Abstract
Background and Aims
During the first peak of the COVID‐19 pandemic, the Preventive Medicine Department and the Occupational Health Department at Hospital Clinic de Barcelona (HCB), a large Spanish referral hospital, developed an innovative comprehensive SARS‐CoV2 Surveillance and Control System (CoSy‐19) in order to preserve patients' and health care workers' (HCWs) safety. We aim to describe the CoSy‐19 and to assess the impact in the number of contacts that new cases generated along this time.
Methods
Observational descriptive study of the findings of the activity of contact tracing of all cases received at the HCB during the first peak of COVID‐19 in Spain (February 25th‐May 3rd, 2020).
Results
A team of 204 professionals and volunteers performed 384 in‐hospital contact‐tracing studies which generated contacts, detecting 298 transmission chains which suggested preventive measures, generated around 22 000 follow‐ups and more than 30 000 days of work leave. The number of contacts that new cases generated decreased during the study period.
Conclusion
Coordination between Preventive Medicine and Occupational Health departments and agile information systems were necessary to preserve non‐COVID activity and workers safety.
Keywords: contact tracing, COVID‐19, Go.Data Software, healthcare associated infection, occupational health, patient safety, SARS‐CoV2
1. INTRODUCTION
The novel coronavirus disease (COVID‐19) caused by SARS‐CoV2 was first detected in Wuhan, China, in late 2019 and then rapidly spread to the rest of the world. 17 The Preventive Medicine Department and the Occupational Health Department at Hospital Clinic, a large Spanish referral hospital, faced three challenges to preserve patients' and health care workers' (HCWs) safety. First, to detect early SARS‐CoV2 cases among patients and HCWs and to identify their contacts to prescribe isolations and quarantines and actively monitor them. Second, to identify and break transmission chains inside the hospital. Finally, to promptly notify SARS‐CoV2 cases to the relevant Surveillance Authority (Catalan Surveillance Agency) which contributes to monitor and control community transmission.
On February 25th, the first SARS‐CoV2 case in Catalonia, Spain, was detected and hospitalized at the Hospital Clínic of Barcelona (HCB). The Preventive medicine and Occupational Health departments developed a comprehensive SARS‐CoV2 Surveillance and Control System (CoSy‐19), which was set up at HCB on March 5th.
We aim to describe the CoSy‐19, its findings in‐hospital transmission during the first months of the first wave of the pandemic, and to assess the impact in the number of contacts that new cases generated along this time.
2. METHODS
2.1. Study design, participants, study period, and setting
This is an observational descriptive study of the findings of the activity of contact tracing of all cases received at the HCB during the first peak of COVID‐19 in Spain (February 25th‐May 3rd, 2020). HCB is a tertiary hospital with over 5500 HCWs. It is a referral center for emerging infectious diseases, including COVID‐19, and specialized patient care in Catalonia, Spain. Before the pandemic, HCB had around 750 beds, 60 of them in Intensive Care Units (ICUs). Between March 5th and May 3rd, the hospital's overall capacity increased up to 942 beds (717 standard hospitalization beds, 158 ICU beds and 67 semi‐ICU beds). During the first weeks of March, Spain suffered a severe shortage of personal protective equipment (PPE) in hospitals. 14 , 15
2.2. Definitions and variables
Cases were classified as confirmed (positive qPCR), probable (clinical and radiologic findings, not microbiologically confirmed), and suspected (compatible symptoms). The CoSy‐19 identified Intra‐hospital contacts among patients and HCWs who had been exposed to a case more than 15 minutes, less than 2 m apart and without proper PPE from a case, probable or confirmed, in the last 48 hours before symptom onset of the case. During the study period, qPCR tests were not always available: all suspected cases were tested until March 14th, 2020. From this date to March 28th, 2020, only HCW and patients with admission criteria were tested, and later on, only HCWs were tested. During the study period, if a contact of a case developed symptoms during the follow‐up, it was considered a secondary case, and defined a chain of transmission together with the primary case. A set of cases linked by chains of transmission was named a cluster. For every case, we registered the reporting date, its classification as HCW or patient, the probable date of onset, and a list of intra‐hospital contacts, either patients or HCWs, with the date of last contact and place of exposure.
