Abstract
A 63-year-old male with mechanical aortic valve replacement presents with Trichosporon mucoides endocarditis. Eosinophilia was noted, which has recently been described in invasive trichosporonosis. He was treated successfully with combination voriconazole and terbinafine therapy. He was deemed not to be a cardiac surgery candidate, due to excessive estimated procedural mortality.
Keywords: Trichosporon, Endocarditis, Prosthetic valve
Highlights
-
•
Trichosporon is a ubiquitous yeast that can cause invasive disease in humans.
-
•
Medical management of fungal endocarditis is reasonable if patient cannot go for surgery.
-
•
Voriconazole and terbinafine can be used in Trichosporon infections with good clinical response.
-
•
Eosinophils may be a non-specific marker of therapeutic response in T. mucoides infections.
1. Introduction
We present a case of invasive Trichosporon mucoides prosthetic valve infective endocarditis in an immunocompetent patient. To our knowledge, only one other case of T. mucoides prosthetic valve endocarditis has been reported [1]. This case shows a satisfactory response to voriconazole and terbinafine dual therapy followed by voriconazole monotherapy for suppression in a patient who is not able to achieve source control for their T. mucoides endocarditis. Eosinophilia may also be an adjunctive clue to diagnosis and treatment response, in invasive trichosporonosis cases.
2. Case
In January 2021, a 63-year-old male who lived in Winnipeg, Manitoba, Canada presented to hospital with three weeks of progressive confusion and weakness. He had a history of rheumatic heart disease requiring bioprosthetic aortic valve replacement and ascending aortic graft repair in 2013, followed by a re-do for valve failure with a mechanical aortic valve placement in 2019. He also had type II diabetes. He was admitted to hospital on day 0 and a urinary tract infection was suspected due to a history of urinary frequency. Urine and blood cultures were drawn prior to initiating Ceftriaxone (2g q24h IV).
On physical examination, notable findings included a temperature of 37.6 °C, heart rate of 133 beats per minute and oxygen saturation of 93% on room air. He had a mechanical click from his aortic valve and a systolic ejection murmur. Onychomycosis was noted. Dermatologic and neurologic examinations were normal.
Complete blood count demonstrated eosinophilia (eosinophil 0.51x109/L, absolute neutrophil of 6.71x109/L) and anemia (hemoglobin 65 g/L). Platelets were within normal limits. Urinalysis was negative for nitrates with 125 leukocytes. Blood cultures returned positive for yeast-like organisms on day +1.
Caspofungin (one dose 70mg IV and 50mg IV daily) was initiated on suspicion of candidemia; Ceftriaxone was stopped. Urine cultures were negative. A computed tomography (CT) demonstrated multiple areas of cerebral infarction concerning for a cardiac embolic source. CT abdomen showed left hydronephrosis with fat stranding, a right wedge shaped hypodensity concerning for possible pyelonephritis, and new splenic infarctions.
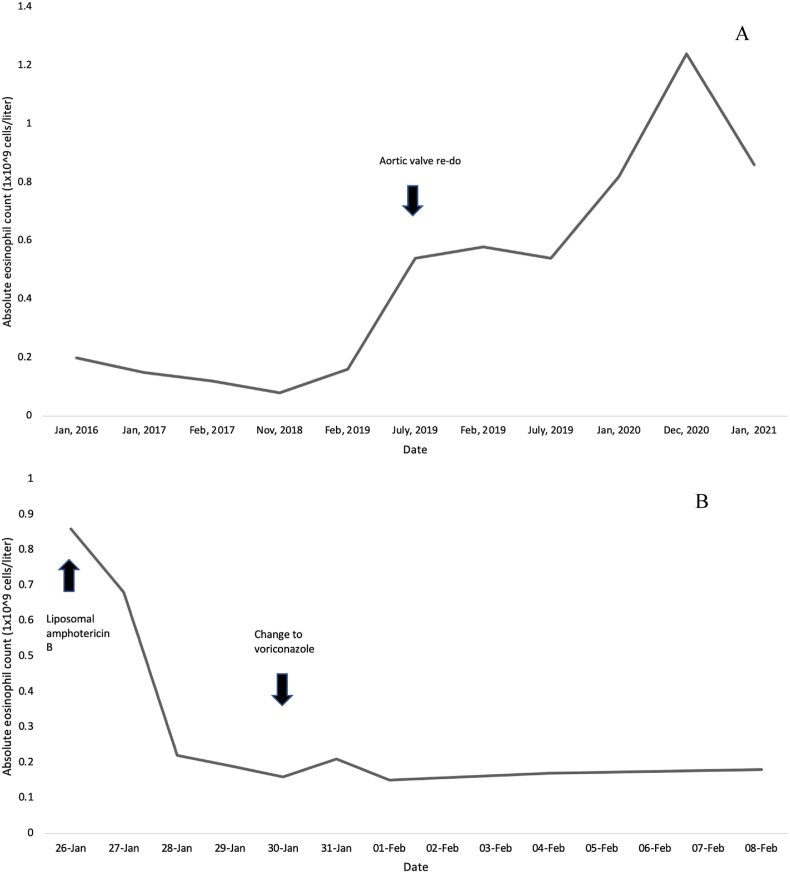
Repeat blood cultures day +1 following Caspofungin initiation were positive again for yeast-like organisms from two sites. On day +3 of admission, Trichosporon mucoides was identified from the first set of cultures by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Billerica, MA, USA). This same species was later identified on the second set of blood cultures (susceptibilities in Table 1). Liposomal amphotericin B (5mg/kg IV daily) was started, and Caspofungin was stopped. Blood cultures drawn one day after amphotericin initiation remained negative. Transthoracic echocardiography (TTE) demonstrated a circumferential aortic valve abscess and an area of echodensity measuring 5x3mm attached to the aortic valve. Toenail cultures were sent, growing a Trichophyton species. Following initiation of amphotericin B, the absolute eosinophils showed a quick decrease and normalization to 0.37x109/L; eosinophils remained within normal limits while on antifungal therapy (Fig. 1).
