Abstract
The objective of this study was to investigate the effects of dietary P levels on the performance of rearing pigeons, and bone characteristics of squabs from 7 to 21 d of age. A total of 192 pairs of adult Silver King pigeons (40 wk of age) were used. The pigeons were randomly allocated to one of 4 treatment groups, each consisting of eight replicates of 6 pigeon pairs per replicate. Dietary treatments included the basal diet (containing 0.3% of P), the basal diet supplemented with 0.2, 0.4, or 0.8% inorganic P. And the dietary Ca content was kept at 1.40% across all treatments. The experimental diets were fed to parent pigeons as corn-soybean complete pellet feed, and squabs fed with crop milk secreted by parent pigeons. Pigeons in the group of 0.4% supplemental non-phytate phosphorus (NPP) had shorter (P = 0.045) oviposition interval than those in the control group and group of 0.8% NPP. When the diet was supplemented with 0.8% of NPP, the least average egg weight was observed (P = 0.006). Female breeding birds had much higher (P < 0.01) Ca, P, and ALP in serum than male ones. At 7-d of age, dietary P supplementation influenced P and Ca content in tibia ash of squabs (P < 0.05). The tibia ash Ca content in the group of 0.2% NPP was the highest among the treatments (P = 0.007). At d 21 of age, both the birds in the group of 0.4 and 0.8% NPP had higher tibia breaking strength (P < 0.01) and tibia ash contents (P < 0.001) compared to the ones in the control group. In conclusion, the P deficiency in the diet of parent pigeons could cause poor bone mineralization of squabs, especially impaired the bone-breaking strength and bone ash content. The 0.8% of NPP supplementation in the diet has a positive influence on mineralization of squabs although production depression was observed. Both P and Ca metabolism of female breeding birds were more active than male ones at earlier time points of rearing period. The desirable supplemental NPP level in diet for breeding pigeon was 0.4% according to the performance data in the present trial. The recommended Ca: P ratio for pigeons, which was different from the optimum value for broilers, needs to be studied in the future.
Key words: phosphorus, breeding pigeon, performance, tibia, squab
INTRODUCTION
Phosphorus (P) plays an important role in nucleic acid synthesis, energy metabolism, muscle function, enzyme activity, lipid metabolism, and bone mineralization (Berndt and Kumar, 2009). The P nutrition for optimal performance and bone development in broilers, ducks and geese have been well documented (Li et al., 2017; Zhu et al., 2018a,b; Xu et al., 2019). Pigeons (Columba livia domestica) as a type of altricial birds, unlike chicken, hatch with unopened eyes and can only be fed by male and female pigeons with ‘crop milk’ for nearly 28 d. Pigeons are widely distributed throughout the whole world (except arctic regions) (Sales and Janssens, 2003). Pigeon meat, or squab, is considered a delicacy in many parts of the world. And the breeding stock is increasing year by year which has made it become the fourth biggest source of poultry-product in China. However, the P requirement of pigeons has not been studied. In the practical production of meat-type pigeon, the P recommendation for broilers is adopted which might not be applicable to this species. Therefore, it is necessary to study the effects of dietary P levels on the performance and development of pigeons.
Bone is a main storage organ for P, which contains about 85% of the body's total P (Cook et al., 1937). Bone formation is highly dependent on the dietary concentrations of P (Liu et al., 2013). It has been shown that low P diets limited the growth of animals, whereas high P intake negatively impacted calcium (Ca) metabolism and bone properties (Roman-Garcia et al., 2010). Many researchers reported that high-P diet increases the concentration of serum P, and decreases the bone mass (Roman-Garcia et al., 2010). The P concentrations in serum and bones can reflect the changes of P homeostasis, and the contents within a normal range are important for normal physiological function and optimal bone mineralization (Proszkowiec-Weglarz and Angel, 2013). Bone characteristics, such as bone ash, are commonly used to examine mineralization of bones for broilers (Bar et al., 2003). And the P metabolic utilization parameters (Ca and P concentrations, and ALP activities in serum and bone) of broilers were the most sensitive ones to dietary P deficiency (Li et al., 2020).
Cereal grains are the major component of pigeon diets and have high P concentrations, most of which is bound as phytate and is considered not biologically available (Vashishth et al., 2017). However, it should be noted that plant feed ingredients, especially wheat, may contain significant endogenous phytase activity (Selle et al., 2003). Phytases can also be produced by different bacteria (Palacios et al., 2008). Some authors have suggested that phytate is hydrolysed by phytases produced by micro-organisms present in the small intestine and particularly in the ceca of broilers (Leytem et al., 2008). Our trial has indicated that the microbiota diversity was higher in crop than in small intestine and rectum of healthy pigeons at 14 and 21 days old (Ji et al., 2020). Therefore, the P metabolism and utilization in pigeons might be different from that in broilers. However, to our knowledge, there are no reports on the effect of different dietary P levels on growth performance, serum metabolites and tibia characteristics of pigeons. In addition, inorganic P supplements are added to the diet to thoroughly fulfill the requirements of birds according to the P recommendation for broilers. The inclusion levels of inorganic P in practice might contribute to increased feed cost and environmental pollution (Gautier et al., 2017). Therefore, the objective of the current study was to investigate the effects of dietary P levels without changing Ca levels on the performance of rearing pigeons, and bone characteristics of squabs from 7 to 21 d of age, so as to get knowledge of P requirement of altricial avian and explore the effects of parental P nutrition on squabs for pigeon breeding.
MATERIALS AND METHODS
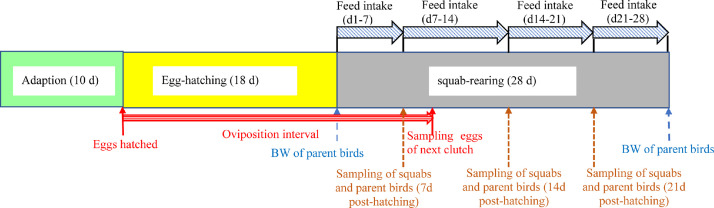
All procedures were approved by the Animal Care and Use Committee of Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences. The sampling design showing the timeline for collecting samples were shown in Figure 1.
Figure 1.
Sampling design showing the timeline for collecting samples of eggs, squabs, and parent pigeons. Egg samples collected from the next clutch during the squab-rearing period of the experiment.
Bird Husbandry
A total of 192 pairs of adult healthy Silver King pigeons (40 wk of age) were obtained from a commercial pigeon farm (Miyun district, Beijing, China). All the female birds were chosen to keep the same oviposition interval at the beginning of the study. The pigeons were randomly allocated to one of 4 treatment groups, each consisting of 8 replicates of 6 pigeon pairs per replicate. Each pair was housed in a cage equipped with a nest and a perch. The 32 replicates (6 cages per replicate) were randomly distributed in the facility. The study lasted for 46 d including the phases of egg-hatching and squab-rearing. Before the experiment began, the birds were fed the control P-deficient diet (containing 0.3% of P) for 10-d adaption. After this adaptive period, the pigeons were fed the experimental treatment diets. The fertile eggs were hatched by parent pigeons and two squabs were assigned to be raised by each one pair of parent pigeon. During the study, caged birds were housed in a room under a 16L:8D lighting cycle, with a mean daily temperature of 15 ± 5°C. Feed was in pellet form and water provided freely.
