Abstract
Cocaine use is commonly associated with increased chronic systemic inflammation. However, the drivers for cocaine use-mediated systemic inflammation are not fully understood. In the current study, we recruited individuals with cocaine use disorder and healthy individuals who did not use cocaine and collected paired saliva and blood samples. The saliva samples were used to assess the oral microbiome, and the plasma samples were evaluated for 33 cytokines and chemokines. Cocaine users exhibited decreased saliva microbial diversities compared to non-users. Streptococcus was the only increased genus in the saliva from cocaine users, whereas several genera were decreased in cocaine users compared to non-users. Notably, cocaine users exhibited increased plasma levels of several monocyte activation markers, including monocyte chemoattractant protein (MCP)-4, macrophage inflammatory protein (MIP)-3✓, macrophage-derived chemokine (MDC), and thymus and activation-regulated chemokine (TARC), all of which were correlated with increased saliva levels of three Streptococcus species. Furthermore, treatment with Streptococcus or its lipoteichoic acid preferentially activated primary human monocytes to produce proinflammatory cytokines and chemokines, such as MIP-3✓ and TARC, in vitro compared to controls. However, monocytes failed to produce these chemokines after exposure to cocaine or cocaine plus bacteria compared to medium or bacteria alone. This study revealed that chronic cocaine use-associated inflammation in the blood may result from increased oral Streptococcus and its effects on myeloid cell activation, but does not result from cocaine directly.
Keywords: cocaine use disorder, saliva microbiome, Streptococcus, plasma inflammation, monocyte activation
Graphical abstract
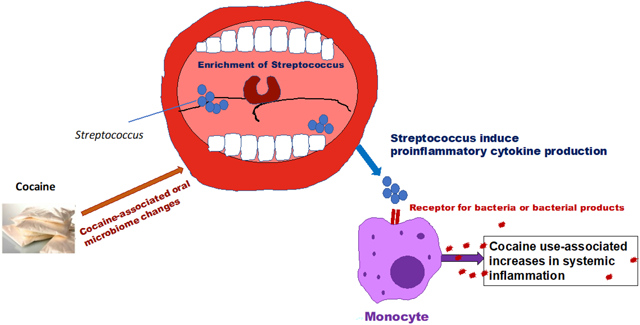
A model for cocaine-associated systemic inflammation via oral microbiome. In the current study, we propose a model of cocaine use-associated systemic inflammation. Cocaine alone does not induce monocyte activation and inflammation. Cocaine use altered oral microbiome with the increases in Streptococcus and systemic translocation. The systemic translocation of Streptococcus or its products activate monocytes via receptors for bacteria or bacterial products (e.g., toll-like receptor 2) to produce proinflammatory cytokines or chemokines.
Introduction
According to the most recent data from the U.S. National Survey on Drug Use and Health, 5.5 million people ages 12 or older were estimated to use cocaine within the past year, representing about 2% of the population in 2019 [1]. Thus, cocaine use disorder is an important public health issue. Recreational use of cocaine commonly occurs via inhalation (smoking) or intranasal and intravenous (IV) administration.
Chronic cocaine use is associated with increases in both systemic and central nervous systemic inflammation [2–7]. However, the drivers for cocaine use-mediated systemic inflammation are not fully understood. Cocaine has been shown to both directly and indirectly affect immune cell functions, thereby inhibiting the body’s immune responses, which can cause an increased risk for infection, especially human immunodeficiency virus (HIV) infection [8]. Cocaine has been reported to influence lymphocyte proliferation, cytokine production, natural killer (NK) cell cytotoxicity, and the phagocytic activity of macrophages and neutrophils [9–13]. A previous study showed that peritoneal and alveolar macrophages isolated from cocaine-exposed mice showed an increase in the respiratory burst compared to macrophages isolated from saline control mice. Furthermore, macrophages exposed to cocaine in vitro failed to induce an increased respiratory burst, suggesting that cocaine stimulates the production of reactive oxygen intermediates which result in changes in macrophage functions in vivo [14]. Another study exposed human neutrophils to various concentrations of cocaine and found that cocaine-exposed neutrophils had a decrease in phagocytosis ability and impaired phagolysosomal acidification [15]. Another study isolated peripheral blood mononuclear cells (PBMCs) from cocaine-dependent subjects infused with cocaine, and after culturing for 48 h, the production of cytokines was examined in the supernatant of the PBMCs. Cocaine infusion increased the production of interferon (IFN)-gamma and decreased IL-10 secretion compared to PBMCs isolated from saline-infused mice [16]. Additionally, cocaine-dependent subjects exhibited a reduction in the capability of monocytes to express both TNF-✓ and IL-6 as compared to control subjects [17]. Furthermore, an acute infusion of cocaine caused a further decrease in the LPS-responsiveness of monocytes, which continued until after cocaine was cleared from the blood [17]. Cocaine has been shown to also affect cells in several ways in vitro, such as inducing vascular endothelial cells to produce the adhesion molecules ICAM-1, VCAM-1, and ELAM-1 (E-selectin), impairing the tight junctions within the endothelial cell lining in the blood-brain barrier (BBB), and enhancing the migration of monocytes through the BBB [18, 19]. Altogether, these findings suggest that cocaine may exert acute anti-inflammatory effects, while having the opposite effect when used chronically. Furthermore, there is a difference between the direct and indirect effects of cocaine. However, whether cocaine directly or indirectly modulates cytokine secretion and systemic inflammation is not fully understood. In addition, the mediators accounting for cocaine use disorder-associated inflammation remain unknown.
In the present study, we collected paired saliva and blood samples from healthy individuals and individuals with cocaine use disorder in order to assess the correlation between the oral microbiome and inflammatory plasma markers in cocaine use disorder. We found oral microbial dysbiosis in humans with cocaine use disorder. We also identified a link between the plasma levels of monocyte activation-related proinflammatory cytokines and enrichment of saliva Streptococcus species in individuals with cocaine use disorder. Furthermore, we used an in vitro primary human monocyte model to understand how exposure to bacteria, bacterial products, and cocaine influences monocyte activation. We isolated primary monocytes from human control subjects and exposed these cells to Streptococcus, lipoteichoic acid, cocaine, or cocaine + Streptococcus. We found that primary human monocytes exposed to Streptococcus were induced to produce proinflammatory cytokines and chemokines. However, primary human monocytes exposed to cocaine or cocaine plus Streptococcus failed to produce these chemokines. The results of our study emphasize the importance of oral health in systemic health and provide insights regarding the health issues of individuals with cocaine use disorder.
Methods
Study Subjects and Cocaine Use
For this study, we recruited eight individuals with cocaine use disorder and ten healthy individuals who did not use cocaine. Individuals with cocaine use disorder were recruited from the Addiction Center at the Medical University of South Carolina (MUSC). This study was approved by the MUSC Institutional Review Boards. All participants provided written informed consent. All participants were aged 18–55 years and fit the criteria for cocaine dependence or abuse [20], with no history of or current psychiatric, neurological, neurodevelopmental disorders or traumatic brain injury, or recent antibiotics or probiotics uses. The controls were matched by age and gender. The cocaine users did not report use of any other drugs of abuse, as verified by chart reviews and urine tests. The enrolled subjects confirmed that their cocaine use was through smoking or intranasal administration, but not through IV administration. The clinical characteristics of crack or powder cocaine users are shown in Supplementary Table 1.