Table 1.
MICa by broth microdilution as described for Candida species in the Clinical and Laboratory Standards Institute - M27 document (CLSI – M27, 4th Ed., 2017). Terbinafine MIC performed separately in San Antonio, Texas.
| Antifungal | MICa (ug/l) |
|---|---|
| Terbinafine | 0.5 |
| Fluconazole | 4 |
| Itraconazole | 0.5 |
| Voriconazole | 0.12 |
| Amphotericin B | 0.5 |
| Micafungin | >16 |
MIC – minimal inhibitory concentration.
Fig. 1.
A: Absolute eosinophil count overtime before and after aortic valve re-do. B: Absolute eosinophil count following initiation of antifungal therapy.
On day +6 of admission to hospital, his creatinine had increased from 97 to 202umol/L; amphotericin B was held, and the patient was switched to voriconazole (IV 4mg/kg twice daily). Shortly after, terbinafine (oral (po) 250mg po daily) was started for synergy. His creatinine eventually stabilized to a new baseline of 200umol/L. Voriconazole was dose adjusted to 300mg po twice daily following a 2-week intravenous course.
In early March (day +50), the Cardiac surgery team determined that he had a high perioperative risk of death and morbidity and declined repeat valve replacement. The patient was discharged in April (day +84) on oral voriconazole and terbinafine. He was followed in the Infectious Disease clinic. Clinically, he has remained afebrile and doing generally well, with no new cardiac or neurologic complications; eosinophils remain within normal limits. Terbinafine was discontinued after more than 4 months of use, due to the patient's clinical stability and concern for his rising cholestatic liver enzymes. Repeat TTE in June and December of 2021 revealed slightly improved size of suspected paravalvular abscess but was otherwise unchanged. He continues to be on voriconazole 300mg po BID and remains clinically well as of December 2021. Given his prosthetic valve fungal endocarditis with suspected paravalvular abscess, which is likely incurable without surgery, he will remain on life-long voriconazole as suppression therapy.
3. Discussion
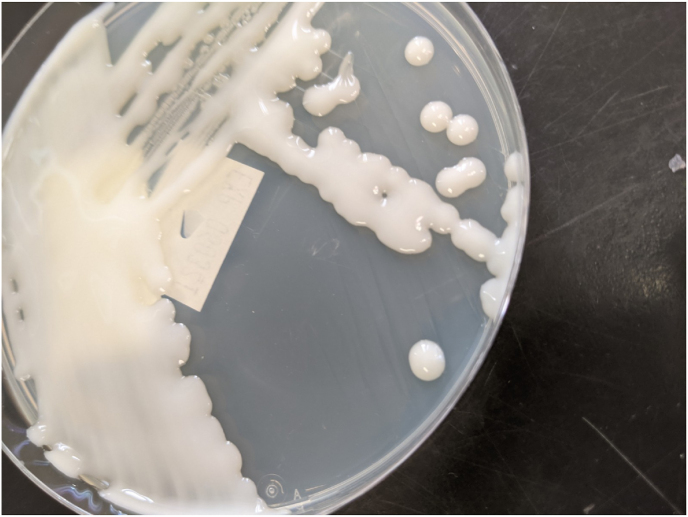
Trichosporon species are basidiomycetous yeasts that are widely distributed in nature [2,3]. Trichosporon sp. commonly colonize the gastrointestinal tract and the skin; the most common clinical manifestation in humans are as the cause of white piedra, an asymptomatic infection causing nodular changes to affected hair shafts. T. asahaii is the most common species associated with disseminated human infections [2,4,5]. Diagnosis of invasive Trichosporon sp. rely on positive cultures or histology with Trichosporon sp. in a patient with clinical signs of infection [3]. On Sabouraud Dextrose Agar, Trichosporon sp. grow as a cream-colored cerebriform yeast (see Fig. 2).