Dietary Treatments
Dietary treatments included the basal diet (containing 0.3% of P), the basal diet supplemented with 0.2, 0.4, or 0.8% inorganic P in the form of feed grade CaHPO4·2H2O (purity ≥98.0%; Beijing Kang Pu Hui Wei Technology Company, Ltd., China). The building sand were washed and used to correct the differences of additive weight among the four treatment diets (Liu et al., 2017). The experimental diets were fed to parent pigeons as corn-soybean complete pellet feed. Squabs were fed with crop milk secreted by parent pigeons. The Ca contents in diets of parent birds were kept at 1.40% in every group. The contents of Ca and P in feed ingredients and diet samples were measured colorimetrically using the methods of EDTA complex titration (National Standards of the People's Republic of China, 2018) and vanadate-molybdate spectrophotometry (Davies et al., 1973; Cao et al., 2021), respectively. Validations of the Ca and P analyses were conducted using soybean powder (GBW 10013 (GSB-4), Institute of Geochemistry and Geophysics Exploring, Chinese Academy of Geological Sciences, China) as a standard reference material. The compositions and nutrient levels of the experimental diets for parent pigeons were shown in Table 1.
Table 1.
Composition and nutrient levels of experimental diets for parent pigeons (as-fed basis).
| Treatment | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Ingredient (%) | ||||
| Corn | 40.0 | 40.0 | 40.0 | 40.0 |
| Pea | 15.0 | 15.0 | 15.0 | 15.0 |
| Wheat | 12.0 | 12.0 | 12.0 | 12.0 |
| Sorghum | 10.0 | 10.1 | 10.1 | 10.1 |
| (46%) Soybean meal | 15.0 | 15.0 | 15.0 | 15.0 |
| Soybean oil | 1.00 | 1.00 | 1.00 | 1.00 |
| Stone powder | 2.60 | 1.90 | 1.25 | 0.00 |
| CaHPO4·2H2O | 0.00 | 1.12 | 2.20 | 4.40 |
| Salt | 0.37 | 0.40 | 0.40 | 0.40 |
| Lysine | 0.20 | 0.20 | 0.20 | 0.20 |
| Methionine | 0.12 | 0.12 | 0.12 | 0.12 |
| Vitamins1 | 0.02 | 0.02 | 0.02 | 0.02 |
| Minerals2 | 0.15 | 0.15 | 0.15 | 0.15 |
| Choline chloride | 0.04 | 0.04 | 0.04 | 0.04 |
| Sand3 | 3.50 | 2.95 | 2.52 | 1.57 |
| Nutrient levels (%) | ||||
| ME (kcal/kg) | 2796.3 | 2796.3 | 2796.3 | 2796.3 |
| CP4 | 15.5 | 15.0 | 15.2 | 15.2 |
| Methionine | 0.25 | 0.25 | 0.25 | 0.25 |
| Lysine | 0.82 | 0.82 | 0.82 | 0.82 |
| Ca4 | 1.40 | 1.50 | 1.42 | 1.40 |
| Total P (TP)4 | 0.30 | 0.54 | 0.77 | 1.28 |
Vitamins provided the following per kilogram: vitamin A, 9,000 IU (retinyl acetate); vitamin D3, 2,400 IU; vitamin E, 24 IU (dl-α-tocopheryl acetate); vitamin K3, 3 mg; vitamin B1, 2.4 mg; vitamin B2, 7.5 mg; vitamin B6, 4 mg; vitamin B12, 0.026 mg; nicotinamide, 26 mg; D-pantothenic acid, 11 mg; folic acid, 1.4 mg; biotin, 0.16 mg.
Minerals provided the following per kilogram: Cu, 15 mg; Fe, 99 mg; Zn, 95 mg; Mn, 97 mg; I, 1.35 mg; Se, 0.45 mg.
Washed building sand with no detectable P and Ca, was used to adjust the amounts of calcium hydrophosphate in each group diet.
CP, Ca, and total P were analyzed, while all others were calculated according to the data reported for broilers (NRC, 1994).
Performance of Parent Pigeons
Egg production per pair were monitored throughout the trial, and the oviposition interval (time between laying period, day) of female birds calculated. The performance data of breeding pigeons were only recorded throughout the rearing phase (4 wk) to not interrupting the egg-hatching of parent birds. Feed intake of adult birds was measured. The remaining feed per cage was weighed every week. From these values, the average feed intake (g/pair·day) and laying rate (%) were determined. Laying rate (%) = layed eggs every day /pigeon pairs × 100% (Chang et al., 2019).
Four pairs of parent pigeons from each treatment were randomly selected for determination of body weight at the beginning and end of the squab-rearing period. The body weights of pigeons were measured after 12-h fasting, and the body weight loss (g/bird) of the adult pigeons during the period calculated. On the 7, 14, and 21-d in rearing period, four pairs of adult birds per treatment were randomly selected for collection of blood samples, respectively. The blood samples were collected from wing vein, immediately centrifuged for analyzing serum Ca, P, and alkaline phosphatase (ALP) activity. The levels of Ca, P, and ALP activity were determined by an automatic biochemistry analyzer (Hitachi 7600, Japan) with commercial kits (Beijing Leadman Biochemical Company, Ltd., China).
Egg Quality
A total of 30 eggs from each treatment were randomly selected to determine egg weight and eggshell quality. Eggshell thickness was measured by an Egg Shell Thickness Gauge (ETG-1061A, Robotmation, Co., Ltd., Tokyo, Japan). Eggshell strength was measured by an Egg Force Reader (FGV-10X, Orka Food Technology, Israel).
Sample Collection and Analysis of Squabs
On the 7, 14, and 21-d post-hatching, 8 squabs per treatment (one for each replicate) were randomly selected, respectively. The birds were killed by cervical dislocation, the crop contents were removed, and then the body mass was recorded. The tibia samples of the selected birds were collected. The right tibia was peeled and frozen at −20°C for analyzing bone strength and the contents of bone ash, tibia ash Ca and P. The left tibia was stored at −20°C for analysis of the ALP activity. The ALP activity in tibia was determined with assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as described by Liu et al. (2017) and Li et al. (2020).
