Highlights
-
•
Radiotherapy has bright side as well as dark side on immune system.
-
•
Radiotherapy does not always synergize with immunotherapy.
-
•
Fully consideration of radiotherapy regimens and schedules is needed to maximize the immunostimulatory effect of radioimmunotherapy.
-
•
Application of new techniques may be a sharp weapon in improving the effect of radioimmunotherapy.
Keywords: Radiotherapy, Immunotherapy, Immune checkpoint inhibitors, Immunosuppression, Synergistic effect
Abbreviations: ICIs, immune checkpoint inhibitors; NSCLC, non-small cell lung cancer; HNSCC, head and neck squamous cell carcinoma; DLNs, draining lymph nodes; TAAs, tumor-associated antigens; DCs, dendritic cells; Tregs, regulatory T cells; MDSCs, myeloid-derived suppressor cells; ORR, objective response rate; ALC, absolute lymphocyte count; OS, overall survival; PFS, progression-free survival; NLR, neutrophil-to-lymphocyte ratio; dsDNA, double-strand DNA; mtDNA, mitochondrial DNA; SBRT, stereotactic body radiotherapy; HIRT, heavy ion radiotherapy
Abstract
The introduction of immunotherapy into cancer treatment has radically changed clinical management of tumors. However, only a minority of patients (approximately 10 to 30%) exhibit long-term response to monotherapy with immunotherapy. Moreover, there are still many cancer types, including pancreatic cancer and glioma, which are resistant to immunotherapy. Due to the immunomodulatory effects of radiotherapy, the combination of radiotherapy and immunotherapy has achieved better therapeutic effects in a number of clinical trials. However, radiotherapy is a double-edged sword in the sense that it also attenuates the immune system under certain doses and fractionation schedules, not all clinical trials show improved survival in the combination of radiotherapy and immunotherapy. Therefore, elucidation of the interactions between radiotherapy and the immune system is warranted to optimize the synergistic effects of radiotherapy and immunotherapy. In this review, we highlight the dark side as well as bright side of radiotherapy on tumor immune microenvironment and immune system. We also elucidate current status of radioimmunotherapy, both in preclinical and clinical studies, and highlight that combination of radiotherapy and immunotherapy attenuates combinatorial effects in some circumstances. Moreover, we provide insights for better combination of radiotherapy and immunotherapy.
Graphical abstract
Introduction
Advances in immunotherapy have highly improved cancer treatment. Immunotherapy, particularly immune checkpoint inhibitors (ICIs), has been transformed to applications in patients with a wide range of advanced-stage malignancies, including metastatic melanoma, late-stage non-small cell lung cancer (NSCLC), Hodgkin's lymphoma, head and neck squamous cell carcinoma (HNSCC), and urothelial carcinoma among others [1]. Although the application of ICIs enables the achievement of long-term response, even complete response, only a minority of patients (approximately 10 to 30%) exhibit long-term response to monotherapy with immunotherapy [2], [3], [4], [5]. Moreover, more than half of patients have indications for radiotherapy [6]. Therefore, it is clinically important to combine radiotherapy with immunotherapy to improve clinical outcomes [7].
Radiotherapy is an important treatment option in a wide range of malignancies. Historically, radiotherapy is developed with empirical clinical observations, which omits the contribution of the immune system in mediating its therapeutic effects [8]. The therapeutic effects of radiotherapy are mainly attributed to induction of DNA damage, which triggers tumor cell cycle arrest and cell death [9]. Consequently, classical radiotherapy aims at maximizing the radiation dose at the tumor site while reducing damage to normal tissues [10]. It is not until the past two decades that accumulated evidences show that the immune system is involved in radiotherapy [7].
Both radiotherapy and immunotherapy interact with immune system in various aspects. Radiotherapy promotes antitumor immunity by increasing the secretion of tumor associated-antigens, proinflammatory cytokines and chemokines [11]. It also promotes the production of immunosuppressive cytokines, especially in the case of repeated irradiation [12]. Moreover, irradiation of the bone marrow, blood and draining lymph nodes (DLNs) results in serious leukopenia and damage of lymphocytes, which is associated with poorer survival in patients [13]. The therapeutic effects of immunotherapy largely depend on the immune system. Effective killing of cancer cells initiated by immunotherapy needs a series of immunological events, which include processing of tumor-associated antigens (TAAs) by dendritic cells (DCs), presentation of TAAs to T cells, priming and activation of effector T cell responses, trafficking of T cells and infiltrating into the tumor bed, and recognizing and eliminating of tumor cells [14]. Under certain circumstance, radiotherapy synergizes with immunotherapy and augments antitumor response, thereby improving clinical outcomes [15]. However, not all patients show enhanced efficacies of radioimmunotherapy, compared to immunotherapy alone [16]. The state of immune system is correlated with the prognosis of patients treated with radioimmunotherapy [17,18]. Radioimmunotherapy is promising in augmenting therapeutic effect of cancer, but further optimization is still needed.
Local and system immune state are the basis of immunotherapy
The local and systemic immune state largely determine the effects of immunotherapy. Based on spatial distribution of CD8+ T cells in the tumor microenvironment, immunophenotypes can be divided into inflamed tumors, immune-excluded tumors and immune desert tumors [19]. Since therapeutic PD-1 blockade induced tumor regression requires pre-existing CD8+ T cells [20], only in inflamed tumors that PD-1/PD-L1 inhibition exerts excellent responses. In contrast, immune-excluded tumors and immune desert tumors are associated with immune escape from PD-1/PD-L1 inhibition [19]. The mechanisms through which immune-excluded and immune desert tumors drive immune suppression are not fully understood.
Systemic immunity is associated with elimination of residual tumors and distant metastases. Study has showed that absolute lymphocyte count (ALC) after radiotherapy is a predictor for prognostic outcomes of patients with multiple tumor species. Patients with low ALC after radiotherapy are correlated with poorer overall survival (OS) and progression-free survival (PFS) [17]. Lower ALC post radiotherapy is also correlated with lower abscopal response rates [17]. Neutrophil-to-lymphocyte ratio (NLR) is also suggested to be an available biomarker for predicting survival outcomes in patients with advanced melanoma treated with nivolumab. Patients with baseline NLR<5 are associated with better OS and PFS [18].
The clinical success of immunotherapy is both astounding and unsatisfactory. Although immunotherapy has gained remarkable success in various cancers, low response rate is an urgent problem to be solved at present [19]. To achieve better outcomes and enable more people to benefit from immunotherapy, immunotherapy is usually combined with radiotherapy and/or chemotherapy, simultaneously or successively [21]. Traditional therapies including radiotherapy exert significantly impact on tumor microenvironment and systemic immunity [22]. Understanding of the complex immunogenic effects of radiotherapy contributes to better designs for synergistic cancer therapies [22].
Radiotherapy influences antitumor immunity in several aspects
Radiotherapy can be used as a curative or palliative treatment option. Approximately half of tumor patients receive radiotherapy during their treatment. It is either used alone or in combination with other treatments such as surgery, chemotherapy and immunotherapy [21,23,24]. On one hand, radiotherapy enhances immunogenicity of tumor cells and improves antitumor immunity. On the other hand, radiotherapy may heighten both local and systemic immunosuppression in patients in some circumstances.
