Abstract
Guard cell turgor pressures in epidermal peels of broad bean (Vicia faba) were measured and controlled with a pressure probe. At the same time, images of the guard cell were acquired using confocal microscopy. To obtain a clear image of guard cell volume, a fluorescent dye that labels the plasma membrane was added to the solution bathing the epidermal peel. At each pressure, 17 to 20 optical sections (each 2 μm thick) were acquired. Out-of-focus light in these images was removed using blind deconvolution, and volume was estimated using direct linear integration. As pressure was increased from as low as 0.3 MPa to as high as 5.0 MPa, guard cell volume increased in a saturating fashion. The elastic modulus was calculated from these data and was found to range from approximately 2 to 40 MPa. The data allow inference of guard cell osmotic content from stomatal aperture and facilitate accurate mechanistic modeling of epidermal water relations and stomatal functioning.
Stomatal aperture in leaves is controlled by the turgor pressures of the guard cells (Pg) and the surrounding epidermal cells (Pe). Increases in Pg open the pore and increases in Pe close the pore, but the exact roles of these two parameters in determining aperture are complex (Franks et al., 1995, 1998). Equal increases in guard cell and epidermal turgor pressure generally close the pore; thus, epidermal cells have a “mechanical advantage” over guard cells (Glinka, 1971; Edwards et al., 1976; Franks et al., 1998). Although some progress has been made toward understanding the relationships between aperture, Pg, and Pe (Meidner and Edwards, 1975; Franks et al., 1995, 1998), less is known about how various perturbations affect Pg and Pe. Pg and Pe are functions of their respective water potentials (Ψg and Ψe) and osmotic pressures (πg and πe). Therefore, efforts to understand and predict the effects of environmental perturbations on stomatal aperture should focus on Ψg, Ψe, πe, and πg. The value of πg is known to vary widely in response to several stomatal effectors (Bearce and Kohl, 1970; Humble and Raschke, 1971; MacRobbie, 1980) and appears to be a major determinant of stomatal aperture in most species. The value of πe may remain relatively constant as stomata respond to environmental factors (Shackel, 1987; Nonami et al., 1990) or, as in the stomata of many grass species, it may vary inversely with πg (Raschke and Fellows, 1971).
The pressure-volume relationship for guard cells is of central importance in the characterization of πg because for any given Ψg and guard cell solutecontent, Pg is ultimately determined by guard cell volume. The only published data (of which we are aware) for pressure-volume relationships in guard cells show a sigmoidal relationship between pressure and volume in broad bean (Vicia faba; Raschke and Dickerson, 1972; Raschke et al., 1972). In those studies guard cell volume was estimated with a conventional light microscope, and Pg was calculated from the water potential of the surrounding medium, from the estimated volume of the guard cell, and from the osmotic pressure at incipient plasmolysis (Pg = 0). The sigmoidal relationship reported in that study contrasts with pressure-volume relationships for other types of plant and algal cells that have been measured using the cell pressure probe (Steudle et al., 1977; Husken et al., 1978; Tomos et al., 1981; Tomos and Leigh, 1999). These measurements were made by observing the meniscus between the oil and cell sap (Husken et al., 1978; Steudle, 1993). However, the large range of volumes over which guard cells operate make this approach impractical for guard cells. Franks et al. (1995, 1998) recently determined pressure-aperture relationships for guard cells of several species, including broad bean using the cell pressure probe to control Pg. By filling the cells with silicone oil from the probe, they were able to achieve large volume and pressure changes, but the elasticity of the probe was too great to allow volume measurements from the probe alone.
Our goal in this study was to determine pressure-volume relationships for guard cells of broad bean. Our approach was to use the cell pressure probe to control guard cell pressure and volume, and to simultaneously measure guard cell volume with a confocal microscope. Use of the confocal microscope enabled guard cell volume to be measured with a high degree of precision while being completely independent of the elastic properties of the pressure probe.
