Summary
High-quality growth of MoS2-xNx films is realized on single-crystal c-Al2O3 substrates by the pulsed laser deposition (PLD) in ammonia rendering highly stable and tunable 1Tʹ/2H biphasic constitution. Raman spectroscopy reveals systematic enhancement of 1Tʹ phase component due to the incorporation of covalently bonded N-doping in MoS2 lattice, inducing compressive strain. Interestingly, the film deposited at 300 mTorr NH3 shows ∼80% 1Tʹ phase. The transport measurements performed on MoS2-xNx films deposited at 300 mTorr NH3 display very low room temperature resistivity of 0.03 mΩ-cm which is 100 times enhanced over the undoped MoS2 grown under comparable conditions. A triboelectric nanogenerator (TENG) device containing biphasic MoS2-xNx film as an electron acceptor exhibits a clear enhancement in the output voltage as compared to the pristine MoS2. Device architecture, p-type N doping in MoS2 lattice, favorably increased work-function, multiphasic component of MoS2, and increased surface roughness synergistically contribute to superior TENG performance.
Subject areas: Materials science, Materials synthesis, Nanomaterials
Graphical abstract

Highlights
-
•
MoS2-xNx films grown on c-Al2O3 and ITO/PET by pulsed laser deposition in NH3
-
•
p-type doping with high conductivity and 1T’+2H dual polymorph state is realized
-
•
Increased work-function of MoS2-xNx films over pristine MoS2 is realized
-
•
Impressive Triboelectric Nanogenerator application is demonstrated with MoS2-xNx
Materials science; Materials synthesis; Nanomaterials
Introduction
Transition metal chalcogenides (TMCs) have rapidly acquired the status of technology materials of interest during the past decade because of their immensely interesting application-worthy physical properties (Jariwala et al., 2014)(Sebastian et al., 2021) (Barani et al., 2021) (Zhang et al., 2019)(Lee et al., 2017) (Briggs et al., 2019) (Mak et al., 2019), (Shin et al., 2012). Amongst the various materials belonging to this class, MoS2 has perhaps been the most investigated one in recent years, a remarkable feature of MoS2 being its intrinsic ability to support multiple polymorphs(Acerce et al., 2017). The polymorphs of MoS2 afford wide bandgap tunability, with 1Tʹ phase being metallic and its polymorphs being metastable semiconductors with bandgap from <0.8 meV to 1.9 eV (Zhao et al., 2018). This aspect has brought MoS2 to the center stage along with graphene and other 2D carbides and nitrides from the standpoint of both fundamental research and technological applicability.
Structurally, a monolayer of MoS2 consists of a layer of three atoms in zigzag configuration, where one Mo atom is sandwiched between the two S atoms and the layers are stacked with very weak van der Waals forces. The primary polymorphs of MoS2 are 1T (trigonal phase), 1H and 2H (hexagonal phase), and 3R (rhombohedral phase). Amongst these phases, the 1T phase is the least stable because of its thermodynamically metastable nature and tends to form the more stable 2H phase by reorganizing its stacking layers and geometry. The metastable 1T phase can also transform to other polymorph intermediates such as 1Tʹ, 1Tʹʹ, and 1Tʹʹʹ via Jahn-Teller distortions (Zhao et al., 2018). Indeed, doping of octahedral and tetrahedral geometry in VDW in TMDCs drives several factors such as registry of interlayer stacked atoms, tilting of octahedrons, bond length, and bond angle, which cause structural distortions leading to the superlattices of a0× 2a0 dimer chain (1Tʹ), 2a0× 2a0 diamond shape (1Tʹʹ) and √3a0×√3a0 trimerized clustering (1Tʹʹʹ) (Zhao et al., 2018). A well-established method of formation of 1T phase from 2H phase of MoS2 is the alkali ion (Li+, Na+, and K+) intercalation route.
Controllable in-situ doping of 2D materials is highly desirable for high-performing heterojunction devices to control the electronic structure, device parameters, and overall device functionality. In this regard, many strategies have been developed to enhance the MoS2 electronic properties such as molecular surface doping by oleylamine (Rosa et al., 2016), polyethylenimine (Du et al., 2013), and benzyl viologen (Kiriya et al., 2014) for effective electronic charge transfer (Tang and Jiang, 2015). Nevertheless, the noncovalent bonding behavior of these doped molecules is volatile, which makes it difficult to control the doping concentration over time and stabilize the desired phase(s). Doping of transition elements (Re, W, Pd, Cu, Co, Ni, Fe, Nb etc.) (Gao et al., 2020) (Fu et al., 2020), (Martinez et al., 2018), noble metal elements (Ag, Au and Pt) (Wang et al., 2019b), (Mombrú et al., 2018), non-metals (O, N, and Cl) (Yang et al., 2014) (Tang et al., 2020), (Azcatl et al., 2016) or vacancies (Mo and S) (Feng et al., 2014), as well as intercalation (Li, Na, and K) (Zou et al., 2020), (Liu et al., 2018) composite formation (MoS2/Graphene, MoS2/h-BN, etc) (Wang et al., 2019a), (Parmar et al., 2019) and substrate-induced strain effect (c-Al2O3, SrTiO3, LaAlO3 SrLaAlO4, etc) (Parmar et al., 2021a), have been shown to greatly enhance the electronic properties of MoS2. While MoS2 is technically an intrinsic semiconductor, practical materials exhibit an n-type semiconductor character owing to the naturally present S vacancies (Zhang et al., 2021). This also hinders the realization of their bipolar nature for a variety of applications in the domain of electronics and optoelectronics, although some successes have been achieved in this context with interesting consequences. For instance, substituting Mo in MoS2 lattice with Nb transforms the intrinsic n-type semiconducting behavior to p-type with increased hole density (3.1 × 1020 cm−3) and high mobility (8.5 cm2V−1 s−1) (Das et al., 2015). Also, the growth windows of polymorphic TMCs using non-metal doping have so far remained relatively unexplored. Interestingly, N doping is one of the facile methods that greatly enhance the device performance by introducing finite and enhanced density of states at the Fermi-level, thereby providing high carrier concentration. For instance, Li at el. calculated that the introduction of 3% strain due to N doping in N-Mo-S lattice leads to 2H to 1Tʹand 2H to 1T phase transition because of geometrical changes, charge injection and strain (Li and Li, 2018).
The chemical vapor deposition method employed to dope TMD thin films requires toxic precursors, high temperatures, and also generates toxic by-products; and the growth of epitaxial film by this method is non-trivial (Frisenda et al., 2018). Mechanical and chemical synthesis protocols provide flaky forms, whereas chemical exfoliation requires intercalation of alkali metal ion or foreign element to synthesize the desirable 1Tʹ phase. This chemical synthesis route is also very time-consuming and requires further washing and processing to create an application-worthy thin film platform. Covalent doping via the substitution of metal and chalcogen atoms is a viable approach to achieve stable and controllable doping in these material systems.
Pulsed laser deposition (PLD) is a unique stoichiometry-transferring single-target thin film deposition technique that allows the growth of multi-functional, phase tunable, and stable thin-film growth, with the possibility of ambient control to achieve non-metal doping (with N, S, O) and pressure control to realize vacancy doping (Parmar et al., 2021b). The substrate temperature is another key parameter that can enable film quality, dopant, and defect control. Further, the PLD technique involves several laser-related process parameters such as laser energy density, pulse frequency, number of laser ablating shots, and so on. for film quality and thickness control (Wang et al., 2014) (Siegel et al., 2015) (Serrao et al., 2015), (Serna et al., 2016)
Ubiquitously, there are several ways to modify the intrinsic properties of materials for high performing TENG devices such as dielectric constant, work function, electrical poling, composites and chemical doping (Kim et al., 2020). Since MoS2 is ranked higher in the negative triboelectric series, it has definitely become interesting to understand the triboelectric charging behavior of MoS2 in TENG devices (Seol et al., 2018). The TENG output response is often associated with the effective work functions that govern the charging polarity of the materials, which can be effectively tuned by chemical doping (Kim et al., 2020). Seol et al. have reported that AuCl3doped MoS2 exhibits superior Voc in TENG as compared to benzyl viologen (BV)-doped MoS2 because of their differences in work-functions which represent p-type and n-type chemical doping, respectively. The p-type AuCl3 doped MoS2 increases the effective work function, making it more electronegative in the triboelectric series, while the BV doping decreases the effective work-function of MoS2 making it more tribo-positive and lowers the Voc (Seol et al., 2018). Thus, the p-type covalent N doping in MoS2 was performed to increase the effective work-function of the MoS2-xNx system. Here, we have used chemical doping toward manipulation of work function, type of charges, electron density, and bonding in N doped MoS2which has been carefully examined by several characterizations, and its detailed analysis toward material intrinsic properties for TENG enhanced performance is presented.
