Abstract
Objective
Patients with end stage renal failure who require haemodialysis suffer morbidity and mortality due to vascular access. Bioengineered human acellular vessels (HAVs) may provide a haemodialysis access option with fewer complications than other grafts. In a prospective phase II trial from 2012 to 2014 (NCT01744418), HAVs were implanted into 40 haemodialysis patients at three sites in Poland. The trial protocol for this “first in man” use of the HAV contemplated only two years of follow up, and the trial results were initially reported in 2016. In light of the retained HAV function seen in many of the patients at the two year time point, follow up for patients who were still alive was extended to a total of 10 years. This interim follow up report, at the long term time point of five years, assessed patient and conduit status in those who continued routine dialysis with the HAV.
Methods
HAVs are bioengineered by culturing human vascular smooth muscle cells on a biodegradable polymer matrix. In this study, patients with patent HAV implants at 24 months were followed every three months, starting at month 27 through to month 60, or at least five years post-implantation. This report contains the follow up functional and histological data on 29 of the original 40 patients who demonstrated HAV function at the 24 month time point.
Results
Eleven patients completed at month 60. One patient maintained primary patency, and 10 maintained secondary patency. Secondary patency was estimated at 58.2% (95% confidence interval 39.2–73.1) at five years, after censoring for deaths (n = 8) and withdrawals (n = 1). No HAV conduit infections were reported during the follow up period.
Conclusion
This phase II long term follow up shows that the human acellular vessel (HAV) may provide durable and functional haemodialysis access for patients with end stage renal disease.
Keywords: Blood vessel prosthesis, Haemodialysis, Regenerative medicine, Tissue engineering, Vascular access
Highlights
-
•
This long term follow up assessed conduit status in patients who continued dialysis with an HAV.
-
•
At month 60, one patient maintained primary patency, and 10 maintained secondary patency.
-
•
Secondary patency was estimated at 58.2% at five years, after censoring for deaths and withdrawals.
-
•
No HAV conduit infections were reported during follow up.
-
•
The HAV provides long term, durable and functional haemodialysis access for patients with ESRD.
Introduction
End stage renal disease (ESRD) patients who are not candidates for autogenous fistulas or whose fistulas have failed, rely on synthetic arteriovenous (AV) grafts (e.g., expanded polytetrafluoroethylene [ePTFE]) for vascular access. However, synthetic grafts have higher infection and stenosis rates than AV fistulas.1, 2, 3, 4, 5, 6 Loss of primary unassisted patency in ePTFE grafts occurs in up to 75% of patients by one year,6 and long term patency rates are approximately 27% at five years.7 Graft and fistula failure can force patients to rely on central venous catheters, which are associated with higher rates of infection, all cause mortality, and cardiovascular events.8,9
Biological alternatives may offer improved benefits over other grafts; however, to date, no biological conduits (e.g., xenografts such as Artegraft™ [LeMaitre Vascular, Burlington, MA, USA] and ProCol™ [LeMaitre]) have gained wide clinical adoption.3,10, 11, 12 The human acellular vessel (HAV), a novel bioengineered alternative for dialysis access, has been reported previously.13, 14, 15, 16, 17, 18
The clinical use of the HAV was reported in 60 ESRD subjects over one year (40 in Poland, 20 in the USA) from two phase II trials (NCT01744418 and NCT01840956).13 Since then, the patients have continued routine dialysis with the HAV. A long term follow up to 10 years post-implantation was added for the original Polish 40 patient cohort (NCT01744418). (For the 20 patient US cohort, Institutional Review Board approval for an extended follow up beyond two years was not requested.) The objective of this report was to provide data on the long term function of HAVs that were implanted into haemodialysis subjects from the Polish trial (NCT0174418), for at least five years of follow up. To the best of the authors’ knowledge, this is the longest follow up of any engineered human connective tissue used in a clinical setting.
