia-De La Torre I., Gordon P., Hayashi T., Katz J.D., Kohsaka H., Lachenbruch P.A., Lang B.A., Li Y., Oddis C.V., Olesinka M., Reed A.M., Rutkowska-Sak L., Sanner H., Selva-O'Callaghan A., Wook Song Y., Vencovsky J., Ytterberg S.R., Miller F.W., Rider L.G., Lundberg I.E., International t.E.r. Myositis Classification Criteria Project consortium, S. the Juvenile Dermatomyositis Cohort Biomarker, and Repository, EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. 2017;3 doi: 10.1136/rmdopen-2017-000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundberg I.E., Tjarnlund A. Response to: '2017 EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: little emphasis on autoantibodies, why?' by Malaviya. Ann. Rheum. Dis. 2018;77:e78. doi: 10.1136/annrheumdis-2017-212709. [DOI] [PubMed] [Google Scholar]
- 29.Barsotti S., Dastmalchi M., Notarnicola A., Leclaire V., Dani L., Gheorghe K., Ekholm L., Bottai M., Tjarnlund A., Lundberg I.E. Performance of the new EULAR/ACR classification criteria for idiopathic inflammatory myopathies (IIM) in a large monocentric IIM cohort. Semin. Arthritis Rheum. 2020;50:492–497. doi: 10.1016/j.semarthrit.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Luu Q., Day J., Hall A., Limaye V., Major G. External validation and evaluation of adding MRI or extended myositis antibody panel to the 2017 EULAR/ACR myositis classification criteria. ACR Open Rheumatol. 2019;1:462–468. doi: 10.1002/acr2.11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casal-Dominguez M., Pinal-Fernandez I., Pak K., Huang W., Selva-O’Callaghan A., Albayda J., Casciola-Rosen L., Paik J.J., Tiniakou E., Mecoli C.A., Lloyd T.E., Danoff S.K., Christopher-Stine L., Mammen A.L. Performance of the 2017 EULAR/ACR classification criteria for inflammatory myopathies in patients with myositis-specific autoantibodies. Arthritis Rheumatol. September 2 2021 doi: 10.1002/art.41964. Epub ahead of print. PMID: 34480833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoogendijk J.E., Amato A.A., Lecky B.R., Choy E.H., Lundberg I.E., Rose M.R., Vencovsky J., de Visser M., Hughes R.A. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul. Disord. 2004;14:337–345. doi: 10.1016/j.nmd.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Mammen A.L., Allenbach Y., Stenzel W., Benveniste O., Group E.t.W.S. 239th ENMC international workshop: classification of dermatomyositis, Amsterdam, The Netherlands, 14-16 December 2018. Neuromuscul. Disord. 2020;30:70–92. doi: 10.1016/j.nmd.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Rose M.R., Group E.I.W. 188th ENMC international workshop: inclusion body myositis, 2-4 December 2011, Naarden, The Netherlands. Neuromuscul. Disord. 2013;23:1044–1055. doi: 10.1016/j.nmd.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Mahler M., Malyavantham K., Seaman A., Bentow C., Anunciacion-Llunell A., Sanz-Martínez M.T., Viñas-Gimenez L., Selva-O’Callaghan A. Profiling of myositis specific antibodies and composite scores as an aid in the differential diagnosis of autoimmune myopathies. Diagnostics. 2021;11:2246. doi: 10.3390/diagnostics11122246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tansley S.L., Li D., Betteridge Z.E., McHugh N.J. The reliability of immunoassays to detect autoantibodies in patients with myositis is dependent on autoantibody specificity. Rheumatology. 2020;59:2109–2114. doi: 10.1093/rheumatology/keaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vulsteke J.B., De Langhe E., Claeys K.G., Dillaerts D., Poesen K., Lenaerts J., Westhovens R., Van Damme P., Blockmans D., De Haes P., Bossuyt X. Detection of myositis-specific antibodies. Ann. Rheum. Dis. 2019;78:e7. doi: 10.1136/annrheumdis-2017-212915. [DOI] [PubMed] [Google Scholar]
