Abstract
The brown planthopper (BPH), Nilaparvata lugens, is an important pest that affects rice (Oryza sativa) production in Asia. The flavone tricin (5,7,4′-trihydroxy-3′,5′-dimethoxy flavone) is a valuable secondary metabolite commonly found in rice plants that can defend rice plants against infestation by BPH. BPH damage can reduce the metabolic level of tricin in rice. Our preliminary transcriptome research results showed that BPH salivary protein 7 (NlSP7), is highly responsive to tricin stimuli. However, the function of NlSP7 in mediating the interaction between the rice plant and the BPH is unknown. In this study, we cloned the NlSP7 gene in N. lugens and found that its mRNA level was greater in the presence of high tricin content than low tricin content, regardless of whether the BPHs were fed a rice plant diet or an artificial diet containing 100 mg/L tricin. Knocking down NlSP7 resulted in BPH individuals spending more time in the non-penetration and pathway phase, and less time feeding on the phloem of rice plants. These changes decreased BPH food intake, feeding behavior, and fitness, as well as the tricin content of the rice plants. These findings demonstrate that the salivary protein 7 of BPH functions as an effector for tricin metabolism in rice.
Subject terms: RNAi, Gene expression analysis
Introduction
Herbivorous insects obtain nutrients from host plants. As sessile organisms, many plants have evolved chemical defense mechanisms to resist these herbivorous insects1. For example, plants can initiate defense mechanisms when they detect insect oral secretions or signals from damaged plant cells. Many secondary metabolites produced by plants result from this co-evolutionary struggle between herbivores and plants2. Recent evidence indicates that saliva-secreted proteins can mediate host plants’ defense responses3. The saliva of herbivorous insects thus plays a key role in plant–insect interactions and in mediating the defenses of host plants4.
The brown planthopper (BPH), or Nilaparvata lugens Stål (Hemiptera: Delphacidae), is a major rice (Oryza sativa L.) pest in Asia5. Rice plants have evolved a variety of physical and chemical defenses against the BPH. Tricin (5,7,4′-trihydroxy-3′,5′-dimethoxy flavone) is a valuable secondary metabolite that is widely distributed in the stems, leaves, and hulls of many gramineous plants, including rice6. Previous studies show that tricin has an ecological function against the BPH by eliciting anti-feeding or anti-oviposition behavior7,8. In rice plants, the flavone tricin 5-O-glucoside is a probing stimulant for the white-backed planthopper (Sogatella furcifera Horvath). Other study has shown that tricin of BPH-resistant rice variety IR36 significantly reduces mortality rates of the rice and reduces honeydew weights of BPH, tricin also inhibits their oviposition and feeding behaviors9. Electrical penetration graph (EPG) data show that tricin significantly inhibits in vivo and in vitro movement of the BPH stylus6.
The BPH is a typical phloem sap-sucking insect. The first salivary protein identified in BPHs was β-glucosidase10. Rice plants treated with β-glucosidase react with increased salicylic acid, ethylene, and hydrogen peroxide levels and decreased jasmonic acid content11. Other salivary proteins, such as salivary sheath protein (NlShp), salivary endo-b-1,4-glucanase (NlEG1), mucin-Like Protein (NlMul), and anther mucin-Like Protein (NlMLP), are shown to play roles in the BPH’s salivary sheath formation and feeding12–15. NlEG1 degrades cellulose in the plant cell wall to allow the stylet of a BPH to reach the phloem14. NlMul-deficient BPHs exhibit disordered development and sometimes death16. In plants, NlMLP induces cell death, expression of defense-related genes, and callose deposition15. However, less is known about the role of salivary effectors of BPHs in mediating the chemical defenses of rice.
In this study, we aim to fill the knowledge gap by exploring the function of the NlSP7 salivary protein in mediating the interaction between rice plants and BPHs. Specifically, we assess whether NlSP7 acts as an effector for mediating tricin metabolism in rice plants. Our previous study has shown that the relationship between rice plants and BPHs is an ideal model to reveal plants’ defense mechanisms at the molecular level and the effects of secondary metabolites on BPHs17. The results of this study can help in developing novel BPH-resistant rice cultivars.
Results
Characterization of NlSP7
Using the proteomics database established for BPH by our laboratory18, we obtained a homologous fragment (312 base pairs) based on alignments with known insect transcriptome sequences. The open reading frame of the sequence was amplified via PCR with gene-specific primers. The complete sequence (678 base pairs) of the cDNA was obtained by RACE, and it comprised a 242-bp 5′ untranslated region, 355-base pair open reading frame, and 81-base pair 3′ untranslated region (Fig. 1A). The predicted protein comprised 117 amino acid residues with a theoretical molecular mass of 12.88 kDa. We designated this sequence as NlSP7 (GenBank accession no. KU365966.1) and NlSP7 sequences showed significant similarities with no similar protein of other insect species.
Figure 1.
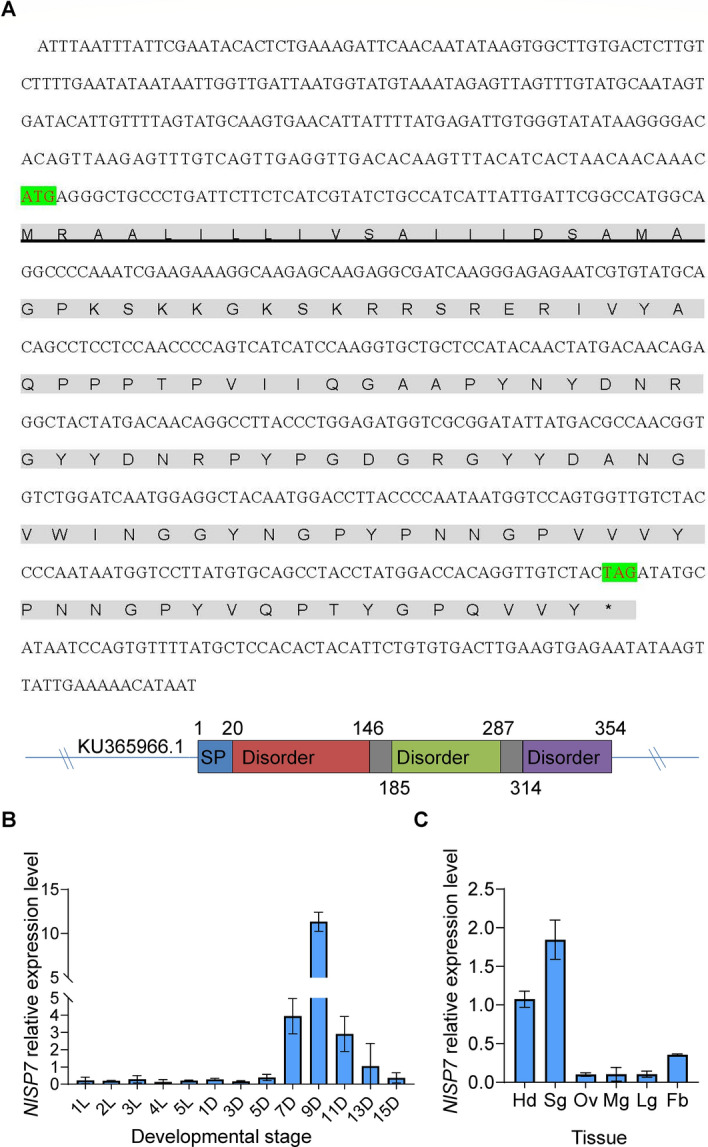
Characterization of NlSP7. (A) CDS sequence of NlSP7. The solid underline indicates the signal peptide as predicted by SignalP 5.0. The asterisk (*) indicates the stop codon. The different short-repeat regions are indicated by different colors. (B,C) The bottom panels show the mean transcript levels ± SD (n = 30) of NlSP7 in whole bodies at various developmental stages (B) and in different tissues (C). RNA was extracted from nymphs at 1–5 days and female brachypterous brown planthoppers reared on Rathu Heenati rice at 1, 3, 5, 7, 9, 11, 13, and 15 days. Hd, head; Sg, salivary gland; Ov, ovary; Mg, midgut; Lg, leg; Fb, fat body was extracted from BPH adults.
