Abstract
Altered approach motivation is hypothesized to be critical for the maintenance of depression. Computer-administered approach-avoidance training programs to increase approach action tendencies toward positive stimuli produce beneficial outcomes. However, there have been few studies examining neural changes following approach-avoidance training. Participants with major depressive disorder were randomized to an approach-avoidance training (AAT) manipulation intended to increase approach tendencies for positive social cues (n = 13) or a control procedure (n = 15). We examined changes in neural activation (primary outcome) and connectivity patterns using Group Iterative Multiple Model Estimation during a social reward anticipation task (exploratory). A laboratory-based social affiliation task was also administered following the manipulation to measure affect during anticipation of real-world social activity. Individuals in the AAT group demonstrated increased activation in reward processing regions during social reward anticipation relative to the control group from pre- to post-training. Following training, connectivity patterns across reward regions were observed in the full sample and connectivity between the medial prefrontal cortex and caudate was associated with anticipatory positive affect before the social interaction. Preliminary evidence of differential connectivity patterns between the two groups also emerged. Results support models whereby modifying approach-oriented behavioral tendencies with computerized training lead to alterations in reward circuitry (NCT02330744).
Keywords: depression, reward, neuroimaging, cognitive bias modification
Introduction
Depression is a common mental health disorder associated with significant functional disability (Pincus and Pettit, 2001; Kessler et al., 2003; Dunlop et al., 2005). Essential features of major depressive disorder (MDD) include loss of interest or pleasure, diminished energy and hopelessness (American Psychiatric Association, 2013). Together, these symptoms point to potential abnormalities in the approach system—a set of biobehavioral processes that motivate the individual to seek out positive, rewarding outcomes (American Psychiatric Association, 2013). Neural substrates linked to the approach system include the basal ganglia involved in reward signaling, orbitofrontal and medial prefrontal cortex (PFC) that modulates behavioral responses and decision-making, as well as a broader network involved in salience processing and action planning, including the amygdala, insula and anterior cingulate (Chau et al., 2004; Knutson and Greer, 2008; Haber and Knutson, 2010). Individuals with MDD show deficits in approach motivation and reward sensitivity (Trew, 2011; Dillon et al., 2014; Nusslock and Alloy, 2017); they are less likely to seek out rewarding experiences (Hopko and Mullane, 2008); are less behaviorally responsive to reward than are non-depressed individuals (Pizzagalli et al., 2008) and display abnormal neural responsivity (Chau et al., 2004; Pizzagalli et al., 2009; Treadway and Zald, 2011), including attenuated activation in fronto-striatal circuits during reward processing (Pizzagalli et al., 2009; Dillon et al., 2014; Hoflich et al., 2019). Identifying ways to directly modify basic mechanisms of impaired approach motivation and reward responsiveness in MDD may inform new or complementary treatment approaches.
Cognitive behavioral therapy is a first-line psychosocial treatment for MDD that incorporates exercises to modify thinking and/or behavioral patterns that may partially address approach system dysfunction (Cuijpers et al., 2007, 2008; Mazzucchelli et al., 2009; Ekers et al., 2014). For example, behavioral activation exercises emphasize structured increases in overt behaviors that are likely to bring about reinforcing environmental contingencies (Hopko et al., 2003). Several neuroimaging studies point to treatment-related changes supporting the malleability of approach system functioning (Dichter et al., 2009, 2010; Mori et al., 2017; Shiota et al., 2017; Yokoyama et al., 2018). However, existing interventions are not universally effective and outcomes regulated by the approach system (e.g. positive affect) appear difficult to change (Craske et al., 2016; Dunn et al., 2020). Given the predominance and impact of approach-related deficits in depression, exploring innovative ways of targeting the approach system may have clinical utility.
Computer-based paradigms that target approach-oriented behavioral tendencies are an alternative way to reduce clinical symptoms. Behavioral assessment of implicit approach-avoidance tendencies was developed based on evidence that positively evaluated stimuli typically automatically elicit motor approach behaviors, whereas negative stimuli trigger avoidance (Rinck and Becker, 2007). Standard approach-avoidance behavioral assessments display valenced stimuli and ask the participant to pull a joystick (arm flexion; approach) or push it away (arm extension; avoidance) with faster approach vs avoidance movements typically seen for positive stimuli (Cacioppo et al., 1993; Taylor and Amir, 2012). Using this paradigm, maladaptive automatic approach-avoidance tendencies characterized by diminished approach of positive cues are apparent in anxiety and depression (Heuer et al., 2007; Vrijsen et al., 2013; Radke et al., 2014a; Bartoszek and Winer, 2015; Fleurkens et al., 2018; Struijs et al., 2018; Loijen et al., 2020). The paradigm can be adapted for training purposes by establishing a contingency between stimulus valence and required responses to encourage the repeated approach of positive cues (Amir et al., 2013; Kakoschke et al., 2017). There is evidence that AAT can manipulate automatic approach action tendencies of dysphoric individuals (Vrijsen et al., 2018), with initial data suggesting potential clinical efficacy for reducing depression symptoms (Becker et al., 2019). If effective, AAT training holds promise as a complementary approach to standard interventions that can be applied to enhance approach behavior.
Assessment of approach-avoidance tendencies during functional magnetic resonance imaging (fMRI) in participants with major depression points to deficits in reward circuitry during approach of positive social cues (Derntl et al., 2011). A behavioral manipulation like the AAT that targets approach system functioning would thus be anticipated to exert its effects through key reward-related fronto-striatal regions. To date, information about neural mechanisms of AAT are limited to effects observed in relation to alcohol cues in individuals with alcohol use disorders (Wiers et al., 2011, 2015). However, the question of how AAT training exerts its clinical influence in depression, and specifically how the approach system is engaged during training, remains unanswered.
The goal of this study was to use a single-session experimental AAT as an initial step toward understanding training-related changes in brain function during social reward processing in MDD. We utilized an AAT procedure designed to increase approach for positive social cues by requiring participants to repeatedly pull pictures of faces displaying positive expressions toward them using a joystick. As these procedures were intended to modify evaluative responses to positive social cues, anticipation of social reward was measured during fMRI before and after the AAT using a well-established measure of reward processing [Social Incentive Delay task (SID); Spreckelmeyer et al., 2009]. Prior work suggests that individuals with MDD show hypoconnectivity within fronto-striatal regions, including less connectivity between the ventromedial PFC and striatal regions implicated in detecting and hedonic responding to rewards (Manelis et al., 2016; Young et al., 2016). Moreover, functional connectivity involving reward processing regions predicts real-world relationships between approach behaviors and positive affect, suggesting that it may provide a complementary source of clinically relevant information (Heller et al., 2020). Thus, both neural activity and functional connectivity were explored in order to capitalize on the potential for connectivity data to better capture neural differences (Camara et al., 2009). We assessed social approach functioning by administering a social affiliation task in the laboratory with a trained confederate following the AAT (Aron et al., 1997; Taylor and Amir, 2012). In keeping with previous findings that neural hyporesponse to positively valenced social stimuli is observed in depression and is improved with treatment (Schaefer et al., 2006), we hypothesized that AAT would enhance approach system functioning, indexed by greater responsivity of reward circuitry during social reward anticipation (medial PFC, striatum and amygdala). We conducted exploratory analyses to evaluate whether individuals in AAT vs the control group differed in terms of strength or number of connections in fronto-striatal regions following the experimental manipulation and whether connectivity was associated with behavioral indicators from the social interaction task (ClinicalTrials.gov: NCT02330744).
