Abstract
Purpose of Review
COVID-19 is highly contagious; since it was first identified, the virus has rapidly spread to more than 100 countries and was declared a pandemic on March 11, 2020, by the World Health Organization (WHO). This disease presents several challenges when managing patients with leukemia. We review the information about chronic myeloid leukemia (CML) and COVID-19: risk factors, prognosis, and the role of tyrosine kinase inhibitors (TKIs).
Recent Findings
At present, we find no data suggesting that patients with CML-chronic phase (CML-CP) are at higher risk of infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) than the general healthy population. TKIs had been proposed to fight the SARS-CoV-2-related disease (COVID-19).
Summary
CML patients should continue receiving their TKIs if they have COVID-19 disease. The role of TKIs as protective factors against SARS-CoV-2 infection in patients with CML should be confirmed by large-scale epidemiologic studies.
Keywords: COVID-19, Chronic myeloid leukemia, Tyrosine kinase inhibitors, Imatinib, Treatment, Pandemic
Introduction
In December 2019, several cases of a new respiratory illness were reported leading to the detection of the coronavirus outbreak, which is believed to have originated in Wuhan, in the Hubei province of the People’s Republic of China [1].
A new, confirmed viral strain was then identified, in January 2020, and subsequently was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—related to SARS-CoV identified in the 2002–2003 outbreak [2]. Subsequently, SARS-CoV2 (COVID-19) has been transmitted from Wuhan to the rest of the world at such a magnitude that the World Health Organization (WHO) declared a pandemic on March 11, 2020 [3]. As of April 16, 2021, 138,276,238 cases were reported, and 2,973,050 people died, according to figures from Johns Hopkins University [4].
Oncology patients have not been spared from this infectious disease, and the first epidemiological study reported SARS-CoV-2 infection rates in oncology patients of 0.7% compared to non-cancer subjects (0.37%) (February 2020) with the subsequent emergence of multiple retrospective cohorts with coincident conclusions [5]. The meta-analysis by Desai et al. showed a prevalence of COVID-19 in patients with active malignancies of 2%, suggesting that cancer patients are a population with increased susceptibility to SARS-CoV-2 infection [6•].
It should be pointed out that in subgroup analysis, according to the type of neoplasm, hematological neoplasms stand out as those with the highest risk of death, as shown by De Joode et al. [7].
In a case–control study carried out in the USA, in September 2020, patients with hematological malignancies were more likely infected by COVID-19, finding significance in those diagnosed with acute lymphoblastic leukemia, essential thrombocythemia, acute myeloid leukemia, and multiple myeloma, with worse results in those with recent diagnosis (hospitalization, 51.9%; death, 14.8%) [8].
Subsequently, Vijenthira et al. performed a meta-analysis of hospitalized patients with hematological malignancies, where the risk of death detected was 34%, associated with age over 60 years [9]. Another cohort of 697 patients analyzed reported a median age of 72 years. Sixty percent of the patients were male, 69% diagnosed with lymphoid neoplasms (N = 479), and 31% with myeloid neoplasms (N = 218); according to the severity of the respiratory disease, up to 62% presented severity criteria. At the end of the follow-up, 33% had died (N = 230). Similarly, age and more than two comorbidities related to higher mortality, particularly in these cohort patients with acute myeloid leukemia and myelodysplastic syndrome, presented fatal outcomes (44% and 42%, respectively) in contrast to relatively lower rates in patients with Philadelphia (Ph) negative myeloproliferative neoplasms (19%) and CML (13%) [10••].
Risk and Impact of COVID-19 in Patients with Chronic Myeloid Leukemia
The available data on COVID-19 in patients with chronic myeloid leukemia (CML) is very limited but suggest that CML does not increase the risk of complications; however, there are reports of severe complications such as respiratory distress, febrile neutropenia, hemolysis, and hemophagocytosis—all of these in patients with particular characteristics in the evolution and control of the neoplastic disease (accelerated phase, blastic phase) or comorbidities [11–13].