The tibia bone strength was measured by a 3-point bending test (Texture Analyzers TA.XT plus, Stable Micro Systems Company, UK) as described by Jiang et al. (2016). The tibia bone of the squabs was put on a fulcrum point with a width of 20.5 mm apart. Loading point was located in the midpoint of fulcrum points. The value of breaking force was determined by the shear test at a speed of 30 mm min−1 with a 50-kg loading cell until fracture occurred. The ultimate breaking force of the tibia bone was obtained according to the load-deformation curve recorded by computer.
After the bone breaking strength was analyzed, the tibia bone was dried in an oven at 105°C for 24 h. After that, bone ash percentage was determined by ashing the bone in a muffle furnace at 600°C for 24 h (Kakhki et al., 2019; Mohammadigheisar et al., 2020). The contents of Ca and P in tibia ash were analyzed using the same methods as those for feed samples.
Statistical Analysis
Data were analyzed by SPSS 20.0 for Windows (SPSS Inc., Chicago, IL) using a one-way ANOVA using the general linear model procedure except the serum index of parent birds subjected to a two-way ANOVA analysis. The model in the two-way analysis included the main effects of dietary supplementary P level, sex, and their interaction. One replicate was regarded as an experimental unit, while individual bird or egg was the experimental unit for egg, blood and bone parameters. Linear and quadratic effects were evaluated using orthogonal polynomial contrasts to test the responses to increasing dietary NPP inclusion. Differences among means were tested by the least significant difference (LSD) method, and the statistical significance was set at P ≤ 0.05.
RESULTS
Production Performance
The dietary TP levels by analysis on an as-fed basis were 0.30, 0.54, 0.77, and 1.28%, respectively. Dietary P level affected oviposition interval and average egg weight (P < 0.05, Table 2). Pigeons in the group of 0.4% supplemental non-phytate phosphorus (NPP) had shorter (P < 0.05) oviposition interval than those in the control group and group of 0.8% NPP. The NPP level had quadratic influences (P < 0.05) on the laying rate, and there was a tendency that female birds fed with 0.2 and 0.4% NPP supplementation had higher laying rate compared with those in other groups. When the diet was supplemented with 0.8% of NPP, the least average egg weight was observed (P < 0.01). Body weight loss, daily feed intake, laying rate, eggshell thickness, and eggshell strength were not affected by dietary P levels (P > 0.05). Although the body weight of squabs at 21-day-old displayed upward trends when dietary P supplementation was increased from 0 to 0.4%, the difference among treatment groups was not significant. According to the oviposition interval and average egg weight, the optimal dietary NPP supplemental level for breeding pigeons was 0.4%. Additionally, the body mass of squabs tended to decrease in the group of 0.8% NPP (P = 0.074).
Table 2.
Effects of dietary P level on performance of adult pigeons and squabs from 7- to 21-day-old.1
| Item | P supplemental level (%) |
SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | 0.8 | Model | Linear | Quadratic | ||
| Body weight loss (g/bird) | 22.7 | 28.8 | 46.2 | 38.9 | 4.29 | 0.352 | 0.217 | 0.315 |
| Feed intake of parent pigeons (g/pair·day) | ||||||||
| From d 1 to 7 of post-hatch | 94.9 | 92.3 | 95.9 | 95.9 | 1.35 | 0.727 | 0.755 | 0.953 |
| From d 7 to 14 of post-hatch | 155.3 | 168.9 | 158.3 | 156.8 | 3.06 | 0.309 | 0.457 | 0.640 |
| From d 14 to 21 of post-hatch | 186.8 | 191.0 | 195.4 | 174.0 | 3.84 | 0.267 | 0.224 | 0.146 |
| From d 21 to 28 of post-hatch | 184.2 | 181.2 | 174.3 | 167.0 | 4.27 | 0.510 | 0.127 | 0.317 |
| From d 1 to 28 of post-hatch | 156.5 | 159.0 | 156.0 | 148.4 | 2.33 | 0.444 | 0.161 | 0.271 |
| Oviposition interval (d) | 39.2a | 37.0ab | 36.7b | 39.1a | 0.45 | 0.045 | 0.716 | 0.018 |
| Laying rate (%) | 3.31 | 4.47 | 4.43 | 3.96 | 0.18 | 0.076 | 0.428 | 0.046 |
| Average egg weight (g) | 22.7a | 22.5a | 23.2a | 21.5b | 0.16 | 0.006 | 0.031 | 0.008 |
| Eggshell thickness (mm) | 0.183 | 0.185 | 0.182 | 0.182 | 0.005 | 0.895 | 0.602 | 0.845 |
| Eggshell strength (kg/cm2) | 1.01 | 1.05 | 1.12 | 1.06 | 0.02 | 0.085 | 0.207 | 0.069 |
| Body mass of squabs (7-day-old, g) | 132.7 | 147.2 | 117.4 | 141.7 | 17.0 | 0.310 | 0.939 | 0.727 |
| Body mass of squabs (14-day-old, g) | 315.1 | 321.5 | 328.6 | 308.5 | 16.4 | 0.673 | 0.685 | 0.478 |
| Body mass of squabs (21-day-old, g) | 380.7 | 380.8 | 461.7 | 395.1 | 31.1 | 0.093 | 0.451 | 0.193 |
1Each value represents the mean of 8 replicates.
Means within the same row lacking a common superscript differ (P < 0.05).
Serum Parameters
The serum Ca, P, and ALP of parent pigeons during the experimental period are presented in Table 3. Parental sex had significant effects (P < 0.01) on serum P, Ca, and ALP on d 7 and d 14 (Table 3) of rearing period. Female birds had much higher (P < 0.01) Ca, P, and ALP in serum than male ones. However, the serum parameters of breeding pigeons were not significantly influenced by sex on d 21 (Table 3) except serum Ca.
Table 3.