To investigate the spatio-temporal expression pattern of NlSP7 in 13 different developmental stages (1st, 2nd, 3rd, 4th and 5th instar nymphs, 1st, 3rd, 5th, 7th, 9th, 11th, 13th, 15th days old adults) and various parts of the adults (head, salivary glands, ovaries, midgut, leg, and fat body) of BPH. RT-qPCR showed that NlSP7 transcripts were expressed in all nymph and adult stages (Fig. 1B). The significantly higher expression in adults suggests that NlSP7 plays its most important role in the adult stage. Tissue-specific expression analysis in adults showed that the NlSP7 transcript levels were slightly higher in the salivary glands, followed by the head and fat body, with lowest expression in the ovaries, midgut, and fat body (Fig. 1C).
NlSP7 expression activated by tricin in different rice varieties
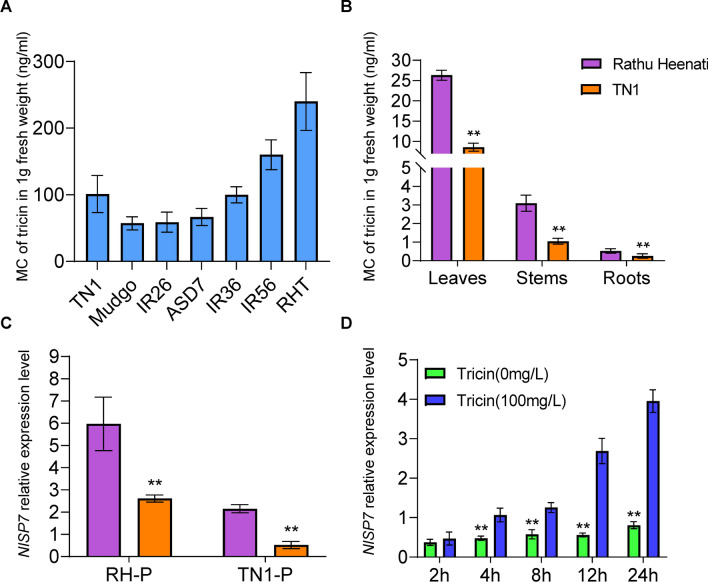
Tricin concentrations in the different rice varieties and organs were different (Fig. 2). The tricin identification results for the TN1, Mudgo, IR26, ASD7, IR36, IR56, and RH varieties were shown in Fig. 2A. The tricin concentrations in the leaves were significantly higher than those in the stems and roots in both the resistant RH and susceptible TN1 varieties. There were no significant differences in the tricin concentrations between the stems and roots. The tricin concentration in the leaves was 28.1 ± 2.6 ng/ml in the RH plant, which was significantly higher than that in the leaves of the TN1 plant (P = 0.002). The tricin concentration in the RH roots differed significantly from that in the TN1 roots (P = 0.004) (Fig. 2B).
Figure 2.
Relationship between NlSP7 and tricin. (A) Tricin mass concentration (MC) (ng/ml) analysis results at the three-leaf stage of seven rice varieties: Taichung Native 1 (TN1), Mudgo, IR26, ASD7, IR36, IR56, and Rathu Heenati (RH) carrying different genes resistant to brown planthoppers. (B) Tricin mass concentration of tricin in the root, stem and leaf parts of RH and TN1 rice varieties. One g each of fresh root, stem, and leaf was ground, and the content of tricin in each part was measured using liquid chromatography-mass spectrometry. Data are represented as the mean ± SD from three biological replicates. (C) q-PCR was used to determine the expression level of TN1-P and RH-P fed two different varieties of rice plants. (D) Artificial diets wih tricin and without tricin were fed to brown planthoppers, then q-PCR was used to detect the expression level of NlSP7 at 2, 4, 8, 12, and 24 h. The mean amounts of three biological replicates (mean ± SD, n = 10) are shown, ** P < 0.01. (Student’s t-test).
First, the optimal concentration of tricin had been chosed from two different tricin concentrations (50 mg/L and 100 mg/L) (Supplementary Fig. S4). The resistence levels differed significantly for the tricin-resistant RH population of BPHs and tricin-sensitive TN1 population of BPHs. The mean resistence levels were significantly lower for the tricin-sensitive population (P < 0.001) than the tricin-resistant population when feeding on the RH rice variety (control in this study, severity score = 9.0) (Fig. 2C). The NlSP7 gene mRNA expression level was higher in the tricin-resistant BPHs than in the tricin-sensitive population (Fig. 2C). These results suggest that NlSP7 may have a role in BPH feeding and virulence. BPH nymphs fed an artificial diet containing tricin had higher NlSP7 transcript levels from 4 h, compared with those fed a diet without tricin (P < 0.01) (Fig. 2D). Thus, the NlSP7 mRNA levels were upregulated after ingesting an artificial diet containing tricin.
Fitness of BPH
To elucidate the role of NlSP7 in BPHs, we synthesized dsRNA from NlSP7 and injected it into 1-day-old female adults to mediate RNAi (Supplementary Fig. S1). This treatment had strong silencing effects, reducing the NlSP7 transcript level significantly (~ 86%) in the first day after treatment, compared with the levels in the control group, in those injected with dsGFP RNA (P < 0.01), and in those injected with dsNlSP7 (P = 0.001 after injection with dsGFP or dsNlSP7 at 2 days, P < 0.001 after injection with dsGFP or dsNlSP7 at 3 days).
