Summary
Presented here is a copper-catalyzed, aerobic oxidative C-H/C-H cyclization reaction, which occurs by cleaving the C-H and N-H bonds of 3-phenylindoles. A broad range of 3-phenylindoles can be well tolerated to produce the indole-containing polycyclic aromatic hydrocarbons (PAH) in good to excellent yields. An evaluation of the reaction mechanism is enabled by the isolation of the di- and tri-indole intermediates, highlighting the role of the substrate for this catalytic reaction. The results of these controlled experiments and kinetic studies provide solid experimental support for a self-catalysis reaction, which has rarely been observed in oxidative C-H activation reactions. Additional mechanistic studies indicate that the substrate for this reaction accelerates by the following mechanism: The substrate combines with the Cu catalyst to transform the less active di-indole intermediate into a tri-indole intermediate. This intermediate is quickly converted into the desired product along with regeneration of the substrate copper complex.
Subject areas: Chemistry, Chemical engineering, Catalysis
Graphical abstract

Highlights
-
•
A self-catalytic cycle in a copper-catalyzed aerobic oxidative cyclization reaction
-
•
Tri-indole intermediates enable an evaluation of the reaction mechanism
-
•
3-Phenylindole accelerated the cyclization of di-indole intermediate
Chemistry; Chemical engineering; Catalysis
Introduction
The development of new reactions is a fundamental and continuous goal for chemistry overall, but remains challenging in synthetic organic chemistry. In this context, catalysis has been established as one of the most useful and powerful tools for identifying and engineering new chemical reactions. In general, the reactants are usually activated by one—or more than one—catalyst, which is neither consumed nor produced during the course of reaction. However, the rapid development of catalysis has shown that in some reaction systems, the product works as either a catalyst or a co-catalyst, thereby promoting the desired reaction. This is known as auto-catalysis and it enables the development of unprecedented transformations that are not possible through other methods (Bissette and Fletcher, 2013; Soai et al., 1995; Lutz et al., 2005; Kawasaki et al., 2009; Matsumoto et al., 2016, 2017; Barrios-Landeros et al., 2008; Giri and Hartwig, 2010; Semenov et al., 2018; Flegeau et al., 2011). Moreover, the reactant may also act as either an activator or co-activator to facilitate the desired transformations; this is known as self-catalysis (Sawato et al., 2019). The synthetic potential of autocatalysis has been exemplified in organometallic reactions (Semenov et al., 2018; Flegeau et al., 2011). However, compared to the auto-catalysis, the organometallic reactions involving a self-catalytic cycle are rare (Li et al., 2018; Wang et al., 2019; Bachmann et al., 2008; MacLeod et al., 2010; Liu et al., 2019; Rodrigues et al., 2021).
Indoles and their derivatives have garnered substantial synthetic interest as a result of their presence as an important structural motif in myriad natural products and pharmaceuticals (Cacchi and Fabrizi, 2011; Lancianesi et al., 2014). Numerous methodologies have been developed to access substituted indole derivatives (Li et al., 2010; Liang et al., 2010; Li et al., 2011; Li et al., 2019; Ozaki et al., 2013; Cheng et al., 2016; Stuart and Fagnou, 2007; Nishino et al., 2012; Cambeiro et al., 2015). One of the most expedient synthetic strategies toward the creation of functionalized indoles has been developed through direct C-H functionalization of the indole core (Ozaki et al., 2013; Cheng et al., 2016; Stuart and Fagnou, 2007; Nishino et al., 2012; Cambeiro et al., 2015). In this context, the electrophilic metalation (Phipps et al., 2008)29 and 1,2-migratory metalation (Lane et al., 2005; Grimster et al., 2005) have proven to be important strategies for the regioselective cleavage of the C-H bonds at the C-2 and C-3 position of indoles. Building on such an efficient C-H bond activation strategy, substantially useful and practical processes have been further developed for the synthesis of functionalized indole derivatives (Ferreira and Stoltz, 2003; Jiao and Bach, 2011; Shi et al., 2009). On the other hand, the selective cleavage of the N-H bond of indoles has become an important and fundamental step in the catalytic functionalization of indoles. This process is useful in creating a direct N-H functionalization of the indoles via the formation of C-N bonds. Moreover, a fundamental step of forming a new C-C bond is provided in the metal-mediated dearomatization of indoles at the C-3 position (Zhuo et al., 2014). Besides, the N-H bond of indoles could be facilely cleaved in the presence of a stoichiometric amount of strong base. That said, the processes for the cleavage of the N-H bonds in the presence of either neutral or even acidic conditions for electrophilic metalation are rare and challenging (Tsuchimoto et al., 2008; Tsuchimoto et al., 2011; Huang et al., 2016; Morimoto et al., 2010; Ackermann et al., 2012). In light of these impediments, we were inspired by the importance of indole-containing polycyclic aromatic hydrocarbons (PAH) in the synthesis of agrochemicals, pharmaceuticals, and natural products (Knölker and Reddy, 2002; Jacob, 2008; Suzuki et al., 2018) to develop an effective N-H bond cleavage strategy. The goal was to identify one that was compatible with electrophilic metalation and to allow new reactions to be established via sequential C-H and N-H bond activation. Herein, we report a copper-catalyzed oxidative coupling and cyclization reaction with oxygen as the sole oxidant via sequential cleavage of C-H and N-H bonds (Figure 1) (Wendlandt et al., 2011; Allen et al., 2013; Guo et al., 2015; Tang et al., 2018; Li, 2009; Yeung and Dong, 2011; Liu et al., 2015; Cho et al., 2011; Yang et al., 2017; Girard et al., 2014). Additional mechanistic studies disclosed that a surprising self-catalysis process took place, enabling a facile N-H bond cleavage process in the presence of acid.
Figure 1.
Self-catalysis involved Cu-Catalyzed oxidative coupling and cyclization of 3-arylindoles
Results and discussion
Reaction mechanism
Our investigation began with the palladium-catalyzed C-H functionalization of indole with bromobenzene. The 3-phenylindole 1a was obtained in 80% yield on a gram scale (Bellina et al., 2008), which was then treated by O2 in TFA at 50 °C for 8.0 h in the presence of a catalytic amount of Cu(OAc)2. The desired cyclization product 2a was obtained in 85% yield (Figure 2). The homo-coupling and cyclization product 2a might form via the cleavage of three C-H bonds and one N-H bond, which revealed that the N-H bond cleavage was even compatible with the C-H bond activation in our catalytic system. Further optimized reaction conditions led us to the finding that the reactivity was significantly affected by the nature of the counter-ion of the copper salts (see supplemental information). High yields were achieved in reactions using Cu(OAc)2, Cu(CF3CO2)2, Cu(OPiv)2, or Cu(acac)2 as the catalyst; no reaction occurred when either CuCl2 or CuBr2 were used. These results indicated that a basic counter-ion (e.g., OAc−, CF3CO2−, or t-BuCH2CO2−) was essential to promote the copper-mediated C-H bond cleavage via a CMD process (García-Cuadrado et al., 2007; Gorelsky et al., 2008). The CF3CO2H (TFA) was the solvent of choice; notably, no reaction took place in other tested solvents. Oxygen was not essential for the reaction since good yield was still obtained when a stoichiometric amount of Cu(OAc)2 was utilized under a nitrogen atmosphere. This result suggested that O2 only functioned as the oxidant to recycle the copper-catalyst.
Figure 2.
The functionalization of the indole derivatives
Controlled experiments
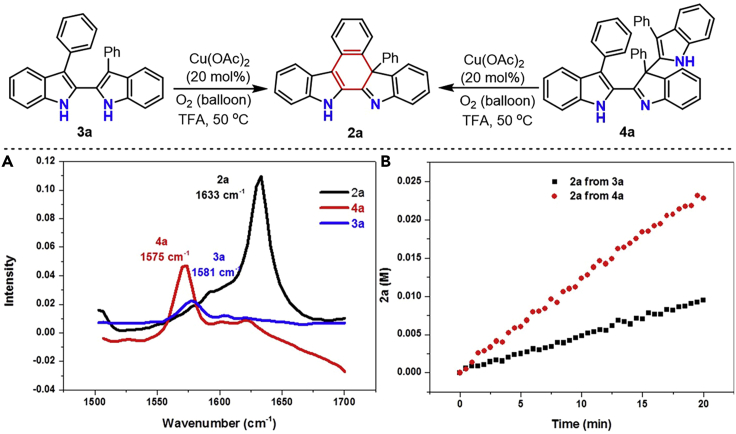
With the optimized reaction condition identified, we next focused our attention on gaining insight into the reaction mechanism. The kinetic analysis of the reaction of 1a under the optimized reaction conditions was monitored by in situ IR and GC-analysis. As shown in Figure 3, the total reaction profile showed that the generation of the cyclization product 2a did not parallel with the consumption of 1a. The decay rate of the substrate 1a was much faster than the rate of the formation of the desired product 2a, which strongly suggested that some unknown intermediates might be involved in the reaction system. These results were intriguing and led to isolate the plausible intermediates by implementing controlled experiments. As expected, both a tri-indole intermediate 4a and di-indole intermediate 3a were obtained in 64% and 6% yields, respectively, when the reaction proceeded at 30 °C for 1.0 h under identical reaction conditions. Prolonging the reaction time from 1.0 h to 1.5 h had a positive impact on the yields of tri-indole intermediate 4a and product 2a. The structures of the di-indole intermediate 3a and tri-indole intermediate 4a were unambiguously confirmed by single-crystal X-ray diffraction analysis (Figure 4A). Further controlled experiments demonstrated that both 3a and 4a could be converted into the desired cyclization product 2a in the presence of O2 under the catalysis of Cu(OAc)2 at 50 °C. However, only 16% and 18% yields were observed when the above two reactions were conducted in the presence of a N2 atmosphere (Figures 4B and 4C). This finding indicated that O2 was not likely involved in the cyclization process, but just acted only as a terminal oxidant to recycle the copper catalyst. In addition, the 128% yield of 2a obtained from the reaction of 4a revealed that all the indole-moieties contained in 4a could be converted to the cyclization product 2a. The cross tri-indole intermediate five was achieved in 29% yield when 3a was treated with chlorine-containing 3-phenylindole 1o at 30°C for 2 h under otherwise identical reaction conditions (Figure 4D). This result revealed that tri-indole intermediate 4a should be formed via the oxidative coupling of the di-indole intermediate 3a and 3-phenylindole 1a.
Figure 3.
The Kinetic Analysis of the Reaction of 1a
(A) The 3D FTIR profile of the standard reaction.
(B) Reaction profile of the standard reaction. 1a (1.0 mmol), Cu(OAc)2 (20 mol %), O2 (balloon), TFA (2.0 mL), 50 °C, 3 h.
Figure 4.
Controlled experiments
The unusual reactivity of the tri-indole intermediate 4a prompted us to pursue the underlying mechanism for this reaction. We postulated that two potential pathways (Figure 5) might be responsible for the present reaction. Path I only involved the intermediate 3a, where the cyclization product 2a was directly generated from the di-indole intermediate 3a via oxidative C-H and N-H bond cleavage. In pathway II, both 3a and 4a intermediates were formed. Importantly, the desired cyclization product 2a was produced from the 4a via C-C and C-H bond cleavages. Further analysis of the two reaction pathways along with the above controlled experiments allowed us to conclude that k2 should be larger than k4 and k3 because the isolated yield of 4a was much higher than those of both 2a and 3a. Thus, once the relationship of k3 and k4 was determined, the two reaction pathways would be differentiated.
Figure 5.
Possible reaction pathways for the formation of 2a
Kinetic studies of 3a and 4a
To distinguish the feasibility of paths I and II, kinetic studies for the two catalytic reactions using either 3a or 4a as a starting material under standard reaction conditions were conducted. This was achieved by monitoring the reactions using in situ IR. The reaction profile shown in Figure 6 demonstrated that the initial rate (k3) of the reaction with 4a was much faster than that of the reaction with 3a (k4). In comparison, the reaction rate of 4a was more than two times faster than that of 3a (k3>2.5 k4). Collectively, these results suggested that path II was the main pathway for this oxidative coupling/cyclization reaction.
Figure 6.
The Reaction Profile of 3a and 4a
(A) The standard spectrum of 2a, 3a and 4a.
(B) The initial rate of intermediate 3a and 4a as determined by in situ IR. Reaction condition: 3a or 4a (0.30 mmol), Cu(OAc)2 (20 mol %), O2 (balloon), TFA (5.0 mL), 50 °C.
The accelerated role of substrate
As can be seen from Figure 7, the self-catalysis should be observed in path II because substrate 1a or its derivative was released from the cyclization reaction of intermediate 4a. Moreover, the rate of transformation for 3a to 2a should be accelerated by either the substrate or its derivative. To test the catalytic effect, 4-methoxy-3-phenyl-1H-indole 1g was chosen as a co-catalyst for the reaction with 3a as the starting material. This was because the infrared characteristic absorption peak of cyclization product 2a was quite different from that of 2g (the cyclization product of 1g, Figure 7A). The kinetic studies monitored by in situ IR were conducted by the addition of either 10 mol % or 100 mol % of 1g to the reaction system. As shown in Figure 7B, the initial reaction rate of 3a was obviously slower than in the presence of 1g. In addition, the acceleration effect of 1g was evident and the cyclization rate of 3a was greatly enhanced in the presence of 10 mol % or 100 mol % amount of 1g. This experiment was fully consistent with our assumption and provided strong evidence to support the hypothesis that the self-catalysis cycle resided in the oxidative cyclization reaction.
Figure 7.
The Accelerated Role of Substrate 1g
(A) The standard spectrum of 2a and 2g.
(B) Kinetic plots of the reaction in 3a (0.30 mmol), Cu(OAc)2 (20 mol %), O2 and TFA (5.0 mL), the reaction in 3a (0.30 mmol), 4-methoxy-3-phenyl-1H-indole 1g (0.03 mmol), Cu(OAc)2 (20 mol %), O2 and TFA (5.0 mL) and the reaction in 3a (0.30 mmol), 4-methoxy-3-phenyl-1H-indole 1g (0.30 mmol), Cu(OAc)2 (20 mol %), O2 and TFA (5.0 mL)
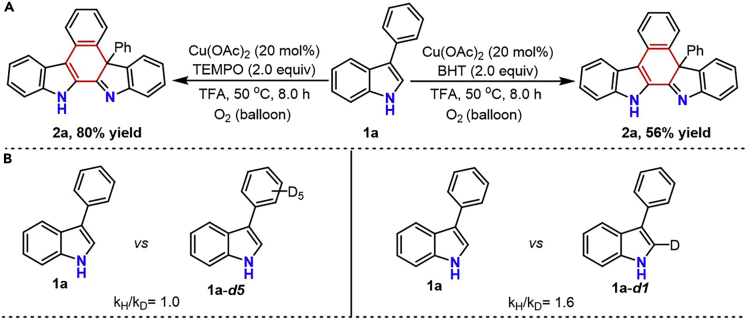
Radical trapping and KIE experiments
After identifying the logical reaction pathway and confirming the existence of self-catalysis in the present reaction, we next conducted additional controlled experiments to gain insight into the possible mechanism of the bond-cleavage and formation. When the radical scavenger TEMPO was introduced into the standard reaction, the desired product was obtained in 80% yield (Figure 8A). In addition, the desired product 2a was still obtained in 56% yield, even when BHT was introduced into this oxidative coupling system (Figure 8A). These data illustrated that the radical process was not involved in this transformation. Parallel experiments were further conducted with 1a, 1a-d5 and 1a-d1 to examine the kinetic isotope effects. The observed KIE values (kH/kD = 1.0 and kH/kD = 1.6) revealed that the C-H bond cleavage events occurring in this reaction were not involved in the rate-limiting step (Figure 8B).