Methods
Participants
The sample consisted of 32 individuals who met diagnostic criteria for MDD according to the Mini International Neuropsychiatric Interview (MINI Version 7.0.0.0 (Sheehan, 2014)). Participants were recruited through clinical referrals as well as posted announcements in community and online settings (e.g. ResearchMatch.org). Participants were required to be between the ages of 18 and 55 and to score 10 or higher on the Patient Health Questionnaire-9 (Kroenke et al., 2001). Exclusion criteria were used to ensure that participants could safely complete the study procedures and to minimize confounding interpretations of our results: (i) pharmacological treatments that could affect brain functioning; (ii) concurrent psychotherapy, or empirically supported treatments for anxiety or depression in the past 6 weeks; (iii) active suicidal ideation; (iv) history of major neurological disorder or moderate-to-severe traumatic brain injury; (v) moderate alcohol or marijuana use disorder (past year); mild substance use disorder (all other drugs in past year); (vi) bipolar I or psychotic disorders and (vii) characteristics that compromise MRI safety. Forty-two individuals were assessed and 32 were randomized at the scan visit. Sample size was determined using a power calculation (power > 0.80 for two-sided P < 0.05) for detecting a between-within analysis of variance interaction term with a large effect size range (d = 1.2–1.6), which was based on our earlier work using AAT, which found a large effect (Taylor et al., 2013; d = 1.58, Taylor et al., 2014) as well as earlier work that found large neural effects of brief cognitive bias modification treatments ranging from d = 0.9 to 1.3 (Britton et al., 2015; Wiers et al., 2015). Recruitment occurred from January 2015 to April 2017 and ended when the project target was met. The current data reflect the primary outcomes measured for the trial; additional secondary outcomes were measured as reported in the trial preregistration and will be reported elsewhere. The final sample included 28 individuals after attrition and quality control checks (described below and Figure 1 CONSORT diagram). The project was approved by the UCSD Human Research Protection Program.
Fig. 1.
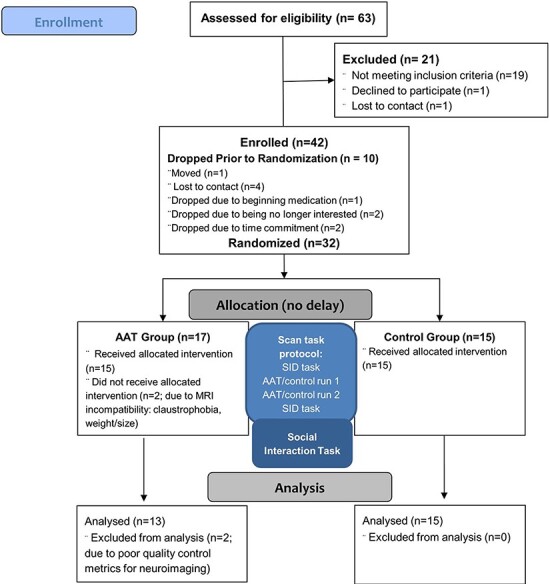
CONSORT diagram.
Procedure
The primary aim was to evaluate the neural correlates of a single-session approach/avoidance training manipulation completed during MRI. Potential participants provided written informed consent and then completed a baseline eligibility session, followed by a session that included questionnaire measures, fMRI and out-of-scan social interaction task. The following questionnaire assessments were administered:
Depression severity
Participants completed the Beck Depression Inventory-II (BDI-II) (Beck et al., 1996) to assess depression symptoms during the past 2 weeks.
Positive and negative affect
Participants completed the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988), a 20-item self-report measure of positive and negative affect over the past week.
Anhedonia
Participants completed the Mood and Symptom Anxiety Questionnaire (MASQ); the MASQ-Anhedonic Depression subscale [MASQ-AD (Clark and Watson, 1991)] was used to assess symptoms of anhedonia during the past week (e.g. ‘felt like nothing was very enjoyable’).
Anxiety
Participants completed the State Trait Anxiety Inventory-Trait [STAI-T (Spielberger et al., 1983)] to measure general anxiety.
Experimental manipulation: fMRI approach/avoidance training.
During fMRI, participants viewed face images (Tottenham et al., 2009) on a computer screen and were instructed to move a joystick in response to the color of the border surrounding each image. Response instructions were linked to the border color, rather than the content of the images, which facilitates training. Face types included positive or neutral expressions (Ferrari et al., 2018). The pull motion made the image ‘zoom’ (i.e. become larger) to give the appearance of approach. Participants moved the joystick to the right as a control motion (Taylor and Amir, 2012), which did not alter the size of the image (Figure 2). To experimentally manipulate automatic action tendencies, a contingency was set between positive facial expressions and approach behaviors in the active AAT condition but not in the control condition such that the majority of positive images (92%) were presented with a green border that indicated an instruction to pull, whereas the minority of neutral facial expression pictures (8%) were presented with a beige border and associated right movement instruction. In the control condition there was no contingency between instruction type and positive vs neutral pictures (i.e. 50% pull across both picture types). Prior to training, participants completed 12 practice trials that utilized different stimuli than those used during training. All participants saw four male and four female faces from the NimStim set displaying positive (happy) and neutral expressions during the training phase. During each training session, participants completed two runs of 96 trials per facial expression (∼15 min). Participants were randomized (parallel group 1:1 allocation) to complete the AAT training or control (2 runs) using a computerized random number generation that created a condition code corresponding to either the AAT training or control condition. Approach bias was indexed by faster reaction times to approach vs move positive cues to the right on the AAT task. Experimenters and participants were blind to which condition number was assigned to AAT vs the control condition.
Fig. 2.
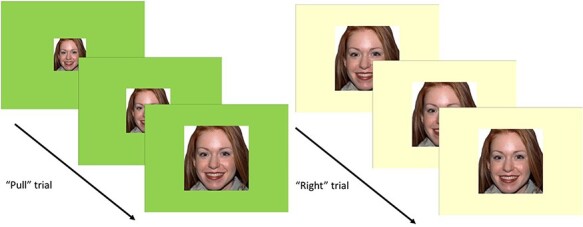
Depiction of AAT task trials.
Social incentive delay task.
Participants completed the SID (Spreckelmeyer et al., 2009) to measure pre–post change in social reward anticipation across the AAT and control groups. In the task, participants were given the opportunity to either gain social reward or avoid social punishment (within separate trial blocks) following a successful reaction to a target symbol. Distinct cues preceding the target symbol indicated to the participant whether to anticipate a social reward, punishment or a neutral outcome. Low and high levels of reward or punishment were designated via one or three lines, respectively, inside the appropriate cue (Figure 3). Social reward and punishment were presented in the form of pictures of individuals with varying intensities of positive (smiling) and negative (angry) facial expressions, respectively. A blurred facial control stimulus served as the neutral cue. Participants gained low and high levels of social reward if their reaction to the target symbol was performed on time (i.e. hit response during the target display) and received the control stimulus if they reacted too slowly (i.e. miss response after the target disappeared). Reward and punishment cues were presented in two separate blocks of 54 trials counterbalanced across participants; within each block, the order of cues was pseudo-randomized. Each trial began with a cue on the center of the screen (250 ms display), followed by a delay period (2250–2750 ms, jittered), and the target symbol (presented for 250 ms at the start of the task and individually adjusted thereafter depending on participant performance). The reward or neutral outcome was presented on the screen for 1650 and 300 ms after the target onset. The task difficulty was adjusted based on the participant’s reaction time and approximate hit rate of 66%. Analyses focused specifically on change in responses to social reward anticipation in line with prior work and given the proposed mechanism of the AAT training procedures, i.e. inducing more positive valuations of target social cues (smiling faces).
Fig. 3.
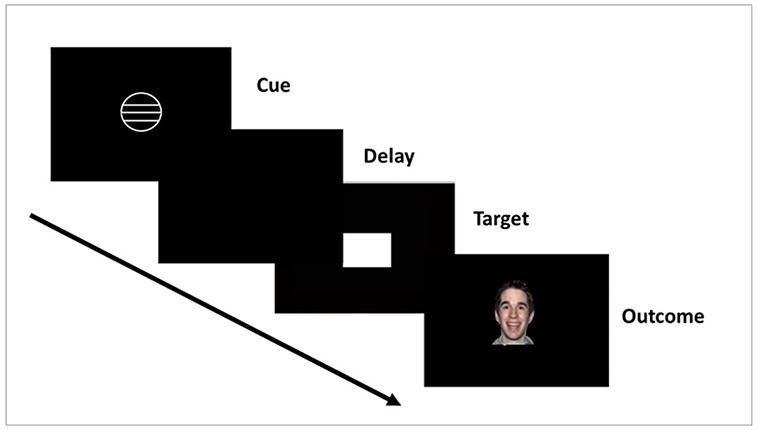
Depiction of the SID task.
Social interaction task.