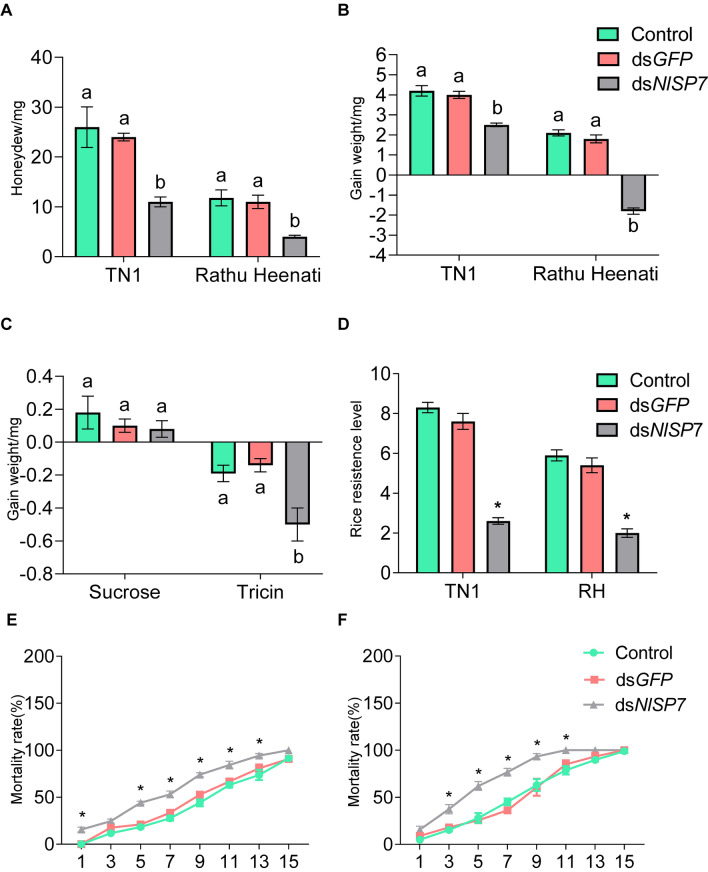
BPHs subjected to the NlSP7 RNAi treatment also exhibited less feeding activity, as indicated by significantly lower excretions of honeydew, compared with the two control groups (P < 0.001 for control and dsNlSP7; P = 0.003 for dsGFP and dsNlSP7), as shown in Fig. 3A. The NlSP7-RNAi BPHs also had lower weight gain values, regardless of whether they were fed RH rice plants (P < 0.001 for control and dsNlSP7, P = 0.001 for dsGFP and dsNlSP7) or TN1 rice plant (Fig. 3B), and smaller weight gain values (P = 0.006 for the control and dsNlSP7, P = 0.003 for dsGFP and dsNlSP7), as shown in Fig. 3C. Furthermore, silencing NlSP7 reduced the virulence of BPH (Fig. 3D)15. Compared with the two control groups, BPHs injected with dsNlSP7 had significantly lower survival rates from 3 to 11 days after microinjection (P = 0.003, P = 0.003, respectively), and most died on RH rice by 9 days after microinjection (Fig. 3E). Similar results were fed an artificial diet containing tricin (100 mg/L) (Fig. 3F). The BPHs injected with dsNlSP7 fed on TN1 rice, the mortality rate was no difference between the BPH injected with dsNlSP7 and the control group, which were feeding on artificial diet without tricin. (Supplementary Fig. S2 and S3).These results indicate that silencing the NlSP7 gene significantly reduces feeding and performance of BPHs on rice plants.
Figure 3.
RNA interference (RNAi) effects on feeding of brown planthoppers (BPHs) exposed to three different treatments (control, injected with dsGFP, injected with dsNlSP7) and resistance levels of different rice varieties. (A) On Taichung Native 1 (TN1) and Rathu Heenati (RH) rice varieties, the mean honeydew amounts excreted at 24 h by a female adult BPH that received three treatments. The experiment was repeated with 20 replicates. (B) Weight gain of BPHs fed TN1 and RH rice varieties after injection with different dsRNA treatments. (C) Mean weight of BPHs fed LDS-sucrose and LDS-tricin (mean ± SD, n = 20). Letters indicate significant differences (P < 0.01, Duncan’s multiple range test). (D) Rice resistance levels of BPHs fed TN1 and RH rice varieties. The experiment was repeated three times. Data are represented as the mean ± SD (P < 0.01, Duncan’s multiple range test). (E) mortality rate of BPHs fed RH rice, repeated 10 times, respectively. (F) mortality rate of BPHs fed an artificial diets with 100 mg/l tricin, repeated 10 times. Asterisks (*) indicate significant difference (P < 0.05, Student’s t-test).
Impairment of BPH feeding
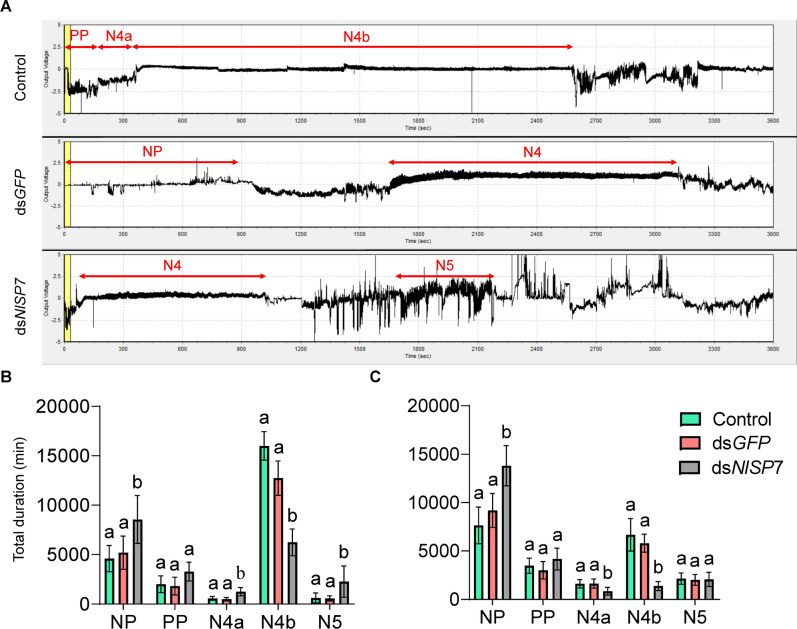
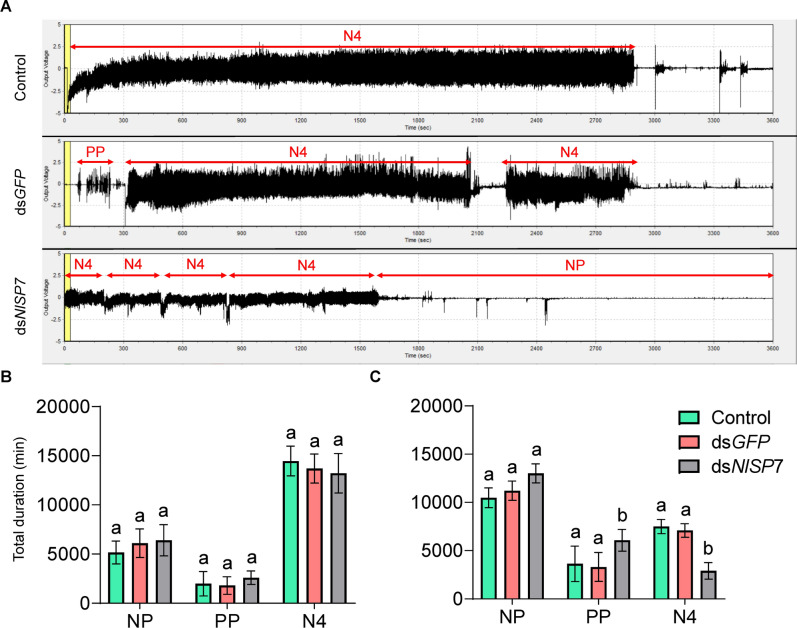
To determine the effect of NlSP7 on BPH feeding, we used the EPG technique to profile the feeding behavior of piercing–sucking insects19. Five main feeding phases can be distinguished by EPG: (1) the non-penetration phase, (2) the pathway phase (including penetration, salivation, stylet movement, and extracellular activity near the phloem), (3) the intracellular phase of activity in the phloem, (4) the phloem sap ingestion phase, and (5) the xylem phase. The top of Fig. 4A shows the representative EPG traces obtained from BPHs, showing the different phases. As shown in Fig. 4B, after NlSP7 knockdown in female adults, the EPG waveforms obtained over 6 h on TN1 rice plants showed that the duration of intracellular activity in the phloem exceeded those of the dsGFP and control groups (P = 0.001), and the duration of the N4b did the duration decrease, other waveforms have increased in varying degrees, the non-penetration (NP) the intracellular activity in the phloem region(N4a) and the xylem phase(N5) increased, the pathway phase (PP) was unchanged for dsNlSP7-treated insects (P < 0.001, P = 0.011, P = 0.001, P = 0.007, respectively). On RH rice plants, the duration of the non-penetration phase increased significantly (P < 0.001), whereas the durations of the intracellular activity in the phloem and phloem sap ingestion phases decreased significantly over the 6-h period (P = 0.017, P < 0.001, respectively), as shown in Fig. 4C. These findings indicate that BPH individuals spent less time feeding on rice plants