Abstract
A major barrier to successful pancreatic cancer (PC) treatment is the surrounding stroma, which secretes growth factors/cytokines that promote PC progression. Wnt and tenascin C (TnC) are key ligands secreted by stromal pancreatic stellate cells (PSCs) that then act on PC cells in a paracrine manner to activate the oncogenic β-catenin and YAP/TAZ signaling pathways. Therefore, therapies targeting oncogenic Wnt/TnC cross talk between PC cells and PSCs constitute a promising new therapeutic approach for PC treatment. The metastasis suppressor N-myc downstream-regulated gene-1 (NDRG1) inhibits tumor progression and metastasis in numerous cancers, including PC. We demonstrate herein that targeting NDRG1 using the clinically trialed anticancer agent di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC) inhibited Wnt/TnC-mediated interactions between PC cells and the surrounding PSCs. Mechanistically, NDRG1 and DpC markedly inhibit secretion of Wnt3a and TnC by PSCs, while also attenuating Wnt/β-catenin and YAP/TAZ activation and downstream signaling in PC cells. This antioncogenic activity was mediated by direct inhibition of β-catenin and YAP/TAZ nuclear localization and by increasing the Wnt inhibitor, DKK1. Expression of NDRG1 also inhibited transforming growth factor (TGF)-β secretion by PC cells, a key mechanism by which PC cells activate PSCs. Using an in vivo orthotopic PC mouse model, we show DpC downregulated β-catenin, TnC, and YAP/TAZ, while potently increasing NDRG1 expression in PC tumors. We conclude that NDRG1 and DpC inhibit Wnt/TnC-mediated interactions between PC cells and PSCs. These results further illuminate the antioncogenic mechanism of NDRG1 and the potential of targeting this metastasis suppressor to overcome the oncogenic effects of the PC-PSC interaction.
Keywords: pancreatic cancer, NDRG1, Wnt, TnC, tumor microenvironment
Abbreviations: AnxA2, annexin A2; DKK, Dickkopf; DpC, di (2-pyridyl) ketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone; Dp44mT, di(2-pyridyl) ketone 4,4-dimethyl-3-thiosemicarbazone; IHC, immunohistochemistry; NDRG1, N-myc downstream regulated gene 1; PC, pancreatic cancer; PSC, pancreatic stellate cells; TAZ, transcriptional coactivator with PDZ-binding motif; TnC, tenascin C; YAP, yes-associated protein
Pancreatic cancer (PC) is one of the deadliest tumors and is predicted to become the second leading cause of cancer mortality in Western countries by 2030 (1). Sadly, there has been little improvement in PC outcomes for 50 years, with a 5-year survival rate of 10% (1). PC treatment is challenged by disease heterogeneity, late presentation, and pronounced drug resistance (1). The development of innovative therapies is critical, and this can only be achieved through an intensive understanding of the cellular and molecular mechanisms involved in the oncogenesis of this tumor.
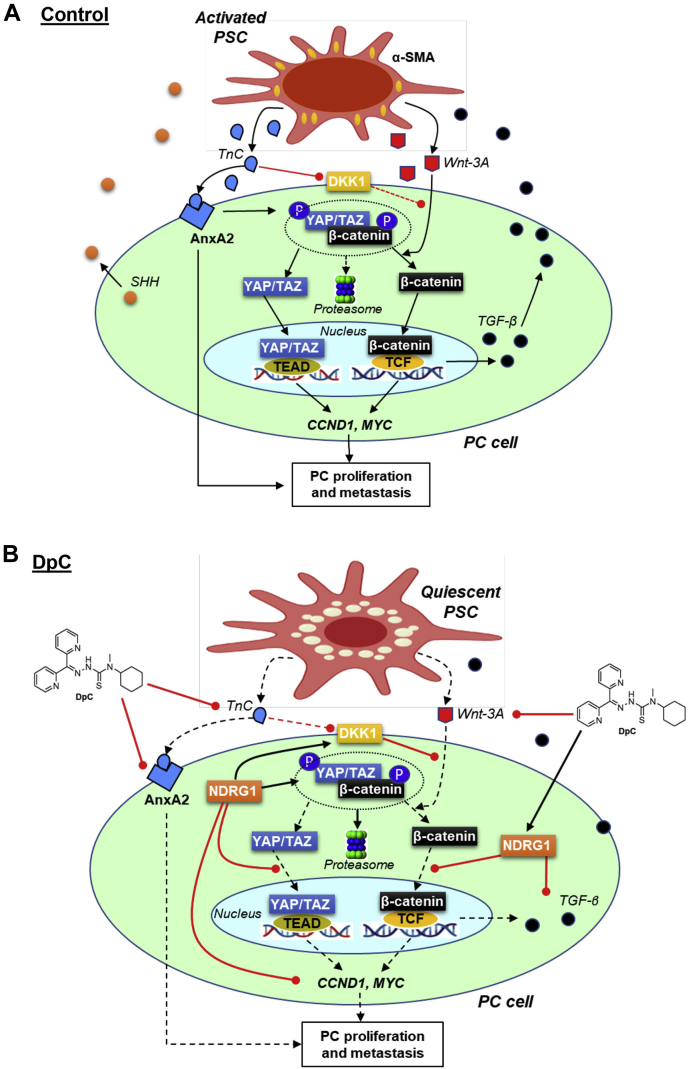
PC is characterized by abundant stroma, including cancer-associated fibroblasts, immune cells, endothelial cells, and the extracellular matrix (2, 3, 4). Cancer-associated fibroblasts are primarily responsible for the deposition of the extracellular collagen and matrix, which leads to fibrous desmoplasia that harbors tumor-promoting properties (5, 6). This desmoplastic reaction mediates drug resistance, immune escape, tumor progression, invasion, and metastasis of PC (7, 8). The major precursors for cancer-associated fibroblasts in PC are the pancreatic stellate cells (PSCs), which are activated by PC cells and transform from a quiescent phenotype to an active myofibroblast phenotype that produces a plethora of tumor-promoting ligands and extracellular matrix proteins (3, 9, 10) (Fig. 1A).
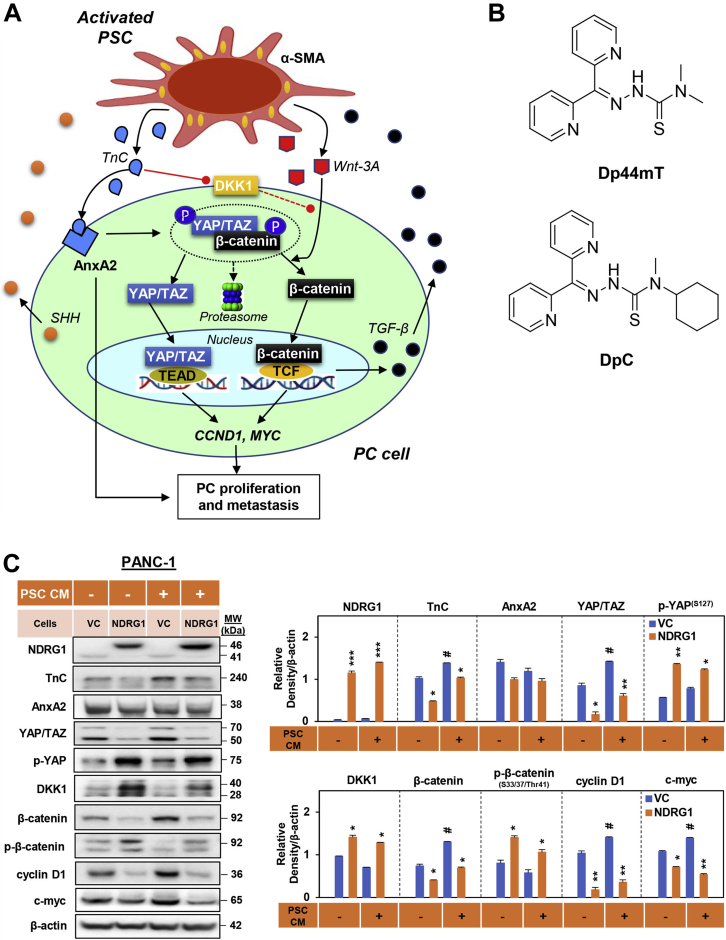
Figure 1.
NDRG1 expression reduced TnC, YAP/TAZ, β-catenin, cyclin D1,and c-myc in PANC-1 cells.A, schematic of the oncogenic signaling pathways involved in pancreatic cancer cell (PC) and pancreatic stellate cell (PSC) bidirectional oncogenic signaling. Activated PSCs induce β-catenin and YAP/TAZ signaling in PC cells. Activation of PSCs results in increased TnC secretion, which acts on PC cells to activate AnxA2 and promote PC proliferation and metastasis. In addition, TnC downregulates DKK1 promoter activity to decrease the expression of this Wnt antagonist. This leads to dissociation of the cytoplasmic β-catenin destruction complex, enabling activation of both β-catenin and YAP/TAZ signaling. Once released from the destruction complex, β-catenin and YAP/TAZ translocate to the nucleus inducing CCND1 and MYC expression, which promotes PC progression. PC cells activate PSCs by secreting sonic hedgehog (SHH) (31, 32, 33) and transforming growth factor-β (TGF-β) that promotes oncogenic activation of PSCs, enabling increased production of TnC and ligands including Wnt-3A. These interactions are part of the oncogenic, bidirectional cross talk between PC and PSC cells. B, line drawings of the structures of Dp44mT and DpC. C, Western blot analysis of PANC-1 pancreatic cancer cells examining the effect of NDRG1 overexpression versus the vector control (VC) on the levels of NDRG1, TnC, AnxA2, YAP/TAZ, p-YAP, DKK1, β-catenin, p-β-catenin, cyclin D1, and c-myc after a 24 h incubation in the presence or absence of PSC-conditioned medium (PSC CM). β-actin was used as a protein-loading control. Blots are representative of three independent experiments. Densitometry results are mean ± SD (n = 3 experiments). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 denote statistical significance comparing NDRG1 overexpression to the respective VC. #p < 0.05 comparing PSC CM treatment with the respective control medium-treated cells.
One of the key stromal factors contributing to PC progression is the tenascin C (TnC) extracellular matrix glycoprotein, which is secreted by activated PSCs and binds to its receptor, AnxA2, on PC cells (Fig. 1A). This process promotes a more mesenchymal, stem cell-like phenotype in PC cells, as well as their proliferation, angiogenesis, and metastasis (11, 12, 13, 14, 15). TnC is a major component of the cancer-specific matrix, and its expression is linked to poor prognosis in several cancers, including PC (14, 16). The oncogenic effects of TnC have also been attributed to its ability to potently activate oncogenic Wnt/β-catenin and YAP/TAZ signaling in PC cells (15, 17, 18, 19, 20, 21) (Fig. 1A). The ability of TnC to activate these latter signaling pathways was demonstrated to occur via the TnC-mediated inhibition of DKK1, which is an inhibitor of Wnt signaling (14, 22).
A major effector of Wnt signaling is β-catenin, which can be found bound to E-cadherin at the adherens junction or sequestered in the cytoplasm by the destruction complex (23, 24, 25, 26). This complex facilitates β-catenin proteasomal degradation and is composed of multiple proteins, including adenomatous polyposis coli (APC), Axin, GSK3, and YAP/TAZ (23, 24, 25, 26) (Fig. 1A). Upon binding of Wnt ligands to lipoprotein receptor-related protein 6 (LRP6) and frizzled, YAP/TAZ is physically dislodged from the destruction complex, leading to the release of β-catenin and its nuclear translocation (27, 28, 29). Once in the nucleus, β-catenin binds to TCF/Lef transcription factors to regulate the transcription of various genes (e.g., CCND1, MYC) that promote PC proliferation and progression (Fig. 1A) (27, 28).
Upon its release from the destruction complex, YAP/TAZ also accumulates in the nucleus, where it can promote oncogenesis via binding to TEA domain transcription factor 1 (TEAD) to promote transcription of genes including CCND1 and MYC (27, 28, 29) (Fig. 1A). Hence, in the presence of TnC and Wnt ligands, both β-catenin and YAP/TAZ escape degradation and accumulate in the nucleus to promote PC progression (27, 28). Targeting the regulatory mechanisms that mediate TnC, Wnt/β-catenin, and YAP/TAZ cross talk between PC cells and PSCs could be a potential therapeutic approach to inhibit stromal-mediated PC proliferation and metastasis (30).
Notably, PC cells themselves can activate PSCs by secreting sonic hedgehog (SHH) (31, 32, 33) and transforming growth factor-β (TGF-β) (34) (Fig. 1A). Both SHH and TGF-β promote the oncogenic activation of PSCs, enabling increased production of extracellular matrix proteins, such as TnC and ligands such as Wnt-3A (12, 13, 35). This mechanism forms the basis of the oncogenic, bidirectional cross talk between PC cells and PSCs. It is vital to disrupt such oncogenic intercellular communication to overcome PC-mediated desmoplasia, particularly the formation of a collagen and matrix “shell” that protects tumors from chemotherapy and leads to cancer progression (36).
The metastasis suppressor, N-myc downstream-regulated gene-1 (NDRG1), was demonstrated by multiple laboratories to inhibit tumor growth, proliferation, angiogenesis, and metastasis in numerous cancers, including PC (37, 38, 39, 40, 41, 42). As part of its mechanism of action, NDRG1 inhibits the epithelial to mesenchymal transition of cancer cells by promoting β-catenin expression at the plasma membrane (to facilitate adherens complex formation) and decreasing both the nuclear expression and activation of β-catenin (43, 44, 45, 46, 47). Significantly, NDRG1 expression can also decrease SHH production by PC cells, leading to decreased PSC activation (48). As such, NDRG1 is a promising therapeutic target for PC treatment.
The expression of NDRG1 can be potently upregulated by a novel class of thiosemicarbazone anticancer agents, including di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT; Fig. 1B), and di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC; Fig. 1B) (39, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56). These agents are metal-binding ligands that demonstrate marked antitumor and antimetastatic activity in vitro and in vivo (39, 40, 46, 49, 50, 51, 52, 53, 54). Both Dp44mT and DpC inhibit multiple oncogenic signaling pathways through the upregulation of NDRG1 that is mediated via hypoxia-inducible factor-1α-dependent and -independent mechanisms (39, 40, 41, 45, 55, 56, 57). Dp44mT was also demonstrated to decrease the expression of Wnt target genes, which was shown to occur via β-catenin degradation (58). Further, Dp44mT and DpC induce cytotoxicity through their ability to form redox-active complexes when bound to iron or copper, leading to reactive oxygen species (ROS) generation (51, 59, 60). In fact, the second-generation compound, DpC, was shown to be highly potent and selective against a pathologically relevant orthotopic PC xenograft in vivo, being more effective than the standard PC chemotherapeutic, Gemcitabine (Gem) (48). Of interest, DpC entered a multicenter Phase I clinical trial (NCT02688101) for the treatment of advanced and resistant cancers in 2016 (61), which has now been completed.
