Abstract
Aging and inflammation both contribute pivotally to cardiovascular (CV) and cerebrovascular disease, the leading causes of death and disability worldwide. The concept of inflamm-aging recognizes low-grade inflammatory pathways observed in the elderly that contribute to CV risk. Understanding the mechanisms that link inflammation and aging could reveal new therapeutic targets and offer options to cope with the growing aging population worldwide. This review reports recent scientific advances in the pathways through which inflamm-aging mediates age-dependent decline in CV function and disease onset and considers critically the translational potential of such concepts into everyday clinical practice.
Keywords: inflamm-aging, vascular aging, inflammation, cardiovascular disease
Condensed abstract
Aging and inflammation both contribute pivotally to cardiovascular and cerebrovascular disease, the leading cause of death and disability worldwide. The concept of inflamm-aging highlights low-grade inflammation observed in the elderly that contributes to cardiovascular (CV) risk. Understanding the mechanisms that link inflammation and aging could uncover new therapeutic targets and offer options to cope with the growing aging population worldwide.
Recent clinical trials proved the efficacy of anti-inflammatory therapies such as colchicine and canakinumab in secondary prevention of cardiovascular (CV) afflictions (1,2). Accordingly, inflamm-aging has recently emerged as a potential mediator of age-dependent CV disease. This narrative review reports the most recent advances on inflamm-aging and the pathways through which it mediates age-dependent decline in CV function and onset of disease. We place the scientific advances into the context of clinical practice.
Inflamm-aging
Aging is defined as the time-related deterioration in physiological functions necessary for survival and fertility and has been considered inevitable. Aging can accompany frailty and hasten development of certain diseases. In the early 2000s the description and investigation of population clusters (i.e. blue zones) in Sardinia, Italy showing high prevalence of healthy centenarians paved the way for the research into mechanisms associated with “successful” aging (3). Nowadays, aging is accounted among the most complex biological processes, driven by a multitude of different intertwined mechanisms which the trans-NIH Geroscience interest group have clustered in seven pillars. Among them, inflammation has recently emerged as a critical mediator of several CV afflictions (1).
With inflammation being a determinant of aging and playing a central role in most age-related chronic disorders, the next step was to investigate the two processes with an integrative approach. Franceschi et al. then coined the term “inflamm-aging” to describe the chronic, low-grade, systemic inflammation which develops with age in the absence of overt infections (thus, sterile inflammation). Inflamm-aging occurs with a broad deregulation of several inflammatory molecules and the defective functioning cells of both innate and adaptive immunity which impairs the ability to mount an appropriate response to immunogenic stimuli (4).
Inflamm-aging still lacks clinically defined and universally accepted diagnostic criteria. In 2019, Alpert et al. reported on cell-subset dynamics in a longitudinal cohort of 135 healthy individuals for 9 years showing that immune-cell frequencies changed at substantially different rates with young people having more stable patterns (5). Specifically, they identified steady-state levels toward which a cell subset converged with time introducing the concept of an “older adult homeostasis”. The description of a trajectory in cell subset distribution over the lifespan led to a proposed quantification of the immune-aging process: the IMM-AGE score. To obviate the need for high-dimensional cytometry data, they reported a gene signature that estimates IMM-AGE and showed the ability to predict all-cause mortality (5). Biomarkers of inflammation rise in the serum of elders. Yet, such observations lack consistency, as variable trajectories of pro-inflammatory cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α – have been recorded with age (6). Age can also augment anti-inflammatory mediators and healthy centenarians show enhanced anti-inflammatory molecule expression that can counterbalance the deleterious effect of inflamm-aging (7). This issue was previously investigated by untargeted proteomics that defined a combination of biomarkers of inflammation and hemostasis that could discriminate the presence of an unhealthy phenotype in the elderly (8). Another investigation of the blood immunome in 1,001 individuals aged 8-96 defined an inflammatory aging clock (i.e. iAge) developed via deep machine learning. Such an inflamm-aging metric predicted multimorbidity, immunosenescence, frailty and cardiovascular aging in different cohorts and identified the chemokine (C-X-C motif) ligand 9 (CXCL9) as the strongest contributor to the inflammatory clock (9). Circulating extracellular microvesicles (EMVs) can serve as systemic messengers involved in inflammation regulation and reflecting the state of the cell from which they originate. EMVs derived from activated endothelium, platelets, and immune cells are increased in liquid biopsies of community-dwelling octogenarians with unsuccessful aging and cognitive decline compared to those found in healthy aging octogenarians (10). Unraveling the mechanisms that drive the age-dependent activation of inflammation could lead to the development of effective therapeutic strategies. Several processes are currently undergoing both experimental and clinical investigations including immunosenescence, garbaging, chronic infections, dysbiosis, metaflammation, clonal hematopoiesis of indeterminate potential, epigenetic, genetic, and differences according to sex (Central Illustration).
Central illustration: Targeting inflamm-aging to cope with age-related cardio- and cerebrovascular diseases.