with high tricin levels after NlSP7 knockdown. The EPG distinguishes three main feeding stages in artificial diets, the non-penetration (NP), the pathway phase (PP) and the artificial diets ingestion (N4) (Fig. 5A). After NlSP7 knockdown in female adults, there are no obvious change in the EPG waveforms obtained over 6 h on artificial diets without tricin (Fig. 5B), and the EPG waveforms obtained over 6 h on artificial diets with tricin showed that the duration of pathway phase activity in the phloem exceeded those of the dsGFP and control groups (P = 0.001), the N4 duration reduced (Fig. 5C). These results of artificial diets indicate that BPH individuals spent less time feeding on artificial diets with tricin levels after NlSP7 knockdown.
Figure 4.
Reduced feeding on Taichung Native 1 (TN1) and Rathu Heenati (RH) rice varieties among three groups of brown planthoppers (the order is control, injected with dsGFP, injected with dsNlSP7). (A) Overall typical view of electrical penetration graph waveforms generated by feeding behavior of the three groups of brown planthoppers. (B,C) one-day-old brachypterous female adults in the three treatment groups. NP, nonpenetration; PP, pathway phase (N1 + N2 + N3), including penetration initiation (N1), salivation and stylet movement (N2), and extracellular activity near the phloem (N3); N4a, intracellular activity in the phloem region; N4b, phloem sap ingestion; N5, xylem phase. Duration of wavelength when each insect feeds on TN1 or RH rice. Electrical penetration graphs were recorded for 6 h per insect. Different letters indicate significant differences among the three treatment groups. Testing of each group was repeated 15 times (P < 0.01, Duncan’s multiple range test).
Figure 5.
Brown planthoppers in three groups (the order is control, injected with dsGFP, injected with dsNlSP7) exhibit reduced feeding on two artificial diets (0 mg/l tricin and 100 mg/l tricin). (A) Overall typical view of electrical penetration graph waveforms generated by the feeding behavior of brown planthoppers on artificial diets. (B,C) data collected from 1-day-old brachypterous female adults with different treatments. NP, nonpenetration; PP, pathway phase (N1 + N2 + N3), including penetration initiation (N1), salivation and stylet movement (N2), and extracellular activity near the phloem (N3); N4, sucrose or tricin solution ingestion. B the duration of each wavelength when feeding on artificial diets without tricin (0 mg/l) and C artificial diets with tricin (100 mg/l) is shown. Electrical penetration graph data were recorded for 6 h per insect. Different letters indicate significant differences among the three treatment groups. Testing of each group was repeated 15 times (P < 0.01, Duncan’s multiple range test).
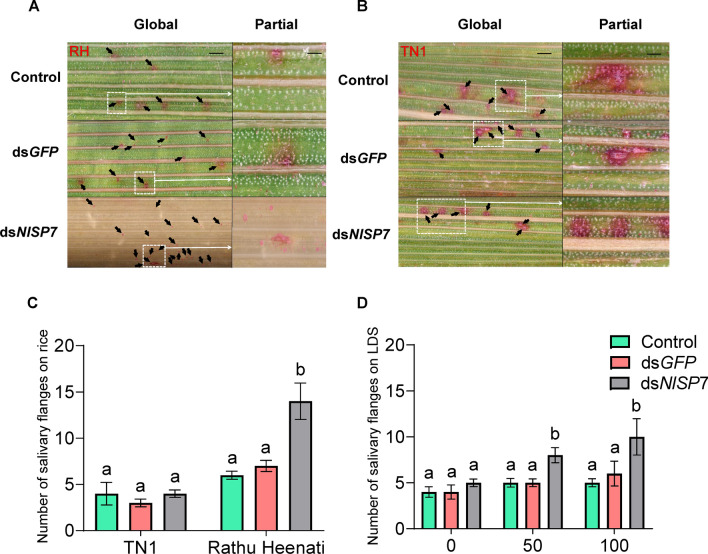
Furthermore, BPHs fed an artificial diet high in tricin produced significantly more salivary flanges than those in the control group and those fed an artificial diet containing low tricin levels (P < 0.001) (Fig. 6D). In addition, the number of salivary flanges differed significantly between the two rice plant varieties. BPHs produced 250% more salivary flanges on RH plants than on TN1 plants after NlSP7 knockdown (Fig. 6A,B). Moreover, comparing salivary flanges among the control group, the numbers of salivary flanges were sparser per unit area and deeper on TN1 plants than on RH plants (Fig. 6C).
Figure 6.
Number of salivary flanges created by brown planthoppers fed rice or an artificial diet, across different treatment groups (control, injected with dsGFP, injected with dsNlSP7). (A,B) images of salivary flanges produced on Rathu Heenati (RH) and Taichung Native 1 (TN1) rice varieties. The global of graphs, bars = 200 μm. The partial of graphs, bars = 50 μm. (C) Salivary flanges created by brown planthoppers fed different rice varieties. (D) Salivary flanges from brown planthoppers fed artificial diets with different levels of tricin. Bars with different letters are significantly different among different tricin levels. Data are represented as means ± SD, with 30 replications (P < 0.01, Duncan’s multiple range test).