The current study mechanistically dissects the effect of NDRG1, as well as the NDRG1-inducing agents, Dp44mT and DpC, on TnC-mediated Wnt/β-catenin and YAP/TAZ signaling pathways in the cross talk between PC cells and PSCs in vitro and in vivo. We demonstrate for the first time that NDRG1 and DpC potently inhibit Wnt/β-catenin and YAP/TAZ levels and their downstream signaling, while also attenuating TnC production in both PC cells and PSCs. These effects were accompanied by reduced PSC activation that could be mediated by decreased TGF-β synthesis by PC cells. Importantly, using a clinically relevant in vivo orthotopic PC mouse model, we further demonstrated that DpC downregulates TnC, β-catenin, and YAP/TAZ, while potently increasing NDRG1 expression in PC tumors. These results highlight the exciting potential of targeting NDRG1 to overcome the oncogenic and prometastatic effects of the PC–stroma interaction to result in an innovative therapy that demonstrates a multifaceted mechanism of action.
Results
NDRG1 overexpression inhibits PSC-mediated β-catenin signaling in PC cells
As oncogenic effectors, both β-catenin and YAP/TAZ are highly expressed in PC (62, 63, 64, 65, 66, 67, 68) and promote PC progression in response to Wnt signaling (63, 69). Further, TnC relieves the inhibitory effect of DKK1 on WNT signaling, enhancing its activation (14). This response leads to the dissociation of β-catenin from the destruction complex and the nuclear translocation of both β-catenin and YAP/TAZ (Fig. 1A) (27). To mechanistically dissect how NDRG1 affects the expression of these molecules in PC, we examined the effect of NDRG1 expression on TnC, its receptor AnxA2, β-catenin, YAP/TAZ and their downstream targets, cyclin D1, and c-myc, as well as the WNT inhibitor, DKK1. These studies using the human PANC-1, MIAPaCa-2, and AsPC-1 PC cell types were performed in the absence or presence of conditioned medium from primary cultures of human PSCs (PSC CM).
The PANC-1 cells were stably transfected to overexpress NDRG1 and compared with cells transfected with the empty vector, i.e., vector control (VC) cells (Fig. 1C). Notably, incubation of PANC-1 VC cells with PSC CM significantly increased the expression of TnC, YAP/TAZ, β-catenin, cyclin D1, and c-myc relative to these cells not treated with PSC CM (Fig. 1C). These observations demonstrated the oncogenic activity of PSC CM on PC cells. The overexpression of NDRG1 in PANC-1 cells significantly decreased TnC, YAP/TAZ, β-catenin, cyclin D1, and c-myc levels in PANC-1 cells in the absence and presence of PSC CM (Fig. 1C). Further, the phosphorylation of YAP (S127) and β-catenin (S33/37/Thr41) that inhibits their oncogenic activity (27, 28, 70, 71, 72), as well as the levels of the WNT antagonist, DKK1 (14, 22), was significantly increased by NDRG1 overexpression in the absence and presence of PSC CM (Fig. 1C). Notably, NDRG1 expression had no significant effect on AnxA2 levels in these cells.
These studies above were further confirmed using another PC cell type, namely MIAPaCa-2 PC cells stably transfected to overexpress NDRG1, with this metastasis suppressor significantly decreasing TnC, YAP/TAZ, β-catenin, cyclin D1, and c-myc in the absence and presence of PSC-CM (Fig. S1A). In addition to the NDRG1 overexpression studies, the effect of silencing NDRG1 was also examined using the AsPC-1 cells. Silencing NDRG1 in AsPC-1 PC cells using siNDRG1 led to a marked and significant decrease of NDRG1 expression compared with the negative control siRNA (siControl;Fig. S1B). This decrease in NDRG1 expression led to a significant increase in YAP/TAZ, cyclin D1, and c-myc in the absence or presence of PSC CM in AsPC-1 cells, with β-catenin being significantly increased in the absence of PSC CM only (Fig. S1B). Notably, NDRG1 silencing significantly decreased DKK1 and the inhibitory phosphorylation of β-catenin, namely p-β-catenin(S33/37/Thr41), in AsPC-1 cells, particularly in the presence of PSC CM (Fig. S1B). On the other hand, the inhibitory phosphorylation of p-YAP(S127) was significantly reduced by NDRG1 silencing only in the presence of PSC CM (Fig. S1B). These studies demonstrated that silencing NDRG1 in AsPC-1 cells generally had the opposite effect to NDRG1 overexpression in PANC-1 (Fig. 1C) and MIAPaCa-2 cells (Fig. S1A).
Collectively, these studies indicate that NDRG1 expression inhibits the oncogenic effects of PSC CM on PC cells, leading to inhibition of β-catenin and YAP/TAZ signaling.
NDRG1 overexpression decreases both cytoplasmic and nuclear levels of β-catenin and YAP/TAZ in PC cells
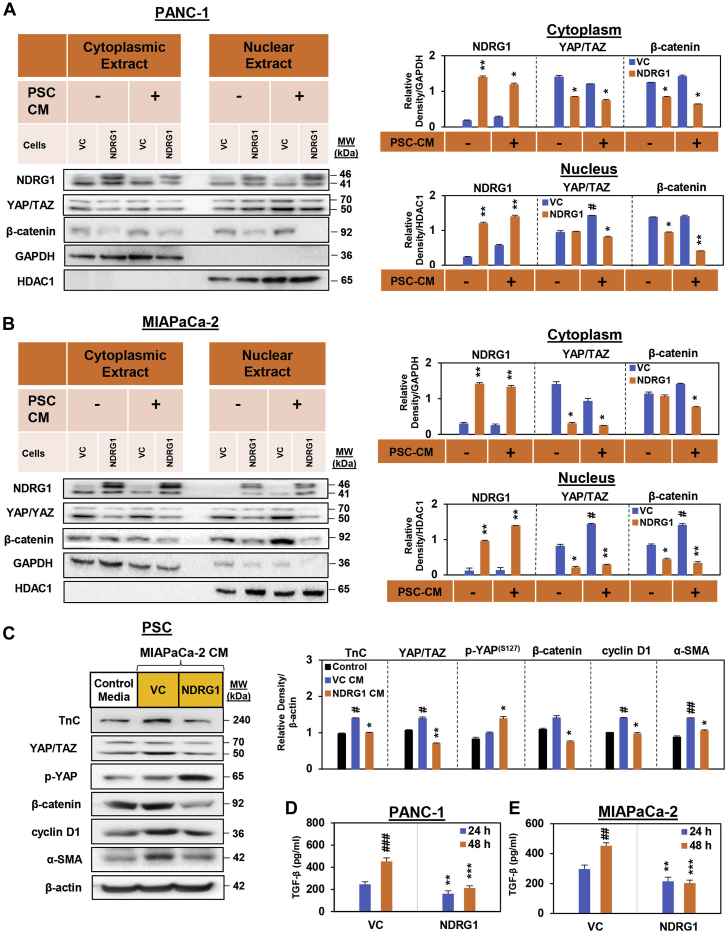
Nuclear translocation of β-catenin and YAP/TAZ is crucial for their ability to promote PC proliferation and metastasis (Fig. 1A) (27). Considering that NDRG1 expression decreased expression of β-catenin, YAP/TAZ, and their downstream targets (Figs. 1C and S1A), it was also important to assess the cellular localization of these proteins in response to NDRG1 overexpression (Fig. 2, A and B). To examine the potential effect of NDRG1 expression on the intracellular localization of β-catenin and YAP/TAZ, PANC-1 and MIAPaCa-2 cells were stably transfected to overexpress NDRG1 and incubated with or without PSC CM for 24 h (Fig. 2, A and B). The cytoplasmic and nuclear proteins were then extracted and assessed for NDRG1, β-catenin, and YAP/TAZ expression via Western blot. NDRG1 was detected in both cytoplasmic and nuclear fractions, with generally higher overall cytoplasmic expression relative to nuclear levels in PANC-1 (Fig. 2A) and MIAPaCa-2 cells (Fig. 2B). Notably, incubation with PSC CM did not significantly affect NDRG1 expression in either cell type. Examining YAP/TAZ, its cytoplasmic levels were significantly reduced by NDRG1 overexpression in both PANC-1 and MIAPaCa-2 cells when compared with the relevant VC cells, regardless of PSC CM. Notably, PSC CM induced a significant increase in nuclear YAP/TAZ levels in both PANC-1 and MIAPaCa-2 cells, with this effect being significantly reduced upon NDRG1 overexpression (Fig. 2, A and B).
Figure 2.
NDRG1 attenuates PSC-mediated nuclear localization of YAP/TAZ and β-catenin in PC cells. NDRG1 expression in (A) PANC-1 cells and (B) MIAPaCa-2 pancreatic cancer (PC) cells decreases both cytoplasmic and nuclear levels of β-catenin and YAP/TAZ in the presence of pancreatic stellate cell-conditioned medium (PSC CM). Western blot analysis of (A) PANC-1 and (B) MIAPaCa-2 pancreatic cancer cells examining the effect of NDRG1 overexpression versus the vector control (VC) on cytoplasmic and nuclear levels of NDRG1, YAP/TAZ, and β-catenin after a 24 h incubation in the presence or absence of PSC-conditioned medium (PSC CM). GAPDH and HDAC1 were loading controls for the cytoplasmic and nuclear extracts, respectively. Blots are representative of three independent experiments. Densitometry results are mean ± SD (n = 3). ∗p < 0.05; ∗∗p < 0.01 denote statistical significance comparing NDRG1 overexpression with the respective VC. #p < 0.05 denotes statistical significance comparing PSC CM treatment with control medium alone. C, Western blot analysis of PSCs incubated with either control medium or conditioned medium from MIAPaCa-2 cells (MIAPaCa-2 CM) overexpressing NDRG1 or the VC and then assessed for TnC, YAP/TAZ, p-YAP, β-catenin, cyclin D1, or α-SMA expression. β-actin was used as a protein-loading control. Blots are representative of three independent experiments. Densitometry results are mean ± SD (n = 3). ∗p < 0.05; ∗∗p < 0.01 denotes statistical significance comparing NDRG1 CM-treated PSCs with the respective VC CM-treated PSCs. #p < 0.05; ##p < 0.01 denotes statistical significance comparing VC CM treatment with the respective control. D and E, TGF-β secretion from PANC-1 (D) and MIAPaCa-2 (E) cells into the overlying medium was examined via ELISA after a 24 or 48 h incubation. Results are mean ± SD (n = 3). ∗∗p < 0.01; ∗∗∗p < 0.001 denotes statistical significance comparing NDRG1 overexpressing to the respective VC cells. ##p < 0.01; ###p < 0.001 denote statistical significance comparing the 24 to 48 h time point.
Examining β-catenin, NDRG1 overexpression again decreased both cytoplasmic and nuclear β-catenin levels in both PANC-1 cells compared with the relevant VC, although this effect was more potent and significant in the presence of PSC CM (Fig. 2A). A similar, but less pronounced effect of NDRG1 overexpression on β-catenin expression was also observed in MIAPaCa-2 cells, and even though PSC CM induced a significant increase in nuclear β-catenin, this effect was robustly inhibited by NDRG1 overexpression (Fig. 2B). Overall, these findings indicate that NDRG1 expression inhibits the PSC CM-mediated nuclear accumulation of β-catenin and YAP/TAZ.
NDRG1 overexpression inhibits PC-mediated TnC and YAP/TAZ expression in PSCs
Considering that the above studies focused on how PC cells are affected by PSC CM, we next assessed the opposite interaction by examining how PSCs are influenced by PC cells (Fig. 2C). In particular, experiments examined whether NDRG1 levels in PC cells can influence their effect on PSC’s. To achieve this, MIAPaCa-2 NDRG1 overexpressing cells and their counterparts transfected with the VC were incubated with serum-free medium for 24 h/37 °C, with the conditioned medium (CM) then being collected. This CM was then incubated with primary cultures of PSCs for 24 h/37 °C and compared with serum-free medium not incubated with MIAPaCa-2 cells. The PSCs were then examined for the expression of TnC, YAP/TAZ, p-YAP(S127), β-catenin, cyclin D1, as well as the marker of activated PSCs, α-smooth muscle actin (α-SMA) (73) (Fig. 2C).
Incubating PSCs with CM from VC cells significantly increased the expression of TnC, YAP/TAZ, cyclin D1, and α-SMA when compared with PSCs incubated with control medium (Fig. 2C). This observation suggested that factors produced by MIAPaCa-2 cancer cells can promote TnC and YAP/TAZ signaling in PSCs, as well as PSC activation, as demonstrated previously (30, 74). However, when PSCs were incubated with CM from NDRG1 overexpressing cells, these latter effects were reversed, there being a significant decrease in TnC, YAP/TAZ, β-catenin, cyclin D1, and α-SMA expression relative to PSCs incubated with CM from VC cells (Fig. 2C). While inhibitory p-YAP(S127) levels were not significantly altered by CM from VC cells, in contrast, CM from MIAPaCa-2 cells overexpressing NDRG1 resulted in a significant increase in p-YAP(S127) in PSCs (Fig. 2C). This finding suggested that NDRG1 overexpression in MIAPaCa-2 PC cells impairs their ability to promote TnC, YAP/TAZ, and their downstream signaling in PSCs, leading to decreased PSC activation.
To further explore this interesting latter result, we next assessed the effect of NDRG1 expression on the ability of PC cells to secrete TGF-β, as this cytokine plays a significant role in facilitating PSC activation (34, 75, 76, 77). In fact, TGF-β secreted by PC cells has been reported to promote the myofibroblast cancer-associated fibroblast phenotype in PSCs, with this being responsible for extracellular matrix biogenesis and desmoplasia in the PC tumor microenvironment (4, 78, 79). Overexpression of NDRG1 in PANC-1 and MIAPaCa-2 cells led to a significant reduction in TGF-β secretion by these cell types at both the 24 and 48 h time points (Fig. 2, D and E). This observation indicates that NDRG1 overexpression impairs the ability of these PC cells to secrete TGF-β, which may then result in the decreased PSC activation mediated by CM derived from NDRG1 overexpressing MIAPaCa-2 cells (Fig. 2C).
NDRG1 overexpression inhibits Wnt-3A-mediated activation of YAP/TAZ and β-catenin in PC cells
As shown above, NDRG1 overexpression decreased β-catenin and YAP/TAZ expression and its downstream signaling in response to PSC CM in PC cells (Figs. 1C and 2, A and B). PSCs secrete numerous growth factors that influence PC progression, with Wnt-3A being a major cytokine responsible for β-catenin and YAP/TAZ activation (4, 80). Notably, Wnt-3A secreted by activated PSCs acts on cancer cells to promote nuclear expression and the subsequent oncogenic activity of β-catenin and YAP/TAZ (27, 81). Hence, we next examined using confocal immunofluorescence microscopy how NDRG1 overexpression in PANC-1 and MIAPaCa-2 cells affected β-catenin and YAP/TAZ nuclear localization in response to an 8 h incubation with Wnt-3A (20 ng/ml; Fig. 3).
Figure 3.
NDRG1 expression decreases Wnt-3A-mediated β-catenin and YAP/TAZ nuclear expression in PANC-1 and MIAPaCa-2 pancreatic cancer cells. Confocal immunofluorescence microscopy images of (A) PANC-1 cells and (B) MIAPaCa-2 cells examining the effect of NDRG1 overexpression versus the vector control (VC) on β-catenin (red) and YAP/TAZ (red) expression in the absence or presence of Wnt-3A (20 ng/ml/8 h). Nuclei were stained with DAPI (blue). Images were analyzed with ImageJ: (i) colocalization with DAPI; and (ii) fluorescence intensity for β-catenin and YAP/TAZ. Scale bar: 25 μm; Magnification: 63×. Image J analysis utilized 16 to 51 cells over three experiments with results being presented as mean ± SD (n = 3). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 comparing NDRG1 overexpressing cells with the respective VC. #p < 0.05; ##p < 0.01 comparing incubation with Wnt-3A relative with cells incubated with control medium alone.
