Abstract
Background:
Early-life metabolic derangements in HIV-exposed uninfected (HEU) infants have been reported.
Methods:
Pregnant women with HIV and HIV-uninfected pregnant women were enrolled with their newborns in a US cohort from 2011–15. We measured cord insulin, C-peptide, and metabolic cytokines of HEU and HIV-unexposed uninfected (HUU) newborns using ELISA and metabolites, lipid subspecies, and eicosanoids via liquid chromatography/mass spectrometry. Linear regression was employed to assess the association of intrauterine HIV/ART with insulin and C-peptide. Graphical lasso regression was used to identify differences between metabolite/lipid subspecies networks associated with C-peptide.
Results:
Of 118 infants, 56 were HEU, ART-exposed. In adjusted analyses, mean cord insulin (β=0.295, p=0.03) and C-peptide (β=0.522, p<0.01) were significantly higher in HEU vs. HUU newborns. HEU neonates exhibited primarily positive associations between complex lipids and C-peptide, indicative of fuel storage, and augmented associations between cord eicosanoids and cytokines. HUU neonates exhibited negative associations with lipids and C-peptide indicative of increased fuel utilization.
Conclusion:
Higher cord insulin and C-peptide in HEU vs. HUU newborns as well as differences in cord metabolites, metabolic-related cytokines, and eicosanoids may reflect a propensity for fuel storage and an inflammatory milieu suggestive of fetal metabolic changes associated with in utero HIV/ART exposure.
Keywords: insulin, in utero HIV exposure, antiretroviral exposure, metabolomics, lipidomics, cord blood
Introduction
Current theories propose that fetal programming and the in utero milieu have durable effects on an individual’s long-term metabolic health, highlighting the critical role of maternal-fetal cross-talk in shaping the metabolic health of children throughout the lifespan 1. For the growing worldwide population of children born to women living with HIV (WLHIV), estimated to be close to 15 million in 2018 2, in utero exposures are especially complex and include maternal HIV infection as well as antiretroviral therapy (ART).
HIV infection and its treatments have been shown to be associated with metabolic perturbations including obesity, unfavorable body composition, insulin resistance, type 2 diabetes, and increased cardiovascular risk in adolescents and adults 3–5. Women of childbearing age or pregnant, living with HIV, and receiving ART are at risk for these cardiometabolic disorders, and it is still largely unknown whether this population may impart a different metabolic imprint on their offspring.
Metabolic derangements from in utero HIV and/or ART (HIV/ART) exposure have been reported in infants who are HIV/ART-exposed uninfected (HEU) 6–8, including poor intrauterine growth 9, mitochondrial toxicity 10, altered intermediary metabolism and fuel utilization 7, and dysregulated metabolic pathways suggesting a pro-inflammatory intrauterine environment 11. Mitochondrial toxicity and alterations in associated intermediary metabolism, which include cellular metabolic pathways such as glycolysis, the Krebs cycle, fatty acid oxidation, and amino acid metabolism, have been reported in HEU children, but few studies have reported on fetal or cord blood outcomes in HEU neonates, such as cord blood insulin, C-peptide, or the metabolome and lipidome. Cord blood insulin and C-peptide levels are markers of insulin sensitivity and higher circulating concentrations have been shown to be associated with poorer postnatal growth and adiposity 12–14 The objective of our study was to evaluate whether in utero HIV/ART exposure is associated with higher cord blood insulin and C-peptide levels, as well as whether cord blood metabolites and lipid subspecies associated with insulin and C-peptide, or eicosanoid networks related to the inflammatory state, are significantly different between the cord blood of HEU and HIV-unexposed uninfected (HUU) neonates.
Materials and Methods:
Study Population
Pregnant WLHIV and HIV-uninfected pregnant women and their infants were enrolled at 12 – 34 weeks gestational age (GA) from 2011–2015 at the Mount Sinai Hospital ambulatory obstetrics-gynecology practice. The practice provides multidisciplinary high-risk care to pregnant WLHIV and routine obstetrical care to HIV-uninfected pregnant women from the same geographic catchment area. HIV-uninfected pregnant women were recruited from the midwifery service where low-risk women with uncomplicated pregnancies receive care. Pregnancies with multiple gestations, ending in spontaneous/therapeutic abortions or intra-uterine fetal demise (IUFD), or resulting in an infant with HIV infection were excluded from the final analyses. All participants provided written informed consent. This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai.
Primary Outcome
We measured cord blood insulin and C-peptide levels using Milliplex ® (MilliporeSigna, Burlington, MA) MAP multiplex assays with Luminex ® (Luminex Corporation, Austin, TX) instrumentation. Each sample was assayed in duplicate; all samples were tested from the same subject on the same plate and maintained at −80°C until the time of testing.
Primary Exposure of Interest
Newborns were classified as: HEU vs. HUU as determined by maternal serological HIV ELISA testing and ART history per self-report and medical- and pharmacy-record review.
Covariates and Measurements
Potential maternal and newborn confounders for cord blood insulin and C-peptide were collected and included: maternal socio-demographics; illicit substance, alcohol, and tobacco use during pregnancy; pre-pregnancy body mass index (BMI); infant sex; family history of diabetes (maternal or paternal relatives of the newborn); newborn GA at delivery determined via ultrasound dating; and birth weight. Information regarding maternal immunological status, including CD4 cell count and HIV RNA level, as well as ART history, was collected in WLHIV.
Physiological Measurements
Maternal gestational diabetes mellitus (GDM) was determined using a two-step glucose screen per standard clinical practice during the period of the study 15. Newborn length, weight, and head circumference were measured using a recumbent stadiometer, manual scale, and paper tape measure, respectively, by one of two trained research assistants.
Biochemical Measurements
Widely-targeted small metabolite (WTSM) screening (600+ small polar metabolites), widely-targeted lipidomic (WTL) profiling (1300+ lipid species in 26 lipid classes), and widely-targeted eicosanoid (WTE) screening (120+ eicosanoids) were performed with cord blood plasma samples. Extraction of small metabolites from plasma was performed in 80% methanol/water and that of lipids in 90% ethanol/water. Extraction of eicosanoids was performed using a solid-phase extraction (SPE) column, eluted in 100% methanol, and dried before dissolving and injecting in the loading buffer. The assays were performed with Waters Aquity® Ultra Performance Liquid Chromatography coupled with ABSciex® 6500+ QTrap Mass Spectrometer (UPLC-MS) in a Multiple Reaction Monitoring (MRM) mode with a gradient appropriate for each column. The ACE® pentafluorophenyl (PFP) column was used for the WTSM assay, a Waters Charged Surface Hybrid (CSH) Fluorophenyl column for the WTL assay, and a Waters BEH Shield RP18 column for the WTE assays. MultiQuant® (ABSciex) software was used for data analysis including metabolite identification and quantification (relative to internal standards). Adipokines (adiponectin, leptin, and resistin), inflammatory cytokines [interleukin (IL)-6, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and IL-10] and ghrelin were measured using Milliplex® (MilliporeSigma, Burlington, MA) MAP multiplex assays with Luminex® (Luminex Corporation, Austin, TX) instrumentation as described above.