Radiotherapy induces both local and systemic antitumor immunity
Radiotherapy induces antitumor immunity to achieve tumor control both in residual tumor site and non-irradiated metastasis (Fig. 1). Ionizing radiation promotes the release of double-stranded DNA (dsDNA) from the nucleus, increases mitochondrial outer membrane permeabilization and triggers the exposure of mitochondrial DNA (mtDNA) in the cytosol [25]. Both dsDNA and mtDNA are potent mediators in initiation of the cGAS-STING pathway and subsequent transcription of type I IFN [26,27]. Type I IFN signaling is essential in activation of DCs, thereby promoting the priming of T cells and tumor control [28]. Accumulation of dsDNA within tumor-derived exosomes after radiotherapy also promotes the recruitment of DCs and directly induces type I IFN response in DCs [29], which further promoting the recruitment of CD8+ T cells and providing the third signal for T cell activation [30]. To escape from attack by T cells, MHC class I molecules are either lacking or poorly expressed in many cancer cells. Radiotherapy upregulates MHC class I molecules on the surface of tumor cells and enhances the generation of TAAs, which broadens the pool of available antigens for presentation [31]. Moreover, the exposure of calreticulin on cell surface, secretion of HMGB1 and ATP enhances immunogenicity and drives immune cell infiltration, thereby promoting immune responses in tumor microenvironment [32,33]. Calreticulin on the cell surface acts as an “eat-me” signal and promotes phagocytosis of tumor cells by macrophage [34], it also induces the production of proinflammatory cytokines including IL-6 and TNF-α in T-helper cells [35]. HMGB1 is crucial for induction of tumor antigen-specific CD8+ T cells by activating Toll-like receptors and the RAGE-NF-κB signal pathway [36]. Increased extracellular ATP is sensed by the purinergic receptor, P2 × 7, which regulates the inflammasome and promotes the secretion of IL-1β and IL-18 [37]. Radiotherapy also increases the abundance of tumor-infiltrating immunostimulatory cells, and stimulates the release of pro-inflammatory mediators from tumor cells and stromal cells [38]. Release of chemokines, such as CXCL9, CXCL10, CXCL11 and CXCL16, leads to the infiltrations of DCs, macrophages and T cells [39]. Recruited antigen-presenting cells including DCs uptake tumor debris, traffic it to lymph nodes to present antigens and prime T cells, thereby increasing the diversity of TCR repertoire and T cell clonality [40]. Collectively, by changing tumor cell phenotypes, triggering hallmark release of immunostimulatory DAMPs, and increasing the number of proinflammatory immune cells, radiotherapy enhances the susceptibility of tumor cells to T‑cell‑mediated antitumor effects and promotes recognition and elimination of cancer cells [41].
Fig. 1.
Radiotherapy induces antitumor immune response in several aspects. (1.5-column). a. Irradiation of local tumor induces DNA DSBs and genomic instability, which induce cell death and cellular senescence. b. Radiotherapy increases the expression of MHC class I and induces the exposure of calreticulin in the cell surface. c. dsDNA and mtDNA initiate cGAS-STING signaling pathway and promote the transcription and secretion of type I IFN. d. Type I IFN stimulates the receptors on tumor cells, DCs and T cells, thus remodeling inflammatory microenvironment. e. Radiotherapy increases the secretion of DAMPs, including HMGB1 and ATP, which enhance immunogenicity and drive the recruitment of immune cells. f. Extracellular dsDNA also initiates cGAS-STING signaling pathway in DCs. g. Radiotherapy promotes the release of TAAs. DCs uptake TAAs and play the role of antigen presentation. h. DCs present antigens and prime T cells, thus increase the diversity of TCR repertoire and T cell clonality. i. Activated T cells recognize and kill residual tumor cells. j. Radiotherapy stimulates tumor cells and stromal cells and initiates the production and release of chemokines, such as CXCL9, CXCL10, CXCL11 and CXCL16. k. Chemokines including CXCL9, CXCL10, CXCL11 and CXCL16 lead to the infiltration of DCs, macrophages and T cells, further promoting inflammatory tumor microenvironment. l. Activated T cells potentially attack nonirradiated metastases in the distance. DSBs, double-strand breaks; dsDNA, double-strand DNA; mtDNA, mitochondrial DNA; IFN, interferon; IFN-R, IFN receptor; DC, Dendritic cell; DAMP, damage-associated molecular pattern; HMGB1, high mobility group box-1; TLRs, toll-like receptors; TCR, T cell receptor.
Radiotherapy also elicits immune-mediated systemic tumor regression in some circumstances [42]. Radiotherapy induced DNA damage and generation of proinflammatory cytokines contributes to tumor heterogeneity and plasticity [43]. Increased immunogenicity converts local tumor into in situ vaccine and stimulates antitumor T-cell immune responses [43]. As a result, activated effector T cells mediate the elimination of cancer cells in irradiated lesions and potentially attack nonirradiated metastases, an effect commonly referred to as abscopal effect [44]. Abscopal effect are rare and unpredictable when only radiotherapy is adopted. Between 1969 and 2014, only 46 cases of abscopal effect were reported [45]. Concomitant application of radiotherapy with immunostimulants or immunotherapy significantly increases the frequency of abscopal effect in patients [46,47]. Further optimization of radiotherapy combined with immunotherapy for the purpose of boosting abscopal effect has great clinical application prospects.
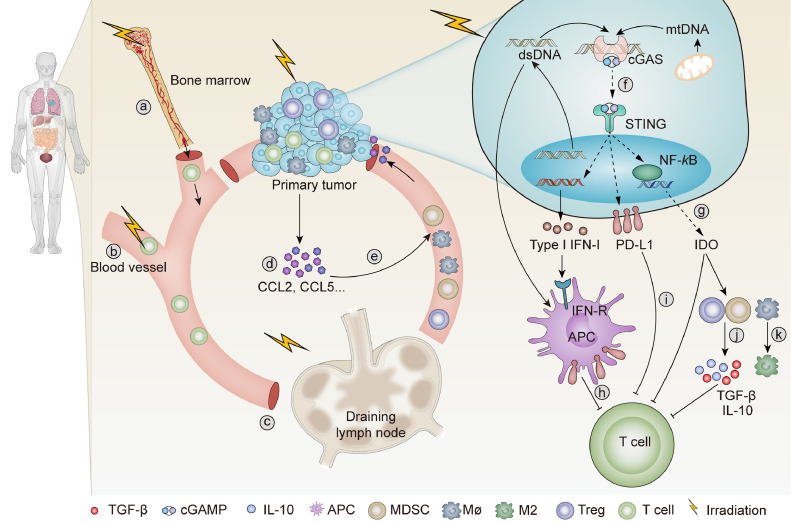
Radiotherapy triggers immunosuppression and lymphopenia
Immunogenic dose of radiotherapy leads to accumulation of dsDNA in cancer cells, which activates cGAS/STING signaling to promote the transcription of type I IFN gene. However, IFN signaling also exerts detrimental effects that orchestrate therapeutic resistance. Repeated irradiation of tumor cells induces chronic type I IFN and IFN-stimulated gene expression, thus mediating radiation resistance and metastatic dissemination through multiple inhibitory pathways [12,48,49]. Both IFN-γ and type I IFN are responsible for upregulation of PD-L1 on tumor cells, which further inducing T-cell exhaustion and resistance to antitumor immunity [50,51]. IDO is also upregulated by type I IFN and IFN-γ, and acts as an immunosuppressive factor [52]. Moreover, activated STING signaling enhances the mobilization of Tregs, MDSCs and abrogates tumor immunogenicity [53,54]. Local radiotherapy upregulates the secretion of CCL2 and CCL5, which are associated with recruitment of Tregs and monocytes [55,56]. Recruited monocytes activate Tregs in a TNF-α-dependent manner, which dampens the efficacy of radiotherapy and mediates tumor immune resistance [55]. By secreting IL-10 and TGF-β, Tregs enhance the immunosuppressive effects of MDSCs and suppress the function of effector T cells [57,58].