We used a web-based, self-administered Timeline Followback (TLFB) Method Assessment [21] to assess the frequency and quantity of cocaine use within the 90 days prior to the study visit. We also assessed the following questions: whether the participants used cocaine (yes/no), days using per month, days using per month during heaviest use, form at first use pattern (route of administration when first starting), form at heaviest use pattern, or form at current use pattern, age of dependence onset, age during heaviest use, quantity used per day during heaviest use, total years of use, and years of abstinence (Supplementary Table 1). Drug screens were performed using the onTrak test cup, an in vitro diagnostic test for the qualitative detection of drugs or drug metabolites in urine. Results of urine screenings were used to substantiate self-reports of cocaine use.
Psychiatric Disorder Diagnostic/Descriptive Assessment
For individuals with cocaine use disorder, we assessed exclusionary psychiatric diagnoses using appropriate Mini-International Neuropsychiatric Interview (M.I.N.I.) modules, as described in our previous study [22]. Briefly, the M.I.N.I. is a brief structured interview to assess current diagnoses based on the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V). The M.I.N.I. only assesses current diagnoses, whereas a more thorough history of substance use was needed in this study, and as such, the substance use module of the Structured Clinical Interview for DSM-V was used for substance use disorder diagnoses.
Microbiome Analysis
The method has been described in previous studies [23–25]. Briefly, bacterial DNA was extracted from saliva using a QIAamp DNA microbiome Kit, according to the manufacturer’s protocols (Qiagen). The V4 variable region of the bacterial 16S ribosomal RNA (16S rRNA) gene was amplified using polymerase chain reaction primers 515F/806R with Illumina adapters and golay indices on the 3’ end and amplified using Phusion High-Fidelity PCR Master Mix (New England BioLabs) with 10 μg BSA under the following conditions: 94°C for 3.5 minutes, followed by 30 cycles of 95°C for 30 seconds, 50°C for 30 seconds, and 72°C for 90 seconds, and a final elongation step at 72°C for 10 minutes. Sequencing was performed on the MiSeq using v2 2 × 250 base pair kit (Illumina, Inc) at the Microbial Analysis, Resources, and Services (University of Connecticut, Storrs, CT). Sequences were processed using the Quantitative Insights Into Microbial Ecology (QIIME) 1 pipeline [26] and demultiplexed. Poor-quality sequences were removed using the default settings of the QIIME 1 script split_libraries.py (minimum average quality score = 25, minimum/maximum sequence length = 200/1,000 base pairs; no ambiguous base calls and no mismatches allowed in the primer sequence). After demultiplexing and quality filtering, sequences were clustered into de novo operational taxonomic units (OTUs) based on 97% sequence similarity, and representative sequences were assigned taxonomy based on fully sequenced microbial genomes (IMG/GG GreenGenes) [26]. Chimeric sequences were identified using ChimeraSlayer [27]; sequences failing the alignment and singleton OTUs were removed.
Plasma Levels of 33 Inflammation Biomarkers
All plasma assays were conducted following the manufacturer’s protocol for the Human Chemokine Panel 1 V-PLEX Plus and Human Proinflammatory Panel kits (Meso Scale Diagnostics, Rockville, MD). Each multiplex array was scanned using a MESO QuickPlex SQ 120. Discover Workbench 4.0 software (Meso Scale Diagnostics) was used to analyze the arrays and quantify the biomarker concentrations based on standard curves, using the manufacturer’s supplied standards.
Monocyte Proinflammatory Cytokine Production in Response to Streptococcus in Vitro
Human peripheral blood mononuclear cells (PBMCs) from healthy donors were used in this study. PBMCs were cultured in RPMI-1640 containing 1% autologous human serum for 1 hour at 37°C. The cells were treated with various concentrations of different bacterial strains (1.25 × 106 or 5 × 106 bacterial cells/mL of Actinomyces odontolyticus, Streptococcus parasanguinis, Streptococcus anginosus) or 2 ng/mL of lipopolysaccharide (LPS) as a control in the presence of GolgiStop (Monensin) and GolgiPlus (1 μL/mL) for 6 hours. The expression of cell surface glycoprotein CD14 and intracellular cytokines in monocytes were analyzed using flow cytometry and FlowJo. The following fluorochrome-labeled monoclonal antibodies (clones) from BD were used: anti-CD14, anti-TNF-✓, IL-1 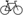 , and IL-6, and isotype control antibodies.
, and IL-6, and isotype control antibodies.
Monocyte Chemokine Production in Response to Streptococcus and Cocaine in Vitro
Frozen PBMCs from eight heathy subjects were thawed in presence the of 20 μg/mL DNase and cultured in the RPMI-1640 medium supplemented with 10% fetal bovine serum, 50 μg/mL penicillin/streptomycin, and 1 mM sodium pyruvate for 1 hour. Total monocytes were isolated with a pan monocyte isolation kit (Miltenyi Biotec, Bergisch Gladback, Germany). The monocytes were cultured with the S. parasanguinis (BEI Resources) or lipoteichoic acid (LTA) from S. pyogenes (Sigma) in the presence of cocaine (Sigma) for 48 hours. The final concentration of S. parasanguinis, LTA from S. pyogenes, and cocaine were 1.25 × 106 bacterial cells/mL, 1.25 × 106 bacterial cells/mL, and 1 μM, respectively. The dose of cocaine was used based on a previous study [28]. The chemokine monocyte chemo-attractant protein-4 (MCP-4), macrophage-derived chemokine (MDC), macrophage inflammatory protein-3 alpha (MIP-3α), and thymus and activation-regulated chemokine (TARC) levels were evaluated in cell culture supernatants. These chemokine levels were measured simultaneously using U-PLEX Biomarker Group 1 (hu) Assay kits (Meso Scale Diagnostics, Rockville, Maryland) according to the manufacturer’s instructions.
Statistical Analysis
OTU raw counts were normalized to the relative abundances, and taxa of the same type were aggregated at the phylum, class, order, family, genus, and species levels. The non-parametric Mann-Whitney U test was performed in QIIME 1 to compare the abundances, and P values were adjusted for multiple comparisons using the false discovery rate (FDR). The microbiome species alpha-diversity index (α-diversity) within each sample was determined using the phyloseq package in R (www.r-project.org). To test the significance of the estimated α-diversity, a Wilcoxon rank sum test was performed using R. To test the statistical significance of variances in the microbiome composition between groups, a permutational multivariate analysis of variance using the R vegan package with the function Adonis was performed. To minimize false discoveries, P values were adjusted for multiple comparisons using the Benjamini-Hochberg FDR method.
Results
Decreased Saliva Microbial Diversity in Cocaine Users Compared to Non-Users
We first analyzed the microbiota composition in the saliva samples from cocaine users and non-users. The α-diversity index (or Shannon and Simpson index) was used to indicate the effective number of microbial communities [29]. We found that the α-diversity index of the cocaine user group was significantly lower than that of the control group (Figure 1A), suggesting cocaine use-associated microbial dysbiosis.
Figure 1. Saliva microbiome in cocaine users and non-users.
(A) Alpha-diversity analysis of the saliva microbiome in cocaine users compared to non-users. Bacterial phyla (B) and genera (C) of the saliva associated with cocaine use. (D) A Venn diagram showing OTU clustering in the genus level between cocaine-users. non-users. Non-parametric Mann-Whitney U tests. P values were adjusted for multiple comparisons by FDR.