2.3. Data sources
New positive SARS‐CoV‐2 RT‐qPCR tests for both patients and HCWs and probable non‐confirmed patient cases were registered in the electronic Hospital Information System (HIS), which runs on SAP software. Initially, probable cases were not registered as such by the electronic system in a standardized form, delaying case identification by the CoSy‐19 and the start of the contact tracing. To account for this, and timely identify both confirmed and probable cases, an electronic marker (sticker) was added to the SAP platform. Attending physicians or a positive microbiological result could activate the sticker. These stickers generated a daily list of cases that the Preventive Medicine Department verified. Direct notification by phone or email from HCWs was encouraged.
HCWs were asked to fill in an electronic epidemiological questionnaire with information about their signs, symptoms and close contacts. The questionnaire was programmed in Research Electronic Data Capture, REDCap, 9 and HCWs could access it through their smartphones while waiting to be tested or fill it in later, once they received a positive result. The information they provided had to be confirmed by the contact tracers through a phone call. Contact tracers recorded data on cases and close contacts in spreadsheets before being uploaded to Go.Data software, 8 , 11 developed by WHO for early outbreak investigation, by a data manager and administrative staff. Medical students conducted the contacts' follow‐up through Go.Data dashboards.
2.4. CoSy‐19 description
Before the pandemic, 11 physicians and infection control nurses performed all the infection control activity. On March 15th, the CoSy‐19 was staffed with 45 professionals and 60 volunteers, and since late March, it comprised 64 professionals and 140 volunteers. Volunteers were medical students who spent 6 hours every week to avoid dropouts. Team leaders were duplicated to maintain activity if one of them became ill or a contact. Telework was encouraged when possible. The CoSy‐19 contact tracers were divided in two teams, one led by Preventive Medicine (tracing patients) and the other by Occupational Health (tracing HCWs). They identified the intra‐hospital close contacts (contacts occurring at the hospital) of each case through phone calls and specific searches in the HIS. Cases were immediately notified to the Catalan Surveillance Agency (CSA). In the hospital, eight preventive medicine physicians and five infection control nurses monitored the correct cohorting and isolation of patients who were cases or contacts. These nurses were also responsible for the training of HCWs on Personal Protective Equipment (PPE) use and on hygiene procedures. Medical students followed contacts telephonically for 14 days, from 8 to 20 hours. The CoSy‐19 complied with ECDC recommendations on resource estimation for contact tracing, 4 and it was agile enough to adapt and cope with the surge in cases and the opening of new wards during the epidemic peak. The workflow is shown in Figure 1.
FIGURE 1.
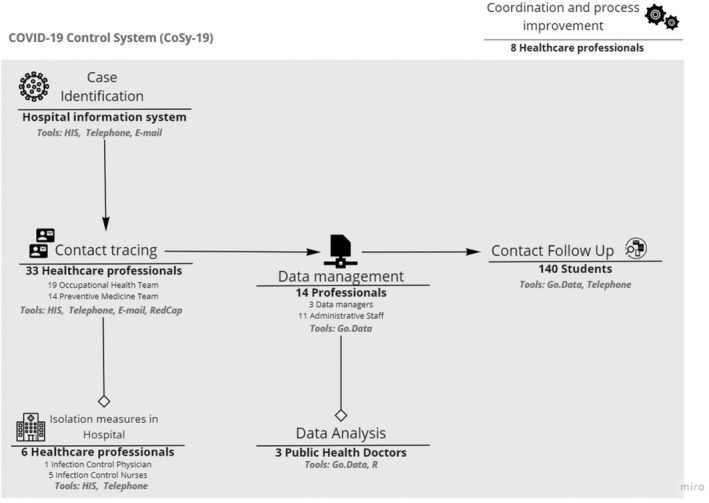
Workflow and technical support system of the CoSy‐19 hospital surveillance and control system with contact tracing for patients and health care workers at Hospital Clínic de Barcelona
2.5. Analysis
We computed the number of contacts generated by each case by the CoSy‐19, along with the number of cases previously registered as contacts. Means and percentages were calculated. Of these transmission chains, we registered how many had a HCW or patient as primary and secondary cases. We plot the daily count of cases that generated contacts in CoSy‐19, and the mean number of contacts by case. We also plot the chains of transmission by date of onset of primary and secondary cases for those whose date of onset had been recorded correctly. We used R Statistical Software version 4.0. 13 Code for the figures is available in the Data S1.