In the absence of Wnt-3A (i.e., (-) Wnt-3A), NDRG1 overexpression resulted in a significant decrease in β-catenin and YAP/TAZ nuclear colocalization in both PANC-1 (Fig. 3Ai) and MIAPaCa-2 cells (Fig. 3Bi) versus the VC. Further, the overall fluorescence intensity of both these proteins was also significantly decreased in NDRG1 overexpressing cells in the absence of Wnt-3A (Fig. 3, Aii and Bii). This finding indicated that the expression levels of β-catenin and YAP/TAZ were attenuated by NDRG1, as demonstrated by the Western blotting analysis in Figure 1C. Incubation of both PANC-1 VC and MIAPaCa-2 VC cells with Wnt-3A (i.e., (+) Wnt-3A) markedly and significantly increased the nuclear levels of β-catenin and YAP/TAZ relative to the VC without Wnt-3A (Fig. 3, Ai and Bi), which is consistent with the ability of Wnt-3A to activate these proteins (27, 81). However, importantly, NDRG1 overexpression suppressed nuclear translocation, leading to significantly decreased nuclear localization of β-catenin and YAP/TAZ in the presence of Wnt-3A in both cell types versus the VC (Fig. 3, Ai and Bi). Incubation of PANC-1 VC cells with Wnt-3A also significantly increased the overall fluorescence intensity of β-catenin and YAP/TAZ versus the VC incubated without Wnt-3A (Fig. 3Aii). In contrast, examining MIAPaCa-2 VC cells, upon incubation with Wnt3A, there was no significant effect on the overall fluorescence intensity of β-catenin and YAP/TAZ relative to the respective VC cells without Wnt3A (Fig. 3Bii). This effect was probably because of the marked increase in nuclear levels of these proteins, which was compensated by a corresponding pronounced decrease in their cytosolic levels, resulting in no significant overall alteration in fluorescence intensity.
Collectively, these results in Figure 1, Figure 2, Figure 3 demonstrate that NDRG1 expression decreased the nuclear and cytoplasmic levels of β-catenin and YAP/TAZ in response to PSC CM or Wnt-3A in PANC-1 and MIAPaCa-2 PC cells. These data indicate the potent ability of NDRG1 expression to inhibit the WNT/β-catenin/YAP/TAZ bidirectional signaling axis at the PC cell–PSC interface.
DpC and Dp44mT inhibit β-catenin and YAP/TAZ signaling in PC cells
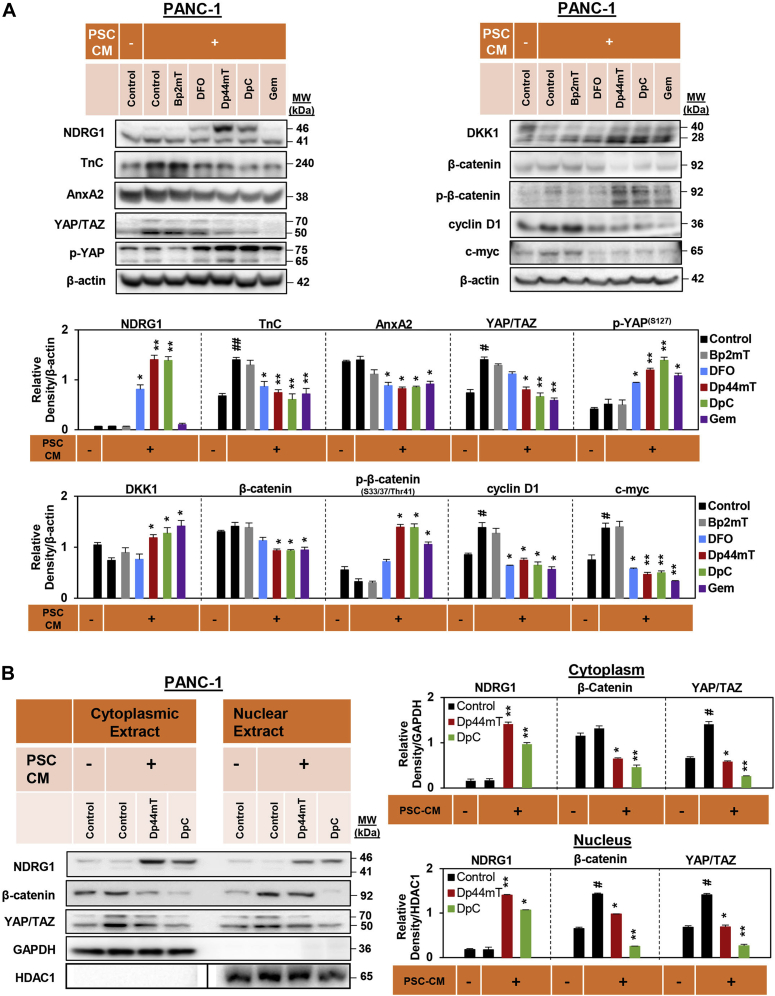
We demonstrate herein that NDRG1 can potently inhibit PSC-mediated activation of oncogenic signaling in PC cells, while also reducing PC-mediated activation of PSCs (Figure 1, Figure 2, Figure 3). These data suggest that NDRG1 is an innovative therapeutic target for PC treatment. To this end, we next examined the novel iron-binding anticancer agents, Dp44mT and DpC, which can potently upregulate NDRG1 expression via hypoxia-inducible factor-1α-dependent and -independent mechanisms (39, 82). We examined the effect of DpC (5 μM) and Dp44mT (5 μM) on β-catenin and YAP/TAZ signaling in the absence or presence of PSC CM using PANC-1, MIAPaCa-2, and AsPC-1 PC cells (Fig. 4A, S2 and S3). These agents were compared with the clinically used iron chelator, DFO (100 μM), as it also binds iron in cancer cells and increases NDRG1 expression, but is not redox-active like Dp44mT and DpC (83, 84, 85). The higher concentration of DFO used in these studies was implemented because of its relatively high hydrophilicity and low membrane permeability, which leads to lower iron chelation efficacy (86, 87). In addition, the activity of Dp44mT and DpC was compared with the negative control compound, Bp2mT (5 μM), which has been specifically designed to have a similar chemical structure to Dp44mT and DpC, but cannot bind metal ions or induce NDRG1 expression (88, 89, 90). The effects of these agents were also compared with the currently used PC therapy, gemcitabine (Gem; 5 μM), in the presence of PSC CM. The ligands DFO, Dp44mT, and DpC significantly upregulated NDRG1 expression in the presence of PSC CM in PANC-1 (Fig. 4A), MIAPaCa-2 (Fig. S2), and AsPC-1 cells (Fig. S3), while Bp2mT and Gem had no significant effect (Figs. 4A, S2 and S3).
Figure 4.
DpC and Dp44mT inhibit pancreatic stellate cell-conditioned medium (PSC CM) induced β-catenin and YAP/TAZ signaling and their nuclear localization in PANC-1 pancreatic cancer cells.A, Western blot analysis of PANC-1 cells incubated for 24 h with control medium, PSC CM, or PSC CM containing either Bp2mT (5 μM), DFO (100 μM), Dp44mT (5 μM), DpC (5 μM), or Gem (5 μM). Blots were examined for NDRG1, TnC, AnxA2, YAP/TAZ, p-YAP, DKK1, β-catenin, p-β-catenin, cyclin D1, and c-myc expression. β-actin was used as the protein-loading control. B, Western blot analysis of PANC-1 cells incubated for 24 h with control medium, PSC CM, or PSC CM containing either Dp44mT or DpC (5 μM). Blots were then assessed for cytoplasmic and nuclear levels of NDRG1, β-catenin, and YAP/TAZ. Both GAPDH and HDAC1 were loading controls for the cytoplasmic and nuclear extract, respectively. For HDAC1, all eight samples were run on the same gel, as for the other blots shown, but without an intervening lane between lanes 4 and 5. This necessitated electronically dividing the blot at this position (shown by black line), so that it aligned with the other blots in (B). Blots are representative of at least three independent experiments. Densitometry results are mean ± SD (n = 3). ∗p < 0.05; ∗∗p < 0.01; comparing each treatment with the PSC CM-treated control. #p < 0.05; ##p < 0.01; comparing the PSC CM-treated control with control medium-treated cells.
Examining PANC-1 cells, PSC CM added to the control significantly upregulated the expression of TnC, YAP/TAZ, cyclin D1, and c-myc relative to control medium without PSC CM (Fig. 4A). This observation demonstrates the oncogenic activity of PSC CM. Importantly, DpC, Dp44mT, and Gem significantly suppressed the ability of PSC CM to upregulate TnC, YAP/TAZ, cyclin D1, and c-myc versus the PSC CM-treated control (Fig. 4A). These agents also significantly decreased AnxA2 and β-catenin levels in the presence of PSC CM relative to the PSC CM-treated control (Fig. 4A). Moreover, Dp44mT, DpC, and Gem significantly increased the levels of antioncogenic p-YAP(S127) and p-β-catenin(S33/37/Thr41) in PSC CM-treated PANC-1 cells versus the PSC CM-treated control (Fig. 4A). Compared with the PSC CM-treated control, DFO also significantly decreased the expression of TnC, AnxA2, cyclin D1, and c-myc, while significantly increasing antioncogenic p-YAP(S127) levels in the presence of PSC CM in PANC-1 cells (Fig. 4A).
Similar effects were also observed using MIAPaCa-2 (Fig. S2) and AsPC-1 (Fig. S3) cells, where relative to the PSC CM-treated control, Dp44mT, DpC, and Gem significantly decreased TnC, AnxA2, YAP/TAZ, β-catenin, cyclin D1, and c-myc in the presence of PSC CM. Considering these latter results, while Gem decreased c-myc expression in MIAPaCa-2 cells (Fig. S2), this effect was not significantly (p > 0.05) different from the PSC CM-treated control. Relative to the PSC CM-treated control, Dp44mT, DpC, and Gem, also significantly increased the levels of antioncogenic p-YAP(S127) and p-β-catenin(S33/37/Thr41) in MIAPaCa-2 and AsPC-1 cells treated with PSC CM (Figs. S2 and S3). Notably, while the WNT inhibitor, DKK1, was only significantly increased by DpC in AsPC-1 cells (Fig. S3), Dp44mT, DpC, and Gem significantly increased DKK1 in PANC-1 (Fig. 4A) and MIAPaCa-2 (Fig. S2) cells.
In summary, these data in Figure 4, Figs. S2 and S3, indicate that DFO, Dp44mT, and DpC, but also the chemotherapeutic agent, Gem, were able to effectively inhibit PSC CM-induced β-catenin and YAP/TAZ signaling in all PC cells examined. The mechanism of Dp44mT and DpC activity was due to their ability to bind cellular metal ions, as the negative control compound, 2-benzoylpyridine 2-methyl-3-thiosemicarbazone (Bp2mT), which cannot bind metals (90, 91), had no significant effect.
DpC and Dp44mT decrease PSC-mediated nuclear expression of β-catenin and YAP/TAZ in PC cells
Considering that Dp44mT and DpC significantly decreased the total protein levels of β-catenin and YAP/TAZ in all three PC cell types (Figs. 4A, S2 and S3), we further investigated their effects on the nuclear localization of these proteins. Cytoplasmic and nuclear fractions of PANC-1 and AsPC-1 cells were obtained following incubation with either DpC or Dp44mT in the absence or presence of PSC CM for 24 h (Figs. 4B and S4).
The expression of NDRG1 was significantly upregulated in PANC-1 (Fig. 4B) and AsPC-1 cells (Fig. S4) upon incubation with Dp44mT or DpC in the cytoplasmic and nuclear fractions. In PANC-1 cells, PSC CM significantly increased β-catenin expression in the nucleus and YAP/TAZ in both the cytoplasm and nucleus compared with the control without PSC CM (Fig. 4B). These stimulatory effects on β-catenin and YAP/TAZ expression were significantly decreased by Dp44mT and especially DpC in the presence of PSC CM (Fig. 4B). Similarly, PSC CM also significantly increased cytoplasmic and nuclear β-catenin and YAP/TAZ levels in AsPC-1 cells compared with the control without PSC CM, with these effects being significantly inhibited by Dp44mT or DpC (Fig. S4).
Overall, these results demonstrate the potent ability of Dp44mT and DpC to upregulate NDRG1 and inhibit PSC-mediated nuclear localization of β-catenin and YAP/TAZ in PC cells, which is vital for their oncogenic transcriptional activation (27, 63, 64, 92, 93).
Dp44mT and DpC inhibit oncogenic signaling in PSCs
Following on from the potent antioncogenic effects of the agents on the PC cell types above (Figs. 4 and S2–S4), we next examined how Dp44mT and DpC affect YAP/TAZ and β-catenin expression in PSCs (Fig. 5). This aspect was essential to assess, as YAP/TAZ and β-catenin signaling are integrally associated with oncogenic activation of PSCs, particularly due to their bidirectional interaction with PC cells (94, 95, 96). We also examined TnC expression, as PSCs are a major source of this oncogenic extracellular matrix protein (12, 97), and also the levels of α-SMA, as it is a marker of activated PSCs (73, 98). Expression of a key antagonist of Wnt/β-catenin signaling, namely DKK1 (99, 100, 101), was additionally assessed in PSCs (Fig. 5A).
Figure 5.