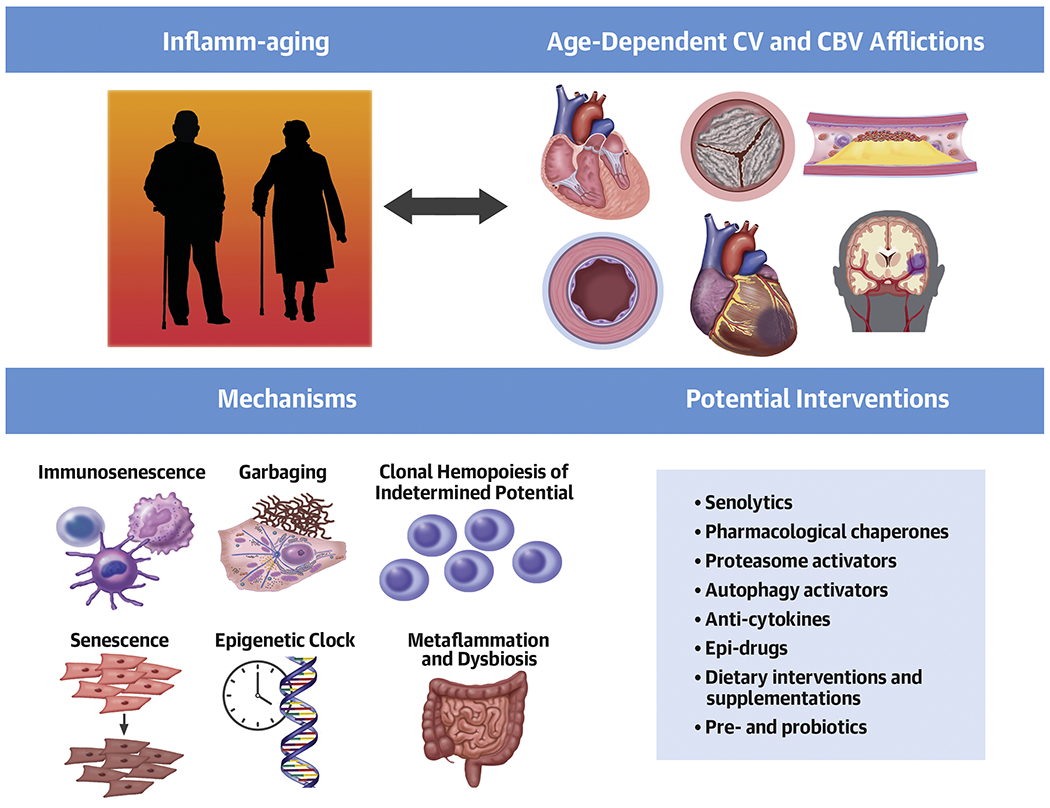
Different interventions targeting inflamm-aging-related pathways are now being tested in the context of age-related conditions. CV: cardiovascular; CBV: cerebrovascular.
Cellular senescence and immunosenescence
The term cellular senescence describes the irreversible arrest of the cell cycle and the acquisition of the so-called Senescence-Associated Secretory Phenotype (SASP). During aging, senescent cells accumulate in most organs including those of the CV system. SASP involves the increased production of reactive oxygen species and the synthesis and release of several inflammatory mediators (11). The SASP influences surrounding cells via paracrine effects and participates in various age-related CV conditions including arterial stiffness, atherosclerosis, and myocardial remodeling. Of interest, emerging evidence suggests that cardiomyocytes can develop an atypical SASP which entails the expression of pro-fibrotic and hypertrophic mediators (12). Countering cellular senescence, agents of a class dubbed senolytics showed beneficial effect in experimental models of CV disease employing aged mice (13).
The concept of cellular senescence has expanded to include the age-dependent functional changes of immune cells termed “immunosenescence”. This multifactorial process relates to the effect of both genetic and environmental factors that increase vulnerability to infectious and chronic diseases. Recent investigations showed that the age-related modification in immune cell distribution and function differs among different populations leading to the definition of the concept of “immunobiography” as the combination of life-long immunogenic stimulation of immune cells influencing the ability to mount appropriate immune responses when older and accounting for the immune heterogeneity observed in the elderly (14). Dysregulation of innate and adaptive immune cells can promote age-related changes in CV function and onset of CV diseases. Figure 1 presents an overview of those alterations.
Figure 1. Immunosenescence and inflamm-aging.
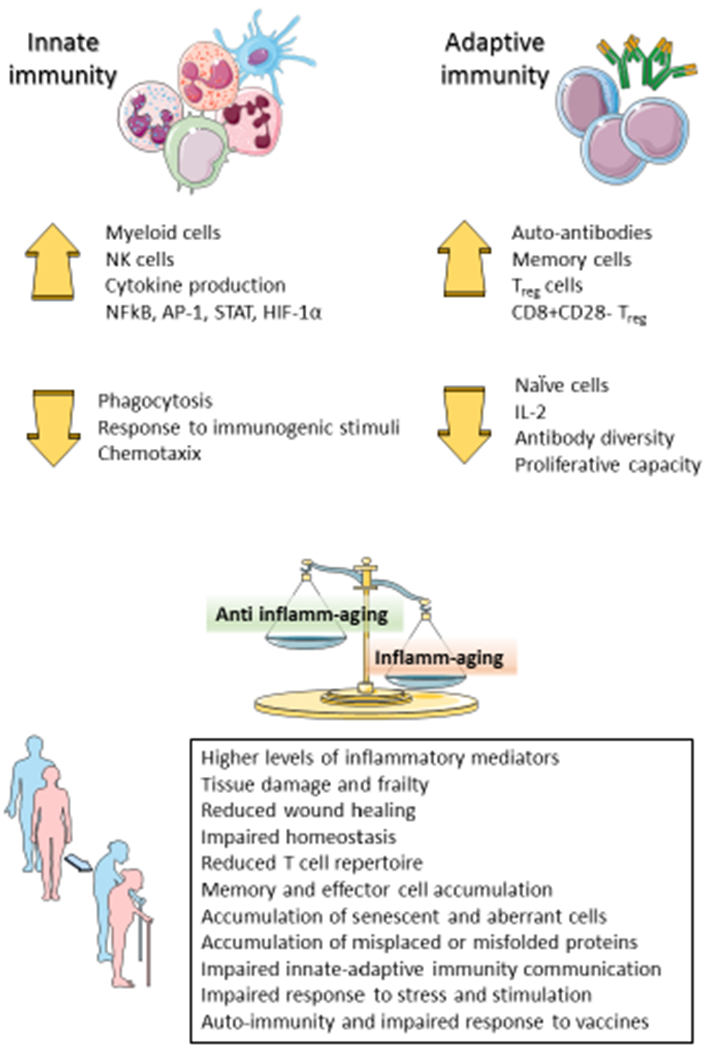
“Immunosenescence” describes the effect of aging on immune cell distribution and characteristics and sustains inflamm-aging.