RESULTS
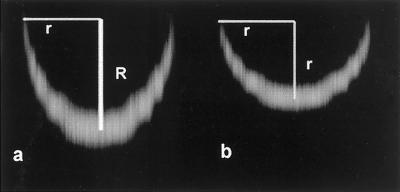
Because the light emitted from the guard cell membranes passed through an aqueous solution, a coverslip, and then a layer of immersion oil before reaching the objective, it was necessary to correct our images for the differences in refractive index among these substances. This correction was accomplished by imaging polystyrene beads (15-μm diameter) that were suspended in water. The lower one-half of the bead (the one-half closest to the objective on the inverted microscope) was distorted by the differences in refractive index mentioned above and therefore did not appear as a half-sphere in the raw images (Fig. 1a). Images of the top one-half of the polystyrene beads were not useful because they were further distorted by the difference in refractive index between the bead and the water in which it was suspended. To empirically correct for the distortion in the images of the lower one-half of the bead, the size of the z-step of the confocal images was adjusted to make the radius in the x-z plane equal to that in the x-y plane (Fig. 1b). This correction factor was determined to be 0.70, which is quite close to the factor of 0.69 determined by White et al. (1996) using a similar method. In these images the diameter of the bead was found to be 14.5 μm, which is sufficiently close to the value of 15 μm reported by the manufacturer to conclude that our imaging and blind deconvolution procedures provided an accurate representation of the material.
Figure 1.
Confocal images of the lower one-half of a spherical polystyrene bead containing a fluorescent dye in the outer layer. Image a is distorted in the z-plane by differences in refractive index among mounting materials. In image b, the pixel spacing in the z-plane has been corrected to make the image of the bead spherical (see text for discussion). The distances R (= 10.36 μ m) and r (= 7.25 μ m) represent the uncorrected and corrected radii, respectively. The image of the top one-half of the bead was further distorted by the light passing through the bead itself, and this portion of the image has been cropped for clarity.
To correct images of guard cells for the distortion discussed above, the pixel spacing in the z-direction was adjusted by a factor of 0.7. In contrast with polystyrene beads, it was possible to obtain accurate and complete three-dimensional confocal images of the guard cells because there was little or no difference in refractive index between the inside of the cell and the water surrounding the cell. Although there was some oil from the probe in the cell, and the refractive index of the oil (1.42) was slightly different from that of the surrounding water (1.33), this apparently caused very little distortion in the images of the guard cells. This conclusion is based on two observations. First, there were no obvious discontinuities or distortions in the outlines of the cells, as there were in the polystyrene beads. Second, images of guard cells that were inflated with oil were virtually identical in overall shape and appearance to those of guard cells that were opened to a similar aperture with light, and hence contained no oil (data not shown, but see images at http://bioweb.usu.edu/kmott). Therefore, it seems probable that some light was distorted by droplets of oil in the cell, but most of the light remained undistorted.
The technical difficulty associated with the simultaneous use of the pressure probe and the confocal microscope limited the number of experiments that yielded useable data. As noted earlier, broad bean guard cells, although ideal for confocal imaging, were difficult to probe because of their small size relative to other plant cells. Further, the considerable time necessary for acquisition of confocal images made it difficult to obtain data for more than a few pressure values before the seal formed by the guard cell membrane around the probe began to leak. This problem was minimized by using relatively large spacing between optical sections, thereby reducing the amount of the time necessary to fully image the guard cell at each pressure. Many confocal images were not useable for volume measurements because of uneven dye distribution in the guard cell membranes. Despite these problems, three fairly complete pressure volume curves for single guard cells were obtained. These curves were generated by starting at the lowest pressure and volume and gradually inflating the cell. Attempts to reduce pressure and volume failed because the tip of the probe became clogged with cellular contents. Therefore, all data are for ascending pressure and volume. For one of these data sets the probe was inserted through the left guard cell and into the right guard cell as described in Franks et al. (1995). In this arrangement, both cells were inflated by the addition of oil to the right cell. However, volume measurements for the left cell (not shown) were highly erratic compared with those of the right cell and two other cells for which the probe was directly inserted, and it seems probable that the left cell did not inflate evenly as oil was added to the cell. Because of this problem and the technical difficulty associated with inserting the probe in this configuration, only single cells were attempted for the rest of the study.
Vital staining revealed that essentially all of guard cells remained alive in the epidermal peels used in this study, but approximately 95% of the epidermal cells were dead (data not shown). In the experiments reported the epidermal cells adjacent to the guard cells being measured were dead.
