Abstract
Background:
In 2015–2016, Mongolia experienced an unexpected large measles outbreak affecting mostly young children and adults. After two nationwide vaccination campaigns, measles transmission declined. To determine if there were any remaining immunity gaps to measles or rubella in the population, a nationally representative serosurvey for measles and rubella antibodies was conducted after the outbreak was over.
Methods:
A nationwide, cross-sectional, stratified, three-stage cluster serosurvey was conducted in November-December 2016. A priori, four regional strata (Ulaanbaatar, Western, Central, and Gobi-Eastern) and five age strata (6 months-23 months, 2–7 years, 8–17 years, 18–30 years, and 31–35 years) were created. Households were visited, members interviewed, and blood specimens were collected from age-appropriate members. Blood specimens were tested for measles immunoglobulin G (IgG) and rubella IgG (Enzygnost® Anti-measles Virus/IgG and Anti-rubella Virus/IgG, Siemens, Healthcare Diagnostics Products, GmbH Marburg, Germany). Factors associated with seropositivity were evaluated.
Results:
Among 4598 persons aged 6 months to 35 years participating in the serosurvey, 94% were measles IgG positive and 95% were rubella IgG positive. Measles IgG seropositivity was associated with increasing age and higher education. Rubella IgG seropositivity was associated with increasing age, higher education, smaller household size, receipt of MMR in routine immunization, residence outside the Western Region, non-Muslim religious affiliation, and non-Kazakh ethnicity. Muslim Kazakhs living in Western Region had the lowest rubella seroprevalence of all survey participants.
Conclusions:
Nationally, high immunity to both measles and rubella has been achieved among persons 1–35 years of age, which should be sufficient to eliminate both measles and rubella if future birth cohorts have ≥ 95% two dose vaccination coverage. Catch-up vaccination is needed to close immunity gaps found among some subpopulations, particularly Muslim Kazakhs living in Western Region.
Keywords: Measles, Rubella, Seroprevalence, Mongolia
1. Introduction
Immunization programs usually monitor population immunity to vaccine-preventable diseases (VPDs) by estimating vaccination coverage, but coverage assessments can be inaccurate because of challenges in determining the denominator (i.e., target population size) and numerator (e.g., variability in reporting doses given to target population, counting doses given to older-aged children outside the target age group). Monitoring population immunity to VPDs through seroprevalence studies can be an additional tool to identify subgroups with higher susceptibility and provide evidence to guide national immunization policies. Standard enzyme-linked immunosorbent assays (ELISAs) that measure immunoglobulin G (IgG) can be used as good proxies for gold-standard assays to assess pre-existing immunity for measles and rubella because they have been shown to be specific [1–5]. The gold-standard tests for measles immunity (i.e., plaque reduction neutralization test (PRNT)) and rubella immunity (i.e., hemagglutination inhibition test) are complicated, time-consuming, and costly [5,6].
In 2014, Mongolia was verified as having eliminated measles based on the absence of measles cases during 2011–2014, high 2-dose measles vaccination coverage that was presumed to equate to high population immunity, and high-quality measles surveillance [7,8]. The road to elimination in Mongolia started in 1973, when monovalent measles vaccine was introduced at age 9 months, with a second dose added at age 24 months in 1989 [9]. In 2009, the schedule was switched from monovalent measles vaccine to measles-mumps-rubella (MMR) vaccine. Over the years, multiple supplementary immunization activities (SIAs) were conducted to close immunity gaps among children who received < 2 routine vaccination doses and to control a large rubella outbreak in the early 2000s (Fig. 1).
Fig. 1.
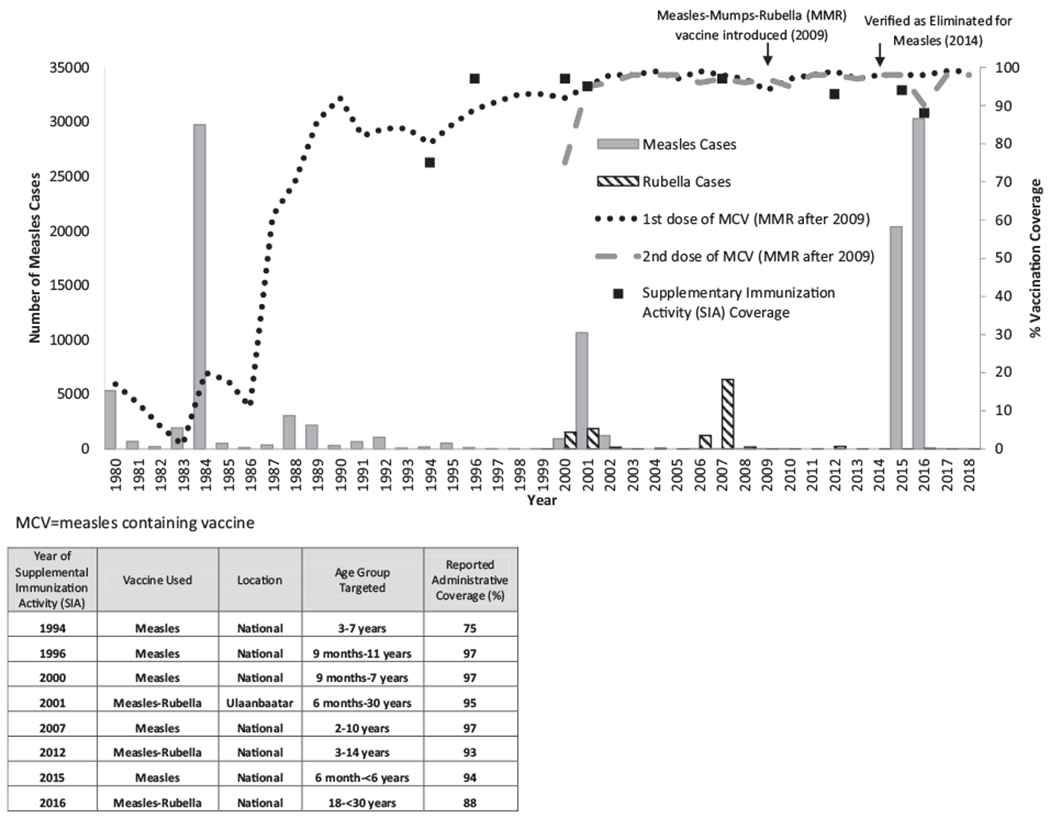
Measles and rubella vaccination coverage and reported measles and rubella cases, Mongolia, 1980–2018 [31].