The social interaction task was an abbreviated version of a previously validated task designed to facilitate closeness between unacquainted partners (Aron et al., 1997). The participant and a trained confederate alternated responses to a series of questions that gradually increased in the depth of self-disclosure they were designed to elicit (Taylor and Amir, 2012; Taylor et al., 2017). Prior to the task, participants were informed that they would be getting to know an assistant who worked in the lab and, once the confederate was present, stated that the purpose of the task was to get to know one another by answering a series of questions about themselves (Aron et al., 1997). Confederates were trained to deliver standardized responses to maintain consistency across participants and to act warmly toward participants using a scripted set of verbal and nonverbal behaviors. Participants completed the following questionnaires in relation to the interaction:
Positive affect
The PANAS-positive affect state scale was administered after instructions were provided about the upcoming social interaction task to provide a measure of anticipatory positive affect to a real-world opportunity for social reward.
Desire for future interaction
The Desire for Future Interaction scale [DFI (Coyne, 1976)] was administered to participants after completion of the task. The DFI has well-established reliability and validity (Voncken and Dijk, 2013). The DFI consists of eight items that assess the extent to which the rater would be willing to engage in a variety of social activities with their interaction partner in the future. The DFI was used as a measure of the participant’s future approach motivation with respect to the social interaction task partner.
Analysis
fMRI acquisition and analysis
Participants were scanned in a 3T General Electric 750 scanner using an 8-channel head array coil. Each scanning session included a three-plane scout scan, a sagittally acquired spoiled gradient recalled sequence for acquiring T1-weighted images (172 slices; thickness: 1 mm; TI = 450 ms, repetition time (TR) = 8 ms, echo time (TE) = 3 ms; matrix: acquired 192X256; field of view (FOV) = 256 cm; flip angle = 12°; sagittal plane) and T2*-weighted axially acquired echo-planar imaging (EPI) scans to measure blood-oxygen-level-dependent (BOLD) signals (parameters: slice thickness = 3 mm; slice spacing = 1 mm; TR = 1.5 s, TE = 32 ms, flip angle = 80°; matrix = 64 × 64, FOV = 240 mm). The SID task was administered over two runs.
Single-subject analysis
Imaging analyses were conducted using Analysis of Functional Images. Standard preprocessing steps were used with the afni.proc.py tool including removal of outlying acquisitions, despiking, slice time correction, co-registration of anatomical and functional scans, spatial smoothing (6-mm half-maximum smoothing kernel) and warping to standardized MNI space. Visual inspection of quality and motion parameters was also conducted and identified two participants with excessive motion who were removed from analysis. Preprocessed time-series data for each individual were analyzed using a multiple regression model containing motion and task response regressors. Specifically, trials were coded on three levels (none, low and high), two type (reward and punishment) and two phase (anticipation and outcome) regressors. Regressors of no interest included motion parameters and the rating phase. Regressors shifted by a hemodynamic waveform (AFNI:waver), and individual preprocessed EPI data were entered into a general linear model.
Whole-brain analyses (primary outcome)
Whole-brain voxel-wise data were entered into a generalized linear model (3dLME) to evaluate regions of significant activation (Cox, 2016). The generalized linear test of interest compared activation across groups (AAT and control) over time (pre and post) to reward cues during anticipation (baseline vs any reward). Permutation testing within AFNI’s 3dClustSim, which computes a three-parameter spatial autocorrelation function from the model residuals using 3dFHWMx to create an optimal smoothing kernel, were used to guard against identifying false-positive activations (voxel-wise a priori probability of 0.001 with corrected cluster-wise activation probability of 0.05). Significant activations with a minimum of 16 contiguous voxels were considered.
GIMME analysis (exploratory outcome)
Connectivity analysis was performed utilizing Group Iterative Multiple Model Estimation (GIMME), a package in R (https://www.nitrc.org/projects/gimme/) that models the directed functional connectivity of fMRI BOLD signal from predefined brain region of interest (ROI)s (Gates and Molenaar, 2012; Yang et al., 2015). GIMME creates functional maps with sufficient model fit (2 or more fit indices) using a data-driven model building/pruning approach to estimate connectivity graphs and determine whether a specific ROI path improves model fit to time-series data, and estimates contemporaneous, lagged, and autoregressive paths among each time series. GIMME first creates a functional network map for the full sample, including group paths only if they are significant for a specified percentage of individuals (75%; Gates and Molenaar, 2012) to create a group-level map of contemporaneous and lagged directed connections that are common to most individuals. After defining the group map, unnecessary group-level paths are pruned and additional individual-level paths are implemented to improve model fit for each participant using Lagrange multiplier test equivalents. Then the common model is pruned by removing paths that are no longer acceptable. We utilized a confirmatory subgroup GIMME [CS-GIMME (Henry et al., 2019)] analysis to explore potential group differences between individuals randomized to AAT vs control. CS-GIMME performs well at subgroup retrieval, even in small datasets (Gates et al., 2017). Correlation matrices were created for each of the individual’s post-training time series (preprocessed with motion and censor parameters regressed out) in the ROIs identified by the task-based group-level analysis. Consistent with earlier work using task-related designs (McCormick, 2014), we extracted measurement occasions when participants were engaged in anticipation of reward. We compared observed values for group-level paths across the AAT and control conditions and also examined potential differences in the number of subgroup-level paths within AAT vs control. We explored brain–behavior relationships by conducting Spearman rank order correlations between the paths identified in the sample and the self-report data from the social interaction task (positive emotion in anticipation of the interaction and desire to interact with one’s partner in the future).
Results
Demographic and behavioral data
Table 1 presents the demographic and clinical characteristics of the sample. There were no statistically significant demographic or clinical differences (i.e. positive affect, depression, anxiety and anhedonia) between participants in conditions at baseline or after the manipulation (Table 1).
Table 1.
Demographic and clinical characteristics
| Patient demographic and clinical characteristics | AAT (n = 13) | Control (n = 15) | Test statistic, P-value |
|---|---|---|---|
| Gender (% female) | 69% | 60% | χ2 = 0.26, P = 0.61 |
| Age, mean (s.d.) | 25.69 (3.61) | 28.60 (8.10) | F(1, 26) = 0.89, P = 0.35, η2 = 0.03 |
| Years of education, mean (s.d.) | 15.61 (1.12) | 15.86 (2.03) | F(1, 26) = 0.16, P = 0.70, η2 = 0.01 |
| Race (%) | χ2 = 2.12, P = 0.83 | ||
| Caucasian | 50% | 33% | |
| Asian-American | 8% | 20% | |
| African-American | 8% | 7% | |
| Mixed race | 25% | 20% | |
| Other | 8% | 13% | |
| Unknown | 0% | 7% | |
| PANAS-PA, mean (s.d.) | 19.84 (6.86) | 19.86 (5.35) | F(1,26) <.01, P = 0.99, η2 <0.01 |
| MASQ-AD, mean (s.d.) | 84.23 (10.02) | 82.73 (10.87) | F(1, 26) = 0.14, P = 0.71, η2 = 0.01 |
| BDI-II, mean (s.d.) | 25.75 (10.66) | 27.50 (6.59) | F(1, 24) = 2.61, P = 0.61, η2 = 0.01 |
| STAI-T, mean (s.d.) | 45.38 (2.56) | 46.53 (5.47) | F(1, 26) = 0.48, P = 0.50, η2 = 0.01 |
| PANAS—social anticipation | 20.08 (8.77) | 19.07 (4.00) | F(1,26) = 0.15, P = 0.70, η2 <0.01 |
| DFI | 40.23 (7.55) | 41.79 (8.81) | F(1,26) = 0.24, P = 0.63, η2 = 0.01 |
| SID hit rate per trial type | |||
| Pre—no reward | 0.46 (0.17) | 0.57 (0.11) | |
| Post—no reward | 0.53 (0.15) | 0.57 (0.07) | |
| Pre—reward | 0.51 (0.12) | 0.60 (0.06) | |
| Post—reward | 0.56 (0.15) | 0.62 (0.08) | |
| AAT bias score reaction time | |||
| Run 1 | 43.38 (82.17) | 33.83 (52.75) | |
| Run 2 | 81.88 (131.17) | 11.13 (63.36) | |
fMRI whole-brain effects
Voxel-wise whole-brain analysis of the interaction effect of time by group on anticipation of social reward revealed several statistically significant clusters spanning reward-related brain regions. Activation in the right medial frontal cortex extending into the anterior frontal pole, left medial and dorsomedial PFC (dmPFC), caudate, left precuneus, right insula, left paracentral lobule and cerebellum increased over time for those in AAT relative to control, while activation in the regions including the postcentral gyrus, precuneus and cingulate decreased (Table 2; Figures 4 and 5). Examination of the means suggested that at post-training, individuals in the AAT group showed significantly greater activation in the right medial frontal gyrus/anterior frontal pole, left medial frontal gyrus and right caudate, and lower activation in the cingulate gyrus relative to controls (see Supplemental Figure S1 for full results of between-group t-tests at pre- and post-timepoints, including effect sizes).