Statistical Analysis
Characteristics of pregnant women and their newborns were compared between groups using t-tests, Wilcoxon rank sum tests, chi-squared tests, or Fisher exact tests. Birth weight-for-age z (WAZ), length-for-age z (LAZ), and head circumference-for-age z (HCAZ) scores were calculated from United States (U.S.) growth standards 16. Insulin and C-peptide were log-transformed to approximate a normal distribution. Data on metabolite levels which were too low to detect were replaced with a value equaling 10% of the lowest detectable value in the dataset (enabling log transformation). Linear regression models were used to assess the association between infant HEU status and cord insulin and C-peptide, with and without adjustment for confounders of interest. Maternal age, GDM, family history of diabetes, pre-pregnancy BMI, C-section delivery, infant sex, preterm birth, and infant birth weight z-score were considered a priori confounders based on prior literature 17. Spearman correlation tests were used to assess the correlations of metabolic-related cytokines (adipokines, inflammatory markers, and ghrelin) with C-peptide. Orthogonal partial least squares discriminant analysis (OPLS-DA) was used to identify separation between HEU and HUU newborns based on metabolite and lipid subspecies, while identifying the metabolites or lipids that are were important for discriminating between groups using a Variable Importance Parameters (VIP) threshold of one 18. We used a graphical lasso approach to visualize differences between HEU and HUU neonates in metabolite networks associated with cord blood C-peptide. Specifically, we first identified the subset of metabolites and lipids associated with cord blood C-peptide in per-analyte multivariable linear regression models adjusting for maternal age, pre-pregnancy BMI, and infant birth weight z-score, using a nominal type I error of 0.05. The graphical lasso was then estimated on this subset using the extended Bayesian information criteria (BIC) to select the tuning parameter 19. Networks were plotted using the R package igraph, with nodes representing metabolites or lipids and edges representing partial correlations, conditional on all other analytes, using a correlation coefficient threshold of 0.2, corresponding to a small/medium effect size 20. In addition, descriptive analyses were employed where a simple Spearman correlation network was plotted to visualize pairwise correlations between eicosanoids, metabolic related/inflammatory cytokines, and C-peptide, using a correlation coefficient of 0.4, corresponding to a medium/large effect size 20. Sensitivity analyses were performed where HEU newborns born to WLHIV with detectable HIV RNA levels (≥ 100 copies/mL) were excluded. Lastly, sub-group univariate analyses of HEU newborns were performed to evaluate whether differences in insulin or C-peptide were present by in utero ART exposure or by maternal HIV RNA level at delivery. For all graphical lasso and Spearman correlation network analyses we chose to focus on modeling C-peptide as an outcome rather than insulin given the longer half-life, negligible hepatic clearance, and constant peripheral clearance of C-peptide21. All other statistical analyses were performed using SAS® 9.3 (Cary, NC).
Results
Characteristics of pregnant women
After excluding two twin pregnancies, two pregnancies which resulted in IUFD, and 10 pregnancies resulting in spontaneous/induced abortions, a total of 118 newborns were included for analysis: 56 HEU and 62 HUU. Median age of WLHIV was higher (26 vs. 23 years, p=<0.01) than that of uninfected women. (Table 1) No differences in maternal race/ethnicity, highest education level, employment status, illicit substance, alcohol or tobacco use in pregnancy, pre-pregnancy BMI, or GDM were noted. Among WLHIV, 24 (42.7%) had a CD4 cell count at enrollment >500 cells/mm3 and 48 (85.7%) had an HIV RNA level <100 copies/mL at delivery. Twenty-nine (52.0%) received protease inhibitor (PI)-based antiretroviral therapy (ART) (atazanavir/ritonavir-, darunavir/ritonavir-, or lopinavir/ritonavir-based), 12 (21.3%) non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART (nevirapine- or rilpivirine-based), five (8.9%) integrase strand transfer inhibitor (INSTI)-based ART (raltegravir-, elvitegravir-, or dolutegravir-based), and nine (16.0%) were on a regimens containing ≥ 3 classes of antiretrovirals (ARVs).
Table 1.
Characteristics of women and infants
| HEU infants n=56 | HUU infants n=62 | p value | |
|---|---|---|---|
|
| |||
| MATERNAL | |||
| Age, years | 26 (23–31) | 23 (21–27) | <0.01 |
| Race/Ethnicity | 0.24 | ||
| White | 1 (1.8) | 2 (3.2) | |
| Black/African American | 33 (58.9) | 25 (40.3) | |
| Hispanic | 19 (33.9) | 30 (48.4) | |
| Other | 3 (5.4) | 5 (8.1) | |
| Highest education level | 0.33 | ||
| Some high school or less | 15 (26.8) | 11 (18.0) | |
| High school diploma or equivalent | 20 (35.7) | 19 (31.2) | |
| Some college or higher | 21 (37.4) | 31 (51.8) | |
| Employment | 16 (28.6) | 24 (38.7) | 0.25 |
| Family history of diabetes | 19 (33.9) | 33 (53.2) | 0.04 |
| Illicit substance or alcohol use in pregnancy | 3 (5.3) | 2 (3.2) | 0.67 |
| Tobacco use in pregnancy | 5 (8.9) | 1 (1.6) | 0.10 |
| Pre-pregnancy BMI, kg/m2 | 26.4 (22.3, 31.5) | 25.0 (22.7, 29.4) | 0.39 |
| CD4 cell count at enrollment, cells/mm3 | ------ | ||
| < 50 | 7 (12.5) | ------ | |
| 51–200 | 11 (19.6) | ------ | |
| 201–500 | 8 (14.3) | ------ | |
| > 500 | 24 (42.7) | ------ | |
| HIV RNA level <100 copies/mL at delivery | 48 (85.7) | ------ | ------ |
| ART during pregnancy | ------ | ||
| NNRTI-baseda | 12 (21.3) | ------ | |
| PI-basedb | 29 (52.0) | ------ | |
| Integrase Inhibitor-basedc | 5 (8.9) | ------ | |
| ≥3 classes of ARVs | 9 (16.0) | ------ | |
| Other | 1 (1.8) | ||
| ART duration in pregnancy, days | 260 (163, 276) | ------ | ------ |
| Gestational Diabetes | 1 (1.8) | 0 (0.0) | 0.29 |
| INFANT | |||
| Preterm (<37 weeks GA) | 5 (8.9) | 3 (4.8) | 0.47 |
| SGA | 12 (15.2) | 6 (7.5) | 0.13 |
| C-section delivery | 38 (67.9) | 16 (25.8) | <0.01 |
| Birth WAZ | −0.50 (−1.10, 0.15) | −0.25 (−0.78, 0.24) | 0.47 |
| Birth LAZ | −0.26 (−0.82, 0.46) | 0.11 (−0.74, 0.60) | 0.28 |
| Birth HCAZ | −0.15 (−0.78, 0.34) | −0.21 (−0.68, 0.30) | 0.77 |
| Cord Blood Metabolic Cytokines | |||
| Adiponectin, μg/mL | 45.0 (32.3, 83.8) | 52.1 (30.2, 73.8) | 0.60 |
| Ghrelin, pg/mL | 13.7 (4.7, 13.7) | 4.4 (2.7, 13.7) | 0.01 |
| Leptin, ng/mL | 11.7 (6.8, 23.2) | 12.4 (7.7, 18.2) | 0.84 |
| Resistin, ng/mL | 101.9 (56.5, 162.8) | 134.7 (81.1, 300.0) | <0.01 |
| IL-6, pg/mL | 13.7 (13.7, 23.0) | 15.9 (7.9, 45.9) | 0.29 |
| IL-10, pg/mL | 1.7 (0.8, 2.8) | 1.5 (0.4, 3.3) | 0.58 |
| TNF-α, pg/mL | 15.0 (11.9, 18.3) | 14.6 (12.1, 17.6) | 0.84 |
| IFN-γ, pg/mL | 0.6 (0.6, 1.0) | 0.6 (0.6, 0.6) | <0.01d |
Continuous variables shown as (Median)(Interquartile Range) and categorical variables shown as (n) (%); p values for continuous variables from Wilcoxon tests and for categorical variables from chi-square or Fisher exact tests as appropriate.
Nevirapine- or Rilpivirine-based
Lopinavir/ritonavir-, Atazanavir-ritonavir-, or Darunavir/ritonavir-based
Raltegravir-, Elvitegravir-, or Dolutegravir-based.
While median values are similar between groups, sum ranks, IQR and standard deviations were not.
