Abstract
Phosphinothricin (PPT) is the active component of a family of environmentally safe, nonselective herbicides. Resistance to PPT in transgenic crops has been reported by nuclear expression of a bar transgene encoding phosphinothricin acetyltransferase, a detoxifying enzyme. We report here expression of a bacterial bar gene (b-bar1) in tobacco (Nicotiana tabacum cv Petit Havana) plastids that confers field-level tolerance to Liberty, an herbicide containing PPT. We also describe a second bacterial bar gene (b-bar2) and a codon-optimized synthetic bar (s-bar) gene with significantly elevated levels of expression in plastids (>7% of total soluble cellular protein). Although these genes are expressed at a high level, direct selection thus far did not yield transplastomic clones, indicating that subcellular localization rather than the absolute amount of the enzyme is critical for direct selection of transgenic clones. The codon-modified s-bar gene is poorly expressed in Escherichia coli, a common enteric bacterium, due to differences in codon use. We propose to use codon usage differences as a precautionary measure to prevent expression of marker genes in the unlikely event of horizontal gene transfer from plastids to bacteria. Localization of the bar gene in the plastid genome is an attractive alternative to incorporation in the nuclear genome since there is no transmission of plastid-encoded genes via pollen.
Bialaphos, a non-selective herbicide, is a tripeptide composed of two l-Ala residues and an analog of Glu known as phosphinothricin (PPT). Bialaphos is toxic to bacteria and plants after intracellular peptidases remove the Ala residues and release active PPT, an inhibitor of Gln synthetase (GS). Inhibition of GS by PPT causes a rapid buildup of intracellular ammonia levels. The associated disruption of chloroplast structure results in inhibition of photosynthesis and plant cell death (Tachibana et al., 1986).
Bialaphos-producing species Streptomyces hygroscopicus and Streptomyces viridochromogenes are protected from PPT toxicity by phosphinothricin acetyltransferase (PAT). PAT is encoded by either the bar (bialaphos resistance; Thompson et al., 1987) or pat (phosphinothricin acetyltransferase; Strauch et al., 1988) genes, and detoxifies PPT by acetylation. The PAT enzymes encoded by these two genes are functionally identical and show 85% identity at the amino acid level (Wohlleben et al., 1988; Wehrmann et al., 1996). PPT resistant crops have been obtained by expressing chimeric bar or pat genes in the cytoplasm from nuclear genes. Herbicide resistant lines have been obtained by direct selection for PPT resistance in tobacco (Nicotiana tabacum cv Petit Havana), potato, Brassica napus, Brassica oleracea (De Block et al., 1987; De Block et al., 1989), maize (Spencer et al., 1990), and rice (Cao et al., 1992). Availability of efficient plastid transformation vectors using the spectinomycin resistance (aadA) gene allowed us to test whether or not bar, when expressed in plastids, confers herbicide resistance (Svab and Maliga, 1993; Zoubenko et al., 1994).
We report here that tobacco plants carrying the bacterial bar gene (b-bar1) in their plastid genome have high levels of PAT activity and are herbicide (PPT) resistant. PAT accumulation could be significantly increased by expressing bar in alternative expression cassettes and by codon modification. Although PAT levels were relatively high, direct selection of transplastomic clones on PPT-containing medium was not possible. Thus, subcellular localization rather than the absolute amount of PAT appears to be critical for direct selection of transgenic clones by PPT resistance.