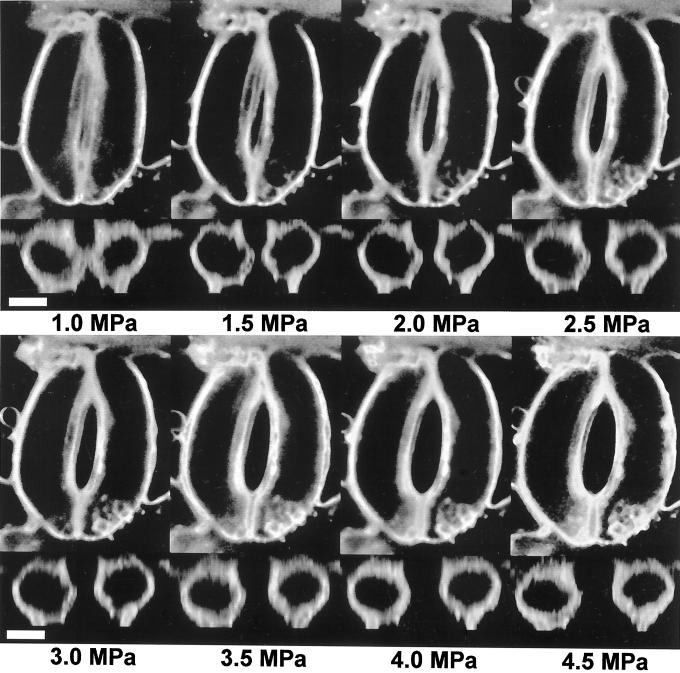
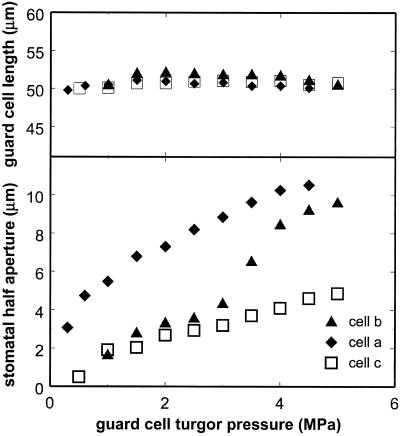
Figure 2 is a composite picture showing medial sections in the x-y and x-z planes for one pair of guard cells at eight different pressures. In the experiment shown in Figure 2 the pressure probe was inserted through the tip of the left guard cell and then into the right guard cell, so both guard cells inflated as oil was added to the cell on the right. In other experiments only one guard cell was inflated, and only that cell changed shape. Guard cell length (Fig. 3a) remained essentially constant as volume increased, but the one-half aperture of the stomatal pore increased with pressure in a nonlinear fashion (Fig. 3b).
Figure 2.
Composite figure showing medial paradermal (x-y plane) and transdermal (x-z plane) images of guard cells as affected by turgor pressure. The pressure probe was inserted through the top of the left cell and into the right cell, and this caused both cells to fill with oil (see “Materials and Methods” for details). Scale bar = 10 μm. Movies showing three-dimensional reconstructions of guard cells can be found at http://bioweb.usu.edu/kmott or www.plantphysiol.org.
Figure 3.
Measurements of overall guard cell length and stomatal one-half aperture as a function of guard cell turgor pressure for three guard cells.
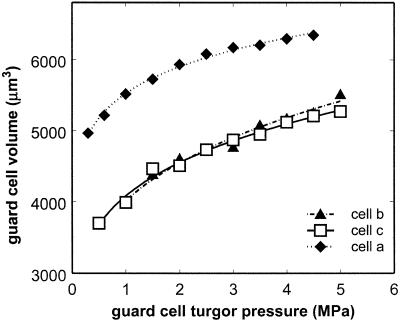
The relationship between pressure and volume for the guard cells was monotonic and negatively curved (Fig. 4), and a simple power function provided the best fit to all three data sets, with r2 values ranging from 0.9837 to 0.9988. The three guard cells for which measurements are shown had qualitatively similar pressure-volume relationships as evidenced by comparable fits with the same model type. However, these three cells varied considerably in absolute volume. Although it is difficult to generalize about the variability of guard cell volumes on the basis of three measured cells, the data presented here suggest that guard cell volumes may vary widely within a leaf of broad bean.
Figure 4.
Guard cell volume (Vg, μm3) as a function of guard cell turgor pressure (Pg, MPa) for three guard cells. Symbols represent direct measurements of Vg (from confocal images) and Pg (with the pressure probe); the lines show the power functions that were fit to the data.