Tricin metabolism mediated by NlSP7
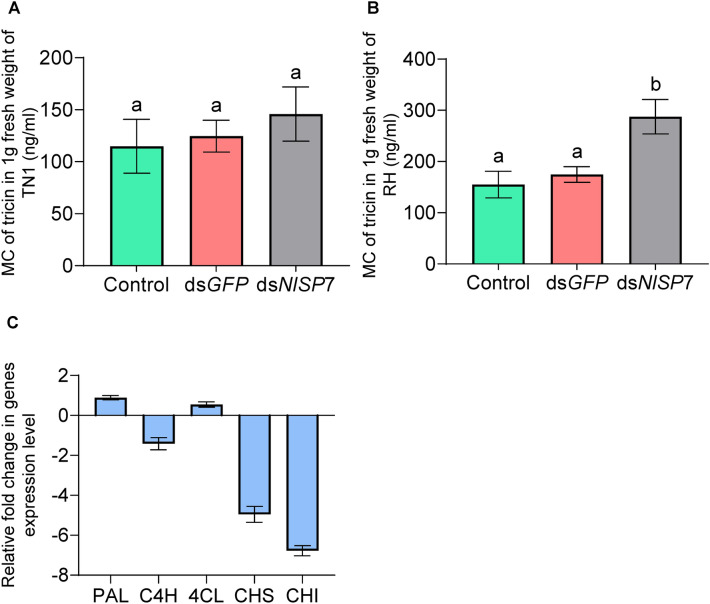
To determine whether the salivary protein NlSP7 influences the production of tricin in rice plants, we investigated tricin levels in rice plants infested by BPH adults, where the ability to produce NlSP7 was silenced or not silenced. The results showed that RH rice plants fed on by control and dsGFP groups BPH had decreased tricin levels than BPH group which injected by dsNLSP7 and RH rice plants fed on by BPH adults had decreased tricin levels compared to RH rice plants not fed on by BPHs, but there is no significant change between the TN1 treatment group and the control group (Fig. 7A,B). After NlSP7 knockdown, the tricin level in RH rice plants increased by 86.36% (P = 0.005) but remained unchanged in TN1 rice plants. Tricin is a secondary metabolite of the phenylalanine pathway in plants, and the phenylalanine pathway starts from phenylalanine. The amino group is removed under the action of phenylalanine ammonia lyase (PAL) to form cinnamic acid. Cinnamic acid adds hydroxyl to form p-coumarin acid under the action of cinnamic acid-4-hydroxylase (C4H), and then forms p-coumarin acid COA under the catalysis of 4-coumarin-CoA ligase (4CL). Chalcone synthase (CHS) catalyzes the formation of chalcone, the isomerization of chalcone to flavanone, which is catalyzed by chalcone isomerase (CHI)20. To further characterize the physiological properties associated with the blocked synthesis of flavonoids induced by NlSP7, we examined the expression levels of PAL, C4H, 4CL, CHS, CHI for the synthesis of flavonoids in RH rice plants fed by BPH for 5 days (Fig. 7C). Our results indicated that the level of tricin induced by NlSP7 shared similarities with the secondary metabolite synthetase induced by the flavonoid pathway marker genes CHS and CHI. Thus, NlSP7 may play important roles in defense-related signal transduction21.
Figure 7.
The effect of brown planthoppers in three groups (control, injected with dsGFP, injected with dsNlSP7) on the tricin content and tricin metabolic pathway genes of different rice varieties. (A) Taichung Native 1 (TN1) and (B) Rathu Heenati (RH) rice varieties fed on by brown planthoppers. After 3 days, the content of tricin was determined by liquid chromatography-mass spectrometry, A same letters above the bars indicate no significant differences between three treatment groups, and B different letters above the bars indicate significant differences between BPHs injected with dsNlSP7 and two control groups (control and dsGFP). Data are represented as the mean ± SD from three biological replicates (P < 0.01, Duncan’s multiple range test). (C) The q-PCR used to determine the expression level of tricin metabolic pathway genes (PAL, C4H, 4CL, CHS, CHI) in rice after being fed on by brown planthoppers. The experiment was repeated three times.
Discussion
Saliva is a complex mixture of biomolecules, and it plays crucial roles in how sap-sucking insects feed on plants12–16. Our experiments demonstrated that the NlSP7 secretory protein in the salivary gland of BPHs is injected into rice plants during feeding. The protein encoded by NlSP7 contains three unknown domains; it is unique to BPHs and exhibits typical tandem amino acid duplication, which is consistent with previous analyses22. In our previous studies, we collected watery and gelling saliva from BPHs to characterize the salivary proteome18. BPH saliva also includes a large effector repertoire involved in its interaction with rice plants, according to previously reported evaluations of the salivary proteome23 and watery salivary proteome24. However, for the first time, our results showed that NlSP7 is involved in the interaction between BPH and defensive secondary metabolites in rice plants.
Tricin is an active secondary metabolic with great potential for combating BPHs by eliciting anti-feeding or anti-oviposition behavior, according to previous studies7,8 and confirmed by our results. In the present study, we prepared a tricin-resistant BPH population that could feed on both RH rice (high tricin content) and TN1 rice (low tricin content). Plant-derived compounds play key roles in insect–plant interactions, and insects can adapt to changes in the levels of these compounds25. NlSP7 may act as an herbivore effector that allows BPH to overcome the defensive effect of tricin in rice plants, as indicated by our results showing that NlSP7 was more highly expressed in the tricin-resistant population than in the tricin-sensitive population. NlSP7 knockdown significantly reduced transcript levels and honeydew quantity by 57.69% and 63.64% in BPHs feeding on TN1 and RH rice varieties, respectively, and weight gain decreased on both the RH rice diet and artificial diet containing 100 mg/L of tricin. The mortality rate reduced by 5% among BPHs fed the RH rice variety (P < 0.01) and by 15% among BPHs fed the artificial diet containing 100 mg/L tricin (P < 0.01). These results are similar to those reported in studies of two other saliva genes, NlShp and NlEG1. Knocking down NlShp by RNA interference inhibited salivary sheath formation, silencing NlEG1 decreases the capacity of BPH to reach the phloem. They have similar results, which will cause brown planthoppers to have difficulty feeding13,14.
Larvae release saliva into plant cells through wound sites created when they feed on the leaves of plants23,26. After NlSP7 gene knockdown in tricin-resistant BPHs, the duration of feeding on rice plants decreased significantly. BPHs fed the high-tricin RH rice variety spent more time in the non-penetration and pathway phases and less time feeding on phloem, thereby decreasing their food intake, weight gain, and survival rate. However, silencing NlSP7 did not affect the early ability of BPH to feed on an artificial diet containing 100 mg/L tricin. These results suggest that NlSP7 facilitates phloem access via cell wall penetration by interacting with tricin.
Our EPG results showed that the duration of intracellular activity in the phloem and phloem sap ingestion phases decreased significantly after NlSP7 knockdown. The durations of the non-penetration and pathway phases increased significantly among BPHs that were fed either rice plant variety or an artificial tricin-rich diet. The number of salivary flanges in the two different plant types and under various tricin levels indicated that ingestion of tricin induced significant increases in salivary flanges. The expression of NlSP7 in the saliva could have affected tricin-induced feeding of BPHs. Similar to other salivary proteins, the expression of NlEG1 in the fat body might be related to the detoxification of plant defense chemicals, as reported previously for some plant cell wall-degrading enzymes in insects27.