Several senolytics have been described, all of them with non-specific targets thus with high potential for broad effect on the senescence of different cell types, but also for unwanted actions. Yet, to date promising preclinical results have emerged with the bcl-XL inhibitor navitoclax and with the combination of dasatinib and quercetin, agents that regulate different survival pathways involved in senescence resistance towards apoptosis (15). The latter combination reduces SASP-associated molecules in patients with idiopathic pulmonary fibrosis or diabetic kidney disease, and trials on CV outcomes are currently ongoing (16).
Garbaging
Despite age-related changes, immune cells still require activation to fuel the inflammatory process. Everyday our body produces considerable cellular debris and modified or misplaced proteins (denoted “garbage”). Ancestral mechanisms can recognize such products including scavenger and pattern recognition receptors and facilitate their clearance through phagocytosis. With age, “garbage” production increases, spurring the formulation of the “garbaging” theory which posits that age disturbs the balance between production and disposal of such waste material. The resulting chronic stimulation of PRRs fuels the release of inflammatory mediators and the development of immunity against autoantigens (17) (Figure 2). Interventions that can remedy this imbalance (e.g. caloric restriction, treatment with rapamycin) showed anti-inflamm-aging features. Similarly, also the ubiquitin-proteasome system might be an anti-aging target since preserved proteosome function associates with human longevity, and proteosome activation can prolong lifespan and reduce age-related afflictions in animal experiments (18). The vast list of modified, misplaced, or misfolded products that can act as damage-associated molecular patterns (DAMPs) and trigger PRR not only includes several cell components such as mitochondrial or extracellular DNA and histones, cardiolipin, ATP and UTP, altered lipids, but also misfolded proteins such as amyloid fibrils. Indeed, amyloid accumulation represents an important link between inflamm-aging and age-related CV and cerebrovascular conditions. Although formerly believed to play role only in rare familial conditions, experimental and clinical evidence now implicate misfolded proteins in common conditions of the elderly such as atherosclerosis, arterial thrombosis, dementia, and heart failure (HF) (18).
Figure 2. Garbaging.
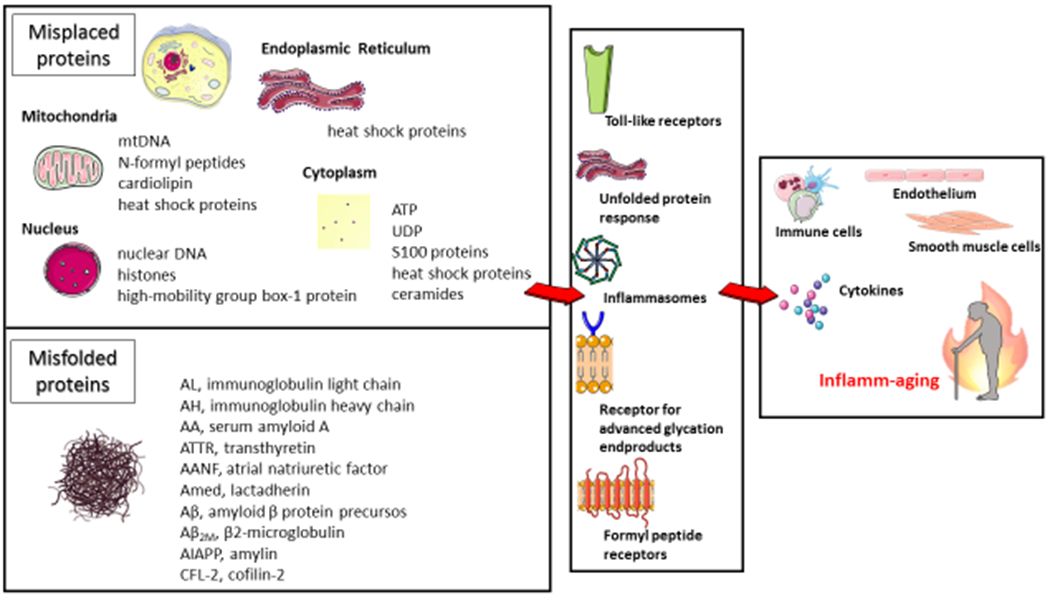
Misplaced and misfolded proteins accumulate with age and are recognized as damage-associated molecular patterns by common receptors eventually fueling inflamm-aging with implications for age-related cardiovascular and cerebrovascular conditions.
Approaches aiming at attenuating cardiac and vascular proteotoxicity by stabilizing protein folding, increasing their clearance, or blunting the unfolded protein response are currently under investigation, and have entered clinical use for treatment of forms of cardiac amyloidosis. Chaperones refold misfolded protein allowing for their disposal, FDA-approved pharmacological chaperones (i.e. 4-phenylbutyrate and tauroursodeoxycholic acid) have been tested in trials for Parkinson and Huntington diseases and showed promising results in experimental myocardial ischemia and remodeling (19). Similarly, positive modulators of heat shock proteins have shown encouraging experimental results in age-related CV disease, although we lack clinical data (20). Drugs promoting autophagy such as rapamycin, chloramphenicol and sulphaphenazole have been tested in experimental aging and CV diseases with promising results (21). Although such results serve as proof of principle, they lack broad translational potential for several reasons. Antibodies against misfolded proteins promoting their clearance may find here another field of application as reflected by the recent (albeit controversial) approval of the monoclonal antibody aducanumab targeting aggregated forms of amyloid beta in patients with Alzheimer’s disease. Several clinical trials are currently evaluating immunotherapies, small interfering RNAs or antisense nucleotide, and gene editing against specific forms of cardiac amyloidosis with promising results. Yet, none of them currently focus on the elderly population leaving this field ripe for investigation.