Lymphopenia is one of the most commonly side effects observed in radiotherapy. Since bone marrow is exquisitely sensitive to radiation, severe marrow injury occurs within the irradiated volume during radiotherapy [59]. Even relatively low radiation doses can induce temporary functional ablation of the bone marrow. When the bone marrow is exposed to moderate radiation doses, it takes several years for active hematopoiesis to be restored. Higher radiation doses can result in irreversible damage [59]. Given that many blood cells in the body have a very short life time, patients who undergo myelosuppression often suffer decreased levels of blood cells and lymphopenia [59,60]. Circulating population of peripheral blood mononuclear cells are also highly sensitive to ionizing radiation [61]. Repeated daily delivery of conventional fraction radiotherapy is cytotoxic enough to deplete migrating immune effector cells. Yovino et al. reported that a single fraction of 2Gy delivered ≥0.5Gy to 4.6% of the total blood cells, while 92.2% of the blood cells received ≥0.5Gy after a typical 2Gy × 30 conventional radiotherapy fraction to brain tumors [13]. Continuous exposure of blood cells to ionizing radiation leads to leukopenia and significantly attenuates the immune system [62]. Another mechanism through which radiotherapy induces lymphopenia is via irradiation of lymphatic organs. Naïve T cells are extremely radiosensitive, even low dose irradiation of lymphoid tissues can lead to rapid p53-mediated apoptosis [63,64]. Elective nodal irradiation is commonly used as conventional remedy [65]. However, irradiation of DLNs contributes to direct lymphatic toxicity and decline of live CD45+ cells within irradiated DLNs [66]. By altering chemokine expression and CD8+ T-cell trafficking, elective nodal irradiation of tumor-associated DLNs also inhibits adaptive immune responses and decreases the combinatorial efficacies of radiotherapy and immune checkpoint blockade [66]. Moreover, radiotherapy-induced secretion of Gal-1 from tumor cells exhibits T-cell proapoptotic activities and are associated with lymphopenia and poor survival outcomes [67].
By upregulating the infiltration of suppressive immune cells and attenuating effector T cells, radiotherapy leads to both local and systemic immunosuppression [68] (Fig. 2). Tumor cell elimination is largely dependent on immunity, both in radiotherapy and immunotherapy, immune cell inhibition hampers antitumor effects in both radiotherapy and radioimmunotherapy [39]. Therefore, appropriate doses and regimens of radiotherapy should be selected to promote immune activation and reduce immune suppression [7]. Since immune cells are required to mediate both local and distant tumor inhibition, protection of lymphocytes should be taken into consideration seriously [22]. To minimize damage to lymphocytes during radiotherapy, exposure to the bone marrow and blood should be minimized. Moreover, both target volumes and total dose of irradiation should be taken into consideration carefully. DLNs are the organ where DCs present antigens and prime T cells, injury of T cells in DLNs potentially weakens adaptive antitumor immunity [63]. The necessity of irradiating DLNs should be carefully considered.
Fig. 2.
Radiotherapy triggers lymphopenia and immunosuppression. (1.5-column). a. Irradiation of bone marrow induces severe myelosuppression. b. Irradiation of blood vessel leads to leukopenia. c. Irradiation if draining lymph nodes restrains adoptive immune response. d. Radiotherapy initiates chemokine production in tumor microenvironment, such as CCL2 and CCL5. e. Increased production of CCL5 and CCL2 drive the infiltration of Tregs, macrophages, and MDSCs. f. Repeated irradiation of tumor cells induces chronic activation of cGAS-STING signaling and promotes immunosuppression. g. Radiotherapy induces the release of IDO, which promote inhibitory immune microenvironment in the tumor site. h. Type I IFN stimulates the expression of PD-L1 on APCs, thus inhibits the function of T cells. i. Radiotherapy increases the expression of PD-L1 on tumor cells, which inhibits the activation of T cells. j. Radiotherapy stimulates the function of MDSCs and Tregs, promotes the secretion of TGF-β and IL-10, thus suppresses the activation of T cells. k. Radiotherapy polarizes macrophages into M2 phenotype. IDO, indoleamine 2,3-dioxygenase; MDSC, myeloid-derived suppressor cell; APC, antigen-presenting cell; Treg, regulatory T cell.
Combination of immunotherapy with radiotherapy
Over the past decade, several immunotherapeutic agents for clinical management of cancer have been approved. The most commonly used immunotherapeutic agents in these trials are anti-PD-1, anti-PD-L1 and anti-CTLA-4. Although a number of clinical trials show increased therapeutic effects, there are still numerous studies that fail to meet the predetermined endpoint for efficacy. Here, we present published clinical trials that investigate the efficacy of radioimmunotherapy in Table 1.
Table 1.
Published clinical trials investigating the efficacy of combination of radiotherapy and immunotherapy.
| NCT ID | Patient population | Experimental treatment | Control or comparator treatment | n | Phase | Patient outcomes |
|---|---|---|---|---|---|---|
| NCT00861614 | Castration-resistant prostate cancer metastatic to bone | SBRT (8Gy × 1) followed by ipilimumab | SBRT (8Gy × 1) | 799 | Phase 3 | Median OS: 11.2 months vs 10 months, p = 0.053 |
| NCT02125461 | Stage III, unresectable NSCLC | CRT plus sequential durvalumab | CRT | 713 | Phase 3 | Median PFS: 17.2 months vs 5.6 months, p = 0.0025 |
| NCT02617589 | Brain Cancer | Nivolumab + RT | TMZ + RT | 560 | Phase 3 | Median OS: 13.40 months vs 14.88 months, p = 0.0037 |
| NCT01295827 | Stage III NSCLC | Nivolumab after standard RT | Nivolumab | 97 | Phase 2 | Median PFS: 4.4 months vs 2.1 months, p = 0.019 |
| NCT02434081 | NSCLC Stage III | Concurrent standard CRT followed by nivolumab | None | 94 | Phase 2 | Median PFS: 12.7 months |
| NCT02336165 | Glioblastoma | Durvalumab + standard RT (2Gy × 30) | Durvalumab | 71 | Phase 2 | Median PFS: 19.9 months vs 13.0 months |
| NCT02684253 | HNSCC | SBRT (9Gy × 3) + nivolumab | Nivolumab | 62 | Phase 2 | ORR: 29% vs 34.5%, p = 0.86 |
| NCT01807065 | mCRPC | Sipuleucel-T +RT (300cGy × 10) | Sipuleucel-T | 51 | Phase 2 | Median PFS: 3.65vs 2.46, p = 0.06 |
| NCT02701400 | Recurrent SCLC | SBRT followed by durvalumab/ tremelimumab | Durvalumab/ tremelimumab | 18 | Phase 2 | Median OS: 5.7 months vs 2.8 months, p = 0.3772 |
| NCT02608385 | Metastatic cancer | SBRT + pembrolizumab | None | 79 | Phase 1 | Median OS: 9.6 months; median PFS: 3.1 months |
| NCT02303990 | Metastatic cancer | Hypofractionated radiotherapy and pembrolizumab | None | 24 | Phase 1 | 1 patient experienced a complete response and 4 had prolonged stable disease |
| NCT02298946 | Colorectal cancer with liver metastases | CTX + SBRT (8Gy × 3) + AMP-224 | CTX + SBRT (8Gy × 1) + AMP-224 | 15 | Phase 1 | No participants experienced a CR or PR |
NSCLC, non-small cell lung cancer; SCLC, small cell lung carcinoma; HNSCC, head and neck squamous cell carcinoma; mCRPC, metastatic castration resistant prostate cancer; SBRT, stereotactic body radiotherapy; CRT, chemoradiotherapy; RT, radiotherapy; TMZ, temozolomide; CTX, cyclophosphamide; SABR, stereotactic ablative radiotherapy; OS, overall survival; PFS, progression-free survival; ORR, objective response rate; CR, complete response; PR, partial response.