Despite these efforts, Mongolia experienced a measles outbreak in 2015–2016, with 53,737 reported cases, which equates to a 2-year incidence of 17,813 per million persons, mostly among children < 6 years of age and young adults 18–29 years of age [10]. To stop the outbreak, a nationwide SIA with monovalent measles vaccine was conducted in May-June 2015, targeting children aged 6 months-5 years (born from May 1, 2009-November 1, 2014). Subsequently, a nationwide SIA with measles-rubella (MR) vaccine was conducted in May-June 2016, targeting persons 18–29 years of age (born from May 1, 1986-May 1, 1998). For both SIAs, target age groups were chosen based on the age distribution of cases in the outbreak and available resources. In order to guide further vaccination efforts to re-achieve measles elimination, a serosurvey was conducted to determine if a measles or rubella immunity gaps existed after the two outbreak response SIAs [11].
2. Materials and methods
A nationwide, cross-sectional, stratified, three-stage cluster serosurvey was conducted in November-December 2016. The design included four regional strata (Ulaanbaatar, Western, Central, and Gobi-Eastern) and five age strata (6–23 months, 2–7 years, 8–17 years, 18–30 years, and 31–35 years). Age groups were defined based on historical vaccination opportunities (routine immunization and SIAs) and on burden of disease during the 2015–2016 measles outbreak. Specifically, those 6–23 months had a high measles burden and were not targeted in the 2015 nor 2016 SIA; 2–7 year-olds were targeted in 2015 SIA; 8–17 year-olds had a relatively low measles incidence and were targeted in historical SIAs; 18–30 year-olds had a very high burden during this outbreak and were targeted in the 2016 SIA; 31–35 year-olds had a high measles burden but less than the 18–30 year olds and were not targeted in the 2016 SIA.
2.1. Sample size
For the four oldest age groups in each region, a minimum effective sample size of 117 was calculated assuming 90% seroprevalence, precision of ± 7.3% and a two-sided α = 0.05. This was inflated to a minimum target of 210 to account for an average cluster size of 7, intra-class correlation = 0.1, 10% non-response, and to enroll seven persons per age-stratum in each of the 30 clusters per region. For the 6–23-month-old group, the precision was set at ± 8%, α = 0.05, the average cluster size to 5, intra-class correlation = 0.1, and 10% non-response resulting in a sample size of 156. To achieve the target sample size, it was estimated that 30 clusters of 60 households (1800 households in total) would need to be sampled in Ulaanbaatar, and 30 clusters of 47 households (1410 households) in each of the other three regional strata, based on the 2010 census. Thus, in each regional stratum, the target enrollment, accounting for 30 clusters, was 996 persons, or 3984 for the total survey.
2.2. Sampling
In each regional stratum, 30 baghs (i.e., villages) were selected from the 2015 national midterm census frame using probability proportional to estimated size, using the number of households as the size measure. For the second stage, households were randomly selected from an updated list of households having at least one person < 36 years old from each selected bagh. For the third stage, a list of eligible individuals, defined as those aged 6 months to 35 years living in the household for at least 6 months, was completed in each selected household. Eligible household members included relatives from different families who lived in the household and members who had temporally left for study or work but were still registered with the household. One person per age stratum per household was randomly selected, so several persons could be selected from a single household. To avoid oversampling in three age strata (2–7 years, 8–17 years and 18–30 years), a random sample of one-third of all households selected had participants enrolled from these age groups. Selected participants were excluded if they could not give blood because of severe illness or hemophilia.
2.3. Data collection
Consent was requested from adults and from parents or caregivers of children before participation in the serosurvey; assent was requested from those 10–17 years of age. After consent was obtained, a brief questionnaire was administered to collect demographic data and vaccination history based on age. For children 12–35 months of age, information on vaccines received during routine immunization services and SIAs was collected. For those from 2 to 7 years of age, data were collected on measles vaccine receipt in the 2015 SIA. For those from 18 to 30 years of age, data were collected on measles-rubella vaccine receipt in the 2016 SIA. If documented vaccination history was not available, vaccination history based on recall was obtained.
2.4. Specimen collection and laboratory testing
Approximately 2 ml of blood were collected from those 6–23 months of age, and 7 ml of blood were collected by venipuncture from those 2 to 35 years old. Serum was separated in the field, then stored, and subsequently transported at 2–8° C to the national laboratory within 7 days of blood collection. All samples were tested by ELISA for measles and rubella IgG (Enzygnost® Anti-measles Virus/IgG and Anti-rubella Virus/IgG, Siemens, Healthcare Diagnostics Products, GmbH Marburg, Germany) as per the manufacturer’s instructions. Test results were interpreted according to manufacturer’s instructions. The following assay cut-off values were used: for measles, samples with titers ≥ 150 mIU/mL were considered positive, which corresponded to 0.1 ΔA (corrected optical density), the lower cut-off for the assay, and 0.1 < ΔA < 0.2, which according to the kit insert is considered equivocal, was considered positive as well; for rubella, titers ≥ 4 mIU/mL were positive, and the same approach for interpreting equivocal results was adopted (ΔA = 0.1 corresponds to 4.0 IU/mL). The rationale for considering measles equivocal results (0.1 < ΔA < 0.2) was based on the correlation found between PRNT and IgG serology, where all sera in the ELISA equivocal range were positive in the PRNT [3,12]. A random sample of 10% of specimens was sent to U.S. Centers for Disease Control and Prevention (US CDC) for measles confirmatory testing (Enzygnost® Anti-measles Virus/IgG, Siemens, Healthcare Diagnostics Products, GmbH Marburg, Germany), where discordance of test results from both laboratories was based on qualitative results. If > 10% of samples tested at US CDC were discordant with results obtained in Mongolia, all samples were to be retested.