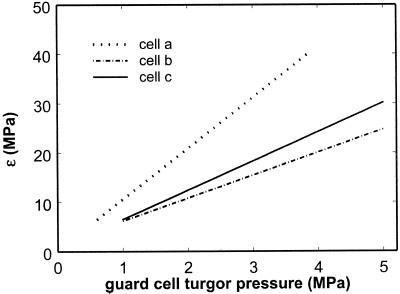
The elastic modulus (ε = VgdPg/dVg) was calculated for each guard cell from a power function that was fit to the pressure-volume relationship, and ε was then plotted against pressure (Fig. 5). For all three cells ε increased with turgor pressure. Although the increase in ε was approximately linear for all three cells, the exact shape of the relationship was dependent on the function used to describe the pressure-volume relationships shown in Figure 5. Therefore, the only robust conclusion that can be drawn from these data is that ε increased monotonically with pressure.
Figure 5.
Moduli of elasticity (εg = Vg × dPg/dVg) for the three guard cells with Pg-Vg curves shown in Figure 4. εg for each cell was determined by differentiation of the power function that was fit to that cell's Vg and Pg measurements.
DISCUSSION
Confocal microscopy has been used several times to investigate the morphology of the stomatal complex, and our images are similar to those in previous studies (Fricker et al., 1997). In any such study, however, it is important to validate the microscopy and imaging procedures using objects of a known shape and dimension (White et al., 1996). In the present study polystyrene beads with a diameter of 15 μm were suspended in water and imaged using the same techniques applied to the guard cells. These images revealed that our system accurately represented the objects in the x-y plane, but because of the differences in refractive index among immersion oil, water, and the coverslip, images were distorted in the z plane. This effect was compensated for by adjusting the pixel spacing in the z plane by a factor of 0.7 (see “Results”).
The general changes in shape and dimensions of the guard cells in this study are similar to those published previously (Willmer and Fricker, 1996), although there is somewhat less upward rotation of the guard cells during opening than previously reported (Weyers and Meidner, 1990). This difference was probably caused by the fact that the epidermal cells in our preparations were dead. The very small differences in overall length of the guard cells found in this study are consistent with earlier studies (see Willmer and Fricker, 1996) that show that guard cell length does not change appreciably as the pore opens. Measurements of one-half aperture are more difficult to compare with literature data because except for cell c, only one of the two guard cells was inflated. Values for the one-half aperture of cell c are consistent with aperture measurements for broad bean reported by Franks et al. (1998), but those of cells a and b are much higher. This is most likely because for cells a and b the absence of counterpressure from the other guard cell allowed the inflating guard cell to bow more extensively than it would have in the presence of counterpressure. Although the relationship between aperture and turgor pressure in cell b appears sigmoidal, the relationships for cells a and c are clearly saturating. The cause of this difference is unknown, but the saturating relationship is consistent with previous data taken at zero epidermal turgor pressure (Franks et al., 1998).
The pressure-volume relationships reported for guard cells of broad bean in this study are critical for interpreting measurements of guard-cell solute content and ion pumping rates in terms of guard-cell turgor pressure and vice versa. Pressure-volume curves for guard cells have been reported in a previous study (Raschke and Dickerson, 1972; Raschke et al., 1972). However, in that study guard cell volume was estimated with a conventional light microscope and Pg was calculated from the water potential of the surrounding medium, the estimated volume of the guard cell, and the osmotic pressure of the bathing solution that induced incipient plasmolysis (Pg = 0). In this study we used the pressure probe to control guard-cell volume independent of the medium outside the cells and to make direct measurements of turgor pressure. We also used confocal microscopy to construct three-dimensional images of individual guard cells and thereby make better estimations of volume than would be possible from traditional light microscopy. Confocal microscopy has been used in the study of guard cells previously (Fricker and White, 1990; Fricker et al., 1997), but this is the first study to measure guard cell volumes using this technique. The guard cell volumes reported in this study are consistent with those reported for broad bean in other studies (Raschke, 1979; Willmer and Fricker, 1996) using light microscopy to estimate volume. They are also similar to the vacuolar volumes estimated for Commelina communis guard cells using confocal microscopy (Fricker and White, 1990).
Our data are not consistent with the pressure-volume relationship measured for guard cells of broad bean in the earlier studies discussed above (Raschke and Dickerson, 1972; Raschke et al., 1972). In that study pressure and volume were found to be sigmoidally related, with positive curvature occurring at Pg values below about 1.2 MPa. In addition, the values for ε reported in that study are much lower than the values calculated in this study, although the relative volume changes are similar. It is possible that the differences in methodology discussed above are responsible for these discrepancies. It is also possible that the differences between our results and those reported earlier (Raschke and Dickerson, 1972) are due to differences in the state of the epidermal cells. All of the epidermal cells adjacent to the guard cells used in this study were dead, and hence, the guard cells were not influenced by epidermal turgor. However, the earlier study did not comment on the state of the epidermal cells in their preparations, so we cannot be certain what influence, if any, the epidermal cells had in their experiments.