A large cohort (N = 740) of hospitalized and non-hospitalized oncohematology patients, conducted in Turkey, had a 4.1% (N = 30) rate of patients with CML and COVID-19 and lower overall mortality [14]. Similar data were reported in an Italian study (March 2020), in a retrospective cohort of 102 patients with hematologic malignancies and COVID-19, where, specifically, chronic myeloid leukemia was documented in 6.7%. [15].
In the study by Breccia et al., data from a large cohort of 6,883 patients with CML were collected, and only 12 confirmed cases with COVID-19 infection were reported, with a prevalence of 0.17%. The results of this survey show that the incidence of COVID-19 infection is extremely low in CML patients treated with tyrosine kinase inhibitors (TKIs) [16••].
Recently, Claudiani et al. showed that serological testing of 161 CML patients and prevalence of infection in CML patients was similar to the general population, suggesting that patients can mount an adequate antibody response against SARS-Cov-2 [17].
The International CML Foundation (iCMLf) reported 110 cases of COVID-19 in CML patients from 20 countries: 61% from Europe, 15% from Asia, 12% from South America, 10% from North America, 2% from Africa, and 1% from Oceania.
Among 110 patients, 93% (N = 102) were symptomatic and 7% (N = 8) asymptomatic. Among the first group of patients, 45% (N = 49) had mild COVID-19, which did not require hospitalization, 17% (N = 19) had moderate symptoms that required hospitalization, 17% (N = 19) had severe symptoms that required admission to the intensive care unit, and the severity was unknown in 14% (N = 15).
The univariate analysis detected the following risk factors for mortality: (1) In patients > 75 years, the mortality rate was 60% vs. 7% in < 75 years (p < 0.001); (2) severity of COVID-19, mortality in patients with severe COVID-19 was 63% vs. 0% in patients with non-severe symptoms (p < 0.001); and (3) treatment with imatinib, mortality 25% vs. 3% in patients with 2nd generation TKI (p = 0.003). It must be taken into account that the group of patients receiving imatinib were older, so it may not be a real risk factor [18].
A study conducted in Hubei, China, involving 530 outpatients diagnosed with chronic myeloid leukemia, whose data was collected by questionnaires, reported COVID-19 prevalence of 0.9% (n = 5). Importantly, 296 (56%) were male, the median age was 44 years, and only 18% were ≥ 60 years. In addition, 100% were in the chronic phase, 15% (N = 81) had complete hematologic response (CHR); 10% (N = 52), complete cytogenetic response (CCyR); and 73% (N = 387), major molecular response (MMR), at the time of the questionnaire. Thus, the covariates associated with a higher risk of developing COVID-19 were exposure to a person infected with SARS-CoV-2 (p = 0.037), not having a complete hematological response (p = 0.003), and other comorbidities (p = 0.024) [19•].
In Brazil, Pagnano et al. reported 28 cases of patients with CML and COVID-19.
The median age was 54 years (24–79), with 44% (N = 13) older than 60. Similar to that reported in the general population, most patients presented at least one comorbidity (60%), the most frequent being: hypertension (25%), diabetes (10.7%), dyslipidemia (7.1%), arterial disease (7.1%), and chronic obstructive pulmonary disease/emphysema (7.1%). Obesity was reported in only 3.6% of cases. The authors concluded that the most severe cases of COVID-19 occurred in patients who did not have MMR [20].
Relevant Aspects in the Treatment of the Patient with CML that Requires Management for COVID-19
It is widely known that, when a patient with a recent diagnosis of CML starts treatment with a TKI, hematological adverse events may occur (anemia, neutropenia, thrombocytopenia, or even pancytopenia). These events are more common in the first weeks of treatment, and most are resolved. However, it is important to consider that a period of neutropenia can increase the risk of severe COVID-19 [21•].
