Abstract
Purpose
To characterize delivery features and explore effectiveness of telehealth-based cancer rehabilitation interventions that address disability in adult cancer survivors.
Methods
A systematic review of electronic databases (CINAHL Plus, Cochrane Library: Database of Systematic Reviews, Embase, National Health Service’s Health Technology Assessment, PubMed, Scopus, Web of Science) was conducted in December 2019 and updated in April 2021.
Results
Searches identified 3,499 unique studies. Sixty-eight studies met inclusion criteria. There were 81 unique interventions across included studies. Interventions were primarily delivered post-treatment and lasted an average of 16.5 weeks (SD = 13.1). They were most frequently delivered using telephone calls (59%), administered delivered by nursing professionals (35%), and delivered in a one-on-one format (88%). Risk of bias of included studies was primarily moderate to high. Included studies captured 55 measures of disability. Only 54% of reported outcomes had data that allowed calculation of effect sizes ranging -3.58 to 15.66.
Conclusions
The analyses suggest small effects of telehealth-based cancer interventions on disability, though the heterogeneity seen in the measurement of disability makes it hard to draw firm conclusions. Further research using more diverse samples, common measures of disability, and pragmatic study designs is needed to advance telehealth in cancer rehabilitation.
Implications for Cancer Survivors
Telehealth-based cancer rehabilitation interventions have the potential to increase access to care designed to reduce disability across the cancer care continuum.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11764-022-01181-4.
Keywords: Telehealth, Cancer rehabilitation, Neoplasm, Function, Intervention, Disability
Introduction
Cancer-related disability is a pervasive consequence of the disease itself as well as oncology-directed treatment. For the purposes of this paper, cancer-related disability is defined as limitations associated with roles, routines, functional activities, or societal participation [1] or reductions in well-being. Nearly 55% of adult cancer survivors report restrictions in their ability to perform instrumental activities of daily living and 37% report restrictions in their ability to perform basic activities of daily living [2]. Survivors may also experience significant challenges in returning to employment due to functional limitations [3]. The negative influence of disability spans many different types of cancer [2] and can also affect a person’s ability to tolerate cancer-related treatment and health-related quality of life [4].
The multidisciplinary field of cancer rehabilitation seeks to reduce disability and improve functioning among survivors [5]. Evidence from reviews supports the utilization of cancer rehabilitation interventions along the oncology care continuum [6–10]; however, rates of referral and participation are low [11, 12]. Although multifactorial, two factors are consistently implicated in low utilization [12, 13]. First, there are not enough rehabilitation specialists who have expertise in oncology [14]. Second, patients perceive they have limited time and energy to access rehabilitation services, particularly if they need to travel long distances to do so [15].
Telehealth-based cancer rehabilitation interventions may help to overcome patient-, provider-, and health system-related barriers which limit access to care [3, 16]. Telehealth is defined as “the delivery of health care services, where distance is a critical factor, by all health care professionals using information and communication technologies, for the exchange of valid information for…treatment, and prevention of disease and injuries, [and] research and evaluation… in all the interests of advancing the health of individuals and their communities” [17, p. 9]. To develop robust cancer rehabilitation programs and train providers in the delivery of interventions, it is important to examine the characteristics and the effectiveness of existing telehealth-based interventions that address disability.
The purpose of this systematic review is to characterize intervention delivery features and evaluate the effectiveness of telehealth-based cancer rehabilitation interventions addressing disability among adult cancer survivors. The results of this systematic review will help to inform future telehealth-based cancer rehabilitation intervention development, testing, and implementation.
Methods
A study protocol was developed a priori using the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) checklist [18]. The protocol was registered on PROSPERO (CRD42020150791) prior to data collection. The 2020 PRISMA statement and checklist guided the manuscript development [19].
Information sources and search strategy
A biomedical librarian (AAL) developed a comprehensive search strategy in consultation with co-authors. The original search was run in December 2019 and updated in April 2021 using seven citation and abstract databases: CINAHL Plus (Ebscohost), Cochrane Library: Database of Systematic Reviews (Wiley & Sons), Embase (Elsevier), National Health Service’s Health Technology Assessment (University of York), PubMed (US National Library of Medicine), Scopus (Elsevier), and Web of Science: Core Collection (Clarivate Analytics). Searches were limited to original research articles and systematic reviews published in English between 1994 and 2021. Keywords and controlled vocabularies (e.g., MeSH, EMTREE, CINAHL Subject Headings) were used to describe telehealth, rehabilitation, and cancer (see Supplementary File Table 1 for final search strategies used). EndNote X9.3.3 (Clarivate Analytics) was used to collect, manage, and identify duplicate citations. Individuals conducting the study selection process hand searched the bibliographies of literature reviews to identify relevant studies not captured in the search.