2.5. Data management/analysis
Data were collected on tablets with pre-set electronic data entry forms. Data were analyzed with STATA® SE 14.1 (College Station, TX, USA) and SAS v9.4 (Cary, NC, USA). Sampling weights were calculated based on the sampling probability of each participant, adjusted for non-response, and the underlying population structure from the 2010 census. Estimation of proportions and 95% logit confidence intervals (CI) using the Taylor series method accounted for the regional stratification, first stage clusters, and sampling weights. Percent differences between sub-populations and a referent group, and corresponding 95% CI, were calculated to assess whether seroprevalence varied in the population. A 95% CI of the difference that did not include zero was considered statistically significant. Descriptive results were given for some subpopulations that were programmatically relevant; however, the sample in this survey was too small to obtain reliable estimates. Of note, results for 6–11 month-olds were removed or presented separately in some analyses because some of them were ineligible to receive the first dose of MMR and thus a lower seroprevalence was expected among this age group.
2.6. Human subjects’ rights and ethics
Informed consent was obtained from participants or caregivers before testing. The study protocol was approved by the Medical Ethics Committee of the Ministry of Health of Mongolia and the Ethics Review Committee at the WHO Regional Office for the Western Pacific. This activity was reviewed in accordance with CDC human research protection procedures and was determined to be research, but CDC involvement did not constitute engagement in human subjects’ research; therefore, it did not require CDC IRB approval.
3. Results
Of the 6030 selected households, 136 (2%) had moved or were absent, so 5894 (98%) households were visited. Of these, members in 156 (3%) refused participation, or the household had no adult respondent present at the time of the survey, or the household was excluded for another reason. Of the remaining 5738 households, 3420 (60%) households had at least one eligible individual; the household questionnaire was completed for 3404 (99.5%) of these households. Among 15,893 age-eligible household members, 5834 (37%) were selected; of these, 5732 (98%) completed the interview, 4632 (79%) had their blood collected, and 4598 (79%) had blood tested (Fig. 2). Differences existed between serosurvey participants and nonparticipants (both those who did not complete the questionnaire and those who did not have IgG results) (Table 1). Participants were more likely to be female, live in rural areas, and be less educated. Among 12–35 month old children, participants were more likely to have vaccination cards and have received ≥ 1 dose of MMR vaccine through routine immunization services; and among age-eligible persons, participants were more likely to be vaccinated in the 2015 and 2016 SIAs. Participants were less likely to be 6–23 months or 18–30 years of age, live in Ulaanbaatar, or be Khalkh ethnicity (Table 1).
Fig. 2.
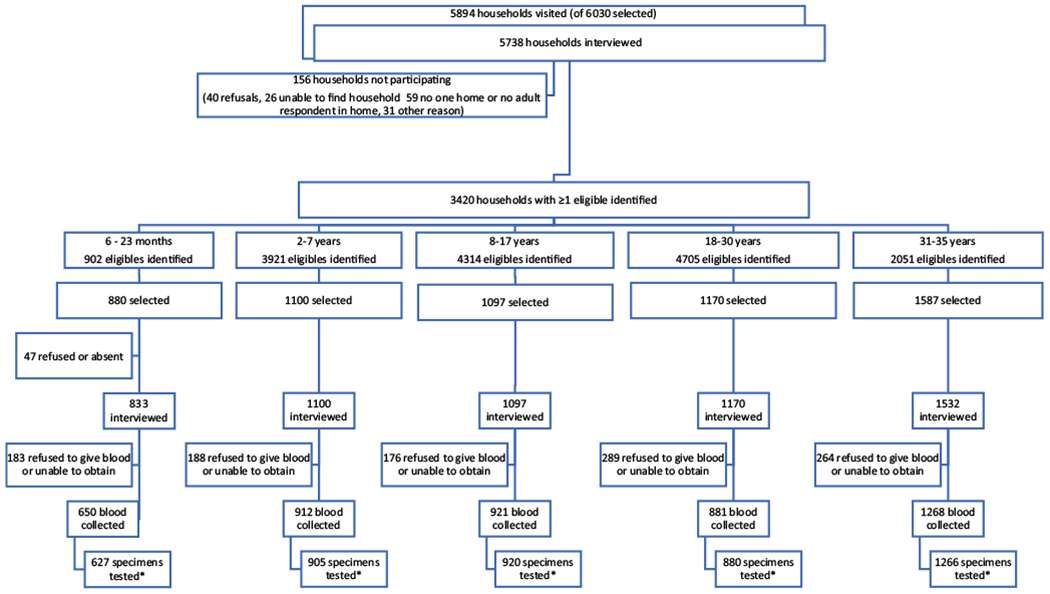
Participation by households and individuals in the interview and in blood collection by age strata, Mongolia, 2016.
*Collected blood could not be tested because the quantity of serum was insufficient for testing
Table 1.
Characteristics of selected individuals (n = 5834) who participated and did not participate in the serosurvey. Mongolia, 2016.