Materials and methods
Study design and participants
Forty patients (aged 18–80 years) with ESRD were enrolled at three hospitals in Poland and assessed at 24 months.13 Patients in need of haemodialysis access and who were not suitable candidates for an autogenous fistula were enrolled in this phase II study; patients were enrolled in two cohorts based on cannulation at two months (cohort 1) and one month (cohort 2) post-implantation.
Patients with patent HAVs at month 24 were invited to participate in long term follow up assessments, including reported patency, interventions, revisions, and histological assessment when appropriate (no HAV underwent intentional investigational biopsy for histology) every three months (±4 weeks), starting at month 27. Patients discontinued follow up if they suffered a loss of secondary patency, underwent complete conduit removal or abandonment, or if a censoring event (i.e., death, renal transplantation, or withdrawal) resulted in discontinued dialysis with the HAV. Radiological and ultrasound assessments were not pre-specified, but were collected for some subjects at physician discretion. All patients who continued long term follow up retained use of the HAV for routine haemodialysis access.
The study was conducted according to Good Clinical Practice guidelines and the principles set forth in the Declaration of Helsinki. The ethics committee of each participating clinical centre approved the long term follow up protocol amendment and informed consent form. Patients provided separate written informed consent for long term follow up at the final, month 24 visit of the main study.
Investigational product and implantation
As previously reported, HAVs measured 6 mm × 35–42 cm, and were produced in vitro.13 Vessels were implanted in the upper arm (above the elbow) between December 2012 and April 2014.13
Objectives and statistical analysis
The objectives of this five year follow up evaluation were to assess HAV patency and determine long term function and usability of the vessel for haemodialysis access. Primary patency, primary assisted patency, and secondary patency were previously defined.13 In the event of surgical revision, the conduit was considered to remain patent so long as a portion of the HAV was available for needle cannulation for haemodialysis.
Histological analysis
During routine surgical interventions, small sections of HAVs were explanted and analysed using previously described histological methods.19 Primary antibodies directed against smooth muscle cell (SMC) markers, alpha smooth muscle actin (αSMA) and calponin 1 (CNN1), or endothelial cell markers, CD31 and CD34, were visualised through fluorescently labelled secondary antibodies.
Ultrasound and imaging
After the two year initial study, some of the HAV implants underwent non-study mandated ultrasound or angiographic imaging, at the discretion of the investigator.
Results
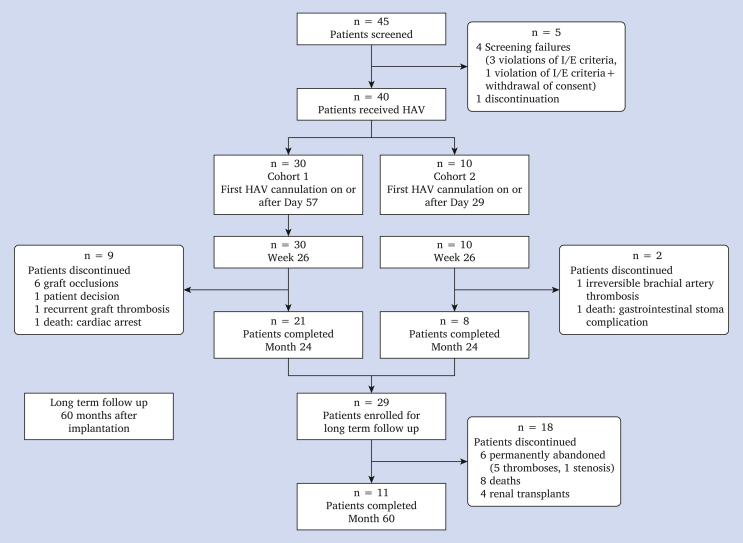
Of the 40 included patients, 29 completed the month 24 visit with patent HAVs and consented to long term follow up. Patient demographics and disposition are shown in Supplementary Table S1 and in Figure 1.
Figure 1.