Fig. 2.
T. mucoides mucoid colonies on Inhibitory Mold Agar (IMA) after 3 days, 30° Celsius, aerobic conditions. (needs colored print).
While invasive trichosporonosis is usually associated with immunosuppression, invasive infections due to T. mucoides have been reported in both immunosuppressed and immunocompetent hosts [1,[6], [7], [8]]. These infections have included fungemia, peritonitis and prosthetic valve endocarditis [1,[6], [7], [8]].
Our patient had eosinophilia since his aortic valve replacement re-do in 2019 (Fig. 1). His eosinophils promptly returned to normal following treatment with appropriate antifungals and continues to remain normal. Eosinophilia may be a non-specific marker for diagnosis of, and treatment response to, invasive trichosporonosis. This finding has recently also been noted in one other case report [9]. Trichosporon can also lead to acute eosinophilic pneumonia [10,11]. This manifests as infiltrates in the lung, symptoms of pneumonia with both eosinophilia in the sputum and blood.
There is a lack of high-quality clinical data informing the optimal treatment of invasive Trichosporon infections. This genus has been observed to be resistant in vitro to all echinocandins [2,4,12]. There have been studies showing high minimal inhibitory concentrations (MIC) and poor clinical response associated with amphotericin B, suggesting this may not be an optimal agent for monotherapy [1,2,4,5]. In one murine model, echinocandin and amphotericin B in combination may have synergistic effect to reduce the burden of the fungus in vivo [12]. Azoles may also be a good alternative in treatment of trichosporonosis: fluconazole, posaconazole and voriconazole have been used with success in previous cases of T. mucoides [1,7,8,13]. Combination therapy with 5-fluorocystine and amphotericin B has also been associated with good clinical response in one case of invasive T. mucoides infection [6]. The use of terbinafine has not been reported in the treatment of Trichosporon infections. It is an allylamine antifungal agent classically used in dermatophyte infections [14]. However, published in vitro studies support extending its use beyond these organisms. Terbinafine, in synergistic combination with azoles, echinocandins, or polyenes, may be particularly beneficial in the treatment of fungi with intrinsic resistance to other antifungals [14]. Several molds with which terbinafine has synergistic effects in vitro with other antifungals therapies have been previously described. These include the following: Scedosporium sp. (terbinafine and itraconazole), Fusarium sp. (terbinafine with either voriconazole, caspofungin or amphotericin B), Pythium, Scopulariopsis brevicularis and Aspergillus (terbinafine and azoles), Rhizopus sp., Absidia sp. and Mucor sp. (terbinafine and voriconazole or amphotericin B) [14]. Terbinafine in combination with azoles has also been shown to have synergistic activity against azole resistant Candida albicans and C. glabrata [14,15]. Terbinafine alone or used together with amphotericin B or fluconazole has synergistic effects to treat Cryptococcus neoformans [16]. Future studies testing synergism of terbinafine and other antifungals for the treatment of Trichosporon can be considered.
In patients with fungal endocarditis, valve replacement is usually necessary. However, as in our patient, this may not always be possible. Oh et al. previously reviewed prior cases of Trichosporon endocarditis in the literature [1]. Among 16 published cases, of which 12 (75%) report a clear mode of treatment, only one case was managed medically without valve replacement [1,17]. This case involved a patient 12-days post-mitral valve replacement surgery who developed Trichosporon cutaneum prosthetic valve endocarditis treated with 42 days of amphotericin B. She had no recurrence after being followed for 5 years. Therefore, while it is uncommon for Trichosporon endocarditis to be managed medically, it may be considered in certain circumstances.
Clinicians should be aware of the existence of rare causes of fungal endocarditis or endovascular infections. This case illustrates T. mucoides prosthetic valve endocarditis receiving medical management with dual therapy with voriconazole and terbinafine with good clinical response. This is the first case of T. mucoides prosthetic valve endocarditis to be managed medically and with the use of terbinafine. Finally, eosinophils may be used as a non-specific marker of therapeutic response in T. mucoides infections.