The growth of MoS2-xNx (anionic doping) thin films examined in this study was accomplished by employing NH3-assisted PLD for in-situ N doping in MoS2 lattice via laser-generated plasma. A comprehensive and rigorous analysis of Raman, XPS, VBS, and XANES spectra for the MoS2-xNx deposited under 10, 100 and 300 mTorr NH3 pressure reveals that substantial enhancement in the 1Tʹ-phase component results for films grown at 300 mTorr NH3 pressure. It can be attributed to the substitution of S with N forming its covalent bonding with the Mo atom. While the possibility of incorporation of some hydrogen cannot be ruled out, we did not find evidence of the same in the set of characterizations employed. The growth pressure also plays a key role in the tunability and stabilization of the 1Tʹ phase. Interestingly, the carrier transport properties also evolve rapidly as a function of doping rendering very high electrical conductivity in films grown at high (300 mTorr) ammonia pressure. We also examined the applicability of such highly nitrogen doped films for the triboelectric nanogenerator (TENG) device by growing them on flexible conducting ITO/PET substrate at 100 °C. Notably such films can be expected to be poly or micro-crystalline, yet the grains could be expected to preserve the virtues of the doping effects. Indeed, the device based on this material demonstrated impressive voltage/current output with the p-type property of the material getting reflected therein. It is important to note that the N doped MoS2 material is in itself very interesting and is an important object of this study. Dry processing routes such as PLD are compatible with clean deposition environments and as such once proper growth parameters are identified, multilayer structures involving other metal oxides, sulfides and nitrides would be feasible. TENG application is used as a proof of concept to elucidate how the nitrogen doping could affect the involvement of such doped 2D material in a device configuration.
Results and discussions
Structural and surface characterization
The Raman spectra for N doped MoS2 grown on single crystal c-Al2O3 (0001) substrate by using PLD in ammonia at pressures of 10, 100, and 300 mTorr are shown in Figure S1. All the spectra were normalized vis-á-vis the high-intensity A1g phonon mode for better comparison, as this mode is present in all cases and is common for both the 1Tʹ and 2H phases of MoS2. It is observed that the 300 mTorr film shows remarkably strong intensity for J2, J3, and E2g phonon modes over the 10 and 100 mTorr cases; and the J2 mode contribution gets smaller in the order of 300 mTorr > 100 mTorr>10 mTorr. All the observed Raman modes are tabulated in Table 1. Two distinct zone center first order sharp peaks at ∼406 cm−1 and ∼382 cm−1 corresponds to A1g (out of plane) and E2g (in-plane) modes, respectively, in the case of pristine MoS2 and 300 mTorr grown MoS2-xNx represent the 2H phase component (Figure 1A). The red-shift in the vibrational frequency in the doped film (Figure 1A, inset) can be attributed to the change in intra and intermolecular force constants of VDW MoS2. The packing of MoS2 lattice is affected by the external defects and dopants that change the intermolecular distances and hence the intermolecular force constants, resulting in the red shift of the A1g phonon mode. Interestingly, the peak splitting of E2g mode into and is significantly high in the 300 mTorr grown MoS2 case, which corresponds to the symmetry breaking of the E2g phonon mode because of N doping (Parmar et al., 2019). Further, a slight increase in FWHM in the doped system is due to the defect states reflecting the structural distortion(s). Interestingly, the disappearance of peak at ∼287 cm−1 (resemble E1g in-plane mode of 2H MoS2) in 300 and 100 mTorr NH3 case depicts the enhancement of 1Tʹ phase MoS2 in the doped films. This implied that because of N doping in MoS2 lattice, the structural distortion occurs that modifies the bond length and angle; and the structure of the 2H MoS2 transforms from 2H to 1T′ phase, which modifies the electronic properties of the MoS2-xNx film, as discussed in the later section.
Table 1.
Obtained room temperature experimental phonon frequencies in Raman spectra of MoS2 grown on c-Al2O3 under 300, 100, 10 mTorr, and pristine MoS2 respectively
| Phonon Mode | 300 mTorr (cm−1) | 100 mTorr (cm−1) | 10 mTorr (cm−1) | MoS2 (cm−1) |
|---|---|---|---|---|
| A1g | ∼405.6 | ∼406.2 | ∼407.2 | ∼407.5 |
| E2g | ∼376.1 | ∼381.8 | ∼381.4 | ∼381.5 |
| E1g | ∼285.4 | ∼287.1 | ∼287.6 | ∼288.2 |
| J1 | ∼148.8 | ∼151 | – | – |
| J2 | ∼197, and ∼222 | ∼227 | ∼225.4 | ∼225.1 |
| J3 | ∼333 | ∼332 | – | – |
Here the intensities of the most intense (A1g) peak are normalized.
Figure 1.
Raman and XPS Spectra of MoS2-xNx thin-film grown on c-Al2O3 under 300 mTorr NH3 pressure and of pristine MoS2/c-Al2O3 case respectively
(A) Inset is the expansion of A1g and E2g mode from the frequency range 350–435 cm−1. A significant enhancement of polymorphic J2 mode and E2g mode splitting corresponds to the co-existence of polymorph and enhanced doping concentration of N in MoS2 lattice.
(B) X-ray photoelectron spectroscopy (XPS) of Mo 3d core for MoS2-xNx/c-Al2O3 (B1) and for pristine MoS2/c-Al2O3 (B2).
(C) X-ray photoelectron spectroscopy (XPS) of N1s + Mo 3p core for MoS2-xNx/c-Al2O3 (C1) and for pristine MoS2/c-Al2O3 (C2).
Ubiquitously, the key spectral signatures noted at 151 cm−1, 196 cm−1, 226 cm−1, and 321 cm−1 correspond to the J1, J2, and J3 modes for the 1Tʹ phase, respectively (Guardia et al., 2014). Here the atomic vibrations of Mo zig-zag chain correspond to J1 and J3 modes and the vibration of S atoms corresponds to the J2 mode. The peak intensity for these modes can be seen to increase with the increase of NH3 pressure and is exceptionally high for the film grown at 300 mTorr ammonia. The high-intensity peak at ∼196 cm−1 (J2) with the broad full-width half maximum (FWHM) represents the defect generated low frequency mode leading to the shortening of the distance between Mo-Mo zig-zag chains (Mignuzzi et al., 2015). Further, the splitting of J2 phonon at ∼197 and ∼222 cm−1 can be understood in terms of the accommodation of compressive strain generated into the film due to N doping, leading to the formation of the distorted octahedron. The peaks at 125 and 150 cm−1 correspond to the combinatorial mode that arises because of the difference of A1g−J2 phonon modes (Ahmed et al., 2020). The significant enhancement in J2 mode and appearance of J1 and J3 mode in 300 mTorr N-H:MoS2 film can be attributed to the generation of compressive strain in the film as the atomic radius of N (65 p.m.) is much smaller as compared to S (100 p.m.). Another possibility of this doublet could be because of the formation of Mo-N bond in presence of high-pressure ammonia, and the vibrational frequency for the same also appears at 350 cm−1, as reported in the literature (Azcatl et al., 2016). Thus, the MoS2-xNx film deposited at 300 mTorr NH3 perfectly fits the picture of the interlayer Mo-S, Mo-N, and intra-layer S-S, S-N, and possibly N-N interactions leading to the formation of high component of the 1Tʹ phase MoS2.