Study inclusion
We initially included studies evaluating cancer rehabilitation interventions that met the following criteria: (1) written in English, (2) had intervention goal(s) included prevention, reduction, or attenuation of decline in disability (i.e., goal of maintaining or improving function in daily roles and routines, preventing or reducing disability, maximizing participation, and/or improving a functional aspect of quality of life); (3) intervention delivered according to the World Health Organization definition of telehealth [17]; (4) included participants with a current or previous cancer diagnosis, (with or without their caregivers); and (5) measured disability, based on the definition by O’Young and colleagues [1], as a primary or secondary outcome. We operationalized the fifth criterion by saying the study needed to measure the impact on function and disability (e.g., social roles, activities of daily living, vocational roles, recreational roles) and/or use of a quality of life measurement tool that assesses ability to function as a component or aspect of quality of life [1]. The initial full text screening revealed a large number of relevant articles (n = 132). To ensure that we were synthesizing literature that addressed our specific research objectives, we added two additional inclusion criteria: (6) utilization of a randomized controlled trial design (to evaluate intervention effectiveness); and (7) inclusion of at least one synchronous interaction between participants and cancer rehabilitation professions (to more closely reflect current billable rehabilitation services).
Studies were excluded if they were as follows: (1) only pharmacological interventions; (2) interventions that addressed solely psychosocial outcomes (e.g., anxiety, depression, distress); (3) measured only impairment-based and symptom severity outcomes; (4) used only qualitative methodology; (5) were any of the following article types: reviews, white papers/editorials, qualitative studies, letters, commentaries, opinions papers, unpublished research; and (6) published prior to 1994.
Selection process
Prior to the study selection process, a pilot test of the study selection and data extraction process was completed with seven research team members (KDL, RB, LP, KC, GC, TM) on a sample set of four citations provided from the initial literature search. Adaptations were made to the data collection process based upon this pilot testing (e.g., addition of items to extract, ability to select more than one option, addition of fields). Reviewers (RB, KDL, JJ, LP, KC, MP, TM, GC, RE, JS, AV, AMF, MF, TK, JB) were trained on data screening procedures through group and individual trainings. Team meetings were held twice during the screening process to discuss and clarify screening procedures.
Covidence (Veritas Health Innovation Ltd., Melbourne, Australia) was used to conduct the two-stage screening process. First, the titles and abstracts of articles were uploaded to Covidence and independently screened by a combination of two reviewers (RB, KDL, JJ, LP, KCW, MP, TM, TK, GC, RE, JS, AV, AMF, MF, JB). Disagreements regarding inclusion between reviewers were resolved through discussion between at least two of the senior project authors (KDL, RSB, LP). Next, the full text articles were independently screened by a combination of two reviewers (RB, KDL, JJ, LP, KCW, MP, TM, TK, GC, RE, JS, AV, AMF, MF, JB) using the same eligibility criteria. Disagreements between the two reviewers were resolved by a third reviewer who was blind to the initial reviewers’ decisions.
Data collection process
A combination of two reviewers (RB, KDL, JJ, LP, KC, MP, TM, GC, RE, AMF, MF, BK, JB) independently extracted data from each included article. Extracted data were entered into a custom-designed Qualtrics survey (Qualtrics, Provo, UT). After completing double-data extraction, the pair of reviewers met to resolve any discrepancies. Final extracted data for each article were compiled into a Microsoft Excel spreadsheet to create a database of results. Each included article was assessed for risk of bias by one co-author (KDL, RB, JJ, LP, or RE) using the Cochrane Collaboration’s Risk of Bias (ROB 2.0) tool [20]. To gain a better understanding of the breadth of intervention delivery and measurement tools used to examine functional disability, we made an a priori decision to not remove any studies with high risk of bias.
Data extraction and analysis
From each article, a combination of two of co-authors (RB, KDL, JJ, LP, KCW, MP, TM, GC, RE, JS, AV, AMF, MF, TK, JB, RE) extracted data pertaining to study designs, sample characteristics, relevant outcome measures (including raw means and standard deviations), and intervention characteristics (see Supplementary File Table 2 for a complete data dictionary). Study design characteristics included primary purpose of study (e.g., feasibility, efficacy or effectiveness, implementation) and primary endpoint. If the authors did not specify a primary endpoint, reviewers selected the assessment that was the closest to the completion of the intervention. Participant characteristics included the following: sex; type(s) of cancer diagnosis; age; race and/or ethnicity; and treatment phase. Telehealth intervention characteristics included the following: intervention name; brief experimental intervention(s) description(s); name and type of comparison or control intervention, discipline of interventionists, and intervention delivery features (format, duration, frequency, mode, dose, and length of session). When available, reviewers extracted raw means and standard deviations associated with baseline and post-intervention timepoints for all identified measures of disability.