Table 2.
Task-based whole-brain group × time interaction activations
| ROI | Volume | x | y | z | T value, P | ROI | BA |
|---|---|---|---|---|---|---|---|
| 1 | 123 | 11 | 61 | 18 | 3.92, 8.9e-5 | Right medial frontal gyrus/anterior frontal pole | 10 |
| 2 | 77 | −1 | 29 | 53 | 3.97, 7.2e-5 | Left dorsomedial frontal cortex | 8 |
| 3 | 44 | −40 | −70 | 36 | 3.85, 1.2e-4 | Left precuneus | 39 |
| 4 | 29 | −4 | 54 | 40 | 4.19, 2.8e-5 | Left medial frontal gyrus | 9 |
| 5 | 25 | 29 | −71 | −23 | 3.65, 2.6e-4 | Cerebellum | 19 |
| 6 | 22 | 10 | 25 | 8 | 3.76, 1.47e-4 | Right caudate | |
| 7 | 17 | −6 | −34 | 81 | 3.81, 1.4e-4 | Left paracentral lobule | |
| 8 | 16 | 42 | 5 | −10 | 3.90, 9.6e-5 | Right insula | 13 |
| 9 | 24 | −9 | 2 | 50 | −4.04, 5.3e-5 | Left cingulate gyrus | 24 |
| 10 | 24 | 18 | −41 | 61 | −3.78, 1.6e-4 | Right postcentral gyrus | 3 |
| 11 | 20 | −27 | −53 | 49 | −3.78, 1.6e-4 | Left precuneus | 7 |
Note: Center of mass coordinates in MNI coordinate space; voxel-wise a priori probability of 0.001 with corrected cluster-wise activation probability of 0.05.
Fig. 4.
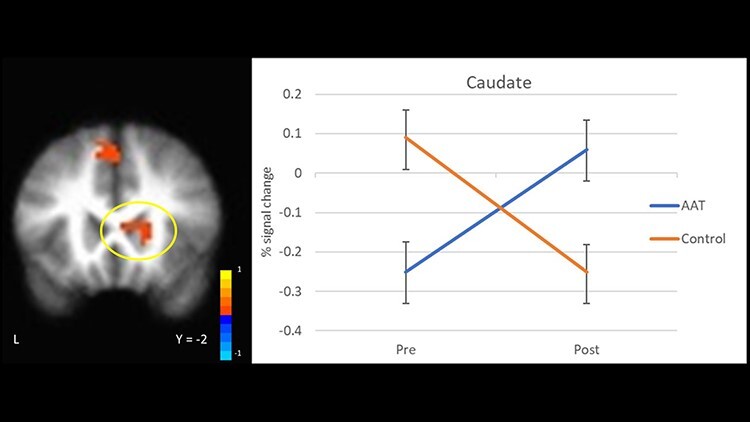
Neural response during social reward anticipation to AAT vs control over time (group × time interaction) in the caudate.
Fig. 5.
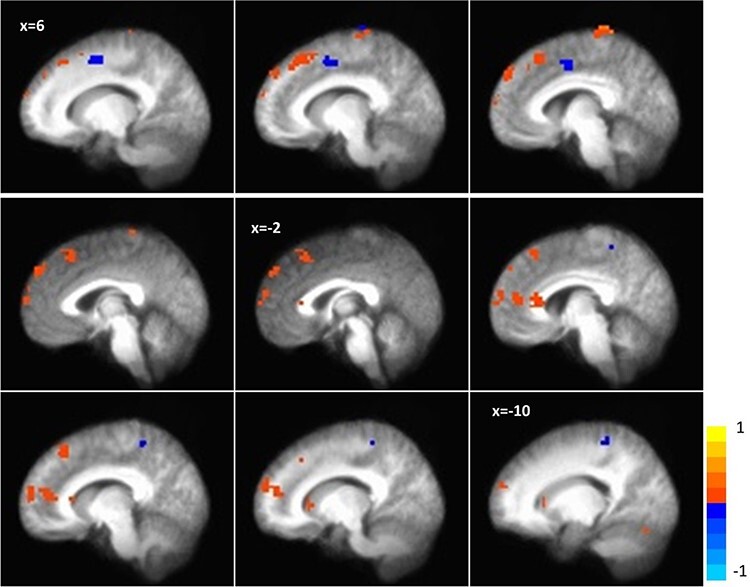
Whole-brain activation during social reward anticipation (group × time interaction).
GIMME results
We first established the connectivity patterns that consistently reflected connections across ROIs during anticipation of social reward across both groups (Figure 5). Connections were observed between the right medial frontal gyrus/anterior frontal pole to the caudate and to the left medial frontal gyrus, as well as dmPFC to the precuneus and to the right medial frontal gyrus/anterior frontal pole (Table 5; Figure 6). Greater connectivity between right medial frontal gyrus/anterior frontal pole and caudate was significantly associated with higher PANAS-positive affect ratings in anticipation of the social interaction (r2 = 0.21, P = 0.02) but no statistically significant relationship with the DFI score was observed (P values >0.1). We then examined group differences in the connectivity graphs for individuals in AAT and control groups to determine if there were differences in connectivity strength, number or patterns following AAT relative to the control condition. We did not observe any differences between the AAT and control groups in the strength of paths that were identified across participants (Table 3). Overall, the groups had a similar number of unique additional paths but different connectivity patterns. Individuals in the AAT condition demonstrated additional paths connecting the precuneus–postcentral gyrus, medial PFC–cerebellum and postcentral gyrus–cingulate (Figure 7A, Table 4). Individuals in the control condition showed additional paths between the cingulate–precuneus, cingulate–postcentral gyrus, right cerebellum–paracentral lobule and dmPFC–cerebellum (Figure 7B, Table 5).
Table 5.
Control group paths from GIMME connectivity analysis
| ROI (#) | M | s.d. |
| (9) Left cingulate gyrus—(11) left precuneus | 0.29 | 0.21 |
| (9) Left cingulate gyrus—(10) right postcentral gyrus | 0.21 | 0.24 |
| (5) Cerebellum—(7) left paracentral lobule | 0.23 | 0.20 |
| (2) Left dorsomedial frontal cortex—(5) cerebellum | 0.21 | 0.15 |
Fig. 6.
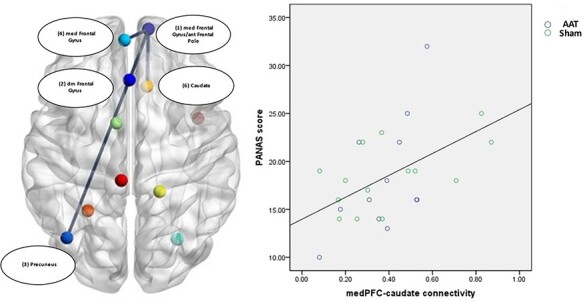
ROI connectivity in the full sample.
Table 3.
Full group paths from GIMME connectivity analysis and between-group statistics
| ROI (#) | M | s.d. | F-stat | P | η2 |
| (1) Right medial frontal gyrus/anterior frontal pole—(6) right caudate | 0.38 | 0.2 | 0.08 | 0.78 | <0.01 |
| (1) Right medial frontal gyrus/anterior frontal pole—(4) left medial frontal gyrus | 0.36 | 0.24 | 0.21 | 0.65 | 0.01 |
| (2) Left dorsomedial frontal cortex—(1) right medial frontal gyrus/anterior frontal pole | 0.31 | 0.18 | 0.01 | 0.94 | <0.001 |
| (2) Left dorsomedial frontal cortex—(3) left precuneus | 0.26 | 0.13 | 0.06 | 0.81 | 0.002 |
| (1) Right medial frontal gyrus/anterior frontal pole (lag)—(1) right medial frontal gyrus/anterior frontal pole | 0.6 | 0.15 | 0.21 | 0.65 | 0.01 |
| (9) Left cingulate (lag)—(9) left cingulate | 0.38 | 0.14 | 0.97 | 0.34 | 0.04 |
| (11) Left precuneus (lag)—(11) left precuneus | 0.46 | 0.16 | 0.09 | 0.76 | <0.01 |
| (3) Left precuneus (lag)—(3) left precuneus | 0.6 | 0.17 | 0.39 | 0.54 | 0.02 |
| (7) Left paracentral lobule (lag)—(7) left paracentral lobule | 0.47 | 0.17 | 0.15 | 0.7 | 0.01 |
| (4) Left medial frontal (lag)—(4) left medial frontal | 0.56 | 0.16 | 0.2 | 0.66 | 0.01 |
| (6) Right caudate (lag)—(6) right caudate | 0.34 | 0.18 | 0.38 | 0.55 | 0.02 |
| (5) Right cerebellum (lag)—(5) right cerebellum | 0.49 | 0.16 | 1.39 | 0.25 | 0.06 |
| (8) Right insula (lag)—(8) right insula | 0.029 | 0.24 | 1.98 | 0.17 | 0.08 |
| (10) Right postcentral (lag)—(10) right postcentral | 0.48 | 0.19 | 0.03 | 0.87 | 0.001 |
| (2) Left Dorsomedial frontal cortex (lag)—(2) left dorsomedial frontal cortex | 0.59 | 0.16 | 1.25 | 0.27 | 0.05 |
Fig. 7.