Effects of sex and dietary P level on serum Ca, P, and ALP of breeding pigeons during squab-rearing period.1
| D 7 post-hatch | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex |
P supplemental level (%) |
P-value |
|||||||||||
| Item | Male | Female | SEM | 0 | 0.2 | 0.4 | 0.8 | SEM | Sex | P level | Sex × P | ||
| Model | Linear | Quadratic | |||||||||||
| Ca (mmol/L) | 1.895b | 5.55a | 0.45 | 3.298 | 3.650 | 4.153 | 3.791 | 0.62 | 0.00 | 0.805 | 0.987 | 0.683 | 0.701 |
| P (mmol/L) | 2.515b | 3.658a | 0.16 | 2.808 | 2.943 | 3.205 | 3.391 | 0.22 | 0.00 | 0.293 | 0.288 | 0.507 | 0.156 |
| ALP (U/L) | 291.3b | 556.6a | 37.7 | 387.0b | 348.5b | 551.8a | 408.6b | 51.2 | 0.00 | 0.05 | 0.721 | 0.435 | 0.029 |
| D 14 post-hatch | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex |
P supplemental level (%) |
P-value |
|||||||||||
| Male | Female | SEM | 0 | 0.2 | 0.4 | 0.8 | SEM | Sex | P level | Sex × P | |||
| Model | Linear | Quadratic | |||||||||||
| Ca (mmol/L) | 1.786b | 4.593a | 0.223 | 2.497b | 3.946a | 3.218ab | 3.097b | 0.31 | 0.00 | 0.021 | 0.917 | 0.663 | 0.006 |
| P (mmol/L) | 2.206b | 3.060a | 0.186 | 1.531b | 2.780a | 2.916a | 3.305a | 0.26 | 0.004 | 0.001 | 0.003 | 0.003 | 0.425 |
| ALP (U/L) | 268.8b | 504.6a | 31.7 | 431.2 | 403.7 | 413.9 | 297.9 | 44.8 | 0.00 | 0.212 | 0.164 | 0.339 | 0.736 |
| D 21 post-hatch | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex |
P supplemental level (%) |
P-value |
|||||||||||
| Male | Female | SEM | 0 | 0.2 | 0.4 | 0.8 | SEM | Sex | P level | Sex × P | |||
| Model | Linear | Quadratic | |||||||||||
| Ca (mmol/L) | 1.771b | 2.975a | 0.387 | 2.908 | 1.980 | 2.195 | 2.410 | 0.55 | 0.046 | 0.701 | 0.736 | 0.478 | 0.521 |
| P (mmol/L) | 2.367 | 3.206 | 0.291 | 3.090 | 2.295 | 2.683 | 3.079 | 0.41 | 0.063 | 0.512 | 0.671 | 0.416 | 0.856 |
| ALP (U/L) | 325.1 | 316.6 | 32.2 | 377.6 | 342.5 | 331.7 | 231.7 | 45.5 | 0.860 | 0.210 | 0.028 | 0.083 | 0.680 |
1Each value represents the mean of 8 breeding pigeons, male half and female half, per treatment.
Means within the same row lacking a common superscript differ (P ≤ 0.05).
On d 7 of rearing period, ALP activity in blood serum was significantly affected (P = 0.05) by dietary P level. Dietary P supplemental level of 0.4% appeared to be adequate. The serum ALP in the group of 0.4% supplemental level was the highest (P = 0.05) among the experimental groups (Table 3). The NPP level had linear influences (P < 0.05) on the serum ALP activity of breeding pigeons on d 21 of post-hatching, and the birds fed with 0.8% NPP supplementation had the lowest ALP. On d 14, P concentration in serum was significantly higher (P = 0.001) for birds in P-supplemented groups when compared with the control group. Serum Ca was also significantly influenced (P = 0.021) by the P level. The highest serum Ca was observed in pigeons fed with 0.2% P-supplemented diet. There was no significant (P > 0.05) difference in serum Ca between control group and 0.8% P-supplemented group (Table 3). An interaction between sex and dietary P level for serum ALP on d 7 (P = 0.029) and serum Ca on d 14 (P = 0.006) were observed (Tables 3 and 4).
Table 4.
Effects of dietary P level on tibia bone characteristics of squabs at 7, 14, and 21-d of age.1
| Item | P supplemental level (%) |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | 0.8 | Model | Linear | Quadratic | ||
| D 7 | ||||||||
| Breaking strength (N) | 9.7 | 16.3 | 11.2 | 15.6 | 1.12 | 0.089 | 0.256 | 0.506 |
| Tibia ash (%) | 32.2 | 34.8 | 32.6 | 35.9 | 0.74 | 0.272 | 0.187 | 0.409 |
| Tibia ash P (%) | 16.2b | 18.6a | 16.7ab | 16.2b | 0.36 | 0.048 | 0.494 | 0.286 |
| Tibia ash Ca (%) | 32.3b | 34.5a | 33.2b | 32.6b | 0.26 | 0.007 | 0.700 | 0.079 |
| ALP (U/g protein) | 9,468 | 8,407 | 9,667 | 9,858 | 1,166 | 0.568 | 0.432 | 0.697 |
| D 14 | ||||||||
| Breaking strength (N) | 60.8 | 67.1 | 66.4 | 63.2 | 1.74 | 0.563 | 0.847 | 0.534 |
| Tibia ash (%) | 41.6 | 42.5 | 42.2 | 43.0 | 0.27 | 0.402 | 0.122 | 0.304 |
| Tibia ash P (%) | 17.2 | 17.2 | 17.1 | 17.2 | 0.13 | 0.994 | 0.963 | 0.981 |
| Tibia ash Ca (%) | 35.0 | 35.0 | 35.5 | 35.2 | 0.13 | 0.47 | 0.453 | 0.498 |
| ALP (U/ g protein) | 3,572 | 3,471 | 3,525 | 3,625 | 906 | 0.998 | 0.918 | 0.983 |
| D 21 | ||||||||
| Breaking strength (N) | 68.5a | 79.9ab | 93.1b | 95.1b | 3.10 | 0.003 | 0.001 | 0.001 |
| Tibia ash (%) | 42.2c | 43.9bc | 45.1b | 47.4a | 0.46 | <0.001 | 0.000 | 0.000 |
| Tibia ash P (%) | 17.3 | 17.4 | 17.3 | 17.5 | 0.07 | 0.569 | 0.344 | 0.529 |
| Tibia ash Ca (%) | 36.2 | 36.1 | 36.2 | 36.2 | 0.06 | 0.954 | 0.707 | 0.921 |
| ALP (U/ g protein) | 3,051a | 2,883a | 3,371a | 1,516b | 471 | 0.003 | 0.005 | 0.002 |
1Each value represents the mean of 8 squabs per treatment.
Means within the same row lacking a common superscript differ (P < 0.05).
Bone Characteristics of Squabs
Influences of dietary P level of parent pigeons on tibia breaking strength, tibia ash, ash Ca, ash P, and tibia ALP activity of squabs are summarized in Table 4. At 7-d of age, dietary P supplemental level influenced P and Ca content in tibia ash (P < 0.05) of squabs. Dietary P level did not affect (P > 0.05) all the measured bone indices of squabs at d 14. At d 21 of age, both the birds in the group of 0.4 and 0.8% supplemental P levels had higher tibia breaking strength (P < 0.01) and tibia ash contents (P < 0.001) compared to the ones in the control group. Tibia ash content was the greatest in the group of 0.8% NPP supplementation, while the ALP activity in tibia was the lowest in this group (P = 0.003).
DISCUSSION
Pigeon squabs have been considered profitable sources of poultry meat as they grow more rapidly than broiler and quail with minimum input (Salesa and Janssensa, 2003). Squabs have a high growth rate which is attributed to the special ‘crop milk’ feeding. Considering that the ‘crop milk’ is produced by parent pigeons and regurgitated to squabs, fast-growing squabs might be susceptible to the P deficiency in diet of breeding pigeons. This is the first study on the role of P on the production performance of breeding pigeons and bone development of squabs. Results from the current study showed that both dietary P deficiency and inclusion beyond bird requirement negatively affected pigeon performance.