The chemical structure and chemical components of pristine and doped MoS2 were further studied by the XPS technique. Figures 1B1, B2 and 1C1, C2 display the XPS spectra of pristine MoS2 and MoS2-xNx (grown at 300 mTorr ammonia pressures) films. Based on previous reports (Yang et al., 2019), (Hu et al., 2018) N doped MoS2 (397-399 eV), MoN (395 eV), and Mo2N (397 eV) related signatures correspond to specific Mo-N bonding/coordination. Therefore, the broad peak at around 397.3 eV observed in Figure 1C2 can be assigned to Mo-N non-specific bonding. Further, the systematic shift toward the lower binding energy is found to be related to the increased doping concentration due to the increase in the NH3 pressure from 10, 100, to 300 mTorr (Figures S2, S3, and S4). Therefore, it is important to calculate the concentration of N (Nc) in MoS2-xNx lattice. We quantitatively calculated the Nc of the doped films grown at NH3 pressures of 100 and 300 mTorr, and the corresponding values were found to be 18.5%, and 25%, respectively. This represents significant degree of nitrogen incorporation, more of an anionic alloy. The percentage was obtained by the ratio of integrated intensities of N 1s core to Mo 3d5/2 (Azcatl et al., 2016).
The doublet structure of the Mo 3d and S 2p spectra illustrated in Figures 1B1, B2 and 1C1, C2 reflect the characteristic spin-orbit splitting between Mo 3d3/2 and Mo 3d5/2 states at 232.6 and 229.5 eV respectively (Li et al., 2017), and for S 2p1/2 and S 2p3/2, states at 163.7 and 162.5 eV, respectively. The sharp peaks of Mo 3d core for pristine MoS2 are indicative of the good local crystallinity of the samples. The shift of ∼0.5 eV toward the lower binding energy of the doped system reflects the presence of polymorphism due to N doping and consequent possible decrease in the effective charge state of Mo. The defective structure with non-metal dopants causes displacement of the Mo4+ 3d peaks toward the lower binding energies in the Mo 3d spectra (Pal et al., 2017). The percentage of 1Tʹ and 2H was also been calculated by assigning the peak in regions 229 and 232 eV, as shown in Figure 1B1, B2. The 300 mTorr case shows significantly higher fraction of (∼80%) 1Tʹ phase of MoS2, which is the metallic phase. Notably, the peak at 229.5 eV also corresponds to the Mo-N bonding which is at about the same location as that of Mo-S bonds of 2H phase MoS2 (Azcatl et al., 2016). Therefore, this XPS signature will also have the corresponding Mo-N contribution in 100 and 300 mTorr cases that cannot be neglected. Similarly, in Figure 1C2 the consecutive shift toward the lower binding energy in S 2p1/2 and 2p3/2 spin-orbit splitting for 100 and 300 mTorr MoS2 films again reflects the enhancement of 1Tʹ phase with the increase of dopant concentration. All the peaks were corrected with the reference binding energy of C (Figure S5). All these results indicate that the doping of non-metals brings about dramatic microstructural changes and the modification of strain state by altering the bond lengths and bond angles.
AFM and HRTEM characterisation
The atomic force microscopic (AFM) topography in Figures S6A and S6B shows the increase in mean roughness from 2.78 nm to 5.2 nm in MoS2-xNx over pristine MoS2. In order to get an idea about the local structure development during initial film growth, we recorded High-resolution transmission electron microscopy (HRTEM) images on ultrathin films of MoS2 and MoS2-xNx deposited directly on carbon-coated holy grids by giving only 30 shots at room temperature. It may be noted that these data do not correspond to the films grown at higher temperature which are expected to be structurally far superior on larger length scales. The pristine MoS2 film was prepared under Ar atmosphere, whereas the MoS2-xNx film was deposited under 300 mTorr NH3 atmosphere. It is very clear from the obtained FFT image (Figures S7A and S7B) that the pristine MoS2 has common honeycomb lattice corresponding to the 2H phase, whereas the co-existence of 1Tʹ and 2H phases is evident in the case of the MoS2-xNx film, with trigonal lattice reflecting the 1Tʹ phase.
XANES characterization
The X-ray absorption near edge spectra (XANES) is a powerful tool to reveal microstructural details and electronic information on metal coordination sites. The formation of MoS2-xNx species under NH3 pressure was therefore confirmed by synchrotron-based XANES. We have analyzed the N K-edge, Mo M-edge, and S L-edge of MoS2-xNx films and the Mo and S edges for the pristine MoS2 films grown on c-Al2O3. The Nitrogen K-edge X-ray absorption energy is about 400 eV (Figure 2A), and the main absorption edge is observed at ∼405 eV (Reinholdt et al., 2021). Importantly, the Mo M3-edge also occur at ∼400 eV that arises from 3p→4d transition (George et al., 2009), (Lajaunie et al., 2015) The substantially enhanced intensity of the near 400 eV signature in the case of MoS2-xNx as compared to pristine MoS2 suggests addition of the Mo N and N K edge contributions. Further, in S L2,3-edge the spin orbit splitting occur in the range of 164–168 eV, as shown in Figure 2B (George et al., 2009). In addition, a comparative analysis based on the observed shape, peak area, and intensities provides information on the local bonding environment responsible for M edge, N edge, and L edge positions (Cai et al., 2015). The shoulder peak in N K-edge indicates that there is substantially extended energy states distribution which corresponds to Mo 4d electrons in the MoS2-xNx case, further indicating the incorporation of the 1Tʹ phase in MoS2-xNx (Cai et al., 2015; Lajaunie et al., 2015). According to the crystal field theory (CFT), the 2H semiconductor MoS2 is due to the symmetry-induced splitting of Mo 4d orbitals of MoS6 D3h group into fully occupied Mo 4dz2, Mo 4dxy, Mo 4dx2−y2, and unoccupied Mo 4dxz and Mo 4dyz (Zhao et al., 2018). On the other hand, in the case of 1Tʹ MoS2 semi-metallic character stems from the octahedral splitting of 4d orbital into triple triple-degenerate t2g (dxy, dxz, dyz) and double-degenerate eg sets, with the t2g orbitals partly occupied by the two 4d electrons. The N K-edges also reflects the high content of N in the MoS2-xNx case. From the S L edge, it is divulged that in the MoS2-xNx case, the feature at 166.5 eV arising because of the transition between S 2p to S 4d hybridized with Mo 5p states, is broadened and comprises two features. The additional feature compared to undoped MoS2 indicates the mixing of S 4d and N 2p states via Mo 5p states. The lower content of S in the MoS2-xNx case due to N substitution over pristine MoS2 is clearly seen from the drop in intensity of the S L2,3-edge (Figure 2B). (Cai et al., 2015) The above observations corroborate well with the noticeable changes in the electronic band structure due to the formation of 1Tʹ phase upon N doping in MoS2 lattice.
Figure 2.
X-ray near edge spectra for MoS2-xNx films grown on c-Al2O3 substrate
(A) N, K edge and Mo M edge are depicted from 390 to 430 eV.
(B) S L-edge XANES in the range of 164–168 eV energy.