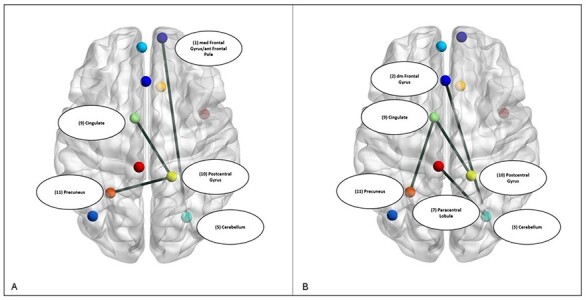
ROI connectivity in the AAT (A) and control (B) subgroups.
Table 4.
AAT group paths from GIMME connectivity analysis
| ROI (#) | M | s.d. |
| (11) Left precuneus—(10) right postcentral gyrus | 0.32 | 0.18 |
| (1) Right medial frontal gyrus/anterior frontal pole—(5) cerebellum | 0.30 | 0.23 |
| (10) Right postcentral gyrus—(9) left cingulate gyrus | 0.31 | 0.12 |
Discussion
This study sought to examine neural mechanisms that might account for effects of computerized AAT on approach system functioning in individuals with MDD. Consistent with hypotheses, individuals in the AAT training condition showed increased activation in the striatum and medial PFC extending into the anterior frontal pole during social reward anticipation relative to those in the control condition. Additional reward-related regions (parietal cortex and insula) were also differentially activated by the training vs control. Irrespective of group assignment, neural activation during social reward anticipation was characterized by connectivity across these reward regions, with outward hubs from the right medial frontal gyrus/anterior frontal pole and dmPFC. The magnitude of connectivity between a region of the medial PFC extending into the anterior frontal pole and striatum was associated with self-reported positive affect during anticipation of the laboratory social interaction, suggesting that this path could be relevant to understanding real-world social responsivity. The AAT and control group each demonstrated additional unique group connections across ROIs, but a similar number of paths. Taken together, findings suggest that one potential effect of AAT may be enhanced engagement of reward-related circuitry in individuals with MDD and that distinct patterns of neural connectivity can be observed across AAT vs a control comparator.
Individuals with MDD demonstrate dysfunction of approach system functioning measured across biobehavioral domains, including blunted affective response to pleasant cues and rewards (Henriques and Davidson, 2000; Sloan and Sandt, 2010), implicit approach action tendencies (Wang et al., 2006; Seidel et al., 2010; Radke et al., 2014b; Bartoszek and Winer, 2015), reward learning (Pizzagalli et al., 2008) and neural responsivity (Schaefer et al., 2006; Derntl et al., 2011; Treadway and Zald, 2011; Zhang et al., 2013; Nusslock et al., 2015). Approach-related neural systems include fronto-striatal circuitry implicated in pleasure and reward processing (Berridge et al., 2009; Haber and Knutson, 2010; Kringelbach and Berridge, 2010), which operate via dopaminergic projections along mesolimbic and mesocortical signaling pathways connecting midbrain nuclei to the ventral striatum and to cortical regions (e.g. medial PFC, insular cortex and anterior cingulate cortex) (Treadway and Zald, 2011; Nusslock and Alloy, 2017). Observations of hyporesponsiveness in key reward processing regions during anticipation of reward (e.g. ventral striatum) (Forbes et al., 2009; Smoski et al., 2009; Admon and Pizzagalli, 2015; Arrondo et al., 2015) and abnormalities in fronto-striatal connectivity (Furman et al., 2011; Manelis et al., 2016; Quevedo et al., 2017) in individuals with MDD highlight this circuit as a potential neurobiological marker and treatment target.
Enhanced neural responsivity in fronto-striatal regions to social reward anticipation during the SID task for those in AAT vs control provides preliminary evidence of malleability of reward-related circuitry with repeated practice. Earlier work demonstrates that anticipation of reward is associated with the recruitment of striatal and PFC regions in healthy individuals (Ernst et al., 2004; Liu et al., 2011). We observed that individuals with depression who completed AAT showed increased activity in the caudate as compared to those completing control training. The caudate is considered to be a key hub for reward-related processing (Chau et al., 2004; Haber and Knutson, 2010; Treadway and Zald, 2011; Pizzagalli, 2014) and may have particular importance for shaping goal-directed behavior based on expectancies (Grahn et al., 2008). Increased activation was also observed in an anterior region of the frontal pole, a region thought to guide attention and behavior in line with internal goals (Orr et al., 2015), evaluate relationships between external stimuli and the self (e.g. self-relatedness) (Phan et al., 2004; Lemogne et al., 2012) and make inferences about the knowledge and beliefs of others (cognitive theory of mind processes) (Abu-Akel and Shamay-Tsoory, 2011; De La Vega et al., 2016). To the extent that hypoactivation reflects deficits in processing socially rewarding stimuli, changes following AAT may reflect amelioration of reward responsivity deficits. It may also reflect changes in social approach orientation potentially via changes in self-referential evaluation of positive social cues. We observed a relative decrease in activation in regions including the cingulate and a region of the preceuneus incorporating the intraparietal sulcus following AAT as compared to the control. Both regions are implicated in adaptive responding to conflict and inhibitory functioning (Botvinick et al., 2004; Osada et al., 2019). It is possible that reduced activation in these regions reflects a shift in reward perceptions, such that the anticipation of receiving positive social feedback resulted in diminished response conflict following repeated AAT wherein positive faces were repeatedly paired with approach behavior.
The results of GIMME connectivity analysis suggest that AAT vs control training results in different patterns of correlation across reward-related regions and highlight the interconnectivity of fronto-striatal regions while anticipating social reward cues. In particular, the observed connectivity between the medial PFC/anterior frontal pole and caudate across both groups aligns with earlier work demonstrating that reward anticipation is associated with frontal-striatal connectivity (Mayer et al., 2011; Cohen et al., 2012). PFC–caudate activity alongside connections bilaterally across medial PFC regions were noted for individuals irrespective of randomization, suggesting that these pathways are common during the anticipation condition of the SID task. We observed that greater communication between the medial PFC/anterior frontal pole and striatum when anticipating positive social rewards in the SID task was associated with more positive emotion when anticipating a real-world social interaction. Individuals with MDD have been shown to demonstrate hypoconnectivity between the medial PFC and striatum (Young et al., 2016); our observed findings point to a potentially important role of this frontal-striatal connection in social approach outcomes in depression. This region of the medial PFC is a core node in the default mode network that plays a central role in self-referential thinking, while the caudate is a key component of salience detection. Connectivity in these regions during anticipation of social reward during the SID may point to a greater perceived self-relevance of impending positive social cues (e.g. the positive feedback is for them) or a perceived link between the social reward and their actions or characteristics that makes the cues more salient. Translating to the social interaction, individuals who are more inclined to anticipate and link positive social outcomes to themselves via self-referential processing may experience greater positive emotions prior to socializing. In supplemental exploratory analyses using group randomization data, GIMME analysis identified divergent patterns of communication between ROIs across the AAT and control groups. The control condition reflected patterns with central cingulate activation and cerebellar activation, while the AAT group was characterized by connectivity involving the postcentral gyrus and medial PFC to the cerebellum. These patterns may reflect greater integration of reward processing circuitry with motor responses in the AAT vs control group (e.g. facilitated responding to reward based on practiced action tendencies during the AAT). Future replication will be needed to clarify the role of differential connectivity in clinical outcomes.