Figure 8.
Radical trapping and KIE experiments
Kinetic behavior of both 4a and Cu(OAc)2
To gain further insight into the mechanism, we then inspected the kinetic behavior of both 4a and Cu(OAc)2 in transformation shown in Figure 9. Initial reaction rates were then measured by varying the concentrations of 4a and the copper catalyst. These experiments revealed a zero-order dependence of the rate on the concentration of Cu(OAc)2. Moreover, a first-order dependence of the rate on the concentration of 4a was also observed. This result indicated that 4a, instead of the copper-catalyst, was involved in the rate-limiting step.
Figure 9.
Kinetic behavior of both 4a and Cu(OAc)2
(A) Plot of initial rates with Cu(OAc)2 showing zero-order dependence. Reaction condition: 4a (0.30 mmol), Cu(OAc)2 (2.5 mol%-25 mol %), O2 (balloon), TFA (5.0 mL), 50 °C.
(B) Plot of initial rates with intermediate 4a showing first-order dependence. Reaction condition: 4a (0.05–0.50 mmol), Cu(OAc)2 (18 mg), O2 (balloon), TFA (5.0 mL), 50 °C.
Catalytic cycle
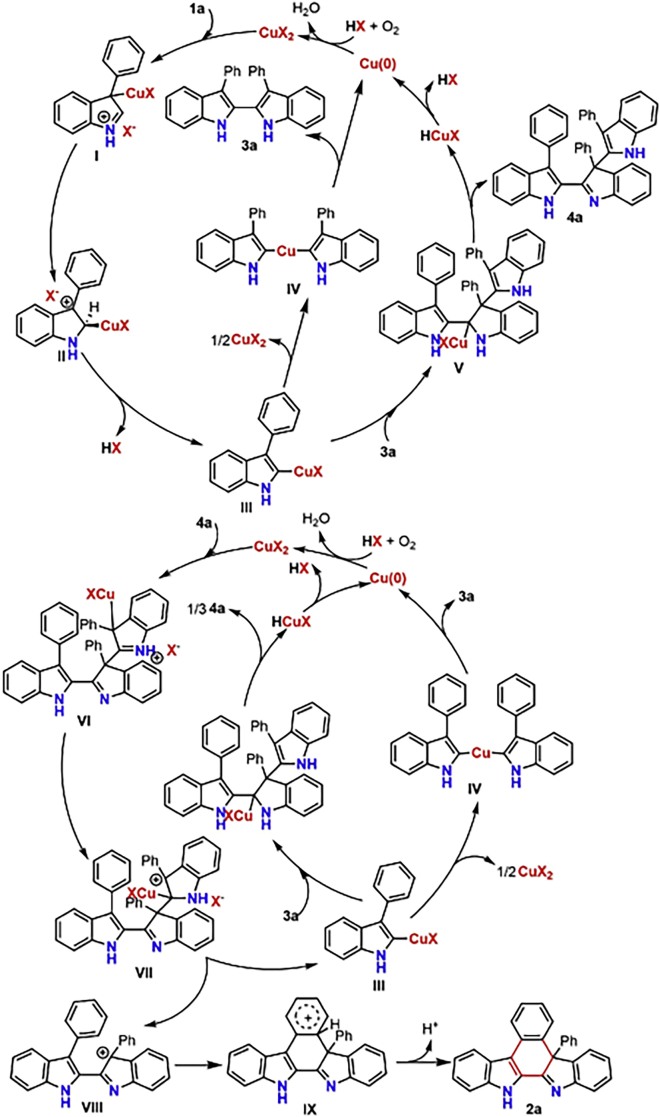
On the basis of the above results, a tentative mechanism for the copper-catalyzed oxidative homo-coupling and cyclization of 3-phenylindole 1a is illustrated in Figure 10. The first catalytic cycle is responsible for the formation of the intermediate 4a, which is a key intermediate for the present reaction. A secondary catalytic cycle is proposed for the mechanism responsible for the formation of the cyclization product 2a from the key intermediate 4a. First, electrophilic metalation of 3-phenylindole 1a with CuX2 occurs to produce the intermediate I, which undergoes 1,2-migratory metalation to form intermediate II under acidic conditions. Cleavage of the C-H bond gives intermediate III, which undergoes self-transmetalation to generate intermediate IV by releasing a half mole amount of CuX2. This is then followed by reductive elimination to give the dimerized intermediate 3a and a half mole amount of Cu(0). Alternatively, intermediate IV might be formed by reaction of intermediate III with 3-phenylindole 1a via electrophilic substitution. The intermediate 3a reacts with intermediate III via migratory insertion to generate intermediate V, which then undergoes β-hydride elimination to generate the trimerization intermediate 4a and HCuX. HCuX then converts into Cu(0), which is oxidized to CuX2 by O2 to furnish the first catalytic cycle. The trimerization intermediate 4a would be captured by CuX2 via electrophilic metalation to enter the second catalytic cycle to form intermediate VI, which would be then followed by 1,2-migratory metalation to produce intermediate VII. Fragmentation of the intermediate VII occurs to release the cationic intermediate VIII, followed again by the regeneration of the copper-contained intermediate III. Intermediate III is then transferred to one-third of a mole amount of trimerization intermediate 3a via the same mechanism as detailed in the first catalytic cycle. This will again be transformed into the cyclization product captured by copper to then enter the next catalytic cycle. Finally, a Friedel-Crafts reaction of cationic intermediate VIII takes place to release product 2a, which is a rate-limiting step for the reaction. The most important step of this reaction is when the copper complex III acts as the catalyst to promote the dimerized-intermediate 3a to form the trimerization-intermediate 4a. The trimerization-intermediate 4a is converted into the desired cyclization product by releasing the copper complex III to furnish the self-catalytic cycle.
Figure 10.
Plausible reaction mechanism
Scope of the reaction
After the elucidation of the reaction mechanism, we next explored the reaction scope and functional group tolerance of this oxidative coupling and cyclization process. A wide range of 3-arylindoles have been prepared via the palladium-catalyzed C-H functionalization reactions, which have been subjected to standard reaction conditions. As shown in Figure 11, a variety of 3-phenylindoles with substituents on either the phenyl or indole-ring proceeded well in the presence of Cu(OAc)2 under aerobic oxidation conditions. These reactions led to the corresponding cyclization products (2a-2x) in 35–92% yields. It appeared that the electronic nature of the substituents on the benzene ring of the 3-phenyl moiety had a strong influence on subsequent reactivity. The electron-rich phenyl ring exhibited high reactivity to give the corresponding products (2a, 2b, and 2e) in 75–86% yields. In contrast, relatively lower reactivities were observed for the substrates with electron-poor phenyl rings (2c and 2d). Moreover, the steric hindrance of the phenyl ring exerted a deleterious effect on the efficiency of this transformation, with the 2-substituted phenyl-ring giving a lower conversion rate (2a vs 2f). Collectively, these results provided further evidence in support of the Friedel-Craft reaction being rate-limiting step of this transformation. Next, the reaction was evaluated with substrates containing substituents on the indole ring. Electron-donating and -withdrawing substituents on the indole ring were tolerated in the reaction, and the electronic nature of the substituent on the indole-ring had no strong influence on reactivity (2g-2x). For example, different substituents such as-F,-Cl, -Me, and-OMe at the C5 position of the indole were competent partners, allowing the expedient synthesis of the desired polycyclic aromatic hydrocarbons in good to excellent yields (2i-2o). Notably, good functional group compatibility was also observed with a tolerance of substrates bearing electron-donating and-withdrawing substituents at the C6 position of the indole ring, affording the final products in 53–90% yields (2p-2x). The reaction was sensitive to steric hindrance, with 4-substituted indoles giving a relatively lower yield (2g and 2h). The structures of 2i and 2j were unambiguously identified by single-crystal X-ray analysis. Importantly, the oxidative cyclization of 3-phenylindole could be conducted on a gram scale (1.63 g, 77% yield). Finally, when 3-(benzo[b]thiophen-3-yl)-1H-indole (1y) was carried out under standard condition, the indole-containing polycyclic aromatic hydrocarbons 2y was obtained in 54% yield. The molecules listed in Table 1exhibit interesting photo-physical properties. The different emission bands from 515 nm to 599 nm were observed by tuning the substituent group (see supplemental information).
Figure 11.
Substrate Scope of 3-Arylindoles
Reaction conditions: 1 (0.60 mmol), Cu(OAc)2 (20 mol %), O2 (balloon), TFA (2.0 mL), 50 °C, 8.0 h. Isolated yield.
In summary, we have disclosed an unusual self-catalysis process for the Cu-catalyzed oxidative coupling and cyclization reaction via C-H and N-H bond activation with O2 as the sole oxidant. This unique self-catalytic process enabled the preparation of synthetically valuable indole-containing polycyclic aromatic hydrocarbons by sequential cleavage of C-H and N-H bonds under mild and operationally convenient conditions. The successful isolation and comparison of the reactivities of the di- and tri-indole intermediates led to the counterintuitive observation that the two indole-moieties containing cyclization product was directly generated from the tri-indole intermediate rather than the di-indole intermediate. The formation of the tri-indole intermediate from the reaction of the di-indole intermediate and indole-copper complex, along with the generation of the desired cyclization product and regeneration of the indole-copper complex from the tri-indole intermediate, accounted for this self-catalysis. There will likely be many additional examples in which either the substrate or its derivative operates as a catalyst. The present study should stimulate our ideas for the further development of organometallic catalysis.
Limitations of the study
3-Arylindoles were not applicable in the construction of indole-containing polycyclic aromatic hydrocarbons by a copper-catalyzed aerobic oxidative cross cyclization.
STAR★Method
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Pd(OAc)2 | Energy Chemical | Cas: 3375-31-3 |
| Cu(OAc)2 | Aladdin | Cas: 142-71-2 |
| Pd(TFA)2 | Energy Chemical | Cas: 42196-31-6 |
| Bis[(2-diphenylphosphino)phenyl] ether | Energy Chemical | Cas: 166330-10-5 |
| K2CO3 | Aladdin | Cas: 584-08-7 |
| Triphenylphosphine | J&K Scientific | Cas: 603-35-0 |
| Trifluoroacetic acid | Aladdin | Cas: 76-05-1 |
| Indole | Energy Chemical | Cas: 204-420-7 |
| BnBu3NCl | Aladdin | Cas: 23616-79-7 |
| Bromobenzene | Energy Chemical | Cas: 108-86-1 |
| 6-Chloroindole | Energy Chemical | Cas: 17422-33-2 |
| 4-Bromofluorobenzene | Aladdin | Cas: 460-00-4 |
| 5-Fluoroindole | Aladdin | Cas: 399-52-0 |
| 5-Chloroindole | Energy Chemical | Cas: 17422-32-1 |
| 5-Methylindole | Energy Chemical | Cas: 614-96-0 |
| 6-Methylindole | Energy Chemical | Cas: 3420-02-8 |
| 6-Fluoroindole | Energy Chemical | Cas: 3420-02-8 |
| 4-Methoxy-1H-indole | Energy Chemical | Cas: 4837-90-5 |
| 4-Bromotoluene | Aladdin | Cas: 106-38-7 |
| 4-Bromochlorobenzene | Aladdin | Cas: 106-39-8 |
| 4-Bromobiphenyl | Energy Chemical | Cas: 92-66-0 |
| 5-Bromo-1,2,3-trimethoxybenzene | Alfa Aesar | Cas: 2675-79-8 |
| LiOH·H2O | Energy Chemical | Cas: 1310-66-3 |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Jianming Liu (jmliu@htu.cn).
Materials availability
All materials generated in this study are available in the article and supplementl information or from the lead contact without restriction upon reasonable request.
Method details
General information
All chemicals and solvents were used as received without further purification. Neutral aluminum oxide was purchased from Sinopharm Chemical Reagent Co., Ltd. 1H and 13C NMR data were recorded with Bruker Advance III (400 MHz) or (600 MHz) spectrometers. All chemical shifts (δ) are reported in ppm and coupling constants (J) are reported in Hz. High resolution mass spectra (HRMS) of the substrates, intermediate and products were obtained using a Bruker Daltonics micro TOF-Q spectrometer. Melting points were measured by a melting point apparatus equipped with a thermometer and used uncorrected. All reactions were monitored by thin-layer chromatography (TLC) through GF254 silica gel-coated plates. The operando IR experiments were recorded on a Mettler Toledo React IR 15 spectrometer using a diamond comb.
Synthesis of 3-arylindoles
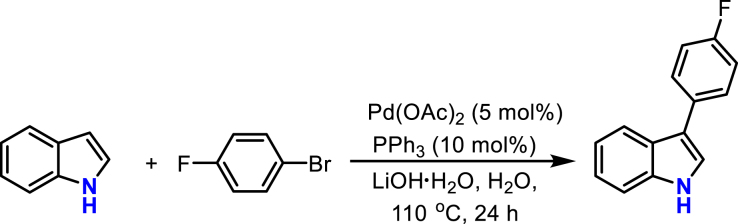
Producer (A) (Bellina et al., 2008): A 25 mL flame-dried Schlenk tube was charged with indole (117 mg, 1.0 mmol), Pd(OAc)2 (11.2 mg, 0.05 mmol), K2CO3 (415 mg, 3.0 mmol), and PPh3 (0.10 mmol, 26.2 mg). The Schlenk tube was purged three times with N2. Then, toluene (4.0 mL) and bromobenzene (189 mg, 1.2 mmol) were injected into the Schlenk tube with a syringe under a N2 atmosphere. The contents of Schlenk tube were then allowed to stir at 110 °C for 24 h. After cooling to room temperature, the solvent was concentrated in vacuum, and the residue was purified by chromatography on silica gel with petroleum ether/ethyl acetate (petroleum ether/ethyl acetate = 50:1, v/v) as the eluent to afford the desired product in 80% yield.
Producer (B) (Chen et al., 2014): A 25 mL flame-dried Schlenk tube was charged with indole (117 mg, 0.75 mmol), Pd(OAc)2 (11.2 mg, 0.05 mmol), LiOH·H2O (126 mg, 3.0 mmol), and PPh3 (26.2 mg, 0.10 mmol). Then, H2O (2.0 mL) and 4-bromofluorobenzene (210 mg, 1.2 mmol) were injected into the Schlenk tube. The reaction mixture was degassed via the freeze–thaw method. The contents of Schlenk tube were then allowed to stir at 110 °C for 24 h. After cooling to room temperature, the solvent was concentrated in vacuum, and the residue was purified by chromatography on silica gel with petroleum ether/ethyl acetate (petroleum ether/ethyl acetate = 50:1, v/v) as the eluent to afford the desired product in 65% yield.