TKIs are metabolized by cytochrome-P450, CYP3A4 isoenzyme, and therefore caution should be exercised when other drugs are indicated, because interactions between drugs are very likely [21•].
In some patients, chloroquine/hydroxychloroquine was used as a treatment option for COVID-19. Imatinib can increase the serum levels of these drugs, because it is a competitive inhibitor of CYP2D6 [21•].
The most used first-line treatment for CML is imatinib. Chloroquine can increase the adverse events of this TKI due to the inhibition of P-glycoprotein, and, on the other hand, it can decrease intracellular imatinib by inhibiting hOCT1, which could cause treatment failure. Chloroquine may also increase the adverse events of dasatinib due to P-glycoprotein inhibition [21•].
A non-hematological adverse event in common with all TKs used for the treatment of CML is that they can prolong the QTc interval, which can lead to torsades de pointes and sudden death [21•].
Chloroquine and azithromycin are two drugs that can also prolong the QTc interval, so CML patients with a TKI and receiving these drugs for COVID-19 should be closely monitored with serial electrocardiograms and serum electrolytes [21•].
CML patients with COVID-19 should not discontinue TKI, but as previously mentioned, drug interactions should be considered [21•].
Activity of Tyrosine Kinase Inhibitors Against Coronavirus
One of the proposed treatments for SARS-Cov-2 (COVID-19)–related disease has been imatinib [22••].
The cause of COVID-19 is an RNA virus. One of the most important viral proteins is found on its surface. It is known as the spike protein (S), which is essential for the fusion of the virus and its entry into human cells, which is carried out through the angiotensin-converting enzyme 2 (ACE2) receptor. Recently, another viral receptor called dipeptidyl peptidase 4 (DPP4), also known as CD26, was described. This receptor allows the virus to block autophagy, which impairs the host’s immune response and maintains the hyperinflammatory status [22••]
Taking into account that ABL1 kinase controls cytoskeletal rearrangement, the inhibitory function of Abl1 by imatinib could affect the adequate performance of actin, which allows the virus to enter and fuse with the cell [22••].
An in vitro investigation showed that the activity of imatinib against the coronavirus takes place in the early stages of infection; after internalization, it inhibits the fusion of virions at the endosomal membrane [23].
The protective function of imatinib that has been reported for COVID-19 (blocking fusion) could prevent the endocytosis necessary for viral activation of different viral species [16••].
There are studies in mice with sepsis and acute lung injury, which suggest that imatinib may reduce pulmonary edema, prevent histological damage, and improve endothelial barrier dysfunction, which may be the result of decreased release of proinflammatory cytokines, such as interleukin-6 and tumor necrosis factor-alpha [24].
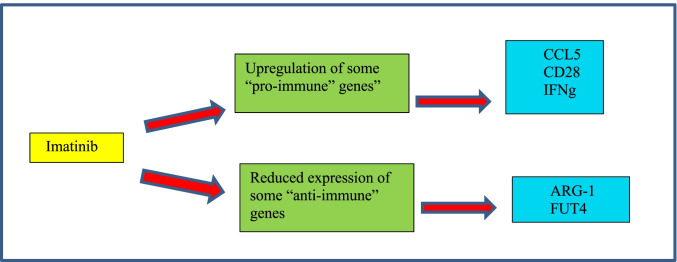
An interesting study of Galimberti et al. who analyzed the expression of 770 genes related to inflammation and immunity (NanoString technology), in samples of 5 patients with CML before starting treatment with imatinib and 6 months after taking this treatment, had the purpose of detecting the immunological role of this TKI on viral infections [22••].
The researchers detected 58 genes deregulated by imatinib, of which 18 were upregulated and 40 downregulated. Of the total of 58 deregulated genes, 34.5% (N = 520) of their function was significantly correlated with the immune or antiviral response [22••].