Patient disposition (CONSORT diagram). HAV = human acellular vessel; I/E = inclusion/exclusion.
At month 60, total HAV exposure was 133.5 subject years for all patients, including 119.9 subject years for the 29 patients who entered into long term follow up. Overall, 18 of the 29 patients discontinued between months 24 and 60 due to permanent vessel abandonment (n = 6), death (n = 8), or renal transplantation (n = 4). There were no indications that the HAV was related to any reported patient death.
At month 60, 11 patients continued to be actively contacted, including 10 who retained patency and continued to dialyse using the HAV. One patient had received a kidney transplant that was not yet fully functional, and remained in the study with the HAV being followed, as future haemodialysis remained a possibility.
Twenty-two (76%) of the 29 enrolled patients lost primary patency during the main portion of the study (at or before month 24). At month 60, one patient maintained primary patency, and 10 maintained secondary patency.
The percentage of patients reported to have required procedures at each contact from month 27 to month 60 ranged from 6.3% to 30.3% for thrombosis and from 6.3% to 26.9% for stenosis. The mean number of interventions ranged from 0.1 to 0.7 at each contact and were comparable over time. Rates for all interventions across all visits ranged from 0.86 to 2.31 per subject year.
There were no reports of HAV conduit infection during the follow up period from month 24 to month 60. Infection at the puncture site was recorded in two subjects, and was unrelated to the HAV.
One kidney transplant was reported prior to month 24, and four patients (14%) received kidney transplants during follow up, ranging from 3.1 to 4.6 years after implantation of the HAV. Evaluation of prior panel reactive antibody (PRA) values that were obtained during the first 24 months of follow up showed that none of the patients demonstrated any new elevations in PRA values directed against major histocompatibility complex (MHC)-I or MHC-II at six months post-implantation. The occurrence of successful kidney transplantation after up to 4.6 years of HAV use may suggest a lack of immune response in patients dialysing long term with an HAV, although patient numbers were small in this dataset.
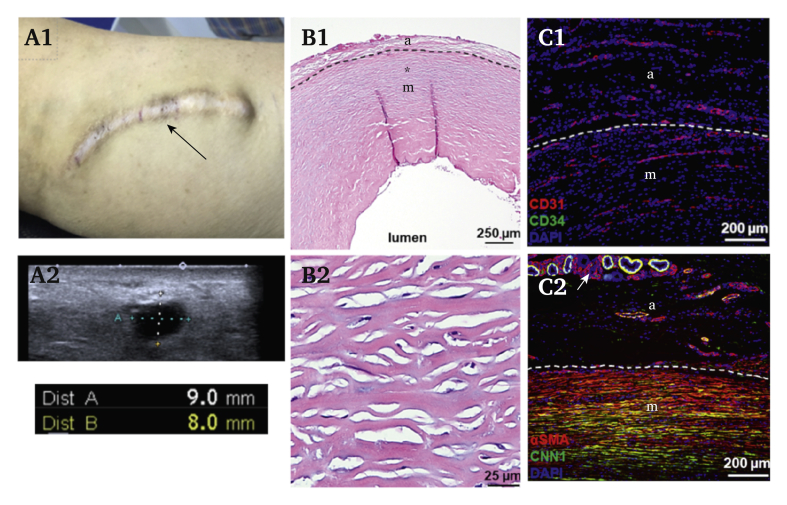
Ultrasound and imaging data were collected for three patients who were using the HAV for dialysis during follow up: a 49 year old woman, a 78 year old woman, and a 43 year old woman at 6, 6.5, and 5.5 years after HAV implantation, respectively (representative images in Fig. 2; note, data were taken beyond month 60 but were included as they provide the longest term data available). In two patients, follow up ultrasounds indicated minimal dilatation of the HAV (5.8–7 mm inner diameter). In one patient, after 6.5 years, varying levels of dilatation were noted through the conduit length, with the inner diameter ranging from 8 to 16 mm in cannulation zones. However, the conduit maintained structural integrity and was functional for haemodialysis.