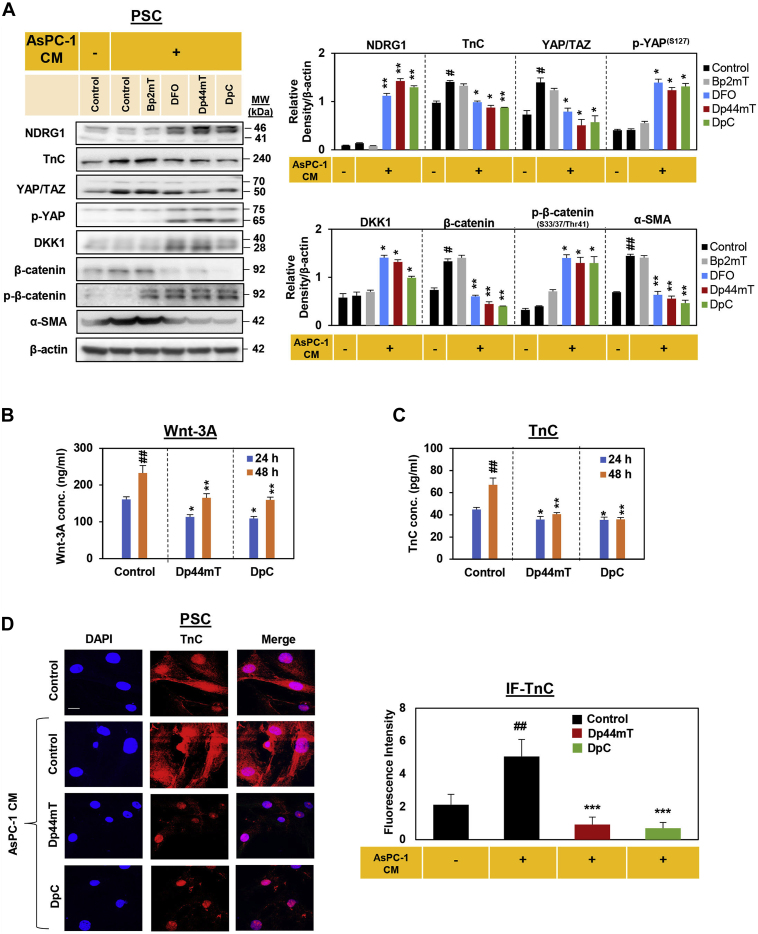
DpC and Dp44mT decrease pancreatic stellate cell (PSC) activation by conditioned medium from AsPC-1 pancreatic cancer cells (AsPC-1 CM) leading to decreased Wnt-3A and TnC secretion.A, Western blot of PSCs incubated for 24 h in control medium, AsPC-1 CM, or AsPC-1 CM together with either Bp2mT (5 μM), DFO (100 μM), Dp44mT (5 μM), or DpC (5 μM). The blots were then assessed for NDRG1, TnC, YAP/TAZ, p-YAP, DKK1, β-catenin, p-β-catenin and α-SMA expression. β-actin was used as a protein-loading control. Blots are representative of three independent experiments. Densitometry results are mean ± SD (n = 3). ∗p < 0.05; ∗∗p < 0.01 denote statistical significance comparing each treatment with the AsPC-1 CM-treated control. #p < 0.05, ##p < 0.01 denote statistical significance comparing the AsPC-1 CM-treated control with the untreated control. B and C, PSCs were incubated with control medium or this medium containing Dp44mT or DpC (5 μM) for 24- or 48-h and assessed for secretion of (B) Wnt-3A or (C) TnC via ELISA. Results are mean ± SD (n = 3). ∗p < 0.05; ∗∗p < 0.01 comparing each treatment to the respective untreated control. ##p < 0.01 comparing the 24 to 48-h time points. D, confocal immunofluorescence microscopy images of PSCs incubated for 24 h with control medium alone, AsPC-1 CM, or AsPC-1 CM together with either Dp44mT or DpC (5 μM) and then assessed for TnC (red) expression. Nuclei were stained using DAPI (blue). Scale bar: 25 μm; Magnification: 63×. Images are representative from three independent experiments. Image J analysis utilized 14 to 21 cells over three experiments with results being presented as mean ± SD (n = 3). ∗∗∗p < 0.01 comparing each treatment with the AsPC-1 CM-treated control. ##p < 0.01 comparing the AsPC-1 CM-treated control with control medium-treated cells.
We initially investigated the effect of Bp2mT (negative control; 5 μM), DFO (100 μM), Dp44mT (5 μM), or DpC (5 μM) on PSCs by incubating them with these agents for 24 h. Notably, these studies were performed in the absence or presence of conditioned medium obtained from AsPC-1 cancer cells (AsPC-1 CM), as tumor cells promote PSC activation (30, 48, 74). Incubation of control PSCs with AsPC-1 CM had no significant effect on NDRG1 expression relative to when control cells were incubated in the absence of AsPC-1 CM (Fig. 5A). However, DFO, Dp44mT, and DpC potently and significantly increased NDRG1 expression in PSCs in the presence of AsPC-1 CM relative to the AsPC-1 CM-treated control. The addition of AsPC-1 CM to the control significantly increased the expression of TnC, YAP/TAZ, β-catenin, and α-SMA, which is consistent with AsPC-1 CM activating these oncogenic signaling pathways in PSCs (Fig. 5A). Incubation of PSCs with DFO, Dp44mT, or DpC was able to markedly reverse this stimulatory activity of AsPC-1 CM, significantly decreasing TnC, YAP/TAZ, β-catenin, and α-SMA expression in PSCs relative to the control incubated with AsPC-1 CM (Fig. 5A). In addition, incubation of PSCs with DFO, DpC, or Dp44mT significantly increased the antioncogenic effectors, p-YAP(S127), DKK1, and p-β-catenin (S33/37/Thr41), when compared with the control cells incubated with AsPC-1 CM alone.
Overall, these studies demonstrated the potent ability of Dp44mT and DpC to upregulate NDRG1 and inhibit β-catenin and YAP/TAZ signaling not only in PC cells (Figs. 4, S3 and S4), but also the PSCs (Fig. 5A), where these proteins are involved in PSC activation (102, 103).
To further dissect the mechanism of the antioncogenic activity of Dp44mT and DpC, studies examined whether Dp44mT and DpC affect the secretion of Wnt-3A and TnC from PSCs using ELISA assays. As shown in Figure 5, B and C, both Dp44mT and DpC significantly decreased Wnt-3A and TnC secretion by PSCs after a 24- and 48-h incubation. This decreased secretion of Wnt-3A and TnC was not due to any alteration in the viability of PSCs. In fact, our studies using these primary cultures of PSCs in vitro and in vivo demonstrate that they are not sensitive to the antiproliferative activity of these thiosemicarbazones (48). This resistance is probably because Dp44mT and DpC demonstrate selective anticancer activity against tumor cells versus mortal (nonmalignant) cell types such as PSCs (50, 51, 90, 104). Examining cellular TnC expression in PSCs by confocal immunofluorescence microscopy further revealed that while AsPC-1 CM significantly increased TnC levels in PSCs, this effect was markedly inhibited in the presence of DpC (5 μM) and Dp44mT (5 μM), which significantly decreased TnC expression in the presence of AsPC-1 CM (Fig. 5D). Overall, these studies confirm the potent ability of Dp44mT and DpC to attenuate the PC cell-mediated oncogenic activation of PSCs.
DpC inhibits oncogenic cross talk between PC cells and PSCs in three-dimensional spheroids
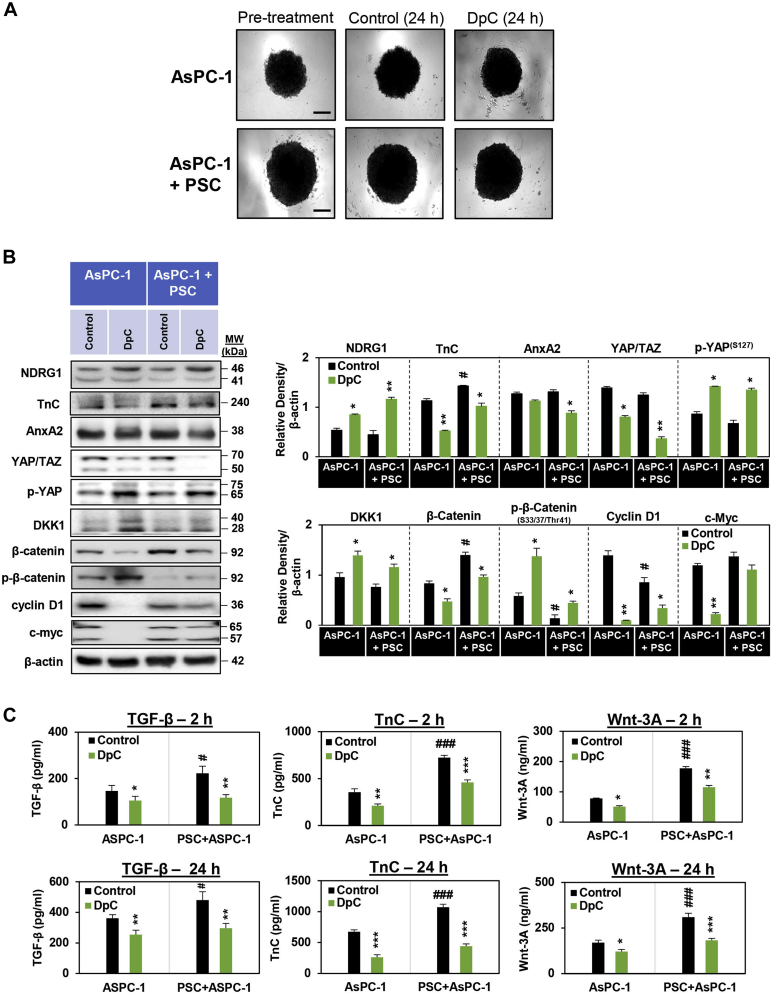
To mitigate the high attrition rate of new cancer therapies reaching the clinics, improving preclinical models is an important approach (105, 106, 107). In this regard, three-dimensional tumor cell cultures in vitro are of interest, as they have higher similarity to cancers in vivo and better represent the tumor cell microenvironment than two-dimensional monolayers (108, 109, 110). Therefore, we assessed the effect of DpC on TnC/AnxA2, β-catenin, and YAP/TAZ signaling in three-dimensional spheroids consisting of either AsPC-1 cells alone or AsPC-1 cells directly cocultured with PSCs in a 1:1 ratio (i.e., AsPC-1 + PSC spheroids; Fig. 6A). The spheroids were formed over 7 days, after which they were treated for 24 h with either the vehicle control or DpC (5 μM) (Fig. 6A). Spheroids composed of AsPC-1 + PSCs always grew larger than those composed of AsPC-1 cells alone, demonstrating their pathophysiological relevance (48). For both AsPC-1 spheroids and AsPC-1 + PSC spheroids, DpC significantly increased NDRG1 expression relative to the control (Fig. 6B). Compared with untreated control AsPC-1 spheroids, the control AsPC-1 + PSC spheroids had significantly higher expression of TnC and β-catenin, suggesting increased oncogenic cross talk between these two cell types (Fig. 6B).
Figure 6.
DpC inhibits β-catenin and YAP/TAZ signaling in three-dimensional spheroids composed of either AsPC-1 pancreatic cancer (PC) cells alone or AsPC-1 PC cells and pancreatic stellate cells (PSCs).A, images of AsPC-1 and AsPC-1 + PSC spheroids taken at day 7 of coculture (pretreatment) and following a 24 h incubation with the vehicle control or DpC. Images taken using ZEISS AxioCam MRm Observer Z.1 Microscope (scale bar = 20 μm, 5× magnification; Zeiss). B, Western blot of AsPC-1 or AsPC-1 + PSC spheroids incubated for 24 h with control medium or control medium-containing DpC (5 μM). The blots were then assessed for NDRG1, TnC, AnxA2, YAP/TAZ, p-YAP, DKK1, β-catenin, p-β-catenin, cyclin D1, and c-myc expression. β-actin was used as a protein-loading control. Blots are representative of three independent experiments. C, AsPC-1 and AsPC-1 + PSC spheroids were incubated with control medium or this medium containing DpC (5 μM) for 2- or 24-h, and this medium examined for TGF-β, TnC, or Wnt-3A secretion via ELISA. Results are mean ± SD (n = 3). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 comparing each treatment with the respective untreated control. #p < 0.05; ###p < 0.001 comparing AsPC-1 + PSC spheroids with AsPC-1 spheroids.
Importantly, DpC significantly decreased the expression of TnC, YAP/TAZ, β-catenin, and cyclin D1 in both AsPC-1 and AsPC-1 + PSCs spheroids versus the relative controls (Fig. 6B). This agent also significantly downregulated AnxA2 in AsPC-1 + PSC spheroids and c-myc in AsPC-1 spheroids (Fig. 6B). Furthermore, DpC also significantly upregulated levels of antioncogenic p-YAP(S127), DKK1, and p-β-catenin(S33/37/Thr41) in AsPC-1 and AsPC-1 + PSCs spheroids versus the relative controls (Fig. 6B). These results demonstrate that DpC inhibits YAP/TAZ and β-catenin signaling in direct three-dimensional cultures of AsPC-1 cells and cocultures of AsPC-1 + PSCs. Additionally, these data using spheroids confirm our findings implementing two-dimensional culture with thiosemicarbazones using PSCs incubated with AsPC-1 CM (Fig. 5) and multiple PC cell types incubated with PSC CM (Figs. 4 and S2–S4).
Apart from investigating cellular protein expression, we also examined the overlying coculture medium for the secretion of key mediators of PC-PSC cross talk from AsPC-1 and AsPC-1 + PSC spheroids, including TGF-β, TnC, and Wnt-3A, using ELISA assays (Fig. 6C). The control AsPC-1 + PSC cocultured spheroids had significantly greater levels of TGF-β, TnC, and Wnt-3A in the overlying medium versus control AsPC-1 spheroids after incubations of 2- or 24 h (Fig. 6C). Notably, DpC significantly decreased the secretion of these ligands by AsPC-1 and particularly the AsPC-1 + PSC spheroids following 2- or 24-h incubations (Fig. 6C). These data further demonstrate the potential of DpC to inhibit oncogenic cross talk between PC and PSCs that is important in the pathogenesis and treatment resistance of PC (36).
DpC decreases the expression of TnC, β-catenin, and YAP/TAZ in vivo
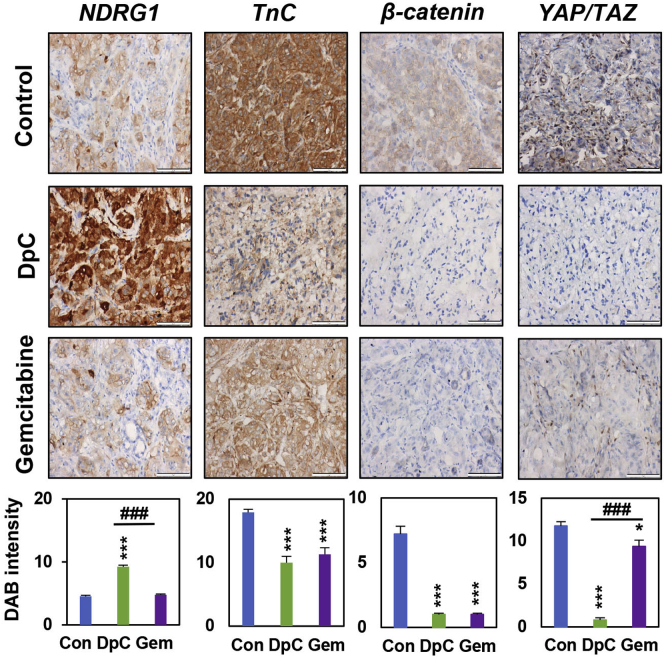
Considering the ability of DpC to decrease the expression of TnC, β-catenin, and YAP/TAZ in vitro using two-dimensional cultures and in three-dimensional spheroids described above, we assessed its effect on these proteins in vivo (Fig. 7). In this study, we utilized an established, pathologically relevant, orthotopic model of PC that also incorporates human PSCs (48, 111). A mixture of AsPC-1 PC cells and PSCs (1:1 ratio) was directly injected into the tail of the pancreas of Balb c nu/nu mice, as described previously (48). This ratio of PC cells to PSCs was chosen based on earlier studies using the same in vivo model, as it replicates early cancer development and progression (111, 112, 113). One week following tumor implantation that is required for the development of primary tumors, mice were treated for 2 weeks with either the vehicle control, DpC (10 mg/kg) or Gem (75 mg/kg). This treatment with DpC significantly inhibited primary tumor growth by approximately 40% relative to the control, prevented fusion of the tumor and the spleen that was evident in the control and Gem-treated samples, and also significantly suppressed metastasis to the brain, lungs, liver, and kidney (48). Treatment with DpC also markedly reduced the collagen content in tumor sections, demonstrating its ability to inhibit desmoplasia (48). The resultant primary tumor specimens from the current investigation were then examined for the expression of TnC, β-catenin, and YAP/TAZ using immunohistochemistry (Fig. 7).
Figure 7.