ART=antiretroviral therapy; BMI=Body Mass Index; GA=gestational age; HCAZ=Head Circumference-for-Age Z score; HEU=HIV-exposed uninfected; HUU=HIV-unexposed uninfected; IFN-Ɣ= Interferon gamma; IL=Interleukin; LAZ=Length-for-Age Z score; NNRTI=Non-nucleoside reverse transcriptase inhibitor, PI=Protease Inhibitor; SGA=Small for Gestational Age; TNF-α=Tumor necrosis factor alpha; WAZ=Weight-for-Age Z score
Characteristics of newborns and cord blood metabolic cytokines
A higher number of HEU newborns were born via C-section delivery compared to HUU newborns (63.3 vs 20.0%, p<0.01). The most common reasons for C-section delivery among HEU newborns included: prevention of HIV infection, repeat C-section, patient desire, non-reassuring fetal heart tones or failure to descend, new-onset intrauterine growth retardation, failed induction, maternal anatomic anomalies, breech presentation, and pre-eclampsia. No differences between groups were observed in rates of preterm birth; small-for-gestational (SGA) outcome; and birth anthropometrics.
Several metabolic cytokines differed between groups. Median ghrelin levels were higher (13.7 vs 4.4 pg/mL, p<0.01), but median resistin levels were lower (101.9 vs 134.7 ng/mL, p<0.01) in HEU vs. HUU. No differences in adiponectin, leptin, or IL-6 were noted between groups. (Table 1)
Measures of cord insulin and C-peptide and correlations with metabolic cytokines in newborns
In univariate analyses, median insulin (14.6 vs 12.6 mIU/mL, p=0.03) and C-peptide (1.04 vs 0.88 ng/mL, p<0.01) levels were higher in HEU compared to HUU newborns. (Table 2) This relationship persisted even after adjusting for maternal age, maternal GDM, family history of diabetes, maternal pre-pregnancy BMI, C-section delivery, infant sex, infant preterm birth, infant birth WAZ (ß=0.295, p=0.03 for insulin outcome; ß=0.522, p<0.01 for C-peptide outcome). In univariate subgroup analysis of HEU newborns, cord blood insulin and C-peptide levels were similar between those exposed to in utero PI-based ART vs. NNRTI-based ART (13.49 vs. 19.98, p=0.16 for insulin and 0.99 vs. 1.53, p=0.16 for C-peptide, respectively). Leptin correlated positively with C-peptide in both HEU and HUU newborns (rho=0.64, p<0.01 and rho=0.26, p=0.04, respectively). However, IL-6 correlated positively with C-peptide in HEU, but not HUU newborns (rho=0.29, p=0.05 vs rho=0.08, p=0.52 respectively), while resistin correlated negatively with C-peptide in HUU, but not HEU newborns (rho= −0.40, p<0.01 vs rho= −0.04, p=0.77 respectively). No correlations with C-peptide were observed for adiponectin, ghrelin, TNF-α, or IFN-γ in either group.
Table 2.
Unadjusted and adjusted Associations of in utero HIV/ART exposure with cord blood insulin and C-peptide
| Model Outcome | HEU (median) (IQR) | HUU (median) (IQR) | Unadjusted Coefficient | p value | Adjusted Coefficient | p value |
|---|---|---|---|---|---|---|
|
| ||||||
| Insulin | 14.6a (10.9, 23.8) | 12.6a (8.8, 19.1) | 0.248 | 0.05 | 0.305 | 0.03 |
| C-peptide | 1.04b (0.80,1.56) | 0.88b (0.48, 1.15) | 0.334 | 0.02 | 0.421 | 0.02 |
All models with outcomes log-transformed and adjusted for maternal age, maternal gestational diabetes, family history of diabetes, maternal pre-pregnancy body mass index, C-section delivery, infant sex, infant preterm birth, and infant birth weight z score
μIU/mL
ng/mL
ART=antiretroviral therapy; HEU=HIV/ART exposed uninfected; HUU=HIV/ARV unexposed uninfected; IQR=interquartile range
Characteristics of the metabolome and lipidome in HEU vs HUU newborns
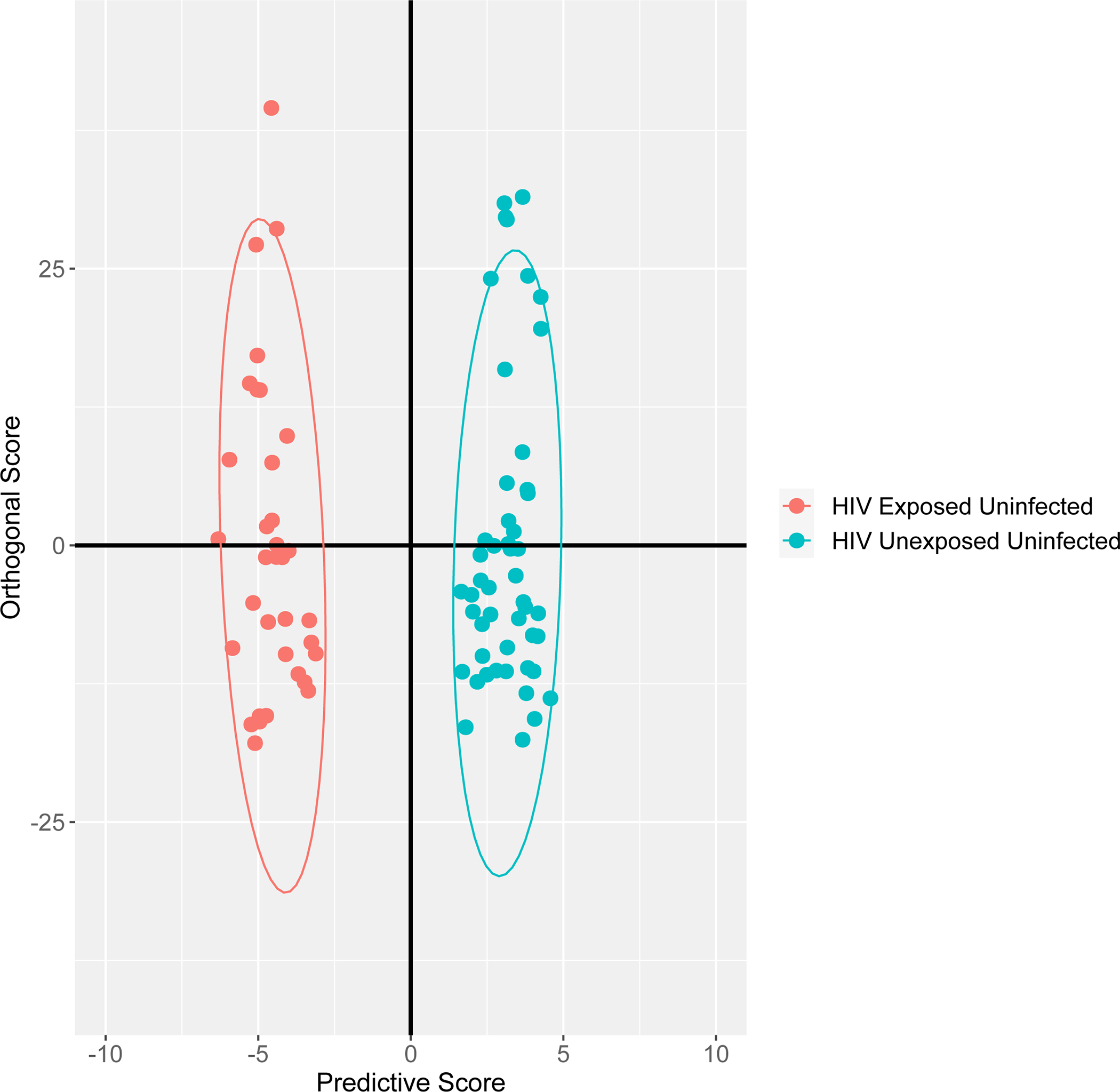
OPLS-DA showed metabolites/lipid subspecies that could discriminate between HEU and HUU newborns. (Figure 1) Long-chain monounsaturated phosphatidylglycerols (PG), polyunsaturated free fatty acids (PUFA), polyunsaturated (mainly) phosphatidylethanolamines (PEs) and phosphatidylcholines (PC), saturated or monounsaturated triacylglycerol (TAGs) and ceramides, as well as taurocholate and glycocholate species (bile acids) were identified as most important for distinguishing between HEU and HUU groups. (Supplemental Table) In sensitivity analyses, excluding HEU newborns born to women with detectable HIV RNA at delivery (≥100 copies/mL), results were similar.