Dietary treatments that were supplemented with 0.40% NPP were able to support optimum production performance, such as shorter oviposition interval, higher laying rate and heavier egg weight, of breeding pigeons when compared with birds receiving 0.8% supplementary P. However no differences in the above measured indices were observed between the two groups supplemented with 0.20 and 0.40% P. It is implied that the appropriate dietary P supplemental level for breeding pigeons at rearing period might be between 0.20 and 0.40% NPP. The production depression in pigeons due to dietary high (0.80%) NPP supplementation was first reported in the present study. It has been reported that the final BW, ADG, and ADFI were significantly decreased in goslings fed the diet supplemented with 0.80% NPP at the age from 1 to 21 d of age (Zhu et al., 2018b). The imbalance Ca: NPP ratio was able to form insoluble complexes and then lowered the utilization of mineral and energy (Plumstead et al., 2008). However, no negative effects on growth were observed in broilers fed the diets with NPP of 0.88% (Orban et al., 1990) and 1.15% (Bailey et al., 1986) at the starter period. The above inconsistent results suggested that both pigeons and geese might have less tolerance for dietary NPP excess than broilers. The NPP requirement for pigeons has not been reported before. In the present trial, body weight loss and daily feed intake of parent birds, eggshell thickness, eggshell strength, and body mass of squabs were not affected by dietary P levels. The results showed that breeding pigeons were not susceptible to P deficiency in diet, which was in agreement with the low tolerance for dietary P excess.
In the practical production, an over-supplementation of Ca, mostly as limestone or shell meal, is widely adopted in grit meal for pigeons, while the P addition in grit is usually scarce. A total of 11 commercial products of grit meal have been analyzed in our lab. The contents of Ca in these samples were high with the highest value being 29.4%. The P contents were severely low in most of the products, ranging between 0.02 and 1.96%. Therefore, the ratios of Ca to P in these grit products were more than 100, indicating the serious lack of P in grit of pigeons (Zhang et al., 2021). Although grains, the major component of pigeon diets, can offer P to birds, most of P is bound as phytate and is considered not biologically available. In addition, the Ca: P ration between 2: 1 and 1: 1 is generally acceptable for poultry (Driver et al., 2005; Rao et al., 2006). So it is interesting that no deleterious effects of low dietary P have been reported in pigeon production. Two reasons might explain this phenomenon. The contents of Fe in the above grit samples were as high as 1.28 ± 0.7% (Zhang et al., 2021), indicating that one reason might be the antagonism of high Fe concentration with Ca utilization. Another reason might be the ability of pigeon to obtain P from the degradation of dietary phytate by diverse microbial communities in the crop, which had the highest microbial diversity among GI tract (Ji et al., 2020). Zeller et al. (2016) reported a synergistic effect of intrinsic and supplied phytase in the crop of broilers. Additionally, the intestinal- and dietary-derived endogenous phytase activity has also been shown to be responsible for phytate-P hydrolysis in broilers (Morgan et al., 2015). Further studies are needed to investigate the above hypotheses.
Blood Ca and P concentrations within a narrow physiological range are sensitive to reflect the body Ca and P metabolism (Veum, 2010). In the current study, sex has significant effects on serum P, Ca, and ALP activity of parent pigeons at earlier time points of rearing period (d 7 and 14). The serum parameters were much higher in female pigeons than those of male birds, indicating that both P and Ca metabolism of female fowl were more active than male ones. The effect of sex on the blood P contents of pigeons has been reported in a previous study (Zheng et al., 2020). This result might be due to the egg production characteristics of female pigeons. Interestingly, Xie et al. (2017) reported the weight loss of male parent pigeons during chick rearing was significantly higher than that of female parent pigeons. This phenomenon was also observed in the present trial (data not included). Researchers suggested that male parental care makes a more important contribution in rearing the young, especially during periods when it is difficult for the female to do so alone (Johnson et al., 1992). However, the relative contribution and mechanism of male and female parent pigeons in brooding and rearing need more research. There was no significant effect of sex on serum P contents in pigeons on d 21 of post-hatching. While the serum Ca on d 21 was significantly influenced by sex. The changing pattern of serum indices may be related with the laying cycle of female pigeons. Typically, pigeons have a 2-egg clutch and low egg yield because of long interclutch period (Ren et al., 2021). Female pigeons normally lay eggs of next clutch during the earlier time of rearing period. This might explain the sexual effect on serum P, Ca, and ALP activity at earlier time points of rearing period.
The activity of ALP is involved in the process of Ca and P deposition, and closely related to the rate of skeletal mineralization of birds (Tilgar et al., 2008). In the present study it was found that serum ALP activity of adult birds was significantly decreased as dietary NPP supplemental level increased from 0.4 to 0.8% on d 7 of rearing period, and the birds fed with 0.8% NPP supplementation had the lowest serum ALP activity on d 21 of post-hatching. The results were in agreement with the previous study of broilers (Liu et al., 2017) and geese (Zhu et al., 2018b). It is suggested that dietary higher P levels have inhibitory effect on the transfer of P from bone storage pools to blood (Marks et al., 2010). The decline of serum ALP was also observed on d 14 after hatching when the dietary NPP addition level increased to 0.80%. However, the differences were not significant. Pigeons fed diet added with 0.40% NPP presented the highest serum ALP among all the treatments on d 7 of post-hatching. This indicated that the dietary 0.40% NPP supplemental level might be sufficient for breeding pigeons.
The result of this experiment showed that serum P concentration was significantly increased in the groups with NPP supplementation compared to the control group. Wang et al. (2018) reported that serum P level of chickens was increased in 0.80% NPP addition group while decreased in 0.15% addition group, compared to 0.40% one. Subsequently, a low serum P concentration leads to the activation of osteoclasts that, in turn, leads to increased bone resorption for maintaining a normal serum P level and simultaneously increasing serum Ca level (Proszkowiec-Weglarz and Angel, 2013). Broilers fed with the P-deficient diet with adequate Ca had lower serum P level and higher serum Ca level (Li et al., 2020). While the serum Ca concentrations in the present trial were also lower in the P-deficient control group than those of 0.20% NPP addition group at d 14 after hatching. However both the P and Ca contents in serum of birds in the P-deficient control group increased to the normal level at d 21 after hatching, which is in agreement with the reports on broilers (Proszkowiec-Weglarz and Angel, 2013; Li et al., 2020). In this study, dietary Ca content was kept at 1.40% across all treatments with varying NPP content resulting in different dietary Ca and P ratios. Pigeons that were fed the diets supplemented with 0.20 and 0.40% NPP (Ca: NPP ratio of 7 and 3.5, respectively), presented higher serum Ca contents and better performance in this experiment. As suggested previously, a 2:1 Ca-to-NPP ratio is recommended for broilers while a wider Ca: NPP ratio can decrease nutrient retention (Rao et al., 2006). The disparity in balanced Ca: NPP ratio between broilers and pigeons may be due to many differences in physiological characteristics. Therefore, it is necessary to study not only the P requirements but also the appropriate Ca and P ratio for pigeons in the future.