Figure 2.
Six years of haemodialysis using a human acellular vessel. (A1) Skin over the human acellular vessel (HAV), which has been used for haemodialysis for approximately six years (subject 02-010); black arrow indicates a cannulation zone subjected to needle punctures over the years. (A2) Ultrasound images of the vessel from the same patient, with the conduit diameter being 8–9 mm, greater than the original implantation inner diameter of 6 mm (note cursors extend outside the vessel lumen). (B1, B2) HAV cross sections at 122 weeks. (C1) Immunofluorescence staining in samples explanted at 200 weeks for endothelial progenitor marker CD34 (green) and the endothelial maker CD31 (red) demonstrate CD31+ endothelial cells with neovascularisation present in both the adventitial and medial layer of the HAV. (C2) Immunofluorescence staining for alpha smooth muscle actin (αSMA; red) and calponin 1 (CNN1; green), markers associated with vascular smooth muscle cells, in samples explanted at 200 weeks. In (C1) and (C2) images, nuclei (blue) were counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
Two HAV segments were obtained from two subjects: at 28 months (explanted near the venous anastomosis) from a 59 year old woman due to a vascular graft thrombosis from a central vein occlusion, and at 46 months (explanted at the mid HAV site) from a 35 year old man during a cannulation site aneurysm resection. The 28 month and 46 month explants are shown in Figure 2. In explanted samples, there was no evidence of vessel wall thinning, degradation, lymphocytic infiltration associated with immunological recognition, or evidence of multinucleated foreign body giant cells associated with a chronic host inflammatory response. These observations are consistent with prior publications documenting a re-populating vascular implant that does not stimulate immunity and maintains its physical structure over time.13,14,20
Haematoxylin and eosin staining revealed progressive re-population of the HAV with host cells after implantation (Fig. 2). An expanding adventitial layer with neovascularisation, as well as a cellular medial layer, are established over time within the once acellular HAV. Immunohistochemical staining identified a substantial number of αSMA+ cells consistent with vascular SMCs, primarily within the medial layer of the vessel and in surrounding microvascular structures (Fig. 2C). The density, circumferential elongation, and co-expression of the mature contractile SMC marker CNN1 were notably increased with implant duration, as shown in the 46 month explant tissue sample. CD31+ (platelet endothelial cell adhesion molecule-1) expression, often associated with vascular endothelial cells was highly expressed within the 46 month explants. The CD31+ expression was mostly observed in cells forming microvessels within the adventitial and medial layers of the HAV but not within the endothelial layer of the 46 month explant samples evaluated. Whether luminal CD31+ cells existed previously but were displaced during angioplasty/thrombectomy and surgical intervention is unknown.
Discussion
The goal of this long term follow up was to assess the functional durability of the HAV when used as an access conduit. Durability for dialysis is a clinically important measure, given that access failures lead to morbidity, hospitalisation, and sometimes death in ESRD patients.8
In this long term follow up report, patients with a patent HAV access for haemodialysis were followed quarterly for at least five years post-implantation. This report contains long term follow up data from the phase II, “first in man” experience with the HAV used for haemodialysis access.13 Patients were implanted from December 2012 to May 2014. At the time of study design and enrolment, because it was not known whether the HAV would be durable when repeatedly punctured for haemodialysis access, the original protocol specified 24 months of follow up. However, at 24 months, well over half of the patients continued using the HAV for haemodialysis, and follow up was extended to obtain information on long term clinical durability. This report is an interim summary of HAV function in the first 40 patients in Poland to receive the HAV for haemodialysis access, five years after implantation. For patients with a patent HAV at five years, follow up will continue up to 10 years.