The elicitation of tricin production in rice plants as a defense reaction by NlSP7 shares common features with the immune responses of well-known effectors and pathogen-associated molecular processes. It also may be recognized by plant pattern recognition receptors, which trigger plant defensive responses. The rice plant’s defense response to BPHs involves secondary metabolites, which is a common response triggered by insect feeding4,28,29. Our results suggest that the increased duration of the non-penetration and pathway phases induced by NlSP7 silencing were dependent on the tricin content. We consider that plants might respond to NlSP7 via a conserved upstream component of plant signaling pathways. The expression of NlSP7 decreased the expression levels of the flavonoid pathway marker genes CHS and CHI. CHS is a chitinase gene associated with flavonoid-dependent defenses, and it is induced by elicitors30. The isomerization of naringenin chalcone to synthesize naringenin flavanone, which is catalyzed by CHI31. These findings suggest that the defense responses triggered by NlSP7 are associated with the flavonoid signaling pathway. Inhibition of the flavonoid signaling pathway promotes the synthesis of lignin, which leads to increased thickening of plant cell walls32. A previous study showed that BPHs feeding on the RH rice variety could lead to thickening of the plant cell wall33. Increasing the thickness of the cell wall on the sieve plates might occlude the sieve tubes enough to directly inhibit continuous feeding by BPHs28. It is thus reasonable to suggest that phloem plugging also might cause dsNlSP7 to perform poorly in BPHs. Further research is required to address this question.
The results obtained in this study indicate that NlSP7 may be an effector involved in the interactions between rice plants and BPHs. The expression level of NlSP7 was higher in the salivary gland of the tricin-resistant BPH population. After NlSP7 knockdown, the amount of honeydew produced by dsRNA-treated BPHs feeding on TN1 and RH rice varieties decreased by 57.69% and 63.64%, respectively, and the virulence scores decreased by 56.16% and 75.84%, respectively. NlSP7 also plays an important role in BPH feeding, as we found that the durations of sucking and phloem feeding decreased by 47.42% and 79.36%, respectively, when BPHs fed on the RH rice variety. The protein encoded by NlSP7 can form a complex with interacting partners when secreted into rice plants.
We found that NlSP7 could influence the tricin content in rice. NlSP7 may activate rice defense responses by affecting the expression of flavonoid biosynthesis genes in plants. Further studies are needed to identify the interacting partner of NlSP7 in rice plant varieties and to determine the role of the complex. The novel molecular interaction identified in this study may provide new insights on the interaction between BPH and defensive secondary metabolites in rice. Further investigation is needed to assess the usefulness of this interaction in rice agriculture.
Materials and methods
Insects and plant materials
Rice variety Taichung Native 1 (TN1) is a BPH-susceptible rice variety with a low concentration of tricin. Rathu Heenati (RH) has a high tricin content6, all plant materials to comply with relevant institutional, national, and international guidelines and legislation. Both varieties of seeds were obtained from the experimental nursery of the Plant Protection Research Institute, Guangdong Agricultural Academy of Science, China. All seeds for experiments were germinated on filter paper, placed in a Petri dish, and transferred to a pot (7 cm in diameter) containing multi-purpose compost. The plants were then maintained in a growth chamber at 27 ± 0.5 °C with a relative humidity of 70 ± 5% and a 12-h light/dark cycle. Plants aged 30 days were used in the experiments.
The N. lugens populations used for collecting secreted saliva in this study originated from Guangdong Agricultural Academy of Science, Plant Protection Research Institute, in Guangzhou, Guangdong Province, China. The tricin-resistant population was fully reared for at least 35 generations and maintained on RH rice plants. The insects were reared at 27 ± 0.5 °C at a relative humidity of 70 ± 5%. Host plants were replaced weekly. Only 1-day-old adult BPH females were selected for use in experiments.
Standards and reagents
Tricin, high-performance liquid-chromatography grade ethyl acetate (> 99.8%), and high-performance liquid-chromatography grade methanol (> 99.9%) were purchased from Merck; MS grade methanol (> 99.9%) from Thermo Fisher Scientific (Waltham, MA, USA); and analytical reagent methanol from Donghong Chemical Factory (Guangzhou, China). The reagents used for molecular experiments were purchased from TransGen Biotech and Genstar (Guangzhou, China). 2,4,5–7-tetra bromo fluorescein disodium salt was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (China).
Reverse transcription PCR and sequence analysis of NlSP7
Newly molted 1-day-old brachypterous female adult BPHs were anesthetized with carbon dioxide for 15 s and dissected in phosphate-buffered saline (pH 7.4). The head, salivary gland, midgut, ovary, leg, and fat body were carefully dissected from 100 individuals under a stereomicroscope (Leica S8AP0, Wetzlar, Germany) before placing the tissues directly in 200 μL of TRIzol for total RNA extraction. After preparing 30 replicate BPHs, total RNA was isolated using a TRIzol Total RNA Isolation Kit (Takara, Dalian, China) according to the manufacturer’s instructions. The total RNA concentration was quantified, and 1,000 ng of RNA was used for reverse transcription in 20 μL of reaction volume using a TransScript® II One-Step gDNA Removal and cDNA Synthesis SuperMix kit (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. Next, 5′ and 3′ rapid amplifications of cDNA ends (RACE) was conducted to amplify NlSP7 cDNA using the 5′-full RACE kit and 3′-full RACE core set (TaKaRa, Kyoto, Japan) according to the manufacturer's instructions. Supplemental Table S1 shows the specific primers for NlSP7.
PCR products were cloned into carriers using a pEASY®-Blunt Zero Cloning Kit (TransGen Biotech,Beijing, China) and sequenced by IGEBio Technology Ltd. (Guangzhou, China). The full-length cDNA sequence of NlSP7 was assembled from the sequencing results and verified by PCR using the primers shown in Table S1. The sequence with a signal peptide was submitted to TMHMM Server version 2.0 to predict transmembrane domains (http://www.cbs.dtu.dk/services/TMHMM/). SignalP 5.0 was used to predict the presence of signal peptides (http://www.cbs.dtu.dk/services/SignalP/). Identified N. lugens sequences were verified using BLASTX to search for similar sequence in National Center for Biotechnology Information (NCBI) protein database.
Real-time quantitative PCR (RT-qPCR)
Real-time quantitative PCR analyses were conducted with reverse transcription cDNA. The RT-qPCR analyses were using CFX96 Real-Time PCR Detection System (Bio-Rad,USA) using 2 × RealStar Green Fast Mixture. The pcr program uses a two-step method: initial denaturation at 95 °C for 2 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 15 s. Nlactin (GenBank:EU179848) were used as the reference gene to normalize gene transcript levels (Table S1). The relative gene transcript levels and interference efficiency were calculated using the 2-△△CT method.
RNAi
Double-stranded RNA (dsRNA) synthesis was conducted based on the cloned NlSP7 sequences. A nucleotide sequence (around 300 bp long) specific to the target gene was cloned into the pEASY® Blunt Zero vector (TransGen Biotech, Guangzhou, China) and submitted for sequencing to IGE Biotechnology Ltd. (Beijing, China). Aequorea victoria green fluorescent protein (GFP) was used as a control. PCR products were used as templates for dsRNA synthesis using a MEGAscript T7 transcription kit (Thermo Fisher, Waltham, MA, USA). Supplemental Table S1 shows the specific primers used to generate these DNA templates.