Radiotherapy synergizes with immunotherapy and potently augments antitumor immunity
Most cancer cannot induce strong endogenous immune responses to eradicate cancer and is not sensitive enough to achieve curative outcomes from the adopted synthetic immune approaches alone [69]. Ionizing irradiation directly kills tumor cells and is able to augment tumor-specific immunity. However, local relapses often occur when radiotherapy is administrated alone [70]. Since radiotherapy has marked immunostimulatory effects [71], it provides a theoretical basis for combining immunotherapy with different forms of radiotherapy.
Combining of local radiotherapy with immunotherapy effectively enhances the therapeutic effects of tumors. Preclinical studies on HNSCC have revealed that by translating tumor microenvironments into an inflamed landscape, radiotherapy renders unresponsive HNSCC tumors sensitive to PD-L1 inhibition [72]. Radiotherapy and anti-PD-L1 antibody synergistically reduce the accumulation of tumor-infiltrating MDSCs, relieve T cells from the suppressive tumor immune microenvironment and amplify antitumor effects [73]. Radioimmunotherapy also induces strong antitumor responses outside of the irradiated area which is mediated by CD8+ T cells [45]. Through integration of immunotherapy with radiotherapy, patients can achieve local control and regression of metastasis. In addition, the combination of radiotherapy and anti-PD-L1 induces immunological memory effects when completely responsive mice are challenged with reimplanted tumor cells [73]. Since recurrence and distant metastasis are the leading causes of cancer-related death [74], generation of memory immune response can potently preventing tumor recurrence and metastasis, thus prolonging survival time of patients. Moreover, combination of radiotherapy with dual ICIs further enhances antitumor immune response in non-redundant immune mechanisms, in which radiation enhances the TCR repertoire diversity of intertumoral T cells, anti-CTLA-4 predominantly inhibits Tregs and anti-PD-L1 reverses T cell exhaustion [75].
Clinical studies also manifest inspiring survival benefits for patients in treatment of radioimmunotherapy. Nivolumab after radiotherapy in patients with advanced NSCLC resulted in longer median PFS (4.4 months vs 2.1 months, p = 0.019) and OS (10.7 months vs 5.3 months, p = 0.026) than in patients without previous radiotherapy [15]. In this study, patients received any radiotherapy at any timepoint before pembrolizumab were grouped to previous radiotherapy, without subgroup of conventional radiotherapy or hypofractionated radiotherapy. It is more often to meet predetermined clinical benefits when immunotherapy is combined with hypofractionated radiation. For example, stereotactic body radiotherapy (SBRT) of 8Gy for 3 times preceding pembrolizumab treatment in patients with metastatic NSCLC improved ORR from 18 to 36% (p = 0.07) at 12 weeks compared with patients in pembrolizumab alone group, with no increase in treatment-related toxic effects [76]. The combination of anti-CTLA-4 antibody ipilimumab and radiotherapy (6 Gy × 5 or 9.5 Gy × 3) in chemotherapy-refractory metastatic NSCLC represented 18% of ORR in enrolled patients [77]. Functional analysis showed increased level of IFN-β in serum and rapid expansion of CD8+ T cells [77], which is in concordant with studies in mice.
In conclusion, radioimmunotherapy remodels the suppressive tumor immune microenvironment and promotes generation of T cell responses that recognize and eradicate cancer cells, both in irradiated tumor sites and distant metastasis. Synergistic effects are more likely to occur when immunotherapy is in combination with SBRT, which is associated with the stimulatory effect on immune system of high-dose, hypofractionated radiation [78]. It is prospective to achieve optimal results by utilizing synergistic effects of immunotherapy and radiotherapy.
Radiation fails to enhance combinatorial effects between radiotherapy and immunotherapy in some circumstances
Immunotherapy eliminates cancer cells by stimulating antitumor immune responses, which largely rely on activation of CD8+ T cells or adopted immune cells. Radiotherapy may potently dampen curative effects of immunotherapy by upregulating infiltration of suppressive immune cells and directly damaging circulating lymphocytes, including CD8+ T cells. Therefore, radiotherapy and immunotherapy do not always show synergetic effect with each other.
Although anti-PD-1 antibody combined with 8Gy × 2 irradiation enhances primary and abscopal tumor control in a CD8+ T-cell dependent manner and reversed adaptive immune resistance [79], anti-PD-1 antibody following 2Gy × 10 irradiation suppresses expression of IFN in tumor-specific CD8+ T cells within DLN and do not enhance primary tumor control or survival [79]. The lack of effect may be due to lymphopenia and immune suppression induced by low-dose daily fractionated radiotherapy. Elective nodal irradiation is frequently used in treatment of localized tumors, aiming at addressing potential subclinical nodal micrometastases. However, when combined with anti-CTLA-4 antibody, elective nodal irradiation contributes to immunosuppressive tumor immune microenvironment and shows no prolongation of survival time compared with monotherapy of anti-CTLA-4 antibody [80]. Moreover, although radiotherapy combined with anti-CTLA-4 leads to tumor regression, there is concurrent upregulation of PD-L1 expression, which induces T-cell exhaustion and drug resistance [75].
Consistent with preclinical studies, there are several clinical trials that show no combinatorial effects between radiotherapy and immunotherapy. A phase 3 trial involving prostate cancer shows that in patients who progressed after docetaxel treatment, radiotherapy of 8Gy combined with ipilimumab exhibits no significant differences of OS compared to radiotherapy alone group [81]. Preclinical evidence suggests that hypofractionated ionizing radiation significantly enhances tumor control when combined with ICIs [79]. However, clinical trial which aims at evaluating the synergistic effects of nivolumab and SBRT (9Gy × 3) in HNSCC failed to improve ORR and there was no improvement in abscopal effect observed until 30 months [16]. Cellular immunotherapy is highly effective against a number of malignancies [82]. As the first autologous cellular immunotherapy approved by FDA, sipuleucel-T represented a 4.1-month improvement in median survival compared with placebo [83]. However, sipuleucel-T following radiotherapy of 300cGy × 10 in metastatic castration resistant prostate cancer showed comparable median PFS with sipuleucel-T alone group. Moreover, cumulative antigen presenting cells and IFN-γ+ T cells are even higher in Sipuleucel-T alone group [84].