Figure 1. Orthogonal partial least squares discriminant analysis showing group discrimination of metabolites and lipid subspecies between HIV/ART-exposed uninfected and HIV/ART-unexposed uninfected neonates.
Metabolic networks associated with cord blood C-peptide
Graphical lasso demonstrated different networks and relationships of metabolites and lipid subspecies with cord blood C-peptide when comparing HEU and HUU neonates (Figure 2).Among HEU neonates there was more heterogeneity in network clusters associated with C-peptide, with the vast majority of metabolites showing a positive association, suggesting a propensity for fuel storage. Networks of LPEs, LPCs and PCs, TAGs, and several amino acids and organic acids were positively associated with C-peptide among HEU neonates. Uridine diphosphate glucose (UDPG), glutathione, tauro-muricholate, and glycothiocholate were positively associated with C-peptide among HEU, but not HUU, neonates.
Figure 2. Graphical lasso regression plots demonstrating metabolite and lipid subspecies networks associated with cord C-peptide by in utero HIV/ART exposure status.
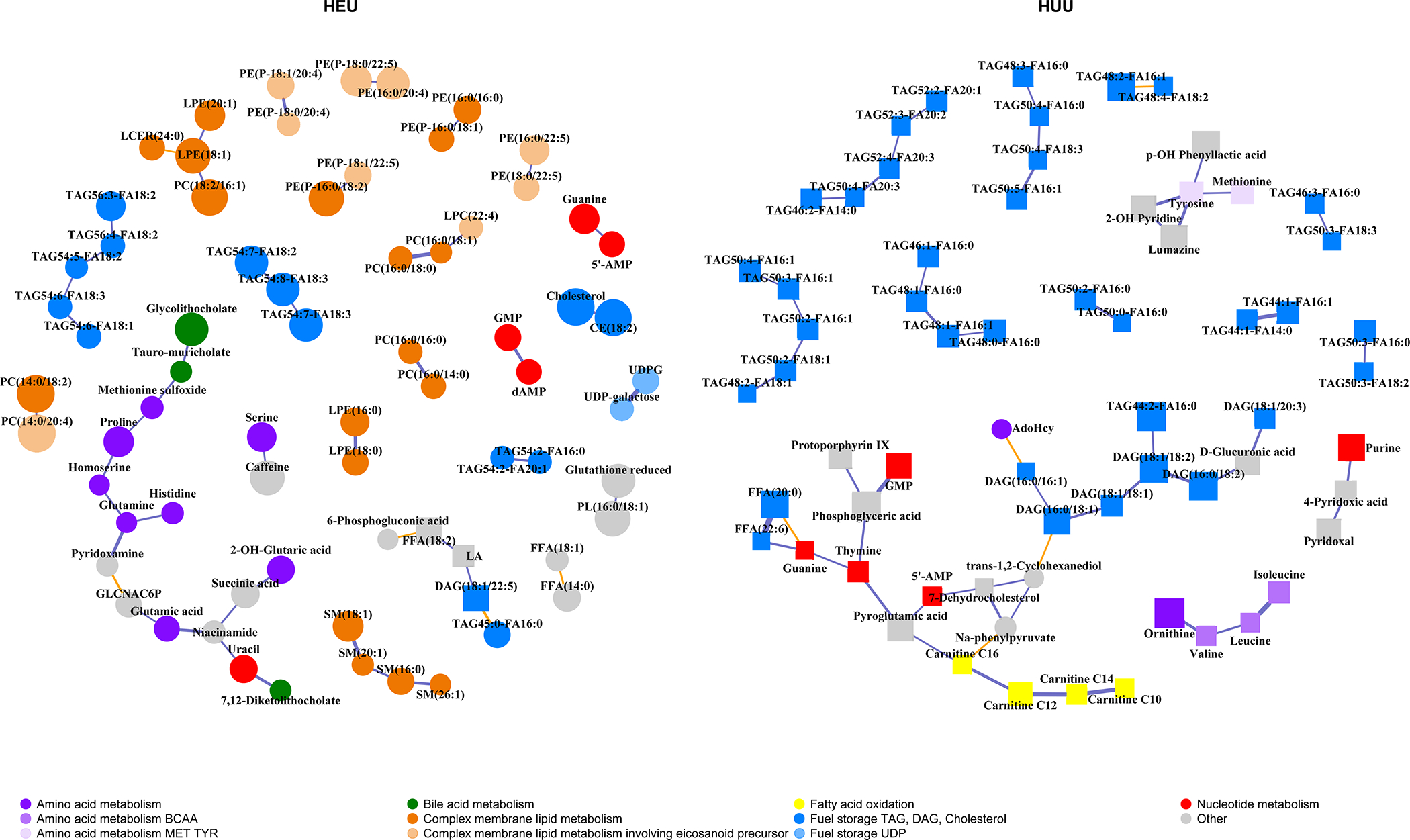
Analytes shown are associated with cord C-peptide after adjusting for maternal age, pre-pregnancy BMI, and infant birth weight z-score. Circle nodes=positive association with C-peptide, i.e., fuel storage; Square nodes=negative association with C-peptide, i.e., fuel utilization; Size of the node indicates the strength of the association with C-peptide; Blue lines=positive partial correlation between nodes; Orange lines=negative partial correlation between nodes; Thickness of edges/lines indicates strength of partial correlation conditional on all other analytes.
AMP=adenosine monophosphate; CE=cholesterol ester; DAG=diacylglycerol/diglyceride; GLCNAC6P= N-acetyl-glucosamine 6-phosphate; GMP=guanine monophosphate; FFA=free fatty acid; LA=linoleic acid; LCER=lactosyl ceramide; LPC= lysophosphatidylcholine; LPE=lysophosphatidylethanolamine; PC=phosphatidylcholine; PE=phosphatidylethanolamine; PL=phospholipid not PE, PC, LPC, LPE, or ceramide class; SM=sphingomyelin; TAG=triacylglycerol/Triglyceride; UDP=uridine diphosphate; UDPG=uridine diphosphate glucose
Among HUU neonates, networks associated with C-peptide were primarily those of TAGs, medium- and long-chain ACs (C10, C12, C14, and C16), branched-chain amino acids (BCAAs) (valine, isoleucine, and leucine), and several amino acids, all of which were negatively associated with C-peptide, suggesting a propensity for fuel utilization, unlike the predominantly positive associations observed in HEU neonates. In addition, the TAG networks that were found to be associated with C-peptide differed not only in the directionality of association, but also in the composition: TAG networks associated with C-peptide among HEU neonates were largely longer in chain length and polyunsaturated, while those among HUU neonates were shorter chain and more monounsaturated. Among HEU neonates, several networks positively associated with C-peptide contained eicosanoid precursors [phosphatidylethanolamine (PE) 18:0/20:4 and PE 18:1/20:4 network, PE 16:0/20:4 and PE 18:0/22:5 network, and the TAG network containing TAG 54:7/18:2, TAG 54:7/18:3, and TAG 54:8/18:3]. However, such an association was absent in HUU neonates.
In sensitivity analyses, excluding HEU neonates born to women with detectable HIV RNA levels at delivery, differences in metabolite networks associated with C-peptide remained stark between HEU vs. HUU neonates. (Supplemental Figure) Networks among HEU neonates were more sparse and still overwhelmingly positively correlated with C-peptide, compared to networks observed in HUU neonates (all negatively correlated with C-peptide).
Correlations of eicosanoids with metabolic-related cytokines and inflammatory markers
In Spearman correlation network plots between metabolic-related cytokines, C-peptide, and eicosanoids, we observed many more pairwise correlations between metabolic cytokines and eicosanoids among HEU vs. HUU neonates. (Figure 3) In particular, among HEU neonates, adiponectin showed strong correlations with more than half of the 31 immunomodulatory targets of eicosanoids, their PUFA precursors, and acyl-amino acids, with leptin having two such correlations. This differed from correlations of eicosanoids with metabolic-related cytokines among HUU neonates where adiponectin was only negatively correlated with one target eicosanoid and leptin with two more of these targets. In addition, eicosanoids among HEU neonates showed a much larger network of correlations with inflammatory cytokines such as IL-6, TNF-α, and IFN-γ compared to HUU neonates.