Work-function and valance band maxima (UPS and VBS)
The work function values of MoS2-xNx and pristine MoS2 films were measured by UV photoelectron spectroscopy. The work function (Φ) of the heterostructures is calculated by:
| Φ = հν - Εonset(Equation 1) |
where hv = 21.22 eV is the incident photon energy and Εonset is the onset energy of the secondary electrons obtained from the extrapolation of the slope drawn at the leading edge of the spectra to the baseline as shown in Figure 3A. From the equation, the work functions were estimated to be 4.67 and 4.85 eV for MoS2/c-Al2O3 and MoS2-xNx/c-Al2O3 respectively. (Tao et al., 2014) The covalent N doping in MoS2 lattice effectively engineers the work-function. The n-type behavior of pristine MoS2 is attributed to sulfur vacancies and its significant depletion by N doping in the case of MoS2-xNx films can cause the enhancement of the work function.
Figure 3.
Electronic property exploration by UV photoelectron spectroscopy, valance band maxima, temperature dependent-resistivity and I-V characteristics of pristine MoS2 and MoS2-xNx films grown on c-Al2O3
(A) Secondary electron cutoff vs kinetic energy plot for work function calculation.
(B) Valance band spectra of pristine MoS2 and MoS2-xNx; films grown on c-Al2O3, representing the Fermi-level overlapping for MoS2-xNx (300 mTorr NH3 pressure).
(C) Temperature-dependent carrier transport of 20 nm MoS2 and MoS2-xNx films grown on c-Al2O3 (0001) substrate, resistivity shows a highly conducting nature of MoS2-xNx at room temperature.
(D) I-V characteristics of Au/MoS2/c-Al2O3 and Au/MoS2-xNx/c-Al2O3 heterostructure.
The hybridization of Mo 4d and S 3p orbitals leads to the formation of valance band states. In 2H MoS2, the conduction band and valance band are mainly composed of Mo 4d orbitals. The noticeable Mo 4d states across the Fermi energy in MoS2-xNx suggests the formation of 1Tʹ MoS2 and hence with more conducting nature. Figure 3B compares the VBS spectra of MoS2-xNx/c-Al2O3 grown at 300 mTorr and pristine MoS2-xNx/c-Al2O3case. Figure S8 compares the same for different pressures. The extraction of the Valance band maxima (VBM) positions was done by taking the intersection of the linear extrapolation of the prominent edge in VBS spectra. The electronic states at ∼1.8 eV belong to the Mo 4dz2 band of 2H MoS2 which is below the Fermi energy, implying the intrinsic n-type doping. The well-established hybridization of Mo 4d and S 3p suggests the long-range in-plane ordering of MoS2. (Tao et al., 2015) The progressive shift in the valence band edge toward the Fermi energy with increase of NH3 growth pressure suggests the change in the electronic structure. For MoS2-xNx/c-Al2O3 grown at 300 mTorr the observed VBM position is located at −0.1 eV with reference to the Fermi energy rendering finite density of states at EF and hence higher conductivity. This comes about by the increased 1Tʹ component with the nitrogen doping concentration. The binding energy of S 2p, Mo 3d and N 1s core (primary peaks), along with work-function and VBM of MoS2/c-Al2O3 and MoS2-xNx/c-Al2O3 films are tabulated for comparison in Table 2.
Table 2.
Obtained binding energies for MoS2 and MoS2-xNx films grown on c-Al2O3 substrate
| MoS2/c-Al2O3 | MoS2-xNx/c-Al2O3 | |
|---|---|---|
| S 2p3/2 | ∼162.5 eV | ∼162.1 eV |
| Mo 3d5/2 | ∼229.5 eV | ∼228.7 eV |
| N 1s | – | ∼397 eV |
| Work-function | ∼4.67 eV | ∼4.85 eV |
| Valance band maxima | ∼1.8 eV | ∼‒0.13 eV |
Electronic transport properties (resistivity and I-V characteristics)
Doping of 2D materials often leads to new phenomena and is often considered as a proposition to enhance the electronic transport properties. Specifically, enhancing the electrical conductivity of MoS2 films by doping is always interesting for enhancing its potential application-worthiness. Heavy doping can even cause significant changes to the band edges and work function, thereby boosting the functionality. Because our doped film consist of a high content of 1Tʹ phase rendered by N substitution at the sulfur site, the investigation of electrical transport is very interesting in this case. Indeed, the temperature-dependent in-plane four-probe resistivity measurement on 300 mTorr grown MoS2-xNx thin-film shows an extremely (by a factor of about 500) low room temperature resistivity as compared to that of pristine (undoped) MoS2 film. Because we used different NH3 pressures to grow the films leading to differing N doping concentrations, the intra-atomic interaction and the degree of covalent bonding between N and Mo should differ, leading to distinct differences in the d-electron hopping (Parmar et al., 2019), (Park et al., 2015) We measured the electrical resistivity of MoS2-xNx thin films comprising >80% 1Tʹ+20% 2H phase and pure 2H MoS2 phase film on c-Al2O3. The data over the 200–300 K regime are plotted and compared in Figure 3C. Indeed, the room temperature resistivity (ρ) shows much-enhanced conductivity for the MoS2-xNx/c-Al2O3 (0001) thin films (∼0.036 mΩ-cm) with respect to the pristine 2H phase MoS2/c-Al2O3 (0001) thin films (∼16.8 mΩ-cm). Further, the pressure dependent comparison of T-dependent carrier transport of pristine MoS2/c-Al2O3 (0001) and MoS2-xNx/c-Al2O3 (0001) deposited under different NH3 pressure (10, 100, and, 300 mTorr) depicts the lowest resistivity in the 300 mTorr case (Figure S9). It is quite interesting that although the mixed-phase film shows >80% 1Tʹ phase (which is much above the percolation threshold), it does not show a fully metallic (i.e., decrease in resistivity while lowering the temperature) feature. Because it contains <20% 2H phase (dielectric proximity), electron localization in the semiconducting regions (along with grain boundary scattering effects) may be the contributing factor for this observation.
The low-temperature resistivity data can be best fitted with the Mott-variable range hopping (Mott-VRH) model (i.e. ln σ∝ 1/T1/(d+1)) where σ is the conductivity and d the dimension, signifying the important role of Anderson localization due to intrinsic disorder effect (possible structural defects, vacancies, and dislocations to lower the strain energy) for this insulating-like behavior (Figure S10) (Cho et al., 2021), (Zhou et al., 2016) In addition, much lower activation energy of MoS2-xNx (∼30.6 meV) is observed over that of pristine MoS2 (∼91.3 meV) (Figure S11) which also indicates the modification in the electronic correlation and consequent enhancement in electrical conductivity (Mitterreiter et al., 2021). Moreover, reported theoretical calculations reveal that the structural distortion-induced changes in crystal field splitting opens up a gap of ∼100 meV for the semiconducting 1Tʹ phase (Pal et al., 2017).
In addition, we have also examined the I-V characteristics of heavily doped MoS2-xNx thin films and compared the current with respect to pure 2H MoS2 film. The in-plane current was measured across Au/MoS2-xNx and Au/MoS2 interfaces on the application of ±15 V sweep voltage (Figure 3D). The channel length was kept ∼0.6 μm between the two Au contacts. It is important to note here that the doped films show three order enhancement in the current as compared with pristine 2H MoS2. This can be attributed to the fact that the enhancement in the 1Tʹ phase via covalent doping provides a smooth electronic transport through the grain boundaries. Generally, the transport in the polymorphic MoS2 is driven by the tunneling of electrons and percolation from the metal-semiconductor grain boundaries of the 1Tʹ phase and 2H phase. However, the highly doped system provides a uniform 1Tʹ channel with pertinent electronic transport across the electrode.