Newly molted 1-day-old brachypterous female adults were first anesthetized with carbon dioxide for 15 s and then injected with approximately 100 ng of dsRNA using a Nanoject II Auto-Nanoliter Injector (Drummond Scientific, Broomall, PA, USA). After injection, the treated BPHs were reared on RH rice at 27 ± 0.5 °C at a relative humidity of 70 ± 5% for 3 days. The injected BPHs were used in the experiments.
Electrical penetration graph (EPG) recording
EPG was performed using a Giga-8 DC EPG amplifier (Wageningen Agricultural University, Wageningen, The Netherlands). At 1 h before the EPG experiments, the 1-day-old brachypterous female adult BPHs were provided with water on filter paper, then anesthetized with CO2 for 20 s6. Newly molted 1-day-old brachypterous female adults were selected from the insect cages and subjected to one of three different treatments: control, injection with double-stranded GFP (dsGFP), or injection with dsNlSP7), with 15 replicates for each treatment. BPHs were fixed using a negative pressure device.
One end of a 3-cm length of gold wire (diameter = 12.5 μm) was connected to an amplifier through the EPG probe. The other end was connected to the dorsum of the BPH with conductive silver glue (Wageningen Agricultural University). A copper electrode (length = 10 cm, diameter = 2 mm) was inserted into the soil in the RH or TN1 planting substrate to establish the other part of the electrical circuit. After a starvation period of 30 min, the BPH was placed on a rice plant. All EPG experiments were recorded for 6 h under continuous light in a climate-controlled room. The 6 h of continuous EPG data starting from the beginning of feeding were analyzed using EPG Stylet + software (Wageningen Agricultural University, 2012).
The artificial diet system comprised a transparent and open cylindrical container (diameter = 6 cm, height = 5 cm) with a double layer of Parafilm PM-996 (Bemis, Oshkosh, WI, USA) covering one end of the container6. The experimental treatments tested two concentrations: 100 mg/L tricin and no tricin. One end of a copper wire was embedded in the solution, and the other end was wrapped around the electrode. The insect electrode and experimental control conditions were used to obtain the reference EPG data for a plant.
BPH bioassays for honeydew and weight gain
Honeydew is used to measure BPH feeding activity. Honeydew was collected as described by Pathak et al.34. One-day-old brachypterous female adults were injected with NlSP7 or GFP dsRNA or not injected (control) and placed on RH rice plants. After 3 days, the active BPHs were placed into Parafilm bags (6 cm × 5 cm) fixed on the stem of an RH or TN1 rice plant and maintained for 24 h in a climate-controlled environment at 27 ± 0.5 °C with a relative humidity of 70 ± 5% and a 12-h light/dark photoperiod. Twenty replicates were prepared.
To assess the effects of NlSP7 knockdown on feeding, BPHs subjected to three treatments (control, injected with dsGFP, or injected with dsNlSP7) were allowed to feed on different rice plant varieties or on an artificial diet. A single rice plant was placed in a round plastic tube (length = 10 cm, diameter = 2 cm) with a hole to facilitate breathing. The treated BPHs were weighed and placed in the tubes. After feeding for 24 h, the BPHs were removed and weighed again. Changes in weight were determined for BPHs fed an artificial diet and those subjected to the three treatments. Data were collected in the same manner as for the rice-fed BPHs. Twenty replicates were prepared for each treatment.
Salivary flange measurements
A single rice plant was placed in a round plastic tube (length = 10 cm, diameter = 2 cm). One-day-old female adult BPHs subjected to one of three treatments (control, injected with dsGFP, or injected with dsNlSP7) were placed in plastic tubes sealed with sponges. The BPHs were maintained in a climate-controlled environment at 27 ± 0.5 °C at a relative humidity of 70 ± 5% and a 12-h light/dark photoperiod. After 24 h, BPHs and the rice stalks were removed. The rice stalks were soaked in Eosin Y for 12 h before observing the salivary flanges on the stalks under a stereoscope. The salivary flanges from BPHs fed an artificial diet were observed directly under a microscope. Thirty replicates were prepared for each treatment.
Liquid chromatography-mass spectrometry
Liquid chromatography and mass spectrometry analyses were conducted using a DGU-20A3 (Shimadzu, Kyoto, Japan) quaternary pump equipped with an autosampler. A Supelco Discovery C18 XDB-C18 (Agilent, Santa Clara, CA, USA) column (4.6 × 50 mm, 1.8 μm) was used at ambient temperature with a sample injection volume of 10 μL. The elution gradient comprised a binary solvent system with H2O (solvent A) and mass spectrometry-grade methanol (solvent B) at a constant flow rate of 800 μL/min. Mass spectrometry and tandem mass spectrometry experiments were performed using a hybrid triple quadrupole/linear ion trap API4000 Q-Trap liquid chromatography mass spectrometer (Applied Biosystems, Carlsbad, CA, USA)6.
Statistical analysis
All data in this article are expressed as the mean ± standard deviation. The content of tricin in different parts of different rice varieties and the expression levels of NlSP7 in different BPH populations (e.g., those fed on TN1 or RH rice varieties) were determined using Student’s t-test. Duncan’s multiple range test was used to analyze the expression level of BPH after feeding on artificial diets with tricin for 2, 4, 8, 12 and 24 h; the bioassay of BPH after RNA interference; and the changes in tricin content after feeding on different rice varieties. All statistical tests were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Supplementary Information
Acknowledgements
We thank Professor Wenqing Zhang (School of Life Sciences, Sun Yat-sen University, China) and Doctor Yangshuo Dai (School of Life Sciences, Sun Yat-sen University, China) for their guidances with our experiments. This work was supported by the National Natural Science Foundation of China (Grant No. 32172507) and the Guangdong Provincial Natural Science Foundation of China (Grant No. 2015A030313573).
Author contributions
Z.F.Z., L.Y.Y., and W.J.W. conceived and supervised the project. Z.F.Z. and L.Y.Y. designed the experiments. G.G. and L.Y.Y. performed most of the experiments. Y.F.L., H.X.X., Y.F.L., Y.Z., and W.J.W. performed some of the experiments. Z.F.Z., L.Y.Y., and G.G. analyzed data and wrote the manuscript. All authors read and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Gu Gong and Long-Yu Yuan.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07106-6.