We used descriptive statistics to summarize characteristics of study designs, participant characteristics, relevant outcome measures, and intervention characteristics. Most often, the unit of analysis was the study, for example, when describing the proportion of studies that used one delivery method versus multiple delivery methods or the average duration of each experimental intervention. Additionally, we used the value list for some variables as the unit of analysis, for example, to describe the proportion of telephone delivery included in the grand sum of all delivery methods reported across all studies.
Standardized effect sizes were used to determine the threshold of outcome clinical significance. For studies that reported raw group means and standard deviations at baseline and the study-specific primary endpoint, effect sizes (Cohen’s d) were computed using standardized mean differences [21]. Between-group effect sizes were estimated by the differences between the intervention mean changes in functional disability in each intervention and control group divided by the pooled standard deviation (SD) at baseline [21]. If the study did not report a primary endpoint, the effect size of the follow-up closest to the post-intervention assessment was selected for comparison to reduce heterogeneity in data collection. Magnitude of effect size was categorized as negligible (< 0.2), small (0.2–0.5), medium (0.5–0.8), or large (> 0.8) [21]. Forest plots were developed to illustrate both the data for each specific study and the pooled estimates. A separate forest plot was presented for each subgroup of studies representing the most commonly included outcome measures. The mean difference and 95% confidence interval for each study in intervention and control groups were used to calculate the pooled mean difference. Q statistics with an I2 index were used to assess heterogeneity between studies. An I2 index of 50% or greater and a p value less than 0.1 were taken to indicate significant statistical heterogeneity [22]. In cases of statistical heterogeneity, a random effects model was applied. When there was no evidence of heterogeneity, the results of fixed effects models were presented. Statistical analyses were performed in R (Version 3.6.3) using the META and METASENS packages.
Results
Literature search and study selection
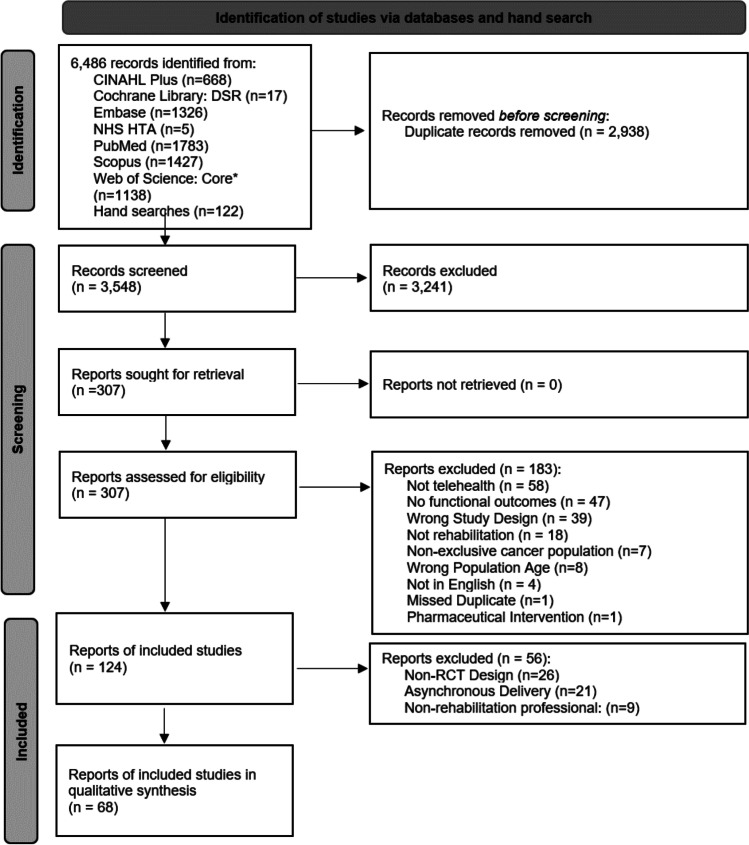
The literature and hand searches yielded 6,437 records of which 2,938 were duplicates and 3,499 were screened (Fig. 1). After the title and abstract screening was completed, 3,192 records were excluded and 307 proceeded to full text screening. One hundred thirty-two articles were included in the first-level of full-text review. The second level of full-text review specified articles that were randomized controlled trials, synchronously delivered, and administered by a rehabilitation professional. Of the 64 excluded during the second-level of full text screening, 26 were not randomized controlled trials, 21 did not deliver care synchronously, nine did not include a rehabilitation professional, and eight did not focus exclusively on adult cancer survivors. The final sample for analysis included 68 studies.
Fig. 1.