Triboelectric nanogenerator performance
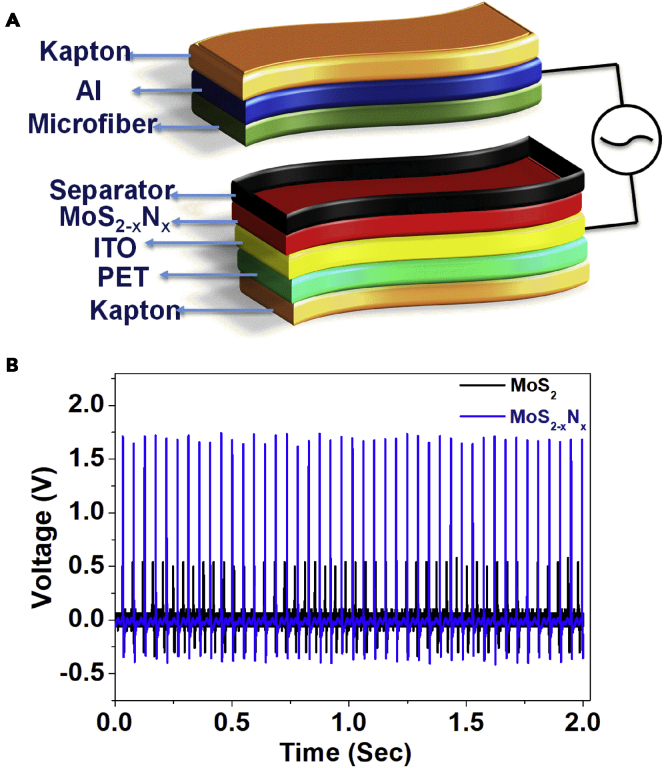
VDW-based TENGs represent an interesting mechano-electronic device (Lim et al., 2019). Herein, each VDW layer acts as an active electrode layer toward inter- and intra-layer charge generation and transfer. The strong interlayer coupling in VDW plays an important role in the charge generation/transfer. With the incorporation of defects, dopants, and charge distributions, this device functionality can be interesting and could generate superior performance. We therefore fabricated a TENG device using MoS2-xNx and pristine MoS2 films as an active material electrode in a vertical contact separation mode TENG design. A 20 nm film was coated on ∼1.3 × 1.3 cm2 area ITO/PET for this purpose. A 1-mm thick 3M double-sided tape boundary was used to maintain a distance between the top and bottom layers as depicted in Figure 4A. The MoS2 or MoS2-xNx film on ITO coated PET and microfiber separator in the middle were assembled with the aluminum tape upside down as top contact (Figure 4A) where the microfiber is composed of cellulose, a commercially available microfiber paper (α-cellulose content >98%) which is an environmentally friendly material. The device was laminated with a Kapton tape allowing some space for electrode connections to prevent damage to the device from the external environment. Then the Cu wires were soldered to the conducting sides of the top and bottom electrodes. A mechanical input of 10 N (measured by PASCO force sensor) was applied at 21 Hz frequency. During measurement, the Cu wires were connected to the oscilloscope to record the open-circuit voltage. A remarkably enhanced open-circuit value obtained for the pristine MoS2 and MoS2-xNx is in the ratio of ∼1:3. The obtained peak to peak voltages were ∼0.8 and ∼2.7 V for pristine MoS2 and MoS2-xNx, respectively (Figure 4B). The enhanced Voc in doped films can result from polymorphism with high 1Tʹ component and very high electrical conductivity. The peak-to-peak voltage and current density were also calculated to obtain the overall MoS2-xNx TENG efficacy under application of different external loads of 0.1, 0.4, 0.8, 1, 10, 20, 30, and 40 MΩ resistance (Figure S12) and the corresponding signal is shown in Figure S13. It is to be noted here that the asymmetric behavior in alternating output voltage in the vertical contact-separation mode can be observed because of the lower charge pushing from one to another electrode (Chen et al., 2020). In this mode when the upper electrode separates, the voltage is mainly associated with the dielectric film. Thus, the output voltage is much higher in the upper electrode than the former electrode. The power density was also obtained to realize the net output powering capability of TENG for the different sets of loads (Figure S14). Figure S15 shows the cross-section FESEM image of MoS2-xNx films grown on a flexible ITO-coated PET substrate.
Figure 4.
Triboelectric nanogenerator device architecture and output voltage performance of MoS2-xNx and pristine MoS2TENG
(A) Schematic of a TENG device containing MoS2-xNx film grown on flexible ITO coated PET substrate.
(B) The continuous open-circuit voltage (Voc) of MoS2-xNx and pristine MoS2 TENG devices was recorded at 21-Hz frequency.
Cellulose fibers are bio-degradable, lightweight, and high strength materials and have a high propensity to lose an electron because of the presence of abundant oxygen atoms, making it a prominent material for a tribo-positive layer (Chen et al., 2020). Here, the effective work-function of the MoS2 and MoS2-xNx films was calculated via UV photoelectron microscopy, which was the main factor for deciding upon its use in the triboelectric charging behavior. It is observed that the MoS2-xNx films exhibit higher effective work function as compared to pristine MoS2 film. Thus, the p-type Nitrogen doping in MoS2 leads to the enhancement of work-function making it more negative in the triboelectric series. Thus, the triboelectric charging features are successfully modified by N doping in the MoS2 lattice that change the effective work-function.
Under the application of external impulse-force, the physical contact between MoS2-xNx/microfiber/Al is instantly established and contact charging occurs. While triboelectricity basically embodies the contact charging process, the charge transfer has to take place between the two interfacing materials to generate a voltage across and drive currents across. This intrinsically involves their propensity to give and accept the charge; a consideration that lays the foundation for the existence of the triboelectric series. Moreover, because the currents have to flow through the bulk for compensating the charge imbalances resulting from the contact charging, the electronic band structures of the participating materials also come into play. There are situations where the electronic character of the two materials differs by way of being a metal, semiconductor, or insulator and this causes further complications of electronic currents and depolarization currents. Thus, the whole process is rather complex to elucidate in simple terms. In our case, the microfiber and Al hold different triboelectric polarity. When both the layers brought into contact, the electrons are exchanged the effective differential work function. Thus, the MoS2-xNx TENG performance enhancement over pristine MoS2 TENG can be attributed to the synergy of the device architecture, p-type N doping in MoS2 lattice, favorably increased work-function, multiphasic component of MoS2 with enhanced conducting component 1T′ and increased surface roughness enhancing the trapped charge kinetics via locally enhanced field.
Conclusion
In summary, we report on the growth, properties, and applicability of high-quality thin films of MoS2-xNx on single-crystal c-Al2O3 substrates under ammonia ambient by the pulsed laser deposition (PLD) technique. We find that the films exhibit 1Tʹ/2H biphasic constitution, with the 1Tʹ component increasing dramatically with enhanced growth pressure of ammonia (∼80% 1Tʹ phase @ 300 mTorr growth pressure). Raman characterization reveals that the enhancement of the 1Tʹ phase component can be attributed to the incorporation of covalently bonded N in the MoS2 lattice inducing compressive strain. Transport measurements show progressive drop in resistivity with enhanced ammonia growth pressure and the MoS2-xNx films grown at 300 mTorr NH3 show extremely low room temperature resistivity of 0.03 mΩ-cm which is two orders of magnitude lower than the undoped MoS2. In a triboelectric nanogenerator (TENG) device the biphasic MoS2-xNx film grown at 300 mTorr NH3 pressure serves as an electron acceptor and exhibits a 3-fold enhancement in the output voltage as compared to the pristine MoS2. Increased work-function due to highly conducting covalent p-type N doping in multiphasic MoS2 with increased roughness and device assembly synergistically contributes to this superior TENG performance.