3. RESULTS
Until May 3rd, 1980 SARS‐CoV2 cases were detected in patients (1278 RT‐qPCR confirmed cases, 527 probable cases, and 176 suspect cases) and 516 in HCWs. A total of 384 cases (15.4%) generated one or more in‐hospital contacts. One thousand seven hundred and eighty four contacts (443 patients and 1341 HCWs) were identified. Of these contacts, 233 developed symptoms and became cases (13.1%; 205 confirmed, 5 probable, and 23 suspected based on mild symptoms). These cases who were contacts before were registered in 298 transmission chains. Most of these relations were among HCWs (189), from HCWs to patients (58), from patients to HCWs (27), and among patients (24). Most contacts who became cases were classified as contacts of a single case (182), although there were cases that were categorized as contacts of 2 cases (39), 3 cases (10), and 4 cases (2). Epidemic curves of daily new cases detected by CoSy‐19, new cases which generated contacts, and the daily average of contacts per case are shown in Figure 2. Figure 3 shows a timeline of transmission chains detected by CoSy‐19 as a function of the onset date of the 171 chains of transmission and 70 clusters among the 232 cases with information on the date of onset. The CoSy‐19 generated around 22 000 follow‐ups. Quarantines for cases and contacts among HCWs resulted in more than 30 000 days of work leave. At the same time, infection control instructions on cohorting and isolation were included in patient cases' and contacts' electronic medical records.
FIGURE 2.
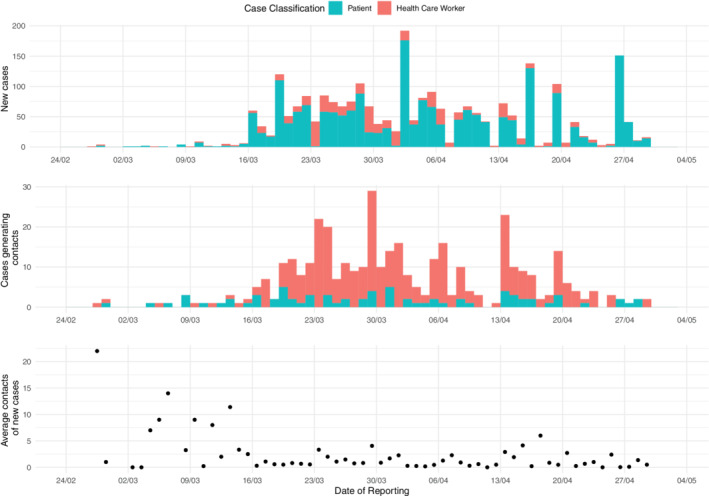
From top to bottom: Daily new cases, cases generating contacts, and average contacts of new cases of HCB (February 25th‐May 3rd) at CosSy‐19
FIGURE 3.