References
- 1.Howe GA, Jander G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 2.Becerra JX. The impact of herbivore-plant coevolution on plant community structure. Proc. Natl. Acad. Sci. USA. 2007;104:7483–7488. doi: 10.1073/pnas.0608253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu H, et al. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc. Natl. Acad. Sci. PNAS. 2019;116:490–495. doi: 10.1073/pnas.1714990116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogenhout SA, Bos JI. Effector proteins that modulate plant-insect interactions. Curr. Opin. Plant Biol. 2011;14:422–428. doi: 10.1016/j.pbi.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Ge Y, et al. Silencing of mir156 confers enhanced resistance to brown planthopper in rice. Planta. 2018;248:813–826. doi: 10.1007/s00425-018-2942-6. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Cui B, Zhang Y. Electrical penetration graphs indicate that tricin is a key secondary metabolite of rice, inhibiting phloem feeding of brown planthopper, Nilaparvata lugens. Entomol. Exp. Appl. 2015;156:14–27. doi: 10.1111/eea.12307. [DOI] [Google Scholar]
- 7.Adjei-Afriyie F, et al. Probing stimulants from the rice plant towards the smaller brown planthopper, Laodelphax striatellus (Fallen) (Homoptera: Delphacidae) Z. Naturforsch. C. J. Biosci. 2000;55:1038–1043. doi: 10.1515/znc-2000-11-1232. [DOI] [PubMed] [Google Scholar]
- 8.Adjei-Afriyie F, Kim CS, Takemura M, Ishikawa M, Horiike M. Isolation and identification of the probing stimulants in the rice plant for the white-back planthopper, Sogatella furcifera (Homoptera: Delphacidae) Biosci. Biotechnol. Biochem. 2000;64:443–446. doi: 10.1271/bbb.64.443. [DOI] [PubMed] [Google Scholar]
- 9.Ling B, Dong H, Zhang M, Xu D, Wang J. Potential resistance of tricin in rice against brown planthopper Nilaparvata lugens (Stal) Acta Ecol. Sin. 2007;27:1300–1306. doi: 10.1016/S1872-2032(07)60031-6. [DOI] [Google Scholar]
- 10.Sōgawa K. Studies on the salivary glands of rice plant leafhoppers. Jpn. J. Appl. Entomol. Zool. 1965;9:275–290. doi: 10.1303/jjaez.9.275. [DOI] [Google Scholar]
- 11.Wang X, et al. B-glucosidase treatment and infestation by the rice brown planthopper Nilaparvata lugens elicit similar signaling pathways in rice plants. Chin. Sci. Bull. 2008;53:53–57. doi: 10.1007/s11434-008-0048-4. [DOI] [Google Scholar]
- 12.Ye W, et al. A salivary EF-hand calcium-binding protein of the brown planthopper Nilaparvata lugens functions as an effector for defense responses in rice. Sci. Rep. 2017;7:40498. doi: 10.1038/srep40498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang HJ, et al. A salivary sheath protein essential for the interaction of the brown planthopper with rice plants. Insect Biochem. Mol. Biol. 2015;66:77–87. doi: 10.1016/j.ibmb.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Ji R, et al. A salivary endo-β-1,4-glucanase acts as an effector that enables the brown planthopper to feed on rice. Plant Physiol. 2017;173:1920–1932. doi: 10.1104/pp.16.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shangguan X, et al. A mucin-like protein of planthopper is required for feeding and induces immunity response in plants. Plant Physiol. 2018;176:552–565. doi: 10.1104/pp.17.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang HJ, Liu CW, Xu HJ, Bao YY, Zhang CX. Mucin-like protein, a saliva component involved in brown planthopper virulence and host adaptation. J. Insect Physiol. 2017;98:223–230. doi: 10.1016/j.jinsphys.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Zhang CX, Chen R, He SY. Challenging battles of plants with phloem-feeding insects and prokaryotic pathogens. Proc. Natl. Acad. Sci. USA. 2019;116:23390–23397. doi: 10.1073/pnas.1915396116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong G, et al. Salivary proteomics response of rice brown planthopper Nilaparvata lugens (Stål) to stimulation of tricin, a key rice insect-resistant compound. J. Environ. Entomol. 2019;41:50–61. [Google Scholar]
- 19.Yuan L-Y, Hao Y-H, Chen Q-K, Pang R, Zhang W-Q. Pancreatic triglyceride lipase is involved in the virulence of the brown planthopper to rice plants-sciencedirect. J. Integr. Agric. 2020;19:2758–2766. doi: 10.1016/S2095-3119(20)63188-4. [DOI] [Google Scholar]
- 20.Eloy NB, et al. Silencing chalcone synthase in maize impedes the incorporation of tricin in lignin and increases in lignin content. Plant Physiol. 2017;173:998–1016. doi: 10.1104/pp.16.01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Zhang J, Wang Z, Zhu Q, Wang W. Remobilization of carbon reserves in response to water deficit during grain filling of rice. Field Crops Res. 2001;71:47–55. doi: 10.1016/S0378-4290(01)00147-2. [DOI] [Google Scholar]
- 22.Jing S, et al. Genomics of interaction between the brown planthopper and rice. Curr. Opin. Insect Sci. 2017;19:82–87. doi: 10.1016/j.cois.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Huang HJ, et al. Screening and functional analyses of Nilaparvata lugens salivary proteome. J. Proteome Res. 2016;15:1883–1896. doi: 10.1021/acs.jproteome.6b00086. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, et al. Marker assisted pyramiding of two brown planthopper resistance genes, Bph3 and Bph27 (t), into elite rice. Rice (N Y) 2016;9:27. doi: 10.1186/s12284-016-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmonds MS. Flavonoid-insect interactions: recent advances in our knowledge. Phytochemistry. 2003;64:21–30. doi: 10.1016/S0031-9422(03)00293-0. [DOI] [PubMed] [Google Scholar]
- 26.Ji R, et al. Comparative transcriptome analysis of salivary glands of two populations of rice brown planthopper, Nilaparvata lugens, that differ in virulence. PLoS ONE. 2013;8:e79612. doi: 10.1371/journal.pone.0079612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calderón-Cortés N, Quesada M, Watanabe H, Cano-Camacho H, Oyama K. Endogenous plant cell wall digestion: a key mechanism in insect evolution. Annu. Rev. Ecol. Evol. Syst. 2012;43:45–71. doi: 10.1146/annurev-ecolsys-110411-160312. [DOI] [Google Scholar]
- 28.Hao P, et al. Herbivore-induced callose deposition on the sieve plates of rice: an important mechanism for host resistance. Plant Physiol. 2008;146:1810–1820. doi: 10.1104/pp.107.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonaventure G. Perception of insect feeding by plants. Plant Biol (Stuttg) 2012;14:872–880. doi: 10.1111/j.1438-8677.2012.00650.x. [DOI] [PubMed] [Google Scholar]
- 30.Naessens E, et al. A secreted MIF cytokine enables aphid feeding and represses plant immune responses. Curr. Biol. 2015;25:1898–1903. doi: 10.1016/j.cub.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 31.Dong NQ, Lin HX. Contribution of phenylpropanoid metabolism to plant development and plant-environment interaction. J. Integr. Plant Biol. 2021;63:180–209. doi: 10.1111/jipb.13054. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, Dodgen LK, Conkle JL, Gan J. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: A review. Sci. Total Environ. 2015;536:655–666. doi: 10.1016/j.scitotenv.2015.07.129. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, et al. A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat. Biotechnol. 2015;33:301–305. doi: 10.1038/nbt.3069. [DOI] [PubMed] [Google Scholar]
- 34.Pathak PK, Heinrichs EA. Selection of biotype populations 2 and 3 of Nilaparvata lugens 1 to resistant rice varieties 2. Environ. Entomol. 1982;11:347–371. doi: 10.1093/ee/11.1.85. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.