RESULTS
Transplastomic Tobacco Plants with an Engineered bar Gene
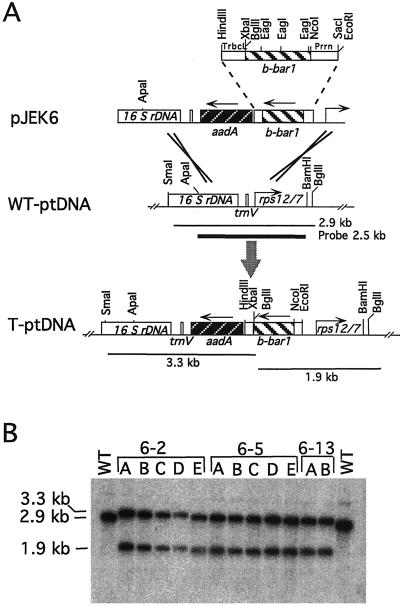
The plastid bar gene with the bacterial coding sequence (b-bar1) was created by inserting the bacterial bar coding region into a plastid expression cassette. This cassette consists of a plastid rRNA operon promoter with a synthetic ribosome binding site (Prrn) and the plastid rbcL gene 3′-untranslated region (TrbcL) for stabilization of the mRNA. The b-bar1 gene was cloned into the plastid transformation vector pPRV111B carrying a selectable spectinomycin resistance (aadA) gene. This plasmid, pJEK6, was introduced into plastids by the biolistic process. The b-bar1 gene integrated into the plastid genome by two homologous recombination events via the plastid targeting sequences (Fig. 1A). Selection for spectinomycin resistance eventually yielded cells with uniformly transformed plastid genome populations, which were then regenerated into plants. Integration of b-bar1 and aadA was verified by DNA gel-blot analysis (Fig. 1B). Three independently transformed lines, Nt-pJEK6-2, Nt-pJEK6-5, and Nt-pJEK6-13 were selected for further study.
Figure 1.
Introduction of the b-bar1 gene into the tobacco plastid genome. A, Map of the plastid targeting region in plasmid pJEK6 and of the cognate regions in the wild-type (wt) and transplastomic (T) plants. Map positions are shown for: the b-bar1 gene; aadA, the selectable spectinomycin resistance gene; and 16SrDNA and rps12/7, plastid genes (Shinozaki et al., 1986). Arrows indicate direction of transcription. Map position of the probe (2.5 kb) is marked by a heavy line; the wild-type (2.9-kb) and transgenic (3.3-kb, 1.9-kb) fragments generated by SmaI and BglII digestion are marked by thin lines. B, DNA gel blot confirms integration of b-bar1 into the tobacco plastid genome. Data are shown for transplastomic lines Nt-pJEK6-2A through E, Nt-pJEK6-5A through E and Nt-pJEK6-13A and B, and the wild-type parental line. SmaI-BglII digested total cellular DNA was probed with the 2.5-kb ApaI-BamHI plastid targeting sequence (A).
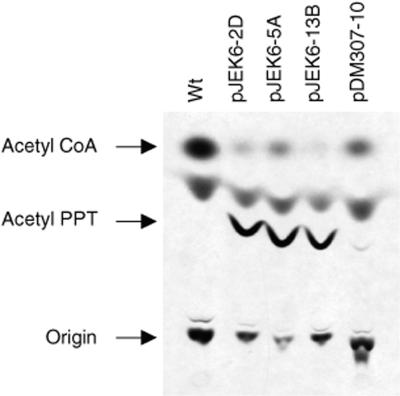
To determine if b-bar1 is expressed in chloroplasts, leaf extracts were assayed for PAT activity. Conversion of PPT into acetyl-PPT indicated PAT activity in each of the three tested transplastomic lines (Fig. 2). As a control, an extract was tested from a plant in which the bar gene was expressed in the nucleus (Nt-pDM307-10 plant, transformed with plasmid pDM307) (Cao et al., 1992). Since the transplastomic lines were selected by the linked spectinomycin resistance (aadA) gene, we were interested to test if accumulation of PAT confers herbicide resistance. Resistance in tissue culture was tested by callus induction from leaf segments on PPT-containing medium. We have found that the transplastomic lines were resistant up to 100 mg L−1 PPT, the highest concentration tested. In contrast, leaf sections from wild-type plants were sensitive to 4 mg L−1 PPT, a concentration that induces bleaching and completely blocks callus proliferation.
Figure 2.