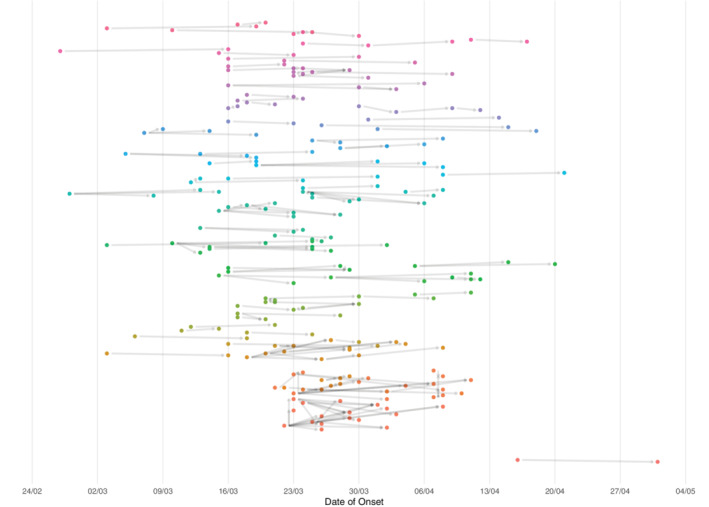
Timeline of the chains of transmission identified through CoSy‐19 at HCB by date of onset during the study period (February 25th‐May 3rd, 2020). Every dot represents a case, and each color a different cluster
4. DISCUSSION
In this study, we describe CoSy‐19, a hospital surveillance and control system designed specifically for COVID‐19. Only a few systems have been described in the medical literature. 2 , 6 The rapid scale‐up of resources and information tools allowed to detect and interrupt intrahospital transmission during the beginning of the epidemic through isolation and quarantines of cases and contacts. The work leave days reduced the number of available HCWs substantially when the number of admitted patients increased. The detection of the context of transmission allowed structural measures to prevent it, which impacted in the contacts produced by every intrahospital case.
The high number of cases detected results from the high community transmission in Spain during the study period. Many measures (respiratory hygiene, PPE training, cleaning, and waste management procedures and standard operating procedures for COVID‐19 patient management, etc.) were already in place before the first cases arrived at the hospital. PPEs were not always available during the first weeks in non‐COVID areas, partially explaining the number of clusters detected. As a result of the detection of intra‐hospital transmission chains, additional infection preventive measures were implemented to further prevent the number of contacts, including restructuring hospital areas, conducting RT‐qPCR tests to screen patients before being admitted to non‐COVID‐19 wards, making changes in common areas for HCWs and raising awareness about the importance of physical distancing among professionals. These measures could explain that the number of contacts generated by each case decreased over time.
Overall, the CoSy‐19 attained four key achievements. First, it helped to reduce the risk of SARS‐CoV2 infection in patients, 1 , 16 who are more likely to have complications than the general population. Second, it reduced HCWs risk by detecting and isolating carriers and their contacts at real risk. 7 Third, areas of SARS‐CoV2 transmission were quickly identified, which allowed the implementation of tailored interventions in the hospital. Finally, agile management of information and prompt notification to the CSA allows contact tracing at a community level.
Based on epidemic modelling, active surveillance may need to be maintained beyond 2022 10 to ensure early detection and response. A high coverage of in‐hospital contact tracing and follow‐up is only possible if the processes are standardized, coordinated between the Preventive Medicine and Occupational Health departments and staffed with the necessary human resources at all times. 2 , 3 The development and maintenance of in‐hospital organizational structures such as the CoSy‐19 are then crucial. Cooperative and interdisciplinary approaches are necessary and should come together with a profound knowledge of the hospitals' organization. This understanding is critical in organizing resources in an emergency in the most efficient way and defining how to integrate this functioning for more extended periods. Systems like the CoSy‐19 should be designed according to the context, particularly for each organizational healthcare unit. While challenging, cooperation between healthcare centers should be encouraged, and digitalization of contact tracing and follow‐ups are necessary for its maintenance.