In conclusion, radiotherapy may weaken the efficacy of immunotherapy in some circumstances. Whether immunotherapy synergizes with radiotherapy is related to multiple factors. The immunomodulatory effect of radiotherapy should be well considered when it is combined with immunotherapy. There is a great need of additional investigation to determine the optimal radiotherapy regimens, targeted checkpoints, and patient cohort to fully evaluate therapeutic effect of radioimmunotherapy.
Enhancing the synergistic effects of radiotherapy and immunotherapy by protecting the immune system
To optimize antitumor effects of radioimmunotherapy, several aspects should be considered, including exploration of appropriate radiotherapy regimens, consideration of appropriate schedules when immunotherapy is combined with radiotherapy and reduction of the immunosuppressive effects of radiotherapy.
Choice of appropriate radiotherapy regimens
To optimize the effects of radioimmunotherapy, radiotherapy regimens should be designed to include two factors: dose and fraction. Conventional radiotherapy is empirically applied by clinicians to achieve local tumor control and tolerable toxicity. However, even tolerable lymphopenia attenuates the therapeutic effects of radiotherapy combined with immunotherapy. Conventional radiotherapy is developed without considering the potential therapeutic effects of the immune system, which usually results in adverse effects on the immune system and is not conducive for immunotherapy [10]. Hypofractionated radiation enhances antitumor immunity when combined with ICIs and results in significant tumor control [79]. Moreover, hypofractionated radiation also has superioreffects compared to single large dose radiotherapy.
Studies have reported improved efficacies in terms of the combination of hypofractionated radiation and immunotherapy. As noted above, anti-PD-1 antibody combined with 8Gy × 2, rather than 2Gy × 10 enhanced primary and abscopal tumor control [79]. Local radiotherapy at a single dose of 20Gy was as effective as 8Gy × 3 and 6Gy × 5. However, only fractionated radiotherapy significantly improves tumor growth both in primary and secondary tumor sites when combined with anti-CTLA-4 antibodies [85]. Single radiation of 12Gy combined with anti-PD-L1 synergistically promoted antitumor immunity by markedly reducing MDSCs and increasing the infiltration and priming of CD8+ T cells in breast cancer mouse model [86]. Moreover, 12Gy radiotherapy of oral cancer polarized macrophages towards an M2 phenotype [87]. We suspect that this contradictory effect may be associated with types of tumors.
There is a specific therapeutic window for radiotherapy regimen when combined with ICIs. The final effects of radioimmunotherapy depends on combined effects of the intricate impact on the immune system. Hypofractionated radiation is more suitable when combined with immunotherapy. However, clinical trials of radiotherapy combined with immunotherapy are usually based on classical regimens. Studies have investigated the synergistic effects of immunotherapy with different doses and fractions of radiotherapy. The optimal regiment of radiotherapy when combined with immunotherapy still need to be explored.
Explore the schedule when immunotherapy is combined with radiotherapy
Effect of immunotherapy administration schedule was reported to have significant affections on tumor inhibition treated with radiotherapy. Concurrent administration of radiotherapy and anti‑PD‑L1 treatment, rather than sequential treatment is associated with long‑term tumor control [88]. Administration of G-CSF can rapidly reverse irradiation-induced neutropenia [89], which reduces radiotherapy-associated toxicity, along with the potential for improving the effects of immunotherapy. Blockade of TGFβ offers a strategy for generating in situ tumor vaccine effects and was shown to extend survival of mice administered with a combination of anti-PD-1 antibody and radiotherapy [90]. Administration of immunomodulatory drugs during radioimmunotherapy may augment the therapeutic effects in patients.
Studies indicated that concomitant, but not sequential treatment of radiotherapy and immunotherapy is more likely to induce better prognostic outcomes in preclinical models. However, the KEYNOTE-001 phase 1 trial revealed that patients receiving any form of radiotherapy prior to pembrolizumab exhibited prolonged PFS [15]. Moreover, concurrent administration of nivolumab with SBRT did not improve ORR versus nivolumab along arm in patients with metastatic HNSCC [16]. The optimal sequence may be associated with multiple factors, such as the type of immunotherapy, regimen of radiotherapy, tumor features and differences between individuals. All of these factors should be taken into account for better prognostic effects.
Reduction of the field of radiotherapy
As earlier mentioned, intact cancer immunity cycle is essential in immunotherapy. It is important for radioimmunotherapy to protect lymphocytes during radiotherapy. Moreover, abscopal effect are highly dependent on activated immune system. To reduce the negative effects of radiotherapy on immune cells, it is necessary to avoid the irradiation of lymph nodes, bone marrow and blood.
In the past two decades, IMRT as well as other technologies have enabled the application of more conformal doses in radiotherapy, making it possible to reduce radiation doses of non-malignant tissues while ensuring tumoricidal doses to local tumors. Conventional radiotherapy regimens in various indications such as NSCLC, cervical cancer, HNSCC are generally formulated with a full dose of 50–70Gy to the tumor site and 45–50Gy to DLNs for the purpose of prophylactic coverage [91]. Since DLNs constitute major platforms for cross priming of T cells, and T cells in lymphoid tissues are extremely sensitive to radiotherapy, irradiation of tumor free lymph nodes can perturb the initiation of antitumor immune responses. In a previous study, nasopharyngeal carcinoma patients with tumor free level Ib lymph nodes showed rare regional lymph node recurrence when treated with level Ib-sparing IMRT [92]. Therefore, prophylactic lymph node irradiation is not necessary in patients with negative cervical lymph nodes. The protection of lymph nodes should be highly considered.
T cells in blood circulation and bone marrow are exquisitely radiation sensitive. It is necessary to reduce the exposure of blood vessels and bone marrow during radiotherapy. Aforementioned clinical trial in HNSCC exhibited no statistically significant differences in ORR and no improvement in abscopal effects until 30 months when SBRT was given between the first and second dose of nivolumab. This outcome may be partly attributed to the injury of lymphocytes. The irradiated volume should be precisely designed.
Partial tumor irradiation may be a prospective choice in the future. Schäfer et al. presented that in patients with early breast cancer who had undergone breast-conserving surgery, partial breast irradiation was not inferior in local control and OS compared to whole breast irradiation [93]. Larger irradiation volumes are not positively correlated with improved disease control. Reducing the volume of radiotherapy may provide better outcomes when combined with immunotherapy.
Applications of new radiotherapy techniques
The development of new technologies is also an excellent option to minimize the side-effects of radiotherapy to the immune system. Proton radiotherapy is able to decrease the dose delivered to nonmalignant tissues, it enables the delivery of tumoricidal doses to the target [94], and induces comparable up-regulation of histocompatibility leukocyte antigen as well as TAAs. In addition, proton radiotherapy increases the exposure of calreticulin on the surface of tumor cells, which increases the killing effects of cytotoxic T lymphocytes to tumor cells [95]. It is promising to combine immunotherapy with proton radiotherapy.
Heavy ion radiotherapy (HIRT) is becoming a cutting-edge technology for malignant tumors. HIRT enables changes in energy and superimposition of multiple Bragg peaks, thereby providing a more accurate treatment [96]. Carbon ion radiation therapy is associated with significantly higher OS, compared to proton radiotherapy and photon-based IMRT [97]. During treatment, HIRT will significantly reduce lymphocyte damage. Consequently, HIRT enhances the role of immune system in radioimmunotherapy.