Limitations of the study
In this study, we have fabricated, optimized, and characterized MoS2-xNx films using pulsed laser deposition (PLD) in NH3 ambient. In addition, we have elucidated the role of N in the MoS2 lattice, which is responsible for enhancing its electronic properties via modulating the chemical and structural properties. While the possibility of incorporation of some hydrogen cannot be ruled out during the deposition in NH3-assisted PLD, we did not find evidence of the same in the set of characterizations employed. However, a solid-state Nuclear Magnetic Resonance (NMR) can possibly bring out the role of Hydrogen in the MoS2-xNx lattice, as H bonding can contribute to the changes of the electronic bands. Also, PLD is difficult to scale up; therefore, after the basic results are established by PLD studies, one could perform a follow-up with scalable sputtering or PVD techniques.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Molybdenum di sulfide (99.9%) | Sigma-Aldrich | CAS# 1317-33-5 |
| Aceton, 99.9% | Sigma Aldrich | CAS# 67-64-1 |
| c-Al2O3 Substrate (99.99%) | MTI | Product# ALC25D05C1 |
| ITO PET sheets | Sigma Aldrich | Product# 639303-1EA |
| Aluminum Foil Tape | 3M | Product# 3363 |
| Copper Foil Tape | 3M | Product# 1182 |
| Microfiber paper | Sigma Aldrich | Product# WHA2200070 |
| Double sided tape | 3M | Product# GPH-110GF |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Satishchandra Ogale (satishogale@iiserpune.ac.in)
Materials availability
This work is an experimental study of thin-film growth and nanogenerators and there is no new code generated.
Experimental model and subject details
Molybdenum di-sulfide (MoS2, 99.9%) was purchased from Sigma-Aldrich. The chemical was used without further purification. Ammonia-Argon gas cylinder (30% Ammonia Bal Argon Pressure 20 Bar/cm2 Purity- 99.9%) was used for the deposition of MoS2-xNx thin films. The c-Al2O3 substrates were cut into 5x5 mm2 area using diamond cutter and sintered at 1000⁰C to obtain atomically flat substrate followed by washing away residual dust/particle with sonicating under acetone and deionized water at room temperature for 15 min each. They were then blow dried in Ar air before loading into the PLD chamber. The ITO coated PET was directly used for deposition without any treatment.
Method details
MoS2-xNx thin-film growth
In this work, we have grown 50 nm thin-films of MoS2-xNx under NH3 pressures of 1 mTorr, 10 mTorr, 100 mTorr, and 300 mTorr respectively by Pulsed Laser Deposition (PLD) (KrF, λ = 248). Here we have used c-Al2O3 substrates for the detailed characterization and ITO coated PET for nanogenerator application. The MoS2-xNx films were deposited at 400°C and 150°C on c-Al2O3 and ITO-coated PET substrates, respectively. To make the c-Al2O3 substrate atomically flat, the annealing treatment was given at 1000°C for 1 h in air before the deposition. The distance between the polycrystalline MoS2target to the substrate was ∼40 mm. The substrate temperature was 400°C for all depositions under ammonia-argon gas mixture (30% ammonia) at 5 Hz frequency by using 1.5 J/cm2 laser energy density. Pristine MoS2 films were deposited under vacuum with the base pressure of 10-6 mbar. All films were cooled down naturally.
Structural and chemical characterizations
Raman spectra for all MoS2 thin-film samples were recorded at ∼2.33 eV (532 nm) excitation energy laser. Atomic force microscopy (AFM) topography images of thin films were taken using Nanosurf AFM (Switzerland). X-ray photoelectron spectroscopy (XPS) was performed in an ultra-high vacuum chamber (2x10-9 mBar) by using an Al Kαx-ray source with 6 mA beam current on a Thermo-Fisher Scientific Instrument, UK, where beam spot size on the thin-film samples were ∼400 μm. High-resolution transmission electron microscopy (HRTEM) images were recorded using UHR FEG-TEM microscopic instrument, operating at 200 kV accelerating voltage. To record the absorption spectrum of MoS2-xNx thin film, an ultraviolet-visible (UV–vis) spectrophotometer (LAMBDA 950, PerkinElmer) was used. Field emission scanning electron microscopy (FESEM) images of the electrodes were recorded using a JEM-2100F (JEOL, Japan) instrument.
X-ray absorption spectroscopy measurement of N K-edges was collected in total electron yield (TEY) mode of MoS2-xNx characterization was done using synchrotron radiation source at RRCAT, Indore at ‘polarized light soft X-ray absorption spectroscopy beamline’ (BL-01) in INDUS-2, respectively. Before recording the XAS spectra, the thin film surface was sputtered using high energy Argon ions inside the chamber where base pressure was maintained at ∼1 x 10-10 mbar in order to remove any surface contamination.
Electrical characterization
Electrical measurements were performed in standard AC transport four-probe method by using a Quantum Design Physical Property Measurement System (PPMS), over the temperature range of T = 5-300 K, where the dimensions of MoS2 and MoS2-xNx film was 2x5 mm2.
Nanogenerator output performance
Keithley multimeter (DMM7510 7.5 multimeter) was used to measure open circuit voltage at 10 MΩ input impedance at 10 N impact force with 21 Hz frequency. For the impact force and frequency source, we used home-made modified sewing machine setup. We measured force using PASPORT Force Sensor. Voltage and current were also measured on Keithley multimeter (DMM7510 7.5 multimeter) with variable resistance.
Quantification and statistical analysis
The TENG data were collected on a Kickstart, an oscilloscope testing system (DMM7510). Figures were produced by Origin from the raw data.
Additional resources
Any additional information about the thin-film fabrication, TENG fabrication, tests and data reported in this paper is available from the lead contact on request.
Acknowledgment
Swati Parmar would like to thank CSIR for SRF fellowship. Satish Ogale and R. Boomishankar would like to thank DST Nanomission Thematic unit project (SR/NM/TP/13/2016) for funding support. We would like to thank Prof. Surjeet Singh and Ms. Dibyata Rout for providing the facility for the R-T measurement.
Author contributions
S.P. and S.O. developed the idea and design the experiments, S.P. performed and analyzed the thin film growth, characterization, and device fabrication. N.P. carried out the TENG device testing and B.R. helped with the analysis. M.W. helped in the FESEM characterization. R.C. helped in providing the XANES experiment and analysis. S.P. and S.O. co-wrote the paper. All the authors contributed to the discussion and analysis.
Declaration of interests
The authors declare no competing interests.
Published: March 18, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.103898.
Contributor Information
Ramamoorthy Boomishankar, Email: boomi@iiserpune.ac.in.