In our setting, CoSy‐19 became a very human‐dependent system requiring many professionals and organizational efforts involving several departments. Moreover, human resources limitations led to the prioritization of contact identification and follow‐up over data collection in the initial stages, and many variables about initial cases (ie, date of onset) were not recorded. Besides, not all processes were standardized at the same time. For example, backward contact tracing was only formalized after a few weeks of functioning which might have compromised transmission detection. However, while the scarcity of PPE, lack of testing, and an overwhelming number of admissions threatened in‐hospital care, the system started working in less than a week and coped with the highest peak of pandemic activity. From summer 2021, Go.Data was replaced by an inhouse solution embedded in our own HIS, 5 , 8 , 9 given that Go.Data requires multiple manual steps and does not allow automation of the follow‐ups. The concepts of isolation, quarantine, case or close contact should become familiar to everyone, a communication campaign to patients and HCWs would facilitate the use of electronic self‐notification tools (eg, to identify contacts) and semi‐automated follow‐up. 12
5. CONCLUSION
Hospital systems like CoSy‐19 are crucial to avoid new cases and contacts in patients and HCWs, help restructure the response in the light of detected transmission and guarantee safe hospital care.
FUNDING
No funding was received for this work. The research of Joaquim Puig is funded by the grants PGC2018‐098676‐B‐100 and 2017SGR1049. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Anna Llupià.
Data Curation: Anna Llupià, Laura Granés, Victòria Olivé, Lourdes Baron‐Miras, Isabel Torà, Clara Marin, Jaume Grau, Inmaculada Soriano, Elena Roel, Marta García‐Diez, Maria López‐Toribio, Caterina Guinovart, Paula Fernández‐Torres, Andreu Prat, Beatriz Julieta Blanco‐Rojas, Maria de Arquer, Marta Tortajada, Anna Vilella.
Investigation: Anna Llupià, Laura de la Torre‐Pérez, Laura Granés, Lourdes Baron‐Miras, Isabel Torà, Clara Marin, Inmaculada Soriano, Elena Roel, Marta García‐Diez, Maria López‐Toribio, Joaquim Puig, Caterina Guinovart, Gemina Santana, Alberto García‐Basteiro, Anna Vilella, Antoni Trilla.
Writing – original Draft Preparation: Anna Llupià, Laura de la Torre‐Pérez, Isabel Torà, Joaquim Puig, Caterina Guinovart.
Writing – review & editing: Anna Llupià, Laura Granés, Victòria Olivé, Lourdes Baron‐Miras, Clara Marin, Jaume Grau, Inmaculada Soriano, Elena Roel, Marta García‐Diez, Maria López‐Toribio, Joaquim Puig, Caterina Guinovart, Gemina Santana, Paula Fernández‐Torres, Alberto García‐Basteiro, Andreu Prat, Beatriz Julieta Blanco‐Rojas, Maria de Arquer, Sonia Barroso, Marta Tortajada, Pilar Varela, Anna Vilella, Antoni Trilla.
All authors have read and approved the final version of the manuscript.
Anna Llupià had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
TRANSPARENCY STATEMENT
The lead author (manuscript guarantor) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ETHICS STATEMENT
After consultation in April 2020, the hospital ethics committee stated that ethics review was not necessary because the study met the criteria for the description of operational improvement activities and was exempt from ethics review.
Supporting information
Data S1 Supporting Information.
ACKNOWLEDGEMENTS
The organization of CoSy‐19 was possible thanks to more than one hundred people. We would like to thank the following: Preventive Medicine Nurses, breaking the chains of transmission. Microbiology Department, keeping our eyes open. Occupational Health Department, taking care of our health care workers. Administrative staff, always available and taking care of all of us. Preventive Medicine Specialists, doing the work we could not do. Infectious diseases Department, hands‐on with in‐hospital SARS‐CoV2 contacts. Redcap implementers, saving time. Directors, making things easy. Brand new doctors, learning fast. Cleaner, the most important person in this pandemic. Infrastructures, providing time and space. Legal advice, always in charge. Technical support, always there when needed. EETAC at UPC, testing the tools. Project managers, supporting and spreading the methodology. IT department, making it happen. Documentation System, acting fast. Dermatology Department, the elite squad. Partners and data scientists. And our rescuers, the wonderful medical students of University of Barcelona.