Producer (C) (Joucla et al., 2010): A 50 mL flame-dried Young-type tube was charged with indole (87.8 mg, 1.0 mmol), Pd(TFA)2 (16.6 mg, 0.05 mmol) and bis[(2-diphenylphosphino)phenyl] ether (53.9 mg, 0.10 mmol). The Schlenk tube was purged three times with O2. Then, toluene (2.0 mL) and 4-phenylcyclohexan-1-one (87 mg, 0.50 mmol) were injected into the Schlenk tube with a syringe under an O2 atmosphere. The contents of Schlenk tube were then allowed to stir at 130 °C for 40 h. After cooling to room temperature, the residue was concentrated in vacuum, and was purified by chromatography on silica gel with petroleum ether/ethyl acetate (petroleum ether/ethyl acetate = 50:1, v/v) as the eluent to afford the desired product in 62% yield.
Producer (D): A 25 mL flame-dried Schlenk tube was charged with 6-chloro-1H-indole (227 mg, 1.0 mmol), Pd(OAc)2 (11.2 mg, 0.05 mmol), K2CO3 (415 mg, 3.0 mmol), and BnBu3NCl (62.4mg, 0.20 mmol). The Schlenk tube was purged three times with N2. Then, toluene (4.0 mL) and p-bromotoluene (205 mg, 1.2 mmol) were injected into the Schlenk tube with a syringe under a N2 atmosphere. The contents of Schlenk tube were then allowed to stir at 110 °C for 24 h. After cooling to room temperature, the solvent was concentrated in vacuum, and the residue was purified by chromatography on silica gel with petroleum ether/ethyl acetate (petroleum ether/ethyl acetate = 50:1, v/v) as the eluent to afford the desired product in 76% yield.
Reaction profile of the standard reaction
-
(a)
A 25 mL flame-dried Schlenk tube was charged with Cu(OAc)2 (18.4 mg, 0.10 mmol) and 3-phenylindole (193 mg, 1.0 mmol). The Schlenk tube was evacuated and filled with O2 (balloon). Then, TFA (5.0 mL) and n-dodecane (170 mg, 1.0 mmol) were injected into the Schlenk tube with a syringe under an O2 atmosphere. The contents of Schlenk tube were then allowed to stir at 50 °C for a given time. The reaction mixture was determined by GC.
-
(b)
A self-prepared three-necked micro reactor was charged with Cu(OAc)2 (18.4 mg, 0.10 mmol) and 3-phenylindle 1a (193 mg, 1.0 mmol). The Schlenk tube was evacuated and filled with O2 (balloon). Then TFA (5.0 mL) was added via a syringe. After complete dissolution of the catalysts and substrate at 50°C, the mixture was recorded by React IR. After 3.0 h, the reaction was stopped. Then, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as the eluent to afford the desired product in 52% yield.
General procedure for the synthesis of intermediates 3a and 4a
A 25 mL flame-dried Schlenk tube was charged with Cu(OAc)2 (10.9 mg, 0.06 mmol) and 3-phenylindole (116 mg, 0.60 mmol). The Schlenk tube was evacuated and filled with O2 (balloon). Then, TFA (2.0 mL) was injected into the Schlenk tube with a syringe under an O2 atmosphere. The contents of Schlenk tube were then allowed to stir at 30 °C for 1.0 h, for 1.5 h. After cooling to room temperature, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4, and concentrated in vacuum. The residue was purified by chromatography on silica gel with n-hexane/ethyl acetate as the eluent to afford the desired products.
The cyclization of intermediate 3a in the presence of O2 or N2
-
(a)
A 25 mL flame-dried Schlenk tube was charged with Cu(OAc)2 (10.9 mg, 0.06 mmol) and intermediate 3a (115.2 mg, 0.30 mmol). The Schlenk tube was evacuated and filled with O2 (balloon). Then, TFA (2.0 mL) was injected into the Schlenk tube with a syringe under an O2 atmosphere. The contents of Schlenk tube were then allowed to stir at 50 °C for 8.0 h. After cooling to room temperature, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4, and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as the eluent to afford the desired product in 61% yield.
-
(b)
A 25 mL flame-dried Schlenk tube was charged with Cu(OAc)2 (10.9 mg, 0.06 mmol) and intermediate 3a (115.2 mg, 0.30 mmol). The Schlenk tube was evacuated and filled with N2. TFA (2.0 mL) was injected into the Schlenk tube with a syringe under a N2 atmosphere. Then the tube was degassed by freeze-pump-thaw using liquid N2. The contents of Schlenk tube were then allowed to stir at 50 °C for 8.0 h. After cooling to room temperature, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as the eluent to afford the desired product in 16% yield.
The cyclization of intermediate 4a in the presence of O2 or N2
-
(a)
A 25 mL flame-dried Schlenk tube was charged with Cu(OAc)2 (7.3 mg, 0.04 mmol) and intermediate 4a (115.2 mg, 0.20 mmol). The Schlenk tube was evacuated and filled with O2 (balloon). Then, TFA (2.0 mL) was injected into the Schlenk tube with a syringe under an O2 atmosphere. The contents of Schlenk tube were then allowed to stir at 50 °C for 8.0 h. After cooling to room temperature, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4, and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as the eluent to afford the desired product in 128% yield.
-
(b)
A 25 mL flame-dried Schlenk tube was charged with Cu(OAc)2 (7.3 mg, 0.04 mmol), intermediate 4a (115.2 mg, 0.20 mmol) and TFA (2.0 mL). Then the tube was degassed by freeze-pump-thaw using liquid N2. The contents of Schlenk tube were then allowed to stir at 50 °C for 8.0 h. After cooling to room temperature, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4, and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as the eluent to afford desired product 2a in 18% yield.
General procedure for the synthesis of intermediates 5 and 6
A 25 mL flame-dried Schlenk tube was charged with Cu(OAc)2 (10.9 mg, 0.06 mmol), intermediate 3a (115.2 mg, 0.30 mmol) and 5-chloro-3-(4-methoxyphenyl)-1H-indole 1o (77 mg, 0.30 mmol). The Schlenk tube was evacuated and filled with O2 (balloon). Then, TFA (2.0 mL) was injected into the Schlenk tube with a syringe under an O2 atmosphere. The contents of Schlenk tube were then allowed to stir at 30 °C for 2.0 h. After cooling to room temperature, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4, and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/dichloromethane as the eluent to afford the desired product in 29% yield of 5, 29% yield of 2a, and 38% yield of 6.
The initial rate of intermediate 3a and 4a
-
(a)
A self-prepared three-necked micro reactor was charged with Cu(OAc)2 (10.9 mg, 0.06 mmol) and intermediate 3a (115 mg, 0.30 mmol). The reactor was allowed to be vacuumed and purged with oxygen (balloon) three times. Then TFA (5.0 mL) was added via a syringe. After complete the dissolution of catalysts and substrate at 50 °C, the mixture was recorded by React IR. After 2.0 h, the reaction was stopped. Then, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as the eluent to afford the desired product.
-
(b)
A self-prepared three-necked micro reactor was charged with Cu(OAc)2 (10.9 mg, 0.06 mmol) and intermediate 4a (173 mg, 0.30 mmol). The reactor was allowed to be vacuumed and purged with oxygen (balloon) three times. Then TFA (5.0 mL) was added via a syringe. After complete dissolution of the catalysts and substrate at 50 °C, the mixture was recorded by React IR. After 2.0 h, the reaction was stopped. Then, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4, and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as the eluent to afford the desired product.
Kinetic plots of the reaction of 3a in the presence of 1g
-
(a)
Anself-prepared three-necked micro reactor was charged with Cu(OAc)2 (10.9 mg, 0.06 mmol) and intermediate 3a (115 mg, 0.30 mmol). The reactor was allowed to be vacuumed and purged with oxygen (balloon) for three times. Then TFA (5 mL) was added via a syringe. After complete dissolution of catalysts and substrate at 50 °C, the mixture was recorded by React IR. After 2.0 h, the reaction was stopped. Then, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as eluent to afford the desired product.
-
(b)
A self-prepared three-necked micro reactor was charged with Cu(OAc)2 (0.06 mmol), intermediate 3a (115 mg, 0.30 mmol) and 4-methoxy-3-phenyl-1H-indole 1g (6.7 mg, 0.03 mmol). The reactor was allowed to be vacuumed and purged with oxygen (balloon) for three times. Then TFA (5.0 mL) was added via a syringe. After complete dissolution of the catalysts and substrate 50 °C, the mixture was recorded by React IR. After 2.0 h, the reaction was stopped. Then, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as the eluent to afford the desired product .
-
(c)
Anself-prepared three-necked micro reactor was charged with Cu(OAc)2 (10.9 mg, 0.06 mmol), intermediate 3a (115 mg, 0.30 mmol) and 4-methoxy-3-phenyl-1H-indole 1g (67 mg, 0.30 mmol). The reactor was allowed to be vacuumed and purged with oxygen (balloon) three times. Then TFA (5.0 mL) was added via a syringe. After complete dissolution of the catalysts and substrate 50 °C, the mixture was recorded by React IR. After 2.0 h, the reaction was stopped. Then, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as the eluent to afford the desired product.
The oxidative cyclization of 3-phenylindole in the presence of TEMPO
A 25 mL flame-dried Schlenk tube was charged with Cu(OAc)2 (10.9 mg, 0.06 mmol), TEMPO (93.6 mg, 0.60 mmol) and 3-phenylindole (116 mg, 0.60 mmol). The Schlenk tube was evacuated and filled with O2 (balloon). Then, TFA (2.0 mL) was injected into the Schlenk tube with a syringe under an O2 atmosphere. The contents of Schlenk tube were then allowed to stir at 50 °C for 8.0 h. After cooling to room temperature, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as the eluent to afford the desired product in 80% yield.
The oxidative cyclization of 3-phenylindole in the presence of BHT
A 25 mL flame-dried Schlenk tube was charged with Cu(OAc)2 (10.9 mg, 0.06 mmol), BHT (132 mg, 0.60 mmol) and 3-phenylindole (116 mg, 0.60 mmol). The Schlenk tube was evacuated and filled with O2 (balloon). Then, TFA (2.0 mL) was injected into the Schlenk tube with a syringe under an O2 atmosphere. The contents of Schlenk tube were then allowed to stir at 50 °C for 8.0 h. After cooling to room temperature, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 10:1, v/v) as the eluent to afford the desired product in 56% yield.
The preparation of 3-(phenyl-d5)-1H-indole
A 25 mL flame-dried Schlenk tube was charged with indole (193 mg, 1.0 mmol), Pd(OAc)2 (11.2 mg, 0.05 mmol), K2CO3 (415 mg, 3.0 mmol), and PPh3 (26.2 mg, 0.10 mmol). The reactor was allowed to be vacuumed and purged with N2 three times. Then, toluene (4.0 mL) and d5-bromobenzene (194 mg, 1.2 mmol) were injected into the Schlenk tube with a syringe under a N2 atmosphere at room temperature. The reaction mixture was stirred at 110 °C for 24 h. After cooling to room temperature, the residues were concentrated in vacuum. Then the residues were purified by chromatography on silica gel with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 50:1, v/v) as the eluent to afford the desired product in 70% yield.
The preparation of 3-phenyl-1H-indole-2-d
A 25 mL flame-dried Schlenk tube was charged with 2-d-indole (194 mg, 1.0 mmol), Pd(OAc)2 (11.2 mg, 0.05 mmol), K2CO3 (415 mg, 3.0 mmol), and PPh3 (26.2 mg, 0.10 mmol). The reactor was allowed to be vacuumed and purged with N2 three times. Then, toluene (4.0 mL) and bromobenzene (189 mg, 1.2 mmol) were injected into the Schlenk tube with a syringe under N2 atmosphere at room temperature. The reaction mixture was stirred at 110 °C under N2 atmosphere for 24 h. After cooling to room temperature, the residues were concentrated in vacuum. Then the residues were purified by chromatography on silica gel with n-hexane/ethyl acetate (n-hexane/ethyl acetate = 50:1, v/v) as the eluent to afford the desired product in 50% yield.
Kinetic isotope effect experiment
General procedure: A self-prepared three-necked micro reactor was charged with Cu(OAc)2 (18.4 mg, 0.10 mmol) and 3-phenylindole (193 mg, 1.0 mmol). The reactor was allowed to be vacuumed and purged with oxygen (balloon) three times. Then TFA (5.0 mL) was injected into the Schlenk tube with a syringe. After complete dissolution of the catalysts and substrate at 50 °C, the mixture was recorded by React IR. After 3.0 h, the reaction was stopped. The standard IR spectra of 2a are shown in Figures S12 and S10. The peak of product (2a) at 1632 cm-1 could be observed.
-
(a)
A self-prepared three-necked micro reactor was charged with Cu(OAc)2 (18.4 mg, 0.10 mmol) and 3-phenylindole (193 mg, 1.0 mmol). The reactor was allowed to be vacuumed and purged with oxygen (balloon) three times. Then, TFA (5.0 mL) was injected into the Schlenk tube with a syringe under an O2 atmosphere. After complete dissolution of the catalysts and substrate at 50 °C, the mixture was recorded by React IR. After 2.0 h, the reaction was stopped. Finally, the profiles of relative concentrations vs time for desired product 2a could be obtained to analyze the initial rate of reaction (Figure S14).
-
(b)
A self-prepared three-necked micro reactor was charged with Cu(OAc)2 (18.4 mg, 0.10 mmol) and d5-3-phenylindole (198 mg, 1.0 mmol). The reactor was allowed to be vacuumed and purged with oxygen (balloon) three times. Then, TFA (5.0 mL) was injected into the Schlenk tube with a syringe under an O2 atmosphere. After complete dissolution of the catalysts and substrate at 50 °C, the mixture was recorded by React IR. After 2.0 h, the reaction was stopped. Finally, the profiles of relative concentrations vs time for the final product could be obtained to analyze the initial rate of reaction (Figure S15).
-
(c)
A self-prepared three-necked micro reactor was charged with Cu(OAc)2 (18.4 mg, 0.10 mmol) and 3-phenylindole-2-d (194 mg, 1.0 mmol). The reactor was allowed to be vacuumed and purged with oxygen (balloon) for three times. Then, TFA (5.0 mL) was injected into the Schlenk tube with a syringe under an O2 atmosphere. After complete dissolution of catalysts and substrate at 50 °C, the mixture was recorded by React IR. After 2.0 h, the reaction was stopped. Finally, the profiles of relative concentrations vs time for final product 2a could be obtained to analyze the initial rate of reaction (Figure S16).
Kinetic studies of intermediate Cu(OAc)2 and 4a
Order in Cu(OAc)2
The order of Cu(OAc)2 was determined by investigating the initial rate of reaction with different concentrations of Cu(OAc)2. Using the above-mentioned general procedure, a self-prepared three-necked micro reactor was charged with intermediate 4a (173 mg, 0.30 mmol) and Cu(OAc)2 (2.5 mol%-25 mol%). The reactor was allowed to be vacuumed and purged with oxygen (balloon) three times. Then TFA (5.0 mL) was added via a syringe. After complete dissolution of the catalysts and substrate at 50 °C, the mixture was recorded by React IR. After 2.0 h, the reaction was stopped. Finally, the profiles of relative concentrations vs time for product 2a could be obtained to analyze the initial rate of reaction. The standard IR spectra of 2a and intermediate 4a are shown in Figure S9. As shown in Figure S10, the reaction rate was independent of the concentration of Cu(OAc)2 and a zero-order dependence on catalyst concentration in this range of concentrations of intermediate 4a was observed.