Figure 3. Spearman correlation networks between cord metabolic cytokines, eicosanoids, and C-peptide by in utero HIV/ART exposure status.
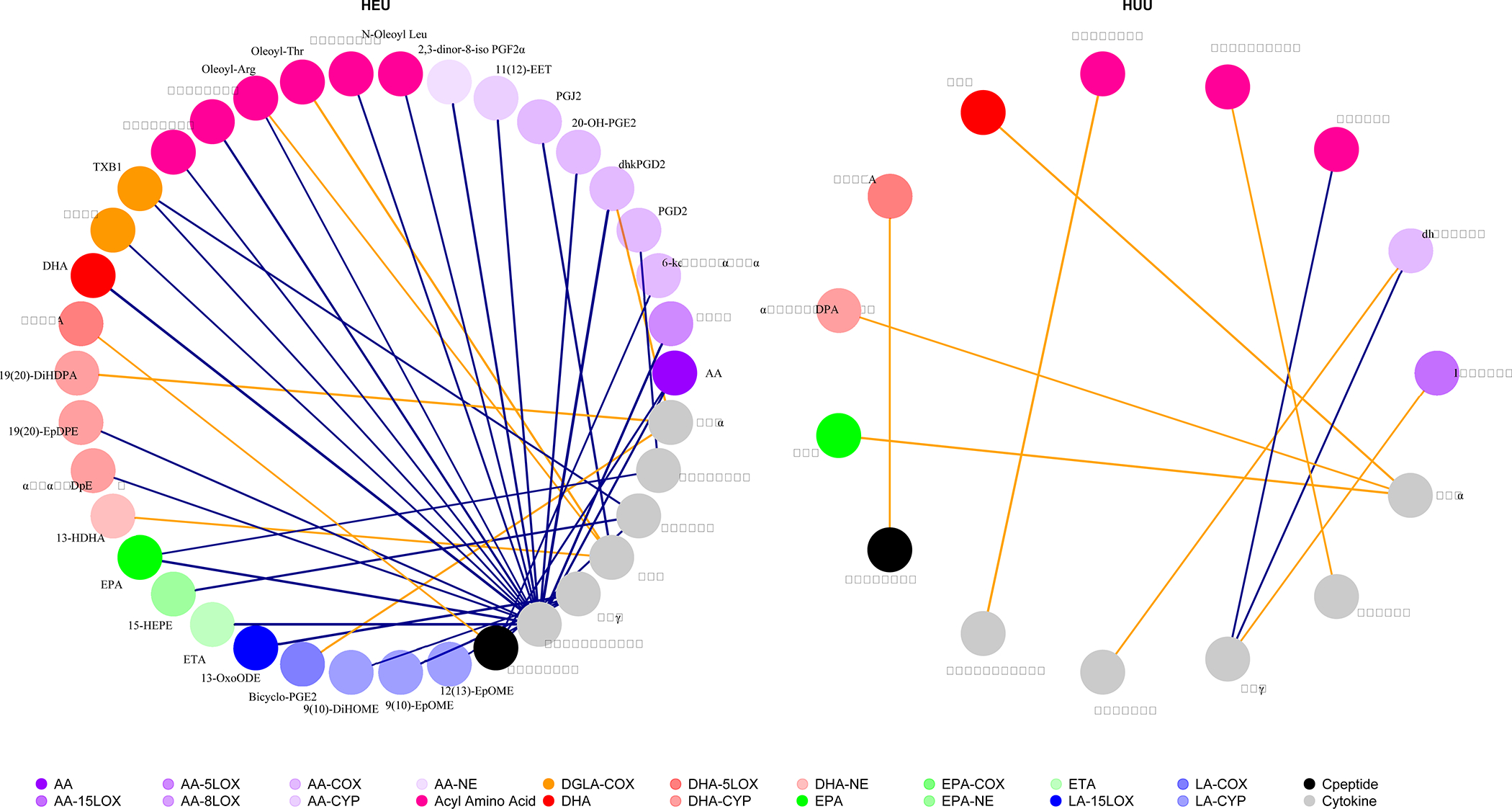
Colors denote eicosanoid pathways listed above; Similar shades denote related eicosanoid pathways with related eicosanoid precursors; Purple lines=positive association between nodes; Orange lines=negative association between nodes.
AA=arachidonic acid; Ala=alanine; Arg=arginine; COX=cyclooxygenase; CYP=cytochrome P-450; DGLA=dihomo-γ-linolenic acid; DHA=docosahexaenoic acid; diHOME= dihydroxy-octadecenoic acid; EET=epoxyeicosatrienoic acid; EPA=eicosapentaenoic acid; EpDPE= epoxydocosapentaenoic; EpOME=epoxyoctadecenoic acids; ETA=eicosatetraenoic acid; HDHA= hydroxydocosahexaenoic acid; HDPA= hydroxydocosapentaenoic acid HEPE=hydroxyeicosapentaenoic acid; HETE= hydroxyeicosatrienoic acid; IFNγ=interferon gamma; IL-6=interleukin 6; LA=linoleic acid; Leu=leucine; LOX=lipooxygenase; LTE=leukotriene; NAGABA= N-arachidonoyl-γ-aminobutyric acid; ODE=octadecadienoic acid; Palm=palmitoyl; PGD=prostaglandin D; PGE=prostaglandin E; PGF=prostaglandin F; PGJ=prostaglandin J; Thr=threonine; TNFα=tumor necrosis factor alpha; TX=thromboxane; Val=valine
Discussion
In our cohort, we found that compared to HUU newborns, HEU newborns had higher cord blood insulin and C-peptide and significantly different relationships of metabolites and lipid subspecies with C-peptide in cord blood. Moreover, differences in the cord blood metabolome and lipidome were significant and contributed to separation between HEU and HUU groups. To our knowledge, this is the first study in HEU neonates to evaluate neonatal metabolic health using widely targeted metabolomics and lipidomics techniques on cord blood.
Only one other published study has evaluated cord blood insulin levels in the context of in utero HIV/ARV exposure 22. This small study compared cord blood insulin levels between HEU newborns exposed to zidovudine (AZT)/lamivudine (3TC)/nelfinavir (NFV) vs. those exposed to AZT monotherapy vs. HUU, and found that those exposed to AZT/3TC/NFV had significantly lower levels of cord blood insulin compared to HUU newborns. These results differ from our findings and may be partially explained by differences in in utero ARV exposure as well as their smaller sample size, approximately one-third that of our study. In addition, while a little over half of the HEU newborns in our study were exposed to PI-based ART, none of our HEU newborns had in utero exposure to the PI, NFV.
Fetal insulin and C-peptide: a determinant of metabolic health for newborns
Insulin is known to stimulate an increase in adipose mass, and therefore fetal hyperinsulinemia associated with maternal GDM, an insulin-sensitive state, often results in a large-for-gestational-age neonate 23. It is noteworthy that, though the HEU newborns in our study had higher levels of cord blood insulin and C-peptide compared to HUU newborns, they were not larger in size (and, in fact, had lower birth WAZ and LAZ, but not statistically significantly so) compared to HUU newborns. Fetal hyperinsulinemia is positively associated with neonatal fat mass and may be a marker for later adiposity. Some studies have demonstrated a positive association between cord blood insulin and neonatal weight and adipose mass 13, as well as infant skinfold thickness at 1 year of age 14.
HEUs have different relationships between cord blood C-peptide and inflammatory markers compared to HUU newborns.
Among HEU but not HUU neonates, cord blood IL-6, an important inflammatory marker, was positively associated with C-peptide. Elevated IL-6 has been shown to be an independent predictor of diabetes in HIV-uninfected adults 24–27 as well as adults with HIV 28. In the adaptive and innate immune systems, IL-6 is involved in the augmentation of inflammation 29,30 and affects glucose homeostasis by its action on skeletal muscle cells, adipocytes, hepatocytes, and pancreatic endocrine cells 31. The positive association of cord blood IL-6 with insulin and C-peptide in the HEU newborns suggests a possible heightened role of inflammation as a mediator of fetal compensatory fuel utilization which governs glucose homeostasis that is not present in HUU newborns.