PRISMA flow diagram
Risk of bias
A risk of bias assessment was completed for all included studies utilizing the Cochrane Collaboration’s Risk of Bias (ROB 2.0) tool [20]. Studies were assessed across five domains describing bias associated with (1) randomization; (2) effects of intervention assignment; (3) missing outcome(s) data; (4) measurement of outcome; and (5) selection of reported results. Overall risk of bias was rated as low, some concerns, or high. These results are summarized in Supplementary Materials Table 3. Overall, included studies had the following risk of bias ratings: some concerns of bias (n = 29, 43%), followed by high concern (n = 21, 31%), and low risk of bias (n = 18, 26%). Contributors to bias in studies rated as some concern and high concern (n = 50) were most commonly: bias in effects of intervention assignment (n = 30, 60%) and/or randomization (n = 26, 52%), followed by measurement of outcome (n = 18, 36%), selection of reported results (n = 11, 22%), and/or missing outcome data (n = 8, 16%).
Study sample and design
Table 1 describes the study samples. Across the 68 studies, sample sizes ranged from seventeen to 641 participants (mean: 154 individuals, SD: 160). Thirty studies (44%) exclusively enrolled females, ten studies (15%) enrolled only males, and the remaining studies (n = 28, 41%) enrolled both male and female survivors. Fifty studies (74%) enrolled participants of one cancer type. Within these studies, the three most prominent cancer types were breast (n = 30, 60%), prostate (n = 8, 16%), and colorectal (n = 4, 8%). Diagnoses ranged from one to eleven in studies that did not limit enrollment based on cancer type. Thirty-four of the 68 studies (50%) included participants that were post-primary treatment completion only whereas 23 studies (34%) enrolled patients during active cancer treatment. Only 5 studies (7%) included participants who were in varying phases across the cancer care continuum (e.g., participants currently receiving treatment and post-treatment completion). Of the 38 studies that reported race or ethnicity (56%), study samples were overwhelmingly White, non-Hispanic participants (mean: 81%, SD: 25).
Table 1.
Included studies (n = 68) participant characteristics
| Authors, (year) | Sample size | Cancer type(s) included | Treatment status | Age (years), mean (SD) | % White |
|---|---|---|---|---|---|
| Abrahams (2017) [23] | 132 | Breast | Post-treatment | Intervention: 52.5 (8.2) | Not reported |
| Allard (2007) [24] | 117 | Breast | During primary treatment | Total sample: 53.6 (10.17) | Not reported |
| Anderson (2006) [25] | 57 | Breast, prostate, multiple myeloma, lung | During primary treatment | Total sample: 52 | 72% |
| Aranda (2006) [26] | 105 | Breast | Palliative |
Intervention: 57 (median); Control: 55 (median) |
Not reported |
| Ashing (2016) [27] | 39 | Breast | Post-treatment | Total sample: 55.5 (13.1) | 0% |
| Ashing-Giwa (2008) [28] | 23 | Cervical | Post-treatment | Total sample: 49.75 (10.9) | Not reported |
| Badger (2013) [29] | 80 | Breast | During primary treatment | Total sample: 47.34(10.5) | 0% |
| Bailey (2004) [30] | 39 | Prostate | Pre-treatment | Total sample: 75.4 | 86% |
| Barsevick (2004) [31] | 396 | Breast, lung, lymphoma, colorectal, genital (endometrial, ovarian, prostate, testicular) | During primary treatment | Total sample: 56.3 (12.5) | 91% |
| Basen-Engquist (2020) [32] | 37 | Breast | During primary treatment |
Intervention: 49.6 (13.3); Control: 49.2 (9.2) |
59% |
| Bennett (2007) [33] | 56 | Breast. Other | Post-treatment |
Intervention: 55.5 (8.9); Control: 60.1 (11.0) |
98% |
| Bruera (2013) [34] | 190 | Gastrointestinal, lung, breast, genitourinary, melanoma, hematologic, other | Palliative | Total sample: 57.5 (median), range 24–84 | 72% |
| Burns (2017) [35] | 82 | Head and neck | During primary treatment |
Intervention: 64 (SD 7.58); Control: 65 (SD 7.45) |
Not reported |
| Chambers (2012) [36] | 740 | Prostate | Pre-treatment |
Age at diagnosis: Intervention: 63.34 (7.59); Control: 63.43 (7.46) |