Order in intermediate 4a
The order in intermediate 4a was determined by investigating the initial rate of reaction with different concentrations of Cu(OAc)2. Using the above-mentioned general procedure, a self-prepared three-necked micro reactor was charged with intermediate 4a (0.05–0.50 mmol) and Cu(OAc)2 (18 mg). The reactor was allowed to be vacuumed and purged with oxygen (balloon) three times. Then TFA (5.0 mL) was added via a syringe. After complete dissolution of the catalysts and substrate at 50 °C, the mixture was recorded by React IR. After 2.0 h, the reaction was stopped. Finally, the profiles of relative concentrations vs time for product 2a could be obtained to analyze the initial rate of reaction. As shown in Figure S11, the initial reaction rate was affected by the concentration of intermediate 4a. The result revealed a first-order dependence of the rate on the concentration of intermediate 4a.
General procedure for the synthesis of the cyclization products 2a
A 25 mL flame-dried Schlenk tube was charged with Cu(OAc)2 (10.9 mg, 0.06 mmol) and 3-phenylindole (116 mg, 0.60 mmol). The Schlenk tube was evacuated and filled with an O2 (balloon). Then, TFA (2.0 mL) was injected into the Schlenk tube with a syringe under an O2 atmosphere. The contents of Schlenk tube were then allowed to stir at 50 °C for 8.0 h. After cooling to room temperature, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×25 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide (n-hexane/ethyl acetate = 10:1, v/v) to afford the desired product in 85% yield.
Experimental procedure for the gram scale synthesis of 2a
A 100 mL flame-dried Schlenk tube was charged with Cu(OAc)2 (199.8 mg, 1.1 mmol) and 3-phenylindole (2.123 g, 11 mmol). The Schlenk tube was evacuated and filled with O2 (balloon). Then, TFA (30 mL) was injected into the Schlenk tube with a syringe under an O2 atmosphere. The contents of Schlenk tube were then allowed to stir at 50 °C for 24 h. After cooling to room temperature, the residue was concentrated in vacuum. The residue was neutralized by 20% NaOH solution and then the aqueous phase was extracted by CH2Cl2 (5×100 mL). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was purified by chromatography on neutral aluminum oxide (n-hexane/ethyl acetate = 10:1, v/v) to afford the desired product in 77% yield.
Characterization data of substrates
3-Phenyl-1H-indole (1a)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a yellow solid, which is a known compound (Bellina et al., 2008), 154 mg, 80% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.36 (br, 1H), 7.89 (d, J = 8.0 Hz, 1H), 7.72–7.68 (m, 3H), 7.49 (d, J = 8.0 Hz, 1H), 7.43 (t, J = 8.0 Hz, 2H), 7.25–7.10 (m, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 137.4, 136.4, 129.2, 127.0, 125.7, 125.5, 123.9, 121.9, 120.1, 119.5, 116.2, 112.5. ESI-MS (M): 193.
3-(p-Tolyl)-1H-indole (1b)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a white solid, which is a known compound (Chen et al., 2014), 151 mg, 73% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.28 (br, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.63 (d, J = 4.0 Hz, 1H), 7.58 (d, J = 8.0 Hz, 2H), 7.47 (dd, J = 8.0, 4.0 Hz, 1H), 7.23 (d, J = 8.0 Hz, 2H), 7.16 (t, J = 8.0 Hz, 1H), 7.09 (t, J = 8.0 Hz, 1H), 2.33 (s, 3H); 13C{1H} NMR (400 MHz, DMSO-d6) δ 137.4, 134.7, 133.5, 129.8, 126.9, 125.6, 123.4, 121.8, 119.9, 119.5, 116.2, 112.4, 21.2. ESI-MS (M): 207.
3-(4-Chlorophenyl)-1H-indole (1c)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a white solid, which is a known compound (Joucla et al., 2010), 154 mg, 68% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.43 (br, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.74–7.70 (m, 3H), 7.49–7.45 (m, 3H), 7.18 (t, J = 8.0 Hz, 1H), 7.11 (t, J = 8.0 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 137.4, 135.3, 130.1, 129.2, 128.4, 125.2, 124.3, 122.1, 120.3, 119.4, 114.9, 112.6. ESI-MS (M): 227.
3-(4-Fluorophenyl)-1H-indole (1d)
The title compound was prepared according to the general procedure (B) and purified by flash column chromatography to give a white solid, which is a known as compound (Chen et al., 2013), 137 mg, 65% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.35 (br, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.73–7.66 (m, 3H), 7.46 (d, J = 8.0 Hz, 1H), 7.28–7.23 (m, 2H), 7.16 (t, J = 8.0 Hz, 1H), 7.10 (t, J = 8.0 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 160.8 (d, J = 242 Hz), 137.3, 132.8 (d, J = 3.0 Hz), 128.8 (d, J = 8.1 Hz), 125.4, 123.8, 121.9, 120.1, 119.3, 116.0 (d, J = 20.2 Hz), 115.2, 112.5. ESI-MS (M): 211.
3-([1,1'-Biphenyl]-4-yl)-1H-indole (1e)
The title compound was prepared according to the general procedure (C) and purified by flash column chromatography to give an orange solid, which is a known compound (Chen et al., 2014), 167 mg, 62% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.43 (br, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.82–7.77 (m, 3H), 7.71 (d, J = 8.0 Hz, 4H), 7.52 (d, J = 8.0 Hz, 1H), 7.46 (t, J = 6.0 Hz, 2H), 7.35 (t, J = 8.0 Hz, 1H), 7.22–7.13 (m, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 140.5, 137.5, 137.4, 135.7, 129.4, 127.6, 127.5, 127.4, 126.8, 125.5, 124.1, 122.0, 120.2, 119.6, 115.7, 112.6. ESI-MS (M): 269.
3-(3, 4, 5-Trimethoxyphenyl)-1H-indole (1f)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a white solid, which is a known compound (Bellina et al., 2008), 243 mg, 86% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.32 (br, 1H), 7.90 (d, J = 8.0 Hz, 1H), 7.69 (d, J = 4.0 Hz, 1H), 7.46 (d, J = 8.0 Hz, 1H), 7.18–7.09 (m, 2H), 6.94 (s, 2H), 3.87 (s, 6H), 3.71 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 153.7, 137.3, 136.1, 132.1, 125.5, 123.9, 121.9, 120.1, 119.5, 116.5, 112.4, 104.5, 60.6, 56.4. ESI-MS (M): 283.
4-Methoxy-3-phenyl-1H-indole (1g)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a yellow oil, which is a known compound (Joucla et al., 2010), 152 mg, 68% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.27 (br, 1H), 7.75 (d, J = 8.0 Hz, 2H), 7.67 (t, J = 4.0 Hz, 1H), 7.47–7.43 (m, 4H), 7.27–7.24 (m, 1H), 6.91 (d, J = 8.0 Hz, 1H), 3.84 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 154.6, 136.6, 132.7, 129.3, 126.9, 125.9, 125.6, 124.5, 116.2, 113.2, 112.1, 101.5, 55.9. ESI-MS (M): 223.
4-Methoxy-3-(p-tolyl)-1H-indole (1h)
The title compound was prepared according to the general procedure (B) and purified by flash column chromatography to give a white solid, 145 mg, 61% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.23 (br, 1H), 7.45–7.43 (m, 2H), 7.26 (d, J = 4.0 Hz, 1H), 7.14 (d, J = 8.0 Hz, 2H), 7.07–7.02 (m, 2H), 6.55–6.51 (m, 1H), 3.75 (s, 3H), 2.32 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 154.4, 138.9, 134.4, 134.0, 129.5, 128.6, 123.2, 122.6, 117.3, 115.4, 105.6, 100.4, 55.3, 21.2. HRMS, calculated for C16H16NO (M + H+): 238.1226, found 238.1221.
5-Methyl-3-phenyl-1H-indole (1i)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a yellow solid, which is a known compound (Chen et al., 2013), 161 mg, 78% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.22 (br, 1H), 7.71–7.69 (m, 3H), 7.63 (d, J = 4.0 Hz, 1H), 7.43 (t, J = 8.0 Hz, 2H), 7.37 (d, J = 8.0 Hz, 1H), 7.24–7.20 (m, 1H), 7.01–6.99 (m, 1H), 2.43 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 136.6, 135.8, 129.2, 128.6, 127.0, 125.8, 125.6, 123.9, 123.5, 119.1, 115.8, 112.1, 21.9. ESI-MS (M): 207.
5-Methyl-3-(p-tolyl)-1H-indole (1j)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a yellow solid, which is a known compound (Yang et al., 2017), 161 mg, 73% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.14 (br, 1H), 7.64 (d, J = 1.6 Hz 1H), 7.57–7.55 (m, 3H), 7.34 (d, J = 8.0 Hz, 1H), 7.23 (d, J = 8.0 Hz, 2H), 6.98 (dd, J = 8.4, 1.6 Hz, 1H), 2.42 (s, 3H), 2.33 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 135.7, 134.6, 133.6, 129.8, 128.4, 126.9, 125.8, 123.4, 123.4, 119.1, 115.7, 112.1, 21.9, 21.2. ESI-MS (M): 221.
3-(4-Methoxyphenyl)-5-methyl-1H-indole (1k)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a yellow solid, which is a known compound (Bellina et al., 2008), 159 mg, 67% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.10 (br, 1H), 7.62–7.58 (m, 3H), 7.51 (d, J = 4.0 Hz, 1H), 7.34 (dd, J = 8.2, 2.2 Hz, 1H), 7.02–6.97 (m, 3H), 3.78 (s, 3H), 2.42 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 157.7, 135.7, 129.0, 128.3, 128.1, 125.9, 123.4, 123.0, 119.0, 115.6, 114.7, 112.0, 55.5, 21.9. ESI-MS (M): 237.
5-Fluoro-3-(p-tolyl)-1H-indole (1l)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a white solid, which is a known compound (Yamaguchi et al., 2017), 144 mg, 64% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.41 (br, 1H), 7.72 (d, J = 4.0 Hz, 1H), 7.57–7.54 (m, 3H), 7.47 (q, J = 4.0 Hz, 1H), 7.23 (d, J = 8.0 Hz, 2H), 7.01 (td, J = 9.2, 2.4 Hz, 1H), 2.32 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 157.9 (d, J = 232 Hz), 134.9, 134.1, 132.9, 129.9, 126.8, 125.6 (d, J = 10.1 Hz), 125.5, 116.4 (d, J = 5.1 Hz), 113.4 (d, J = 10.1 Hz), 110.0 (d, J = 26.3 Hz), 104.2 (d, J = 23.2 Hz), 21.2. ESI-MS (M): 225.
5-Fluoro-3-(4-methoxyphenyl)-1H-indole (1m)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a white solid, 161 mg, 67% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.36 (br, 1H), 7.66 (d, J = 2.0 Hz, 1H), 7.59–7.56 (m, 2H), 7.55–7.43 (m, 2H), 7.03–6.97 (m, 3H), 3.78 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 157.9 (d, J = 232 Hz), 157.9, 156.7, 134.0, 128.3, 128.0, 125.7 (d, J = 9.1 Hz), 125.1, 114.8 (d, J = 5.1 Hz), 113.4 (d, J = 10.1 Hz), 109.9 (d, J = 26.3 Hz), 104.2 (d, J = 24.2 Hz), 55.5. HRMS, calculated for C15H13FNO (M + H+): 242.0976, found 242.0973.
5-Chloro-3-(p-tolyl)-1H-indole (1n)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a white solid, 174 mg, 72% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.52 (br, 1H), 7.84 (s, 1H), 7.71 (s, 1H), 7.56–7.49 (m, 3H), 7.24–7.16 (m, 3H), 2.31 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 135.8, 135.1, 132.7, 129.9, 127.0, 126.6, 125.2, 124.8, 121.8, 118.6, 116.1, 113.9, 21.2. HRMS, calculated for C15H13ClN (M + H+): 242.0731, found 242.0730.
5-Chloro-3-(4-methoxyphenyl)-1H-indole (1o)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a white solid, 164 mg, 64% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (br, 1H), 7.83 (d, J = 4.0 Hz, 1H), 7.66 (d, J = 2.4 Hz, 1H), 7.59–7.56 (m, 2H), 7.50 (d, J = 8.0 Hz, 1H), 7.17 (dd, J = 8.6, 2.2 Hz, 1H), 7.02–6.99 (m, 2H), 3.77 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 158.0, 135.8, 128.3, 128.0, 126.7, 124.8, 124.7, 121.8, 118.6, 116.0, 114.8, 113.9, 55.5. HRMS, calculated for C15H13ClNO (M + H+): 258.0680, found 258.0675.
6-Methyl-3-phenyl-1H-indole (1p)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a yellow solid, which is a known compound (Chen et al., 2013), 147 mg, 71% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.19 (br, 1H), 7.77 (d, J = 8.0 Hz, 1H), 7.70 (dd, J = 8.0, 4.0 Hz, 2H), 7.61 (d, J = 4.0 Hz, 1H), 7.42 (t, J = 8.0 Hz, 2H), 7.28 (s, 1H), 7.22 (t, J = 8.0 Hz, 1H), 6.95 (dd, J = 8.0, 4.0 Hz, 1H), 2.43 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 137.9, 136.6, 131.0, 129.2, 126.8, 125.6, 123.5, 123.1, 121.9, 119.3, 116.1, 112.2, 21.8. ESI-MS (M): 207.
6-Methyl-3-(p-tolyl)-1H-indole (1q)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give an orange solid, which is a known compound, 157 mg, 71% yield (Hsieh and Dong, 2009). 1H NMR (400 MHz, DMSO-d6) δ 11.10 (br, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.56 (d, J = 8.0 Hz, 2H), 7.53 (d, J = 2.4 Hz, 1H), 7.22 (d, J = 8.0 Hz, 3H), 6.92 (dd, J = 8.0, 4.0 Hz, 1H), 2.41 (s, 3H), 2.32 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 137.8, 134.6, 133.6, 130.8, 129.8, 126.7, 123.5, 122.7, 121.7, 119.2, 116.0, 112.2, 21.8, 21.2. ESI-MS (M): 221.
3-(4-Methoxyphenyl)-6-methyl-1H-indole (1r)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a yellow solid, 175 mg, 74% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.08 (br, 1H), 7.72 (dd, J = 8.2, 2.2 Hz, 1H), 7.60 (dd, J = 8.4, 1.6 Hz, 2H), 7.50 (t, J = 2.0 Hz, 1H), 7.27 (d, J = 4.0 Hz, 1H), 7.01–6.99 (m, 2H), 6.93 (d, J = 8.0 Hz, 1H), 3.78 (s, 3H) 2.43 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 157.7, 137.8, 130.8, 129.0, 128.0, 123.6, 122.2, 121.6, 119.2, 115.9, 114.7, 112.1, 55.5, 21.8. HRMS, calculated for C16H16NO (M + H+): 238.1226, found 238.1225.