PAT assay confirms b-bar1 expression in tobacco plastids. PAT activity was determined by conversion of PPT into acetyl-PPT using radiolabeled 14C-acetyl-CoA. Data are shown for transplastomic lines Nt-pJEK6-2D, Nt-pJEK6-5A and Nt-pJEK6-13B, nuclear transformant Nt-pDM307-10 and wild type (wt). The unmarked spot is an acetyl-CoA degradation product.
Tobacco Plants Expressing bar in Their Plastid Genome Are Herbicide Resistant
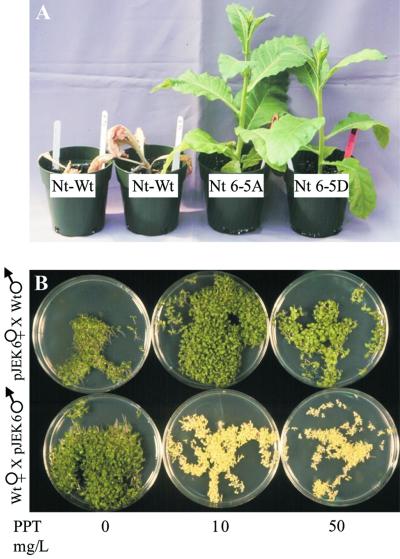
Plants regenerated in tissue culture were transferred to the greenhouse. Herbicide resistance was tested by spraying the plants with Liberty, a commercial formulation of PPT. Wild-type plants died within 2 weeks after treatment with the recommended field dose (2%), whereas transplastomic plants flowered and set seed (Fig. 3A). Herbicide resistance was tested by germinating seedlings on sterile medium containing PPT. Selfed seed progeny of each of the three lines was uniformly resistant when germinated on 10, 50, and 100 mg L−1 PPT (data not shown). Seedlings from reciprocal crosses of Nt-pJEK6-5A and wild-type plants are shown in Figure 3B. The seedlings were resistant if the transplastomic plant was used as the female parent, whereas they were sensitive if the transplastomic plant was used as the pollen parent as there is no pollen transmission of plastids.
Figure 3.
Transplastomic tobacco plants are herbicide resistant. A, Wild-type and pJEK6-transformed plants 2 weeks after Liberty treatment (5 mL, 2% [v/v] solution). B, Seeds from reciprocal crosses with Nt-pJEK6-5A plants germinated on 0, 10, and 50 mg L−1 PPT. Wt × pJEK6-5A, progeny from cross with transplastomic plant used as pollen parent; pJEK6-5A × Wt, progeny from cross with transplastomic plant used as maternal parent.
Synthetic s-bar Gene Is Expressed Well in Plastids but Poorly in E. coli
Expression of the bacterial b-bar1 gene conferred herbicide resistance in tissue culture and in the greenhouse. However, these clones were obtained by selection for spectinomycin resistance (aadA) encoded in the vector. Tobacco leaves bombarded with the same plasmid and selected on PPT-containing medium did not yield transplastomic clones. Therefore, we set out to test whether or not increasing bar expression levels will facilitate recovery of transplastomic clones by herbicide resistance. There are codon usage differences in plastids and bacteria. To enhance bar expression, a synthetic bar gene (s-bar) was created mimicking the codon usage of highly expressed plastid photosynthetic genes. The overall GC content of the b-bar1 gene was 67.9%, compared with 45.8% GC of the s-bar gene. The s-bar coding region was expressed from an improved promoter, PrrnLatpB+DS (Kuroda and Maliga, 2001) but had the same 3′-untranslated region (TrbcL) and was cloned into the same plastid vector (pPRV111B) as the b-bar1 gene (plasmid pKO3). The s-bar-coding region in plasmid pKO3 has a 23-amino acid C-terminal extension due to a 1-bp deletion close to the stop codon. As a control, a second bacterial bar gene (b-bar2) was expressed in the same cassette as the s-bar gene (plasmid pKO18; GC content 66.3%). The s-bar and b-bar2 genes were introduced into the tobacco plastid genome by selection for spectinomycin resistance and tested for PAT accumulation.