Focusing on these 20 genes, 9 genes showed decreased expression during the administration of imatinib, highlighting arginase 1 (ARG-1), complement C3a receptor 1 (C3AR1), carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM1), gelsolin (GSN), nectin 1, and fucosyltransferase 4 (FUT4), which usually have a proviral function [22••].
On the other hand, 11 genes had an increased expression as a result of the administration of imatinib, such as CD28, interferon-gamma (IFN gamma), CC motif chemokine ligand 5 (CCL5), CC chemokine receptor type 5 (CCR5), and Toll-like receptor 3 (TLR3), which could exert an “antiviral” role, by allowing the immune control of the host against the viral attack [22••].
Recent research on CD28 in COVID-19 disease has shown that its expression on CD8 + T cells is lower in severe patients, compared to those with mild COVID-19.
In the case of chemokine CCL5, a mouse model showed that imatinib causes overexpression of this cytokine in NK cells and therefore its hyperactivity can make animals resistant to viral infection. TLR3 overexpression has previously been reported to protect neutropenic mice from meningoencephalitis; thus, it may also play a positive role in COVID-19. And finally, the proimmune action of IFN gamma is well known in Fig. 1 [22••].
Fig. 1.
The effects that TKIs have on the CML models
Currently, Emadi et al. are conducting a double-blind, randomized, and controlled study at the University of Maryland Medical Center, Baltimore, USA, with the objective of evaluating the efficacy and safety of imatinib (400 mg daily for 14 days) plus the best conventional care (BCC), as compared with placebo, in adult patients (≥ 18 years of age), hospitalized and with COVID-19. The researchers are expecting a better outcome in the imatinib arm—this is a result of the decrease in SARS-Cov2/cell fusion and the inhibitory activity of imatinib that can interfere with the release or replication of the virus. The study’s patient recruitment started on June 15, 2020 [25].
In the Republic of Turkey, Basci et al. reported the outcome of 16 patients diagnosed with CML and COVID-19 on treatment with TKI and compared it with a control group (N = 48). They found in the group that received imatinib: lower rates of admission to the intensive care unit (ICU) and ventilatory mechanical support and shorter hospital stays; however, they did not obtain statistical significance. The mortality rate in patients receiving TKI was 6.3% vs. 12.8% in the control group [26].
Other studies have been registered on the “ClinicalsTrials.gov” website and are ongoing, with imatinib as monotherapy (NCT04357613), or in combination with baricitinib, lopinavir/ritonavir (NCT04346147), or hydroxychloroquine (NCT04356495) [22••].
Conclusions
The susceptibility found throughout the pandemic of patients with malignant neoplastic diseases to COVID-19 has prompted the international community to study and report the characteristics of this particular population, pathological background, clinical behavior, and outcome. From these studies, the authors found that patients with hematologic malignancies in the context of COVID-19 infection had a higher rate of complications and mortality compared to the general population. However, the behavior of patients with a diagnosis of chronic myeloid leukemia differs from these findings since the evidence so far indicates that this disease does not increase the risk of complications, leading to the premise that particularly in this hematological neoplasm the subjects can mount an adequate immune response to viral infection. The prognosis of these patients depends on multiple factors, such as disease stage, response to treatment with TKIs, physical and functional status, comorbidities, and age over 60 years (already demonstrated as a significant predictor of mortality in COVID-19).
Many challenges exist in caring for oncology patients during the COVID-19 pandemic, including balancing the benefits of oncology-directed treatment with the implicit increased risk of severe COVID-19 infection.
The patients with CML-chronic phase do not indicate to delay or hold the treatment (TKIs).
To date, no evidence consistently supports any treatment for COVID-19 patients, so identifying new therapeutic options seems crucial in the management of this disease. Imatinib has also shown antiviral, immunomodulatory, and endothelium-protective properties which may be potentially helpful in COVID-19.
Further studies are needed to find risk factors and to detect the most vulnerable subgroups, as well as the actual role of imatinib in COVID-19.