Microbiome, nutrition and metaflammation
Comprised of trillions of microorganisms in close contact with our intestinal mucosae, the microbiome furnishes a lifelong source for inflammatory stimuli whose potential role in healthy aging or age-related CV diseases has only recently emerged. The composition of the intestinal microbiome changes with aging. Bacteria phyla more abundant in younger individuals may possess anti-inflammatory properties (i.e. Firmicutes) and undergo progressive replacement by facultative anaerobes whose abundance shows strong correlation with circulating levels of pro-inflammatory cytokines such as IL-6 and IL-8 (22). Recent evidence suggests that Christensenellaceae characterize the gut microbiota of supercentenarians (a family that belongs to the Firmicutes phylum) and is highly heritable showing consistent associations with host health (23).
Moreover, the age-related reduction in mucosal barrier function favors the absorption of pro-inflammatory components such as lipopolysaccharide (LPS) mediating chronic low grade endotoxemia and activation of the immune system (24). By modulating inflammation, the gut microbiota may participate in the pathophysiology of many cardiovascular conditions. We recently reported that in mice with endotoxemia, treatment with a specific antibody neutralizing IL-1β effectively reduced arterial thrombogenicity by acting on NET-associated tissue factor (25). Accordingly, gut microbiota modulation may furnish an attractive strategy for healthy aging that can be pursued by dietary approaches (e.g. Mediterranean diet, omega-3 fatty acid supplementation, phenols), administration of pre- and probiotics or even microbiome transplantation. For example, we showed that dietary supplementation with alpha-linolenic acid – a cheap, environment-friendly, and abundantly available plant-derived omega-3 fatty acid – can modulate the gut microbiota composition of aged mice with direct effects on levels of metabolites such as trimethyl amine oxide and short chain fatty acids and reduced levels of mediators of inflammation (26,27). Clinical studies confirmed the anti-inflammatory effects of omega-3 fatty acids in different age ranges including the elderly. Yet, their effect on age-related CV conditions remains clinically unproven (28).
Nutrition can not only influence a healthy microbiome, but may also contribute to inflamm-aging according to the “metaflammation theory” (29). Abundant experimental evidence supports the relationship between dietary intake and inflammation. In immune cells, dietary fatty acids induce the activation of Toll-like receptors, with transcription of NF-κB-dependent genes, NLRP3 inflammasome assembly, and consequent production and activation of pro-inflammatory cytokines. Yet, adipocytes comprise the core of the metaflammation due to ischemic conditions and endoplasmic reticulum stress. Dysfunctional adipocytes increase the production of pro-inflammatory adipocytokines such as tumor necrosis factor, leptin, and resistin at the expense of anti-inflammatory adiponectin. In the elderly, adipose tissue undergoes extensive changes, with reduced pre-adipocyte differentiation, increased fibrosis, accumulation of senescent cells and accumulation of immune cells (30). Consequently, its secretory profile fuels inflamm-aging: leptin resistance increases the level of such pro-inflammatory adipocytokine while the “adiponectin paradox” associates increased levels of adiponectin with cardiovascular and overall mortality, distinct from observations in younger individuals (31). Of interest such a paradox was also observed in patients with age-related CV diseases such as HF (32). Caloric restriction or other pro-longevity dietary interventions as well as regular physical activity can ameliorate adipose tissue function, increase adiponectin sensitivity, and reduce the levels of pro-inflammatory mediators with beneficial effects on age-related afflictions (33). In general, healthy lifestyle behaviors (i.e. diet, physical activity, and weight control) help to preserve physiological body composition, resist age-associated sarcopenia, can benefit the immune system, metabolism, and limit inflammation (reviewed in (34,35)).
Clonal Hematopoiesis of Indeterminate Potential
Clonal hematopoiesis of indeterminate potential (CHIP) has recently emerged as an important link between inflammation, aging and cardiovascular disease. CHIP is defined as the presence of somatic mutations in a subset of leukemia driver genes in hematopoietic stem cells that give rise to clones with a variant allele fraction in peripheral blood of at least 2% in individuals without hematological malignancies. The diagnosis of CHIP requires DNA sequencing of blood (36). Somatic mutations accumulate with age; thus, CHIP is rare in young individuals, more common in people above 60 years and involves over 1 in 10 people aged over 70. People with CHIP have a relatively low risk of developing hematologic malignancies (<1%/year) but a markedly increased risk of developing atherosclerosis, thrombotic events, and HF. In older individuals with no history of CV disease the risk of incident coronary artery disease was almost doubled in those carrying CHIP, while CHIP was four times more prevalent in patients with early-onset myocardial infarction than in age-matched healthy controls (37). Furthermore, the presence of CHIP mutations was associated with disease progression or higher mortality in patients with HF (38,39). The quest for molecular mechanisms that explain the link between CHIP and CV disease demonstrated the involvement of inflamm-aging as CHIP associates with increased levels of cytokines such as IL-1β, IL-6 and IL-8 (40), all involved in the development of CV afflictions (41).
A very recent study underscored this concept by demonstrating a highly inflamed transcriptome in circulating monocytes and T cells of patients with HF and DNMT3A CHIP potentially contributing to the aggravation of chronic HF (42). Furthermore, the high thrombotic risk of patients with a JAK2V617F mutation relates to the leukocyte rather than platelet count, and their neutrophils showed increased propensity towards generation of pro-thrombotic neutrophil extracellular traps (43). Animals bearing CHIP mutations demonstrated augmented atherosclerosis and HF (44). Similarly, experimental HF studies demonstrated that genetic or CRISPER-based deletion of Tet2 or Dnmt3a in bone marrow increased cardiac hypertrophy and fibrosis as well as reduced ventricular function (45).
In myeloid cells, Tet2 supresses inflammation by inhibiting NLRP3 inflammasome activation while stimulated murine macrophages missing Tet2 showed increased expression of several pro-inflammatory mediators (46). Dnmt3a exhibits similar anti-inflammatory effects although the mechanisms are less well characterized (42). Transplantation of Jak2V617F bone marrow increased atherosclerosis in mice, while mice harboring such mutation showed augmented venous thrombosis likely related to increased NET formation (43). Lastly, the relationship between inflammation and CHIP is bi-directional with several inflammatory mediators, including IL-1β, being associated with increased risk of developing clonal hematopoiesis (47).