The general shape of the pressure-volume curves reported here is qualitatively similar to those reported for other plant cells. However, the physical nature of guard cells is such that they span much greater ranges of pressure and volume than other plant cells (compare with Steudle et al., 1977; Husken et al., 1978). The large changes in relative volume and pressure reported in our study are realistic for guard cells because of the large and rapid changes in osmotic pressure (πg) that occur in these cells. For example, a turgor pressure of about 4.0 MPa was necessary to produce maximum aperture in Tradescantia virginiana and broad bean (Franks et al., 1998), and a similar value of Pg for maximum aperture in broad bean was estimated from measurements of guard cell osmotic pressure (Raschke, 1979). It should be noted, however, that much lower pressure ranges for complete opening of stomata in broad bean and T. virginiana have also been reported (Meidner and Edwards, 1975).
One of the first studies to provide data on ε for individual cells of higher plants (Husken et al., 1978) reported values of ε from 0.2 to 2.5 MPa in tissue cells of peppers and noted the now widely observed pressure dependence of ε. Similar studies since then have reported estimates of ε for higher plant cells spanning a considerable range. For example, values of ε in epidermal, subsidiary, and mesophyll cells of T. virginiana have been measured between 0.6 and 24 MPa (Zimmermann et al., 1980). Our data are consistent with these values except at very high turgor pressures where ε approached 40 MPa. These very high values of ε are at least partly due to the extremely high turgor pressures achieved in this study.
The positive dependence of ε on Pg found in this study has been observed many times in the literature. In this study the relationship between ε versus Pg was found to be approximately linear, which is consistent with other measurements on cells of higher plants (Steudle et al., 1977, 1982; Tyerman et al., 1989). However, for giant algal cells this relationship was found to be negatively curved (Zimmermann and Steudle, 1974, 1975). One difference between our calculation of ε and those in previous studies is that our calculations were made from the first derivative of the function that was fitted to the pressure versus volume data, and they therefore accurately reflect changes in volume. In many previous estimates of ε, the change in volume of the cell was small enough to be negligible and was therefore taken as a constant (Steudle et al., 1977). However, to obtain an accurate estimate of ε in guard cells, it is necessary to account for volume changes because these changes are very large relative to total cell volume.
Guard cell pressure-volume dynamics have been analyzed on the basis of the theory of polymer elasticity (Sharpe et al., 1987). Using the sigmoidal pressure-volume curves reported in a previous study (Raschke and Dickerson, 1972), two different modes of behavior in different pressure domains were distinguished. The first mode is isotropic expansion, which occurs at lower pressures and is characterized by roughly equal stretching of the cell wall in the longitudinal and tangential directions. During the isotropic mode, the dependence of Pg on Vg is positively curved (Sharpe et al., 1987). The second mode, anisotropic expansion, occurs at higher volumes and pressures where the radial wall thickenings have reached the limits of their elasticity. Most additional expansion occurs longitudinally (Sharpe et al., 1987) and the dependence of Pg on Vg is negatively curved. The data presented here show uniformly negative curvature in the dependence of Pg on Vg, suggesting that guard cell expansion was anisotropic within the range of Vg and Pg studied here.
A similar relationship was observed in T. virginiana (monotonic with negative curvature) between pressure and stomatal aperture in the absence of counterforce generated by epidermal turgor (Franks et al., 1995, 1998). However, in the presence of epidermal turgor this relationship took on a sigmoidal form in accordance with models of the pressure volume relationship for guard cells (Sharpe et al., 1987). These different pressure-aperture relationships may reflect an effect of epidermal turgor or volume on the relationship between guard cell volume and pressure. It is unfortunate that we were unable to obtain simultaneous measurements of pressure and volume in epidermal peels with intact epidermal cells, so we can only speculate on the issue. It seems possible, however, that if guard cell expansion is indeed isotropic at very low pressures in the absence of epidermal turgor, epidermal turgor could extend this region to higher guard cell pressures by physically limiting the paradermal expansion of guard cells. Further study is required to test this hypothesis.