- 38.Bundell C., Rojana-Udomsart A., Mastaglia F., Hollingsworth P., McLean-Tooke A. Diagnostic performance of a commercial immunoblot assay for myositis antibody testing. Pathology. 2016;48:363–366. doi: 10.1016/j.pathol.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Espinosa-Ortega F., Holmqvist M., Alexanderson H., Storfors H., Mimori T., Lundberg I.E., Ronnelid J. Comparison of autoantibody specificities tested by a line blot assay and immunoprecipitation-based algorithm in patients with idiopathic inflammatory myopathies. Ann. Rheum. Dis. 2019;78:858–860. doi: 10.1136/annrheumdis-2018-214690. [DOI] [PubMed] [Google Scholar]
- 40.Piette Y., De Sloovere M., Vandendriessche S., Dehoorne J., De Bleecker J.L., Van Praet L., Vander Mijnsbrugge A.S., De Schepper S., Jacques P., De Keyser F., Smith V., Bonroy C. Pitfalls in the detection of myositis specific antibodies by lineblot in clinically suspected idiopathic inflammatory myopathy. Clin. Exp. Rheumatol. 2020;38:212–219. [PubMed] [Google Scholar]
- 41.Ronnelid J., Helmers S.B., Storfors H., Grip K., Ronnblom L., Franck-Larsson K., Nordmark G., Lundberg I.E. Use of a commercial line blot assay as a screening test for autoantibodies in inflammatory myopathies. Autoimmun. Rev. 2009;9:58–61. doi: 10.1016/j.autrev.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Ghirardello A., Rampudda M., Ekholm L., Bassi N., Tarricone E., Zampieri S., Zen M., Vattemi G.A., Lundberg I.E., Doria A. Diagnostic performance and validation of autoantibody testing in myositis by a commercial line blot assay. Rheumatology. 2010;49:2370–2374. doi: 10.1093/rheumatology/keq281. [DOI] [PubMed] [Google Scholar]
- 43.Mahler M., Betteridge Z., Bentow C., Richards M., Seaman A., Chinoy H., McHugh N. Comparison of three immunoassays for the detection of myositis specific antibodies. Front. Immunol. 2019;10:848. doi: 10.3389/fimmu.2019.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavazzana I., Richards M., Bentow C., Seaman A., Fredi M., Giudizi M.G., Palterer B., Pratesi F., Migliorini P., Franceschini F., Satoh M., Ceribelli A., Mahler M. Evaluation of a novel particle-based assay for detection of autoantibodies in idiopathic inflammatory myopathies. J. Immunol. Methods. 2019;474:112661. doi: 10.1016/j.jim.2019.112661. [DOI] [PubMed] [Google Scholar]
- 45.Cavazzana I., Fredi M., Ceribelli A., Mordenti C., Ferrari F., Carabellese N., Tincani A., Satoh M., Franceschini F. Testing for myositis specific autoantibodies: comparison between line blot and immunoprecipitation assays in 57 myositis sera. J. Immunol. Methods. 2016;433:1–5. doi: 10.1016/j.jim.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Vulsteke J.B., Satoh M., Malyavantham K., Bossuyt X., De Langhe E., Mahler M. Anti-OJ autoantibodies: rare or underdetected? Autoimmun. Rev. 2019;18:658–664. doi: 10.1016/j.autrev.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Hodgkinson L.M., Wu T.T., Fiorentino D.F. Dermatomyositis autoantibodies: how can we maximize utility? Ann. Transl. Med. 2021;9:433. doi: 10.21037/atm-20-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiorentino D.F., Gutierrez-Alamillo L., Hines D., Yang Q., Casciola-Rosen L. Distinct dermatomyositis populations are detected with different autoantibody assay platforms. Clin. Exp. Rheumatol. 2019;37:1048–1051. [PMC free article] [PubMed] [Google Scholar]
- 49.Pinal-Fernandez I., Pak K., Casal-Dominguez M., Hosono Y., Mecoli C., Christopher-Stine L., Mammen A.L. Validation of anti-Mi2 autoantibody testing by line blot. Autoimmun. Rev. 2020;19:102425. doi: 10.1016/j.autrev.2019.102425. [DOI] [PubMed] [Google Scholar]