At the phylum level of the saliva microbiome, the relative abundance of Firmicutes was significantly increased and the relative abundance of Proteobacteria was significantly decreased in cocaine users compared to non-users (Figure 1B). To better understand the effect of cocaine on the microbial composition, we analyzed the association of cocaine exposure with the enrichment of bacteria at the genus level. Chronic cocaine exposure was associated with an increased abundance of Streptococcus, and a decreased abundance of Actinobacillus, Campylobacter, Fusobacterium, Haemophilus, Mannheimia, Neisseria, and Porphyromonas compared to non-users (Figure 1C). Moreover, some Streptococcus-related species were increased, while some Neisseria-related species were decreased in cocaine users compared to non-users (Supplementary Figure S1). No differences were observed between heavy users (> 4 days/week) and light users (≤ 4 days/week), short-term users and long-term users, and powder and crack cocaine users (Supplementary Table 1). A Venn diagram is showing OTU clustering between cocaine-users and non-users (Figure 1D).
Plasma Levels of Inflammation in Cocaine Users Compared to Non-Users
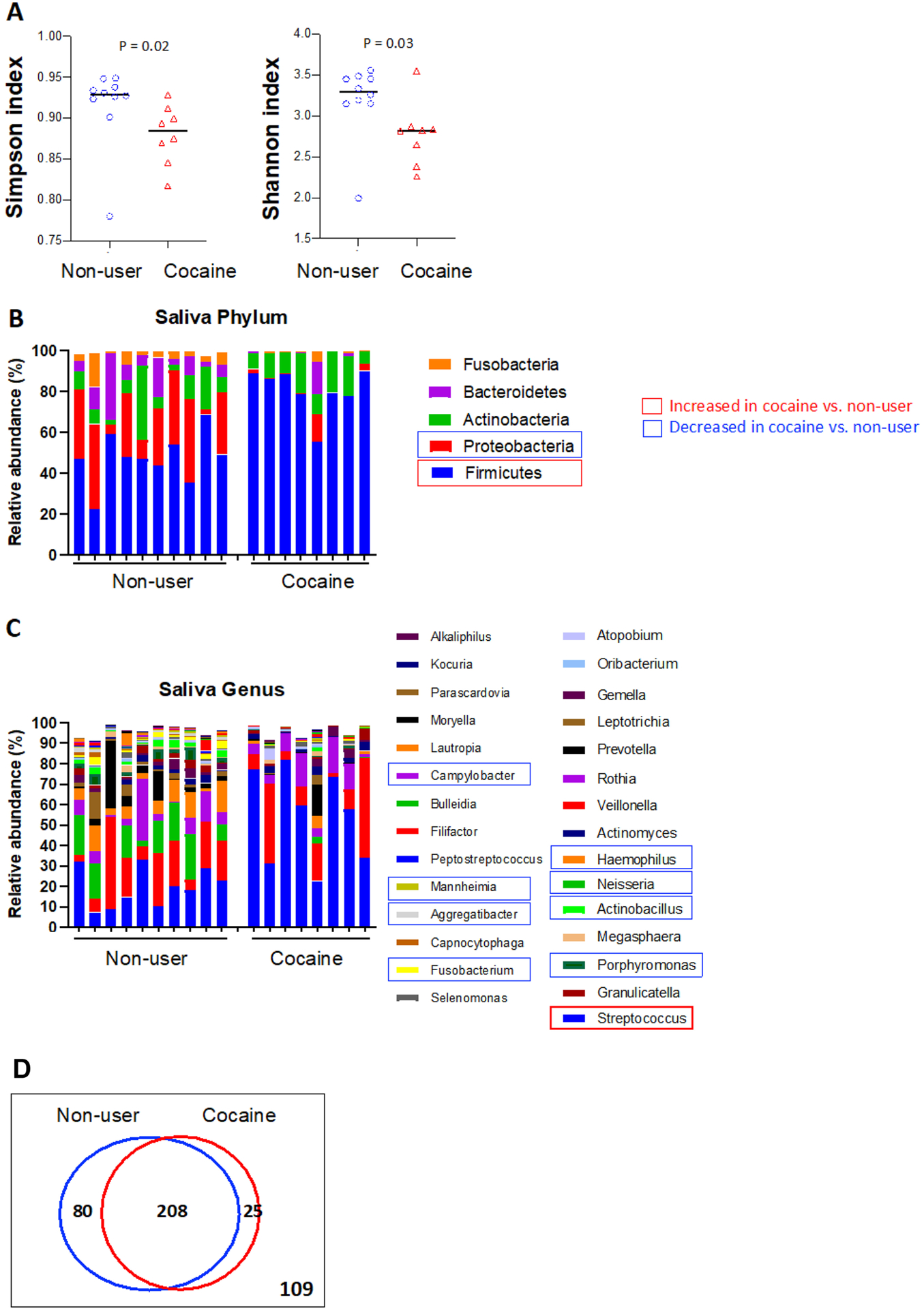
Next, in order to determine whether chronic cocaine use disorder is associated with systemic inflammation, we examined the plasma levels of a panel of 33 soluble markers for inflammation. Notably, plasma levels of MCP-4, MIP-3α, MDC, and TARC were increased (Figures 2A–2D), and plasma levels of IL-10, IL-4, IL12p70, IL12IL23p40, and IL-27 were decreased (Figures 2E–2I) in cocaine users compared to non-users. These data indicated that cocaine use disorder is associated with proinflammatory responses relevant to monocyte/macrophage activation. Furthermore, many inflammatory cytokines were shown to have similar levels between cocaine users and controls (Supplementary Figure S2).
Figure 2. Protein expression of proinflammatory plasma biomarkers in cocaine users compared to non-users.
Median plasma levels of inflammatory cytokines MCP-4, MIP-3α, MDC, TARC, IL-10, IL-4, IL12p70, IL12IL23p40, and IL-27 in cocaine users and non-users were evaluated using Human Proinflammatory Panel kits. Statistical analyses were performed using unpaired Mann-Whitney U tests with a P < 0.05 denoting a significant effect of cocaine usage.
Correlations Between the Oral Microbiome and Inflammatory Plasma Markers
In order to assess whether cocaine use-associated plasma inflammation is mediated by a specific microbiome (e.g., increased Streptococcus related to cocaine use), we analyzed correlations between the oral microbiome and plasma levels of inflammatory cytokines. Intriguingly, plasma levels of MCP-4, MDC, TARC, or MIP-3α were directly correlated with the relative abundance of Streptococcus australis, Streptococcus parasanguinis, and Streptococcus gordonii in the saliva and inversely correlated with the levels of Neisseria cinerea, Neisseria mucosa, and Neisseria flavescens in cocaine users (Figure 3). These correlations were not demonstrable in the non-users (data not shown). These results revealed a link between cocaine use-associated oral microbiota dysbiosis and systemic inflammation as related to monocyte activation.
Figure 3. Correlations between the oral microbiome and inflammatory plasma markers.