Differential relationships between metabolic cytokines (resistin, leptin, and adiponectin) and cord blood C-peptide by in utero HIV/ARV exposure status
Relationships of metabolic cytokines with C-peptide in cord blood also differed by HEU/HUU status in our study, reflecting potential alterations in the pathways governing fuel homeostasis when HIV and ARVs are present in the in utero environment.
The negative association that we observed between cord blood resistin and C-peptide in HUU newborns, though paradoxical from what has been observed in adult studies, has been observed with other studies of umbilical cord adipokines in the newborns of women with diabetes 32,33. This inverse relationship may reflect an immunomodulatory effect of C-peptide on resistin-producing immune cells 34. High fetal insulin environments have been shown to suppress resistin, a hormone which restricts adipose mass 35. In addition to the direct action of insulin on adipose tissue, this may also explain why adipose mass increases and large-for-gestational-age newborns more often result from pregnancies complicated by GDM. However, the HEU newborns in our study did not show this association between cord blood resistin and C-peptide, despite having higher insulin and C-peptide levels and lower resistin levels than HUU newborns, indicating that the hormonal metabolic pathways involved in glucose metabolism may be altered in HEU newborns compared to HUU newborns.
For both HEU and HUU newborns, we observed a positive association between leptin and C-peptide, but no association between adiponectin and C-peptide. The positive association between cord blood leptin levels and insulin has also been reported by other similar studies 36. This relationship could be explained in part by the amount of adipose mass present in our neonates as leptin is released by adipose tissue. However, we did not measure adiposity in our neonates. The lack of association between adiponectin and insulin in cord blood of newborns is in contrast to what has been reported in adults with and without HIV where adiponectin is inversely associated with risk for insulin resistance 37 and Type 2 diabetes 38, but in line with other studies in neonates and in early postnatal life 39,40. Adiponectin in the fetal circulation comes from both adipose tissue and vascular cells 41, unlike in adults where it is exclusively secreted by adipose tissue and, as a result, higher concentrations are seen in cord blood than in adults 42. Some have postulated that the extremely high adiponectin levels in cord blood compared to adult levels (often two or three times higher in cord blood and similar to levels we observed in our study) preclude the ability to observe a strong relationship between adiponectin and insulin sensitivity due to a “ceiling effect” of the adiponectin and inherently lower levels of insulin and C-peptide in neonates compared to adults 39.
HEU and HUU newborns demonstrate contrasting network relationships between fuel utilization and insulin
The notable differences in cord metabolites and lipid subspecies networks associated with C-peptide further indicate alterations in the fetal metabolic environment among HEU newborns. Few studies have compared HEU and HUU cord blood metabolite networks in relation to cord blood insulin or C-peptide, but one recent small study comparing cord blood metabolomics in HEU vs. HUU newborns found that fetal lipid metabolism was perturbed in HEU newborns, which was associated with differences in inflammation between HEU and HUU newborns 11. While this study did not evaluate the relationship of cord blood metabolites and lipid subspecies with insulin, it noted differences in cord PCs and PEs between HEU and HUU newborns which were consistent with our findings. The Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study evaluated cord blood small metabolite signatures of C-peptide and found several medium- and long-chain ACs to be negatively associated with C-peptide in those with Northern European and Afro-Caribbean ancestry. This is consistent with our observations in HUU neonates, but not in our HEU neonates. Specifically, we found a large network including ACs C10, C12, C14, and C16 which were inversely associated with C-peptide among HUU neonates. We also noted in this same network that the branched-chain amino acids (BCAAs) (valine, isoleucine, and leucine) were negatively associated with C-peptide among HUU neonates, a finding that is opposite to what we have previously observed in infants at 6 weeks of life 7 and in children and adults later in life 43,44, suggesting that the relationships between metabolic pathways and insulin or C-peptide in the in utero environment are very different from those postnatally and in adulthood.
The fact that we observed higher cord blood insulin and C-peptide in HEU neonates as well as different metabolite and lipid signature networks associated with cord blood C-peptide in our network analyses stratified by HEU vs. HUU groups, indicates that fuel homeostasis is different between HEU and HUU newborns. We have previously shown dysregulated fuel utilization in Cameroonian HEU infants compared to HUU infants at 6 weeks of age through targeted metabolomics techniques 7.
Networks positively associated with C-peptide were more heterogeneous, comprised of PEs, LPEs, LPCs, PCs (associated with complex lipid membrane metabolism) TAGs,/DAGs, (associated with fuel storage), bile acids, amino acids, and metabolites associated with purine metabolism. In addition, these networks were almost always positively associated with C-peptide among HEU newborns, reflecting fuel storage. However, networks associated with C-peptide in HUU newborns were primarily TAGs and small metabolites including amino acids and ACs, and almost all negatively associated with C-peptide, indicative of fuel utilization. The negative association of DAGs and TAGs with C-peptide in HUU newborns likely reflects increased flux through metabolic pathways in response to insulin and C-peptide, and overall upregulated fuel utilization that is more sensitive to insulin and C-peptide levels than that which occurs in HEU newborns. For example, among HUU neonates, we see that long-chain fatty ACs see Figure 2) also have a negative association with C-peptide, suggesting enhanced beta-oxidation of fatty acids is occurring.
In contrast, HEU newborns show an overwhelming positive association of networks of LPEs, LPCs and PCs, TAGs, and several amino acids and organic acids with C-peptide, a lack of AC association with C-peptide, and a positive association of the uridine 5’-diphosphoglucose and uridine 5’-diphosphogalactose network with C-peptide, all of which suggests fuel storage and perhaps a tissue-specific impairment in fuel utilization. The positive association of LPEs, LPCs and PCs, TAGs with C-peptide may indicate insulin stimulation of hepatic lipogenesis/phospholipid formation and/or secretion. The differences in the composition of the TAG networks associated with C-peptide between HEU and HUU neonates were also striking in that the latter contained a higher predominance of shorter chain-length TAGs as well as FA’s 16:0 and 18:0 which reflect lipogenesis from palmitate, palmitoleate, stearate, or oleate. TAG networks associated with C-peptide among HEU newborns, however, tended to have longer chain lengths with several composed of eicosanoid precursors. This may suggest that HEU neonates have higher levels of inflammation (which is absent in the HUU neonates). In addition, at a pancreatic-cell level, there may be an amino acid interaction which may raise C-peptide/insulin levels, but insulin-stimulated whole-body amino acid disposal at a skeletal muscle level in the HEU neonate could be blunted 45. The positive association of the uridine 5’-diphosphoglucose network with C-peptide in HEU newborns (but absent in HUU newborns) suggests higher glycogen storage in HEU vs. HUU newborns; the positive association of methionine sulfoxide with C-peptide may suggest that increased reactive oxygen species production in HEU vs. HUU neonates compensates for the positive association of C-peptide with reduced glutathione.
A propensity for inflammation in HEU newborns
Not only did we observe more eicosanoid precursors within TAG networks associated with C-peptide in HEU cord blood, but we also demonstrated that eicosanoids (largely pro-inflammatory) have a significantly larger and more complex network of correlations with metabolic-related cytokines among our HEU vs. HUU newborns. This suggests that adipose-immune cell interactions were more common in HEU newborns which may reflect a more inflammatory in utero metabolic milieu for HEU compared to HUU newborns, likely a result of maternal HIV and ART use. A small cord blood study of HEU and HUU neonates evaluated the immune metabolome and observed higher levels of pro-inflammatory metabolites, lysophospholipids, and cytokines in HEU neonates demonstrating an upregulated fetal inflammatory metabolome indicative of metabolic stress 11.