Llupià A, de la Torre‐Pérez L, Granés L, et al. SARS‐CoV2 hospital surveillance and control system with contact tracing for patients and health care workers at a large reference hospital in Spain during the first wave: An observational descriptive study. Health Sci Rep. 2022;5:e513. doi: 10.1002/hsr2.513
Funding information Departament d'Innovació, Universitats i Empresa, Generalitat de Catalunya, Grant/Award Number: 2017SGR1049; Ministerio de Ciencia e Innovación, Grant/Award Number: PGC2018‐098676‐B‐100
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions. The computer code to generate the graphics and analyze the data is available as a supplementary material.
REFERENCES
- 1. Abbas M, Robalo Nunes T, Martischang R, et al. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10(1):7. doi: 10.1186/s13756-020-00875-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng VC, Wong SC, Tong DW, et al. Multipronged infection control strategy to achieve zero nosocomial coronavirus disease 2019 (COVID‐19) cases among Hong Kong healthcare workers in the first 300 days of the pandemic. Infect Control Hosp Epidemiol. 2021;19:1‐10. doi: 10.1017/ice.2021.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi UY, Kwon YM, Kang HJ, et al. Surveillance of the infection prevention and control practices of healthcare workers by an infection control surveillance‐working group and a team of infection control coordinators during the COVID‐19 pandemic. J Infect Public Health. 2021;14(4):454‐460. doi: 10.1016/j.jiph.2021.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Centre for Disease Prevention and Control . Resource estimation for contact tracing, quarantine and monitoring activities for COVID‐19 cases in the EU/EEA (ECDC Technical Report) 2020:1–9.
- 5. Ferretti L, Wymant C, Kendall M, et al. Quantifying SARS‐CoV‐2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368(6491):eabb6936. doi: 10.1126/science.abb6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gan WH, Lim JW, Koh D. Preventing intra‐hospital infection and transmission of coronavirus disease 2019 in health‐care workers. Saf Health Work. 2020;11(2):241‐243. doi: 10.1016/j.shaw.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia‐Basteiro AL, Moncunill G, Tortajada M, et al. Seroprevalence of antibodies against SARS‐CoV‐2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11(1):3500. doi: 10.1038/s41467-020-17318-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Go.Data . Global Outbreak Alert & Response Network. Geneva: WHO; 2020. [Google Scholar]
- 9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS‐CoV‐2 through the postpandemic period. Science. 2020;368(6493):860‐868. doi: 10.1126/science.abb5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Llupià A, Garcia‐Basteiro A, Puig J. Still using MS Excel? Implementation of the WHO Go.Data software for the COVID‐19 contact tracing. Health Sci Rep 2020;3(2):e164. . doi: 10.1002/hsr2.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pryor R, Atkinson C, Cooper K, et al. The electronic medical record and COVID‐19: is it up to the challenge? Am J Infect Control. 2020;48(8):966‐967. doi: 10.1016/j.ajic.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. R Core Team . R: A Language and Environment for Statistical Computing (Manual). Vienna, Austria; 2020.
- 14. Rada AG. Covid‐19: the precarious position of Spain's nursing homes [published correction appears in BMJ. 2020 Apr 21;369:m1586]. BMJ. 2020;369:m1554. doi: 10.1136/bmj.m1554 [DOI] [PubMed] [Google Scholar]
- 15. Tabah A, Ramanan M, Laupland KB, et al. Personal protective equipment and intensive care unit healthcare worker safety in the COVID‐19 era (PPE‐SAFE): an international survey [published correction appears in J Crit Care. 2021 Jun;63:280‐281]. J Crit Care. 2020;59:70‐75. doi: 10.1016/j.jcrc.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong SCY, Kwong RT, Wu TC, et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020;105(2):119‐127. doi: 10.1016/j.jhin.2020.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization . Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (Covid‐19). Geneva: World Health Organisation; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions. The computer code to generate the graphics and analyze the data is available as a supplementary material.