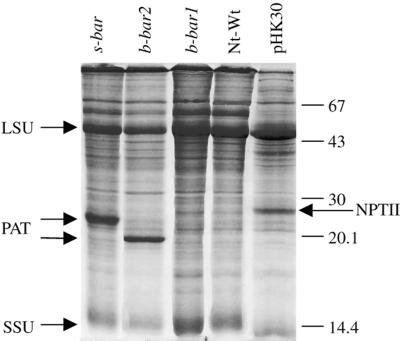
PAT accumulation in plants transformed with the s-bar (pKO3) and b-bar2 (pKO18) genes was significantly higher than in plants transformed with b-bar1 (pJEK6) since extracts of s-bar and b-bar2 plants contain a distinct PAT band, whereas no distinct band is visible in the b-bar1 extract (Fig. 4.). Having confirmed higher levels of PAT accumulation from the plastid s-bar and b-bar2 genes than from b-bar1, we attempted direct selection of transplastomic clones by herbicide (PPT) resistance (4 mg L −1). Thus far no transplastomic clones were obtained by the protocols described for transformation with nuclear bar genes.
Figure 4.
PAT accumulation in tobacco leaves from plastid b-bar1 (pJEK6-5A), b-bar2 (pKO18-3), and s-bar (pKO3-24) genes. Proteins were separated by SDS-PAGE and stained with Coomassie Blue R250. As control, a sample from non-transformed (Wt) plants was run. Position of PAT, NPTII, and the large (LSU) and small (SSU) Rubisco subunits is marked by arrows. Larger size of PAT in the Nt-pKO3-24 plant is due to a C-terminal extension. Reference for protein levels is NPTII (7% [w/v] of total soluble cellular protein) in the Nt-pHK30 extract (Kuroda and Maliga, 2001).
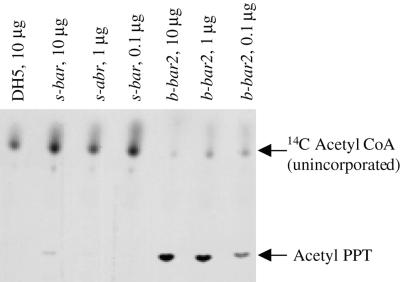
Certain codons are rarely used in E. coli and, as a consequence, heterologous mRNAs containing these codons are poorly translated (Kane, 1995; Makrides, 1996). Therefore, we compared expression of s-bar, with the plastid codon usage, and b-bar2, with the bacterial codon usage, in E. coli (Fig. 5). Although the two plasmids were present at comparable copy number per cell, E. coli carrying the bacterial b-bar2 gene (plasmid pKO18) had a 100-fold higher PAT activity than the E. coli carrying s-bar, as determined by dilution of protein extracts from b-bar2-expressing bacteria. Thus, E. coli is unable to efficiently translate the s-bar gene, which is well expressed in chloroplasts.
Figure 5.
PAT activity in E. coli carrying s-bar (pKO3), b-bar2 (pKO18), and in non-transformed DH5 α bacteria. Note dilution series. Unincorporated 14C-acetyl-CoA and labeled acetyl-PPT are indicated with arrows.
DISCUSSION
We report here that expression of the bacterial bar gene in tobacco plastids confers herbicide resistance. The bacterial bar gene has a high GC content (68.3%; GenBank accession no. X17220), whereas plastid genes have a relatively high AT content. The average GC content of tobacco plastid genes is 27.9% for genetic system genes and 30.9% for photosynthetic genes (Shimada and Sugiura, 1991) (GenBank accession no. Z00044). Differences in the GC content are also reflected in the codon usage biases. Although its GC content is high, data presented here indicate that in chloroplasts the bacterial b-bar1 gene is expressed well enough to confer resistance to field level (2%) PPT applications. PAT levels in chloroplasts could be significantly boosted after introduction of the bacterial bar gene into a more efficient cassette (b-bar2), suggesting that codon modification that lowers GC content is not necessary to obtain high expression levels in chloroplasts. Efficient translation of the somatotropin protein from an unmodified human cDNA in chloroplasts confirms this observation (Staub et al., 2000).