Satishchandra Ogale, Email: satishogale@iiserpune.ac.in, satishogale@tcgcrest.org.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
No new code was generated during the course of this study.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Acerce M., Akdoğan E.K., Chhowalla M. Metallic molybdenum disulfide nanosheet-based electrochemical actuators. Nature. 2017;549:370–373. doi: 10.1038/nature23668. [DOI] [PubMed] [Google Scholar]
- Ahmed T., Bellare P., Debnath R., Roy A., Ravishankar N., Ghosh A. Thermal history-dependent current relaxation in hBN/MoS2 van der Waals dimers. ACS Nano. 2020;14:5909–5916. doi: 10.1021/acsnano.0c01079. [DOI] [PubMed] [Google Scholar]
- Azcatl A., Qin X., Prakash A., Zhang C., Cheng L., Wang Q., Lu N., Kim M.J., Kim J., Cho K., et al. Covalent nitrogen doping and compressive strain in MoS2 by remote N2 plasma exposure. Nano Lett. 2016;16:5437–5443. doi: 10.1021/acs.nanolett.6b01853. [DOI] [PubMed] [Google Scholar]
- Barani Z., Kargar F., Ghafouri Y., Ghosh S., Godziszewski K., Baraghani S., Yashchyshyn Y., Cywiński G., Rumyantsev S., Salguero T.T., et al. Electrically insulating flexible films with quasi-1D van der Waals fillers as efficient electromagnetic shields in the GHz and sub-THz frequency bands. Adv. Mater. 2021;33:2007286. doi: 10.1002/adma.202007286. [DOI] [PubMed] [Google Scholar]
- Briggs N., Subramanian S., Lin Z., Li X., Zhang X., Zhang K., Xiao K., Geohegan D., Wallace R., Chen L.-Q., et al. A roadmap for electronic grade 2D materials. 2D Mater. 2019;6:022001. [Google Scholar]
- Cai L., He J., Liu Q., Yao T., Chen L., Yan W., Hu F., Jiang Y., Zhao Y., Hu T., et al. Vacancy-induced ferromagnetism of MoS2nanosheets. J. Am. Chem. Soc. 2015;137:2622–2627. doi: 10.1021/ja5120908. [DOI] [PubMed] [Google Scholar]
- Chen A., Zhang C., Zhu G., Wang Z.L. Polymer materials for high-performance triboelectric nanogenerators. Adv. Sci. 2020;7:2000186. doi: 10.1002/advs.202000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C., Wong J., Taqieddin A., Biswas S., Aluru N.R., Nam S., Atwater H.A. Highly strain-tunable interlayer excitons in MoS2/WSe2heterobilayers. Nano Lett. 2021;21:3956–3964. doi: 10.1021/acs.nanolett.1c00724. [DOI] [PubMed] [Google Scholar]
- Das S., Demarteau M., Roelofs A. Nb-doped single crystalline MoS2 field effect transistor. Appl. Phys. Lett. 2015;106:173506. [Google Scholar]
- Du Y., Liu H., Neal A.T., Si M., Ye P.D. Molecular doping of multilayer MoS2field-effect transistors: reduction in sheet and contact resistances. IEEE Electron.Device Lett. 2013;34:1328–1330. [Google Scholar]
- Feng L.-P., Su J., Liu Z.-T. Effect of vacancies on structural, electronic and optical properties of monolayer MoS2: a first-principles study. J. Alloys Compd. 2014;613:122–127. [Google Scholar]
- Frisenda R., Navarro-Moratalla E., Gant P., Pérez De Lara D., Jarillo-Herrero P., Gorbachev R.V., Castellanos-Gomez A. Recent progress in the assembly of nanodevices and van der Waals heterostructures by deterministic placement of 2D materials. Chem. Soc. Rev. 2018;47:53–68. doi: 10.1039/c7cs00556c. [DOI] [PubMed] [Google Scholar]
- Fu S., Kang K., Shayan K., Yoshimura A., Dadras S., Wang X., Zhang L., Chen S., Liu N., Jindal A., et al. Enabling room temperature ferromagnetism in monolayer MoS2 via in situ iron-doping. Nat. Commun. 2020;11:2034. doi: 10.1038/s41467-020-15877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Suh J., Cao M.C., Joe A.Y., Mujid F., Lee K.-H., Xie S., Poddar P., Lee J.-U., Kang K., et al. Tuning electrical conductance of MoS2 monolayers through substitutional doping. Nano Lett. 2020;20:4095–4101. doi: 10.1021/acs.nanolett.9b05247. [DOI] [PubMed] [Google Scholar]
- George S.J., Drury O.B., Fu J., Friedrich S., Doonan C.J., George G.N., White J.M., Young C.G., Cramer S.P. Molybdenum X-ray absorption edges from 200 to 20,000eV: the benefits of soft X-ray spectroscopy for chemical speciation. J. Inorg. Biochem. 2009;103:157–167. doi: 10.1016/j.jinorgbio.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia L., Paredes J.I., Munuera J.M., Villar-Rodil S., Ayán-Varela M., Martínez-Alonso A., Tascón J.M.D. Chemically exfoliated MoS2nanosheets as an efficient catalyst for reduction reactions in the aqueous phase. ACS Appl. Mater. Inter. 2014;6:21702–21710. doi: 10.1021/am506922q. [DOI] [PubMed] [Google Scholar]
- Hu C., Yuan C., Hong A., Guo M., Yu T., Luo X. Work function variation of monolayer MoS2 by nitrogen-doping. Appl. Phys. Lett. 2018;113:041602. [Google Scholar]
- Jariwala D., Sangwan V.K., Lauhon L.J., Marks T.J., Hersam M.C. Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides. ACS Nano. 2014;8:1102–1120. doi: 10.1021/nn500064s. [DOI] [PubMed] [Google Scholar]
- Kim D.W., Lee J.H., Kim J.K. Material aspects of triboelectric energy generation and sensors. NPG Asia Mater. 2020;12:6. [Google Scholar]
- Kiriya D., Tosun M., Zhao P., Kang J.S., Javey A. Air-stable surface charge transfer doping of MoS2 by benzyl viologen. J. Am. Chem. Soc. 2014;136:7853–7856. doi: 10.1021/ja5033327. [DOI] [PubMed] [Google Scholar]
- Lajaunie L., Boucher F., Dessapt R., Moreau P. Quantitative use of electron energy-loss spectroscopy Mo-M2,3 edges for the study of molybdenum oxides. Ultramicroscopy. 2015;149:1–8. doi: 10.1016/j.ultramic.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Lee J., Wang Z., Xie H., Mak K.F., Shan J. Valley magnetoelectricity in single-layer MoS2. Nat. Mater. 2017;16:887–891. doi: 10.1038/nmat4931. [DOI] [PubMed] [Google Scholar]
- Li J., Kang J., Cai Q., Hong W., Jian C., Liu W., Banerjee K. Boosting hydrogen evolution performance of MoS2 by band structure engineering. Adv. Mater. Inter. 2017;4:1700303. [Google Scholar]
- Li L., Li Z. Structural phase transition barrier of N-doped MoS2 with charge injection. Mater.Res. Express. 2018;6:016308. [Google Scholar]
- Lim K.-W., Peddigari M., Park C.H., Lee H.Y., Min Y., Kim J.-W., Ahn C.-W., Choi J.-J., Hahn B.-D., Choi J.-H., et al. A high output magneto-mechano-triboelectric generator enabled by accelerated water-soluble nano-bullets for powering a wireless indoor positioning system. Energy Environ. Sci. 2019;12:666–674. [Google Scholar]
- Liu L., Wu J., Wu L., Ye M., Liu X., Wang Q., Hou S., Lu P., Sun L., Zheng J., et al. Phase-selective synthesis of 1T′ MoS2 monolayers and heterophase bilayers. Nat. Mater. 2018;17:1108–1114. doi: 10.1038/s41563-018-0187-1. [DOI] [PubMed] [Google Scholar]
- Mak K.F., Shan J., Ralph D.C. Probing and controlling magnetic states in 2D layered magnetic materials. Nat. Rev. Phys. 2019;1:646–661. [Google Scholar]
- Martinez L.M., Delgado J.A., Saiz C.L., Cosio A., Wu Y., Villagrán D., Gandha K., Karthik C., Nlebedim I.C., Singamaneni S.R. Magnetic and electrocatalytic properties of transition metal doped MoS2 nanocrystals. J. Appl. Phys. 2018;124:153903. [Google Scholar]
- Mignuzzi S., Pollard A.J., Bonini N., Brennan B., Gilmore I.S., Pimenta M.A., Richards D., Roy D. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B. 2015;91:195411. [Google Scholar]