Not reported |
| Chan (2020) [37] | 202 | Prostate | During primary treatment, post-primary treatment | Total sample: 70 (median) | 92.6% |
| Cheville (2013) [38] | 66 | Lung, colorectal | During primary treatment |
Intervention group: 63.8 (12.5); Control group: 65.5 (8.9) |
100% |
| Cheville (2018) [39] | 516 | Stage IIIC or IV solid tumors, multiple myeloma, myelodysplastic syndrome, or Stage IIIC or IV lymphoma were enrolled | During primary treatment | Total sample: 65.6 (11.1) | 95.3% |
| Cheville (2019) [40] | 516 | "Pathologically confirmed stage IIIC or IV solid or hematologic cancer" | During primary treatment | Total sample: 65.6 (11.1) | Not reported |
| Chow (2020) [41] | 41 | Leukemia, lymphoma, other | Post-treatment | Total sample: 45.1 (median) | 78% |
| Clark (2013) [42] | 131 | Brain, gastrointestinal, head and neck, lung, other | During primary treatment | Total sample: 58.7 (10.6) | 97% |
| Dennett (2018) [43] | 46 | Breast, prostate, non-Hodgkin’s lymphoma, colorectal, gynecological, liver, pancreas, Hodgkin's lymphoma, kidney, lung, acute myeloid leukemia | During primary treatment | Total sample: 59 (12) | Not reported |
| Djuric (2011) [44] | 40 | Breast | Pre-treatment | Intervention non-completers 52.0 (6.9); Intervention completers 52.3 (9.5) | 99% |
| Dong (2019) [45] | 50 | Breast | Post-treatment |
Intervention: 48.00 (5.54); Control: 51.63 (7.49) |
Not reported |
| Dong (2020) [46] | 44 | Breast | Post-treatment |
Intervention: 50.4 (7.4); Control: 52 (8.5) |
Not reported |
| Dos Santos (2020) [47] | 167 | Breast, digestive, hematologic | Post-treatment | Total sample: 51 (median) | Not reported |
| Duijts (2012) [48] | 422 | Breast | Post-treatment | Total sample: 48.2 (5.6) | Not reported |
| Ferguson (2016) [49] | 47 | Breast | Post-treatment | Total sample: 54.6 (12.12) | 100% |
| Fjell (2020) [50] | 149 | Breast | During treatment | Intervention: 48 (10.6); control: 50 (11.6) | Not reported |
| Freeman (2015) [51] | 118 | Breast | Post-treatment |
Live delivery: 55.44 (8.08); Telemedicine: 55.57 (9.88); Waitlist Ctrl: 55.28 (7.90) |
87% |
| Galiano-Castillo (2017) [52] | 81 | Breast | Post-treatment | Total sample: 48.3 (8.8) | Not reported |
| Galiano-Castillo (2016) [53] | 81 | Breast | Post-treatment | Intervention: 47.4 (9.6); control: 49.2 (7.9) | Not reported |
| Gell (2020) [54] | 66 | Breast, cervical, endometrial, lung, leukemia, lymphoma, urinary, melanoma, rectal, oral, ovarian, prostate | Post-treatment | Total sample: 61.4 (9.0) | 98.5% |
| Gordon (2015) [55] | 410 | Colorectal | During primary treatment | Total sample: 66.3 (10.1) | Not reported |
| Hawkes (2013) [56] | 410 | Colorectal | Post-treatment | Total sample: 66.35 | Not reported |
| Hawkins (2017) [57] | 300 | Prostate | During primary treatment |
Experimental: 60.71 (7.54) Control 1: 59.44 (6.40); Control 2: 60.53 (7.44); |
Not reported |
| Hayes (2013) [58] | 194 | Breast | During primary treatment | Not reported | Not reported |
| Hegel (2011) [59] | 29 | Breast | During primary treatment | Total sample: 52.6 (9.4) | 100% |
| Hung (2014) [60] | 37 | Lymphoma, myeloma | Post-treatment |
Intervention: 57.5 (9.8); Usual care: 59.9 (9.2) |
Not reported |
| Jensen (2012) [61] | 95 | Prostate | Post-treatment |
Intervention: 64.1 (62.5–65.8); Control: 62.5 (60.9–64.2) |
Not reported |
| Kelleher (2021) [62] | 31 | Colorectal | Post-treatment | Total sample: 59.6 (10.5) | 96.8% |
| Kim (2020) [63] | 326 | Breast | During treatment | Total sample: 52 | 87.0% |
| Koller (2018) [64] | 39 | Gastrointestinal, breast, gynecological, lung, multiple myeloma, other | Palliative | Total sample: 56.6 (10.6) | Not reported |
| Lahart (2016) [65] | 80 | Breast cancer | Post-treatment | Total sample: 53.6 (9.4) | 78% |
| Lee (2019) [66] | 96 | Prostate | Post-treatment |
Intervention: 69.06 (7.21); Control: 69.82 (7.73) |
Not reported |
| Ligibel (2012) [67] | 121 | Breast, colon, rectal | Post-treatment | Total sample: 54 (995) | 91.7% |