Evidence suggests that existing treatments for depression might normalize approach system functioning (Dichter et al., 2009); however, those treatments target evaluative processes explicitly in comparison to AAT programs. AAT programs intervene on a specific component of approach system functioning by requiring individuals to repeatedly implement approach behaviors in the context of positive social stimuli, thus changing valuation and approach tendencies. AAT has shown promise as a method for modifying approach behaviors in samples with psychopathology (Loijen et al., 2020); yet to date, knowledge about neurobiological effects of AAT on approach systems and the relationships between these systems and outcomes has been limited. Our results offer initial evidence that completing AAT vs control paradigms exerts neural effects on a novel task assessing social reward anticipation. Behavioral modification used in the AAT program may elicit changes via modification of neural substrates involved in processing social reward; however, future work examining treatment-related change with AAT is needed to fully elucidate neural mechanisms in the context of clinical intervention.
These data have a number of caveats. The sample was small. Future replication will be necessary to have confidence in the robustness and replicability of observed effects. While incentive delay tasks have been shown to produce reliable activations in regions including the striatum (Wu et al., 2014; Elliott et al., 2020), the use of a small sample combined with the high variability in neuroimaging data leads to the potential for measurement error to influence findings, making replication critical. Moreover, a larger sample size would permit the examination of brain–behavior relationships within the training and control groups separately, as well as more nuanced analysis of effects within individuals with depression, who are likely to display heterogeneity in approach system functioning (e.g. anhedonic symptoms). The current study was designed to examine neural changes during a relatively brief experimental manipulation. Controlled single-session paradigms may be useful in early stages of intervention development to isolate neural circuits that are and are not engaged in the absence of clinical symptom change, but the data cannot definitely speak to what neural changes might be observed over the course of AAT administered as an intervention program. Results comparing groups on outcomes obtained in the context of the social interaction task immediately following the 1-session program did not show statistically significant differences, pointing to a potential need for longer administrations to shift emotional/motivational reactivity and symptoms. Social cues were used in the current version of the AAT and SID consistent with prior literature in depressed samples (Radke et al., 2014a), and thus future research is needed to evaluate reward response to other types of stimuli. Parameters of the AAT training and control programs (i.e. using a control condition with 50/50 contingency, using visually enlarging images) were selected based on the prior literature to match what would typically be administered in the course of a clinical trial. However, using an active control wherein individuals only pull stimuli (both positive and neutral) may not be truly inert. For example, there is evidence in non‐clinical samples that implicit approach training for neutral faces shifts the valence of that stimuli in a positive direction (Woud et al., 2011), suggesting that approaching both positive and neutral faces—without trained avoidance—may provide clinical benefit. We observed in our data that in some brain regions changes occurred in both groups in opposite directions, suggesting that the control condition may have exerted a different but still impactful effect [see for example (Blackwell et al., 2017; Tiggemann and Kemps, 2020) on the impact of control group selection in cognitive bias modification trials]. Despite these limitations, the current study points to potential neural targets underlying AAT in MDD. Taken together, these data reveal that AAT programs could operate via enhancing activity in or communication across reward-related circuitry, which may relate to clinical and behavioral outcomes. The current data suggest that AAT may be a viable method for restoring neural functioning in reward-related circuitry in individuals with depression.
Supplementary Material
Acknowledgements
We would like to thank the many individuals who helped make this research possible: Taylor Smith and Sarah Dowling for conducting diagnostic interviews and overseeing project management; Karalani Cross for overseeing project management and Carl Bolano, Kevin Carlis, Michelle Chang, Joanna Chen, Melody Chen, Christina Cui, Vivi Dang, Angelica Estrada, Alyson Johnson, Sanskruti Kakaria, Sarah Knapp, Stephanie Lee, Mercy Lopez, Gregory Pak, Jasmine Rai, Atiyeh Samadi, Rachel Storer, Aaron Tay, Sarah Tran and Stephanie Zepeda for their help with recruitment, screening, data collection and management.
Contributor Information
Jessica Bomyea, Center of Excellence for Stress and Mental Health, VA San Diego Healthcare System, San Diego, CA 92161, USA; Department of Psychiatry, University of California, San Diego, San Diego, CA 92037, USA.
Soo-Hee Choi, Department of Psychiatry, Seoul National University Hospital, Seoul, Republic of Korea; Department of Psychiatry, Seoul National University College of Medicine and Institute of Human Behavioral Medicine, SNU-MRC, Seoul, Republic of Korea.
Alison Sweet, Department of Psychiatry, University of California, San Diego, San Diego, CA 92037, USA.
Murray Stein, Department of Psychiatry, University of California, San Diego, San Diego, CA 92037, USA; School of Public Health, University of California, San Diego, San Diego, CA, USA; Psychiatry Service, VA San Diego Healthcare System, San Diego, CA, USA.
Martin Paulus, Laureate Institute for Brain Research, Tulsa, OK 74136, USA.
Charles Taylor, Department of Psychiatry, University of California, San Diego, San Diego, CA 92037, USA.
Funding
This work was supported by the Brain and Behavioral Research Foundation Young Investigator Grant awarded to Dr C.T. (#21695) and VA CSR&D CX001600 awarded to Dr J.B.
Conflict of interest
Dr M.P. is an advisor to Spring Care, Inc., a behavioral health startup, and he has received royalties for an article about methamphetamine in UpToDate. Dr C.T. declares that in the past 3 years he has been a paid consultant for Homewood Health and receives payment for editorial work for UpToDate. Dr M.S. has in the past 3 years been a paid consultant for Acadia Pharmaceuticals, Aptinyx, Bionomics, Genentech, GW Pharma, Janssen, Nobilis Therapeutics and Oxeia Biopharmaceuticals. J.B., A.S. and S.-H.C. declare no conflicts of interest. These data have not been previously published.
Supplementary data
Supplementary data are available at SCAN online.
Author contributions
C.T. designed the study and wrote the protocol. C.T., J.B. and S.-H.C. managed literature searches and statistical analyses. All authors contributed to and have approved the final manuscript.