The ALP enzyme, which is localized in the plasma membrane of osteoblasts before extracellular release, correlates with bone reabsorption and the release of minerals (Golub and Boesze-Battaglia, 2007). The ALP activity can be used as a general indicator of skeletal development in vertebrate animals, and bone ALP activity predicted the rate of mineralization of leg (Tilgar et al., 2008). An increase in ALP activity is usually associated with poor bone mineralization (Saraç and Saygıli, 2008). The present study found that tibia ALP activity decreased as dietary supplemental NPP increased to 0.8%. The data of the present study is in agreement with the result of broilers. It has been reported that dietary P deficiency impaired the bone development of broilers by increasing tibia ALP activity but decreasing tibia ash P contents (Li et al., 2020). The tibia strength increased with decreasing tibia ALP activity in the squabs at d 21 of age. Results from the present study indicated that the 0.8% of NPP supplementation in the diet of breeding pigeons promoted the bone mineralization of squabs, but reduced the performance of parent pigeons. Previous studies have also suggested that the NPP requirement should be higher for maximal bone mineralization than for growth, and meeting the P needs for maximum bone characteristics provide sufficient P to maximize BW of broilers (Yan et al., 2006; Persia and Saylor, 2007; Liu et al., 2017). In the current study, although the 0.8% of NPP supplemental level in the diet of parent pigeons has a positive influence on bone mineralization of squabs, the body mass tended to decrease indicating poor growth of squabs. The disparity in poor performance and increased bone mineralization of squabs in the group of 0.8% P addition might also indicate the low tolerance of pigeons for dietary P excess. Exact reasons remain to be further studied.
About 80% of P was stored in the skeleton as hydroxyapatite (Proszkowiec-Weglarz and Angel, 2013). In the present trial, fat was not extracted from the bones prior to ash analysis. Studies have confirmed that bone ash results were reliable and comparable regardless of fat extraction (Garcia and Dale, 2006; Yan et al., 2006). Many studies have demonstrated that dietary P plays a critical role in the bone development of broilers. It was found that P deficiency could cause poor mineralization of broilers from 1 to 21 d of age, especially impaired the bone-breaking strength and bone ash content, which were regarded as good indices to reflect overall skeletal health (Bradbury et al., 2014; Liu et al., 2017). Tibia ash percentage has been the traditional criterion to measure the degree of bone mineralization in poultry. The results from the current study demonstrated that tibia ash content of squabs at d 21 of age increased with increased NPP level in diets fed to parent birds. Tibia ash contents were maximal in 0.8% group when the squabs were at d 21 of age. Similar reports showed that broilers fed diet with 0.34% NPP had higher tibia ash content than those fed diet with 0.19% NPP from 22 to 42 d of age (Jiang et al., 2016). In addition, squabs in the control group had the lowest bone breaking strength. Dietary P supplementation increased tibia breaking strength of squabs at d 21 of age, which is similar to the result of tibia ash. As expected, dietary P supplementation increased P content in tibia of squabs at d 7 of age. However, the content of tibia P was not affected by dietary NPP level as the age of squabs increased. This result was consistent with the results of Yang et al. (Yang et al., 2020), who reported that dietary NPP had no effect on tibia P content of broilers from 22 to 42 d of age. Our data in the present study suggested that P homeostasis of squabs might develop with age. Dietary NPP supplementation at 0.2% increased tibia ash Ca content of squabs aged 7 d. Our result was agreed with a previous study that dietary P deficiency decreased Ca content in tibia of broilers (Yang et al., 2020). In our trial, dietary NPP supplementation at 0.2% increased not only tibia Ca but also tibia P content of squabs aged 7 d, which was partly disagreement with previous reports that the content of tibia P of broilers was not affected by dietary NPP (Akter et al., 2016; Yang et al., 2020). A research suggested that high P intake negatively affect Ca metabolism and induced the loss of bone mineralization in broilers (Proszkowiec-Weglarz and Angel, 2013). Nonetheless, the negative effect of 0.8% NPP supplementation in the diet of parent pigeons on bone Ca contents of squabs were not observed in our study. The reason might be the adequate Ca supplementation in all treatment groups. Adequate Ca supplementation can prevent the bone loss of rats induced by a high-P diet (Katsumata et al., 2015). In addition, the effects of dietary P levels for breeding pigeons on P absorption of squabs are being studied in our lab.
In conclusion, the P deficiency in the diet of parent pigeons could cause poor mineralization of squabs from 1 to 21 d of age, especially impaired the bone-breaking strength and bone ash content. The 0.8% of NPP supplementation in the diet has a positive influence on bone mineralization of squabs although production depression was observed. Both P and Ca metabolism of female breeding birds were more active than male ones at earlier time points of rearing period. The desirable dietary NPP supplemental level for breeding pigeon was 0.4% according to the performance data in the present trial. The recommended Ca: P ratio for pigeons needs to be studied in the future, which was different from the optimum value for broilers.
ACKNOWLEDGMENTS
This study was financially supported by grants [XMS201906, XMS201705] from Institute of Animal Science and Veterinary, Beijing Academy of Agriculture and Forestry Sciences to Dr. Feng Ji. The technical advice by Prof. Li Sufen (Hebei Normal University of Science and Technology, Hebei, China), Prof. Li Sufen (Hebei Normal University of Science and Technology, Hebei, China), Dr. Liao Xiudong (Chinese Academy of Agricultural Sciences, Beijing, China) and Dr. Liu Songbai (Guangdong Wen's Foodstuffs Group Corporation Ltd, Guangdong, China).