Nuclear gene transformants have been identified by direct selection for PPT resistance (10 mg L−1), whereas bombardment with the plastid b-bar1 gene and selection for PPT resistance did not yield transplastomic clones (data not shown). Initially we assumed that a low level of PAT produced by the few transformed plastid genome copies was insufficient to facilitate recovery of transplastomic clones. However, increasing PAT output per transgene copy by using more efficient translation control signals and optimizing codon usage did not enable direct selection either. Therefore, we speculate that subcellular localization rather than the absolute amount of PAT is critical for direct selection of transgenic clones.
The target of PPT is the GS enzyme (“Introduction”). There are two forms of GS in plants: cytosolic GS (GS1), occurring in the cytoplasm of leaf and non-photosynthetic cells, and chloroplastic GS (GS2), present in the chloroplasts of photosynthetic tissues (Cren and Hirel, 1999). We speculate that the relatively low expression level of the plastid-localized GS2, as compared with the cytoplasmic GS1, in tissue culture cells and inefficient inactivation of PPT in the cytoplasm by the plastid-localized PAT are the reason for the lack of recovery of transplastomic clones by direct selection. However, resistance to PPT is obtained in tissue culture cells once the inactivating enzyme (PAT) is expressed at sufficiently high levels due to the increase in the number of the transformed plastid genome copies.
Higher levels of PAT in the leaves are expected to confer higher levels of PPT resistance. However, even the Nt-pJEK6 plants, which accumulate PAT at the lowest level (no distinct band in Fig. 4) had field level PPT tolerance. Tissue cultures and seedlings of all plastid-transformed lines were resistant to 100 mg L−1 PPT; no tests were performed at higher PPT levels. High-level PAT accumulation does not seem to be detrimental as high-expressing plants are fertile and have normal phenotypes.
It is interesting that optimizing the bar gene codon usage for chloroplast expression resulted in significantly reduced PAT activity when the gene was expressed in E. coli. Codons, which are rare in E. coli, are known to be problematic for efficient translation of heterologous genes (Kane, 1995; Makrides, 1996). The triplet frequency per thousand nucleotides for AGA and AGG is the lowest in E. coli, reflecting low abundance of the tRNA required for translation of these Arg codons. The minor Arg tRNA Arg(AGG/AGA) has been shown to be a limiting factor in the bacterial expression of several mammalian genes. The co-expression of the ArgU (dnaY) gene, which encodes for tRNA Arg(AGG/AGA), resulted in high level production of the target protein (Makrides, 1996). The bacterial bar gene has 14 Arg codons, none of which are the rare AGA/AGG codons, whereas the s-bar gene has five of the rare Arg codons. It appears that greatly reduced s-bar expression in E. coli is due to codon optimization for expression in chloroplasts.
Horizontal gene transfer from transgenic plants to a bacterial pathogen occurs, if at all, at an extremely low frequency (Schluter et al., 1995; Dröge et al., 1998; Sylvanen, 1999). If the transferred gene is not expressed, it is not advantageous to the recipient organism and is likely to be lost. Codon modification reported here for the bar gene may be a practical new approach to reduce the likelihood of horizontal gene transfer to specific hosts as long as significant differences in codon usage exist. Furthermore, localization of bar in the plastid genome has the advantage of preventing dissemination of bar due to lack of pollen transmission of chloroplasts in most crops (Daniell et al., 1998). Exceptional transfer of paternal plastids has been shown even in species with a strict maternal inheritance in a specialized tissue culture system (Medgyesy et al., 1986; Avni and Edelman, 1991). The failure of direct selection of transplastomic clones by PPT resistance indicates that cells with a few bar copies are PPT sensitive. Thus, should such an exceptional transfer occur, the few paternal plastid bar genes are unlikely to confer herbicide resistance, making the plastid bar genes an attractive alternative to nuclear bar counterparts.