FLASH radiotherapy makes it possible to deliver doses at ultra-high dose rate (> 40Gy/s), compared with conventional dose rate applied in clinic, which is about 5Gy/min [98]. Consequently, it reduces normal tissue toxicities and synchronously maintains local tumor control. By providing higher dose rate, FLASH radiotherapy reduces treatment duration. The short exposure time significantly reduces irradiation of circulating immune cells [99]. The protection of circulating immune cells provides foundation for combining FLASH radiotherapy with immunotherapy.
Studies on these new techniques are sparse. There is a need to determine whether patients benefit more from immunotherapy in combination with proton radiotherapy, HIRT or FLASH radiotherapy.
Conclusion
The efficacy of immunotherapy is highly dependent on the immune system. Combination of immunotherapy and other treatment methods should take full consideration of the immunomodulatory effects. Radiotherapy is an ideal companion due to its stimulatory effects on the immune system. However, radiotherapy exert profound immunomodulatory effects and not all radiotherapy regimens collaborate with immunotherapy. Understanding the underlying immunological mechanisms of radiotherapy will be critical in improving the synergistic effects of radiotherapy and immunotherapy. Available evidences prove that simultaneous administration of hypofractionated radiation with immunotherapy is more likely to enhance the therapeutic effects. Reduction of irradiation volumes also displays potential on improving outcomes in patients. Moreover, technological breakthroughs of radiotherapy technology provide an important opportunity to create maximal therapeutic effects of radioimmunotherapy. More innovative preclinical and clinical evidences are urgently needed to potentiate the therapeutic effect in patients.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81874222, and 82073354), and the China Postdoctoral Science Foundation (Grant No. 2020M682431)
CRediT authorship contribution statement
Danyi Zhai: Investigation, Software, Visualization, Writing – original draft. Dandan An: Investigation, Visualization. Chao Wan: Funding acquisition, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing. Kunyu Yang: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Contributor Information
Chao Wan, Email: wanc@hust.edu.cn.
Kunyu Yang, Email: yangkunyu@hust.edu.cn.
References
- 1.Bagchi S., Yuan R., Engleman E.G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. Jan 24. [DOI] [PubMed] [Google Scholar]
- 2.Andrews L.P., Yano H., Vignali D.A.A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat. Immunol. 2019;20(11):1425–1434. doi: 10.1038/s41590-019-0512-0. Nov. [DOI] [PubMed] [Google Scholar]
- 3.Schadendorf D., Hodi F.S., Robert C., et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015;33(17):1889–1894. doi: 10.1200/JCO.2014.56.2736. Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer R.J., Escudier B., McDermott D.F., et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya S., Asaithamby A. Repurposing DNA repair factors to eradicate tumor cells upon radiotherapy. Transl. Cancer Res. 2017;6:S822–S839. doi: 10.21037/tcr.2017.05.22. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrera F.G., Bourhis J., Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J. Clin. 2017;67(1):65–85. doi: 10.3322/caac.21358. Jan. [DOI] [PubMed] [Google Scholar]
- 8.Cascade P.N., Leibel S.A. Decision-making in radiotherapy for the cancer patient: the American college of radiology appropriateness criteria project. CA Cancer J. Clin. 1998;48(3):146–150. doi: 10.3322/canjclin.48.3.146. May-Jun. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya S., Asaithamby A. Repurposing DNA repair factors to eradicate tumor cells upon radiotherapy. Transl. Cancer Res. 2017;6(Suppl 5):S822–S839. doi: 10.21037/tcr.2017.05.22. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cascade P.N. Setting appropriateness guidelines for radiology. Radiology. 1994;192(1):50A–54A. Jul. [PubMed] [Google Scholar]
- 11.Golden E.B., Frances D., Pellicciotta I., Demaria S., Helen Barcellos-Hoff M., Formenti S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weichselbaum R.R., Ishwaran H., Yoon T., et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc. Natl. Acad Sci. U. S. A. 2008;105(47):18490–18495. doi: 10.1073/pnas.0809242105. Nov 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yovino S., Kleinberg L., Grossman S.A., Narayanan M., Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Investig. 2013;31(2):140–144. doi: 10.3109/07357907.2012.762780. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. Jul 25. [DOI] [PubMed] [Google Scholar]
- 15.Shaverdian N., Lisberg A.E., Bornazyan K., et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. doi: 10.1016/S1470-2045(17)30380-7. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBride S., Sherman E., Tsai C.J., et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. 2021;39(1):30. doi: 10.1200/Jco.20.00290. Jan 1-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen D.W., Verma V., Patel R.R., Barsoumian H.B., Cortez M.A., Welsh J.W. Absolute lymphocyte count predicts abscopal responses and outcomes in patients receiving combined immunotherapy and radiation therapy: analysis of 3 phase 1/2 trials. Int. J. Radiat. Oncol. 2020;108(1):196–203. doi: 10.1016/j.ijrobp.2020.01.032. Sep 1. [DOI] [PubMed] [Google Scholar]
- 18.Capone M., Giannarelli D., Mallardo D., et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer. 2018;6 doi: 10.1186/s40425-018-0383-1. Jul 16ARTN 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegde P.S., Chen D.S. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35. doi: 10.1016/j.immuni.2019.12.011. Jan 14. [DOI] [PubMed] [Google Scholar]
- 20.Tumeh P.C., Harview C.L., Yearley J.H., et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. Nov 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meric-Bernstam F., Larkin J., Tabernero J., Bonini C. Enhancing anti-tumour efficacy with immunotherapy combinations. Lancet. 2021;397(10278):1010–1022. doi: 10.1016/S0140-6736(20)32598-8. Mar 13. [DOI] [PubMed] [Google Scholar]
- 22.Yu W.D., Sun G., Li J., Xu J., Wang X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019;452:66–70. doi: 10.1016/j.canlet.2019.02.048. Jun 28. [DOI] [PubMed] [Google Scholar]
- 23.Hennequin C., Guillerm S., Quero L. Combination of chemotherapy and radiotherapy: a thirty years evolution. Cancer Radiother. 2019;23(6–7):662–665. doi: 10.1016/j.canrad.2019.07.157. Oct. [DOI] [PubMed] [Google Scholar]
- 24.Bernier J., Poortmans P.M. Surgery and radiation therapy of triple-negative breast cancers: from biology to clinics. Breast. 2016;28:148–155. doi: 10.1016/j.breast.2016.05.014. Aug. [DOI] [PubMed] [Google Scholar]
- 25.Patrushev M., Kasymov V., Patrusheva V., Ushakova T., Gogvadze V., Gaziev A. Mitochondrial permeability transition triggers the release of mtDNA fragments. Cell. Mol. Life Sci. 2004;61(24):3100–3103. doi: 10.1007/s00018-004-4424-1. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng L.F., Liang H., Xu M., et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type i interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamazaki T., Galluzzi L. Mitochondrial control of innate immune signaling by irradiated cancer cells. Oncoimmunology. 2020;9(1) doi: 10.1080/2162402X.2020.1797292. Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnette B.C., Liang H., Lee Y., et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–2496. doi: 10.1158/0008-5472.Can-10-2820. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamond J.M., Vanpouille-Box C., Spada S., et al. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol. Res. 2018;6(8):910–920. doi: 10.1158/2326-6066.Cir-17-0581. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim J.Y.H., Gerber S.A., Murphy S.P., Lord E.M. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol. Immunother. 2014;63(3):259–271. doi: 10.1007/s00262-013-1506-7. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Limbergen E.J., De Ruysscher D.K., Pimentel V.O., et al. Combining radiotherapy with immunotherapy: the past, the present and the future. Br. J. Radiol. 2017;90(1076) doi: 10.1259/bjr.20170157. ARTN 20170157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zitvogel L., Kepp O., Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140(6):798–804. doi: 10.1016/j.cell.2010.02.015. Mar 19. [DOI] [PubMed] [Google Scholar]