Declarations
Conflict of Interest
Nancy Delgado and Anahí Torres declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Leukemia
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Rafiq D, Batool A, Bazaz M. Three months of COVID -19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:e2113. doi: 10.1002/rmv.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashour H, Elkhatib W, Rahman M, Elshabrawy H. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9:186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Who.int. 2021. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. [online] Available at: <https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020> [Accessed 16 April 2021]. (2021)
- 4.Johns Hopkins Coronavirus Resource Center. 2021. COVID-19 Map - Johns Hopkins Coronavirus Resource Center. [online] Available at: <https://coronavirus.jhu.edu/map.html> [Accessed 16 April 2021].
- 5.Yu J, Ouyang W, Chua M, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan. China JAMA Oncology. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.•Desai A, Sachdeva S, Parekh T, Desai R. COVID-19, and cancer: lessons from a pooled meta-analysis. JCO Global Oncology. 2020;6:557-559. 10.1200/go.20.00097. This editorial highlights the increase in COVID-19 in cancer patients. [DOI] [PMC free article] [PubMed]
- 7.De Joode K, Dumoulin D, Tol J, Westgeest H, Beerepoot L, van den Berkmortel F, et al. Dutch Oncology COVID-19 consortium: outcome of COVID-19 in patients with cancer in a nationwide cohort study. Eur J Cancer. 2020;141:171–184. doi: 10.1016/j.ejca.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Berger N, Xu R. When hematologic malignancies meet COVID-19 in the United States: infections, death, and disparities. Blood Rev. 2021;47:100775. doi: 10.1016/j.blre.2020.100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vijenthira A, Gong I, Fox T, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.•• García-Suárez J, de la Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. Journal of Hematology & Oncology. 2020;13: 1–13. 10.1186/s13045-020-00970-7. This article describes the poor prognostic factors (age over 60 years and two or more comorbidities) in oncohematological patients with COVID-19, as well as lower mortality rates in those with chronic myeloid leukemia. [DOI] [PMC free article] [PubMed]
- 11.Abdalhadi A, Alshurafa A, Alkhatib M, Abou Kamar M, Yassin M. Confirmed coronavirus disease-19 (COVID-19) in a male with chronic myeloid leukemia complicated by febrile neutropenia and acute respiratory distress syndrome. Case Reports in Oncology. 2020;13:569–577. doi: 10.1159/000508378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mounir L, Mehdi S, Kawtar F, Akram M, Youness E, Meryem T, et al. Severe COVID-19 infection in a patient with a blastic transformation of chronic myeloid leukemia and severe treatment-induced immunosuppression: a case report. Pan African Med J. 2020;37:1–5. doi: 10.11604/pamj.supp.2020.37.1.25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorà F, Chiusolo P, Laurenti L, Innocenti I, Autore F, Alma E, et al. SARS CoV 2 infection in chronic myelogenous leukemia: severe hematological presentation. Transfus Apheres Sci. 2020;59:102881. doi: 10.1016/j.transci.2020.102881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yigenoglu T, Bascı S, Dal M, Korkmaz S, Turgut B, Altuntas F. The outcome of COVID-19 in patients with hematological malignancy. J Med Virol. 2020;93:1255–1255. doi: 10.1002/jmv.26607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cattaneo C, Daffini R, Pagani C, Salvetti M, Mancini V, Borlenghi E, et al. Clinical characteristics and risk factors for mortality in hematologic patients affected by COVID-19. Cancer. 2020;126:5069–5076. doi: 10.1002/cncr.33160. [DOI] [PubMed] [Google Scholar]