| Non-participants |
Participants in serosurvey |
|||||
|---|---|---|---|---|---|---|
| n | N | % | n | N | % | |
| Total | 1236 | 5834 | 21% | 4598 | 5834 | 79% |
| Sex | ||||||
| Male | 244 | 1236 | 20% | 1010 | 4598 | 22% |
| Female | 185 | 1236 | 15% | 1136 | 4598 | 25% |
| Unknown1 | 807 | 1236 | 65% | 2452 | 4598 | 53% |
| Age | ||||||
| 6–11 months | 161 | 1236 | 13% | 154 | 4598 | 3% |
| 12–23 months | 92 | 1236 | 7% | 473 | 4598 | 10% |
| 2–7 years | 195 | 1236 | 16% | 905 | 4598 | 20% |
| 8–17 years | 177 | 1236 | 14% | 920 | 4598 | 20% |
| 18–30 years | 290 | 1236 | 23% | 880 | 4598 | 19% |
| 31–35 years | 321 | 1236 | 26% | 1266 | 4598 | 28% |
| Region | ||||||
| Western | 203 | 1236 | 16% | 1164 | 4598 | 25% |
| Central | 155 | 1236 | 13% | 1116 | 4598 | 24% |
| Govi-Eastern | 136 | 1236 | 11% | 1147 | 4598 | 25% |
| Ulaanbaatar | 742 | 1236 | 60% | 1171 | 4598 | 25% |
| Residence | ||||||
| Urban | 896 | 1236 | 72% | 2218 | 4598 | 48% |
| Rural | 340 | 1236 | 28% | 2380 | 4598 | 52% |
| Religion | ||||||
| No religion | 400 | 1236 | 32% | 1378 | 4598 | 30% |
| Buddhist | 699 | 1236 | 57% | 2660 | 4598 | 58% |
| Muslim | 102 | 1236 | 8% | 387 | 4598 | 8% |
| Other | 34 | 1236 | 3% | 173 | 4598 | 4% |
| Unknown | 1 | 1236 | 0% | – | – | – |
| Ethnic group | ||||||
| Khalkh | 998 | 1236 | 81% | 3456 | 4598 | 75% |
| Kazakh | 108 | 1236 | 9% | 415 | 4598 | 9% |
| Buriad | 20 | 1236 | 2% | 85 | 4598 | 2% |
| Other | 109 | 1236 | 9% | 642 | 4598 | 14% |
| Unknown | 1 | 1236 | 0% | – | – | – |
| Highest education level (or maternal education level for children) | ||||||
| No education | 151 | 1236 | 12% | 797 | 4598 | 17% |
| Primary/lower secondary | 174 | 1236 | 14% | 1074 | 4598 | 23% |
| Higher secondary/vocational | 298 | 1236 | 24% | 804 | 4598 | 17% |
| Diploma/tertiary | 217 | 1236 | 18% | 660 | 4598 | 14% |
| Unknown | 396 | 1236 | 32% | 1263 | 4598 | 27% |
| Household size | ||||||
| 1–3 members | 324 | 1236 | 26% | 1030 | 4598 | 22% |
| 4–5 members | 681 | 1236 | 55% | 2731 | 4598 | 59% |
| ≥6 members | 230 | 1236 | 19% | 837 | 4598 | 18% |
| Unknown | 1 | 1236 | 0% | – | – | – |
| Card available to review vaccination data (among 12–35 months) | 45 | 169 | 27% | 368 | 616 | 60% |
| Number of measles doses delivered by routine immunization (among 12–35-month-olds) | ||||||
| 0 doses | 17 | 169 | 10% | 71 | 616 | 12% |
| 1 dose | 44 | 169 | 26% | 361 | 616 | 59% |
| 2 doses | 27 | 169 | 16% | 117 | 616 | 19% |
| Unknown | 81 | 169 | 48% | 67 | 616 | 11% |
| Vaccinated in 2015 SIA (among those age-eligible) | 101 | 428 | 24% | 786 | 1403 | 56% |
| Vaccinated in 2016 SIA (among those age-eligible) | 121 | 290 | 42% | 651 | 880 | 74% |
Data was collected electronically, and there was a problem where many responses were unable to be recorded properly.
For quality assurance, 460 samples were randomly tested for measles IgG at US CDC, of which 20 (4%) were discordant, and thus the results from in-country testing were considered valid. Nationally, of 4598 persons tested, 4188 [94% (95% CI: 93%-95%)] were measles IgG positive (Table 2). Measles IgG seroprevalence among 2–7 year-olds, 18–30 year-olds, and 31–35 year-olds was significantly higher (5%–8%) than among 12–23 month-olds. Among children aged 8–17 years, however, measles IgG seroprevalence was only 89% (95% CI: 87%–92%), not statistically different from the seroprevalence among 12–23 month-olds. Those with at least a higher secondary education level were more likely to be measles IgG seropositive. Among 12–35 month-old children, those vaccinated with ≥ 1 dose of measles vaccine had higher measles IgG seropositivity (94%) than those who were unvaccinated (87%), but the difference was not statistically significant. Of 786 age-eligible children who were vaccinated in the 2015 SIA, 739 [96% (95% CI: 92%–98%)] were measles IgG seropositive, compared with 297 [84% (95% CI: 72%–92%)] of 344 age-eligible children who were not vaccinated in the 2015 SIA (difference 11%, 95% CI: 3%–20%). Among 18–30-year-old participants, there was no difference in measles IgG seropositivity among those who were vaccinated in the 2016 SIA (98%) compared to those who were not (96%). Measles IgG seroprevalence did not differ by region, rural/urban residence, household size, religion or ethnicity.
Table 2.
Measles and rubella immunoglobulin G (IgG) by age, region, and sociodemographic factors – Mongolia, 2016 (n = 4598).