To date, no therapies are available to blunt CV risk in patients with CHIP. The pathophysiological role of specific cytokines together with the ready availability of approved anti-cytokine drugs should stimulate randomized clinical trials to allocate such therapy based on the presence of CHIP.
Genetic, epigenetic and methylation clock
Genetic complement contributes importantly to lifetime CV risk, influences the onset of inflamm-aging and determines lifespan. Genome-wide association studies (GWAS) performed with Italian and Chinese centenarians included polymorphisms in the IL6 gene locus among the top genetic variants correlated with longevity and accounting for up to 1% of variance in lifespan (48,49). Indeed, circulating levels of IL-6 identified, together with those of soluble tumor necrosis factor-α receptor-1, as best NF-κB-related predictors of inflammatory risk in the InCHIANTI study. The resulting inflammation index score was tested in a large study with elderly subjects and resulted as the best predictor of 10-year all-cause mortality (50). Mendelian randomization studies and GWAS support the causality of IL-6 signaling in ischemic stroke, atherosclerosis, and coronary artery disease (51). Recently, a GWAS meta-analysis including more than 350’000 individuals included IL6 locus among the susceptibility genes underlying calcific aortic valve stenosis, implying a role for inflamm-aging also for this age-related CV condition (52).
We and others previously reported how the genes involved in the regulation of aging (i.e. aging and longevity genes), also mediate the age-related decline of CV function and onset of diseases through common molecular pathways including inflammation (11). Aging influences such genes through different mechanisms including epigenetic regulation. During aging, epigenetic patterns undergo profound modifications in response to environmental stimuli with effects on several age-dependent conditions, including CV diseases. Age-associated remodeling of the epigenetic signature includes loss of heterochromatin, modification of epigenetic markers at specific genomic locations and increased epigenetic variability. Reduced DNA and histone methylation results in loss of heterochromatin at repetitive sequences which is a hallmark of cell senescence. Hypomethylated DNA from older donors displays higher immunogenicity and activates immune cells through highly conserved DAMP receptors including members of the Toll-like family (53). Indeed, in people aged >90 years plasma levels of unmethylated cell-free DNA associated positively with markers of inflamm-aging and frailty scores (54). Furthermore, assessment of gain or loss of DNA epigenetic markers such as methyl groups revealed consistent changes associated with aging (55). Computational models of such reproducible modifications have yielded high estimation power on the age and known as epigenetic (or methylation) clocks (56). Age-dependent hypo- or hypermethylation is frequently seen in the promoter region of different inflammation mediators leading to their increased expression (57). DNA methylation changes are tissue-specific, whole blood epigenetic clocks integrated with known age-related changes in blood cell composition have shown correlation with several markers of inflamm-aging including CRP, TNF-α and IL-6 (58). Changes in DNA methylation patterns contribute to the pathophysiology of hypertension, atherosclerosis, and ischemic cardio- or cerebrovascular diseases both experimentally and clinically. Accordingly, chronological and biological age estimation by different methylation clocks can predict aging characteristics and healthspan (59).
Several compounds with rejuvenating properties including metformin, senolytics and NAD+ inducers, were reported to regulate epigenetic modifications. Yet, despite promising experimental results, to date novel drugs that target specifically epigenetic processes (also known as epi-drugs) have undergone limited clinical investigation due to their broad range of actions rising concerns for unwanted effects. Apabetalone is an epi-drug that inhibits the family of epigenetic reader proteins called bromo- and extraterminal domain protein (BET) which regulate the assembly of transcription complexes. This drug showed anti-inflammatory and anti-atherosclerotic properties in animals as well as an adequate safety profile in phase I and II clinical trials (60). Yet, despite its anti-inflammatory properties (61), apabetalone did not meet the primary endpoint in the recent BETonMACE trial that investigated secondary CV prevention in patients with diabetes (62). In 2019, a pilot trial investigating thymus regeneration by growth hormone in combination with dehydroepiandrosterone and metformin in 9 healthy donors suggested that epigenetic aging can be reversed with volunteers showing a 2-year decrease in epigenetic vs. chronological age even six months after treatment discontinuation (63). Confirmation of such preliminary findings will require rigorous randomized clinical trials.
Sex-related aspects
Aging differs substantially between sexes. Women tend to live longer than men and show lower biological age when assessed through defined biomarkers (64). Such a difference reflects on sex-specific trajectories for most of age-dependent diseases but often not sex-specific diagnostic and therapeutic approaches. Such paradox prompted international quests for increased representation of female animals and women in experimental and clinical studies as part of the broader momentum towards personalized and precision medicine (65). Many of the abovementioned mechanisms show sexual dimorphism. A very recent study reports on higher number of senescent cells in male mice as compared to females, a difference that only narrows late in life (66). Shorter telomeres in males and increased capacity for regeneration and proliferation of female stem cells may contribute to these differences (67). Similarly, female tissues demonstrate higher beneficial activity of proteosomes in maintaining homeostasis by removing defective proteins compared to those of male mice (68). Again, age may reduce such differences (69).
Epigenetic clocks show sex-specific trends at all ages, with men showing higher epigenetic ages than women (70). Most tissues show such effects and in women earlier menopause associates with increased epigenetic age (71). In a recent metanalysis of epigenome-wide association studies, the age-by-sex interaction of methylation patterns found significant differences in methylation levels and variability across the sites. Furthermore, individuals with successful vs. unsuccessful aging showed peculiar sex-specific alterations in top methylated genes with male centenarians showing “feminization” of the methylation profiles (72). Also, CHIP occurs more frequently in men as compared to women, and early menopause associates with greater incidence of CHIP (73).