DpC treatment decreases the expression of TnC, β-catenin, and YAP/TAZ in orthotopic pancreatic cancer (PC) tumors in nude mice. Mice orthotopically implanted in the pancreas with a 1:1 mixture of AsPC-1-luc PC cells and pancreatic stellate cells (PSCs) were treated with the vehicle control, DpC (10 mg/kg, three times/week via oral gavage), or Gemcitabine (Gem; 75 mg/kg, two times/week via intraperitoneal (i.p.) injection). Treatments were administered for 2 weeks, followed by 1 week recovery. Primary tumors were assessed for: NDRG1, TnC, β-catenin, and YAP/TAZ expression via immunohistochemistry (IHC; scale bar = 50 μm; 40×). Results are mean ± S.E.M (n = 7 mice; three images/specimen). ∗p < 0.05; ∗∗∗p < 0.001 comparing each treatment with the respective vehicle control (Con). ###p < 0.001 comparing DpC with Gem.
As demonstrated in Figure 7, DpC significantly upregulated tumor NDRG1 expression in vivo, while Gem did not significantly affect this metastasis suppressor versus the control. Treatment with DpC significantly decreased TnC, β-catenin, and YAP/TAZ expression in PC tumors in vivo relative to the vehicle control (Fig. 7). Treatment with Gem also similarly reduced the levels of these latter oncogenic signaling proteins in vivo. However, of note, Gem was markedly and significantly less effective at decreasing YAP/TAZ levels when compared with DpC (Fig. 7). These results in vivo validate our in vitro observations and demonstrate the potent inhibitory effect of DpC on PC-PSC cross talk, which leads to decreased levels of TnC, β-catenin, and YAP/TAZ in tumor cells.
Discussion
The major contributor to PC progression and resistance to current chemotherapies is the bidirectional oncogenic signaling between PC cells and PSCs, which promotes desmoplasia (48, 76). PC cells secrete ligands such as SHH and TGF-β that then activate neighboring PSC cells and induce the production of cytokines, growth factors, and ECM proteins that promote PC growth and progression (Fig. 8A; (5, 6)). We recently identified that the metastasis suppressor NDRG1 and NDRG1-inducing thiosemicarbazones (i.e. Dp44mT, DpC) can potently reduce SHH production by PC cells and desensitize them to ligands such as HGF and IGF-1 that are produced by activated PSCs (48). DpC also inhibited PSC activation in vitro and reduced desmoplasia in vivo (48). The current study mechanistically dissected for the first time the effects of the metastasis suppressor, NDRG1, and the NDRG1-inducing thiosemicarbazones on more recently identified key mediators of cross talk between PC cells and PSCs, namely TGF-β, TnC, Wnt/β-catenin, and YAP/TAZ (12, 97, 114, 115, 116). In fact, TnC is highly elevated in invasive PC and produced by activated PSCs (35, 117), leading to activation of oncogenic Wnt/β-catenin and YAP/TAZ pathways (Fig. 1A) (14, 27, 118).
Figure 8.
Schematic of how DpC affects the oncogenic signaling pathways involved in pancreatic cancer cell (PC) and pancreatic stellate cell (PSC) bidirectional oncogenic signaling.A, activated PSCs induce β-catenin and YAP/TAZ signaling in PC cells (see the Legend for Fig. 1A for a full description). B, upon DpC treatment, NDRG1 is upregulated in PC cells. This response inhibits β-catenin phosphorylation and prevents its entry into the nucleus, where it would normally promote the expression of pro-proliferative proteins (e.g., cyclin D1, c-myc, etc.) and cytokines (e.g., TGF-β). NDRG1 also inhibits YAP/TAZ entry into the nucleus to prevent its ability to induce proproliferative gene expression. The decreased production of TGF-β by PC cells prevents their ability to activate adjacent PSCs, which results in a quiescent state, leading to reduced production of TnC and Wnt-3A by these PSCs. This effect further inhibits downstream β-catenin and YAP/TAZ signaling in PC cells, thereby disrupting cross talk between PC cells and PSCs.
Following our discovery that NDRG1 inhibits SHH production by PC cells, we hypothesized that NDRG1 may attenuate PSC activation (48). In the current study, we present direct evidence demonstrating the NDRG1-mediated inhibition of PSC activation, which was found to be mediated by disrupting the interconnected TnC, YAP/TAZ, Wnt/β-catenin, and TGF-β signaling cascades that play major roles in PC-PSC cross talk (24, 117).
We demonstrate for the first time that NDRG1 reduced TnC levels in both PC and PSC cells. TnC is mainly synthesized by PSCs and influences PC progression by binding to AnxA2 on PC cells (Fig. 8A; (12, 35, 119, 120)). The expression of TnC in PC cells was found to enhance cell viability and migration (97). Hence, our studies in vitro and in vivo examining the ability of NDRG1 and NDRG1-inducing thiosemicarbazones to decrease TnC expression in PC cells, as well as TnC secreted by PSCs, suggest potent inhibition of TnC-mediated oncogenic activity.
The expression of NDRG1 also potently reduced both YAP/TAZ and β-catenin expression, nuclear localization, and downstream signaling in PC cells (Fig. 8B). This was further demonstrated by the increased inhibitory phosphorylations of β-catenin (S33/37/Thr41) and YAP/TAZ (S127) via NDRG1, which promotes their degradation (27, 28, 70, 71, 72). The Wnt inhibitor, DKK1, was also markedly increased by NDRG1 expression in PC cells, which is likely due to the decreased TnC levels, as this latter protein is an inhibitor of DKK1 (14) (Fig. 8B). Importantly, these effects were observed both in the absence and presence of PSC CM, which along with TnC also contains other growth factors and ligands, such as Wnt, which promote β-catenin and YAP/TAZ oncogenic signaling (27, 114). In fact, we demonstrate herein that NDRG1 expression also effectively inhibited Wnt-3A-mediated nuclear localization of both β-catenin and YAP/TAZ (Fig. 8B). Hence, NDRG1 potently inhibited PSC-mediated Wnt/β-catenin and YAP/TAZ signaling pathways in PC cells, further demonstrating the ability of NDRG1 to inhibit PC-PSC cross talk.
These effects were accompanied by reduced production of TGF-β by PC cells overexpressing NDRG1. As TGF-β is a direct transcriptional target of the β-catenin pathway (121), inhibition of Wnt/β-catenin is a likely mechanism by which NDRG1 impairs TGF-β production by PC cells. We hypothesized that the reduced TGF-β production by PC cells in response to NDRG1 was responsible for the attenuated activation of surrounding PSCs. This proposition was confirmed herein by the reduced levels of PSC activation marker α-SMA in PSC cells that were incubated with conditioned media from NDRG1-overexpressing PC cells. Further, these “NDRG1 conditioned” PSCs also had reduced levels of YAP/TAZ and β-catenin proteins, which further suggests their reduced activation, as these latter proteins are vital for PSC activation (24, 116). The mechanism by which NDRG1 reduced TnC production by PSCs can also be linked to its effects on TGF-β, as the ability of PSCs to produce TnC was found to be driven by TGF-β (35, 77).
TGF-β has been well established to promote PC-PSC cross talk, with numerous studies demonstrating that PC-derived TGF-β can promote activation of fibroblasts into cancer-associated fibroblasts, with anti-TGF-β antibodies inhibiting this effect (78, 122, 123, 124). TGF-β signaling also influences other signaling pathways that promote PC-PSC cross talk, including the SHH pathway. In fact, TGF-β can directly promote GLI1 expression, a key facilitator of downstream SHH signaling, in an SHH-independent manner (125). This analysis provides further insights into the inhibitory effect of NDRG1 on PC-PSC cross talk, which is likely mediated via reduced production of both SHH (48) and TGF-β ligands by PC cells.
We further demonstrated that DpC, which is well known to induce NDRG1 expression (39, 48, 53, 56, 89), was also able to: (i) inhibit expression of TnC and its receptor, AnxA2, in PC cells; (ii) decrease β-catenin and YAP/TAZ expression and suppress their downstream signaling in PC cells; (iii) increase the inhibitory phosphorylation of β-catenin and YAP/TAZ at S33/37/Thr41 and S127, respectively; and (iv) inhibit cancer-cell-mediated PSC activation and secretion of TnC and Wnt-3A using three-dimensional spheroids consisting of cocultured PC cells and PSCs (Fig. 8B). Notably, DpC also inhibited TnC, β-catenin, and YAP/TAZ levels in vivo in a pathologically relevant, orthotopic PC xenograft, being markedly more potent at decreasing YAP/TAZ levels than the current PC therapy, Gem.
The mechanisms by which DpC is able to exert these multifaceted effects are likely to occur through its effects on NDRG1, as demonstrated previously (46, 56, 126). In fact, treatment with DpC mirrored many of the effects induced by NDRG1 alone. Considering that DpC and NDRG1 reduced TGF-β production by PC cells, this is likely to be responsible for the subsequent reduction in PSC-derived TnC levels. The reduced levels of PSC-derived Wnt3A in response to DpC may also be linked to the reduced PC-derived SHH and PSC GLI1 expression, as demonstrated in our recent study (48). In fact, the GLI transcription factors, which are activated by SHH signaling, can induce Wnt genes in cancer (127). The ability of DpC to inhibit AnxA2 was likely independent of NDRG1, as this metastasis suppressor did not affect AnxA2 levels. Notably, recent studies have demonstrated that AKT/mTOR signaling promotes AnxA2 expression in pancreatic cancer cells (128). Our laboratories have also recently demonstrated that DpC potently inhibits AKT activation in PC cells (48). Hence, we hypothesize that the ability of DpC to inhibit AnxA2 may be mediated via its inhibitory effects on AKT signaling.
Overall, these results strongly support our recent findings that DpC is a potent inhibitor of PC-PSC cross talk and desmoplasia (48), demonstrating a novel unexplored mechanism of action of this agent and its ability to inhibit TGF-β, TnC, Wnt/β-catenin and YAP/TAZ signaling in PC. This further highlights the potential of DpC as an innovative PC therapy (39).
Current clinical trials of Wnt inhibitors have shown promising outcomes in treating Wnt-dependent cancers, including PC (129, 130, 131, 132). However, there are still no clinically available drugs that target Wnt/β-catenin signaling for PC treatment. Further, TnC inhibition has also been explored in PC, with the development of several humanized antibodies to target tumor-specific expression of TnC (i.e., F16-SIP) (133). However, while the antibody, F16-SIP, led to a dramatic reduction of tumor volume when combined with chemotherapy in PC, the tumors later relapsed (134). Thus, more effective and multifaceted inhibitors of TnC are required.
The current investigation demonstrates that the innovative anticancer agent, DpC, inhibits PC-PSC cross talk in a multifaceted manner, including inhibition of SHH, HGF/c-MET, IGF-1/IGFR, TGF-β, Wnt/β-catenin, TnC, and YAP/TAZ signaling in PC and PSC cells. Hence, this thiosemicarbazone provides a unique, multitargeted approach for treating aggressive PC that remains highly intractable to standard chemotherapies. This is important to consider, as traditional strategies of anticancer drug design adopting a single molecular target leads to selective pressure on tumor cells resulting in drug resistance (135). Further, to date, all current therapeutic strategies have shown dismal activity against PC, with the disease demonstrating an abysmal poor 5-year survival rate of 10% (1).
In conclusion, we demonstrate that NDRG1 is a promising molecular target that can potently inhibit the interactions between PSCs and PC cells via its effects on the oncogenic TGF-β, TnC, Wnt/β-catenin, and YAP/TAZ signaling pathways. Uniquely, to comprehensively suppress these pathways, not only do NDRG1 and the NDRG1-inducing agents decrease the expression of oncogenic effectors (i.e., TnC, YAP/TAZ, AnxA2, cyclin D1, and c-myc), but these also beneficially enhance inhibitor activity (i.e., DKK, p-YAP(S127), p-β-catenin(S33/37/Thr41)) to negate the oncogenic potential of these signaling networks. The current studies also reveal a novel therapeutic approach using NDRG1-targeting agents such as the clinically trialed agent, DpC (61), which demonstrate a multifaceted mechanism of action by inhibiting multiple oncogenic signaling pathways that could more effectively inhibit PC progression and improve patient outcomes.
Experimental procedures
Ethics approval
Studies using primary cultures of human PSCs were approved by the South Eastern Sydney Local Health District Human Research Ethics Committee (13/023-HREC/13/POWH/65). Animal studies were approved by the University of Sydney Animal Ethics Committee (#2018–1433). All studies abide by Declaration of Helsinki principles.
Cell culture
Human AsPC-1, PANC-1, and MIAPaCa-2 PC cells were purchased from the American Type Culture Collection and maintained as described previously (39). AsPC-1 cells stably transfected with a luciferase reporter construct (AsPC-1-Luc) were purchased from the Japanese Collection of Research Bioresources Cell Bank (JCRB; JCRB# 1454). All of these cell types were authenticated based on viability, recovery, growth, morphology, and cytogenetic analysis (i.e., antigen expression, DNA profile, and isoenzymology) by the provider. PANC-1 and MIAPaCa-2 cells were stably transfected to overexpress NDRG1 and were compared with the relative VC-transfected counterparts, as described previously (40, 47).
Approval to prepare human PSCs from human PC tumors was obtained through the South Western Sydney Clinical School, University of New South Wales (The South Eastern Sydney Local Health District Human Research Ethics Committee [13/023 (HREC/13/POWH/65)]). These PSCs were initially isolated by the outgrowth method from resected pancreatic tissues obtained from three different patients with PC (136). The purity of PSCs yield was assessed by morphology and immunostaining for: (1) glial fibrillary acidic protein (GFAP) that is a PSC selective marker; (2) α-smooth muscle actin (α-SMA), which is a PSC activation marker; and (3) the absence of staining for the cancer cell marker, cytokeratin, using established methods (137, 138). These primary cultures of PSCs were maintained in Iscove's Modified Dulbecco’s Medium (IMDM) medium with 10% FCS.
Cell treatments
DFO (Sigma-Aldrich) was dissolved in medium to a final concentration of 100 μM. The thiosemicarbazones, Dp44mT, DpC, and the negative control compound, Bp2mT, were synthesized and characterized using standard methods (50, 51, 90, 91, 104), dissolved in DMSO at a concentration of 10 mM, then diluted in medium (final [DMSO]: ≤0.1%) to a concentration of 5 μM. The cytotoxic agent, Gemcitabine (Gem; Eli Lilly), was dissolved in medium and then used at 5 μM. All agents were incubated with cells for 24 h/37 °C, unless indicated otherwise. The Wnt ligand, Wnt-3A (20 ng/ml; R&D Systems), was incubated with cells for 8 h/37 °C (27).
Protein extraction and Western analysis
Total protein was extracted and Western analysis was performed via standard procedures using the antibodies outlined in Table S1 (47).
ELISA assays
Cells were seeded at 500,000 cells/well on 6-well plates overnight followed by treatment with either control medium or this medium containing Dp44mT (5 μM) or DpC (5 μM) for 2-, 24-, or 48-h/37 °C. The overlying cell conditioned medium was then collected and analyzed for TnC, Wnt-3A, and TGF-β levels using the human TnC ELISA kit (Cat. #: ab213831; Abcam Australia), human Wnt-3A ELISA kit (Cat. #: MBS3802292; MyBioSource), and human TGF-β ELISA kit (Cat. #: BMS249-4; Thermo Fisher Scientific), respectively.
Gene silencing by small interfering RNA (siRNA)
The siRNA specific for NDRG1 (siNDRG1; Cat. #4392422; Life Technologies) was compared with a negative control siRNA (siControl; Life Technologies). The siRNA was transiently transfected into AsPC-1 cells using Lipofectamine 2000 (Life Technologies) following the manufacturer's instructions and incubated for 72 h/37 °C.