| Number of samples tested |
Measles
|
Rubella
|
|||||
|---|---|---|---|---|---|---|---|
| # IgG positive | Weighted % (95% CI) | Difference in seroprevalence compared to reference group (95% CI) | # IgG positive | Weighted % (95% CI) | Difference in seroprevalence compared to reference group (95% CI) | ||
| Total | 4598 | 4188 | 94 (93–95) | N/A | 4292 | 95 (94–96) | N/A |
| Age | |||||||
| 6–11 months | 154 | 73 | 47 (35–59) | N/A | 65 | 39 (27–51) | N/A |
| 12–23 months | 473 | 408 | 90 (85–93) | REF | 426 | 93 (89–96) | REF |
| 2–7 years | 905 | 852 | 95 (92–96) | 5 (2–9) | 819 | 92 (89–95) | −1 (−4–2) |
| 8–17 years | 920 | 822 | 89 (87–92) | 0 (−5–4) | 873 | 94 (92–96) | 1 (−3–5) |
| 18–30 years | 880 | 863 | 98 (96–99) | 8 (4–12) | 867 | 98 (97–99) | 5 (2–9) |
| 31–35 years | 1266 | 1170 | 95 (93–96) | 5 (1–9) | 1242 | 99 (98–99) | 5 (2–9) |
| Region | |||||||
| Western | 1164 | 1040 | 93 (91–94) | REF | 1026 | 91 (86–94) | REF |
| Central | 1116 | 1035 | 95 (93–96) | 2 (0.2–4) | 1047 | 95 (93–97) | 4 (2–9) |
| Gobi-Eastern | 1147 | 1034 | 93 (91–95) | 0 (−2–3) | 1105 | 98 (96–98) | 7 (3–11) |
| Ulaanbaatar | 1171 | 1079 | 93 (91–95) | 0 (−2–3) | 1114 | 96 (94–98) | 5 (1–9) |
| Residence | |||||||
| Urban | 2218 | 2035 | 94 (92–95) | REF | 2106 | 96 (95–97) | REF |
| Rural | 2380 | 2153 | 94 (93–95) | 0 (−2–2) | 2186 | 94 (92–95) | −3 (−4 – −0.2) |
| Religion | |||||||
| Muslim | 387 | 334 | 91 (86–94) | REF | 293 | 81 (74–86) | REF |
| Buddhist | 2660 | 2442 | 95 (93–96) | 3 (0–8) | 2522 | 96 (95–97) | 15 (10–21) |
| No religion | 1378 | 1262 | 93 (91–95) | 2 (−2–6) | 1315 | 96 (94–97) | 15 (10–21) |
| Other | 173 | 150 | 91 (85–95) | 0 (−7–7) | 162 | 97 (95–99) | 17 (11–23) |
| Ethnic group | |||||||
| Kazakh | 415 | 361 | 93 (91–95) | REF | 318 | 83 (77–88) | REF |
| Khalkh | 3456 | 3155 | 94 (92–95) | 0 (−2–3) | 3279 | 96 (95–97) | 13 (7–19) |
| Buriad | 85 | 77 | 96 (92–98) | 3 (0–7) | 81 | 97 (91–99) | 14 (8–21) |
| Other | 642 | 595 | 95 (93–96) | 1 (−1–4) | 614 | 98 (96–99) | 15 (9–21) |
| Highest education level (or maternal education level for children) | |||||||
| No education | 797 | 711 | 90 (87–93) | REF | 752 | 95 (93–97) | REF |
| Primary/lower secondary | 1074 | 975 | 93 (91–95) | 3 (−1–7) | 1040 | 96 (93–97) | 0 (−2–4) |
| Higher secondary or technical /vocational | 804 | 768 | 97 (95–98) | 7 (3–11) | 792 | 98 (95–99) | 3 (0.2–6) |
| Diploma/tertiary | 660 | 648 | 98 (94–99) | 8 (4–11) | 652 | 100 (99–100) | 4 (2–6) |
| Household size | |||||||
| 1–3 members | 1030 | 958 | 95 (94–97) | REF | 993 | 98 (96–99) | REF |
| 4–5 members | 2731 | 2,487 | 94 (92–95) | −2 (−4–0) | 2,560 | 96 (94–97) | −2 (−4 - −0.1) |
| ≥6 members | 837 | 743 | 92 (88–95) | −3 (−7–0) | 739 | 92 (88–95) | −5 (−8 – −2) |
| MMR vaccination (routine immunization among 12-35 m) by card or recall | |||||||
| 0 doses | 71 | 56 | 87 (77–93) | REF | 48 | 78 (60–89) | REF |
| 1 + dose | 479 | 435 | 94 (90–96) | 7 (−1–16) | 453 | 96 (92–98) | 18 (4–32) |
| Unknown | 57 | 47 | 83 (58–94) | N/A | 45 | 81 (51–94) | N/A |
| Measles vaccination (supplemental immunization activity in 2015 among 6m-6y) | |||||||
| No vaccinated | 344 | 297 | 84 (72–92) | REF | 300 | 83 (67–92) | REF |
| Vaccinated (card or recall) | 786 | 739 | 96 (92–98) | 11 (3–20) | 724 | 97 (93–99) | 14 (2–26) |
| MR vaccination (supplemental immunization activity in 2016 among 18–30 y) | |||||||
| Not vaccinated | 132 | 126 | 96 (90–99) | REF | 130 | 99 (95–100) | REF |
| Vaccinated (card or recall) | 651 | 641 | 98 (96–99) | 2 (−2–6) | 642 | 99 (96–99) | 0 (−2–2) |
REF = reference group.
N/A = not applicable.
Note seroprevalence among 6–11 month olds was not compared to the referent group as it is known that many in this group are not age-eligible for vaccination and thus there should be a lower seroprevalence in this group.
Overall, 4292 [95% (95% CI: 94%–96%)] of 4598 persons tested were rubella IgG positive (Table 2). Multiple factors were significantly associated with rubella IgG seropositivity. Rubella IgG seroprevalence among persons in all age strata from 12 months-35 years was ≥ 92%. Compared to rubella IgG seroprevalence among 12–23-month-olds (93%), seroprevalence was significantly higher among those 18–30 (98%) and 31–35 years old (99%) (5% higher, 95% CI: 2%–9%). Those with a higher education level had a 3–4% higher rubella seroprevalence than those with no education (95%). Among children 12–35 months of age, 453 [95% (95% CI: 92%–98%)] of 479 who received at least one dose of vaccine were rubella IgG seropositive, compared to 48 [78% (95% CI: (60%–89%)] of 71 who had no MMR doses (difference = 18%, 95% CI: 4%–32%). Among those children eligible for the 2015 SIA, 724 [97% (95%CI 93%–99%)] of 786 who received a 2015 SIA dose of measles vaccine were rubella IgG seropositive; 300 [83% (95% CI: 67%–92%)] of 344 who were not vaccinated in the 2015 SIA were rubella IgG seropositive (difference = 14%, 95% CI: 2%–26%) (Table 2). Rubella IgG seropositivity was highest (98%) in households with 1–3 members. Rubella IgG seroprevalence was higher in Ulaanbaatar (96%), Gobi-Eastern (98%), and Central (95%) Regions compared with Western Region (91%). Higher rubella IgG seroprevalence occurred among those who were Buddhist (96%), and those who reported no religion affiliation (96%), than among Muslims (81%). Khalkhs (96%), Buriads (97%), and persons of other ethnicity (98%) had a higher rubella IgG seroprevalence compared with Kazakhs (83%).