6-Fluoro-3-phenyl-1H-indole (1s)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a white solid, which is a known compound (Chen et al., 2014), 150 mg, 68% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.40 (br, 1H), 7.83 (q, J = 5.3 Hz, 1H), 7.68–7.66 (m, 3H), 7.42 (t, J = 8.0 Hz, 2H), 7.26–7.22 (m, 2H), 6.98–6.92 (m, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 159.3 (d, J = 236 Hz), 137.3 (d, J = 13.1 Hz), 135.9, 129.3, 127.0, 126.0, 124.4 (d, J = 3.0 Hz), 122.4, 120.6 (d, J = 10.1 Hz), 116.4, 108.5 (d, J = 24.2 Hz), 98.3 (d, J = 25.3 Hz). ESI-MS (M): 211.
6-Fluoro-3-(p-tolyl)-1H-indole (1t)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a white solid, 169 mg, 75% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.35 (br, 1H), 7.81 (q, J = 4.0 Hz, 1H), 7.62 (d, J = 2.4 Hz, 1H), 7.57–7.54 (m, 2H), 7.24–7.21 (m, 3H), 6.96–6.91 (m, 1H), 2.33 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 159.3 (d, J = 236 Hz), 137.3 (d, J = 12.1 Hz), 135.0, 133.0, 129.8, 126.9, 123.9 (d, J = 3.0 Hz), 122.5, 120.6 (d, J = 10.1 Hz), 116.4, 108.3 (d, J = 24.2 Hz), 98.2 (d, J = 24.2 Hz), 21.2. HRMS, calculated for C15H13FN (M + H+): 226.1027, found 226.1024.
6-Fluoro-3-(4-methoxyphenyl)-1H-indole (1u)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a yellow solid, 159 mg, 66% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.40 (br, 1H), 7.68 (d, J = 4.0 Hz, 1H), 7.62–7.58 (m, 3H), 7.50 (q, J = 4.0 Hz, 1H), 7.06–6.99 (m, 3H), 3.77 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 158.0 (d, J = 232 Hz), 157.9, 134.0, 128.3, 128.1, 125.8 (d, J = 10.1 Hz), 125.0, 116.4 (d, J = 5.1 Hz), 114.8, 113.3 (d, J = 10.1 Hz), 110.0 (d, J = 26.3 Hz), 104.1 (d, J = 24.2 Hz), 55.5. HRMS, calculated for C15H12FNO (M + H+): 242.0976, found 242.0973.
6-Chloro-3-phenyl-1H-indole (1v)
The title compound was prepared according to the general procedure (A) and purified by flash column chromatography to give a yellow solid, which is a known compound (Joucla et al., 2010), 154 mg, 67% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (br, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 8.0 Hz, 2H), 7.52 (d, J = 4.0 Hz, 1H), 7.43 (t, J = 8.0 Hz, 2H), 7.27–7.23 (m, 1H), 7.10 (dd, J = 8.6, 1.8 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 137.8, 135.7, 129.3, 127.0, 126.6, 126.1, 124.9, 124.3, 120.9, 120.4, 116.5, 112.0. ESI-MS (M): 227.
6-Chloro-3-(p-tolyl)-1H-indole (1w)
The title compound was prepared according to the general procedure (D) and purified by flash column chromatography to give a yellow solid, 183 mg, 76% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.42 (br, 1H), 7.82 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 2.8 Hz, 1H), 7.57–7.53 (m, 2H), 7.49 (d, J = 4.0 Hz, 1H), 7.25–7.22 (m, 2H), 7.09 (dd, J = 8.0, 4.0 Hz, 1H), 2.33 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 137.7, 135.1, 132.7, 129.9, 127.0, 126.5, 124.5, 124.3, 120.9, 120.2, 116.4, 111.9, 21.2. HRMS, calculated for C15H13ClN (M + H+): 242.0731, found 242.0732.
6-Chloro-3-(4-methoxyphenyl)-1H-indole (1x)
The title compound was prepared according to the general procedure (D) and purified by flash column chromatography to give a white solid, 182 mg, 71% yield. 1H NMR (400 MHz, DMSO-d6) δ 11.37 (br, 1H), 7.79 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 4.0 Hz, 1H), 7.59–7.56 (m, 2H), 7.49–7.48 (m, 1H), 7.08 (dd, J = 8.0, 2.0 Hz, 1H), 7.01–6.99 (m, 2H), 3.78 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 158.0, 137.7, 128.2, 128.1, 126.5, 124.4, 124.1, 120.8, 120.1, 116.3, 114.8, 111.9, 55.6. HRMS, calculated for C15H13ClNO (M + H+): 258.0680, found 258.0683.
Characterization data of intermediates
3,3'-Diphenyl-1H,1'H-2,2'-biindole (3a)
1H NMR (400 MHz, DMSO-d6) δ 11.57 (br, 2H), 7.64 (d, J = 8.0 Hz, 2H), 7.41 (d, J = 8.0 Hz, 2H), 7.19 (t, J = 6.0 Hz, 10H), 7.11–7.06 (m, 4H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 136.8, 135.3, 128.9, 128.6, 127.3, 127.0, 125.9, 122.6, 120.2, 119.3, 116.5, 112.2. HRMS, calculated for C28H21N2 (M + H+): 385.1699, found 385.1695.
3,3',3''-Triphenyl-1H,1''H,3'H-2,2':3',2''-terindole (4a)
1H NMR (400 MHz, DMSO-d6) δ 11.35 (br, 1H), 10.35 (br, 1H), 7.54 (d, J = 12.0 Hz, 1H), 7.49 (d, J = 8.0 Hz, 1H), 7.39–7.24 (m, 13H), 7.17–7.12 (m, 4H), 6.98–6.87 (m, 6H), 6.78 (d, J = 8.0 Hz, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 172.0, 154.5, 145.0, 140.3, 137.0, 136.2, 135.3, 134.4, 130.9, 130.3, 130.1, 129.4, 129.1, 128.6, 128.4, 128.1, 128.0, 127.6, 127.5, 127.3, 126.7, 126.4, 124.3, 124.1, 122.3, 121.3, 121.1, 120.6, 120.0, 119.8, 119.1, 116.3, 113.1, 111.9, 68.5. HRMS, calculated for C42H30N3 (M + H+): 576.2434, found 576.2430.
5''-chloro-3''-(4-methoxyphenyl)-3,3'-diphenyl-1H,1''H,3'H-2,2':3',2''-terindole (5)
1H NMR (400 MHz, DMSO-d6) δ 11.40 (br, 1H), 10.18 (br, 1H), 7.54–7.47 (m, 2H), 7.32–7.23 (m, 12H), 7.21–7.13 (m, 4H), 7.08–7.01 (m, 2H), 6.96 (t, J = 8.0 Hz, 1H), 6.62 (d, J = 8.0 Hz, 2H), 6.46–6.43 (m, 2H), 3.60 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 171.9, 158.2, 154.5, 145.0, 139.7, 137.0, 135.1, 134.6, 132.2, 131.4, 130.7, 130.7, 129.3, 128.8, 128.4, 128.3, 127.9, 127.5, 127.1, 126.6, 126.6, 125.5, 124.4, 124.2, 124.0, 122.1, 121.3, 121.2, 120.5, 119.9, 118.1, 115.4, 113.5, 113.1, 113.1, 68.4, 55.3. HRMS, calculated for C43H31ClN3O (M + H+): 640.2150, found: 640.2153.
5,5'-Dichloro-3,3'-bis(4-methoxyphenyl)-1H,1'H-2,2'-biindole (6)
1H NMR (600 MHz, DMSO-d6) δ 11.72 (br, 2H), 7.54 (s, 2H), 7.42 (d, J = 12.0 Hz, 2H), 7.18 (dd, J = 6.0, 6.0 Hz, 2H), 7.07 (d, J = 12.0 Hz, 4H), 6.80 (d, J = 6.0 Hz, 4H), 3.70 (s, 6H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 158.1, 135.1, 129.9, 128.1, 128.1, 126.6, 124.8, 122.7, 118.4, 116.3, 114.4, 113.7, 55.5. HRMS, calculated for C30H23Cl2N2O2 (M + H+): 513.1131, found: 513.1134.
3-(Phenyl-d5)-1H-indole
1H NMR (400 MHz, DMSO-d6) δ 11.37 (br, 1H), 7.88(dd, J = 8.0, 4.0 Hz, 1H), 7.69 (d, J = 2.8 Hz, 1H), 7.47 (d, J = 8.0 Hz, 1H), 7.17 (td, J = 8.0, 4.0 Hz, 1H), 7.10 (td, J = 8.0, 4.0 Hz, 1H).
3-Phenyl-1H-indole-2-d
1H NMR (600 MHz, CDCl3) δ 8.21 (d, J = 12.0 Hz, 1H), 7.91 (t, J = 9.0 Hz, 3H), 7.68 (t, J = 9.0 Hz, 2H), 7.53 (t, J = 9.0, 1H), 7.47–7.43 (m, 3H).
4b-phenyl-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole-d4
1H NMR (400 MHz, DMSO-d6) δ 12.40 (br, 1H), 8.15 (d, J = 8.0 Hz, 1H), 7.88 (d, J = 4.0 Hz, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.49–7.42 (m, 2H), 7.29 (td, J = 6.0, 4.0 Hz, 2H), 7.19 (t, J = 8.0 Hz, 1H).
Characterization data of products
4b-phenyl-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2a)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 97.4 mg, 85% yield; m.p. 282–284°C. 1H NMR (400 MHz, DMSO-d6) δ 12.42 (br, 1H), 8.15 (q, J = 4.0 Hz, 2H), 8.04 (d, J = 8.0 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.50–7.42 (m, 3H), 7.32–7.26 (m, 3H), 7.19 (t, J = 8.0 Hz, 1H), 7.11 (t, J = 6.0 Hz, 2H), 7.04 (t, J = 6.0 Hz, 1H), 6.84 (d, J = 8.0 Hz, 2H); 13C{1H}NMR (101 MHz, DMSO-d6) δ 176.9, 156.5, 142.4, 142.3, 139.8, 136.9, 133.8, 129.9, 129.2, 129.1, 128.8, 128.0, 127.5, 126.8, 126.2, 126.0, 125.6, 125.6, 125.1, 123.9, 121.6, 121.6, 121.6, 118.8, 113.4, 67.8. HRMS, calculated for C28H18N2Na (M + Na+): 405.1362, found 405.1360.
6-Methyl-4b-(p-tolyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2b)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 92.3 mg, 75% yield; m.p. 286–289°C. 1H NMR (600 MHz, DMSO-d6) δ 12.33 (br, 1H), 8.12 (d, J = 6.0 Hz, 1H), 8.02 (d, J = 6.0 Hz, 1H), 7.89 (d, J = 6.0 Hz, 1H), 7.84 (s, 1H), 7.72 (d, J = 6.0 Hz, 1H), 7.48 (d, J = 12.0 Hz, 1H), 7.43 (t, J = 6.0 Hz, 1H), 7.30–7.26 (m, 3H), 7.17 (t, J = 6.0 Hz, 1H), 6.89 (d, J = 6.0 Hz, 2H), 6.71 (d, J = 6.0 Hz, 2H), 2.41 (s, 3H), 2.05 (s, 3H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 177.2, 156.6, 142.6, 139.7, 139.4, 137.2, 136.8, 134.9, 130.9, 129.7, 129.5, 129.1, 128.9, 128.6, 126.6, 126.2, 126.0, 125.5, 124.9, 123.9, 121.6, 121.4, 119.0, 113.3, 67.5, 21.5, 20.7. HRMS, calculated for C30H23N2 (M + H+): 411.1856, found 411.1857.
6-Chloro-4b-(4-chlorophenyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2c)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 47.4 mg, 35% yield; m.p. 284–285°C. 1H NMR (600 MHz, DMSO-d6) δ 12.50 (br, 1H), 8.14 (q, J = 8.0 Hz, 2H), 7.96 (d, J = 6.0 Hz, 2H), 7.76 (d, J = 6.0 Hz, 1H), 7.55(dd, J = 8.4, 1.8 Hz, 1H), 7.50–7.48 (m, 2H), 7.35–7.30 (m, 2H), 7.24–7.22 (m, 2H), 7.21 (d, J = 6.0 Hz, 1H), 6.81 (d, J = 12.0 Hz, 2H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 175.6, 156.4, 141.4, 140.5, 139.8, 138.6, 132.8, 132.5, 129.9, 129.6, 129.6, 129.4, 129.0, 127.8, 127.7, 127.3, 127.2, 126.1, 125.4, 123.7, 121.9, 121.4, 117.9, 113.6, 66.9. HRMS, calculated for C28H17Cl2N2 (M + H+): 451.0763, found 451.0755.
6-Fluoro-4b-(4-fluorophenyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2d)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 62.7 mg, 50% yield; m.p. 176–179°C. 1H NMR (400 MHz, DMSO-d6) δ 12.47 (br, 1H), 8.18–8.12 (m, 2H), 7.98 (d, J = 8.0 Hz, 1H), 7.84 (dd, J = 12.0, 4.0 Hz, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.50–7.44 (m, 2H), 7.33–7.28 (m, 3H), 7.21–7.17 (m, 1H), 7.00–6.98 (m, 2H), 6.85–6.82 (m, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 176.1, 161.5 (d, J = 245 Hz), 159.8 (d, J = 246 Hz), 156.3, 141.7, 139.8, 139.4 (d, J = 7.1 Hz), 137.8 (d, J = 4.0 Hz), 130.2 (d, J = 3.0 Hz), 129.5, 129.1, 128.0 (d, J = 8.1 Hz), 127.4 (d, J = 8.0 Hz), 127.1, 126.1, 125.2, 123.6, 121.7, 121.4, 118.1, 116.6, 115.6 (d, J = 23.2 Hz), 115.4 (d, J = 21.2 Hz), 113.5, 66.9; 19F NMR (376 MHz, DMSO-d6) δ −115.10, −115.19 ppm. HRMS, calculated for C28H17 F2N2 (M + H+): 419.1354, found 419.1351.
4b-([1,1'-Biphenyl]-4-yl)-6-phenyl-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2e)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 137.8 mg, 86% yield; m.p. 273–276°C. 1H NMR (400 MHz, DMSO-d6) δ 12.50 (br, 1H), 8.29 (s, 1H), 8.24 (d, J = 8.0 Hz, 1H), 8.19 (d, J = 8.0 Hz, 1H), 8.06 (d, J = 8.0 Hz, 1H), 7.80 (q, J = 8.0 Hz, 4H), 7.50 (t, J = 8.0 Hz, 3H), 7.44 (t, J = 8.0 Hz, 3H), 7.41 (d, J = 8.0 Hz, 3H), 7.35–7.30 (m, 4H), 7.27–7.20 (m, 2H), 6.98 (d, J = 8.0 Hz, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 176.6, 156.6, 142.3, 141.2, 140.0, 139.9, 139.8, 139.6, 137.7, 137.0, 133.0, 130.0, 129.6, 129.2, 128.1, 127.9, 127.6, 127.1, 127.0, 126.7, 126.3, 126.2, 125.9, 125.2, 123.9, 121.7, 121.7, 121.6, 118.6, 113.5, 67.6. HRMS, calculated for C40H27N2 (M + H+): 535.2169, found: 535.2170.