| Meneses (2020) [68] | 432 | Breast | Post-treatment | Not reported | 94.2% |
| Meneses (2018) [69] | 40 | Breast | Post-treatment | Total sample: 56.63 (10.26) | 62.5% |
| Meneses (2009) [70] | 53 | Breast | Post-treatment | Total sample: 53.58 (11.55) | 94.3% |
| Meneses (2007) [71] | 256 | Breast | Post-treatment | Total sample: 54.5 (11.58) | 82% |
| Morey (2009) [72] | 641 | Breast, colorectal, prostate | Post-treatment |
Intervention 73.0 (5.0); Control 73.1 (5.1) |
89% |
| Pinto (2005) [73] | 86 | Breast | Post-treatment | Total sample: 53.42 (9.08) | 96.4% |
| Pinto (2013) [74] | 192 | Breast | Post-treatment | Total sample: 60.0 (9.9) | 93.8% |
| Pinto (2013) [75] | 46 | Colorectal | Post-treatment | Total sample: 57.3 (9.7) | 98% |
| Raphaelis (2020) [76] | 153 | Gynecological, gastrointestinal, ears, nose, throat, lung, hematological, bone, breast, Other | During treatment and post-treatment |
Intervention: 58.6; Control: 58.9 |
|
| Sajid (2016) [77] | 19 | Prostate | During primary treatment | Total sample: 70 | 73.7% |
| Scura (2004) [78] | 17 | Prostate | Pre-treatment | Total sample: 66 (8.3) | 59% |
| Silvers (2014) [79] | 21 | Esophageal, stomach | Pre-treatment |
Intervention: 72 (12); Control: 64 (14) |
Not reported |
| Sui (2020) [80] | 200 | Lung | During treatment and post-treatment |
Intervention: 61.37 (11.21); Control: 62.35 (9.98) |
Not reported |
| Syrjala (2018) [81] | 337 | Acute leukemia, chronic myelogenous leukemia, non-Hodgkin’s lymphoma, myelodysplasias, multiple myeloma, Hodgkin’s lymphoma, other | Post-treatment | Total sample: 51 (12) | 94% |
| Temur (2019) [82] | 61 | Breast | Post-treatment |
Intervention group: 47.6 (8.96); Control group: 45.6 (9.03) |
Not reported |
| Vallerand (2018) [83] | 51 | Leukemia, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma | During primary treatment | Total sample: 52.6 (13.7) | Not reported |
| Waller (2021) [84] | 22 | Abdominal | Pre-treatment |
Intervention: 55.5; Control: 61 |
Not reported |
| Wang (2019) [85] | 60 | Oral | During primary treatment | Total sample: 55.98 (11.73) | Not reported |
| Wenzel (2015) [86] | 204 | Cervical | Post-treatment | Total sample: 43 | 51.4% |
| Yanez (2015) [87] | 74 | Prostate | During primary treatment | Total sample: 68.84 (9.23) | 56.8% |
| Yates (2005) [88] | 109 | Breast | During primary treatment | Total sample: 49.4 (9.4) | Not reported |
| Zhang (2015) [89] | 244 | Prostate | Post-treatment |
Support group: 66.8 (7.2); Telephone group: (64.3 (7.3); Control: 64.9 (8.2) |
62.7% |
| Zhou (2020) [90] | 111 | Breast | Pre-treatment and during primary treatment | Intervention: 49.84 (8.85) | Not reported |
The reported primary purpose of included study designs was to test efficacy or effectiveness (n = 49, 72%) followed by feasibility (n = 16, 24%), and implementation (n = 3, 4%) of an intervention. Thirty-two studies (47%) included a usual care control group, whereas seven studies (10%) used waitlist-controls. The remaining studies (n = 29; 43%) included different combinations of active or attention control groups. Studies were conducted in North America (n = 39, 57%), Europe (n = 13, 19%), Australia (n = 10, 15%), and Asia (n = 6, 9%).
Intervention delivery features
Table 2 presents a summary of intervention descriptions and delivery characteristics are presented in Table 2. Most interventions were delivered completely by telehealth methods (n = 44, 65%), although 24 interventions (35%) had an in-person component. Most commonly used intervention delivery methods were telephone calls (n = 61 interventions, 90%), additional in-person component (n = 24, 35%), general internet portals (n = 9, 13%), and activity trackers (n = 9, 13%). Regarding the method of delivery, most of the studies used multiple delivery methods (n = 39, 57%) versus one type of delivery method (n = 29, 43%). The number of delivery methods ranged from 1 to 6, with a mean of 2.0 (SD = 1.1). Almost two-thirds of the studies (n = 40, 59%) used telephone calls as the only delivery method.
Table 2.