Since a lower rubella IgG seropositivity was identified among Muslims and Kazakhs living in the Western region, further analysis of this subgroup was conducted. Among survey participants, over 99% of Muslims were Kazakhs, 93% of Kazakhs were Muslims, and 91% of Muslim Kazakhs lived in the Western Region. Among 353 Kazakh-Muslim participants living in the Western Region, 304 (86%; unweighted) were measles IgG seropositive, and 262 (74%; unweighted) were rubella IgG seropositive. The proportion of Muslim Kazakhs living in the Western Region who were rubella IgG seropositive was approximately 20% lower than the proportion who were seropositive among all other participants living in the Western region [764/811 (94%; unweighted)]. In a descriptive analysis of the Western Region, Muslim Kazakhs were enrolled in only 10 of the 30 clusters in the Western Region. In these clusters, more of the Muslim Kazakh children 1–7 years old were seronegative for rubella IgG compared with other children 1–7 years old in other clusters in the Western Region, while measles IgG seroprevalence was similar (Fig. 3). Among 1–2-year-old Muslim Kazakh children, only 19 (33%) of 57 reported receiving MMR in routine immunization services. Among 1–7 year old Muslim Kazakhs, only 58 (61%) of 95 reported receiving a dose in the 2015 SIA (data not shown).
Fig. 3.
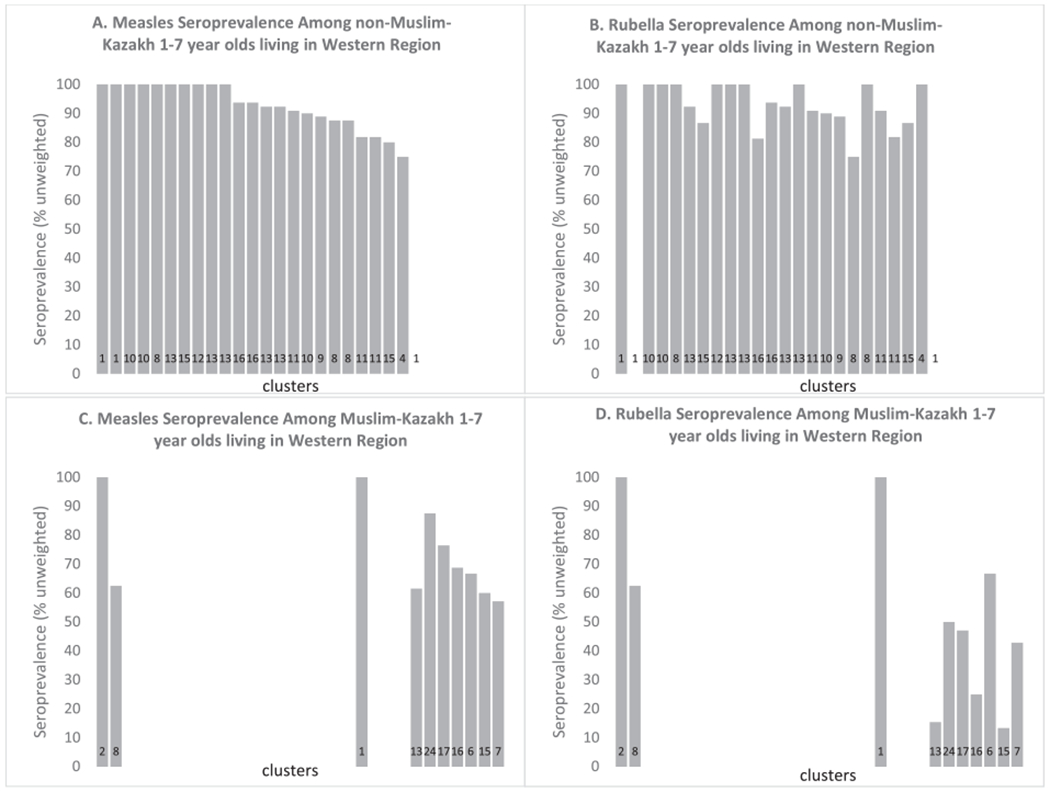
Measles and Rubella seroprevalence among 1–7 year olds living in Western Region, stratified by being Muslim Kazakh (C & D) or not (A & B). The x-axis for each panel is each of the 30 clusters in the Western Region, with the number at the bottom of the bar being the total number of 1–7-year-olds in each cluster who are Muslim Kazakh (C & D) or all others (A & B) (i.e. the denominator). Each bar is the measles (A & C) or rubella (B & D) unweighted seroprevalence for each cluster stratified by being Muslim Kazakh or not, in the Western region.
4. Discussion
High-quality measles and rubella serosurveys are tools that can help determine population immunity gaps, in order to guide vaccination efforts, and to help document that population immunity is sufficient to achieve elimination [5,7,13,14]. This can be valuable especially as reported vaccination coverage can be inaccurate. Based on the R0 for measles, it is estimated that 95% of a population should be immune to eliminate endemic measles virus transmission, while a lower population immunity is needed for rubella elimination, given its lower R0 [15,16]. While this survey did not test individuals aged <6 months and >35 years, population immunity in Mongolia appears to be high enough to eliminate measles and rubella, assuming the low reported incidence of measles among those aged > 35 years during the 2015–2016 measles outbreak is evidence that they are protected from measles, and assuming that a majority of adults are immune to rubella from natural infection (which is often asymptomatic) or from the rubella outbreak response immunization campaign in Ulaanbaatar in 2001.
Overall, population immunity was high for both measles and rubella, but a few notable gaps were seen. Measles antibody seroprevalence in 8–17 year-old children was below the 95% threshold for measles elimination; this age group was not targeted during the 2015 and 2016 SIAs because measles incidence was lower in this age group during the outbreak. School vaccination record checks might be a simple intervention to close any remaining immunity gaps and could be implemented to ensure that school-aged children have received 2-doses of MMR (as well as other vaccines), as has been done successfully in other countries [17–20].