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- Akter M., Graham H., Iji P.A. Response of broiler chickens to different levels of calcium, non-phytate phosphorus and phytase. Br. Poult. Sci. 2016;57:799–809. doi: 10.1080/00071668.2016.1216943. [DOI] [PubMed] [Google Scholar]
- Bailey C.A., Linton S., Brister R., Creger C.R. Effects of graded levels of dietary phosphorus on bone mineralization in the very young poult. Poult. Sci. 1986;65:1018–1020. doi: 10.3382/ps.0651018. [DOI] [PubMed] [Google Scholar]
- Bar A., Shinder D., Yosefi S., Vax E., Plavnik I. Metabolism and requirements for calcium and phosphorus in the fast-growing chicken as affected by age. Br. J. Nutr. 2003;89:51–61. doi: 10.1079/BJN2002757. [DOI] [PubMed] [Google Scholar]
- Berndt T., Kumar R. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology (Bethesda) 2009;24:17–25. doi: 10.1152/physiol.00034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E.J., Wilkinson S.J., Cronin G.M., Thomson P.C., Bedford M.R., Cowieson A.J. Nutritional geometry of calcium and phosphorus nutrition in broiler chicks. Growth performance, skeletal health and intake arrays. Animal. 2014;8:1071–1079. doi: 10.1017/S1751731114001037. [DOI] [PubMed] [Google Scholar]
- Cao S.M., Li T.T., Shao Y.X., Zhang L.Y., Lu L., Zhang R.J., Hou S.S., Luo X.G., Liao X.D. Regulation of bone phosphorus retention and bone development possibly by related hormones and local bone-derived regulators in broiler chicks. J. Anim. Sci. Biotechnol. 2021;12:88. doi: 10.1186/s40104-021-00610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Zhang R., Fu S., Mu C., Tang Q., Bu Z. Effects of different dietary calcium levels on the performance, egg quality, and albumen transparency of laying pigeons. Animlas (Basel) 2019;9:110. doi: 10.3390/ani9030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S.F., Scott K.G., Abelson P. The deposition of radio phosphorus in tissues of growing chicks. Proc. Natl. Acad. Sci. U. S. A. 1937;23:528–533. doi: 10.1073/pnas.23.9.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J.L., Andrews G.S., Miller R., Owen H.G. Comparison of the stannous chloride and vanadate methods for estimation of serum inorganic phosphorus by use of the “SMA 12-60”. Clin. Chem. 1973;19:411–414. [PubMed] [Google Scholar]
- Driver J.P., Pesti G.M., Bakalli R.I., Edwards H.J. Effects of calcium and nonphytate phosphorus concentrations on phytase efficacy in broiler chicks. Poult. Sci. 2005;84:1406–1417. doi: 10.1093/ps/84.9.1406. [DOI] [PubMed] [Google Scholar]
- Garcia A.R., Dale N.M. Foot ash as a means of quantifying bone mineralization in chicks. J. Appl. Poult. Res. 2006;15:103–109. [Google Scholar]
- Gautier A.E., Walk C.L., Dilger R.N. Influence of dietary calcium concentrations and the calcium-to-non-phytate phosphorus ratio on growth performance, bone characteristics, and digestibility in broilers. Poult. Sci. 2017;96:2795–2803. doi: 10.3382/ps/pex096. [DOI] [PubMed] [Google Scholar]
- Golub E.E., Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr. Opin. Ortho. 2007;18:444–448. [Google Scholar]
- Ji F., Zhang D., Shao Y., Yu X., Liu X., Shan D., Wang Z. Changes in the diversity and composition of gut microbiota in pigeon squabs infected with Trichomonas gallinae. Sci. Rep. 2020;10:19978. doi: 10.1038/s41598-020-76821-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Lu L., Li S.F., Wang L., Zhang L.Y., Liu S.B., Luo X.G. An optimal dietary non-phytate phosphorus level of broilers fed a conventional corn-soybean meal diet from 4 to 6 weeks of age. Animal. 2016;10:1626–1634. doi: 10.1017/S1751731116000501. [DOI] [PubMed] [Google Scholar]
- Johnson L.S., Merkle M.S., Kermott L.H. Experimental evidence for importance of male parental care in monogamous house wrens. Auk. 1992;109:662–664. [Google Scholar]
- Kakhki R.A.M., Kiarie E., Heuthorst T., Neijat M., Wornath-Vanhumbeck A. Medullary bone attributes in aged Lohmann LSL-lite layers fed different levels of calcium and top-dressed 25-hydroxy vitamin D3. Can. J. Anim. Sci. 2019;99:138–149. doi: 10.3382/ps/pey446. [DOI] [PubMed] [Google Scholar]
- Katsumata S., Matsuzaki H., Uehara M., Suzuki K. Effects of dietary calcium supplementation on bone metabolism, kidney mineral concentrations, and kidney function in rats fed a high-phosphorus diet. J. Nutr. Sci. Vitaminol. (Tokyo) 2015;61:195–200. doi: 10.3177/jnsv.61.195. [DOI] [PubMed] [Google Scholar]
- Leytem A., Turner B., Thacker P. Phosphorus characterization in feces from broiler chicks fed low-phytate barley diets. J. Sci. Food Agr. 2008;87:1495–1501. [Google Scholar]
- Li X., Zhang D., Bryden W.L. Calcium and phosphorus metabolism and nutrition of poultry: are current diets formulated in excess? Anim. Prod. Sci. 2017;57:2304–2310. [Google Scholar]
- Li T.T., Xing G.Z., Shao Y.X., Zhang L.Y., Li S.F., Lu L., Liu Z.P., Liao X.D., Luo X.G. Dietary calcium or phosphorus deficiency impairs the bone development by regulating related calcium or phosphorus metabolic utilization parameters of broilers. Poult. Sci. 2020;99:3207–3214. doi: 10.1016/j.psj.2020.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.B., Chen D.W., Adeola O. Phosphorus digestibility response of broiler chickens to dietary calcium-to-phosphorus ratios. Poult. Sci. 2013;92:1572–1578. doi: 10.3382/ps.2012-02758. [DOI] [PubMed] [Google Scholar]
- Liu S.B., Liao X.D., Lu L., Li S.F., Wang L., Zhang L.Y., Jiang Y., Luo X.G. Dietary non-phytate phosphorus requirement of broilers fed a conventional corn-soybean meal diet from 1 to 21 d of age. Poult. Sci. 2017;96:151–159. doi: 10.3382/ps/pew212. [DOI] [PubMed] [Google Scholar]
- Marks J., Debnam E.S., Unwin R.J. Phosphate homeostasis and the renal-gastrointestinal axis. Am. J. Physiol. Renal Physiol. 2010;299:F285–F296. doi: 10.1152/ajprenal.00508.2009. [DOI] [PubMed] [Google Scholar]
- Mohammadigheisar M., Shouldice V.L., Torrey S., Widowski T., Kiarie E.G. Research Note: Comparative gastrointestinal, tibia, and plasma attributes in 48-day-old fast- and slow-growing broiler chicken strains. Poult. Sci. 2020;99:3086–3091. doi: 10.1016/j.psj.2020.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N.K., Walk C.L., Bedford M.R., Burton E.J. Contribution of intestinal- and cereal-derived phytase activity on phytate degradation in young broilers. Poult. Sci. 2015;94:1577–1583. doi: 10.3382/ps/pev108. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- National Standards of the People's Republic of China . National Supervision and Inspection Center of Feed Quality; Wuhan, China: 2018. Determination of Calcium in Feeds. GB/T 6436-2018. [Google Scholar]