Summary of intervention description and delivery features
| Author (year) | Brief intervention description | Mode of delivery | Format | Duration and frequency of intervention | Interventionist (discipline) | Comparison condition(s) |
|---|---|---|---|---|---|---|
| Abrahams (2017) [23] | Cognitive-behavioral model of face-to-face and web-based modules reviewing precipitating and perpetuating factors of fatigue |
General internet portal; Telephone calls; In-person |
Individual |
24 weeks Fixed Interactions: 3 |
Psychology | Wait list |
| Allard (2007) [24] | Psychoeducational telephone intervention designed to help women direct their focus of attention toward the functional pathway of coping | Telephone calls | Individual |
2 weeks Fixed Interactions: 2 |
Nursing | Usual care |
| Anderson (2006) [25] | 20-min audiotape included positive mood statements and positive imagery suggestions | Telephone calls | Individual |
2 weeks Not reported Nurses called “periodically” |
Nursing |
Distraction audiotape; Relaxation audiotape; Wait list |
| Aranda (2006) [26] | Cognitive-behavioral techniques and education | Telephone calls | Family dyad or group |
1 week Fixed Interactions: 2 |
Nursing | Usual care |
| Ashing (2016) [27] | Psychoeducation | Telephone calls | Individual |
16 weeks Fixed Interactions: 8 |
Health-related Research Assistant | Usual care |
| Ashing-Giwa (2008) [28] | Problem-focused Cognitive Behavioral Therapy | Telephone calls | Individual |
12 weeks Fixed Interactions: 6 |
Health-related Research Assistant | Usual care |
| Badger (2013) [29] | Enhancing emotional support both through the direct effects of the interaction with the interventionist and mobilizing the survivors’ naturally occurring support | Telephone calls | Family dyad or group |
8 weeks Fixed Interactions: 8 |
Social Work | Telephone health education |
| Bailey (2004) [30] | Watchful waiting intervention to manage uncertainty via cognitive reframing | Telephone calls | Individual |
5 weeks Fixed Interactions: 5 |
Nursing | Usual care |
| Barsevick (2004) [31] | Energy conservation and activity management | Telephone calls | Individual |
3 weeks Fixed Interactions: 3 |
Nursing | Attention control (information on nutrition) |
| Basen-Engquist (2020) [32] | To educate and enhance self-efficacy in the size acceptance approach | Telephone calls | Individual |
25 weeks Fixed Interactions: 20 |
Masters-level Health Educators | Usual care |
| Bennett (2007) [33] | Motivational interviewing to facilitate 30 daily minutes of moderate to vigorous physical activity | Telephone calls | Hybrid |
16 weeks Fixed Interactions: 4 |
Social Work | Two phone calls (brief, social conversations) |
| Bruera (2013) [34] | Methylphenidate + nursing phone intervention | Telephone calls | Individual |
2 weeks Variable |
Nursing | Methylphenidate + control phone intervention; Placebo + nursing phone intervention; Placebo + control phone intervention |
| Burns (2017) [35] | Speech pathology telepractice |
Video Conferencing; Telephone calls; In-Person; Other |
Individual | Not reported | Speech Language Pathology | Usual care |
| Chambers (2012) [36] | Psycho-education, stress management, coaching, decision support and cognitive reframing | Telephone calls | Individual |
20 weeks Fixed Interactions: 5 |
Nursing | Usual care |
| Chan (2020) [37] | Web-based behavioral support with two optional calls with dietician and/or exercise physiologist |
General Internet Portal; Telephone calls; Activity tracker; Text |
Individual |
12 weeks Variable 4 interactions (2 optional calls) |
Dietetics; Exercise Physiology |
Level 1: General information and guidelines via website; Level 2: Level 1 plus personalized diet and exercise prescriptions and videos and survey about progress; Level 3: Level 2 plus Fitbit Alta with physical activity reports and supportive text messages |
| Cheville (2013) [38] | Exercise plus pedometer-based walking program |
Telephone calls; In-Person |
Individual |
8 weeks Fixed Interactions: 5 |
Physical Therapy | Usual care |
| Cheville (2018) [39] | Three Component Model (TCM)-Rehabilitation Services plus conventional TCM pain management |
Telephone calls; Activity trackers |
Individual |
26 weeks Fixed Interactions: 8 |
Physical Therapy; Nursing; MD |
Three component model rehabilitation; Exercise plus pedometer |
| Cheville (2019) [40] | Telerehabilitation with pharmacological pain management arm |
General internet portal; Telephone calls; Activity tracker; In-person |
Individual |
24 weeks Variable |