MATERIALS AND METHODS
Construction of Plasmid pJEK6
The NcoI/XbaI fragment comprising the b-bar1 coding region was generated by PCR amplification of plasmid pDM302 (Cao et al., 1992) with the following primers: P1, 5′-AAACCATGGCACCACAAACAGAGAGCCCAGAACGACGCCC-3′; P2, 5′-AAAATCTAGATCATCAGATCTCGGTGACG-3′. P1 extends the bar coding region by five amino acids, as described for the aadA and neo plastid markers (Carrer et al., 1993; Svab and Maliga, 1993). The PCR fragment was ligated into the EcoRV site of pBluescript II KS+ (Stratagene, La Jolla, CA) to create plasmid pJEK3. The NcoI-XbaI fragment from plasmid pJEK3 was ligated into NcoI-XbaI digested pGS104 plasmid (Serino and Maliga, 1997) to generate plasmid pJEK6. Plasmid pGS104 carries a Prrn-TrbcL expression cassette in a pPRV111B plastid transformation vector to yield plasmid pJEK6 (Fig. 1A).
Construction of Plasmids pKO3 and pKO18
Codon usage for the s-bar gene was designed based on codon usage in photosynthetic genes rbcL, psaA, psaB, psaC, psbA, psbB, psbC, psbD, psbE, and psbF. The s-bar-coding region (NcoI/XbaI fragment) was created by single-step assembly PCR from 28 primers (Stemmer et al., 1995; GenBank accession no. AY028212). Plasmid pKO3, carrying s-bar in the PrrnLatpB+DS/TrbcL cassette, was obtained by replacing the NheI/XbaI fragment in plasmid pHK30 (Kuroda and Maliga, 2001). The s-bar-coding region in plasmid pKO3 has a C deletion 9 bp upstream of the BglII site, resulting in changing the last four amino acids and a 23-amino acid C-terminal extension relative to the bacterial bar genes. The last 30 amino acids of s-bar in pKO3 are VLPLLRSDDLEGIEFLQPGGSTSSRVDISR. Thus, PAT encoded by pKO3 has a 14-amino acid N-terminal extension derived from the PrrnLatpB+DS promoter cassette and a 23-amino acid C-terminal extension. The N-terminal extension, but not the C-terminal extension, is important for high level expression.
The bacterial b-bar2 gene was created by replacing the EagI- BglII internal fragment in s-bar with the 0.5-kb EagI- BglII fragment (EagI digestion was partial) from b-bar1. Since the point mutation is in the EagI- BglII region of s-bar, which was replaced by the b-bar fragment, the b-bar2 gene does not have the C-terminal extension. In vector pKO18 b-bar2 is expressed in the PrrnLatpB+DS/TrbcL cassette, thus it has a 14-amino acid extension derived from the plastid atpB coding region N terminus (Kuroda and Maliga, 2001). Other than the different 5- and 14-amino acid N-terminal extensions, b-bar1 and b-bar2, encode identical PAT proteins.
Plastid Transformation and Characterization of Transgenic Plants
Plastid transformation in tobacco (Nicotiana tabacum cv Petit Havana), general tissue culture procedures and DNA-gel blot analysis of the transformed plastid genomes was carried out as described (Svab and Maliga, 1993; Zoubenko et al., 1994). Plants regenerated from the same line are distinguished by the letters of the alphabet. To test herbicide resistance, wild-type and transgenic plants were sprayed with 5 mL of a 2% (v/v) solution of Liberty (AgrEvo, Wilmington, DE) with an aerosol sprayer.