- Mitterreiter E., Schuler B., Micevic A., Hernangómez-Pérez D., Barthelmi K., Cochrane K.A., Kiemle J., Sigger F., Klein J., Wong E., et al. The role of chalcogen vacancies for atomic defect emission in MoS2. Nat. Commun. 2021;12:3822. doi: 10.1038/s41467-021-24102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombrú D., Faccio R., Mombrú Á.W. Possible doping of single-layer MoS2 with Pt: a DFT study. Appl. Surf. Sci. 2018;462:409–416. [Google Scholar]
- Pal B., Singh A., G S., Mahale P., Kumar A., Thirupathaiah S., Sezen H., Amati M., Gregoratti L., Waghmare U.V., et al. Chemically exfoliated MoS2 layers: spectroscopic evidence for the semiconducting nature of the dominant trigonal metastable phase. Phys. Rev. B. 2017;96:195426. [Google Scholar]
- Park T.-E., Suh J., Seo D., Park J., Lin D.-Y., Huang Y.-S., Choi H.-J., Wu J., Jang C., Chang J. Hopping conduction in p-type MoS2 near the critical regime of the metal-insulator transition. Appl. Phys. Lett. 2015;107:223107. [Google Scholar]
- Parmar S., Biswas A., Kumar Singh S., Ray B., Parmar S., Gosavi S., Sathe V., Janay Choudhary R., Datar S., Ogale S. Coexisting 1T/2H polymorphs, reentrant resistivity behavior, and charge distribution in MoS2−hBN 2D/2D composite thin films. Phys. Rev. Mater. 2019;3:074007. [Google Scholar]
- Parmar S., Biswas A., Ray B., Gosavi S., Datar S., Ogale S. Stabilizing metastable polymorphs of van der Waals solid MoS2 on single crystal oxide substrates: exploring the possible role of surface chemistry and structure. J. Phys. Chem. C. 2021;125:11216–11224. [Google Scholar]
- Parmar S., Das T., Biswas A., Ray B., Debnath B., Gosavi S., Shanker G.S., Datar S., Chakraborty S., Ogale S. N, H dual-doped black anatase TiO2 thin films toward significant self-activation in electrocatalytic hydrogen evolution reaction in alkaline media. Adv. Energy Sustainability Res. 2021;3:2100137. doi: 10.1002/aesr.202100137. [DOI] [Google Scholar]
- Reinholdt P., Vidal M.L., Kongsted J., Iannuzzi M., Coriani S., Odelius M. Nitrogen K-edge X-ray absorption spectra of ammonium and ammonia in water solution: assessing the performance of polarizable embedding coupled cluster methods. J. Phys. Chem. Lett. 2021;12:8865–8871. doi: 10.1021/acs.jpclett.1c02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa C.J.L.d.l., Phillipson R., Teyssandier J., Adisoejoso J., Balaji Y., Huyghebaert C., Radu I., Heyns M., Feyter S.D., Gendt S.D. Molecular doping of MoS2 transistors by self-assembled oleylamine networks. Appl. Phys. Lett. 2016;109:253112. [Google Scholar]
- Sebastian A., Pendurthi R., Choudhury T.H., Redwing J.M., Das S. Benchmarking monolayer MoS2 and WS2 field-effect transistors. Nat. Commun. 2021;12:693. doi: 10.1038/s41467-020-20732-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol M., Kim S., Cho Y., Byun K.-E., Kim H., Kim J., Kim S.K., Kim S.-W., Shin H.-J., Park S. Triboelectric series of 2D layered materials. Adv. Mater. 2018;30:1801210. doi: 10.1002/adma.201801210. [DOI] [PubMed] [Google Scholar]
- Serna M.I., Yoo S.H., Moreno S., Xi Y., Oviedo J.P., Choi H., Alshareef H.N., Kim M.J., Minary-Jolandan M., Quevedo-Lopez M.A. Large-area deposition of MoS2 by pulsed laser deposition with in situ thickness control. ACS Nano. 2016;10:6054–6061. doi: 10.1021/acsnano.6b01636. [DOI] [PubMed] [Google Scholar]
- Serrao C.R., Diamond A.M., Hsu S.-L., You L., Gadgil S., Clarkson J., Carraro C., Maboudian R., Hu C., Salahuddin S. Highly crystalline MoS2 thin films grown by pulsed laser deposition. Appl. Phys. Lett. 2015;106:052101. [Google Scholar]
- Shin B., Zhu Y., Bojarczuk N.A., Chey S.J., Guha S. Control of Interfacial MoSe₂ Layer Thickness in CuZnSnSe₄ Thin Film Solar Cell: 8.9% power conversion efficiency with a TiN diffusion barrier. Appl. Phys. Lett. 2012;161:053903. [Google Scholar]
- Siegel G., Subbaiah Y.P.V., Prestgard M.C., Tiwari A. Growth of centimeter-scale atomically thin MoS2 films by pulsed laser deposition. APL Mater. 2015;3:056103. [Google Scholar]
- Tang J., Wei Z., Wang Q., Wang Y., Han B., Li X., Huang B., Liao M., Liu J., Li N., et al. In Situ oxygen doping of monolayer MoS2 for novel electronics. Small. 2020;16:2004276. doi: 10.1002/smll.202004276. [DOI] [PubMed] [Google Scholar]
- Tang Q., Jiang D.-e. Stabilization and band-gap tuning of the 1T-MoS2 monolayer by covalent functionalization. Chem. Mater. 2015;27:3743–3748. [Google Scholar]
- Tao J., Chai J., Lu X., Wong L.M., Wong T.I., Pan J., Xiong Q., Chi D., Wang S. Growth of wafer-scale MoS2 monolayer by magnetron sputtering. Nanoscale. 2015;7:2497–2503. doi: 10.1039/c4nr06411a. [DOI] [PubMed] [Google Scholar]
- Tao J., Chai J.W., Zhang Z., Pan J.S., Wang S.J. The energy-band alignment at molybdenum disulphide and high-k dielectrics interfaces. Appl. Phys. Lett. 2014;104:232110. [Google Scholar]
- Wang H., Tran D., Qian J., Ding F., Losic D. MoS2/Graphene composites as promising materials for energy storage and conversion applications. Adv. Mater. Inter. 2019;6:1900915. [Google Scholar]
- Wang J., Zhou Q., Lu Z., Gui Y., Zeng W. Adsorption of H2O molecule on TM (Au, Ag) doped-MoS2 monolayer: a first-principles study. Phys. E Low-dimens. Syst. Nanostruct. 2019;113:72–78. [Google Scholar]
- Wang S., Yu H., Zhang H., Wang A., Zhao M., Chen Y., Mei L., Wang J. Broadband few-layer MoS2saturable absorbers. Adv. Mater. 2014;26:3538–3544. doi: 10.1002/adma.201306322. [DOI] [PubMed] [Google Scholar]
- Yang L., Majumdar K., Liu H., Du Y., Wu H., Hatzistergos M., Hung P.Y., Tieckelmann R., Tsai W., Hobbs C., et al. Chloride molecular doping technique on 2D materials: WS2 and MoS2. Nano Lett. 2014;14:6275–6280. doi: 10.1021/nl502603d. [DOI] [PubMed] [Google Scholar]
- Yang Q., Wang Z., Dong L., Zhao W., Jin Y., Fang L., Hu B., Dong M. Activating MoS2 with super-high nitrogen-doping concentration as efficient catalyst for hydrogen evolution reaction. J. Phys. Chem. C. 2019;123:10917–10925. [Google Scholar]
- Zhang X.-X., Lai Y., Dohner E., Moon S., Taniguchi T., Watanabe K., Smirnov D., Heinz T.F. Zeeman-induced valley-sensitive photocurrent in monolayer MoS2. Phys. Rev. Lett. 2019;122:127401. doi: 10.1103/PhysRevLett.122.127401. [DOI] [PubMed] [Google Scholar]
- Zhang X., Liao Q., Kang Z., Liu B., Liu X., Ou Y., Xiao J., Du J., Liu Y., Gao L., et al. Hidden vacancy benefit in monolayer 2D semiconductors. Adv. Mater. 2021;33:2007051. doi: 10.1002/adma.202007051. [DOI] [PubMed] [Google Scholar]
- Zhao W., Pan J., Fang Y., Che X., Wang D., Bu K., Huang F. Metastable MoS2: crystal structure, electronic band structure, synthetic approach and intriguing physical properties. Chemistry. 2018;24:15942–15954. doi: 10.1002/chem.201801018. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Kiriya D., Haller E.E., Ager J.W., Javey A., Chrzan D.C. Compliant substrate epitaxy: Au on MoS2. Phys. Rev. B. 2016;93:054106. [Google Scholar]
- Zou J., Li F., Bissett M.A., Kim F., Hardwick L.J. Intercalation behaviour of Li and Na into 3-layer and multilayer MoS2 flakes. Electrochim.Acta. 2020;331:135284. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
No new code was generated during the course of this study.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.