Conditioned medium from PSCs and AsPC-1 cells
PSC CM was generated by culturing PSCs in serum-free DMEM for 48 h/37 °C. Media was then collected and concentrated using Centricon YM3 filters (Millipore). AsPC-1-conditioned medium (AsPC-1 CM) and conditioned medium from both MIAPaCa-2 VC or MIAPaCa-2 NDRG1 overexpressing cells (MIAPaCa-2 CM) were generated by culturing these cells in RPMI 1640 (AsPC-1) or DMEM (MIAPaCa-2) serum-free medium for 24 h/37 °C.
Cell culture and nuclear-cytoplasmic fractionation
The VC and NDRG1 overexpressing PC cells were incubated with or without PSC CM for 24 h/37 °C. To assess the effect of Dp44mT and DpC, PC cells were incubated with Dp44mT (5 μM) or DpC (5 μM) in the presence or absence of PSC CM for 24 h/37 °C. Then, nuclear-cytoplasmic fractionation was conducted using the NE-PER Nuclear and Cytoplasmic Extraction kit (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Spheroids
AsPC-1 cells were seeded alone (20,000 cells/well) or in a 1:1 ratio with PSCs (20,000 cells/well) and cultured in RPMI 1640 media containing 10% FCS on 96-well plates precoated with 1% agarose (dissolved in PBS) for 7 days/37 °C. Once formed, spheroids were incubated with control medium or this medium containing DpC (5 μM) for 24 h/37 °C, after which protein extraction was performed, as described previously (47).
Immunofluorescence
Immunofluorescence was performed as described in (47) using the antibodies described in Table S2. Images were captured using ZEISS LSM 800 plus Airyscan Laser Confocal Microscope (10 × ; Zeiss). Raw images were analyzed using ImageJ software.
Animal studies
In vivo studies used a well-established, pathologically relevant, orthotopic PC model that demonstrates primary pancreatic tumor growth, metastasis, and desmoplasia (113) to assess the effects of DpC on PC, as described previously (48). Briefly, a mixture of 1 × 106 AsPC-1-Luc and PSCs (all derived from the same patient) was injected directly (1:1 ratio) into the tail of the pancreas of 8 to 10-week-old female BALB/c-nu/nu mice. One week following injection, all mice had tumors of similar size (48, 111) and were randomized into three groups (n = 7/group): (i) control (either the vehicle used to dissolve DpC (30% propylene glycol in 0.9% saline) or the vehicle used to prepare Gem (PBS)); (ii) DpC (10 mg/kg, 3 times/week via oral gavage); and (iii) Gem (75 mg/kg, 2 times/week via intraperitoneal (i.p.) injection), based on previous studies (48, 50, 111). Treatments were administered for 2 weeks, followed by a 1 week recovery prior to euthanasia. Tumor specimens were used to assess the effects of DpC and Gem and WNT, TnC, β-catenin and YAP/TAZ signaling.
Immunohistochemistry
Tumor sections were prepared and stained as described in (48) using antibodies listed in Table S3. IHC staining was quantified using Fiji ImageJ to give a mean DAB intensity (139).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software). For comparison of two experimental conditions, a two-tailed, unpaired Student’s t test was performed using at least three independent experiments. For comparison of more than two datasets, a one-way ANOVA was performed, and the Tukey test was used to correct for multiple comparisons. Statistical significance was set at p < 0.05. Data are presented as mean ± SD or S.E.M., as specified.
Data availability
This study includes no data deposited in external repositories.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We sincerely appreciate the outstanding technical assistance of Mr Mahan Gholam Azad (Centre for Cancer Cell Biology and Drug Discovery, Griffith University) regarding figure preparation.
Author contributions
B. G. and Z. K. conceptualization; B. G., D. R. R., and Z. K. formal analysis; D. R. R. and Z. K. funding acquisition; B. G., F. S. T., S. C. L., S. S., P. J. J., and Z. K. investigation; B. G., F. S. T., S. C. L., S. S., P. J. J., and Z. K. methodology; Z. K. project administration; M. V. A. and D. R. R. resources; D. R. R. and Z. K. supervision; B. G. writing—original draft; D. R. R. and Z. K. writing—review and editing.
Funding and additional information
This work was supported by the Avner Pancreatic Cancer Foundation 2018 Innovation grant, the National Health and Medical Research Council (NHMRC; grant #APP1160968), Cancer Institute of NSW (CINSW; grant #2016/0RFlOl), and University of Sydney grants to Z. K. Z. K. is grateful for a University of Sydney Bridging Fellowship, a National Health and Medical Research Council RD Wright Fellowship [APP1140447], a CINSW Career Development Fellowship [CDF171126], and a University of Sydney Equity Fellowship. B. G. is grateful for his University of Sydney International Scholarship (USydIS). P. J. J. is grateful for Cancer Institute NSW Career Development Fellowship [CDF171147] support. S. S. would like to acknowledge Pankind Foundation for a Collaboration Grant. D. R. R. thanks the NHMRC for Senior Principal Research Fellowships [1062607 and 1159596]; NHMRC Project Grants [APP1144829; APP1128152; APP1144456]; and the Avner Pancreatic Cancer Foundation for a 2017 Innovation Grant.
Edited by Phyllis Hanson
Supporting information
References
- 1.Mizrahi J.D., Surana R., Valle J.W., Shroff R.T. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen M.F., Mortensen M.B., Detlefsen S. Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells. World J. Gastroenterol. 2016;22:2678–2700. doi: 10.3748/wjg.v22.i9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awaji M., Singh R.K. Cancer-associated fibroblasts' functional heterogeneity in pancreatic ductal adenocarcinoma. Cancers. 2019;11:290. doi: 10.3390/cancers11030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosein A.N., Brekken R.A., Maitra A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020;17:487–505. doi: 10.1038/s41575-020-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veenstra V.L., Damhofer H., Waasdorp C., van Rijssen L.B., van de Vijver M.J., Dijk F., Wilmink H.W., Besselink M.G., Busch O.R., Chang D.K., Bailey P.J., Biankin A.V., Kocher H.M., Medema J.P., Li J.S., et al. ADAM12 is a circulating marker for stromal activation in pancreatic cancer and predicts response to chemotherapy. Oncogenesis. 2018;7:87. doi: 10.1038/s41389-018-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon A., Thompson C., Hall B.R., Jain M., Kumar S., Batra S.K. Desmoplasia in pancreatic ductal adenocarcinoma: Insight into pathological function and therapeutic potential. Genes Cancer. 2018;9:78–86. doi: 10.18632/genesandcancer.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnittert J., Bansal R., Mardhian D.F., van Baarlen J., Ostman A., Prakash J. Integrin alpha11 in pancreatic stellate cells regulates tumor stroma interaction in pancreatic cancer. FASEB J. 2019;33:6609–6621. doi: 10.1096/fj.201802336R. [DOI] [PubMed] [Google Scholar]
- 8.Goehrig D., Nigri J., Samain R., Wu Z., Cappello P., Gabiane G., Zhang X., Zhao Y., Kim I.S., Chanal M., Curto R., Hervieu V., de La Fouchardiere C., Novelli F., Milani P., et al. Stromal protein betaig-h3 reprogrammes tumour microenvironment in pancreatic cancer. Gut. 2019;68:693–707. doi: 10.1136/gutjnl-2018-317570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Q., Zhang B., Hu Q., Qin Y., Xu W., Liu W., Yu X., Xu J. The impact of cancer-associated fibroblasts on major hallmarks of pancreatic cancer. Theranostics. 2018;8:5072–5087. doi: 10.7150/thno.26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi H., Enomoto A., Woods S.L., Burt A.D., Takahashi M., Worthley D.L. Cancer-associated fibroblasts in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2019;16:282–295. doi: 10.1038/s41575-019-0115-0. [DOI] [PubMed] [Google Scholar]
- 11.Yoneura N., Takano S., Yoshitomi H., Nakata Y., Shimazaki R., Kagawa S., Furukawa K., Takayashiki T., Kuboki S., Miyazaki M., Ohtsuka M. Expression of annexin II and stromal tenascin C promotes epithelial to mesenchymal transition and correlates with distant metastasis in pancreatic cancer. Int. J. Mol. Med. 2018;42:821–830. doi: 10.3892/ijmm.2018.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley K., Muth S., Jaffee E., Zheng L. Hedgehog signaling stimulates tenascin C to promote invasion of pancreatic ductal adenocarcinoma cells through annexin A2. Cell Adh. Migr. 2017;11:514–523. doi: 10.1080/19336918.2016.1259057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi M., He X., Wei W., Wang J., Zhang T., Shen X. Tenascin-C induces resistance to apoptosis in pancreatic cancer cell through activation of ERK/NF-kappaB pathway. Apoptosis. 2015;20:843–857. doi: 10.1007/s10495-015-1106-4. [DOI] [PubMed] [Google Scholar]
- 14.Saupe F., Schwenzer A., Jia Y., Gasser I., Spenle C., Langlois B., Kammerer M., Lefebvre O., Hlushchuk R., Rupp T., Marko M., van der Heyden M., Cremel G., Arnold C., Klein A., et al. Tenascin-C downregulates wnt inhibitor dickkopf-1, promoting tumorigenesis in a neuroendocrine tumor model. Cell Rep. 2013;5:482–492. doi: 10.1016/j.celrep.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Hendaoui I., Tucker R.P., Zingg D., Bichet S., Schittny J., Chiquet-Ehrismann R. Tenascin-C is required for normal Wnt/beta-catenin signaling in the whisker follicle stem cell niche. Matrix Biol. 2014;40:46–53. doi: 10.1016/j.matbio.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Lowy C.M., Oskarsson T. Tenascin C in metastasis: A view from the invasive front. Cell Adh. Migr. 2015;9:112–124. doi: 10.1080/19336918.2015.1008331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Connell J.T., Sugimoto H., Cooke V.G., MacDonald B.A., Mehta A.I., LeBleu V.S., Dewar R., Rocha R.M., Brentani R.R., Resnick M.B., Neilson E.G., Zeisberg M., Kalluri R. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16002–16007. doi: 10.1073/pnas.1109493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noda N., Minoura H., Nishiura R., Toyoda N., Imanaka-Yoshida K., Sakakura T., Yoshida T. Expression of Tenascin-C in stromal cells of the murine uterus during early pregnancy: Induction by interleukin-1α, prostaglandin E2, and prostaglandin F2α. Biol. Reprod. 2000;63:1713–1720. doi: 10.1095/biolreprod63.6.1713. [DOI] [PubMed] [Google Scholar]
- 19.Sundquist E., Kauppila J.H., Veijola J., Mroueh R., Lehenkari P., Laitinen S., Risteli J., Soini Y., Kosma V.M., Sawazaki-Calone I., Macedo C.C., Bloigu R., Coletta R.D., Salo T. Tenascin-C and fibronectin expression divide early stage tongue cancer into low- and high-risk groups. Br. J. Cancer. 2017;116:640–648. doi: 10.1038/bjc.2016.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami T., Kikuchi H., Ishimatsu H., Iino I., Hirotsu A., Matsumoto T., Ozaki Y., Kawabata T., Hiramatsu Y., Ohta M., Kamiya K., Fukushima M., Baba S., Kitagawa K., Kitagawa M., et al. Tenascin C in colorectal cancer stroma is a predictive marker for liver metastasis and is a potent target of miR-198 as identified by microRNA analysis. Br. J. Cancer. 2017;117:1360–1370. doi: 10.1038/bjc.2017.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen E.A., Menon R., Bailey K.M., Thomas D.G., Van Noord R.A., Tran J., Wang H., Qu P.P., Hoering A., Fearon E.R., Chugh R., Lawlor E.R. Activation of Wnt/beta-catenin in Ewing sarcoma cells antagonizes EWS/ETS function and promotes phenotypic transition to more metastatic cell states. Cancer Res. 2016;76:5040–5053. doi: 10.1158/0008-5472.CAN-15-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kagey M.H., He X. Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. Br. J. Pharmacol. 2017;174:4637–4650. doi: 10.1111/bph.13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia de Herreros A., Dunach M. Intracellular signals activated by canonical Wnt ligands independent of GSK3 inhibition and beta-catenin stabilization. Cells. 2019;8:1148. doi: 10.3390/cells8101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piersma B., Bank R.A., Boersema M. Signaling in fibrosis: TGF-beta, WNT, and YAP/TAZ converge. Front. Med. 2015;2:59. doi: 10.3389/fmed.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z., Liu J., Chu D., Shan Y., Ma G., Zhang H., Zhang X.D., Wang P., Chen Q., Deng C., Chen W., Dimitrov D.S., Zhao Q. A dual-specific IGF-I/II human engineered antibody domain inhibits IGF signaling in breast cancer cells. Int. J. Biol. Sci. 2018;14:799–806. doi: 10.7150/ijbs.25928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bazzichetto C., Conciatori F., Luchini C., Simionato F., Santoro R., Vaccaro V., Corbo V., Falcone I., Ferretti G., Cognetti F., Melisi D., Scarpa A., Ciuffreda L., Milella M. From genetic alterations to tumor microenvironment: The Ariadne's string in pancreatic cancer. Cells. 2020;9:309. doi: 10.3390/cells9020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V., Fassina A., Cordenonsi M., Piccolo S. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Azzolin L., Zanconato F., Bresolin S., Forcato M., Basso G., Bicciato S., Cordenonsi M., Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Bailey P., Chang D.K., Nones K., Johns A.L., Patch A.M., Gingras M.C., Miller D.K., Christ A.N., Bruxner T.J., Quinn M.C., Nourse C., Murtaugh L.C., Harliwong I., Idrisoglu S., Manning S., et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 30.Li X., Wang Z., Ma Q., Xu Q., Liu H., Duan W., Lei J., Ma J., Wang X., Lv S., Han L., Li W., Guo J., Guo K., Zhang D., et al. Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin. Cancer Res. 2014;20:4326–4338. doi: 10.1158/1078-0432.CCR-13-3426. [DOI] [PubMed] [Google Scholar]
- 31.Bailey J.M., Mohr A.M., Hollingsworth M.A. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28:3513–3525. doi: 10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acebron S.P., Karaulanov E., Berger B.S., Huang Y.L., Niehrs C. Mitotic Wnt signaling promotes protein stabilization and regulates cell size. Mol. Cell. 2014;54:663–674. doi: 10.1016/j.molcel.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Bai Y., Bai Y., Dong J., Li Q., Jin Y., Chen B., Zhou M. Hedgehog signaling in pancreatic fibrosis and cancer. Medicine. 2016;95 doi: 10.1097/MD.0000000000002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Principe D.R., DeCant B., Mascariñas E., Wayne E.A., Diaz A.M., Akagi N., Hwang R., Pasche B., Dawson D.W., Fang D., Bentrem D.J., Munshi H.G., Jung B., Grippo P.J. TGFβ signaling in the pancreatic tumor microenvironment promotes fibrosis and immune evasion to facilitate tumorigenesis. Cancer Res. 2016;76:2525–2539. doi: 10.1158/0008-5472.CAN-15-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esposito I., Penzel R., Chaib-Harrireche M., Barcena U., Bergmann F., Riedl S., Kayed H., Giese N., Kleeff J., Friess H., Schirmacher P. Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J. Pathol. 2006;208:673–685. doi: 10.1002/path.1935. [DOI] [PubMed] [Google Scholar]