Our study was limited by the small sample size and single-site study design which may diminish generalizability, but, to our knowledge, is the one of the largest cord blood metabolomic and lipidomic studies in HEU and HUU neonates. There is the possibility that the differences between groups observed in networks of metabolites and lipid subspecies associated with cord blood C-peptide may have been attributed to the small sample size. We were also unable to measure glucose accurately in real-time at the time of the initial cord blood collection, and, therefore, an assessment of insulin sensitivity using glucose and insulin are not available. In addition, the heterogeneity of the in utero ART exposures prevents us from making definitive conclusions regarding the fetal metabolic effects of individual ARVs. While we did not observe differences in cord blood insulin or C-peptide between the two largest groups of in utero ART exposure (PI-based vs. NNRTI-based ART) among HEU newborns in univariate analyses, the small numbers preclude definitive conclusions. Since all WLHIV initiate ART with HIV diagnosis in pregnancy, it is difficult to disentangle the effects of HIV and ART on our outcomes. We were also unable to rigorously assess the contribution of maternal viremia to cord blood outcomes given the small number of women who had a detectable HIV RNA level at delivery, though sensitivity analyses excluding HEU neonates born to WLHIV with detectable HIV RNA levels at delivery revealed similar results. Lastly, as with all observational studies, selection of an ideal comparison group is sometimes difficult. To tackle this challenge, HUU neonates in our study were born to HIV-uninfected pregnant women without any pregnancy complications, but with similar socio-demographic and anthropometric characteristics as the pregnant WLHIV who were enrolled.
In conclusion, HEU newborns appear to exhibit disturbed fuel homeostasis associated with higher cord insulin and C-peptide compared to HUU newborns. The predominance of complex lipid networks positively associated with C-peptide in cord blood and the more prominent association of these metabolites with metabolic cytokines in HEU, but not HUU newborns, suggest altered fetal metabolic programming and fuel utilization, along with a propensity for inflammation associated with in utero HIV/ART exposure. Larger longitudinal studies monitoring the metabolic health of HEU newborns are warranted to understand the long-term implications of these neonatal findings in this important and growing population globally.
Supplementary Material
AA=amino acids; BA=bile acids; CER=ceramides, includes sphingomyelins, DCER, HCER; Chol=cholesterol esters; DAG diacylglycerol/diglyceride; FFA=free fatty acids; HEU=HIV-exposed uninfected; HUU=HIV-unexposed uninfected; LPC= lysophosphatidylcholine; LPE= lysophosphatidylethanolamine; NU=nucleotide; OA=organic acid; PC= phosphatidylcholine; PE=phosphatidylethanolamine; Plip=phospholipid not PE, PC, LPC, LPE or CER class, SM=small metabolites; SD=standard deviation; TAG=triacylglycerol/triglyceride; VIP=Variable Importance Parameter
Analytes shown are associated with cord C-peptide after adjusting for maternal age, pre-pregnancy BMI, and infant birth weight z-score. Circle nodes=positive association with C-peptide, i.e., fuel storage; Square nodes=negative association with C-peptide, i.e., fuel utilization; Size of the node indicates the strength of the association with C-peptide; Blue lines=positive partial correlation between nodes; Orange lines=negative partial correlation between nodes; Thickness of edges/lines indicates strength of partial correlation conditional on all other analytes.
AMP=adenosine monophosphate; CE=cholesterol ester; DAG=diacylglycerol/diglyceride; GLCNAC6P= N-acetyl-glucosamine 6-phosphate; GMP=guanine monophosphate; FFA=free fatty acid; LA=linoleic acid; LCER=lactosyl ceramide; LPC= lysophosphatidylcholine; LPE=lysophosphatidylethanolamine; PC=phosphatidylcholine; PE=phosphatidylethanolamine; PL=phospholipid not PE, PC, LPC, LPE, or ceramide class; SM=sphingomyelin; TAG=triacylglycerol/Triglyceride; UDP=uridine diphosphate; UDPG=uridine diphosphate glucose
Impact Statement:
There is a paucity of studies assessing cord blood and neonatal metabolic health in HIV-exposed uninfected (HEU) newborns, an increasing population worldwide.
Compared to HIV-unexposed uninfected (HUU) newborns, HEU newborns exhibit alterations in fuel homeostasis and an inflammatory milieu associated with in utero HIV/antiretroviral therapy (ART) exposure
The long-term implications of these neonatal findings are as yet unknown, but merit continued evaluation as this important and growing population ages into adulthood.
Acknowledgments:
We would like to thank all the study participants and staff at the Mount Sinai Medical Center Obstetrical Clinic. This work was supported by K23HD070760 and by the Icahn School of Medicine at Mount Sinai Dean’s Office and ConduITS - the Institutes for Translational Sciences (CTSA) (UL1TR001433). Y.Q. was supported by NIDDK P60DK020541. I.J.K. was supported by grants NIDDK P60DK020541 (Einstein DRTC) and NIAID 1U19AI091175 (Einstein CMCR), and had support of a S10 SIG Award for the Sciex 6500+ QTRAP (1S10OD021798-01) which was used to perform the mass spectrometric assay evaluations in this study.
Funding sources:
This work was supported in part by NICHD K23HD070760 and by the Icahn School of Medicine at Mount Sinai Dean’s Office and ConduITS - the Institutes for Translational Sciences (CTSA) (UL1TR001433). Y.Q. is supported by NIDDK P60DK020541. I.J.K. was supported by grants NIDDK P60DK020541 (Einstein DRTC) and NIAID 1U19AI091175 (Einstein CMCR), and the mass spectrometric work at Einstein DRTC-SIMC was supported by a S10 SIG Award for the Sciex 6500+ QTRAP (1S10OD021798-01).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
Consent Statement: All participants provided written informed consent. This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai.
REFERENCES
- 1.Barker DJ Rise and Fall of Western Diseases. Nature 338, 371–372 (1989). [DOI] [PubMed] [Google Scholar]
- 2.Slogrove AL, Powis KM, Johnson LF, Stover J & Mahy M Estimates of the Global Population of Children Who Are Hiv-Exposed and Uninfected, 2000–18: A Modelling Study. Lancet Glob Health 8, e67–e75 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown TT et al. Antiretroviral Therapy and the Prevalence and Incidence of Diabetes Mellitus in the Multicenter Aids Cohort Study. Archives of internal medicine 165, 1179–1184 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Nix LM & Tien PC Metabolic Syndrome, Diabetes, and Cardiovascular Risk in Hiv. Curr HIV/AIDS Rep 11, 271–278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant PM et al. Long-Term Body Composition Changes in Antiretroviral-Treated Hiv-Infected Individuals. AIDS 30, 2805–2813 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jao J & Abrams EJ Metabolic Complications of in Utero Maternal Hiv and Antiretroviral Exposure in Hiv-Exposed Infants. Pediatr Infect Dis J 33, 734–740 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jao J et al. Lower Preprandial Insulin and Altered Fuel Use in Hiv/Antiretroviral-Exposed Infants in Cameroon. J Clin Endocrinol Metab 100, 3260–3269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jao J et al. Lower Mitochondrial DNA and Altered Mitochondrial Fuel Metabolism in Hiv-Exposed Uninfected Infants in Cameroon. AIDS 31, 2475–2481 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JY et al. Highly Active Antiretroviral Therapy and Adverse Birth Outcomes among Hiv-Infected Women in Botswana. J Infect Dis 206, 1695–1705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cote HC et al. Perinatal Exposure to Antiretroviral Therapy Is Associated with Increased Blood Mitochondrial DNA Levels and Decreased Mitochondrial Gene Expression in Infants. J Infect Dis 198, 851–859 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Schoeman JC et al. Fetal Metabolic Stress Disrupts Immune Homeostasis and Induces Proinflammatory Responses in Human Immunodeficiency Virus Type 1- and Combination Antiretroviral Therapy-Exposed Infants. J Infect Dis 216, 436–446 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regnault N et al. Higher Cord C-Peptide Concentrations Are Associated with Slower Growth Rate in the 1st Year of Life in Girls but Not in Boys. Diabetes 60, 2152–2159 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlsen EM et al. The Association between Newborn Regional Body Composition and Cord Blood Concentrations of C-Peptide and Insulin-Like Growth Factor I. PLoS One 10, e0121350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang DL et al. Cord Blood Insulin, Igf-I, Igf-Ii, Leptin, Adiponectin and Ghrelin, and Their Associations with Insulin Sensitivity, Beta-Cell Function and Adiposity in Infancy. Diabet Med 35, 1412–1419 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Carpenter MW & Coustan DR Criteria for Screening Tests for Gestational Diabetes. Am J Obstet Gynecol 144, 768–773 (1982). [DOI] [PubMed] [Google Scholar]