MATERIALS AND METHODS
Plant Material
All measurements were carried out with broad bean (Vicia faba) plants grown in a controlled environment greenhouse in 1-L pots containing a substrate consisting of equal parts perlite, vermiculite, and peat moss. Pots were watered daily to excess with a nutrient solution containing 9.1 mm nitrogen, 1.8 mm phosphorus, 2.7 mm potassium, and 11 μm chelated iron (Peter's 20–10−20, Grace Sierra Horticultural Products, Milpitas, CA). Day and night temperatures were 30°C and 20°C, respectively, and day length was extended to 16 h when necessary with high-pressure sodium lamps, which provided a photon flux density of approximately 500 μmol m−2 s−1 at the top of the plant. The use of broad bean in this work was a compromise between suitability for pressure probe measurements and suitability for confocal microscopy. Tradescantia virginiana, with its much larger guard cells, would have been a better candidate for the pressure probe measurements. However, preliminary experiments with T. virginiana did not yield usable confocal images because the need for direct probe access to the guard cells necessitated inversion of the peel. This placed the subsidiary cells between the objective lens and the guard cells, attenuating light and placing the guard cells beyond the working distance of the objective.
Epidermal Peels and Membrane Labeling
Mature, non-senescent leaves of broad bean were excised from plants, and epidermal peels were prepared as described previously (Franks et al., 1995, 1998). Epidermal peels approximately 5 mm × 10 mm were mounted cuticle up in a well slide by applying a drop of molten “valap” (1:1:1, vaseline:lanolin:paraffin) to each end. The well was quickly filled with 200 μL of bathing solution comprised of 25 mm MES [2-(N-morpholino)ethanesulfonic acid] (pH 6.5, adjusted with NaOH). After the probe was inserted (see below) and immediately prior to the first pressure measurement, a membrane-selective fluorophore, {N-[3-triethylammoniumpropyl]-4-[6-(diethylamino) phenyl] hexatrienyl pyridinium dibromide; T-3166, Molecular Probes, Eugene, OR}, was added to the well slide to achieve a concentration of 0.125 mm. This molecule accumulates in biological membranes and fluoresces at approximately 610 nm when excited with the 488 nm laser of the confocal microscope.
To determine viability of the epidermal cells, a vital stain (fluorescein diacetate, F-1303, Molecular Probes) was applied to the epidermal peels at 10 μm for approximately 5 min before visually examining the cells for dye accumulation.
Guard Cell Pressure Measurements
The pressure probe used in this study has been described previously (Franks et al., 1995, 1998). This probe differs from earlier designs in that it has been specially designed to operate at very high pressures (up to 6.0 MPa), but its principle of operation is the same as the conventional cell pressure probe. To vary pressure within a guard cell the probe was inserted into the end of a guard cell and oil was added or removed from the cell interior (see Franks et al., 1995). Visual observation suggested that cell volume responded within a few seconds. However, at least 1 min was allowed for the cell to osmotically equilibrate before confocal images were acquired. In previous studies (Franks et al., 1995, 1998) it was possible to control the pressure in both guard cells of a stoma simultaneously by inserting the probe through the end of one guard cell and then into the other. This approach was attempted several times in the early phases of this study, but the added difficulty associated with acquiring confocal images made it impractical for most experiments.
Laser Scanning Confocal Microscopy
At each pressure a series of paradermal images (“optical sections” in the focal plane parallel to the epidermis) were collected using a Keller type MRC 1024 krypton/argon laser scanning confocal system (Bio-Rad, Hercules, CA) with an inverted microscope (Diaphot TE300, Nikon, Tokyo). The pinhole diameter for the system was 2 mm and the objective lens was a Nikon 60×, plan apo, oil immersion with a numerical aperture of 1.4. The 488-nm laser line was used for fluorophore excitation. The fluorophore FM 4-64 varies in its emission spectrum depending on its binding state, so two sets of images were collected simultaneously using different filters (HQ598 DF 40 and 680 DF 32). Each image pair was then merged. Paradermal images were collected in automated 2-μm steps from a focal plane approximately 4 μm above the guard cells to a plane approximately 4 μm below the guard cells. Three frames were averaged per section. This relatively large spacing between steps was necessary to allow complete imaging of the guard cells at each pressure in a short period of time. The x-y pixel spacing was 0.128 μm.