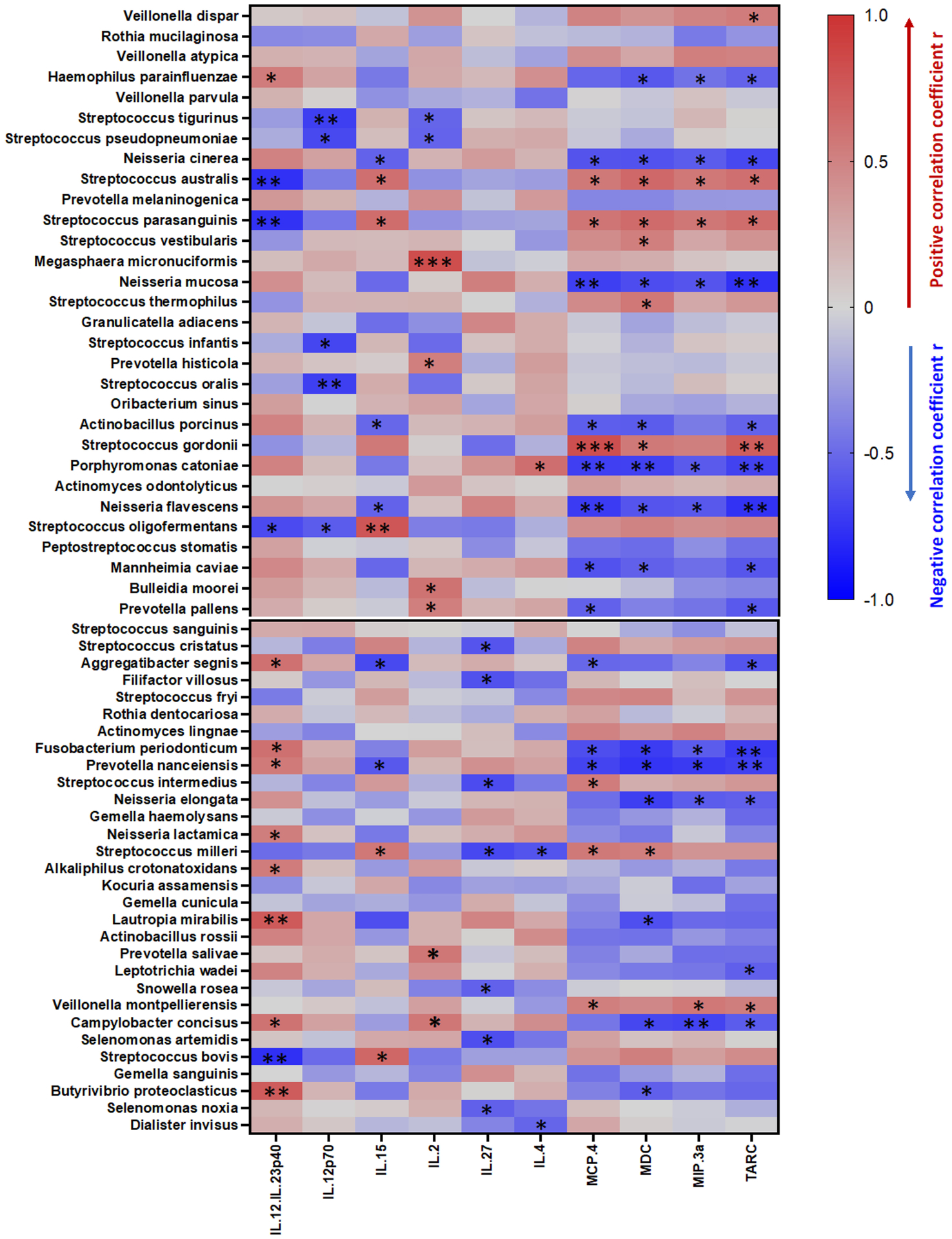
The vertical lines along the scale bar indicate the correlations (red for positive and blue for negative). The relative size of the node was determined by the relative abundance of the microbe. Correlations with adjusted P values less than 0.05 are displayed. * P < 0.05, ** P < 0.01, and *** P < 0.001 denote significant correlations.
Monocyte Activation in Response to Streptococcus But Not to Cocaine in Vitro
In order to assess proinflammatory responses to Streptococcus stimulation, we treated PBMCs from 5 different healthy individuals with two commercially available Streptococcus strains, S. parasanguinis and S. Anginosus, in addition to A. odontolyticus (an orally enriched commensal bacterium) as a control and LPS as a positive control in vitro. As expected, stimulation via Streptococcus bacterial species decreased the surface expression of CD14 and increased the intracellular expression of IL-1β, IL-6, and TNF-α in monocytes compared to the control bacteria (Figure 4). The concentration choice for each bacterium was based on titration assays (Supplementary Figure 3S). These results confirm the ability of Streptococcus to induce proinflammatory cytokine induction in monocytes.
Figure 4. Streptococcus induced intracellular proinflammatory cytokine production in monocytes in vitro.
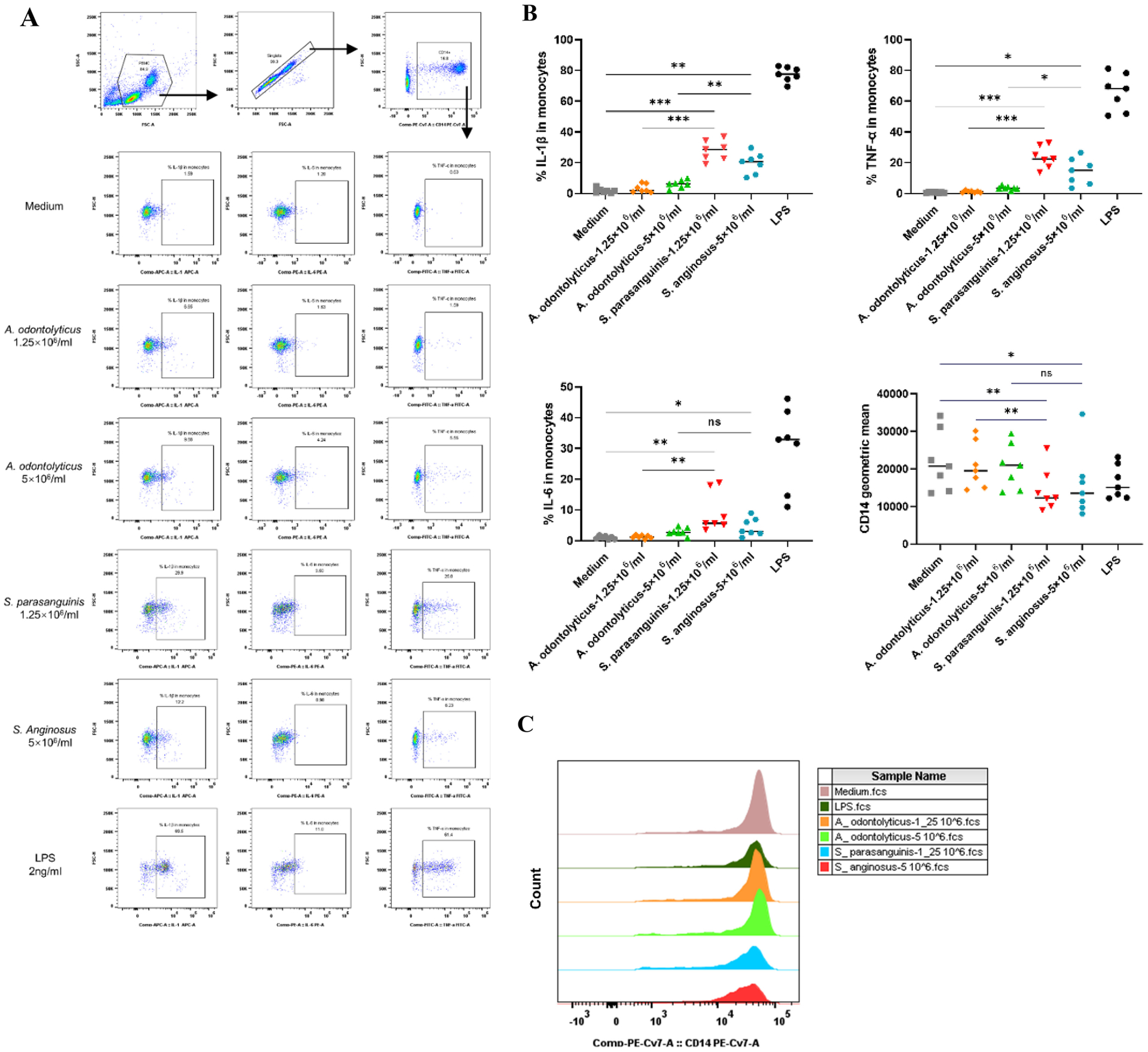
PBMCs from healthy donors were treated with different concentrations of each bacterial strain for 6 h. The surface glycoprotein CD14 and intracellular cytokines in monocytes were measured by flow cytometry. (A) Scatter plots from one representative donor representing the intracellular IL-1β, IL-6 and TNF-α expression in monocytes in response to 1.25 × 106 or 5 × 106 bacterial cells/mL of A. odontolyticus, S. parasanguinis, and S. anginosus or 2 ng/mL of LPS. (B) Median percentages of monocytes expressing IL-1β, IL-6, and TNF-α, and the geometric mean expression of CD14 on monocytes following stimulation with