- 16.Olsen IE, Groveman SA, Lawson ML, Clark RH & Zemel BS New Intrauterine Growth Curves Based on United States Data. Pediatrics 125, e214–224 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Lowe WL Jr. et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (Hapo Fus): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 42, 372–380 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bylesjö M et al. Opls Discriminant Analysis: Combining the Strengths of Pls-Da and Simca Classification. Journal of Chemometrics 20, 341–351 (2006). [Google Scholar]
- 19.Friedman J, Hastie T & Tibshirani R Sparse Inverse Covariance Estimation with the Graphical Lasso. Biostatistics 9, 432–441 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen J A Power Primer. Psychological bulletin 112, 155–159 (1992). [DOI] [PubMed] [Google Scholar]
- 21.Jones AG & Hattersley AT The Clinical Utility of C-Peptide Measurement in the Care of Patients with Diabetes. Diabet Med 30, 803–817 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Beitune P et al. Effect of Maternal Use of Antiretroviral Agents on Serum Insulin Levels of the Newborn Infant. Diabetes Care 28, 856–859 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Sridhar SB, Ferrara A, Ehrlich SF, Brown SD & Hedderson MM Risk of Large-for-Gestational-Age Newborns in Women with Gestational Diabetes by Race and Ethnicity and Body Mass Index Categories. Obstet Gynecol 121, 1255–1262 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spranger J et al. Inflammatory Cytokines and the Risk to Develop Type 2 Diabetes: Results of the Prospective Population-Based European Prospective Investigation into Cancer and Nutrition (Epic)-Potsdam Study. Diabetes 52, 812–817 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Liu S et al. A Prospective Study of Inflammatory Cytokines and Diabetes Mellitus in a Multiethnic Cohort of Postmenopausal Women. Archives of internal medicine 167, 1676–1685 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Pradhan AD, Manson JE, Rifai N, Buring JE & Ridker PM C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus. JAMA 286, 327–334 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Vinitha R et al. Adiponectin, Leptin, Interleukin-6 and Hba1c in the Prediction of Incident Type 2 Diabetes: A Nested Case-Control Study in Asian Indian Men with Impaired Glucose Tolerance. Diabetes Res Clin Pract 109, 340–346 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Betene ADC et al. Interleukin-6, High Sensitivity C-Reactive Protein, and the Development of Type 2 Diabetes among Hiv-Positive Patients Taking Antiretroviral Therapy. J Acquir Immune Defic Syndr 67, 538–546 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamimura D, Ishihara K & Hirano T Il-6 Signal Transduction and Its Physiological Roles: The Signal Orchestration Model. Rev Physiol Biochem Pharmacol 149, 1–38 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Jones SA, Horiuchi S, Topley N, Yamamoto N & Fuller GM The Soluble Interleukin 6 Receptor: Mechanisms of Production and Implications in Disease. FASEB J 15, 43–58 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Kristiansen OP & Mandrup-Poulsen T Interleukin-6 and Diabetes: The Good, the Bad, or the Indifferent? Diabetes 54 Suppl 2, S114–124 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Ng PC et al. Plasma Ghrelin and Resistin Concentrations Are Suppressed in Infants of Insulin-Dependent Diabetic Mothers. J Clin Endocrinol Metab 89, 5563–5568 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Mohamed MH et al. Cord Blood Resistin and Adiponectin in Term Newborns of Diabetic Mothers. Arch Med Sci 6, 558–566 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silswal N et al. Human Resistin Stimulates the Pro-Inflammatory Cytokines Tnf-Alpha and Il-12 in Macrophages by Nf-Kappab-Dependent Pathway. Biochemical and biophysical research communications 334, 1092–1101 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Kim KH, Lee K, Moon YS & Sul HS A Cysteine-Rich Adipose Tissue-Specific Secretory Factor Inhibits Adipocyte Differentiation. J Biol Chem 276, 11252–11256 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Catalano PM, Presley L, Minium J & Hauguel-de Mouzon S Fetuses of Obese Mothers Develop Insulin Resistance in Utero. Diabetes Care 32, 1076–1080 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Addy CL et al. Hypoadiponectinemia Is Associated with Insulin Resistance, Hypertriglyceridemia, and Fat Redistribution in Human Immunodeficiency Virus-Infected Patients Treated with Highly Active Antiretroviral Therapy. J Clin Endocrinol Metab 88, 627–636 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Duncan BB et al. Adiponectin and the Development of Type 2 Diabetes: The Atherosclerosis Risk in Communities Study. Diabetes 53, 2473–2478 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Luo ZC et al. Maternal and Fetal Leptin, Adiponectin Levels and Associations with Fetal Insulin Sensitivity. Obesity (Silver Spring) 21, 210–216 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Basu S et al. In Utero Gender Dimorphism of Adiponectin Reflects Insulin Sensitivity and Adiposity of the Fetus. Obesity (Silver Spring) 17, 1144–1149 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinar H et al. High Molecular Mass Multimer Complexes and Vascular Expression Contribute to High Adiponectin in the Fetus. J Clin Endocrinol Metab 93, 2885–2890 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weyermann M, Beermann C, Brenner H & Rothenbacher D Adiponectin and Leptin in Maternal Serum, Cord Blood, and Breast Milk. Clin Chem 52, 2095–2102 (2006). [DOI] [PubMed] [Google Scholar]
- 43.McCormack SE et al. Circulating Branched-Chain Amino Acid Concentrations Are Associated with Obesity and Future Insulin Resistance in Children and Adolescents. Pediatr Obes 8, 52–61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newgard CB et al. A Branched-Chain Amino Acid-Related Metabolic Signature That Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab 9, 311–326 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis TA, Burrin DG, Fiorotto ML, Reeds PJ & Jahoor F Roles of Insulin and Amino Acids in the Regulation of Protein Synthesis in the Neonate. J Nutr 128, 347S–350S (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AA=amino acids; BA=bile acids; CER=ceramides, includes sphingomyelins, DCER, HCER; Chol=cholesterol esters; DAG diacylglycerol/diglyceride; FFA=free fatty acids; HEU=HIV-exposed uninfected; HUU=HIV-unexposed uninfected; LPC= lysophosphatidylcholine; LPE= lysophosphatidylethanolamine; NU=nucleotide; OA=organic acid; PC= phosphatidylcholine; PE=phosphatidylethanolamine; Plip=phospholipid not PE, PC, LPC, LPE or CER class, SM=small metabolites; SD=standard deviation; TAG=triacylglycerol/triglyceride; VIP=Variable Importance Parameter
Analytes shown are associated with cord C-peptide after adjusting for maternal age, pre-pregnancy BMI, and infant birth weight z-score. Circle nodes=positive association with C-peptide, i.e., fuel storage; Square nodes=negative association with C-peptide, i.e., fuel utilization; Size of the node indicates the strength of the association with C-peptide; Blue lines=positive partial correlation between nodes; Orange lines=negative partial correlation between nodes; Thickness of edges/lines indicates strength of partial correlation conditional on all other analytes.
AMP=adenosine monophosphate; CE=cholesterol ester; DAG=diacylglycerol/diglyceride; GLCNAC6P= N-acetyl-glucosamine 6-phosphate; GMP=guanine monophosphate; FFA=free fatty acid; LA=linoleic acid; LCER=lactosyl ceramide; LPC= lysophosphatidylcholine; LPE=lysophosphatidylethanolamine; PC=phosphatidylcholine; PE=phosphatidylethanolamine; PL=phospholipid not PE, PC, LPC, LPE, or ceramide class; SM=sphingomyelin; TAG=triacylglycerol/Triglyceride; UDP=uridine diphosphate; UDPG=uridine diphosphate glucose