PAT Assay
The PAT assay was performed as described by Spencer et al. (1990). One hundred milligrams of leaf tissue was homogenized in 1 volume of extraction buffer (10 mm Na2HPO4, 10 mm NaCl). The supernatant was collected after spinning in a microfuge for 10 min. Escherichia coli was grown to stationary phase levels (optical density measured at 550 nm > 1.3). Four hundred microliters of lysate was collected and pelleted. The pellet was resuspended in 100 μL of B-PER Reagent (Pierce, Rockford, IL) and microfuged for 1 min. The Protein Assay reagent kit (Bio-Rad Laboratories, Hercules, CA) was used to determine protein concentrations with bovine serum albumin as a reference; PAT activity in leaf extracts was determined using 20 μg of protein. Protein extracts from bacteria were diluted 10-fold (10 μg protein per assay). Added to the protein samples and incubated at 37°C for 30 min were 1 mg mL−1 PPT and 14C-labeled acetyl-coenzyme A (CoA); the entire reaction was spotted onto a TLC plate. Ascending chromatography was performed in a 3:2 mixture of 1-propanol and NH4OH, and radioactivity was detected by exposure to Kodak XAR6 film (Eastman-Kodak, Rochester, NY).
Protein Gel Analysis
One hundred milligrams of leaf tissue was homogenized in 1 volume of extraction buffer (10 mm Na2HPO4, 10 mm NaCl). The supernatant was collected after spinning in a microfuge for 10 min. The Protein Assay reagent kit (Bio-Rad Laboratories) was used to determine protein concentrations using bovine serum albumin as a reference. The proteins were separated in an SDS polyacrylamide gel (SDS-PAGE; 15% [w/v] acrylamide) run at 20 mA for 2.5 h. The gel was stained with Coomassie Blue R250 solution.
ACKNOWLEDGMENTS
We thank Ray Wu for plasmid pDM307 and Hiroshi Kuroda for plasmid pHK30.
Footnotes
This work was supported by the Rice Biotechnology Research Grant from The Rockefeller Foundation, the National Science Foundation (grant nos. MCB 96–30763 and MCB 99–05043), and the Monsanto Company (to P.M.).
LITERATURE CITED
- Avni A, Edelman M. Direct selection for paternal inheritance of chloroplasts in sexual progeny of Nicotiana. Mol Gen Genet. 1991;225:273–277. doi: 10.1007/BF00269859. [DOI] [PubMed] [Google Scholar]
- Cao J, Duan X, McElroy D, Wu R. Regeneration of herbicide resistant transgenic rice plants following microprojectile-mediated transformation of suspension culture cells. Plant Cell Rep. 1992;11:586–591. doi: 10.1007/BF00233098. [DOI] [PubMed] [Google Scholar]
- Carrer H, Hockenberry TN, Svab Z, Maliga P. Kanamycin resistance as a selectable marker for plastid transformation in tobacco. Mol Gen Genet. 1993;241:49–56. doi: 10.1007/BF00280200. [DOI] [PubMed] [Google Scholar]
- Cren M, Hirel B. Glutamine synthetase in higher plants: regulation of gene and protein expression from the organ to the cell. Plant Cell Physiol. 1999;40:1187–1193. [Google Scholar]
- Daniell H, Datta R, Varma S, Gray S, Lee SB. Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat Biotechnol. 1998;16:345–348. doi: 10.1038/nbt0498-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Block M, Botterman J, Vandewiele M, Dockx J, Thoen C, Gossele V, Rao V, Movva N, Thompson C, Van Montagu M. Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J. 1987;6:2513–2518. doi: 10.1002/j.1460-2075.1987.tb02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Block M, De Brouwer D, Tenning P. Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of the bar and neo genes in the transgenic plants. Plant Physiol. 1989;91:694–701. doi: 10.1104/pp.91.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge M, Pühler A, Selbitschka W. Horizontal gene transfer as a biosafety issue: a natural phenomenon of public concern. J Biotechnol. 1998;64:75–90. doi: 10.1016/s0168-1656(98)00105-9. [DOI] [PubMed] [Google Scholar]