- Orban J.I., Roland D.S. Response of four broiler strains to dietary phosphorus above and below the requirement when brooded at two temperatures. Poult. Sci. 1990;69:440–445. doi: 10.3382/ps.0690440. [DOI] [PubMed] [Google Scholar]
- Palacios M.C., Haros M., Rosell C.M., Sanz Y. Selection of phytate-degrading human bifidobacteria and application in whole wheat dough fermentation. Food Microbiol. 2008;25:169–176. doi: 10.1016/j.fm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Persia M.E., Saylor W.W. Effects of broiler strain, dietary nonphytate phosphorus, and phytase supplementation on chick performance and tibia ash. J. Appl. Poult. Res. 2007;15:72–81. [Google Scholar]
- Plumstead P.W., Leytem A.B., Maguire R.O., Spears J.W., Kwanyuen P., Brake J. Interaction of calcium and phytate in broiler diets. 1. Effects on apparent prececal digestibility and retention of phosphorus. Poult. Sci. 2008;87:449–458. doi: 10.3382/ps.2007-00231. [DOI] [PubMed] [Google Scholar]
- Proszkowiec-Weglarz M., Angel R. Calcium and phosphorus metabolism in broilers: effect of homeostatic mechanism on calcium and phosphorus digestibility. J. Appl. Poult. Res. 2013;22:609–627. [Google Scholar]
- Rao S.V.R., Pavani P., Raju M., Reddy M.R. Interaction between dietary calcium and non-phytate phosphorus levels on growth, bone mineralization and mineral excretion in commercial broilers. Anim. Feed Sci. Tech. 2006;131:133–148. [Google Scholar]
- Ren Y., Li X., Han G., Wang M., Xi M., Shen J., Li Y., Li C. Dynamic variations in serum amino acid and the related gene expression in liver, ovary, and oviduct of pigeon during one egg-laying cycle. Poult. Sci. 2021;100:101–184. doi: 10.1016/j.psj.2021.101184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Garcia P., Carrillo-Lopez N., Fernandez-Martin J.L., Naves-Diaz M., Ruiz-Torres M.P., Cannata-Andia J.B. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone. 2010;46:121–128. doi: 10.1016/j.bone.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Sales J., Janssens G.P.J. Nutrition of the domestic pigeon (Columba livia domestica) Worlds Poult. Sci. J. 2003;59:221–232. doi: 10.1093/ps/82.9.1457. [DOI] [PubMed] [Google Scholar]
- Salesa J., Janssensa G.P.J. Nutrition of the domestic pigeon (Columba livia domestica) Worlds Poult. Sci. J. 2003;59:221–232. doi: 10.1093/ps/82.9.1457. [DOI] [PubMed] [Google Scholar]
- Saraç F., Saygıli F. Causes of high bone alkaline phosphatase. Biotechnol. Biotec. Eq. 2008;21:194–197. [Google Scholar]
- Selle P.H., Walker A.R., Bryden W.L. Total and phytate-phosphorus contents and phytase activity of Australian-sourced feed ingredients for pigs and poultry. Aust. J. Exp. Agric. 2003;43:475–479. [Google Scholar]
- Tilgar V., Kilgas P., Viitak A., Reynolds S.J. The rate of bone mineralization in birds is directly related to alkaline phosphatase activity. Physiol. Biochem. Zool. 2008;81:106–111. doi: 10.1086/523305. [DOI] [PubMed] [Google Scholar]
- Vashishth A., Ram S., Beniwal V. Cereal phytases and their importance in improvement of micronutrients bioavailability. 3 Biotech. 2017;7:42. doi: 10.1007/s13205-017-0698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veum T.L. In: Pages 94–111 in Phosphorus and Calcium Utilization and Requirements in Farm Animals. Vitti D.M.S.S., Kebreab E., editors. CABI Wallingford and Cambridge; Massachusetts: 2010. Phosphorus and calcium nutrition and metabolism. Pages 94–111 in Phosphorus and Calcium Utilization and Requirements in Farm Animals. [Google Scholar]
- Wang R.M., Zhao J.P., Wang X.J., Jiao H.C., Wu J.M., Lin H. Fibroblast growth factor 23 mRNA expression profile in chickens and its response to dietary phosphorus. Poult. Sci. 2018;97:2258–2266. doi: 10.3382/ps/pey092. [DOI] [PubMed] [Google Scholar]
- Xie P., Wang X.P., Bu Z., Zou X.T. Differential expression of fatty acid transporters and fatty acid synthesis-related genes in crop tissues of male and female pigeons (Columba livia domestica) during incubation and chick rearing. Br. Poult. Sci. 2017;58:594–602. doi: 10.1080/00071668.2017.1357798. [DOI] [PubMed] [Google Scholar]
- Xu H., Dai S., Zhang K., Ding X., Bai S., Wang J., Peng H., Zeng Q. Dietary phosphorus deficiency impaired growth, intestinal digestion and absorption function of meat ducks. Asian-Australas. J. Anim. Sci. 2019:1897–1906. doi: 10.5713/ajas.18.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F., Keen C.A., Zhang K.Y., Waldroup P.W. Comparison of methods to evaluate bone mineralization. J. Appl. Poult. Res. 2006;14:492–498. [Google Scholar]
- Yang Y., Xing G.C., Li S.F., Shao Y.X., Zhang L.Y., Lu L., Luo X.G., Liao X.D. Effect of dietary calcium or phosphorus deficiency on bone development and related calcium or phosphorus metabolic utilization parameters of broilers from 22 to 42 days of age. J. Integr. Agr. 2020;19:2775–2783. [Google Scholar]
- Zeller E., Schollenberger M., Kuhn I., Rodehutscord M. Dietary effects on inositol phosphate breakdown in the crop of broilers. Arch. Anim. Nutr. 2016;70:57–71. doi: 10.1080/1745039X.2015.1112622. [DOI] [PubMed] [Google Scholar]
- Zhang S., Wang X., Ji F., Wang Z., Shao Y., An Y. Contents of main minerals in grit for pigeons and comparison of pretreatment methods for calcium and phosphorus determination in lab. China Food. 2021:79–83. (in Chinese) [Google Scholar]
- Zheng M., Lv Q., Fu X., Shi F. Biochemical reference intervals for homing pigeons in China. Poult. Sci. 2020;99:3463–3468. doi: 10.1016/j.psj.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.W., Wen J., Jiang X.X., Wang W.C., Yang L. High calcium to phosphorus ratio impairs growth and bone mineralization in Pekin ducklings. Poult. Sci. 2018;97:1163–1169. doi: 10.3382/ps/pex401. [DOI] [PubMed] [Google Scholar]
- Zhu Y.W., Wang C.Y., Wen J., Wang W.C., Yang L. Effect of dietary high non-phytate phosphorus level on growth performance and metabolism of calcium and phosphorus in Lion-head geese. Anim. Feed Sci. Tech. 2018:115–121. [Google Scholar]