References
- Abu-Akel A., Shamay-Tsoory S. (2011). Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia, 49(11), 2971–84. [DOI] [PubMed] [Google Scholar]
- Admon R., Pizzagalli D.A. (2015). Dysfunctional reward processing in depression. Current Opinion in Psychology, 4, 114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N., Kuckertz J.M., Najmi S. (2013). The effect of modifying automatic action tendencies on overt avoidance behaviors. Emotion, 13(3), 478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A., Melinat E., Aron E.N., Vallone R.D., Bator R.J. (1997). The experimental generation of interpersonal closeness: a procedure and some preliminary findings. Personality and Social Psychology Bulletin, 23(4), 363–77. [Google Scholar]
- Arrondo G., Segarra N., Metastasio A., et al. (2015). Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Frontiers in Psychology, 6, 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association. [Google Scholar]
- Bartoszek G., Winer E.S. (2015). Spider-fearful individuals hesitantly approach threat, whereas depressed individuals do not persistently approach reward. Journal of Behavior Therapy and Experimental Psychiatry, 46, 1–7. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Becker E.S., Barth A., Smits J.A.J., Beisel S., Lindenmeyer J., Rinck M. (2019). Positivity-approach training for depressive symptoms: a randomized controlled trial. Journal of Affective Disorders, 245, 297–304. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E., Aldridge J.W. (2009). Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9(1), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell S.E., Woud M.L., MacLeod C. (2017). A question of control? Examining the role of control conditions in experimental psychopathology using the example of cognitive bias modification research. The Spanish Journal of Psychology, 20, E54. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences, 8(12), 539–46. [DOI] [PubMed] [Google Scholar]
- Britton J.C., Suway J.G., Clementi M.A., Fox N.A., Pine D.S., Bar-Haim Y. (2015). Neural changes with attention bias modification for anxiety: a randomized trial. Social Cognitive and Affective Neuroscience, 10(7), 913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J.T., Priester J.R., Berntson G.G. (1993). Rudimentary determinants of attitudes. II: arm flexion and extension have differential effects on attitudes. Journal of Personality and Social Psychology, 65(1), 5–17. [DOI] [PubMed] [Google Scholar]
- Camara E., Rodriguez-Fornells A., Ye Z., Munte T.F. (2009). Reward networks in the brain as captured by connectivity measures. Frontiers in Neuroscience, 3(3), 350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau D.T., Roth R.M., Green A.I. (2004). The neural circuitry of reward and its relevance to psychiatric disorders. Current Psychiatry Reports, 6(5), 391–9. [DOI] [PubMed] [Google Scholar]
- Clark L.A., Watson D. (1991). Tripartite model of anxiety and depression - psychometric evidence and taxonomic implications. Journal of Abnormal Psychology, 100(3), 316–36. [DOI] [PubMed] [Google Scholar]
- Cohen M.X., Bour L., Mantione M., et al. (2012). Top-down-directed synchrony from medial frontal cortex to nucleus accumbens during reward anticipation. Human Brain Mapping, 33(1), 246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W., Reynolds R.C., Taylor P.A. (2016). AFNI and clustering: false positive rates redux. bioRxiv, 152–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J.C. (1976). Depression and the response of others. Journal of Abnormal Psychology, 85(2), 186–93. [DOI] [PubMed] [Google Scholar]
- Craske M.G., Meuret A.E., Ritz T., Treanor M., Dour H.J. (2016). Treatment for anhedonia: a neuroscience driven approach. Depression and Anxiety, 33(10), 927–38. [DOI] [PubMed] [Google Scholar]
- Cuijpers P., van Straten A., Warmerdam L. (2007). Behavioral activation treatments of depression: a meta-analysis. Clinical Psychology Review, 27(3), 318–26. [DOI] [PubMed] [Google Scholar]
- Cuijpers P., van Straten A., Andersson G., van Oppen P. (2008). Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. Journal of Consulting and Clinical Psychology, 76(6), 909–22. [DOI] [PubMed] [Google Scholar]
- de la Vega A., Chang L.J., Banich M.T., Wager T.D., Yarkoni T. (2016). Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. Journal of Neuroscience, 36(24), 6553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Seidel E.M., Eickhoff S.B., et al. (2011). Neural correlates of social approach and withdrawal in patients with major depression. Social Neuroscience, 6(5–6), 482–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G.S., Felder J.N., Petty C., Bizzell J., Ernst M., Smoski M.J. (2009). The effects of psychotherapy on neural responses to rewards in major depression. Biological Psychiatry, 66(9), 886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G.S., Felder J.N., Smoski M.J. (2010). The effects of brief behavioral activation therapy for depression on cognitive control in affective contexts: an fMRI investigation. Journal of Affective Disorders, 126(1–2), 236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon D.G., Rosso I.M., Pechtel P., Killgore W.D., Rauch S.L., Pizzagalli D.A. (2014). Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depression and Anxiety, 31(3), 233–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop D.D., Manheim L.M., Song J., Lyons J.S., Chang R.W. (2005). Incidence of disability among preretirement adults: the impact of depression. American Journal of Public Health, 95(11), 2003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B.D., German R.E., Khazanov G., Xu C.L., Hollon S.D., DeRubeis R.J. (2020). Changes in positive and negative affect during pharmacological treatment and cognitive therapy for major depressive disorder: a secondary analysis of two randomized controlled trials. Clinical Psychological Science, 8(1), 36–51. [Google Scholar]
- Ekers D., Webster L., van Straten A., Cuijpers P., Richards D., Gilbody S. (2014). Behavioural activation for depression; an update of meta-analysis of effectiveness and sub group analysis. PLoS One, 9(6), e100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M.L., Knodt A.R., Ireland D., et al. (2020). What is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychological Science, 31(7), 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., McClure E.B., et al. (2004). Choice selection and reward anticipation: an fMRI study. Neuropsychologia, 42(12), 1585–97. [DOI] [PubMed] [Google Scholar]
- Ferrari G.R.A., Mobius M., Becker E.S., Spijker J., Rinck M. (2018). Working mechanisms of a general positivity approach-avoidance training: effects on action tendencies as well as on subjective and physiological stress responses. Journal of Behavior Therapy and Experimental Psychiatry, 59, 134–41. [DOI] [PubMed] [Google Scholar]
- Fleurkens P., van Minnen A., Becker E.S., et al. (2018). Automatic approach-avoidance tendencies as a candidate intermediate phenotype for depression: associations with childhood trauma and the 5-HTTLPR transporter polymorphism. PLoS One, 13(3), e0193787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Hariri A.R., Martin S.L., et al. (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American Journal of Psychiatry, 166(1), 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D.J., Hamilton J.P., Gotlib I.H. (2011). Frontostriatal functional connectivity in major depressive disorder. Biology of MoodandAnxiety Disorders, 1(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates K.M., Lane S.T., Varangis E., Giovanello K., Guskiewicz K. (2017). Unsupervised classification during time-series model building. Multivariate Behavioral Research, 52(2), 129–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates K.M., Molenaar P.C. (2012). Group search algorithm recovers effective connectivity maps for individuals in homogeneous and heterogeneous samples. NeuroImage, 63(1), 310–9. [DOI] [PubMed] [Google Scholar]
- Grahn J.A., Parkinson J.A., Owen A.M. (2008). The cognitive functions of the caudate nucleus. Progress in Neurobiology, 86(3), 141–55. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., Shi T.C., Ezie C.E.C., et al. (2020). Association between real-world experiential diversity and positive affect relates to hippocampal-striatal functional connectivity. Nature Neuroscience, 23(7), 800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques J.B., Davidson R.J. (2000). Decreased responsiveness to reward in depression. CognitionandEmotion, 14(5), 711–24. [Google Scholar]
- Henry T.R., Feczko E., Cordova M., et al. (2019). Comparing directed functional connectivity between groups with confirmatory subgrouping GIMME. NeuroImage, 188, 642–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer K., Rinck M., Becker E.S. (2007). Avoidance of emotional facial expressions in social anxiety: the approach-avoidance task. Behaviour Research and Therapy, 45(12), 2990–3001. [DOI] [PubMed] [Google Scholar]
- Hoflich A., Michenthaler P., Kasper S., Lanzenberger R. (2019). Circuit mechanisms of reward, anhedonia, and depression. The International Journal of Neuropsychopharmacology, 22(2), 105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopko D.R., Lejuez C.W., Ruggiero K.J., Eifert G.H. (2003). Contemporary behavioral activation treatments for depression: procedures, principles, and progress. Clinical Psychology Review, 23(5), 699–717. [DOI] [PubMed] [Google Scholar]
- Hopko D.R., Mullane C.M. (2008). Exploring the relation of depression and overt behavior with daily diaries. Behaviour Research and Therapy, 46(9), 1085–9. [DOI] [PubMed] [Google Scholar]
- Kakoschke N., Kemps E., Tiggemann M. (2017). Approach bias modification training and consumption: a review of the literature. Addictive Behaviors, 64, 21–8. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., et al. (2003). The epidemiology of major depressive disorder - results from the National Comorbidity Survey Replication (NCS-R). JAMA-Journal of the American Medical Association, 289(23), 3095–105. [DOI] [PubMed] [Google Scholar]