Indeed, the aging immune system shows sex-specific trajectories, with men showing overall more maladaptive changes. Immunosenescence trajectories have very recently undergone tracking in aging men and women. Specifically, aging showed a shared epigenomic signature in terms of declining naïve T cell and increasing monocyte and cytotoxic cell functions with men showing a greater magnitude of changes especially after age 65 (74).
Recent experimental evidence suggests that inflamm-aging associates with maladaptive changes in cardiac structure and function in male, but not female, mice (75). The sexual dimorphism shown in several components of inflamm-aging relate to the different distribution of CV diseases among different sexes and calls for a greater effort in implementing personalized medicine.
Conclusions and future perspectives
Recent investigations unveiled mechanisms by which inflamm-aging influences the onset of CV and cerebrovascular diseases thereby identifying novel therapeutic targets. Inflamm-aging may be considered a syndrome characterized by several derangements involving the immune system, epigenetics, hematopoietic stem cells, macromolecular damage, altered metabolism, cellular senescence, and protein disposal. It is unlikely that a single target will blunt onset and progression of inflamm-aging thus, future efforts to decipher more fully its mediators and identify novel effective therapeutics will require a holistic approach taking into consideration the full spectrum of these biological processes and sex-related differences. In terms of available interventions, inflamm-aging remains to date a relatively understudied area lacking specific clinical trials designed on elderly inflamed populations. Future clinical investigations should aim at answering important unanswered question including: (i) What are the diagnostic criteria for inflamm-aging? (ii) Is age enough to distinguish inflammation from inflamm-aging? (iii) Will specific trials on inflamm-aging population replicate the successful findings of recent anti-inflammatory trials?
Highlights.
Low-grade inflammation in the elderly (“inflammaging”) contributes to several diseases, but a comprehensive clinical definition is lacking.
Clonal hematopoiesis and epigenetic changes underlying inflammation in the elderly may be leveraged to find new therapeutic targets.
Targeting inflammation may reduce the burden of cardiovascular morbidity in an aging population.
Funding
GGC received support for this work from: the Swiss National Science Foundation [310030_175546], the Swiss Heart Foundation, the Alfred and Annemarie von Sick Grants for Translational and Clinical Research Cardiology and Oncology and the Foundation for Cardiovascular Research–Zurich Heart House. GGC is also the recipient of a Sheikh Khalifa’s Foundation Ass. Professorship at the Faculty of Medicine, University of Zurich. LL received support from the Swiss Heart Foundation and the Novartis Foundation for medical-biological Research. PL receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund. FM received support from the “Rete Cardiologica” of Italian Ministry of Health (#2754291).
Disclosures
LL, PL and GGC are coinventors on the International Patent WO/2020/226993 filed in April 2020. The patent relates to the use of antibodies which specifically bind IL-1α to reduce various sequelae of ischemia-reperfusion injury to the central nervous system. LL reports speaker fees outside of this work from Daichi-Sankyo. LB is founder of two spin-offs (Glycardial Diagnostics and Ivestatin Therapeutics), has several patents, has received consultancy fees from Sanofi, and Novartis and speaker fees from Lilly, Pfizer, and AstraZeneca (all unrelated to this work). TFL reports educational and research grants outside this work from Abbott, Amgen, AstraZeneca, Boehringer-Ingelheim, Daichi-Sankyo, Novartis, Servier, Sanofi and Vifor and speaker fees from Amgen and Daichi-Sankyo. PL is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Novartis, Pfizer, Sanofi-Regeneron. PL is a member of scientific advisory board for Amgen, Caristo, Cartesian, Corvidia Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, PlaqueTec, and XBiotech, Inc. PL’s laboratory has received research funding in the last 2 years from Novartis. PL is on the Board of Directors of XBiotech, Inc. PL has a financial interest in Xbiotech, a company developing therapeutic human antibodies. PL’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. FM has nothing to disclose.
Abbreviation list
- CV
cardiovascular
- NIH
National Institute of Health
- IL
interleukin
- TNF
tumor necrosis factor
- CXCL
CXC ligand
- EMV
extracellular microvesicle
- SASP
senescence-associated secretory pattern
- LPS
lipopolysaccharide
- CHIP
clonal hemopoiesis of indetermined potential
- GWAS
genome-wide association study
- DAMP
damage-associated molecular patterns
- BET
bromo- and extraterminal domain
- HF
heart failure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liberale L, Montecucco F, Schwarz L, Luscher TF, Camici GG. Inflammation and cardiovascular diseases: lessons from seminal clinical trials. Cardiovasc Res 2021;117:411–422. [DOI] [PubMed] [Google Scholar]
- 2.Libby P Inflammation in Atherosclerosis-No Longer a Theory. Clin Chem 2021;67:131–142. [DOI] [PubMed] [Google Scholar]
- 3.Poulain M, Pes GM, Grasland C et al. Identification of a geographic area characterized by extreme longevity in the Sardinia island: the AKEA study. Exp Gerontol 2004;39:1423–9. [DOI] [PubMed] [Google Scholar]
- 4.Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016;17:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alpert A, Pickman Y, Leipold M et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med 2019;25:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franceschi C, Capri M, Monti D et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007;128:92–105. [DOI] [PubMed] [Google Scholar]
- 7.Morrisette-Thomas V, Cohen AA, Fulop T et al. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech Ageing Dev 2014;139:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cubedo J, Padro T, Formiga F et al. Inflammation and hemostasis in older octogenarians: implication in 5-year survival. Transl Res 2017;185:34–46 e9. [DOI] [PubMed] [Google Scholar]