Although confocal microscopy eliminates most of the light from other paradermal planes in the specimen, each paradermal image invariably contains some interfering light from other planes. This fluorescent light is emitted from other planes and diverges according to its wavelength and the optical properties of the medium, and the pattern of divergence is called a point spread function. To eliminate this light from the final image, the series of paradermal images was processed by blind deconvolution (AutoDeblur software; AutoQuant Imaging, Watervliet, NY). The process of blind deconvolution does not require prior knowledge of the sample's point spread function because it iteratively searches for the most likely solution based on the noise characteristics of the detector electronics and the optical system (Holmes et al., 1995). Each image was subjected to 10 iterations using the highest resolution setting.
To evaluate the degree to which the confocal imaging and subsequent blind deconvolution procedures produced images that accurately represent the guard cell, these procedures were applied to an object of known shape and dimensions. Spherical polystyrene beads 15 μm in diameter and containing fluorescent dye in the outermost layer (F-7235, Molecular Probes) were suspended in distilled water and optical sections were collected with the confocal microscope using the procedure described for guard cells. These images were then subjected to the same blind deconvolution procedures used for guard cells.
Volume Measurements
The AutoDeblur software was used to interpolate three images parallel to and between each original pair of paradermal images, and then to generate a series of approximately 20 transsectional images (perpendicular to the leaf surface and to the longitudinal axis of the stomatal pore) that were separated by 2 μm. These transsectional images were found to be more conducive to area measurements than the paradermal images. Guard cell area for each transsectional image was determined by tracing the outline of the guard cell by hand, and volume was calculated by direct linear integration (multiplying the area of each slice by its thickness and then summing these slice volumes). The precision (repeatability) of these volume measurements was determined by performing multiple measurements on the same guard cells. Three different guard cells were used and the volume of each was measured five times. The standard deviations of these measurements were 1.65%, 1.45%, and 0.84% of the means for the three cells.
A commercially available data modeling program (CurveExpert) was used to fit volume and pressure measurements to 35 different models and to identify the best fits. Models that predicted zero or negative volume at zero turgor pressure were excluded from further analysis.
ACKNOWLEDGMENTS
We thank Rand Hooper for excellent technical assistance and Jevin West for his work in analyzing the confocal images.
Footnotes
This work was supported by the National Science Foundation (grant no. IBN–9808394 to K.A.M.).
This article contains Web-only data. The supplemental material is available at www.plantphysiol.org.
LITERATURE CITED
- Bearce BC, Kohl HC. Measuring osmotic pressure of sap within live cells by means of a visual melting point apparatus. Plant Physiol. 1970;46:515–519. doi: 10.1104/pp.46.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M, Meidner H, Sheriff DW. Direct measurement of turgor pressure potentials of guard cells: II. The mechanical advantage of subsidiary cells, the spannungsphase, and the optimum leaf water deficit. J Exp Bot. 1976;27:163–171. [Google Scholar]
- Franks PJ, Cowan IR, Farquhar GD. A study of stomatal mechanics using the cell pressure probe. Plant Cell Environ. 1998;21:94–100. [Google Scholar]
- Franks PJ, Cowan IR, Tyerman SD, Cleary AL, Lloyd J, Farquhar GD. Guard cell pressure/aperture characteristics measured with the pressure probe. Plant Cell Environ. 1995;18:795–800. [Google Scholar]
- Fricker M, White N. Volume measurements of guard cell vacuoles during stomatal movements using confocal microscopy. Trans R Microsc Soc. 1990;1:345–348. [Google Scholar]
- Fricker MD, Chow C-M, Errington RJ, May M, Mellor J, Meyer AJ, Tlalka M, Vaus DJ, Wood J, White NS (1997) Quantitative imaging of intact cells and tissues by multidimensional confocal fluorescence microscopy. Experimental Biology Online. (December 4, 1997)
- Glinka Z. The effect of epidermal cell water potential on stomatal response to illumination of leaf discs of Vicia faba. Physiol Plant. 1971;24:476–479. [Google Scholar]