- Kane JF. Effects of rare codon clusters on high-level expression of heterologous proteins in Escherichia coli. Curr Opin Biotechnol. 1995;6:494–500. doi: 10.1016/0958-1669(95)80082-4. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Maliga P. Sequences downstream of the translation initiation codon are important determinants of translation efficiency in chloroplasts. Plant Physiol. 2001;125:430–436. doi: 10.1104/pp.125.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrides SC. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev. 1996;60:512–538. doi: 10.1128/mr.60.3.512-538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medgyesy P, Pay A, Marton L. Transmission of paternal chloroplasts in Nicotiana. Mol Gen Genet. 1986;204:195–198. [Google Scholar]
- Schluter K, Futterer J, Potrykus I. Horizontal gene transfer from a transgenic potato line to a bacterial pathogen (Erwinia chrysanthemi) occurs—if at all—at an extremely low frequency. Biotechnology. 1995;13:1094–1098. doi: 10.1038/nbt1095-1094. [DOI] [PubMed] [Google Scholar]
- Serino G, Maliga P. A negative selection scheme based on the expression of cytosine deaminase in plastids. Plant J. 1997;12:697–701. doi: 10.1046/j.1365-313x.1997.00697.x. [DOI] [PubMed] [Google Scholar]
- Shimada H, Sugiura M. Fine structural features of the chloroplast genome: comparison of the sequenced chloroplast genomes. Nucleic Acids Res. 1991;19:983–995. doi: 10.1093/nar/19.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsabayashi T, Zaita N, Chungwongse J, Obokata J, Yamaguchi-Shinozaki K. The complete sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986;5:2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TM, Gordon-Kamm WJ, Daines RJ, Start WG, Lemaux PG. Bialaphos selection of stable transformants from maize cell culture. Theor Appl Genet. 1990;79:625–631. doi: 10.1007/BF00226875. [DOI] [PubMed] [Google Scholar]
- Staub JM, Garcia B, Graves J, Hajdukiewicz PTJ, Hunter P, Nehra N, Paradkar V, Schlittler M, Carroll JA, Ward D. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat Biotechnol. 2000;18:333–338. doi: 10.1038/73796. [DOI] [PubMed] [Google Scholar]
- Stemmer WP, Crameri A, Ha KD, Brennan TM, Heyneker HL. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene. 1995;164:49–53. doi: 10.1016/0378-1119(95)00511-4. [DOI] [PubMed] [Google Scholar]
- Strauch E, Wohlleben W, Pühler A. Cloning of a phosphinothricin N-acetyltransferase gene from Streptomyces viridochromogenes Tü494 and its expression in Streptomyces lividans and Escherichia coli. Gene. 1988;63:65–74. doi: 10.1016/0378-1119(88)90546-x. [DOI] [PubMed] [Google Scholar]
- Svab Z, Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA. 1993;90:913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvanen M. In search of horizontal gene transfer. Nat Biotechnol. 1999;17:833. doi: 10.1038/12781. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Watanabe T, Sekizawa T, Takematsu T. Action mechanism of bialaphos II: accumulation of ammonia in plants treated with bialaphos. J Pest Sci. 1986;11:33–37. [Google Scholar]
- Thompson CJ, Movva NR, Tizard R, Crameri R, Davies JE, Lauwereys M, Botterman J. Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus. EMBO J. 1987;6:2519–2523. doi: 10.1002/j.1460-2075.1987.tb02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrmann A, Van Vliet A, Opsomer C, Botterman J, Schulz A. The similarities of bar and pat gene products make them equally applicable for plant engineers. Nat Biotechnol. 1996;14:1274–1278. doi: 10.1038/nbt1096-1274. [DOI] [PubMed] [Google Scholar]
- Wohlleben W, Arnold W, Broer I, Hillemann D, Strauch E, Pühler A. Nucleotide sequence of the phosphinothricin N-acetyltransferase gene from Streptomyces viridochromogenes Tü494 and its expression in Nicotiana tabacum. Gene. 1988;70:25–37. doi: 10.1016/0378-1119(88)90101-1. [DOI] [PubMed] [Google Scholar]
- Zoubenko OV, Allison LA, Svab Z, Maliga P. Efficient targeting of foreign genes into the tobacco plastid genome. Nucleic Acids Res. 1994;22:3819–3824. doi: 10.1093/nar/22.19.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]