- 16.•• Breccia M, Abruzzese E, Bocchia M, Bonifacio M, Castagnetti F, Fava C et al. Chronic myeloid leukemia management at the time of the COVID-19 pandemic in Italy. A campus CML survey. Leukemia. 2020;34: 2260-2261. 10.1038/s41375-020-0904-z. Cohort study of patients with Chronic Myeloid Leukemia that emphasizes the low prevalence of COVID-19 infection in patients under treatment with tyrosine kinase inhibitors. [DOI] [PMC free article] [PubMed]
- 17.Claudiani S, Rosadas C, McClure M, Khan M, Tedder RS, Innes AJ, et al. Prevalence of Sars-Cov-2 infection in patients with chronic myeloid leukemia. Blood. 2020;136(Supplement 1):20. doi: 10.1182/blood-2020-142454. [DOI] [Google Scholar]
- 18.Rea D, Mauro MJ, Cortes JE, Jiang Q, Pagnano KB, Ongondi M, et al. COVID-19 in patients (pts) with chronic myeloid leukemia (CML): results from the International CML Foundation (iCMLf) CML and COVID-19 (CANDID) Study. Blood. 2020;136(Supplement 1):46–47. doi: 10.1182/blood-2020-140161. [DOI] [Google Scholar]
- 19.• Li W, Wang D, Guo J, Yuan G, Yang Z, Peter R et al. COVID-19 in persons with chronic myeloid leukemia. Leukemia. 2020;34: 1799-1804. 10.1038/s41375-020-0853-6. This editorial studies the risk factors for developing COVID-19 in patients with chronic myeloid leukemia, particularly those without hematological response or contacts of people infected by SARS COV2.
- 20.Pagnano KBB, Toreli AC, Perobelli LM, Quixada AT, Seguro FS, Bendit I, et al. COVID-19 in chronic myeloid leukemia patients-Brazilian experience. Hematol transfuses Cell Ther. 2020;42(S2):S526–S527. doi: 10.1016/j.htct.2020.10.889. [DOI] [Google Scholar]
- 21.• Ezkazan AE. Chronic myeloid leukemia and the use of tyrosine kinase inhibitors in the days of COVID-19 pandemic. Br J Clin Pharmacol. 2020;86(9): 1790–1792. 10.1111/bcp.14353This article mentions the management of chronic myeloid leukemia with tyrosine kinase inhibitors, along with the medications used to treat SARS-Cov-2, which can be complex and require close monitoring due to the interactions between drugs. [DOI] [PMC free article] [PubMed]
- 22.•• Galimberti S, Petrini M, Baratè C, Ricci F, Balducci S, Grassi S et al. Tyrosine kinase inhibitors play and antiviral action in patients affected by chronic myeloid leukemia: a possible model supporting their use in the fight against SARS-CoV-2. Front Oncol. 2020;10: 1428. 10.3389/fonc.2020.01428. In this work, 770 genes related to inflammation and immunity were analyzed in patients with CML, before and after 6 months of treatment with imatinib, and it was shown that this TKI increases the expression of some anti-viral genes and reduces the expression of some pro-viral genes. [DOI] [PMC free article] [PubMed]
- 23.Demeter J, Weisinger J, Nagy Z. Mild clinical course of COVID-19 infection in chronic myeloid leukemia (CML) patients receiving tyrosine kinase inhibitors (TKIs) without interruption. Mediterr J Hematol Infect Dis. 2021;13(1):e2021022. doi: 10.4084/MJHID.2021.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizzo AN, Sammani S, Esquinca AE, Jacobson JR, Garcia JGN, Letsiou E, et al. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am J Physiol Cell Mol Physiol. 2015;309:1294–1304. doi: 10.1152/ajplung.00031.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emadi A, Chua JV, Talwani R, Bentzen SM, John B. Safety and efficacy of imatinib for hospitalized adults with COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):897. doi: 10.1186/s13063-020-04819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basci S, Ata N, Altuntas F, Yigenoglu TN, Dal MS, Korkmaz S, et al. Outcome of COVID-19 in patients with chronic myeloid leukemia receiving tyrosine kinase inhibitors. J Oncol Pharm Practice. 2020;26(7):1676–1682. doi: 10.1177/1078155220953198. [DOI] [PMC free article] [PubMed] [Google Scholar]