- 36.Neesse A., Michl P., Frese K.K., Feig C., Cook N., Jacobetz M.A., Lolkema M.P., Buchholz M., Olive K.P., Gress T.M., Tuveson D.A. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 37.Hosoi F., Izumi H., Kawahara A., Murakami Y., Kinoshita H., Kage M., Nishio K., Kohno K., Kuwano M., Ono M. N-myc downstream regulated gene 1/Cap43 suppresses tumor growth and angiogenesis of pancreatic cancer through attenuation of inhibitor of kappaB kinase beta expression. Cancer Res. 2009;69:4983–4991. doi: 10.1158/0008-5472.CAN-08-4882. [DOI] [PubMed] [Google Scholar]
- 38.Murakami Y., Hosoi F., Izumi H., Maruyama Y., Ureshino H., Watari K., Kohno K., Kuwano M., Ono M. Identification of sites subjected to serine/threonine phosphorylation by SGK1 affecting N-myc downstream-regulated gene 1 (NDRG1)/Cap43-dependent suppression of angiogenic CXC chemokine expression in human pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2010;396:376–381. doi: 10.1016/j.bbrc.2010.04.100. [DOI] [PubMed] [Google Scholar]
- 39.Kovacevic Z., Chikhani S., Lovejoy D.B., Richardson D.R. Novel thiosemicarbazone iron chelators induce up-regulation and phosphorylation of the metastasis suppressor N-myc down-stream regulated gene 1: A new strategy for the treatment of pancreatic cancer. Mol. Pharmacol. 2011;80:598–609. doi: 10.1124/mol.111.073627. [DOI] [PubMed] [Google Scholar]
- 40.Kovacevic Z., Chikhani S., Lui G.Y., Sivagurunathan S., Richardson D.R. The iron-regulated metastasis suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the PI3K and Ras signaling pathways. Antioxid. Redox Signal. 2013;18:874–887. doi: 10.1089/ars.2011.4273. [DOI] [PubMed] [Google Scholar]
- 41.Dixon K.M., Lui G.Y., Kovacevic Z., Zhang D., Yao M., Chen Z., Dong Q., Assinder S.J., Richardson D.R. Dp44mT targets the AKT, TGF-beta and ERK pathways via the metastasis suppressor NDRG1 in normal prostate epithelial cells and prostate cancer cells. Br. J. Cancer. 2013;108:409–419. doi: 10.1038/bjc.2012.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chekmarev J., Azad M., Richardson D. The oncogenic signaling disruptor, NDRG1: Molecular and cellular mechanisms of activity. Cells. 2021;10:2382. doi: 10.3390/cells10092382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menezes S.V., Fouani L., Huang M.L.H., Geleta B., Maleki S., Richardson A., Richardson D.R., Kovacevic Z. The metastasis suppressor, NDRG1, attenuates oncogenic TGF-β and NF-κB signaling to enhance membrane E-cadherin expression in pancreatic cancer cells. Carcinogenesis. 2019;40:805–818. doi: 10.1093/carcin/bgy178. [DOI] [PubMed] [Google Scholar]
- 44.Ambe C.M., Nguyen P., Centeno B.A., Choi J., Strosberg J., Kvols L., Hodul P., Hoffe S., Malafa M.P. Multimodality management of “borderline resectable” pancreatic neuroendocrine tumors: Report of a single-institution experience. Cancer Control. 2017;24 doi: 10.1177/1073274817729076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin R., Liu W., Menezes S., Yue F., Zheng M., Kovacevic Z., Richardson D.R. The metastasis suppressor NDRG1 modulates the phosphorylation and nuclear translocation of beta-catenin through mechanisms involving FRAT1 and PAK4. J. Cell Sci. 2014;127:3116–3130. doi: 10.1242/jcs.147835. [DOI] [PubMed] [Google Scholar]
- 46.Liu W., Xing F., Iiizumi-Gairani M., Okuda H., Watabe M., Pai S.K., Pandey P.R., Hirota S., Kobayashi A., Mo Y.Y., Fukuda K., Li Y., Watabe K. N-myc downstream regulated gene 1 modulates Wnt-beta-catenin signalling and pleiotropically suppresses metastasis. EMBO Mol. Med. 2012;4:93–108. doi: 10.1002/emmm.201100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Z., Zhang D., Yue F., Zheng M., Kovacevic Z., Richardson D.R. The iron chelators Dp44mT and DFO inhibit TGF-β-induced epithelial-mesenchymal transition via up-regulation of N-Myc downstream-regulated gene 1 (NDRG1) J. Biol. Chem. 2012;287:17016–17028. doi: 10.1074/jbc.M112.350470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geleta B., Park K.C., Jansson P.J., Sahni S., Maleki S., Xu Z., Murakami T., Pajic M., Apte M.V., Richardson D.R., Kovacevic Z. Breaking the cycle: Targeting of NDRG1 to inhibit bi-directional oncogenic cross-talk between pancreatic cancer and stroma. FASEB J. 2021;35 doi: 10.1096/fj.202002279R. [DOI] [PubMed] [Google Scholar]
- 49.Whitnall M., Howard J., Ponka P., Richardson D.R. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc. Natl. Acad. Sci. U. S. A. 2006;103:14901–14906. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lovejoy D.B., Sharp D.M., Seebacher N., Obeidy P., Prichard T., Stefani C., Basha M.T., Sharpe P.C., Jansson P.J., Kalinowski D.S., Bernhardt P.V., Richardson D.R. Novel second-generation di-2-pyridylketone thiosemicarbazones show synergism with standard chemotherapeutics and demonstrate potent activity against lung cancer xenografts after oral and intravenous administration in vivo. J. Med. Chem. 2012;55:7230–7244. doi: 10.1021/jm300768u. [DOI] [PubMed] [Google Scholar]
- 51.Yuan J., Lovejoy D.B., Richardson D.R. Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: In vitro and in vivo assessment. Blood. 2004;104:1450–1458. doi: 10.1182/blood-2004-03-0868. [DOI] [PubMed] [Google Scholar]
- 52.Guo Z.L., Richardson D.R., Kalinowski D.S., Kovacevic Z., Tan-Un K.C., Chan G.C. The novel thiosemicarbazone, di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC), inhibits neuroblastoma growth in vitro and in vivo via multiple mechanisms. J. Hematol. Oncol. 2016;9:98. doi: 10.1186/s13045-016-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kovacevic Z., Menezes S.V., Sahni S., Kalinowski D.S., Bae D.H., Lane D.J., Richardson D.R. The metastasis suppressor, N-myc downstream-regulated gene-1 (NDRG1), down-regulates the ErbB family of receptors to inhibit downstream oncogenic signaling pathways. J. Biol. Chem. 2016;291:1029–1052. doi: 10.1074/jbc.M115.689653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim S.C., Jansson P.J., Assinder S.J., Maleki S., Richardson D.R., Kovacevic Z. Unique targeting of androgen-dependent and -independent AR signaling in prostate cancer to overcome androgen resistance. FASEB J. 2020;34:11511–11528. doi: 10.1096/fj.201903167R. [DOI] [PubMed] [Google Scholar]
- 55.Sun J., Zhang D., Bae D.H., Sahni S., Jansson P., Zheng Y., Zhao Q., Yue F., Zheng M., Kovacevic Z., Richardson D.R. Metastasis suppressor, NDRG1, mediates its activity through signaling pathways and molecular motors. Carcinogenesis. 2013;34:1943–1954. doi: 10.1093/carcin/bgt163. [DOI] [PubMed] [Google Scholar]
- 56.Sun J., Zhang D., Zheng Y., Zhao Q., Zheng M., Kovacevic Z., Richardson D.R. Targeting the metastasis suppressor, NDRG1, using novel iron chelators: Regulation of stress fiber-mediated tumor cell migration via modulation of the ROCK1/pMLC2 signaling pathway. Mol. Pharmacol. 2013;83:454–469. doi: 10.1124/mol.112.083097. [DOI] [PubMed] [Google Scholar]
- 57.Lee J.E., Kim J.H. Valproic acid inhibits the invasion of PC3 prostate cancer cells by upregulating the metastasis suppressor protein NDRG1. Genet. Mol. Biol. 2015;38:527–533. doi: 10.1590/S1415-475738420150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li P., Zheng X., Shou K., Niu Y., Jian C., Zhao Y., Yi W., Hu X., Yu A. The iron chelator Dp44mT suppresses osteosarcoma's proliferation, invasion and migration: In vitro and in vivo. Am. J. Transl. Res. 2016;8:5370–5385. [PMC free article] [PubMed] [Google Scholar]
- 59.Kalinowski D.S., Richardson D.R. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol. Rev. 2005;57:547–583. doi: 10.1124/pr.57.4.2. [DOI] [PubMed] [Google Scholar]
- 60.Jansson P.J., Sharpe P.C., Bernhardt P.V., Richardson D.R. Novel thiosemicarbazones of the ApT and DpT series and their copper complexes: Identification of pronounced redox activity and characterization of their antitumor activity. J. Med. Chem. 2010;53:5759–5769. doi: 10.1021/jm100561b. [DOI] [PubMed] [Google Scholar]
- 61.Jansson P.J., Kalinowski D.S., Lane D.J., Kovacevic Z., Seebacher N.A., Fouani L., Sahni S., Merlot A.M., Richardson D.R. The renaissance of polypharmacology in the development of anti-cancer therapeutics: Inhibition of the “Triad of Death” in cancer by Di-2-pyridylketone thiosemicarbazones. Pharmacol. Res. 2015;100:255–260. doi: 10.1016/j.phrs.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z., Ma Q., Li P., Sha H., Li X., Xu J. Aberrant expression of CXCR4 and β-catenin in pancreatic cancer. Anticancer Res. 2013;33:4103–4110. [PubMed] [Google Scholar]
- 63.Sano M., Driscoll D.R., DeJesus-Monge W.E., Quattrochi B., Appleman V.A., Ou J., Zhu L.J., Yoshida N., Yamazaki S., Takayama T., Sugitani M., Nemoto N., Klimstra D.S., Lewis B.C. Activation of WNT/β-catenin signaling enhances pancreatic cancer development and the malignant potential via up-regulation of Cyr61. Neoplasia. 2016;18:785–794. doi: 10.1016/j.neo.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saukkonen K., Hagström J., Mustonen H., Juuti A., Nordling S., Kallio P., Alitalo K., Seppänen H., Haglund C. PROX1 and β-catenin are prognostic markers in pancreatic ductal adenocarcinoma. BMC Cancer. 2016;16:472. doi: 10.1186/s12885-016-2497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gruber R., Panayiotou R., Nye E., Spencer-Dene B., Stamp G., Behrens A. YAP1 and TAZ control pancreatic cancer initiation in mice by direct up-regulation of JAK-STAT3 signaling. Gastroenterology. 2016;151:526–539. doi: 10.1053/j.gastro.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang Z., Zhou C., Cheng L., Yan B., Chen K., Chen X., Zong L., Lei J., Duan W., Xu Q., Li X., Wang Z., Ma Q., Ma J. Inhibiting YAP expression suppresses pancreatic cancer progression by disrupting tumor-stromal interactions. J. Exp. Clin. Cancer Res. 2018;37:69. doi: 10.1186/s13046-018-0740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tu B., Yao J., Ferri-Borgogno S., Zhao J., Chen S., Wang Q., Yan L., Zhou X., Zhu C., Bang S., Chang Q., Bristow C.A., Kang Y., Zheng H., Wang H., et al. YAP1 oncogene is a context-specific driver for pancreatic ductal adenocarcinoma. JCI Insight. 2019;4 doi: 10.1172/jci.insight.130811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ansari D., Ohlsson H., Althini C., Bauden M., Zhou Q., Hu D., Andersson R. The Hippo signaling pathway in pancreatic cancer. Anticancer Res. 2019;39:3317–3321. doi: 10.21873/anticanres.13474. [DOI] [PubMed] [Google Scholar]
- 69.Santoro R., Zanotto M., Simionato F., Zecchetto C., Merz V., Cavallini C., Piro G., Sabbadini F., Boschi F., Scarpa A., Melisi D. Modulating TAK1 expression inhibits YAP and TAZ oncogenic functions in pancreatic cancer. Mol. Cancer Ther. 2020;19:247–257. doi: 10.1158/1535-7163.MCT-19-0270. [DOI] [PubMed] [Google Scholar]
- 70.Hagen T., Vidal-Puig A. Characterisation of the phosphorylation of beta-catenin at the GSK-3 priming site Ser45. Biochem. Biophys. Res. Commun. 2002;294:324–328. doi: 10.1016/S0006-291X(02)00485-0. [DOI] [PubMed] [Google Scholar]
- 71.Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z.C., et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao B., Li L., Lei Q., Guan K.L. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujita H., Ohuchida K., Mizumoto K., Nakata K., Yu J., Kayashima T., Cui L., Manabe T., Ohtsuka T., Tanaka M. Alpha-smooth muscle actin expressing stroma promotes an aggressive tumor biology in pancreatic ductal adenocarcinoma. Pancreas. 2010;39:1254–1262. doi: 10.1097/MPA.0b013e3181dbf647. [DOI] [PubMed] [Google Scholar]
- 74.Bailey J.M., Swanson B.J., Hamada T., Eggers J.P., Singh P.K., Caffery T., Ouellette M.M., Hollingsworth M.A. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin. Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vogelmann R., Ruf D., Wagner M., Adler G., Menke A. Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-beta1 transgenic mouse model. Am. J. Physiol. 2001;280:G164–G172. doi: 10.1152/ajpgi.2001.280.1.G164. [DOI] [PubMed] [Google Scholar]
- 76.Masamune A., Watanabe T., Kikuta K., Shimosegawa T. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin. Gastroenterol. Hepatol. 2009;7:S48–S54. doi: 10.1016/j.cgh.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 77.Sarper M., Cortes E., Lieberthal T.J., Del Río Hernández A. ATRA modulates mechanical activation of TGF-β by pancreatic stellate cells. Sci. Rep. 2016;6:27639. doi: 10.1038/srep27639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biffi G., Oni T.E., Spielman B., Hao Y., Elyada E., Park Y., Preall J., Tuveson D.A. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019;9:282–301. doi: 10.1158/2159-8290.CD-18-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sperb N., Tsesmelis M., Wirth T. Crosstalk between tumor and stromal cells in pancreatic ductal adenocarcinoma. Int. J. Mol. Sci. 2020;21:5486. doi: 10.3390/ijms21155486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohlund D., Handly-Santana A., Biffi G., Elyada E., Almeida A.S., Ponz-Sarvise M., Corbo V., Oni T.E., Hearn S.A., Lee E.J., Chio I.I., Hwang C.I., Tiriac H., Baker L.A., Engle D.D., et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017;214:579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park H.W., Kim Y.C., Yu B., Moroishi T., Mo J.S., Plouffe S.W., Meng Z., Lin K.C., Yu F.X., Alexander C.M., Wang C.Y., Guan K.L. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Le N.T., Richardson D.R. Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: A link between iron metabolism and proliferation. Blood. 2004;104:2967–2975. doi: 10.1182/blood-2004-05-1866. [DOI] [PubMed] [Google Scholar]
- 83.Richardson D.R. Iron chelators as therapeutic agents for the treatment of cancer. Crit. Rev. Oncol. Hematol. 2002;42:267–281. doi: 10.1016/s1040-8428(01)00218-9. [DOI] [PubMed] [Google Scholar]
- 84.Buss J.L., Torti F.M., Torti S.V. The role of iron chelation in cancer therapy. Curr. Med. Chem. 2003;10:1021–1034. doi: 10.2174/0929867033457638. [DOI] [PubMed] [Google Scholar]