Further efforts are needed in Mongolia to achieve high 2-dose MMR coverage among new birth cohorts and high-risk subpopulations. The small number of survey clusters and sample size for Muslim Kazakhs limited seroprevalence estimates for this subpopulation; however, the data indicate a potential gap in seroprotection for rubella, but not measles, among 1–7 year old Muslin Kazakhs living in the Western Region. This potential rubella immunity gap might be due to a lack of vaccination since the country has had a low rubella incidence for many years. No apparent differences between Muslim Kazakhs living in the Western region and all others were observed for measles antibody seropositivity, but this is not unexpected given the recent measles outbreak that affected the entire country and multiple age cohorts. While only 3.9% of the country is Kazakh, and almost all Kazakhs are Muslim, 76% of persons in Bayan-Ulgig Province, where 9 clusters in this survey were located, identify as Kazakh [21]. In this province the Altai Mountains serve as a physical barrier to access, but it is unknown if this is the biggest barrier to vaccination of this population; special efforts are needed to target this subpopulation for vaccination. Efforts should also be made to understand access and utilization of routine vaccination services in this subpopulation.
The proportions of persons seronegative for measles and rubella antibodies were higher in households where the mother or the participant had minimal or no education. In addition, the proportion of susceptible individuals to rubella increased significantly by household size, which has been observed for other routine immunizations in developing countries [22–25]. It is assumed, but our data cannot substantiate, that large household size and lower education status are associated with lower socioeconomic status in Mongolia. Reasons for the lower seroprevalence among those with less education and larger household size are unknown.
While we did not find an association between MMR vaccination in routine immunization services and measles IgG seropositivity, it is often challenging to document such an association because of a lack of documentation for vaccination. In addition, this study was conducted after a large outbreak, and thus many people who were reported as unvaccinated could have been immune from natural infection or could have received an SIA dose. Additionally, both SIAs were conducted after the outbreak started; thus, it is unknown if seropositive individuals acquired immunity from an SIA dose or from natural infection. However, we did find an expected association of routine MMR immunization with rubella antibody seropositivity. We also found an association between being vaccinated in the 2015 measles SIA and both measles and rubella antibody seropositivity. One potential reason for this finding is that a higher proportion of children who had received a routine dose of MMR also received an SIA dose, as has been seen elsewhere [26–29]. The relationship was not seen when looking at MMR among 12–35 month olds potentially because of the smaller sample size, and misclassification of receipt of vaccination and missing data. We also did not find an association between rubella antibody seropositivity and receiving an MR dose during the 2016 SIA which targeted adults. Many of these adults would be expected to be already immune from natural infection by adulthood, and some might be immune from receipt of rubella vaccine during the 2001 outbreak response SIA in Ulaanbaatar.
This survey has several limitations. First, participation rates were different among certain ages, regions, and ethnic/religious groups and could have led to bias in the results. Participants were more likely to have been vaccinated by routine immunization services and by SIAs, which could lead to a higher seroprevalence in this study compared to the true population seroprevalence. Vaccination data were provided by recall for SIAs and for 40% of routine doses received by children 12–35 months of age, and these data could be inaccurate. This study tested samples only for IgG, which is a conservative measure of immunity, not virus neutralization assays, considered the gold-standard; thus, our findings potentially underestimate population immunity. Protective measles antibody levels are usually considered to be ≥ 120 mIU/ml; however, the ELISA test for measles antibody used has a cutoff of ≥ 150 mIU/ml. Thus, individuals with titers between 120–<150 mIU/ml were considered seronegative but are probably still protected [5,30]. Finally, some subpopulation analyses are limited by their sample size, and caution should be taken when extrapolating these results to the entire population.
A serosurvey can be a valuable tool if designed and implemented well around an answerable question. However, serosurveys have limitations. They cannot replace surveillance for disease. Sample sizes can be quite large to get the proper precision, and thus require a lot of human and financial resources to execute [14]. In this case, this serosurvey was useful in identifying immunity gaps, while reassuring the country that they had closed most of their immunity gap and did not need to conduct another national SIA. In 2017, Mongolia reported only 9 measles cases and 11 rubella cases, with only 1 measles case and 1 rubella case in 2018 [31]. Given their high-quality surveillance and the high population immunity found in this study, evidence suggests that the country is on track to eliminate measles and rubella.
Acknowledgments
We would like to thank Dr Yadamsuren Buyanjargal from Ministry of Health, Davaajargal Davaatseren and Urangoo from the National Statistics Office for all their support and guidance in the implementation and monitoring of the survey. We thank Sodbayar Demberelsuren from the Expanded Programme on Immunization at the World Health Organization Country Office in Mongolia for her advice, commitment and kind assistance. We thank Raydel Anderson, Nobia Williams and Marcus Collins of the Centers for Disease Control and Prevention for providing support for confirmatory testing. Finally, we would like to thank all Expanded Programme on Immunization staff from the Ministry of Health and from the National Center for Communicable Diseases, National Statistics Office enumerators, supervisors, field teams and drivers for their efforts in the selection, recruitment, obtaining consent, data collection and blood specimen acquisition, processing, transport and testing. We would also like to thank the participants for agreeing to participate.
Funding
This work was funded by the Bill and Melinda Gates Foundation (BMGF). BMGF had no direct role in this work.
Footnotes
Credit authorship contribution statement
Francisco Nogareda: Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing - review & editing. Nyamaa Gunregjav: Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing - review & editing. Amarzaya Sarankhuu: Investigation, Methodology, Supervision, Writing - review & editing. Enkhtuya Munkhbat: Investigation, Methodology, Supervision, Writing - review & editing. Enkhbaatar Ichinnorov: Investigation, Methodology, Supervision, Writing - review & editing. Pagbajabyn Nymadawa: Conceptualization, Methodology, Writing - review & editing. Kathleen Wannemuehler: Conceptualization, Data curation, Formal analysis, Methodology, Writing - review & editing. Mick N. Mulders: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing - review & editing. Jose Hagan: Investigation, Methodology, Writing - review & editing. Minal K. Patel: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].Chen MH, Zhu Z, Zhang Y, Favors S, Xu WB, Featherstone DA, et al. An indirect immunocolorimetric assay to detect rubella virus infected cells. J Virol Methods 2007;146:414–8. [DOI] [PubMed] [Google Scholar]
- [2].Lambert ND, Pankratz VS, Larrabee BR, Ogee-Nwankwo A, Chen MH, Icenogle JP, et al. High-throughput assay optimization and statistical interpolation of rubella-specific neutralizing antibody titers. Clin Vaccine Immunol 2014;21:340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cohen BJ, Parry RP, Doblas D, Samuel D, Warrener L, Andrews N, et al. Measles immunity testing: comparison of two measles IgG ELISAs with plaque reduction neutralisation assay. J Virol Methods 2006;131:209–12. [DOI] [PubMed] [Google Scholar]
- [4].Skendzel LP. Rubella immunity. Defining the level of protective antibody. Am J Clin Pathol 1996;106:170–4. [DOI] [PubMed] [Google Scholar]
- [5].World Health Organization. Guidelines on the use of serosurveys in support of measles and rubella elimination. Geneva, Switzerland: Immunizations, Vaccines, and Biologicals; 2019. https://www.who.int/immunization/monitoring_surveillance/burden/laboratory/serosurvey/en/. Accessed: December 13, 2019. [Google Scholar]
- [6].Dimech W, Mulders MN. A review of testing used in seroprevalence studies on measles and rubella. Vaccine 2016;34:4119–22. [DOI] [PubMed] [Google Scholar]
- [7].Framework for verifying elimination of measles and rubella. Wkly Epidemiol Rec 2013; 88: 89–99. [PubMed] [Google Scholar]
- [8].World Health Organization Regional Office for the Western Pacific. Mongolia certified measles free. 2014. http://www.wpro.who.int/mediacentre/releases/2014/20140708/en/. Accessed: April 4, 2018.