Declaration of competing interest
There are none.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Oh T.H., Shin S.U., Kim S.S., Kim S.E., Kim U.J., Kang S.-J., et al. Prosthetic valve endocarditis by Trichosporon mucoides: a case report and review of literature. Medicine (Baltim.) 2020 Oct 9;99(41) doi: 10.1097/MD.0000000000022584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo A.L., Padovan A.C.B., Chaves G.M. Current knowledge of Trichosporon spp. and trichosporonosis. Clin. Microbiol. Rev. 2011 Oct;24(4):682–700. doi: 10.1128/CMR.00003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arendrup M.C., Boekhout T., Akova M., Meis J.F., Cornely O.A., Lortholary O. ESCMID† and ECMM‡ joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 2014 Apr;20:76–98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- 4.Singh S., Capoor M.R., Varshney S., Gupta D.K., Verma P.K., Ramesh V. Epidemiology and antifungal susceptibility of infections caused by Trichosporon species: an emerging non-Candida and non-cryptococcus yeast worldwide. Indian J. Med. Microbiol. 2019 Oct;37(4):536–541. doi: 10.4103/ijmm.IJMM_19_146. [DOI] [PubMed] [Google Scholar]
- 5.Girmenia C., Pagano L., Martino B., D'Antonio D., Fanci R., Specchia G., et al. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J. Clin. Microbiol. 2005 Apr;43(4):1818–1828. doi: 10.1128/JCM.43.4.1818-1828.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kendirli T., Ciftci E., Ince E., Oncel S., Dalgic N., Guriz H., et al. Successful treatment of Trichosporon mucoides infection with lipid complex amphotericin B and 5-fluorocytosine. Mycoses. 2006 May;49(3):251–253. doi: 10.1111/j.1439-0507.2006.01223.x. [DOI] [PubMed] [Google Scholar]
- 7.Padhi S., Dash M., Pattanaik S., Sahu S. Fungemia due to Trichosporon mucoides in a diabetes mellitus patient: a rare case report. Indian J. Med. Microbiol. 2014 Jan;32(1):72–74. doi: 10.4103/0255-0857.124324. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y.T., Yang W.C., Chen T.W., Lin C.C. Trichosporon mucoides peritonitis in a continuous ambulatory peritoneal dialysis patient. Perit Dial Int J Int Soc Perit Dial. 2013 May;33(3):341–342. doi: 10.3747/pdi.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang F., Feng J., Wang L., Jiang L., Sheng L., Wu J., et al. Disseminated Trichosporon asahii infection presenting as eosinophilia in an immunocompetent patient: a case report. Indian J. Med. Microbiol. 2021 Oct;39(4):552–555. doi: 10.1016/j.ijmmb.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa H., Fujimura M., Amaike S., Nishiura Y., Nakagawa-Yoshida K., Suga M., et al. Seasonal chronic cough with sputum eosinophilia caused by Trichosporon cutaneum (Trichosporon asahii) Int. Arch. Allergy Immunol. 1998;116(2):162–165. doi: 10.1159/000023940. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki E., Sugisaki K., Shigenaga T., Matsumoto T., Kita S., Inobe Y., et al. A case of acute eosinophilic pneumonia caused by inhalation of Trichosporon terrestre. Am. J. Respir. Crit. Care Med. 1995 Feb;151(2):541–543. doi: 10.1164/ajrccm.151.2.7842218. [DOI] [PubMed] [Google Scholar]
- 12.Serena C., Pastor F.J., Gilgado F., Mayayo E., Guarro J. Efficacy of micafungin in combination with other drugs in a murine model of disseminated trichosporonosis. Antimicrob. Agents Chemother. 2005 Feb;49(2):497–502. doi: 10.1128/AAC.49.2.497-502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacasse A., Cleveland K.O. Trichosporon mucoides fungemia in a liver transplant recipient: case report and review. Transpl. Infect. Dis. 2009 Apr;11(2):155–159. doi: 10.1111/j.1399-3062.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- 14.Terbinafine Krishnan-Natesan S. A pharmacological and clinical review. Expet Opin. Pharmacother. 2009 Nov;10(16):2723–2733. doi: 10.1517/14656560903307462. [DOI] [PubMed] [Google Scholar]
- 15.Weig M., Müller F.-M.C. Synergism of voriconazole and terbinafine against Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 2001 Mar;45(3):966–968. doi: 10.1128/AAC.45.3.966-968.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerra C.R., Ishida K., Nucci M., Rozental S. Terbinafine inhibits Cryptococcus neoformans growth and modulates fungal morphology. Mem. Inst. Oswaldo Cruz. 2012 Aug;107(5):582–590. doi: 10.1590/s0074-02762012000500003. [DOI] [PubMed] [Google Scholar]
- 17.Madhavan T., Eisses J., Quinn E.L. Infections due to Trichosporon cutaneum, an uncommon systemic pathogen. Henry Ford Hosp. Med. J. 1976;24(1):27–30. [Google Scholar]