- 33.Krysko D.V., Garg A.D., Kaczmarek A., Krysko O., Agostinis P., Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer. 2012;12(12):860–875. doi: 10.1038/nrc3380. Dec. [DOI] [PubMed] [Google Scholar]
- 34.Chao M.P., Jaiswal S., Weissman-Tsukamoto R., et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci. Transl. Med. 2010;2(63) doi: 10.1126/scitranslmed.3001375. Dec 22ARTN 63ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawaria S., Binder R.J. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat. Commun. 2011;2:521. doi: 10.1038/ncomms1524. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apetoh L., Ghiringhelli F., Tesniere A., et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. Sep. [DOI] [PubMed] [Google Scholar]
- 37.Perregaux D.G., McNiff P., Laliberte R., Conklyn M., Gabel C.A. ATP acts as an agonist to promote stimulus-induced secretion of IL-1 beta and IL-18 in human blood. J. Immunol. 2000;165(8):4615–4623. doi: 10.4049/jimmunol.165.8.4615. Oct 15. [DOI] [PubMed] [Google Scholar]
- 38.McLaughlin M., Patin E.C., Pedersen M., et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer. 2020;20(4):203–217. doi: 10.1038/s41568-020-0246-1. Apr. [DOI] [PubMed] [Google Scholar]
- 39.Formenti S.C., Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. JNCI J. Natl. Cancer Inst. 2013;105(4):256–265. doi: 10.1093/jnci/djs629. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudqvist N.P., Pilones K.A., Lhuillier C., et al. Radiotherapy and CTLA-4 blockade shape the TCR repertoire of tumor-infiltrating T Cells. Cancer Immunol. Res. 2018;6(2):139–150. doi: 10.1158/2326-6066.CIR-17-0134. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reits E.A., Hodge J.W., Herberts C.A., et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Ruiz M.E., Vanpouille-Box C., Melero I., Formenti S.C., Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39(8):644–655. doi: 10.1016/j.it.2018.06.001. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grass G.D., Krishna N., Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr. Probl. Cancer. 2016;40(1):10–24. doi: 10.1016/j.currproblcancer.2015.10.003. Jan-Feb. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y., Dong Y.P., Kong L., Shi F., Zhu H., Yu J.M. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018;11 doi: 10.1186/s13045-018-0647-8. Aug 16ARTN 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ngwa W., Irabor O.C., Schoenfeld J.D., Hesser J., Demaria S., Formenti S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer. 2018;18(5):313–322. doi: 10.1038/nrc.2018.6. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golden E.B., Chhabra A., Chachoua A., et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795–803. doi: 10.1016/s1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 47.Ngwa W., Irabor O.C., Schoenfeld J.D., Hesser J., Demaria S., Formenti S.C. Using immunotherapy to boost the abscopal effect. 放疗+免疫治疗增强远隔效应. Nat. Rev. Cancer. 2018;18(5):313–322. doi: 10.1038/nrc.2018.6. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benci J.L., Xu B., Qiu Y., et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167(6):1540–1554. doi: 10.1016/j.cell.2016.11.022. Dec 1e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khodarev N.N., Roizman B., Weichselbaum R.R. Molecular pathways: interferon/stat1 pathway: role in the tumor resistance to genotoxic stress and aggressive growth. Clin. Cancer Res. 2012;18(11):3015–3021. doi: 10.1158/1078-0432.CCR-11-3225. Jun 1. [DOI] [PubMed] [Google Scholar]
- 50.Lee S.J., Jang B.C., Lee S.W., et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580(3):755–762. doi: 10.1016/j.febslet.2005.12.093. Feb 6. [DOI] [PubMed] [Google Scholar]
- 51.Yang X., Zhang X., Fu M.L., et al. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25(1):37–48. doi: 10.1016/j.ccr.2013.12.004. Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmgaard R.B., Zamarin D., Munn D.H., Wolchok J.D., Allison J.P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 2013;210(7):1389–1402. doi: 10.1084/jem.20130066. Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang H., Deng L., Hou Y., et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 2017;8(1):1736. doi: 10.1038/s41467-017-01566-5. Nov 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang D., Xiao-Feng H., Guan-Jun D., et al. Activated STING enhances Tregs infiltration in the HPV-related carcinogenesis of tongue squamous cells via the c-jun/CCL22 signal. Biochim. Biophys. Acta. 2015;1852(11):2494–2503. doi: 10.1016/j.bbadis.2015.08.011. Nov. [DOI] [PubMed] [Google Scholar]
- 55.Mondini M., Loyher P.L., Hamon P., et al. CCR2-dependent recruitment of tregs and monocytes following radiotherapy is associated with TNFalpha-mediated resistance. Cancer Immunol. Res. 2019;7(3):376–387. doi: 10.1158/2326-6066.CIR-18-0633. Mar. [DOI] [PubMed] [Google Scholar]
- 56.Casagrande N., Borghese C., Visser L., Mongiat M., Colombatti A., Aldinucci D. CCR5 antagonism by maraviroc inhibits Hodgkin lymphoma microenvironment interactions and xenograft growth. Haematologica. 2019;104(3):564–575. doi: 10.3324/haematol.2018.196725. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Facciabene A., Motz G.T., Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72(9):2162–2171. doi: 10.1158/0008-5472.Can-11-3687. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strauss L., Bergmann C., Szczepanski M., Gooding W., Johnson J.T., Whiteside T.L. A unique subset of CD4(+)CD25(high) Foxp3(+) T cells secreting interleukin-10 and transforming growth factor-beta 1 mediates suppression in the tumor microenvironment. Clin. Cancer Res. 2007;13(15):4345–4354. doi: 10.1158/1078-0432.Ccr-07-0472. Aug 1. [DOI] [PubMed] [Google Scholar]
- 59.Mauch P., Constine L., Greenberger J., et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995;31(5):1319–1339. doi: 10.1016/0360-3016(94)00430-S. Mar 30. [DOI] [PubMed] [Google Scholar]
- 60.Mac Manus M., Lamborn K., Khan W., Varghese A., Graef L., Knox S. Radiotherapy-associated neutropenia and thrombocytopenia: analysis of risk factors and development of a predictive model. Blood. 1997;89(7):2303–2310. Apr 1. [PubMed] [Google Scholar]
- 61.Bauer M., Goldstein M., Christmann M., Becker H., Heylmann D., Kaina B. Human monocytes are severely impaired in base and DNA double-strand break repair that renders them vulnerable to oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2011;108(52):21105–21110. doi: 10.1073/pnas.1111919109. Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaue D., McBride W.H. T lymphocytes and normal tissue responses to radiation. Front. Oncol. 2012;2:119. doi: 10.3389/fonc.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Ruysscher D., Niedermann G., Burnet N.G., Siva S., Lee A.W.M., Hegi-Johnson F. Radiotherapy toxicity. Nat. Rev. Dis. Prim. 2019;5(1):13. doi: 10.1038/s41572-019-0064-5. Feb 21. [DOI] [PubMed] [Google Scholar]