- Holmes T, Bhattacharyya S, Cooper JA, Hanzel D, Krishnamurthi V, Lin W, Roysam B, Szarowski DH, Turner JN. Light microscopic images reconstructed by maximum likelihood deconvolution. In: Pawley JW, editor. Handbood of Biological Confocal Microscopy. New York: Plenum Press; 1995. pp. 389–402. [Google Scholar]
- Humble GD, Raschke K. Stomatal opening quantitatively related to potassium transport: evidence from electron probe analysis. Plant Physiol. 1971;48:447–453. doi: 10.1104/pp.48.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husken D, Steudle E, Zimmermann U. Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiol. 1978;61:158–163. doi: 10.1104/pp.61.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EAC. Osmotic measurements on stomatal cells of Commelina communis L. J Membr Biol. 1980;53:189–198. [Google Scholar]
- Meidner H, Edwards M. Direct measurements of turgor pressure potentials of guard cells, I. J Exp Bot. 1975;26:319–330. [Google Scholar]
- Nonami H, Schulze E-D, Ziegler H. Mechanisms of stomatal movement in response to air humidity, irradiance and xylem water potential. Planta. 1990;183:57–64. doi: 10.1007/BF00197567. [DOI] [PubMed] [Google Scholar]
- Raschke K. Movements of stomata. In: Feinleib HW, Feinleib ME, editors. Encyclopedia of Plant Physiology. Berlin: Springer-Verlag; 1979. pp. 381–441. [Google Scholar]
- Raschke K, Dickerson M. Changes in shape and volume of guard cells during stomatal movement. Plant Res. 1973;1972:149–153. [Google Scholar]
- Raschke K, Dickerson M, Pierce M. Mechanics of stomatal responses to changes in water potential. Plant Res. 1973;1972:155–157. [Google Scholar]
- Raschke K, Fellows MP. Stomatal movement in Zea mays, shuttle of potassium and chloride ions between guard cells and subsidiary cells. Planta. 1971;1110:296–316. doi: 10.1007/BF00398116. [DOI] [PubMed] [Google Scholar]
- Shackel KA. Direct measurement of turgor and osmotic potential in individual epidermal cells. Plant Physiol. 1987;83:719–722. doi: 10.1104/pp.83.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe PJH, Wu H, Spence RD. Stomatal mechanics. In: Zeiger E, Farquhar GD, Cowan IR, editors. Stomatal Function. Stanford, CA: Stanford University Press; 1987. pp. 91–114. [Google Scholar]
- Steudle E. Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue and organ level. In: Smith JAC, Griffiths H, editors. Water Deficits: Plant Responses from Cell to Community. Oxford: Bios Scientific Publishers; 1993. pp. 5–36. [Google Scholar]
- Steudle E, Zimmermann U, Luttge U. Effect of turgor pressure and cell size on the wall elasticity of plant cells. Plant Physiol. 1977;59:285–289. doi: 10.1104/pp.59.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E, Zimmerman U, Zillikens J. Effect of cell turgor on hydraulic conductivity and elastic modulus of Elodea leaf cells. Planta. 1982;154:371–380. doi: 10.1007/BF00393917. [DOI] [PubMed] [Google Scholar]
- Tomos AD, Leigh RA. The pressure probe: a versatile tool in plant cell physiology. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:447–472. doi: 10.1146/annurev.arplant.50.1.447. [DOI] [PubMed] [Google Scholar]
- Tomos AD, Steudle E, Zimmermann U, Schulze E-D. Water relations of leaf epidermal cells of Tradescantia virginiana. Plant Physiol. 1981;68:1135–1143. doi: 10.1104/pp.68.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman SD, Oats P, Gibbs J, Dracup M, Greenway H. Turgor-volume regulation and cellular water relations of Nicotiana tabacum roots grown in high salinities. Aust J Plant Physiol. 1989;16:517–531. [Google Scholar]
- Wyers J, Meidner H. Methods in Stomatal Research. Harlow: Longman Scientific and Technical; 1990. [Google Scholar]
- White NS, Errington RJ, Fricker MD, Wood JL. Aberration control in quantitative imaging of botanical specimens by multidimensional fluorescence microscopy. J Microsc. 1996;181:99–116. [Google Scholar]
- Willmer C, Fricker M. Stomata. London: Chapman & Hall; 1996. [Google Scholar]
- Zimmermann U, Husken D, Schulze E-D. Direct turgor pressure measurements in individual cells of Tradescantia virginiana. Planta. 1980;149:445–453. doi: 10.1007/BF00385746. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Steudle E. Hydraulic conductivity and volumetric elastic modulus in giant algal cells: pressure and volume dependence. In: Zimmermann U, Dainty J, editors. Membrane Transport in Plants. Berlin: Springer-Verlag; 1974. pp. 65–71. [Google Scholar]
- Zimmermann U, Steudle E. The hydraulic conductivity and volumetric elastic modulus of cells and isolated cell walls of Nitella and Chara spp.: pressure and volume effects. Aust J Plant Physiol. 1975;2:1–12. [Google Scholar]