- 50.Richards M., García-De La Torre I., González-Bello Y.C., Vázquez-Del Mercado M., Andrade-Ortega L., Medrano-Ramírez G., Navarro-Zarza J.E., Maradiaga-Ceceña M., Loyo E., Rojo-Mejía A., Gómez G., Seaman A., Fritzler M.J., Koenig M., Mahler M. Autoantibodies to Mi-2 alpha and Mi-2 beta in patients with idiopathic inflammatory myopathy. Rheumatology. 2019;58:1655–1661. doi: 10.1093/rheumatology/kez092. [DOI] [PubMed] [Google Scholar]
- 51.Hanke K., Brückner C.S., Dähnrich C., Huscher D., Komorowski L., Meyer W., Janssen A., Backhaus M., Becker M., Kill A., Egerer K., Burmester G.R., Hiepe F., Schlumberger W., Riemekasten G. Antibodies against PM/Scl-75 and PM/Scl-100 are independent markers for different subsets of systemic sclerosis patients. Arthritis Res. Ther. 2009;11:R22. doi: 10.1186/ar2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonroy C., Van Praet J., Smith V., Van Steendam K., Mimori T., Deschepper E., Deforce D., Devreese K., De Keyser F. Optimization and diagnostic performance of a single multiparameter lineblot in the serological workup of systemic sclerosis. J. Immunol. Methods. 2012;379:53–60. doi: 10.1016/j.jim.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Alkema W., Koenen H., Kersten B.E., Kaffa C., Dinnissen J.W.B., Damoiseaux J.G.M.C., Joosten I., Driessen-Diks S., van der Molen R.G., Vonk M.C., Smeets R.L. Autoantibody profiles in systemic sclerosis; a comparison of diagnostic tests. Autoimmunity. 2021;54:148–155. doi: 10.1080/08916934.2021.1907842. [DOI] [PubMed] [Google Scholar]
- 54.Platteel A.C.M., Wevers B.A., Lim J., Bakker J.A., Bontkes H.J., Curvers J., Damoiseaux J., Heron M., de Kort G., Limper M., van Lochem E.G., Mulder A.H.L., Saris C.G.J., van der Valk H., van der Kooi A.J., van Leeuwen E.M.M., Veltkamp M., Schreurs M.W.J., Meek B., Hamann D. Frequencies and clinical associations of myositis-related antibodies in The Netherlands: a one-year survey of all Dutch patients. J. Transl. Autoimmun. 2019;2:100013. doi: 10.1016/j.jtauto.2019.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Horebeek N., Vulsteke J.B., Bossuyt X., Claeys K.G., Dillaerts D., Poesen K., Lenaerts J., Van Damme P., Blockmans D., De Haes P., De Langhe E. Detection of multiple myositis-specific autoantibodies in unique patients with idiopathic inflammatory myopathy: a single centre-experience and literature review: systematic review. Semin. Arthritis Rheum. 2021;51:486–494. doi: 10.1016/j.semarthrit.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Cruellas M.G., Viana Vdos S., Levy-Neto M., Souza F.H., Shinjo S.K. Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics. 2013;68:909–914. doi: 10.6061/clinics/2013(07)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ronnelid J., Espinosa-Ortega F., Lundberg I.E. Response to: 'Semi-quantitative analysis of line blot assay for myositis-specific and myositis-associated antibodies: a better performance?' by Cavazzana et al. Ann. Rheum. Dis. 2020;79:e153. doi: 10.1136/annrheumdis-2019-215967. [DOI] [PubMed] [Google Scholar]
- 58.Mahler M., Fritzler M.J. Detection of myositis-specific antibodies: additional notes. Ann. Rheum. Dis. 2019;78:e45. doi: 10.1136/annrheumdis-2018-213153. [DOI] [PubMed] [Google Scholar]
- 59.Infantino M., Manfredi M., Grossi V., Benucci M. Detection of myositis-specific antibodies: additional notes. Ann. Rheum. Dis. 2019;78:e29. doi: 10.1136/annrheumdis-2018-213320. [DOI] [PubMed] [Google Scholar]