- 64.Gudkov A.V., Komarova E.A. The role of p53 in determining sensitivity to radiotherapy. Nat. Rev. Cancer. 2003;3(2):117–129. doi: 10.1038/nrc992. Feb. [DOI] [PubMed] [Google Scholar]
- 65.Jiang L., Zhao X., Meng X., Yu J. Involved field irradiation for the treatment of esophageal cancer: is it better than elective nodal irradiation? Cancer Lett. 2015;357(1):69–74. doi: 10.1016/j.canlet.2014.11.045. Feb 1. [DOI] [PubMed] [Google Scholar]
- 66.Marciscano A.E., Ghasemzadeh A., Nirschl T.R., et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin. Cancer Res. 2018;24(20):5058–5071. doi: 10.1158/1078-0432.Ccr-17-3427. Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuo P., Bratman S.V., Shultz D.B., et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin. Cancer Res. 2014;20(21):5558–5569. doi: 10.1158/1078-0432.Ccr-14-1138. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weichselbaum R.R., Liang H., Deng L.F., Fu Y.X. Radiotherapy and immunotherapy: a beneficial liaison? Nat. Rev. Clin. Oncol. 2017;14(6):365–379. doi: 10.1038/nrclinonc.2016.211. Jun. [DOI] [PubMed] [Google Scholar]
- 69.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hennequin C., Hannoun-Levi J.M., Rozet F. Management of local relapse after prostate cancer radiotherapy: surgery or radiotherapy? Cancer Radiother. 2017;21(6–7):433–436. doi: 10.1016/j.canrad.2017.07.026. Oct. [DOI] [PubMed] [Google Scholar]
- 71.Demaria S., Ng B., Devitt M.L., et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. 2004;58(3):862–870. doi: 10.1016/j.ijrobp.2003.09.012. Mar 1. [DOI] [PubMed] [Google Scholar]
- 72.Oweida A., Lennon S., Calame D., et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology. 2017;6(10) doi: 10.1080/2162402X.2017.1356153. ARTN e1356153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng L., Liang H., Burnette B., et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014;124(2):687–695. doi: 10.1172/JCI67313. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ganesh K., Massague J. Targeting metastatic cancer. Nat. Med. 2021;27(1):34–44. doi: 10.1038/s41591-020-01195-4. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Twyman-Saint Victor C., Rech A.J., Maity A., et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373. doi: 10.1038/nature14292. Apr 16-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Theelen W.S.M.E., Peulen H.M.U., Lalezari F., et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1276–1282. doi: 10.1001/jamaoncol.2019.1478. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Formenti S.C., Rudqvist N.P., Golden E., et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018;24(12):1845. doi: 10.1038/s41591-018-0232-2. Dec-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arnold K.M., Flynn N.J., Raben A., et al. The impact of radiation on the tumor microenvironment: effect of dose and fractionation schedules. Cancer Growth Metastasis. 2018;11 doi: 10.1177/1179064418761639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morisada M., Clavijo P.E., Moore E., et al. PD-1 blockade reverses adaptive immune resistance induced by high-dose hypofractionated but not low-dose daily fractionated radiation. Oncoimmunology. 2018;7(3) doi: 10.1080/2162402X.2017.1395996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marciscano A.E., Ghasemzadeh A., Nirschl T.R., et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin. Cancer Res. 2018;24(20):5058–5071. doi: 10.1158/1078-0432.CCR-17-3427. Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kwon E.D., Drake C.G., Scher H.I., et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oved J.H., Barrett D.M., Teachey D.T. Cellular therapy: immune-related complications. Immunol. Rev. 2019;290(1):114–126. doi: 10.1111/imr.12768. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kantoff P.W., Higano C.S., Shore N.D., et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. Jul 29. [DOI] [PubMed] [Google Scholar]
- 84.Twardowski P., Wong J.Y.C., Pal S.K., Frankel P.H., Franklin K., Junqueira M. Randomized phase II trial of sipuleucel-T immunotherapy preceded by sensitizing radiation therapy and sipuleucel-t alone in patients with metastatic castrate resistant prostate cancer. J. Clin. Oncol. 2017;35(6) doi: 10.1200/JCO.2017.35.6_suppl.222. Feb 20. [DOI] [PubMed] [Google Scholar]
- 85.Dewan M.Z., Galloway A.E., Kawashima N., et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deng L.F., Liang H., Burnette B., et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014;124(2):687–695. doi: 10.1172/Jci67313. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okubo M., Kioi M., Nakashima H., et al. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci. Rep. 2016;6 doi: 10.1038/srep27548. -UkJun 8ARTN 27548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dovedi S.J., Illidge T.M. The antitumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. Oncoimmunology. 2015;4(7) doi: 10.1080/2162402X.2015.1016709. ARTN e1016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidberger H., Hess C.F., Hoffmann W., Reuss-Borst M.A., Bamberg M. Granulocyte colony-stimulating factor treatment of leucopenia during fractionated radiotherapy. Eur. J. Cancer. 1993;29A(14):1927–1931. doi: 10.1016/0959-8049(93)90445-l. [DOI] [PubMed] [Google Scholar]
- 90.Vanpouille-Box C., Diamond J.M., Pilones K.A., et al. TGFbeta is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015;75(11):2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bentzen S.M., Harari P.M., Bernier J. Exploitable mechanisms for combining drugs with radiation: concepts, achievements and future directions. Nat. Clin. Pract. Oncol. 2007;4(3):172–180. doi: 10.1038/ncponc0744. Mar. [DOI] [PubMed] [Google Scholar]
- 92.Chen J., Ou D., He X., Hu C. Sparing level Ib lymph nodes by intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma. Int. J. Clin. Oncol. 2014;19(6):998–1004. doi: 10.1007/s10147-013-0650-6. Dec. [DOI] [PubMed] [Google Scholar]
- 93.Schäfer R., Strnad V., Polgár C., et al. Quality-of-life results for accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation in early breast cancer after breast-conserving surgery (GEC-ESTRO): 5-year results of a randomised, phase 3 trial. Lancet Oncol. 2018;19(6):834–844. doi: 10.1016/s1470-2045(18)30195-5. [DOI] [PubMed] [Google Scholar]
- 94.Huang J.Y., Mehta M. Can proton therapy reduce radiation-related lymphopenia in glioblastoma? Neuro Oncol. 2021;23(2):179–181. doi: 10.1093/neuonc/noaa273. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gameiro S.R., Malamas A.S., Bernstein M.B., et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int. J. Radiat. Oncol. Biol. Phys. 2016;95(1):120–130. doi: 10.1016/j.ijrobp.2016.02.022. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inaniwa T., Furukawa T., Kase Y., et al. Treatment planning for a scanned carbon beam with a modified microdosimetric kinetic model. Phys. Med. Biol. 2010;55(22):6721–6737. doi: 10.1088/0031-9155/55/22/008. Nov 21. [DOI] [PubMed] [Google Scholar]
- 97.Zhang W., Hu W., Hu J., et al. Carbon ion radiation therapy for sinonasal malignancies: promising results from 2282 cases from the real world. Cancer Sci. 2020;111(12):4465–4479. doi: 10.1111/cas.14650. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hughes J.R., Parsons J.L. FLASH radiotherapy: current knowledge and future insights using proton-beam therapy. Int. J. Mol. Sci. 2020;21(18) doi: 10.3390/ijms21186492. Sep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Durante M., Brauer-Krisch E., Hill M. Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br. J. Radiol. 2018;91(1082) doi: 10.1259/bjr.20170628. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]