5,6,7-Trimethoxy-4b-(3,4,5-trimethoxyphenyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2f)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 70.8 mg, 42% yield; m.p. 143–145°C. 1H NMR (600 MHz, DMSO-d6) δ 12.42 (br, 1H), 8.13 (d, J = 6.0 Hz, 1H), 7.99 (dd, J = 6.6, 1.2 Hz, 1H), 7.64 (dd, J = 7.8, 1.2 Hz, 1H), 7.48 (t, J = 6.0 Hz, 2H), 7.39 (td, J = 7.5, 1.8 Hz, 1H), 7.32–7.26 (m, 2H), 7.20 (td, J = 7.5, 1.2 Hz, 1H), 6.15 (s, 2H), 3.99 (s, 3H), 3.85 (s, 3H), 3.64 (s, 3H), 3.48 (s, 3H), 3.46 (s, 6H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 175.5, 156.4, 153.9, 153.3, 153.0, 141.4, 139.9, 139.9, 138.7, 136.9, 130.2, 128.9, 126.5, 125.0, 123.7, 122.9, 121.7, 121.4, 120.8, 118.1, 113.4, 105.7, 103.6, 68.2, 61.9, 60.9, 60.3, 56.4, 56.0. HRMS, calculated for C34H31N2O6 (M + H+): 563.2177, found 563.2179.
4,9-Dimethoxy-4b-phenyl-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2g)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 102.1 mg, 77% yield; m.p. 192–194°C. 1H NMR (400 MHz, DMSO-d6) δ 12.32 (br, 1H), 8.43 (dd, J = 8.0, 4.0 Hz, 1H), 8.10 (dd, J = 7.8, 1.4 Hz, 1H), 7.44 (t, J = 8.0 Hz, 1H), 7.40–7.35 (m, 2H), 7.19–7.14 (m, 2H), 7.11–7.03 (m, 3H), 7.01–6.95 (m, 2H), 6.78–6.76 (m, 2H), 6.64 (d, J = 8.0 Hz, 1H), 3.95 (s, 3H), 3.73 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 177.6, 158.2, 156.3, 154.3, 141.4, 140.3, 137.9, 133.9, 131.1, 129.8, 129.7, 129.2, 128.9, 128.7, 128.4, 127.3, 126.5, 126.1, 125.2, 119.6, 114.7, 114.6, 111.2, 106.3, 101.8, 69.9, 56.3, 55.7. HRMS, calculated for C30H23N2O2 (M + H+): 443.1754, found 443.1749.
4,9-Dimethoxy-6-methyl-4b-(p-tolyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazoe (2h)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 74.7 mg, 53% yield; m.p. 177–179°C. 1H NMR (400 MHz, DMSO-d6) δ 12.21 (br, 1H), 8.30 (d, J = 8.0 Hz, 1H), 7.93 (d, J = 2.0 Hz, 1H), 7.43 (t, J = 8.0 Hz, 1H), 7.33 (d, J = 8.0 Hz, 1H), 7.19–7.14 (m, 2H), 6.97 (t, J = 8.0 Hz, 2H), 6.87 (d, J = 8.0 Hz, 2H), 6.62 (t, J = 6.0 Hz, 3H), 3.94 (s, 3H), 3.78 (s, 3H), 2.33 (s, 3H), 2.08 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 177.9, 158.3, 156.2, 154.3, 141.4, 138.1, 137.4, 136.4, 134.1, 130.9, 130.9, 130.5, 129.7, 129.3, 129.2, 128.7, 128.5, 126.4, 125.9, 119.8, 114.7, 114.5, 111.1, 106.2, 101.6, 69.7, 56.1, 55.6, 21.7, 20.8. HRMS, calculated for C32H27N2O2 (M + H+): 471.2067, found 471.2063.
3,10-Dimethyl-4b-phenyl-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2i)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 105.8 mg, 86% yield; m.p. 189–192°C. 1H NMR (400 MHz, DMSO-d6) δ 12.22 (br, 1H), 8.13 (dd, J = 8.0, 4.0 Hz, 1H), 8.04 (dd, J = 7.8, 1.4 Hz, 1H), 7.93 (s, 1H), 7.68 (d, J = 4.0 Hz, 1H), 7.59 (d, J = 8.0 Hz, 1H), 7.48 (td, J = 7.6, 1.2 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.30 (td, J = 7.6, 1.2 Hz, 1H), 7.23 (d, J = 8.0 Hz, 1H), 7.13–7.09 (m, 3H), 7.07–7.03 (m, 1H), 6.81 (dd, J = 8.0, 4.0 Hz, 2H), 2.44 (s, 3H), 2.38 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 176.0, 154.3, 142.6, 142.5, 138.0, 136.8, 136.3, 134.1, 130.4, 130.0, 129.5, 129.1, 128.7, 128.0, 127.4, 126.8, 126.6, 126.0, 125.4, 125.3, 124.2, 121.0, 121.0, 117.9, 113.0, 67.6, 21.7, 21.6. HRMS, calculated for C30H23N2 (M + H+): 411.1856, found 411.1855.
3,6,10-Trimethyl-4b-(p-tolyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2j)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 116.9 mg, 89% yield; m.p. 178–181°C. 1H NMR (600 MHz, DMSO-d6) δ 12.11 (br, 1H), 8.00 (d, J = 6.0 Hz, 1H), 7.89 (s, 1H), 7.82 (s, 1H), 7.67 (s, 1H), 7.57 (d, J = 6.0 Hz, 1H), 7.33 (d, J = 6.0 Hz, 1H), 7.27 (d, J = 6.0 Hz, 1H), 7.22 (d, J = 6.0 Hz, 1H), 7.09 (d, J = 12.0 Hz, 1H), 6.89 (d, J = 6.0 Hz, 2H), 6.66 (d, J = 6.0 Hz, 2H), 2.43 (s, 6H), 2.39 (s, 3H), 2.07 (s, 3H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 176.2, 154.4, 142.8, 139.7, 138.0, 137.1, 136.6, 136.1, 134.6, 131.2, 130.2, 129.6, 129.4, 129.0, 128.5, 126.8, 126.5, 126.0, 125.3, 124.1, 120.9, 120.9, 118.1, 112.9, 67.3, 21.7, 21.7, 21.5, 20.8. HRMS, calculated for C32H27N2 (M + H+): 439.2169, found 439.2168.
6-Methoxy-4b-(4-methoxyphenyl)-3,10-dimethyl-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2k)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 115.6 mg, 82% yield; m.p. 196–197°C. 1H NMR (400 MHz, DMSO-d6) δ 12.04 (br, 1H), 8.06 (d, J = 12.0 Hz, 1H), 7.89 (s, 1H), 7.64 (s, 1H), 7.56 (t, J = 8.0 Hz, 1H), 7.52 (d, J = 2.4 Hz, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.22 (d, J = 8.0 Hz, 1H), 7.09 (d, J = 8.0 Hz, 1H), 7.04 (dd, J = 8.0, 4.0 Hz, 1H), 6.67 (t, J = 10.0 Hz, 4H), 3.88 (s, 3H), 3.56 (s, 3H), 2.43 (s, 3H), 2.39 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 176.1, 158.5, 156.8, 154.4, 142.7, 139.1, 138.0, 136.1, 134.1, 130.0, 129.4, 128.9, 127.2, 126.7, 126.5, 123.9, 120.9, 118.2, 115.0, 114.5, 112.9, 112.4, 67.0, 55.7, 55.4, 21.7, 21.6. HRMS, calculated for C32H27N2O2 (M + H+): 471.2067, found 471.2065.
3,10-Difluoro-6-methyl-4b-(p-tolyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2l)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 108.4 mg, 81% yield; m.p. 185–188°C. 1H NMR (600 MHz, DMSO-d6) δ 12.43 (br, 1H), 7.96 (dd, J = 8.1, 2.1 Hz, 1H), 7.91–7.87 (m, 2H), 7.84 (s, 1H), 7.72 (q, J = 4.0 Hz, 1H), 7.45 (q, J = 4.0 Hz, 1H), 7.28–7.25 (m, 2H), 7.15 (td, J = 9.0, 2.4 Hz, 1H), 6.92 (d, J = 6.0 Hz, 2H), 6.68 (dd, J = 9.3, 2.1 Hz, 2H), 2.43 (s, 3H), 2.08 (s, 3H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 177.0, 161.3 (d, J = 243 Hz), 158.3 (d, J = 234 Hz), 152.6, 144.5 (d, J = 9.0 Hz), 138.7, 137.1, 136.4, 136.1, 135.2, 130.8, 130.2, 129.8, 129.3, 128.6, 125.9, 125.5, 123.7 (d, J = 10.6 Hz), 122.4 (d, J = 9.1 Hz), 118.9 (d, J = 6.0 Hz), 115.7, 115.5, 114.5 (d, J = 9.1 Hz), 114.1 (d, J = 25.7 Hz), 113.5 (d, J = 27.2 Hz), 106.3 (d, J = 24.2 Hz), 68.0, 21.4, 20.8; 19F NMR (565 MHz, DMSO-d6) δ −115.06, −121.86 ppm. HRMS, calculated for C30H21F2N2 (M + H+): 447.1667, found 447.1663.
3,10-Difluoro-6-methoxy-4b-(4-methoxyphenyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2m)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 114.7 mg, 80% yield; m.p. 169–173°C. 1H NMR (600 MHz, DMSO-d6) δ 12.38 (br, 1H), 8.03 (d, J = 6.0 Hz, 1H), 7.91–7.88 (m, 2H), 7.71 (q, J = 4.0 Hz, 1H), 7.53 (d, J = 3.0 Hz, 1H), 7.45 (q, J = 4.0 Hz, 1H), 7.26 (td, J = 8.4, 2.4 Hz, 1H), 7.14 (td, J = 9.3, 2.4 Hz, 1H), 7.01 (dd, J = 8.7, 2.7 Hz, 1H), 6.72–6.68 (m, 4H), 3.88 (s, 3H), 3.56 (s, 3H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 176.9, 161.3 (d, J = 243 Hz), 158.3 (d, J = 234 Hz), 158.7, 157.1, 152.5, 144.5 (d, J = 7.6 Hz), 138.3, 136.4, 133.1, 130.1, 127.2, 126.9, 125.5, 123.4 (d, J = 10.6 Hz), 122.3 (d, J = 9.1 Hz), 118.9 (d, J = 4.5 Hz), 115.7 (d, J = 24.2 Hz), 114.9, 114.6, 114.4 (d, J = 9.1 Hz), 113.9 (d, J = 25.7 Hz), 113.5 (d, J = 27.2 Hz), 112.9, 106.2 (d, J = 24.2 Hz), 67.7, 55.8, 55.4; 19F NMR (565 MHz, DMSO-d6) δ -115.04, -122.01 ppm. HRMS, calculated for C30H21F2N2O2 (M + H+): 479.1566, found 479.1561.
3,10-Dichloro-6-methyl-4b-(p-tolyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2n)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 107.6 mg, 75% yield; m.p. 196–199°C. 1H NMR (600 MHz, DMSO-d6) δ 12.55 (br, 1H), 8.16 (t, J = 2.1 Hz, 1H), 8.03 (t, J = 1.8 Hz, 1H), 7.99 (d, J = 6.0 Hz, 1H), 7.84 (s, 1H), 7.72 (dd, J = 8.1, 1.8 Hz, 1H), 7.50–7.49 (m, 1H), 7.46 (dd, J = 8.4, 1.8 Hz, 1H), 7.30–7.26 (m, 2H), 6.92 (d, J = 12.0 Hz, 2H), 6.68 (dd, J = 8.7, 1.5 Hz, 2H), 2.44 (s, 3H), 2.08 (s, 3H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 177.3, 155.1, 144.4, 138.5, 138.2, 137.2, 136.2, 135.6, 131.2, 130.4, 129.9, 129.8, 129.4, 129.2, 128.7, 126.3, 126.2, 125.9, 125.8, 125.2, 124.6, 122.7, 120.7, 118.8, 114.9, 67.9, 21.5, 20.8. HRMS, calculated for C30H21Cl2N2 (M + H+): 479.1076, found 479.1080.
3,10-Dichloro-6-methoxy-4b-(4-methoxyphenyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2o)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 140.8 mg, 92% yield; m.p. 185–187°C. 1H NMR (600 MHz, DMSO-d6) δ 12.49 (br, 1H), 8.16 (d, J = 2.4 Hz, 1H), 8.06–8.04 (m, 2H), 7.72 (d, J = 6.0 Hz, 1H), 7.53 (d, J = 2.4 Hz, 1H), 7.50 (dd, J = 8.1, 2.1 Hz, 1H), 7.46 (d, J = 12.0 Hz, 1H), 7.29 (dd, J = 8.7, 2.1 Hz, 1H), 7.03 (dd, J = 8.4, 2.4 Hz, 1H), 6.73–6.68 (m, 4H), 3.89 (s, 3H), 3.57 (s, 3H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 177.3, 158.8, 157.3, 155.1, 144.4, 138.3, 138.2, 132.9, 131.2, 129.6, 129.2, 127.3, 127.2, 126.1, 126.0, 125.2, 125.2, 124.4, 122.7, 120.6, 118.9, 115.2, 114.8, 114.7, 112.8, 67.6, 55.8, 55.4. HRMS, calculated for C30H21Cl2N2O2 (M + H+): 511.0975, found 511.0971.
2,11-Dimethyl-4b-phenyl-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2p)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 98.4 mg, 80% yield; m.p. 208–212°C. 1H NMR (600 MHz, DMSO-d6) δ 12.25 (br, 1H), 8.10 (d, J = 6.0 Hz, 1H), 8.00 (t, J = 9.0 Hz, 2H), 7.71 (d, J = 6.0 Hz, 1H), 7.53 (s, 1H), 7.46 (t, J = 6.0 Hz, 1H), 7.30–7.26 (m, 2H), 7.11–7.01 (m, 5H), 6.82 (d, J = 6.0 Hz, 2H), 2.41 (s, 3H), 2.39 (s, 3H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 177.1, 156.9, 142.7, 140.3, 139.6, 138.6, 137.4, 134.7, 133.9, 129.5, 129.1, 128.7, 128.0, 127.4, 127.2, 126.0, 125.7, 125.5, 125.4, 123.5, 122.0, 121.9, 121.3, 118.8, 113.0, 67.4, 21.9, 21.5. HRMS, calculated for C30H23N2 (M + H+): 411.1856, found 411.1852.
2,6,11-Trimethyl-4b-(p-tolyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2q)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 118.3 mg, 90% yield; m.p. 288–290°C. 1H NMR (400 MHz, DMSO-d6) δ 12.14 (br, 1H), 7.98 (q, J = 4.0 Hz, 2H), 7.79 (d, J = 4.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 1.6 Hz, 1H), 7.25 (d, J = 8.0 Hz, 2H), 7.08 (d, J = 8.0 Hz, 1H), 7.00 (dd, J = 8.0, 4.0 Hz, 1H), 6.88 (d, J = 8.0 Hz, 2H), 6.67 (d, J = 8.0 Hz, 2H), 2.41 (s, 6H), 2.39 (s, 3H), 2.06 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 177.4, 157.0, 140.2, 139.8, 139.7, 138.4, 137.6, 136.6, 134.7, 134.5, 131.0, 129.6, 129.0, 129.0, 128.6, 127.0, 125.9, 125.7, 125.3, 123.2, 121.8, 121.2, 119.0, 112.9, 67.1, 21.9, 21.5, 20.7. HRMS, calculated for C32H27N2 (M + H+): 439.2168, found 439.2172.