A. odontolyticus, S. parasanguinis, S. Anginosus, and LPS. (C) Histograms from one representative donor showing CD14 expression on monocytes treated with different concentrations of A. odontolyticus, S. parasanguinis, S. Anginosus, or LPS. The y-axis indicates the cell count for each population, and the x-axis shows the anti-CD14-PE-Cy7 fluorescence intensity. Statistical analysis was performed using a paired one-way ANOVA test; * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001. A. odontolyticus, Actinomyces odontolyticus; S. parasanguinis, Streptococcus parasanguinis; S. anginosus, Streptococcus anginosus; LPS, lipopolysaccharide.
Furthermore, in order to evaluate chemokine production by monocytes in response to Streptococcus and cocaine exposure in vitro, we isolated monocytes and stimulated with S. parasanguinis or LTA from S. pyogenes in the presence or absence of cocaine for 48 hours. The cocaine concentration (1 μM) shown the optimal dose for induction of chemokines and cytokines by human primary monocytes in vitro [28], was used in the current study. Indeed, Streptococcus induced increased production of MIP-3α and TARC and also slightly increased the production of both MCP-4 and MDC, which did not reach significance (Figure 5). Neither cocaine alone nor cocaine plus Streptococcus changes the level of any of these chemokines compared to medium or bacteria alone, respectively (Figure 5).
Figure 5. Streptococcus-induced chemokine production in isolated human primary monocytes in vitro.
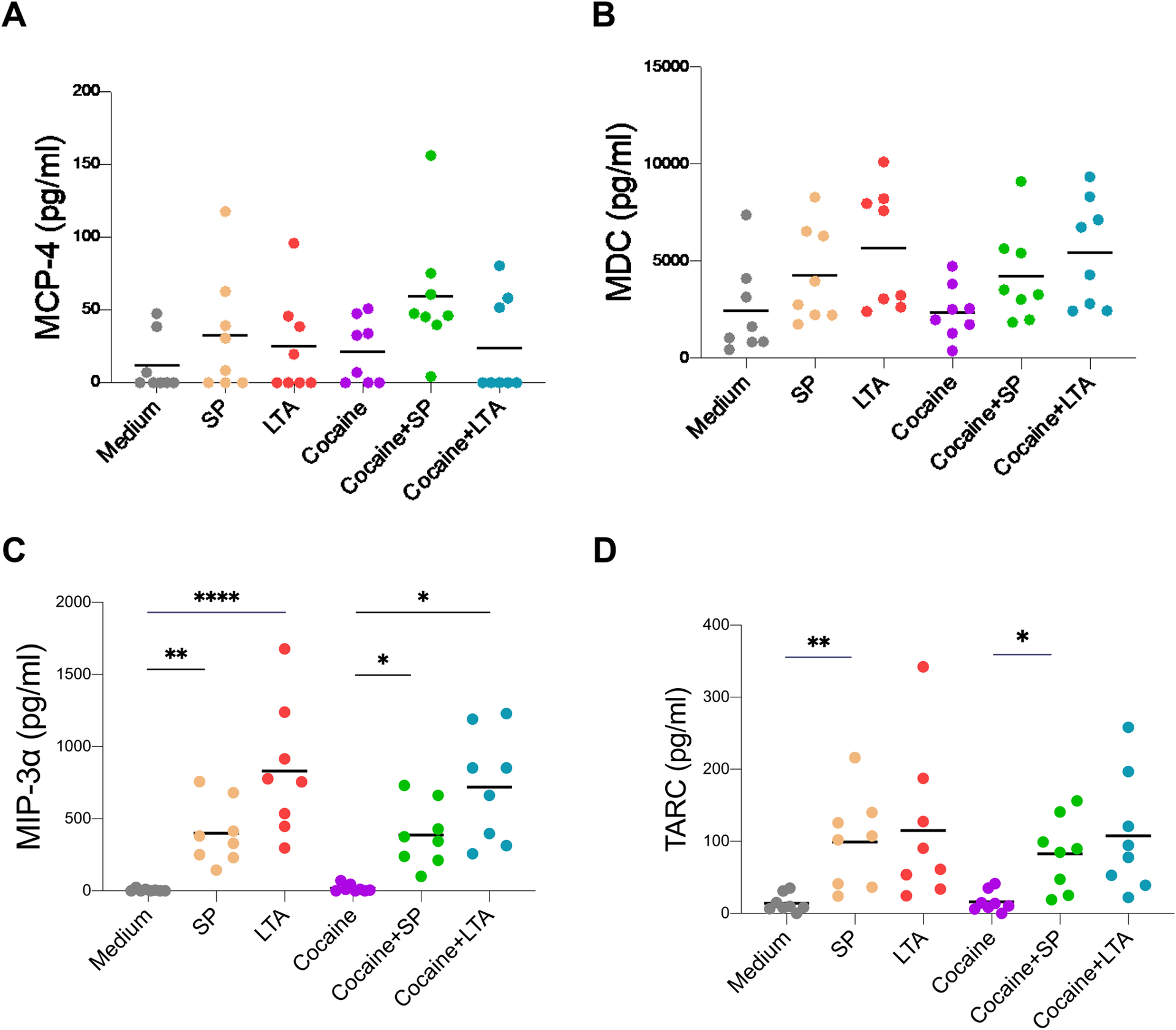
Human monocytes were isolated from PBMCs from eight healthy individuals and cultured with S. parasanguinis (SP, 1.25 × 106 bacterial cells/mL) or LTA from Streptococcus pyogenes or cocaine (1 μM) for 48 h. Levels of MCP-4 (A), MDC (B), MIP-3α (C), and TARC (D) were evaluated in the cell culture supernatants. Paired one-way ANOVA tests were used to determine statistical significance; * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001.