- 9.Sayed N, Huang X, Nguyen K et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat Aging 2021;1:598–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiva-Blanch G, Vilella-Figuerola A, Padro T, Formiga F, Ferrer A, Badimon L. Functional and Cognitive Decline Is Associated With Increased Endothelial Cell Inflammation and Platelet Activation: Liquid Biopsy of Microvesicles in Community-Dwelling Octogenarians. Front Cell Dev Biol 2021;9:716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberale L, Kraler S, Camici GG, Luscher TF. Ageing and longevity genes in cardiovascular diseases. Basic Clin Pharmacol Toxicol 2020;127:120–131. [DOI] [PubMed] [Google Scholar]
- 12.Walaszczyk A, Dookun E, Redgrave R et al. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 2019;18:e12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correia-Melo C, Birch J, Fielder E et al. Rapamycin improves healthspan but not inflammaging in nfkappab1(−/−) mice. Aging Cell 2019;18:e12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawelec G Immune signatures associated with mortality differ in elderly populations from different birth cohorts and countries even within northern Europe. Mech Ageing Dev 2019;177:182–185. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016;15:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier R, Bawamia B, Bennaceur K et al. Telomerase Activation to Reverse Immunosenescence in Elderly Patients With Acute Coronary Syndrome: Protocol for a Randomized Pilot Trial. JMIR Res Protoc 2020;9:e19456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and ‘Garbaging’. Trends Endocrinol Metab 2017;28:199–212. [DOI] [PubMed] [Google Scholar]
- 18.Diteepeng T, Del Monte F, Luciani M. The long and winding road to target protein misfolding in cardiovascular diseases. Eur J Clin Invest 2021;51:e13504. [DOI] [PubMed] [Google Scholar]
- 19.Lynn EG, Lhotak S, Lebeau P et al. 4-Phenylbutyrate protects against atherosclerotic lesion growth by increasing the expression of HSP25 in macrophages and in the circulation of Apoe(−/−) mice. FASEB J 2019;33:8406–8422. [DOI] [PubMed] [Google Scholar]
- 20.Ye S, Luo W, Khan ZA et al. Celastrol Attenuates Angiotensin II-Induced Cardiac Remodeling by Targeting STAT3. Circ Res 2020;126:1007–1023. [DOI] [PubMed] [Google Scholar]
- 21.Quarles E, Basisty N, Chiao YA et al. Rapamycin persistently improves cardiac function in aged, male and female mice, even following cessation of treatment. Aging Cell 2020;19:e13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, Li R, Lin H et al. Enriched taxa were found among the gut microbiota of centenarians in East China. PLoS One 2019;14:e0222763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biagi E, Franceschi C, Rampelli S et al. Gut Microbiota and Extreme Longevity. Curr Biol 2016;26:1480–5. [DOI] [PubMed] [Google Scholar]
- 24.Sovran B, Hugenholtz F, Elderman M et al. Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity. Sci Rep 2019;9:1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberale L, Holy EW, Akhmedov A et al. Interleukin-1beta Mediates Arterial Thrombus Formation via NET-Associated Tissue Factor. J Clin Med 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonetti NR, Liberale L, Akhmedov A et al. Long-term dietary supplementation with plant-derived omega-3 fatty acid improves outcome in experimental ischemic stroke. Atherosclerosis 2021;325:89–98. [DOI] [PubMed] [Google Scholar]
- 27.Saeedi Saravi SS, Bonetti NR, Pugin B et al. Lifelong dietary omega-3 fatty acid suppresses thrombotic potential through gut microbiota alteration in aged mice. iScience 2021;24:102897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason RP, Libby P, Bhatt DL. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler Thromb Vasc Biol 2020;40:1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 2018;14:576–590. [DOI] [PubMed] [Google Scholar]
- 30.Arai Y, Kamide K, Hirose N. Adipokines and Aging: Findings From Centenarians and the Very Old. Front Endocrinol (Lausanne) 2019;10:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bik W, Baranowska-Bik A, Wolinska-Witort E et al. Assessment of adiponectin and its isoforms in Polish centenarians. Exp Gerontol 2013;48:401–7. [DOI] [PubMed] [Google Scholar]
- 32.Van Berendoncks AM, Garnier A, Beckers P et al. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail 2010;3:185–94. [DOI] [PubMed] [Google Scholar]
- 33.Xu XM, Ning YC, Wang WJ et al. Anti-Inflamm-Aging Effects of Long-Term Caloric Restriction via Overexpression of SIGIRR to Inhibit NF-kappaB Signaling Pathway. Cell Physiol Biochem 2015;37:1257–70. [DOI] [PubMed] [Google Scholar]
- 34.Martucci M, Ostan R, Biondi F et al. Mediterranean diet and inflammaging within the hormesis paradigm. Nutr Rev 2017;75:442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liberale L, Montecucco F, Tardif JC, Libby P, Camici GG. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J 2020;41:2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaiswal S, Libby P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol 2020;17:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaiswal S, Natarajan P, Silver AJ et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorsheimer L, Assmus B, Rasper T et al. Association of Mutations Contributing to Clonal Hematopoiesis With Prognosis in Chronic Ischemic Heart Failure. JAMA Cardiol 2019;4:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pascual-Figal DA, Bayes-Genis A, Diez-Diez M et al. Clonal Hematopoiesis and Risk of Progression of Heart Failure With Reduced Left Ventricular Ejection Fraction. J Am Coll Cardiol 2021;77:1747–1759. [DOI] [PubMed] [Google Scholar]
- 40.Abplanalp WT, Mas-Peiro S, Cremer S, John D, Dimmeler S, Zeiher AM. Association of Clonal Hematopoiesis of Indeterminate Potential With Inflammatory Gene Expression in Patients With Severe Degenerative Aortic Valve Stenosis or Chronic Postischemic Heart Failure. JAMA Cardiol 2020;5:1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liberale L, Ministrini S, Carbone F, Camici GG, Montecucco F. Cytokines as therapeutic targets for cardio- and cerebrovascular diseases. Basic Res Cardiol 2021;116:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abplanalp WT, Cremer S, John D et al. Clonal Hematopoiesis-Driver DNMT3A Mutations Alter Immune Cells in Heart Failure. Circ Res 2021;128:216–228. [DOI] [PubMed] [Google Scholar]