6-Methoxy-4b-(4-methoxyphenyl)-2,11-dimethyl-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2r)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 124.1 mg, 88% yield; m.p. 282–286°C. 1H NMR (400 MHz, DMSO-d6) δ 12.08 (br, 1H), 8.03 (d, J = 8.0 Hz, 1H), 7.98 (d, J = 8.0 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.50 (d, J = 4.0 Hz, 2H), 7.22 (s, 1H), 7.08 (d, J = 4.0 Hz, 1H), 7.01 (td, J = 8.0, 4.0 Hz, 2H), 6.69–6.64 (m, 4H), 3.86 (s, 3H), 3.55 (s, 3H), 2.41 (s, 3H), 2.40 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 177.3, 158.5, 156.9, 140.2, 139.7, 139.6, 138.5, 134.5, 134.4, 128.2, 127.1, 127.0, 126.7, 126.3, 125.4, 123.1, 121.8, 121.7, 121.2, 119.1, 114.7, 114.4, 112.9, 112.6, 66.8, 55.7, 55.4, 21.9, 21.5. HRMS, calculated for C32H27N2O2 (M + H+): 471.2067, found 471.2064.
2,11-Difluoro-4b-phenyl-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2s)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 92.8 mg, 74% yield; m.p. 281–283°C; 1H NMR (600 MHz, DMSO-d6) δ 12.64 (br, 1H), 8.18 (q, J = 6.0 Hz, 1H), 8.12 (d, J = 6.0 Hz, 1H), 8.01 (d, J = 6.0 Hz, 1H), 7.88 (t, J = 6.0 Hz, 1H), 7.59 (dd, J = 9.0, 2.4 Hz, 1H), 7.47 (t, J = 6.0 Hz, 1H), 7.31 (t, J = 9.0 Hz, 1H), 7.26 (d, J = 6.0 Hz, 1H), 7.13–7.03 (m, 5H), 6.81 (d, J = 6.0 Hz, 2H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 178.8, 163.1 (d, J = 243 Hz), 160.9 (d, J = 243 Hz), 158.2 (d, J = 12.1 Hz), 142.0, 140.3 (d, J = 12.1 Hz), 138.3, 137.0, 133.1, 130.1, 129.3, 128.9, 128.1, 127.6, 127.0 (d, J = 10.6 Hz), 126.0 (d, J = 10.6 Hz), 125.7, 123.3 (d, J = 10.6 Hz), 120.8, 119.5, 113.1 (d, J = 22.7 Hz), 110.5 (d, J = 25.7 Hz), 109.1 (d, J = 22.7 Hz), 99.2 (d, J = 25.7 Hz), 67.3; 19F NMR (565 MHz, DMSO-d6) δ −113.60, −116.29 ppm. HRMS, calculated for C28H17F2N2 (M + H+): 419.1354, found 419.1352.
2,11-Difluoro-6-methyl-4b-(p-tolyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2t)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 120.4 mg, 90% yield; m.p. 273–276°C. 1H NMR (400 MHz, DMSO-d6) δ 12.45 (br, 1H), 8.15 (q, J = 4.0 Hz, 1H), 7.99 (d, J = 8.0 Hz, 1H), 7.93–7.89 (m, 1H), 7.80 (d, J = 2.0 Hz, 1H), 7.56 (dd, J = 9.2, 2.4 Hz, 1H), 7.26 (d, J = 8.0 Hz, 1H), 7.20 (dd, J = 9.6, 2.4 Hz, 1H), 7.10 (td, J = 8.0, 4.0 Hz, 1H), 7.03 (td, J = 9.2, 2.4 Hz, 1H), 6.90 (d, J = 8.0 Hz, 2H), 6.66 (d, J = 8.0 Hz, 2H), 2.41 (s, 3H), 2.06 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 179.1, 163.0 (d, J = 244 Hz), 160.9 (d, J = 241 Hz), 158.2 (d, J = 11.1 Hz), 140.2 (d, J = 12.1 Hz), 139.1, 138.5 (d, J = 3.0 Hz), 137.2, 136.9, 135.5, 130.1, 129.7 (d, J = 5.6 Hz), 129.2 (d, J = 6.1 Hz), 128.7, 127.0 (d, J = 6.1 Hz), 125.9 (d, J = 11.1 Hz), 125.6 (d, J = 4.0 Hz), 123.3 (d, J = 10.1 Hz), 120.7, 119.8, 112.9 (d, J = 23.2 Hz), 110.3 (d, J = 24.2 Hz), 108.8 (d, J = 24.2 Hz), 99.1 (d, J = 25.3 Hz), 67.0, 21.5, 20.7; 19F NMR (376 MHz, DMSO-d6) δ −113.85, −116.39 ppm. HRMS, calculated for C30H21F2N2 (M + H+): 447.1667, found 447.1661.
2,11-Difluoro-6-methoxy-4b-(4-methoxyphenyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2u)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 131.9 mg, 92% yield; m.p. 277–278°C. 1H NMR (600 MHz, DMSO-d6) δ 12.38 (br, 1H), 8.15 (q, J = 4.0 Hz, 1H), 8.07 (d, J = 6.0 Hz, 1H), 7.90 (q, J = 4.0 Hz, 1H), 7.55 (dd, J = 9.0, 2.4 Hz, 1H), 7.50 (d, J = 2.4 Hz, 1H), 7.19 (dd, J = 9.6, 2.4 Hz, 1H), 7.09 (td, J = 9.0, 2.4 Hz, 1H), 7.04 (td, J = 9.3, 2.4 Hz, 2H), 6.70–6.66 (m, 4H), 3.87 (s, 3H), 3.56 (s, 3H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 179.0, 163.1 (d, J = 243 Hz), 160.9 (d, J = 240 Hz), 158.7, 158.2 (d, J = 10.6 Hz), 157.3, 140.2 (d, J = 12.1 Hz), 139.4, 138.4, 133.6, 128.9, 127.1 (d, J = 4.5 Hz), 126.8 (d, J = 9.1 Hz), 125.4, 123.2 (d, J = 10.6 Hz), 120.6, 119.9, 115.0, 114.6, 112.9 (d, J = 22.7 Hz), 110.2 (d, J = 24.2 Hz), 108.8 (d, J = 24.2 Hz), 99.0 (d, J = 25.7 Hz), 66.7, 55.8, 55.4; 19F NMR (565 MHz, DMSO-d6) δ -113.78, -116.33 ppm. HRMS, calculated for C30H20F2N2NaO2 (M + Na+): 501.1385, found 501.1384.
2,11-Dichloro-4b-phenyl-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2v)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 71.6 mg, 53% yield; m.p. 288–291°C. 1H NMR (600 MHz, DMSO-d6) δ 12.65 (br, 1H), 8.18 (d, J = 6.0 Hz, 1H), 8.12 (d, J = 6.0 Hz, 1H), 8.01 (d, J = 6.0 Hz, 1H), 7.90 (d, J = 12.0 Hz, 1H), 7.81 (s, 1H), 7.50–7.47 (m, 2H), 7.34–7.32 (m, 2H), 7.20 (dd, J = 8.4, 2.1 Hz, 1H), 7.13 (t, J = 9.0 Hz, 2H), 7.08 (t, J = 6.0 Hz, 1H), 6.80 (d, J = 6.0 Hz, 2H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 178.4, 157.8, 141.5, 141.2, 140.3, 136.6, 133.7, 132.8, 130.2, 129.9, 129.4, 129.0, 128.1, 127.8, 127.3, 126.5, 126.2, 125.9, 123.3, 122.6, 122.1, 121.6, 119.4, 112.9, 67.6. HRMS, calculated for C28H17Cl2N2 (M + H+): 451.0763, found 451.0760.
2,11-Dichloro-6-methyl-4b-(p-tolyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2w)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 120.5 mg, 84% yield; m.p. 183–186°C. 1H NMR (400 MHz, DMSO-d6) δ 12.53 (br, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.97 (d, J = 8.0 Hz, 1H), 7.92 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 2.0 Hz, 2H), 7.46 (d, J = 4.0 Hz, 1H), 7.33 (dd, J = 8.0, 2.0 Hz, 1H), 7.26 (dd, J = 4.0, 4.0 Hz, 1H), 7.16 (dd, J = 8.8, 2.0 Hz, 1H), 6.90 (d, J = 8.0 Hz, 2H), 6.65 (d, J = 8.0 Hz, 2H), 2.41 (s, 3H), 2.06 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 178.7, 157.9, 141.3, 140.3, 138.6, 137.1, 136.8, 135.6, 133.6, 129.9, 129.8, 129.8, 129.3, 129.3, 128.7, 127.3, 126.3, 125.9, 125.8, 123.2, 122.5, 121.9, 121.4, 119.7, 112.8, 67.3, 21.5, 20.7. HRMS, calculated for C30H21Cl2N2 (M + H+): 479.1076, found 479.1075.
2,11-Dichloro-6-methoxy-4b-(4-methoxyphenyl)-4b,13-dihydrobenzo[c]indolo[2,3-a]carbazole (2x)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give a yellow solid, 125.5 mg, 82% yield; m.p. 267–270°C. 1H NMR (600 MHz, DMSO-d6) δ 12.48 (br, 1H), 8.13 (d, J = 6.0 Hz, 1H), 8.04 (d, J = 12.0 Hz, 1H), 7.91 (d, J = 12.0 Hz, 1H), 7.78 (d, J = 2.4 Hz, 1H), 7.49 (d, J = 3.0 Hz, 1H), 7.45 (d, J = 1.8 Hz, 1H), 7.32 (dd, J = 8.1, 2.1 Hz, 1H), 7.16 (dd, J = 8.7, 2.1 Hz, 1H), 7.02 (dd, J = 8.7, 2.7 Hz, 1H), 6.69–6.66 (m, 4H), 3.87 (s, 3H), 3.55 (s, 3H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 178.6, 158.7, 157.9, 157.4, 141.2, 140.3, 139.0, 133.6, 133.1, 129.9, 129.0, 127.2, 127.1, 127.1, 126.3, 125.2, 123.1, 122.3, 121.8, 121.4, 119.8, 114.9, 114.7, 112.9, 112.8, 67.0, 55.8, 55.4. HRMS, calculated for C30H21Cl2N2O2 (M + H+): 511.0975, found 511.0976.
15c-(Benzo[b]thiophen-2-yl)-6,15c-dihydrobenzo[4,5]thieno[2,3-c]indolo[2,3-a]carbazole (2years)
The title compound was prepared according to the general procedure and purified by flash column chromatography to give an orange solid, 80 mg, 54% yield; m.p. 112–115°C. 1H NMR (400 MHz, DMSO-d6) δ 12.37 (br, 1H), 8.52 (d, J = 8.0 Hz, 1H), 8.07 (dd, J = 8.0, 4.0 Hz, 3H), 7.90 (d, J = 8.0 Hz, 1H), 7.85 (dd, J = 8.0, 4.0 Hz, 1H), 7.68–7.66 (m, 1H), 7.63–7.55 (m, 2H), 7.50 (td, J = 8.0, 4.0 Hz, 1H), 7.38 (t, J = 8.0 Hz, 1H), 7.27 (t, J = 4.0 Hz, 2H), 7.24–7.20 (m, 2H), 7.15 (td, J = 8.0, 4.0 Hz, 1H), 7.00 (d, J = 8.0 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ 179.8, 156.7, 141.4, 140.8, 138.9, 137.7, 137.4, 137.1, 135.9, 135.7, 132.6, 130.3, 127.9, 127.6, 126.9, 125.8, 125.7, 125.6, 125.0, 124.8, 124.4, 123.9, 123.8, 122.4, 121.8, 121.6, 121.3, 121.2, 113.4, 108.6, 63.3. HRMS, calculated for C32H19N2S2 (M + H+): 495.0984, found: 495.0982.
Acknowledgments
We are grateful for the financial support from National Natural Science Foundation of China (21573057, 21925111), and Natural Science Foundation of Henan Province (212300410052).
Author contributions
J.L. and H.H. contributed to the conception and design of the experiments. J. L., X.W., Z. W., Y. Y., Q. T., and H. L. performed the experiments. H.H and J. L. wrote the manuscript and all authors contributed to data analysis and scientific discussion.
Declaration of interests
The authors declare no competing interests.
Published: March 18, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.103906.
Contributor Information
Jianming Liu, Email: jmliu@htu.cn.
Hanmin Huang, Email: hanmin@ustc.edu.cn.