Eight patients died during the long term follow up, in addition to two who died before 24 months. A total of 10 deaths over 133.5 subject years equates to a mortality rate of 7.5% per patient year, which is below the typical mortality rates reported for ESRD patients.21 Deaths during the study were attributed to known and common causes of death in haemodialysis patients (i.e., cardiac arrest, intestinal stoma complications, sepsis, and other medical comorbidities) and in no case were deemed related to the HAV.
Available imaging supports the durability and structural integrity of the HAV, even at five years post-implantation, although the clinical ultrasound data are limited. From information derived from quarterly surveys, the currently available information indicates no serious safety signals regarding the long term use of the HAV for haemodialysis. There were no reports of conduit infections during follow up, from 24 to 60 months, supporting a low risk of infection with long term use. Of seven patients who suffered permanent HAV abandonment between months 24 and 60, the most frequent cause was thrombosis. No difference in the durability of HAVs that were cannulated either one or two months post-implantation was seen.
HAV remodelling over time, evaluated by histological methods, has been reported previously.13,17,19,20 Additional imaging from explants taken during this follow up continue to support an important integration of host cells over time during dialysis use. Microvessel formation near and within the vessel wall is accompanied by dense re-population by αSMA+ and CNN1+ cells, most pronounced 46 months post-implantation. Cells not only re-populated the HAV, but also remodelled the implant into vascularised adventitial and medial tissue layers. Collectively, this indicates that the HAV evolves after implantation and adopts characteristics similar to the patients' native blood vessels.
Results from this follow up study represent the longest assessment of any engineered functional vascular connective tissue; however, there are limitations. Firstly, patients and their physicians were queried every three months regarding usability of the HAV for dialysis, the occurrence of interventions and failures, and other serious adverse events related to the HAV. Routine imaging was not obtained after 24 months, and routine bloodwork was not done. Hence, this report is primarily a clinical report of the long term functionality of a novel engineered blood vessel when used for dialysis access, and quantitative imaging data are limited. Only patients who had a patent conduit at 24 months underwent long term follow up and are reported in this dataset. Therefore, long term data on patients who had a thrombosed or abandoned HAV were not collected.
The evaluation of the HAV in haemodialysis access is ongoing; there are two phase III trials underway (NCT02644941 and NCT03183245).
In conclusion, the HAV warrants ongoing evaluation as a bioengineered vascular conduit for long term dialysis access. Clinical outcomes in this small patient cohort support durability and low infection risk. Histological evaluations support ongoing host cell remodelling to produce a vessel similar to a native artery. Although limited in patient number, these results support the ongoing randomised controlled clinical studies of the HAV in dialysis access.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ejvsvf.2022.01.003.
Conflicts of interest
L.E.N., J.H.L., and A.J.P. own stock or stock options in Humacyte. The other authors declare no competing interests.
Funding
Funding was provided by Humacyte, Inc.
Acknowledgements
The authors thank Mauricio Berdugo, Kimberly Sauls, Keri Hamilton, Heather Prichard, Robert Kirkton, William Tente, Kaleb Naegeli, Marek Ilzecki MD, PhD, Wojciech Witkiewicz MD, PhD, and Malgorzata Guziewicz.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Almasri J., Alsawas M., Mainou M., Mustafa R.A., Wang Z., Woo K., et al. Outcomes of vascular access for hemodialysis: a systematic review and meta-analysis. J Vasc Surg. 2016;64:236–243. doi: 10.1016/j.jvs.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 2.Akoh J.A., Patel N. Infection of hemodialysis arteriovenous grafts. J Vasc Access. 2010;11:155–158. doi: 10.1177/112972981001100213. [DOI] [PubMed] [Google Scholar]