- 43.Wolach O, Sellar RS, Martinod K et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rauch PJ, Silver AJ, Gopakumar J et al. Loss-of-Function Mutations in Dnmt3a and Tet2 Lead to Accelerated Atherosclerosis and Convergent Macrophage Phenotypes in Mice. Blood 2018;132. [Google Scholar]
- 45.Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K. CRISPR-Mediated Gene Editing to Assess the Roles of Tet2 and Dnmt3a in Clonal Hematopoiesis and Cardiovascular Disease. Circ Res 2018;123:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuster JJ, MacLauchlan S, Zuriaga MA et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017;355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higa KC, Goodspeed A, Chavez JS et al. Chronic interleukin-1 exposure triggers selection for Cebpa-knockout multipotent hematopoietic progenitors. J Exp Med 2021;218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng Y, Nie C, Min J et al. Novel loci and pathways significantly associated with longevity. Sci Rep 2016;6:21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonafe M, Olivieri F, Cavallone L et al. A gender--dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. Eur J Immunol 2001;31:2357–61. [DOI] [PubMed] [Google Scholar]
- 50.Varadhan R, Yao W, Matteini A et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci 2014;69:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Georgakis MK, Malik R, Gill D et al. Interleukin-6 Signaling Effects on Ischemic Stroke and Other Cardiovascular Outcomes: A Mendelian Randomization Study. Circ Genom Precis Med 2020;13:e002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theriault S, Dina C, Messika-Zeitoun D et al. Genetic Association Analyses Highlight IL6, ALPL, and NAV1 As 3 New Susceptibility Genes Underlying Calcific Aortic Valve Stenosis. Circ Genom Precis Med 2019;12:e002617. [DOI] [PubMed] [Google Scholar]
- 53.Agrawal A, Tay J, Yang GE, Agrawal S, Gupta S. Age-associated epigenetic modifications in human DNA increase its immunogenicity. Aging (Albany NY) 2010;2:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jylhava J, Nevalainen T, Marttila S, Jylha M, Hervonen A, Hurme M. Characterization of the role of distinct plasma cell-free DNA species in age-associated inflammation and frailty. Aging Cell 2013;12:388–97. [DOI] [PubMed] [Google Scholar]
- 55.Bacalini MG, D’Aquila P, Marasco E et al. The methylation of nuclear and mitochondrial DNA in ageing phenotypes and longevity. Mech Ageing Dev 2017;165:156–161. [DOI] [PubMed] [Google Scholar]
- 56.Nachun D, Lu AT, Bick AG et al. Clonal hematopoiesis associated with epigenetic aging and clinical outcomes. Aging Cell 2021;20:e13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicolia V, Cavallaro RA, Lopez-Gonzalez I et al. DNA Methylation Profiles of Selected Pro-Inflammatory Cytokines in Alzheimer Disease. J Neuropathol Exp Neurol 2017;76:27–31. [DOI] [PubMed] [Google Scholar]
- 58.Chen BH, Marioni RE, Colicino E et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY) 2016;8:1844–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bergsma T, Rogaeva E. DNA Methylation Clocks and Their Predictive Capacity for Aging Phenotypes and Healthspan. Neurosci Insights 2020;15:2633105520942221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wasiak S, Gilham D, Tsujikawa LM et al. Downregulation of the Complement Cascade In Vitro, in Mice and in Patients with Cardiovascular Disease by the BET Protein Inhibitor Apabetalone (RVX-208). J Cardiovasc Transl Res 2017;10:337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsujikawa LM, Fu L, Das S et al. Apabetalone (RVX-208) reduces vascular inflammation in vitro and in CVD patients by a BET-dependent epigenetic mechanism. Clin Epigenetics 2019;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ray KK, Nicholls SJ, Buhr KA et al. Effect of Apabetalone Added to Standard Therapy on Major Adverse Cardiovascular Events in Patients With Recent Acute Coronary Syndrome and Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2020;323:1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fahy GM, Brooke RT, Watson JP et al. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 2019;18:e13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jylhava J, Pedersen NL, Hagg S. Biological Age Predictors. EBioMedicine 2017;21:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogel B, Acevedo M, Appelman Y et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet 2021;397:2385–2438. [DOI] [PubMed] [Google Scholar]
- 66.Yousefzadeh MJ, Zhao J, Bukata C et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 2020;19:e13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dulken B, Brunet A. Stem Cell Aging and Sex: Are We Missing Something? Cell Stem Cell 2015;16:588–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jenkins EC, Shah N, Gomez M et al. Proteasome mapping reveals sexual dimorphism in tissue-specific sensitivity to protein aggregations. EMBO Rep 2020;21:e48978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pomatto LCD, Wong S, Carney C, Shen B, Tower J, Davies KJA. The age- and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaption and resistance to oxidative stress in Drosophila melanogaster. Aging (Albany NY) 2017;9:1153–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horvath S, Gurven M, Levine ME et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol 2016;17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li X, Ploner A, Wang Y et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. Elife 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yusipov I, Bacalini MG, Kalyakulina A et al. Age-related DNA methylation changes are sex-specific: a comprehensive assessment. Aging (Albany NY) 2020;12:24057–24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Honigberg MC, Zekavat SM, Niroula A et al. Premature Menopause, Clonal Hematopoiesis, and Coronary Artery Disease in Postmenopausal Women. Circulation 2021;143:410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marquez EJ, Chung CH, Marches R et al. Sexual-dimorphism in human immune system aging. Nat Commun 2020;11:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kane AE, Bisset ES, Heinze-Milne S, Keller KM, Grandy SA, Howlett SE. Maladaptive Changes Associated With Cardiac Aging Are Sex-Specific and Graded by Frailty and Inflammation in C57BL/6 Mice. J Gerontol A Biol Sci Med Sci 2021;76:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]