- [9].Rentsen T, Enkhtuya B, Nymadawa P, Kobune F, Suzuki K, Yoshida H, et al. Measles outbreak after a post-honeymoon period in Mongolia, 2001. Jpn J Infect Dis 2007;60:198–9. [PubMed] [Google Scholar]
- [10].Orsoo O, Saw YM, Sereenen E, Yadamsuren B, Byambaa A, Kariya T, et al. Epidemiological characteristics and trends of a Nationwide measles outbreak in Mongolia, 2015–2016. BMC Public Health 2019;19:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hagan JE, Takashima Y, Sarankhuu A, Dashpagma O, Jantsansengee B, Pastore R, et al. Risk factors for measles virus infection among adults during a large outbreak in postelimination era in Mongolia, 2015. J Infect Dis 2017;216:1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wangchuk S, Nogareda F, Tshering N, Khandu L, Pelden S, Wannemuehler K, et al. Measles and rubella immunity in the population of Bhutan, 2017. Vaccine 2019;37:6463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cutts FT, Hanson M. Seroepidemiology: an underused tool for designing and monitoring vaccination programmes in low- and middle-income countries. Trop Med Int Health 2016;21:1086–98. [DOI] [PubMed] [Google Scholar]
- [14].Durrheim DN, Orenstein WA, Schluter WW. Assessing population immunity for measles elimination - The promise and peril of serosurveys. Vaccine 2018;36:4001–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis 2004;189(Suppl 1):S27–35. [DOI] [PubMed] [Google Scholar]
- [16].Moss WJ, Strebel P. Biological feasibility of measles eradication. J Infect Dis 2011;204(Suppl 1):S47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Orenstein WA, Hinman AR. The immunization system in the United States - the role of school immunization laws. Vaccine 1999;17(Suppl 3):S19–24. [DOI] [PubMed] [Google Scholar]
- [18].Vernon TM, Conner JS, Shaw BS, Lampe JM, Doster ME. An evaluation of three techniques improving immunization levels in elementary schools. Am J Public Health 1976;66:457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Goodson JL, Alexander JP, Linkins RW, Orenstein WA. Measles and rubella elimination: learning from polio eradication and moving forward with a diagonal approach. Expert Rev Vaccines 2017;16:1203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zuo S, Cairns L, Hutin Y, Liang X, Tong Y, Zhu Q, et al. Accelerating measles elimination and strengthening routine immunization services in Guizhou Province, China, 2003–2009. Vaccine 2015;33:2050–5. [DOI] [PubMed] [Google Scholar]
- [21].National Statistics Office of Mongolia. 2015 population and housing by-census of Mongolia: National Report. Ulaanbaatar: 2016. http://www.en.nso.mn/content/166. Accessed: July 3, 2018. [Google Scholar]
- [22].Balogun SA, Yusuff HA, Yusuf KQ, Al-Shenqiti AM, Balogun MT, Tettey P. Maternal education and child immunization: the mediating roles of maternal literacy and socioeconomic status. Pan Afr Med J 2017;26:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Forshaw J, Gerver SM, Gill M, Cooper E, Manikam L, Ward H. The global effect of maternal education on complete childhood vaccination: a systematic review and meta-analysis. BMC Infect Dis 2017;17:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Khan MT, Zaheer S, Shafique K. Maternal education, empowerment, economic status and child polio vaccination uptake in Pakistan: a population based cross sectional study. BMJ Open 2017;7. e013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vikram K, Vanneman R, Desai S. Linkages between maternal education and childhood immunization in India. Soc Sci Med 2012;75:331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kearney M, Yach D, Van Dyk H, Fisher SA. Evaluation of a mass measles immunisation campaign in a rapidly growing peri-urban area. South African Medical Journal = Suid-Afrikaanse tydskrif vir geneeskunde 1989;76:157–9. [PubMed] [Google Scholar]
- [27].Portnoy A, Jit M, Helleringer S, Verguet S. Impact of measles supplementary immunization activities on reaching children missed by routine programs. Vaccine 2018;36:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vijayaraghavan M, Martin RM, Sangrujee N, Kimani GN, Oyombe S, Kalu A, et al. Measles supplemental immunization activities improve measles vaccine coverage and equity: Evidence from Kenya, 2002. Health Policy (Amsterdam, Netherlands). 2007;83:27–36. [DOI] [PubMed] [Google Scholar]
- [29].Hu X, Xiao S, Chen B, Sa Z. Gaps in the 2010 measles SIA coverage among migrant children in Beijing: evidence from a parental survey. Vaccine 2012;30:5721–5. [DOI] [PubMed] [Google Scholar]
- [30].Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, et al. Measles antibody: reevaluation of protective titers. J Infect Dis 1990;162:1036–42. [DOI] [PubMed] [Google Scholar]
- [31].World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2019 global summary. 2019. http://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=MNG. Accessed: October 7, 2019.