- Knutson B., Greer S.M. (2008). Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1511), 3771–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L., Berridge K.C. (2010). The functional neuroanatomy of pleasure and happiness. Discovery Medicine, 9(49), 579–87. [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. (2001). The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C., Delaveau P., Freton M., Guionnet S., Fossati P. (2012). Medial prefrontal cortex and the self in major depression. Journal of Affective Disorders, 136(1–2), E1–11. [DOI] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35(5), 1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loijen A., Vrijsen J.N., Egger J.I.M., Becker E.S., Rinck M. (2020). Biased approach-avoidance tendencies in psychopathology: a systematic review of their assessment and modification. Clinical Psychology Review, 77, 101825. [DOI] [PubMed] [Google Scholar]
- Manelis A., Almeida J.R., Stiffler R., Lockovich J.C., Aslam H.A., Phillips M.L. (2016). Anticipation-related brain connectivity in bipolar and unipolar depression: a graph theory approach. Brain, 139(Pt 9), 2554–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R., Mannell M.V., Ling J., Gasparovic C., Yeo R.A. (2011). Functional connectivity in mild traumatic brain injury. Human Brain Mapping, 32(11), 1825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli T., Kane R., Rees C. (2009). Behavioral activation treatments for depression in adults: a meta-analysis and review. Clinical Psychology-Science and Practice, 16(4), 383–411. [Google Scholar]
- McCormick B. (2014). Promoting physical activity and exercise as adjunct treatment of cognitive dysfunction in psychiatric disorders. In: 7th International Scientific Conference on Kinesiology: Fundamental and Applied Kinesiology—Steps Forward, 103–6. [Google Scholar]
- Mori A., Okamoto Y., Okada G., et al. (2017). Behavioral activation can normalize neural hypoactivation in subthreshold depression during a monetary incentive delay task (vol 189, pg 254, 2016). Journal of Affective Disorders, 208, 670. [DOI] [PubMed] [Google Scholar]
- Nusslock R., Walden K., Harmon-Jones E. (2015). Asymmetrical frontal cortical activity associated with differential risk for mood and anxiety disorder symptoms: an RDoC perspective. International Journal of Psychophysiology, 98(2), 249–61. [DOI] [PubMed] [Google Scholar]
- Nusslock R., Alloy L.B. (2017). Reward processing and mood-related symptoms: an RDoC and translational neuroscience perspective. Journal of Affective Disorders, 216, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr J.M., Smolker H.R., Banich M.T. (2015). Organization of the human frontal pole revealed by large-scale DTI-based connectivity: implications for control of behavior. PLoS One, 10(5), e0124797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T., Ohta S., Ogawa A., et al. (2019). An essential role of the intraparietal sulcus in response inhibition predicted by parcellation-based network. Journal of Neuroscience, 39(13), 2509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Taylor S.F., Welsh R.C., Ho S.H., Britton J.C., Liberzon I. (2004). Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. NeuroImage, 21(2), 768–80. [DOI] [PubMed] [Google Scholar]
- Pincus H.A., Pettit A.R. (2001). The societal costs of chronic major depression. The Journal of Clinical Psychiatry, 62(Suppl 6), 5–9. [PubMed] [Google Scholar]
- Pizzagalli D.A., Iosifescu D., Hallett L.A., Ratner K.G., Fava M. (2008). Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. Journal of Psychiatric Research, 43(1), 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A., Holmes A.J., Dillon D.G., et al. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry, 166(6), 702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A. (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review of Clinical Psychology, 10, 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K., Ng R., Scott H., et al. (2017). Ventral striatum functional connectivity during rewards and losses and symptomatology in depressed patients. Biological Psychology, 123, 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke S., Guths F., Andre J.A., Muller B.W., de Bruijn E.R.A. (2014a). In action or inaction? Social approach–avoidance tendencies in major depression. PsychiatryResearch, 219(3), 513–7. [DOI] [PubMed] [Google Scholar]
- Radke S., Guths F., Andre J.A., Muller B.W., de Bruijn E.R.A. (2014b). In action or inaction? Social approach-avoidance tendencies in major depression. Psychiatry Research, 219(3), 513–7. [DOI] [PubMed] [Google Scholar]
- Rinck M., Becker E.S. (2007). Approach and avoidance in fear of spiders. Journal of Behavior Therapy and Experimental Psychiatry, 38(2), 105–20. [DOI] [PubMed] [Google Scholar]
- Schaefer H.S., Putnam K.M., Benca R.M., Davidson R.J. (2006). Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biological Psychiatry, 60(9), 974–86. [DOI] [PubMed] [Google Scholar]
- Seidel E.M., Habel U., Finkelmeyer A., Schneider F., Gur R.C., Derntl B. (2010). Implicit and explicit behavioral tendencies in male and female depression. Psychiatry Research, 177(1–2), 124–30. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V. (2014). The Mini-international Neuropsychiatric Interview, Version 7.0 For DSM-5 (MINI 7.0). Medical Outcomes Systems. [Google Scholar]
- Shiota S., Okamoto Y., Okada G., et al. (2017). Effects of behavioural activation on the neural basis of other perspective self-referential processing in subthreshold depression: a functional magnetic resonance imaging study. Psychological Medicine, 47(5), 877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan D.M., Sandt A.R. (2010). Depressed mood and emotional responding. Biological Psychology, 84(2), 368–74. [DOI] [PubMed] [Google Scholar]
- Smoski M.J., Felder J., Bizzell J., et al. (2009). fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders, 118(1–3), 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists. [Google Scholar]
- Spreckelmeyer K.N., Krach S., Kohls G., et al. (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience, 4(2), 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struijs S.Y., Lamers F., Rinck M., Roelofs K., Spinhoven P., Penninx B.W.J.H. (2018). The predictive value of approach and avoidance tendencies on the onset and course of depression and anxiety disorders. Depression and Anxiety, 35(6), 551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C., Shukla A., Cross K., Amir N., Stein M.B., Paulus M. (2013). Training the brain to abstain from alcohol: towards the neural basis of approach/avoidance training effects in hazardous drinkers. Neuropsychopharmacology, 38, S458–9. [Google Scholar]
- Taylor C.T., Aupperle R.L., Flagan T., et al. (2014). Neural correlates of a computerized attention modification program in anxious subjects. Social Cognitive and Affective Neuroscience, 9(9), 1379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.T., Pearlstein S.L., Stein M.B. (2017). The affective tie that binds: examining the contribution of positive emotions and anxiety to relationship formation in social anxiety disorder. Journal of Anxiety Disorders, 49, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.T., Amir N. (2012). Modifying automatic approach action tendencies in individuals with elevated social anxiety symptoms. Behaviour Research and Therapy, 50(9), 529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiggemann M., Kemps E. (2020). Is sham training still training? An alternative control group for attentional bias modification. Frontiers in Psychology, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., et al. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168(3), 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway M.T., Zald D.H. (2011). Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews, 35(3), 537–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trew J.L. (2011). Exploring the roles of approach and avoidance in depression: an integrative model. Clinical Psychology Review, 31(7), 1156–68. [DOI] [PubMed] [Google Scholar]
- Voncken M.J., Dijk K.F.L. (2013). Socially anxious individuals get a second chance after being disliked at first sight: the role of self-disclosure in the development of likeability in sequential social contact. Cognitive Therapy and Research, 37(1), 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijsen J.N., van Oostrom I., Speckens A., Becker E.S., Rinck M. (2013). Approach and avoidance of emotional faces in happy and sad mood. Cognitive Therapy and Research, 37(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijsen J.N., Fischer V.S., Muller B.W., et al. (2018). Cognitive bias modification as an add-on treatment in clinical depression: results from a placebo-controlled, single-blinded randomized control trial. Journal of Affective Disorders, 238, 342–50. [DOI] [PubMed] [Google Scholar]
- Wang C.E., Brennen T., Holte A. (2006). Decreased approach motivation in depression. Scandinavian Journal of Psychology, 47(6), 505–11. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect—the Panas scales. Journal of Personality and Social Psychology, 54(6), 1063–70. [DOI] [PubMed] [Google Scholar]
- Wiers C.E., Stelzel C., Gladwin T.E., et al. (2015). Effects of cognitive bias modification training on neural alcohol cue reactivity in alcohol dependence. American Journal of Psychiatry, 172(4), 335–43. [DOI] [PubMed] [Google Scholar]
- Wiers R.W., Eberl C., Rinck M., Becker E.S., Lindenmeyer J. (2011). Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychological Science, 22(4), 490–7. [DOI] [PubMed] [Google Scholar]
- Woud M.L., Becker E.S., Rinck M. (2011). Induction of implicit evaluation biases by approach-avoidance training: a commentary on Vandenbosch and De Houwer (this issue). CognitionandEmotion, 25(7), 1331–8. [Google Scholar]
- Wu C.C., Samanez-Larkin G.R., Katovich K., Knutson B. (2014). Affective traits link to reliable neural markers of incwentive anticipation. NeuroImage, 84, 279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Gates K.M., Molenaar P., Li P. (2015). Neural changes underlying successful second language word learning: an fMRI study. Journal of Neurolinguistics, 33, 29–49. [Google Scholar]
- Yokoyama S., Okamoto Y., Takagaki K., et al. (2018). Effects of behavioral activation on default mode network connectivity in subthreshold depression: a preliminary resting-state fMRI study. Journal of Affective Disorders, 227, 156–63. [DOI] [PubMed] [Google Scholar]
- Young C.B., Chen T., Nusslock R., Keller J., Schatzberg A.F., Menon V. (2016). Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Translational Psychiatry, 6, e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.N., Chang S.H., Guo L.Y., Zhang K.L., Wang J. (2013). The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. Journal of Affective Disorders, 151(2), 531–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