- 60.Damoiseaux J., Andrade L.E.C., Carballo O.G., Conrad K., Francescantonio P.L.C., Fritzler M.J., Garcia de la Torre I., Herold M., Klotz W., Cruvinel W.M., Mimori T., von Muhlen C., Satoh M., Chan E.K. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann. Rheum. Dis. 2019;78:879–889. doi: 10.1136/annrheumdis-2018-214436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Infantino M., Tampoia M., Fabris M., Alessio M.G., Previtali G., Pesce G., Deleonardi G., Porcelli B., Musso M., Grossi V., Benucci M., Manfredi M., Bizzaro N. Combining immunofluorescence with immunoblot assay improves the specificity of autoantibody testing for myositis. Rheumatology. 2019;58:1239–1244. doi: 10.1093/rheumatology/key451. [DOI] [PubMed] [Google Scholar]
- 62.Agmon-Levin N., Damoiseaux J., Kallenberg C., Sack U., Witte T., Herold M., Bossuyt X., Musset L., Cervera R., Plaza-Lopez A., Dias C., Sousa M.J., Radice A., Eriksson C., Hultgren O., Viander M., Khamashta M., Regenass S., Andrade L.E., Wiik A., Tincani A., Ronnelid J., Bloch D.B., Fritzler M.J., Chan E.K., Garcia-De La Torre I., Konstantinov K.N., Lahita R., Wilson M., Vainio O., Fabien N., Sinico R.A., Meroni P., Shoenfeld Y. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann. Rheum. Dis. 2014;73:17–23. doi: 10.1136/annrheumdis-2013-203863. [DOI] [PubMed] [Google Scholar]
- 63.Bossuyt X., De Langhe E., Borghi M.O., Meroni P.L. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2020;16:715–726. doi: 10.1038/s41584-020-00522-w. [DOI] [PubMed] [Google Scholar]
- 64.Chan E.K., Damoiseaux J., Carballo O.G., Conrad K., de Melo Cruvinel W., Francescantonio P.L., Fritzler M.J., Garcia-De La Torre I., Herold M., Mimori T., Satoh M., von Muhlen C.A., Andrade L.E. Report of the first international consensus on standardized Nomenclature of antinuclear antibody HEp-2 cell patterns 2014-2015. Front. Immunol. 2015;6:412. doi: 10.3389/fimmu.2015.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahler M., You D., Baron M., Taillefer S.S., Hudson M., Canadian Scleroderma Research G., Fritzler M.J. Anti-centromere antibodies in a large cohort of systemic sclerosis patients: comparison between immunofluorescence, CENP-A and CENP-B ELISA. Clin. Chim. Acta. 2011;412:1937–1943. doi: 10.1016/j.cca.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 66.Infantino M., Palterer B., Biagiotti R., Almerigogna F., Benucci M., Damiani A., Grossi V., Azzurri A., Casprini P., Bacci G., Giudizi M.G., Manfredi M. Reflex testing of speckled cytoplasmic patterns observed in routine ANA HEp-2 indirect immunofluorescence with a multiplex anti-synthetase dot-blot assay: a multicentric pilot study. Immunol. Res. 2018;66:74–78. doi: 10.1007/s12026-017-8974-3. [DOI] [PubMed] [Google Scholar]
- 67.Van Blerk M., Bossuyt X., Humbel R., Mewis A., Servais G., Tomasi J.P., Van Campenhout C., Van Hoovels L., Vercammen M., Damoiseaux J., Coucke W., Van de Walle P. Belgian recommendations on ANA, anti-dsDNA and anti-ENA antibody testing. Acta Clin. Belg. 2014;69:83–86. doi: 10.1179/2295333714Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 68.Damoiseaux J.G., Tervaert J.W. From ANA to ENA: how to proceed? Autoimmun. Rev. 2006;5:10–17. doi: 10.1016/j.autrev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Van Praet J.T., Vander Cruyssen B., Bonroy C., Smith V., Delanghe J., De Keyser F. Validation of a new screening strategy for anti-extractable nuclear antigen antibodies. Clin. Exp. Rheumatol. 2009;27:971–976. [PubMed] [Google Scholar]
- 70.Willems P., De Langhe E., Claessens J., Westhovens R., Van Hoeyveld E., Poesen K., Vanderschueren S., Blockmans D., Bossuyt X. Screening for connective tissue disease-associated antibodies by automated immunoassay. Clin. Chem. Lab. Med. 2018;56:909–918. doi: 10.1515/cclm-2017-0905. [DOI] [PubMed] [Google Scholar]