Supplemental information
Data and code availability
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Ackermann L., Wang L., Lygin A.V. Ruthenium-catalyzed aerobic oxidative coupling of alkynes with 2-aryl-substituted pyrroles. Chem. Sci. 2012;3:177–180. [Google Scholar]
- Allen S.E., Walvoord R.R., Padilla-Salinas R., Kozlowski M.C. Aerobic copper-catalyzed organic reactions. Chem. Rev. 2013;113:6234–6458. doi: 10.1021/cr300527g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissette A.J., Fletcher S.P. Mechanisms of autocatalysis. Angew. Chem. Int. Ed. 2013;52:12800–12826. doi: 10.1002/anie.201303822. [DOI] [PubMed] [Google Scholar]
- Barrios-Landeros F., Carrow B.P., Hartwig J.F. Autocatalytic oxidative addition of PhBr to Pd(PtBu3)2 via Pd(PtBu3)2(H)(Br) J. Am. Chem. Soc. 2008;130:5842–5843. doi: 10.1021/ja711159y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann J., Zierold R., Chong Y.T., Hauert R., Sturm C., Schmidt-Grund R., Rheinländer B., Grundmann M., Gösele U., Nielsch K. A practical, self-catalytic, atomic layer deposition of silicon dioxide. Angew. Chem. Int. Ed. 2008;47:6177–6179. doi: 10.1002/anie.200800245. [DOI] [PubMed] [Google Scholar]
- Bellina F., Benelli F., Rossi R. Direct palladium-catalyzed C-3 arylation of free (NH)-indoles with aryl bromides under ligandless conditions. J. Org. Chem. 2008;73:5529–5535. doi: 10.1021/jo8007572. [DOI] [PubMed] [Google Scholar]
- Cho S.H., Kim J.Y., Kwak J., Chang S. Recent advances in the transition metal-catalyzed twofold oxidative C−H bond activation strategy for C−C and C−N bond formation. Chem. Soc. Rev. 2011;40:5068–5083. doi: 10.1039/c1cs15082k. [DOI] [PubMed] [Google Scholar]
- Chen Y., Guo S., Li K., Qu J., Yuan H., Hua Q., Chen B. Palladium-catalyzed direct denitrogenative C-3-arylation of 1H-indoles with arylhydrazines using air as the oxidant. Adv. Synth. Catal. 2013;355:711–715. [Google Scholar]
- Chen S., Liao Y., Zhao F., Qi H., Liu S., Deng G.-J. Palladium-catalyzed direct arylation of indoles with cyclohexanones. Org. Lett. 2014;16:1618–1621. doi: 10.1021/ol500231c. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Li G., Liu Y., Shi Y., Gao G., Wu D., Lan J., You J. Unparalleled ease of access to a library of biheteroaryl fluorophores via oxidative cross-coupling reactions: discovery of photostable NIR probe for mitochondria. J. Am. Chem. Soc. 2016;138:4730–4738. doi: 10.1021/jacs.5b09241. [DOI] [PubMed] [Google Scholar]
- Cambeiro X.C., Ahlsten N., Larrosa I. Au-catalyzed cross-coupling of arenes via double C−H activation. J. Am. Chem. Soc. 2015;137:15636–15639. doi: 10.1021/jacs.5b10593. [DOI] [PubMed] [Google Scholar]
- Cacchi S., Fabrizi G. Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev. 2011;111:PR215–PR283. doi: 10.1021/cr100403z. [DOI] [PubMed] [Google Scholar]
- Flegeau E.F., Bruneau C., Dixneuf P.H., Jutand A. Autocatalysis for C-H bond activation by Ruthenium(II) complexes in catalytic arylation of functional arenes. J. Am. Chem. Soc. 2011;133:10161–10170. doi: 10.1021/ja201462n. [DOI] [PubMed] [Google Scholar]
- Ferreira E.M., Stoltz B.M. Catalytic C-H bond functionalization with palladium(II): aerobic oxidative annulations of indoles. J. Am. Chem. Soc. 2003;125:9578–9579. doi: 10.1021/ja035054y. [DOI] [PubMed] [Google Scholar]
- Grimster N.P., Gauntlett C., Godfrey C.R.A., Gaunt M.J. Palladium-catalyzed intermolecular alkenylation of indoles by solvent-controlled regioselective C-H functionalization. Angew. Chem. Int. Ed. 2005;44:3125–3129. doi: 10.1002/anie.200500468. [DOI] [PubMed] [Google Scholar]
- Giri R., Hartwig F.J. Cu(I)-amido complexes in the Ullmann reaction: reactions of Cu(I)-amido complexes with iodoarenes with and without autocatalysis by CuI. J. Am. Chem. Soc. 2010;132:15860–15863. doi: 10.1021/ja105695s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cuadrado D., de Mendoza P., Braga A.A.C., Maseras F., Echavarren A.M. Proton-abstraction mechanism in the palladium-catalyzed intramolecular arylation: substituent effects. J. Am. Chem. Soc. 2007;129:6880–6886. doi: 10.1021/ja071034a. [DOI] [PubMed] [Google Scholar]
- Gorelsky S.I., Lapointe D., Fagnou K. Analysis of the concerted metalation-deprotonation mechanism in palladium-catalyzed direct arylation across a broad range of aromatic substrates. J. Am. Chem. Soc. 2008;130:10848–10849. doi: 10.1021/ja802533u. [DOI] [PubMed] [Google Scholar]
- Girard S.A., Knauber T., Li C.-J. The cross-dehydrogenative coupling of Csp3-H bonds: a versatile strategy for C-C bond formations. Angew. Chem. Int. Ed. 2014;53:74–100. doi: 10.1002/anie.201304268. [DOI] [PubMed] [Google Scholar]
- Guo X.-X., Gu D.-W., Wu Z., Zhang W. Copper-catalyzed C−H functionalization reactions: efficient synthesis of heterocycles. Chem. Rev. 2015;115:1622–1651. doi: 10.1021/cr500410y. [DOI] [PubMed] [Google Scholar]
- Hsieh T.H.H., Dong V.M. Indole synthesis: palladium-catalyzed C-H bond amination via reduction of nitroalkenes with carbon monoxide. Tetrahedron. 2009;65:3062–3068. [Google Scholar]
- Huang H., Cai J., Ji X., Xiao F., Chen Y., Deng G.-J. Internal oxidant-triggered aerobic oxygenation and cyclization of indoles under copper catalysis. Angew. Chem. Int. Ed. 2016;55:307–311. doi: 10.1002/anie.201508076. [DOI] [PubMed] [Google Scholar]
- Jiao L., Bach T. Palladium-catalyzed direct 2-alkylation of indoles by norbornene-mediated regioselective cascade C-H activation. J. Am. Chem. Soc. 2011;133:12990–12993. doi: 10.1021/ja2055066. [DOI] [PubMed] [Google Scholar]
- Jacob J. The significance of polycyclic aromatic hydrocarbons as enviromental carcinogens. 35 Years research on PAH—a retrospective. Polycyclic Aromat. Compd. 2008;28:242–272. [Google Scholar]
- Joucla L., Batail N., Djakovitcha L. On Water” Direct and site-selective Pd-catalysed C-H arylation of (NH)-indoles. Adv. Synth. Catal. 2010;352:2929–2936. [Google Scholar]
- Kawasaki T., Matsumura Y., Tsutsumi T., Suzuki K., Ito M., Soai K. Asymmetric autocatalysis triggered by carbon isotope (13C/12C) chirality. Science. 2009;324:492–495. doi: 10.1126/science.1170322. [DOI] [PubMed] [Google Scholar]
- Lutz F., Igarashi T., Kawasaki T., Soai K. Small amounts of a chiral β-amino alcohols reverse the enantioselectivity of chiral catalysts in cooperative asymmetric autocatalysis. J. Am. Chem. Soc. 2005;127:12206–12207. doi: 10.1021/ja054323c. [DOI] [PubMed] [Google Scholar]
- Li H., Zhong J., Vehkamäki H., Kurtén T., Wang W., Ge M., Zhang S., Li Z., Zhang X., Francisco J.S., Zeng X.C. Self-catalytic reaction of SO3 and NH3 to produce sulfamic acid and its implication to atmospheric particle Formation. J. Am. Chem. Soc. 2018;140:11020–11028. doi: 10.1021/jacs.8b04928. [DOI] [PubMed] [Google Scholar]
- Knölker H., Reddy K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002;102:4303–4428. doi: 10.1021/cr020059j. [DOI] [PubMed] [Google Scholar]
- Liu C., Yuan J., Gao M., Tang S., Li W., Shi R., Lei A. Oxidative coupling between two hydrocarbons: an ppdate of recent C−H functionalizations. Chem. Rev. 2015;115:12138–12204. doi: 10.1021/cr500431s. [DOI] [PubMed] [Google Scholar]
- Li C.-J. Cross-dehydrogenative coupling (CDC): exploring C-C bond formations beyond functional group transformations. Acc. Chem. Res. 2009;42:335–344. doi: 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]
- Lane B.S., Brown M.A., Sames D. Direct palladium-catalyzed C-2 and C-3 arylation of indoles: a mechanistic rationale for regioselectivity. J. Am. Chem. Soc. 2005;127:8050–8057. doi: 10.1021/ja043273t. [DOI] [PubMed] [Google Scholar]
- Liu G., Gao J., Zhang H., Qin X., Guan X., Jiang G., Zhang G., Zhang S. A modified pudovik reaction, self-catalysis synthesis of 3-phosphinoylindoles. Tetrahedron Lett. 2019;60:1971–1974. [Google Scholar]
- Lancianesi S., Palmieri A., Petrini M. Synthetic approaches to 3-(2-nitroalkyl) indoles and their use to access tryptamines and related bioactive compounds. Chem. Rev. 2014;114:7108–7149. doi: 10.1021/cr400676v. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang W.-H., Yang S.-D., Li B.-J., Feng C., Shi Z.-J. Oxidative dimerization of N-protected and free indole derivatives toward 3,3-biindoles via Pd-catalyzed direct C-H transformations. Chem. Commun. 2010;46:4553–4555. doi: 10.1039/c0cc00486c. [DOI] [PubMed] [Google Scholar]
- Liang Z., Zhao J., Zhang Y. Palladium-catalyzed regioselective oxidative coupling of indoles and one-pot synthesis of acetoxylated biindolyls. J. Org. Chem. 2010;75:170–177. doi: 10.1021/jo902265s. [DOI] [PubMed] [Google Scholar]
- Li Y.-X., Ji K.-G., Wang H.-X., Ali S., Liang Y.-M. Iodine-induced regioselective C-C and C-N bonds formation of N-protected indoles. J. Org. Chem. 2011;76:744–747. doi: 10.1021/jo1023014. [DOI] [PubMed] [Google Scholar]
- Li T., Yang Y., Li B., Yang P. Cobalt-catalyzed cross-dehydrogenative coupling between N-(2-pyridyl) and free indoles for the dynthesis of unsymmetrical 2,2’-biindoles. Chem. Commun. 2019;55:353–356. doi: 10.1039/c8cc07659f. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Kaimori Y., Uchida M., Omori H., Kawasaki T., Soai K. Achiral inorganic gypsum scts as an origin of chirality through its enantiotopic surface in conjunction with asymmetric autocatalysis. Angew. Chem. Int. Ed. 2017;56:545–548. doi: 10.1002/anie.201610099. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Ozaki H., Harada S., Tada K., Ayugase T., Kawasaki T.H., Soai K. Asymmetric induction by a nitrogen 14N/15N isotopomer in conjunction with asymmetric autocatalysis. Angew. Chem. Int. Ed. 2016;55:15246–15249. doi: 10.1002/anie.201608955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod P.D., Li Z., Li C.-J. Self-catalytic, solvent-free or in/on water protocol: aza-Friedel-Crafts reactions between 3,4-dihydroisoquinoline and 1- or 2-naphthols. Tetrahedron. 2010;66:1045–1050. [Google Scholar]
- Morimoto K., Hirano K., Satoh T., Miura M. Rhodium-catalyzed oxidative coupling/cyclization of 2-phenylindoles with alkynes via C-H and N-H bond cleavages with air as the oxidant. Org. Lett. 2010;12:2068–2071. doi: 10.1021/ol100560k. [DOI] [PubMed] [Google Scholar]
- Nishino M., Hirano K., Satoh T., Miura M. Copper-mediated and copper-catalyzed cross-coupling of indoles and 1,3-azoles: double C−H activation. Angew. Chem. Int. Ed. 2012;51:6993–6997. doi: 10.1002/anie.201201491. [DOI] [PubMed] [Google Scholar]
- Ozaki K., Zhang H., Ito H., Lei A., Itami K. One-shot indole-to-carbazole π-extension by a Pd-Cu-Ag trimetallic system. Chem. Sci. 2013;4:3416–3420. [Google Scholar]
- Phipps R.J., Grimster N.P., Gaunt M.J. Cu(II)-catalyzed direct and site-selective arylation of indoles under mild conditions. J. Am. Chem. Soc. 2008;130:8172–8174. doi: 10.1021/ja801767s. [DOI] [PubMed] [Google Scholar]
- Rodrigues L.L., Micallef A.S., Pfrunder M.C., Truong V.X., McMurtrie J.C., Dargaville T.R., Goldmann A.S., Feist F., Barner-Kowollik C. A self-catalyzed visible light driven thiol ligation. J. Am. Chem. Soc. 2021;143:7292–7297. doi: 10.1021/jacs.1c03213. [DOI] [PubMed] [Google Scholar]
- Shi Z., Ding S., Cui Y., Jiao N. A palladium-catalyzed oxidative cycloaromatization of niaryls with alkynes using molecular oxygen as the oxidant. Angew. Chem. Int. Ed. 2009;48:7895–7898. doi: 10.1002/anie.200903975. [DOI] [PubMed] [Google Scholar]
- Sawato T., Saito N., Yamaguchi M. Chemical systems involving two competitive self-catalytic reactions. ACS Omega. 2019;4:5879–5899. doi: 10.1021/acsomega.9b00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov S.N., Belding L., Cafferty B.J., Mousavi M.P.S., Finogenova A.M., Cruz R.S., Skorb E.V., Whitesides G.M. Autocatalytic cycles in a copper-catalyzed azide-alkyne cycloaddition reaction. J. Am. Chem. Soc. 2018;140:10221–10232. doi: 10.1021/jacs.8b05048. [DOI] [PubMed] [Google Scholar]
- Soai K., Shibata T., Morioka H., Choji K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature. 1995;378:767–768. [Google Scholar]
- Stuart D.R., Fagnou K. The catalytic cross-coupling of unactivated arenes. Science. 2007;316:1172–1175. doi: 10.1126/science.1141956. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Asako T., Itami K., Yamaguchi J. Modular synthesis of heptaarylindole. Org. Biomol. Chem. 2018;16:3771–3776. doi: 10.1039/c8ob00993g. [DOI] [PubMed] [Google Scholar]
- Tang X., Wu W., Zeng W., Jiang H. Copper-catalyzed oxidative carbon-carbon and/or carbon-heteroatom bond formation with O2 or internal oxidants. Acc. Chem. Res. 2018;51:1092–1105. doi: 10.1021/acs.accounts.7b00611. [DOI] [PubMed] [Google Scholar]
- Tsuchimoto T., Matsubayashi H., Kaneko M., Nagase Y., Miyamura T., Shirakawa E. Indium-catalyzed annulation of 2-aryl- and 2-heteroarylindoles with propargyl ethers: concise synthesis and photophysical properties of diverse aryl- and heteroaryl-annulated[a]carbazoles. J. Am. Chem. Soc. 2008;130:15823–15835. doi: 10.1021/ja803954e. [DOI] [PubMed] [Google Scholar]
- Tsuchimoto T., Iwabuchi M., Nagase Y., Oki K., Takahashi H. Indium-catalyzed heteroaryl-heteroaryl bond formation through nucleophilic aromatic substitution. Angew. Chem. Int. Ed. 2011;50:1375–1379. doi: 10.1002/anie.201005750. [DOI] [PubMed] [Google Scholar]
- Wang C.-G., Oh X.Y., Liu X., Goto A. Self-catalyzed living radical polymerization using quaternary-ammonium-iodide-containing monomers. Macromolecules. 2019;52:2712–2718. [Google Scholar]
- Wendlandt A.E., Suess A.M., Stahl S.S. Copper-catalyzed aerobic oxidative C-H functionalizations: trends and mechanistic Insights. Angew. Chem. Int. Ed. 2011;50:11062–11087. doi: 10.1002/anie.201103945. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lan J., You J. Oxidative C−H/C−H coupling reactions between two (hetero)arenes. Chem. Rev. 2017;117:8787–8863. doi: 10.1021/acs.chemrev.6b00567. [DOI] [PubMed] [Google Scholar]
- Yeung C.S., Dong V.M. Catalytic dehydrogenative cross-coupling: forming carbon−carbon bonds by oxidizing two Ccarbon−hydrogen bonds. Chem. Rev. 2011;111:1215–1292. doi: 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]
- Yang R., Yu J.-T., Sun S., Zheng Q., Cheng J. Copper-mediated intramolecular aza-wacker-type cyclization of 2-alkenylanilines toward 3-aryl indoles. Tetrahedron Lett. 2017;58:445–448. [Google Scholar]
- Yamaguchi M., Suzuki K., Sato Y., Manabe K. Palladium-catalyzed direct C3-selective arylation of N-unsubstituted indoles with aryl chlorides and triflates. Org. Lett. 2017;19:5388–5391. doi: 10.1021/acs.orglett.7b02669. [DOI] [PubMed] [Google Scholar]
- Zhuo C.-X., Zheng C., You S.-L. Transition-metal-catalyzed asymmetric allylic dearomatization reactions. Acc. Chem. Res. 2014;47:2558–2573. doi: 10.1021/ar500167f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.