- 85.Donfrancesco A., Deb G., De Sio L., Cozza R., Castellano A. Role of deferoxamine in tumor therapy. Acta Haematol. 1996;95:66–69. doi: 10.1159/000203951. [DOI] [PubMed] [Google Scholar]
- 86.Richardson D., Ponka P., Baker E. The effect of the iron(III) chelator, desferrioxamine, on iron and transferrin uptake by the human malignant melanoma cell. Cancer Res. 1994;54:685–689. [PubMed] [Google Scholar]
- 87.Richardson D.R., Milnes K. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents II: The mechanism of action of ligands derived from salicylaldehyde benzoyl hydrazone and 2-hydroxy-1-naphthylaldehyde benzoyl hydrazone. Blood. 1997;89:3025–3038. [PubMed] [Google Scholar]
- 88.Stacy A.E., Palanimuthu D., Bernhardt P.V., Kalinowski D.S., Jansson P.J., Richardson D.R. Zinc(II)-thiosemicarbazone complexes are localized to the lysosomal compartment where they transmetallate with copper ions to induce cytotoxicity. J. Med. Chem. 2016;59:4965–4984. doi: 10.1021/acs.jmedchem.6b00238. [DOI] [PubMed] [Google Scholar]
- 89.Wangpu X., Lu J., Xi R., Yue F., Sahni S., Park K.C., Menezes S., Huang M.L., Zheng M., Kovacevic Z., Richardson D.R. Targeting the metastasis suppressor, n-myc downstream regulated gene-1, with novel di-2-pyridylketone thiosemicarbazones: Suppression of tumor cell migration and cell-collagen adhesion by inhibiting focal adhesion kinase/paxillin signaling. Mol. Pharmacol. 2016;89:521–540. doi: 10.1124/mol.115.103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kalinowski D.S., Yu Y., Sharpe P.C., Islam M., Liao Y.T., Lovejoy D.B., Kumar N., Bernhardt P.V., Richardson D.R. Design, synthesis, and characterization of novel iron chelators: Structure-activity relationships of the 2-benzoylpyridine thiosemicarbazone series and their 3-nitrobenzoyl analogues as potent antitumor agents. J. Med. Chem. 2007;50:3716–3729. doi: 10.1021/jm070445z. [DOI] [PubMed] [Google Scholar]
- 91.Stacy A.E., Palanimuthu D., Bernhardt P.V., Kalinowski D.S., Jansson P.J., Richardson D.R. Structure-activity relationships of di-2-pyridylketone, 2-benzoylpyridine, and 2-acetylpyridine thiosemicarbazones for overcoming Pgp-mediated drug resistance. J. Med. Chem. 2016;59:8601–8620. doi: 10.1021/acs.jmedchem.6b01050. [DOI] [PubMed] [Google Scholar]
- 92.Morvaridi S., Dhall D., Greene M.I., Pandol S.J., Wang Q. Role of YAP and TAZ in pancreatic ductal adenocarcinoma and in stellate cells associated with cancer and chronic pancreatitis. Sci. Rep. 2015;5:16759. doi: 10.1038/srep16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rozengurt E., Sinnett-Smith J., Eibl G. Yes-associated protein (YAP) in pancreatic cancer: At the epicenter of a targetable signaling network associated with patient survival. Signal Transduct. Target. Ther. 2018;3:11. doi: 10.1038/s41392-017-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R.P., Chaudhry S.I., Harrington K., Williamson P., Moeendarbary E., Charras G., Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenbluh J., Nijhawan D., Cox A.G., Li X., Neal J.T., Schafer E.J., Zack T.I., Wang X., Tsherniak A., Schinzel A.C., Shao D.D., Schumacher S.E., Weir B.A., Vazquez F., Cowley G.S., et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paron I., Berchtold S., Voros J., Shamarla M., Erkan M., Hofler H., Esposito I. Tenascin-C enhances pancreatic cancer cell growth and motility and affects cell adhesion through activation of the integrin pathway. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Apte M., Park S., Phillips P., Santucci N., Goldstein D., Kumar R., Ramm G., Buchler M., Friess H., McCarroll J. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 99.Mao B., Wu W., Davidson G., Marhold J., Li M., Mechler B.M., Delius H., Hoppe D., Stannek P., Walter C., Glinka A., Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 100.Bosada F.M., Devasthali V., Jones K.A., Stankunas K. Wnt/beta-catenin signaling enables developmental transitions during valvulogenesis. Development. 2016;143:1041–1054. doi: 10.1242/dev.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Igbinigie E., Guo F., Jiang S.W., Kelley C., Li J. Dkk1 involvement and its potential as a biomarker in pancreatic ductal adenocarcinoma. Clin. Chim. Acta. 2019;488:226–234. doi: 10.1016/j.cca.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 102.Alsolami M., Kuhns S., Alsulami M., Blacque O.E. ERICH3 in primary cilia regulates cilium formation and the localisations of ciliary transport and sonic Hedgehog signaling proteins. Sci. Rep. 2019;9:16519. doi: 10.1038/s41598-019-52830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferrari N., Ranftl R., Chicherova I., Slaven N.D., Moeendarbary E., Farrugia A.J., Lam M., Semiannikova M., Westergaard M.C.W., Tchou J., Magnani L., Calvo F. Dickkopf-3 links HSF1 and YAP/TAZ signalling to control aggressive behaviours in cancer-associated fibroblasts. Nat. Commun. 2019;10:130. doi: 10.1038/s41467-018-07987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Richardson D.R., Sharpe P.C., Lovejoy D.B., Senaratne D., Kalinowski D.S., Islam M., Bernhardt P.V. Dipyridyl thiosemicarbazone chelators with potent and selective antitumor activity form iron complexes with redox activity. J. Med. Chem. 2006;49:6510–6521. doi: 10.1021/jm0606342. [DOI] [PubMed] [Google Scholar]
- 105.Safavi M., Sabourian R., Abdollahi M. The development of biomarkers to reduce attrition rate in drug discovery focused on oncology and central nervous system. Expert Opin. Drug Discov. 2016;11:939–956. doi: 10.1080/17460441.2016.1217196. [DOI] [PubMed] [Google Scholar]
- 106.Ogilvie L.A., Kovachev A., Wierling C., Lange B.M.H., Lehrach H. Models of models: A translational route for cancer treatment and drug development. Front. Oncol. 2017;7:219. doi: 10.3389/fonc.2017.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adams D.J. The valley of death in anticancer drug development: A reassessment. Trends Pharmacol. Sci. 2012;33:173–180. doi: 10.1016/j.tips.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hickman J.A., Graeser R., de Hoogt R., Vidic S., Brito C., Gutekunst M., van der Kuip H. Three-dimensional models of cancer for pharmacology and cancer cell biology: Capturing tumor complexity in vitro/ex vivo. Biotechnol. J. 2014;9:1115–1128. doi: 10.1002/biot.201300492. [DOI] [PubMed] [Google Scholar]
- 109.Boghaert E.R., Lu X., Hessler P.E., McGonigal T.P., Oleksijew A., Mitten M.J., Foster-Duke K., Hickson J.A., Santo V.E., Brito C., Uziel T., Vaidya K.S. The volume of three-dimensional cultures of cancer cells in vitro influences transcriptional profile differences and similarities with monolayer cultures and xenografted tumors. Neoplasia. 2017;19:695–706. doi: 10.1016/j.neo.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoarau-Véchot J., Rafii A., Touboul C., Pasquier J. Halfway between 2D and animal models: Are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int. J. Mol. Sci. 2018;19:181. doi: 10.3390/ijms19010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pothula S.P., Xu Z., Goldstein D., Merrett N., Pirola R.C., Wilson J.S., Apte M.V. Targeting the HGF/c-MET pathway: Stromal remodelling in pancreatic cancer. Oncotarget. 2017;8:76722–76739. doi: 10.18632/oncotarget.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu Z., Vonlaufen A., Phillips P.A., Fiala-Beer E., Zhang X., Yang L., Biankin A.V., Goldstein D., Pirola R.C., Wilson J.S. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am. J. Pathol. 2010;177:2585–2596. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pothula S.P., Xu Z., Goldstein D., Biankin A.V., Pirola R.C., Wilson J.S., Apte M.V. Hepatocyte growth factor inhibition: A novel therapeutic approach in pancreatic cancer. Br. J. Cancer. 2016;114:269–280. doi: 10.1038/bjc.2015.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang R., Kikuchi A.T., Nakao T., Russell J.O., Preziosi M.E., Poddar M., Singh S., Bell A.W., England S.G., Monga S.P. Elimination of Wnt secretion from stellate cells is dispensable for zonation and development of liver fibrosis following hepatobiliary injury. Gene Expr. 2019;19:121–136. doi: 10.3727/105221618X15373858350141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aguilera K.Y., Dawson D.W. WNT ligand dependencies in pancreatic cancer. Front. Cell Dev. Biol. 2021;9:671022. doi: 10.3389/fcell.2021.671022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Froeling F.E., Feig C., Chelala C., Dobson R., Mein C.E., Tuveson D.A., Clevers H., Hart I.R., Kocher H.M. Retinoic acid–induced pancreatic stellate cell quiescence reduces paracrine Wnt–β-catenin signaling to slow tumor progression. Gastroenterology. 2011;141:1486–1497. doi: 10.1053/j.gastro.2011.06.047. 1497.e1-14. [DOI] [PubMed] [Google Scholar]
- 117.Qian S., Tan X., Liu X., Liu P., Wu Y. Exosomal Tenascin-C induces proliferation and invasion of pancreatic cancer cells by WNT signaling. Onco Targets Ther. 2019;12:3197–3205. doi: 10.2147/OTT.S192218. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.He S., Huang Q., Hu J., Li L., Xiao Y., Yu H., Han Z., Wang T., Zhou W., Wei H., Xiao J. EWS-FLI1-mediated TENASCIN-C expression promotes tumour progression by targeting MALAT1 through integrin α5β1-mediated YAP activation in Ewing sarcoma. Br. J. Cancer. 2019;121:922–933. doi: 10.1038/s41416-019-0608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gong X.G., Lv Y.F., Li X.Q., Xu F.G., Ma Q.Y. Gemcitabine resistance induced by interaction between alternatively spliced segment of tenascin-C and annexin A2 in pancreatic cancer cells. Biol. Pharm. Bull. 2010;33:1261–1267. doi: 10.1248/bpb.33.1261. [DOI] [PubMed] [Google Scholar]
- 120.Wang Z., Wei Q., Han L., Cao K., Lan T., Xu Z., Wang Y., Gao Y., Xue J., Shan F., Feng J., Xie X. Tenascin-c renders a proangiogenic phenotype in macrophage via annexin II. J. Cell. Mol. Med. 2018;22:429–438. doi: 10.1111/jcmm.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Medici D., Hay E.D., Olsen B.R. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol. Biol. Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stylianou A., Gkretsi V., Stylianopoulos T. Transforming growth factor-beta modulates pancreatic cancer associated fibroblasts cell shape, stiffness and invasion. Biochim. Biophys. Acta Gen. Subj. 2018;1862:1537–1546. doi: 10.1016/j.bbagen.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoon H., Tang C.M., Banerjee S., Delgado A.L., Yebra M., Davis J., Sicklick J.K. TGF-beta1-mediated transition of resident fibroblasts to cancer-associated fibroblasts promotes cancer metastasis in gastrointestinal stromal tumor. Oncogenesis. 2021;10:13. doi: 10.1038/s41389-021-00302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haber P.S., Keogh G.W., Apte M.V., Moran C.S., Stewart N.L., Crawford D.H., Pirola R.C., McCaughan G.W., Ramm G.A., Wilson J.S. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am. J. Pathol. 1999;155:1087–1095. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dennler S., Andre J., Alexaki I., Li A., Magnaldo T., ten Dijke P., Wang X.J., Verrecchia F., Mauviel A. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 126.Xi R., Pun I.H., Menezes S.V., Fouani L., Kalinowski D.S., Huang M.L., Zhang X., Richardson D.R., Kovacevic Z. Novel thiosemicarbazones inhibit lysine-rich carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) coisolated (LYRIC) and the LYRIC-induced epithelial-mesenchymal transition via upregulation of N-myc downstream-regulated gene 1 (NDRG1) Mol. Pharmacol. 2017;91:499–517. doi: 10.1124/mol.116.107870. [DOI] [PubMed] [Google Scholar]
- 127.Mullor J.L., Dahmane N., Sun T., Ruiz i Altaba A. Wnt signals are targets and mediators of Gli function. Curr. Biol. 2001;11:769–773. doi: 10.1016/s0960-9822(01)00229-9. [DOI] [PubMed] [Google Scholar]
- 128.Kagawa S., Takano S., Yoshitomi H., Kimura F., Satoh M., Shimizu H., Yoshidome H., Ohtsuka M., Kato A., Furukawa K., Matsushita K., Nomura F., Miyazaki M. Akt/mTOR signaling pathway is crucial for gemcitabine resistance induced by Annexin II in pancreatic cancer cells. J. Surg. Res. 2012;178:758–767. doi: 10.1016/j.jss.2012.05.065. [DOI] [PubMed] [Google Scholar]
- 129.Zhan T., Rindtorff N., Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krishnamurthy N., Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jung Y.S., Park J.I. Wnt signaling in cancer: Therapeutic targeting of wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020;52:183–191. doi: 10.1038/s12276-020-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ram Makena M., Gatla H., Verlekar D., Sukhavasi S., Pandey M.K., Pramanik K.C. Wnt/β-catenin signaling: The culprit in pancreatic carcinogenesis and therapeutic resistance. Int. J. Mol. Sci. 2019;20:4242. doi: 10.3390/ijms20174242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brack S.S., Silacci M., Birchler M., Neri D. Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-C. Clin. Cancer Res. 2006;12:3200–3208. doi: 10.1158/1078-0432.CCR-05-2804. [DOI] [PubMed] [Google Scholar]
- 134.Spenle C., Saupe F., Midwood K., Burckel H., Noel G., Orend G. Tenascin-C: Exploitation and collateral damage in cancer management. Cell Adh. Migr. 2015;9:141–153. doi: 10.1080/19336918.2014.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Noubissi F.K., Goswami S., Sanek N.A., Kawakami K., Minamoto T., Moser A., Grinblat Y., Spiegelman V.S. Wnt signaling stimulates transcriptional outcome of the Hedgehog pathway by stabilizing GLI1 mRNA. Cancer Res. 2009;69:8572–8578. doi: 10.1158/0008-5472.CAN-09-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bachem M.G., Schneider E., Gross H., Weidenbach H., Schmid R.M., Menke A., Siech M., Beger H., Grunert A., Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–432. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- 137.Apte M.V., Haber P.S., Applegate T.L., Norton I.D., McCaughan G.W., Korsten M.A., Pirola R.C., Wilson J.S. Periacinar stellate shaped cells in rat pancreas: Identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vonlaufen A., Phillips P.A., Yang L., Xu Z., Fiala-Beer E., Zhang X., Pirola R.C., Wilson J.S., Apte M.V. Isolation of quiescent human pancreatic stellate cells: A promising in vitro tool for studies of human pancreatic stellate cell biology. Pancreatology. 2010;10:434–443. doi: 10.1159/000260900. [DOI] [PubMed] [Google Scholar]
- 139.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study includes no data deposited in external repositories.