Discussion
The current study provides insights into the potential mechanism of cocaine use-associated oral microbial dysbiosis and systemic inflammation in humans (graphical abstract figure). Smoked and IV dosing of cocaine has a faster and greater rate of abuse liability and toxicity than intranasal or oral routes in rats [30], which may be due to the microbial dysbiosis since chronic inhalation of cocaine can drastically change the microbiota [31]. We found that people with cocaine use disorder had increased Streptococcus as compared to non-users. A previous study demonstrated that drug users had a significant reduction in the buffering capacity of saliva, resulting in an increase in Streptococcus mutans and Lactobacillus [32]. Consistently, studies have reported decreased salivary flow among crack cocaine users [33, 34]. Saliva serves several purposes within the oral cavity, one of which is to provide antimicrobial functions through various proteins and peptides, including lysozyme, lactoperoxidase, lactoferrin, and secretory immunoglobulin A. As such, saliva plays a critical role in shaping and maintaining homeostasis within the oral microbiota [35]. Thus, the increased abundance of Streptococcus species among cocaine users may be due to a decreased salivary flow rate in cocaine users. Further studies are needed to confirm this relationship between cocaine use, salivary flow rate, and Streptococcus species.
A previous study showed increased levels of Th1, Th2, and Th17 cytokines in individuals with cocaine use disorder compared to control individuals [36]. Interestingly, our results showed that cocaine users had an increase in plasma levels of MCP-4, MDC, TARC, and MIP-3α—all chemokines responsible in attracting immune cells, particularly lymphocytes. Our results are consistent with previous studies, which reported that cocaine administration acutely increases the number and activity of several lymphocyte populations, including CD4+ T, CD8+ T, and NK cells [37, 38]. Thus, the increased plasma chemokines we observed in our study may play a role in lymphocyte recruitment in cocaine users. However, it is not clear what cell is producing the chemokines or what tissue or organ is recruiting the lymphocytes. Previous studies have shown that cocaine administration in mice induces a compromised mucosal barrier in vivo [4, 39] and a disrupted intestinal microbiota [4]. One study showed a 6.71-fold increase in the abundance of Streptococcus in the gut microbiota of mice exposed to cocaine [4]. Intriguingly, the relative abundance of S. australis, S. parasanguinis, and S. gordonii in the saliva was directly correlated with increased plasma levels of MCP-4, MDC, TARC, and MIP-3α in cocaine users in our study. Consistently, monocytes produced TARC and MIP-3α in response to Streptococcus bacterium or its product LTA in vitro, and cocaine alone or cocaine plus bacteria did not affect this monocyte activation (Figure 5). The concentration of cocaine used here was 1 μM which was the optimal dose to induce chemokines and cytokines by human primary monocytes in vitro [28]. 1 μM of cocaine concentration was ~2–3-fold higher compared to the peak physiological dose in plasma after cocaine administration in humans in vivo [40]. Thus, it is possible that the increase in chemokines in cocaine users may be a result of the increase abundance of Streptococcus. We present two hypotheses regarding the role of Streptococcus in monocyte activation in cocaine users (see the graphical abstract). First, cocaine disrupts the epithelial barrier, allowing for the translocation of Streptococcus or other bacterial products to the blood, resulting in monocyte activation and production of proinflammatory cytokines or chemokines via bacteria associated receptors (e.g., toll-like receptors). Second, Streptococcus or its products directly activate monocytes or macrophages within the oral cavity, which promotes cell migration to the blood. Both of these hypotheses require further investigation to confirm.
Cocaine has been shown to suppress IL-2-mediated expression of IFN-γ and IL-8 in human mononuclear cells in vitro [41]. Similarly, cocaine inhibits concanavalin A-induced production of IL-2, IL-4, IL-5, IL-10, and IFN-γ by murine spleen cells and LPS-induced production of IL-1β, IL-6, and TNF-α by murine macrophages [42]. In mice, acute or short-term cocaine exposure decreases the ability of spleen cells to produce IL-2, IFN-γ, and IL-4 in response to mitogen stimulation [43]. However, cocaine has been shown to directly activate the NF-κB signaling pathway, resulting in the upregulation of proinflammatory cytokines mainly in THP1 cells in vitro [44]. Nonetheless, direct effects of cocaine are generally considered anti-inflammatory or immunosuppressive. On the contrary, after cocaine administration in mice in vivo, there was increased secretion of IL-6 by splenocytes and peritoneal macrophages, while the production of TNF-α was enhanced in splenocytes but inhibited in macrophages [42]. In another study, cocaine administration resulted in an inflammatory environment in the gut by increasing the levels of multiple proinflammatory cytokines (IL-18 and IL-1β) and chemokines (CCL-2, CCL-7, CXCL-10, and CCL-11) [4]. These findings suggest that the effects of cocaine on immune cells and immune responses in vivo are more complicated than the direct effects of cocaine on immune cells in vitro. In the current study, monocytes were not activated in response to cocaine in vitro. Thus, persistent systemic inflammation associated with chronic cocaine use may be an indirect effect.
Monocytes can migrate across the BBB in pathological conditions and are implicated in the progression of many CNS neurodegenerative diseases [45, 46]. Persistent neuroinflammation and systemic inflammation contribute to neuropathology. Intriguingly, cocaine has been shown to facilitate monocyte trafficking across the BBB, leading to enhanced HIV infection and increased neuropathology [46]. The mechanisms by which cocaine elicits these responses, however, remain poorly understood. Moreover, monocytes from cocaine-dependent humans have reduced capacities to produce TNF-✓ and IL-6 in response to LPS stimulation in vitro compared to those of control subjects [13]. This reduced response of monocytes from cocaine-dependent humans to LPS may result from increased activation of monocytes in vivo, which results in desensitization to stimulation in vitro.
Investigating the mechanism of cocaine use-mediated chronic inflammation is important to patient care. Evidence of monocyte or macrophage activation and inflammatory cytokine production by cocaine directly is rare [28]. In our study, Streptococcus stimulation induced proinflammatory cytokines and chemokines by monocytes in vitro. However, primary human monocytes were not activated by cocaine or the combination of cocaine and target bacteria in vitro. Our results may differ from previous studies [28] because of the heterogeneity of individuals enrolled in each study. Nonetheless, long-term cocaine use has been associated with weight loss, malnutrition, loss of appetite, various gastrointestinal (GI) complications, and reduced blood flow to the GI tract, suggesting that cocaine use causes GI tract dysfunctions [47, 48]. Moreover, changes in the intestinal flora were reported to increase rodents’ sensitivity to cocaine rewards and exercise, suggesting a link between intestinal flora and altered behavioral responses to the drug [49]. Given that the role of the gut microbiome in regulating addictive behaviors is associated with cocaine abuse, it is important to understand the mechanisms of cocaine-mediated disruption of the GI environment.