- 71.Satoh M., Chan E.K., Ho L.A., Rose K.M., Parks C.G., Cohn R.D., Jusko T.A., Walker N.J., Germolec D.R., Whitt I.Z., Crockett P.W., Pauley B.A., Chan J.Y., Ross S.J., Birnbaum L.S., Zeldin D.C., Miller F.W. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64:2319–2327. doi: 10.1002/art.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Musset L., Miyara M., Benveniste O., Charuel J.L., Shikhman A., Boyer O., Fowler R., Mammen A., Phillips J., Mahler M. Analysis of autoantibodies to 3-hydroxy-3-methylglutaryl-coenzyme A reductase using different technologies. J. Immunol. Res. 2014;2014:405956. doi: 10.1155/2014/405956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Damoiseaux J., von Muhlen C.A., Garcia-De La Torre I., Carballo O.G., de Melo Cruvinel W., Francescantonio P.L., Fritzler M.J., Herold M., Mimori T., Satoh M., Andrade L.E., Chan E.K., Conrad K. International consensus on ANA patterns (ICAP): the bumpy road towards a consensus on reporting ANA results. Auto Immun. Highlights. 2016;7:1. doi: 10.1007/s13317-016-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Damoiseaux J. The perspective on standardisation and harmonisation: the viewpoint of the EASI president. Auto Immun. Highlights. 2020;11:4. doi: 10.1186/s13317-020-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Picard C., Vincent T., Lega J.C., Hue S., Fortenfant F., Lakomy D., Humbel R.L., Goetz J., Molinari N., Bardin N., Bertin D., Johanet C., Chretien P., Dubucquoi S., Streichenberger N., Desplat-Jego S., Bossuyt X., Sibilia J., Abreu I., Chevailler A., Fabien N. Heterogeneous clinical spectrum of anti-SRP myositis and importance of the methods of detection of anti-SRP autoantibodies: a multicentric study. Immunol. Res. 2016;64:677–686. doi: 10.1007/s12026-015-8774-6. [DOI] [PubMed] [Google Scholar]
- 76.Fierz W., Bossuyt X. Likelihood ratio approach and clinical interpretation of laboratory tests. Front. Immunol. 2021;12:655262. doi: 10.3389/fimmu.2021.655262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacobs J.F.M., Bossuyt X. Standardization and harmonization of autoimmune diagnostics. Clin. Chem. Lab. Med. 2018;56:1563–1567. doi: 10.1515/cclm-2018-0807. [DOI] [PubMed] [Google Scholar]
- 78.Fierz W., Bossuyt X. Likelihood ratios as value proposition for diagnostic laboratory tests. J. Appl. Lab. Med. 2020;5:1061–1069. doi: 10.1093/jalm/jfaa064. [DOI] [PubMed] [Google Scholar]
- 79.Monogioudi E., Martos G., Hutu D.P., Schimmel H., Meroni P.L., Sheldon J., Zegers I. Standardization of autoimmune testing – is it feasible? Clin. Chem. Lab. Med. 2018;56:1734–1742. doi: 10.1515/cclm-2017-1077. [DOI] [PubMed] [Google Scholar]
- 80.Smolen J.S., Butcher B., Fritzler M.J., Gordon T., Hardin J., Kalden J.R., Lahita R., Maini R.N., Reeves W., Reichlin M., Rothfield N., Takasaki Y., van Venrooij W.J., Tan E.M. Reference sera for antinuclear antibodies. II. Further definition of antibody specificities in international antinuclear antibody reference sera by immunofluorescence and western blotting. Arthritis Rheum. 1997;40 doi: 10.1002/art.1780400304. 413-8. [DOI] [PubMed] [Google Scholar]
- 81.Tansley S.L., Snowball J., Pauling J.D., Lissina A., Kuwana M., Rider L.G., Ronnelid J., McHugh N.J., International Myositis A., Clinical G. Studies Group Myositis Autoantibody Scientific Interest, the promise, perceptions, and pitfalls of immunoassays for autoantibody testing in myositis. Arthritis Res. Ther. 2020;22:117. doi: 10.1186/s13075-020-02210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]