- 3.Katzman H.E., Glickman M.H., Schild A.F., Fujitani R.M., Lawson J.H. Multicenter evaluation of the bovine mesenteric vein bioprostheses for hemodialysis access in patients with an earlier failed prosthetic graft. J Am Coll Surg. 2005;201:223–230. doi: 10.1016/j.jamcollsurg.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 4.Arhuidese I., Orandi B., Nejim B., Malas M. Utilization, patency, and complications associated with vascular access for hemodialysis in the United States. J Vasc Surg. 2018;68:1166–1174. doi: 10.1016/j.jvs.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 5.Beathard G.A. The treatment of vascular access graft dysfunction: a nephrologist's view and experience. Adv Ren Replace Ther. 1994;1:131–147. doi: 10.1016/s1073-4449(12)80044-6. [DOI] [PubMed] [Google Scholar]
- 6.Dixon B.S., Beck G.J., Vazquez M.A., Greenberg A., Delmez J.A., Allon M., et al. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360:2191–2201. doi: 10.1056/NEJMoa0805840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S., Lok C., Arnold R., Rajan D., Glickman M. Comparison of post-creation procedures and costs between surgical and an endovascular approach to arteriovenous fistula creation. J Vasc Access. 2017;18(Suppl. 2):8–14. doi: 10.5301/jva.5000723. [DOI] [PubMed] [Google Scholar]
- 8.Ravani P., Palmer S.C., Oliver M.J., Quinn R.R., MacRae J.M., Tai D.J., et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol. 2013;24:465–473. doi: 10.1681/ASN.2012070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allon M. Dialysis catheter-related bacteremia: treatment and prophylaxis. Am J Kidney Dis. 2004;44:779–791. [PubMed] [Google Scholar]
- 10.Pashneh-Tala S., MacNeil S., Claeyssens F. The tissue-engineered vascular graft-past, present, and future. Tissue Eng Part B Rev. 2016;22:68–100. doi: 10.1089/ten.teb.2015.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAllister T.N., Maruszewski M., Garrido S.A., Wystrychowski W., Dusserre N., Marini, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–1446. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 12.Salacinski H.J., Punshon G., Krijgsman B., Hamilton G., Seifalian A.M. A hybrid compliant vascular graft seeded with microvascular endothelial cells extracted from human omentum. Artif Organs. 2001;25:974–982. doi: 10.1046/j.1525-1594.2001.06716.x. [DOI] [PubMed] [Google Scholar]
- 13.Lawson J.H., Glickman M.H., Ilzecki M., Jaroszynski A., Peden E.K., Pilgrim A.J., et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. 2016;387:2026–2034. doi: 10.1016/S0140-6736(16)00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahl S.L.M., Kypson A.P., Lawson J.H., Blum J.L., Strader J.T., Li Y., et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3:68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 15.Kirkton R.D., Prichard H.L., Santiago-Maysonet M., Niklason L.E., Lawson J.H., Dahl S.L.M. Susceptibility of ePTFE vascular grafts and bioengineered human acellular vessels to infection. J Surg Res. 2018;221:143–151. doi: 10.1016/j.jss.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 16.Niklason L.E., Gao J., Abbott W.M., Hirschi K.K., Houser S., Marini R., et al. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 17.Dahl S.L.M., Koh J., Prabhakar V., Niklason L.E. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659–666. [PubMed] [Google Scholar]
- 18.Poh M., Boyer M., Solan A., Dahl S.L., Pedrotty D., Banik S.S., et al. Blood vessels engineered from human cells. Lancet. 2005;365:2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 19.Kirkton R.D., Santiago-Maysonet M., Lawson J.H., Tente W.E., Dahl S.L.M., Niklason L.E., et al. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutowski P., Gage S.M., Guziewicz M., Ilzecki M., Kazimierczak A., Kirkton R.D., et al. Arterial reconstruction with human bioengineered acellular blood vessels in patients with peripheral arterial disease. J Vasc Surg. 2020;72:1247–1258. doi: 10.1016/j.jvs.2019.11.056. [DOI] [PubMed] [Google Scholar]
- 21.United States Renal Data System . National Institute of Diabestes and Digestive Kidney Diseases; Bethesda, MD: 2018. USRDS Annual Data Report: epidemiology of kidney disease in the United States. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.