This study revealed that chronic cocaine use-associated inflammation in the circulation may result from increased oral Streptococcus and its effects on monocyte activation, but not cocaine directly. There are several limitations in the current study. First, the small sample size prevented us from drawing further conclusions. Second, enrichment of Streptococcus may not be specific to cocaine users. However, we have previously compared the oral microbiome in people with a history of using drugs that primarily affect the oral microenvironment, including cocaine users (either through smoking or intranasal), cannabis smokers, and tobacco smokers. We found that cocaine users had the highest relative enrichment of Streptococcus bacteria in the oral microbiome (~59%) compared to those in cannabis smokers (~35%) [50] or in tobacco smokers (~12%) [51, 52]. Third, there were some outliers presented in the microbiome data (e.g., Figure 1A–1B); they may be caused by the effect of antibiotics (e.g., antibiotics-containing food), even we excluded recent uses of antibiotics and probiotics during recruitment. Nevertheless, our study emphasizes the importance of oral health in systemic health and provides insights into the health issues of individuals with cocaine use disorder.
Supplementary Material
Acknowledgement
This work was supported by grants from the National Institute of Drug Abuse K24DA038240 (McRae-Clark), R01 DA045596 (Fitting), NR016928 (Cong), ONF RE01 and P20NR016605 (Starkweather)—Pilot 3 sub-award (Xu), and R01DA043938 (Stoops).
Conflict of Interest
The authors declare no competing interests. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
References
- 1.Hedegaard Holly S MR, and Garnett Matthew F.: Increase in Drug Overdose Deaths Involving Cocaine: United States, 2009–2018. In. Edited by Prevention CfDCa, vol. NCHS Data Brief No. 384, October 2020. Hyattsville, MD: National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
- 2.Fox HC, D’Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, Sinha R: Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol 2012, 27(2):156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo ML, Liao K, Periyasamy P, Yang L, Cai Y, Callen SE, Buch S: Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy 2015, 11(7):995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chivero ET, Ahmad R, Thangaraj A, Periyasamy P, Kumar B, Kroeger E, Feng D, Guo ML, Roy S, Dhawan P et al. : Cocaine Induces Inflammatory Gut Milieu by Compromising the Mucosal Barrier Integrity and Altering the Gut Microbiota Colonization. Sci Rep 2019, 9(1):12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niu F, Liao K, Hu G, Sil S, Callen S, Guo ML, Yang L, Buch S: Cocaine-induced release of CXCL10 from pericytes regulates monocyte transmigration into the CNS. J Cell Biol 2019, 218(2):700–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonder Haar C, Ferland JN, Kaur S, Riparip LK, Rosi S, Winstanley CA: Cocaine self-administration is increased after frontal traumatic brain injury and associated with neuroinflammation. Eur J Neurosci 2019, 50(3):2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreira FP, Medeiros JR, Lhullier AC, Souza LD, Jansen K, Portela LV, Lara DR, da Silva RA, Wiener CD, Oses JP: Cocaine abuse and effects in the serum levels of cytokines IL-6 and IL-10. Drug Alcohol Depend 2016, 158:181–185. [DOI] [PubMed] [Google Scholar]
- 8.Cabral Guy A: Drugs of Abuse, Immune Modulation, and AIDS. J Neuroimmune Pharmacol 2006, 1(3):280–295. [DOI] [PubMed] [Google Scholar]
- 9.Vaz A, Lefkowitz SS, Lefkowitz DL: Effects of Cocaine on the Respiratory Burst of Murine Macrophages. Advances in Experimental Medicine & Biology 1993, 335:135. [DOI] [PubMed] [Google Scholar]
- 10.Phillips DL, Tebbett IR, Masten S, Shiverick KT: Stimulatory effects of cocaine and its metabolites on IM-9 human B-lymphoblastoid cells. International Journal of Immunopharmacology 1995, 17(1):57. [DOI] [PubMed] [Google Scholar]
- 11.Gan X, Zhang L, Newton T, Chang SL, Fiala M: Cocaine Infusion Increases Interferon-gamma and Decreases Interleukin-10 in Cocaine-Dependent Subjects. Clinical Immunology and Immunopathology 1998, 89(2):181–190. [DOI] [PubMed] [Google Scholar]
- 12.Mukunda BN, Callahan JM, Hobbs MS, West BC: Cocaine Inhibits Human Neutrophil Phagocytosis and Phagolysosomal Acidification In Vitro. Journal of Immunopharmacology 2000, 22(2):373–386. [DOI] [PubMed] [Google Scholar]
- 13.Irwin MR, Olmos L, Wang M, Valladares EM, Motivala SJ, Fong T, Newton T, Butch A, Olmstead R, Cole SW: Cocaine Dependence and Acute Cocaine Induce Decreases of Monocyte Proinflammatory Cytokine Expression across the Diurnal Period: Autonomic Mechanisms. Journal of Pharmacology and Experimental Therapeutics 2007, 320(2):507–515. [DOI] [PubMed] [Google Scholar]
- 14.Vaz A, Lefkowitz SS, Lefkowitz DL: Effects of cocaine on the respiratory burst of murine macrophages. Adv Exp Med Biol 1993, 335:135–142. [DOI] [PubMed] [Google Scholar]
- 15.Mukunda BN, Callahan JM, Hobbs MS, West BC: Cocaine inhibits human neutrophil phagocytosis and phagolysosomal acidification in vitro. Immunopharmacol Immunotoxicol 2000, 22(2):373–386. [DOI] [PubMed] [Google Scholar]
- 16.Gan X, Zhang L, Newton T, Chang SL, Ling W, Kermani V, Berger O, Graves MC, Fiala M: Cocaine infusion increases interferon-gamma and decreases interleukin-10 in cocaine-dependent subjects. Clinical immunology and immunopathology 1998, 89(2):181–190. [DOI] [PubMed] [Google Scholar]
- 17.Irwin MR, Olmos L, Wang M, Valladares EM, Motivala SJ, Fong T, Newton T, Butch A, Olmstead R, Cole SW: Cocaine dependence and acute cocaine induce decreases of monocyte proinflammatory cytokine expression across the diurnal period: autonomic mechanisms. J Pharmacol Exp Ther 2007, 320(2):507–515. [DOI] [PubMed] [Google Scholar]
- 18.Gan X, Zhang L, Berger O, Stins MF, Way D, Taub DD, Chang SL, Kim KS, House SD, Weinand M et al. : Cocaine enhances brain endothelial adhesion molecules and leukocyte migration. Clinical immunology 1999, 91(1):68–76. [DOI] [PubMed] [Google Scholar]
- 19.Kousik SM, Napier TC, Carvey PM: The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Front Pharmacol 2012, 3:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Do LLTN: American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 2011.
- 21.Tomko RL, Baker NL, McClure EA, Sonne SC, McRae-Clark AL, Sherman BJ, Gray KM: Incremental validity of estimated cannabis grams as a predictor of problems and cannabinoid biomarkers: Evidence from a clinical trial. Drug Alcohol Depend 2018, 182:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettersson A, Modin S, Wahlstrom R, Af Winklerfelt Hammarberg S, Krakau I: The Mini-International Neuropsychiatric Interview is useful and well accepted as part of the clinical assessment for depression and anxiety in primary care: a mixed-methods study. BMC Fam Pract 2018, 19(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu W, Luo Z, Alekseyenko AV, Martin L, Wan Z, Ling B, Qin Z, Heath SL, Maas K, Cong X et al. : Distinct systemic microbiome and microbial translocation are associated with plasma level of anti-CD4 autoantibody in HIV infection. Sci Rep 2018, 8(1):12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Z, Li M, Wu Y, Meng Z, Martin L, Zhang L, Ogunrinde E, Zhou Z, Qin S, Wan Z et al. : Systemic translocation of Staphylococcus drives autoantibody production in HIV disease. Microbiome 2019, 7(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogunrinde E, Zhou Z, Luo Z, Alekseyenko A, Li QZ, Macedo D, Kamen DL, Oates JC, Gilkeson GS, Jiang W: A link between plasma microbial translocation, microbiome, and autoantibody development in first-degree relatives of systemic lupus erythematosus patients. Arthritis & rheumatology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI: QIIME allows analysis of high-throughput community sequencing data. Nature Methods 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju F, Zhang T: 16S rRNA gene high-throughput sequencing data mining of microbial diversity and interactions. Applied Microbiology & Biotechnology 2015, 99(10):4119–4129. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Looney D, Taub D, Chang SL, Way D, Witte MH, Graves MC, Fiala M: Cocaine opens the blood-brain barrier to HIV-1 invasion. J Neurovirol 1998, 4(6):619–626. [DOI] [PubMed] [Google Scholar]
- 29.Wagner BD, Grunwald GK, Zerbe GO, Mikulich-Gilbertson SK, Robertson CE, Zemanick ET, Kirk HJ: On the Use of Diversity Measures in Longitudinal Sequencing Studies of Microbial Communities. Frontiers in Microbiology 2018, 9:1037–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dow-Edwards D, Fico TA, Osman M, Gamagaris Z, Hutchings DE: Comparison of oral and subcutaneous routes of cocaine administration on behavior, plasma drug concentration and toxicity in female rats. Pharmacology Biochemistry & Behavior 1989, 33(1):167–173. [DOI] [PubMed] [Google Scholar]
- 31.Cecilia Scorza, Claudia Piccini, Marcela Busi M, Carriquiry A Andrés J, Zunino Pablo: Alterations in the Gut Microbiota of Rats Chronically Exposed to Volatilized Cocaine and Its Active Adulterants Caffeine and Phenacetin. Neurotoxicity research 2018. [DOI] [PubMed] [Google Scholar]
- 32.Mateos-Moreno MV, Del-Rio-Highsmith J, Rioboo-Garcia R, Sola-Ruiz MF, Celemin-Vinuela A: Dental profile of a community of recovering drug addicts: Biomedical aspects. Retrospective cohort study. Med Oral Patol Oral Cir Bucal 2013, 18(4):e671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antoniazzi RP, Sari AR, Casarin M, Moraes CMB, Feldens CA: Association between crack cocaine use and reduced salivary flow. Braz Oral Res 2017, 31:e42. [DOI] [PubMed] [Google Scholar]
- 34.Sordi MB, Massochin RC, Camargo AR, Lemos T, Munhoz EA: Oral health assessment for users of marijuana and cocaine/crack substances. Braz Oral Res 2017, 31:e102. [DOI] [PubMed] [Google Scholar]
- 35.Lynge Pedersen AM, Belstrom D: The role of natural salivary defences in maintaining a healthy oral microbiota. J Dent 2019, 80 Suppl 1:S3–S12. [DOI] [PubMed] [Google Scholar]
- 36.Zaparte A, Schuch JB, Viola TW, Baptista TAS, Beidacki AS, do Prado CH, Sanvicente-Vieira B, Bauer ME, Grassi-Oliveira R: Cocaine Use Disorder Is Associated With Changes in Th1/Th2/Th17 Cytokines and Lymphocytes Subsets. Front Immunol 2019, 10:2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz P, Cleary T, Nassiri M, Steele B: Human T Lymphocyte Subpopulation and NK Cell Alterations in Persons Exposed to Cocaine. Clinical Immunology & Immunopathology 1994, 70(3):0–250. [DOI] [PubMed] [Google Scholar]
- 38.Dyke CV, Stesin A, Jones R, Chuntharapai A, Seaman W: Cocaine increases natural killer cell activity. Journal of Clinical Investigation 1986, 77(4):1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pradhan L, Mondal D, Chandra S, Ali M, Agrawal KC: Molecular analysis of cocaine-induced endothelial dysfunction: role of endothelin-1 and nitric oxide. Cardiovasc Toxicol 2008, 8(4):161–171. [DOI] [PubMed] [Google Scholar]
- 40.Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM: Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology 1999, 21(2):294–303. [DOI] [PubMed] [Google Scholar]
- 41.Mao JT, Huang M, Wang J, Sharma S, Tashkin DP, Dubinett SM: Cocaine Down-Regulates IL-2-Induced Peripheral Blood Lymphocyte IL-8 and IFN-γ Production. Cellular Immunology 1996, 172(2):217–223. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Huang DS, Watson RR: In vivo and in vitro cocaine modulation on production of cytokines in C57BL/6 mice. Life ences 1994, 54(6):401. [DOI] [PubMed] [Google Scholar]
- 43.In vivo cocaine administration influences lymphokine production and humoral immune response. Immunologic Research 1992, 11(1):74–79. [DOI] [PubMed] [Google Scholar]
- 44.Sahu G, Farley K, El-Hage N, Aiamkitsumrit B, Fassnacht R, Kashanchi F, Ochem A, Simon GL, Karn J, Hauser KF et al. : Cocaine promotes both initiation and elongation phase of HIV-1 transcription by activating NF-kappaB and MSK1 and inducing selective epigenetic modifications at HIV-1 LTR. Virology 2015, 483:185–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garre JM, Yang G: Contributions of monocytes to nervous system disorders. J Mol Med (Berl) 2018, 96(9):873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogel DY, Kooij G, Heijnen PD, Breur M, Peferoen LA, van der Valk P, de Vries HE, Amor S, Dijkstra CD: GM-CSF promotes migration of human monocytes across the blood brain barrier. European journal of immunology 2015, 45(6):1808–1819. [DOI] [PubMed] [Google Scholar]
- 47.Gibbons TE, Sayed K, Fuchs GJ: Massive pan-gastrointestinal bleeding following cocaine use. World Journal of Pediatrics 2009, 5(2):149–151. [DOI] [PubMed] [Google Scholar]
- 48.Linder JD, Monkemuller KE, Raijman I, Johnson L, Lazenby AJ, Wilcox CM: Cocaine-associated ischemic colitis. South Med J 2000, 93(9):909–913. [PubMed] [Google Scholar]
- 49.Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ, Ribeiro EA, Russo SJ, Nestler EJ: Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. entific Reports 2016, 6:35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russo C, Ferk F, Misik M, Ropek N, Nersesyan A, Mejri D, Holzmann K, Lavorgna M, Isidori M, Knasmuller S: Low doses of widely consumed cannabinoids (cannabidiol and cannabidivarin) cause DNA damage and chromosomal aberrations in human-derived cells. Arch Toxicol 2019, 93(1):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, Flores SC, Fontenot AP, Ghedin E, Huang L: Comparison of the respiratory microbiome in healthy nonsmokers and smokers. American journal of respiratory and critical care medicine 2013, 187(10):1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J, Peters BA, Dominianni C, Zhang Y, Pei Z, Yang L, Ma Y, Purdue MP, Jacobs EJ, Gapstur SM: Cigarette smoking and the oral microbiome in a large study of American adults. The ISME journal 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