Physical Therapy; Nursing; MD |
Usual care |
| Chow (2020) [41] | Mobile health supported intervention to improve diet and physical activity |
Telephone calls; Text-messaging; General internet portal; Activity Tracker; Facebook group; Mobile application |
Individual |
16 weeks Fixed Interactions: # not reported |
Health-related Research Assistant | Access to the Fitbit tracker and Healthwatch360 app |
| Clark (2013) [42] | Multi-disciplinary counseling intervention to maintain quality of life | Telephone calls | Hybrid |
27 weeks Fixed Interactions: 17 |
Physical Therapy; Psychology; Social Work; Nursing |
Usual care |
| Dennett (2018) [43] | Motivational interviewing plus aerobic and resistance exercise, individual and group education | Telephone calls | Individual |
7 weeks Fixed Interactions: 7 |
Physical Therapy | Oncology rehabilitation of exercise and education |
| Djuric (2011) [44] |
Motivational interviewing; Education and counseling |
Telephone calls | Individual |
52 weeks Variable |
Dietetics | Education, pedometer, log, newsletters |
| Dong (2019) [45] |
Remotely-guided muscle training, endurance training, and education |
Video Conferencing; Instant Messaging or Texting; Mobile Application; Activity Tracker |
Individual |
12 weeks Fixed Interactions: 84 |
Physical Therapy | Usual care (traditional rehabilitation) |
| Dong (2020) [46] |
Remotely-guided muscle training, endurance training, and education |
Video Conferencing; Instant Messaging or Texting; Mobile Application; Activity Tracker |
Individual |
12 weeks Fixed Interactions: 84 |
Physical Therapy | Usual care (traditional rehabilitation) |
| Dos Santos (2020) [47] | Computer-Assisted Cognitive Rehabilitation | Other (computer program) | Individual |
12 weeks Fixed Interactions: 9 |
Neuropsychology |
Telephone calls; Cognitive exercises at home via workbook |
| Duijts (2012) [48] | Cognitive Behavioral Therapy and relaxation exercises |
Telephone calls; In-person |
Group |
6 weeks Fixed Interactions: 6 |
Physical Therapy; Psychology; Social Work |
Physical exercise |
| Ferguson (2016) [49] | Cognitive Behavioral Therapy | Video conferencing | Individual |
8 weeks Fixed Interactions: 8 |
Psychology | Behavioral placebo (supportive therapy) |
| Fjell (2020) [50] | Interaktor: Real-time cancer sequelae monitoring |
Text messaging; Mobile Application; Telephone calls; In-person |
Individual |
18 weeks Variable |
Nursing; Health-related Research Assistant |
Usual care |
| Freeman (2015) [51] |
Education; mind–body connection; imagery techniques |
Video Conferencing; Telephone Calls |
Group |
17 weeks Fixed Interactions: 17 |
Social Work; Medical Physician | In-person delivery |
| Galiano-Castillo (2017) [52] | Tailored exercise program |
Video Conferencing; General Internet Portal; Instant Messaging or Texting; Telephone calls |
Individual |
8 weeks Fixed Interactions: 24 |
Not specified | Education regarding health benefits of physical activity; Written general exercise guidelines |
| Galiano-Castillo (2016) [53] | Online system that facilities remote rehabilitation and consists of public and private interfaces with updated information about breast cancer |
Video Conferencing; General Internet Portal; Instant Messaging or Texting; Telephone calls |
Individual |
8 weeks Fixed Interactions: 24 |
Not specified | Written recommendations |
| Gell (2020) [54] | Health coaching |
Instant Messaging or Texting; Telephone calls; Activity Tracker |
Individual |
8 weeks Fixed Interactions: 4 |
Not specified | Fitbit only |
| Gordon (2015) [55] | Health coaching | Telephone calls | Individual |
24 weeks Fixed Interactions: 11 |
Not specified | Usual care |
| Hawkes (2013) [56] | Health coaching | Telephone calls | Individual |
24 weeks Fixed Interactions: 12 |
Psychology; Nursing; Health-related Research Assistant |
Usual care |
| Hawkins (2017) [57] | Comprehensive Health Enhancement Support System + Mentor | Telephone calls | Individual |
24 weeks Fixed Interactions: 10 |
Human Cancer Information Mentor |
Mentor only; CHESS only |
| Hayes (2013) [58] | Exercise | Telephone calls | Individual |
32 weeks Fixed Interactions: 16 |
Exercise Physiologist |
Telephone delivered exercise intervention; Usual care |
| Hegel (2011) [59] | Cognitive Behavioral Therapy | Telephone calls | Individual |
6 weeks Fixed Interactions: 6 |
Occupational Therapy | Usual care |
| Hung (2014) [60] | Nutrition and exercise counseling | Telephone calls | Individual |
12 weeks Variable |
Dietetics; Exercise Physiology |
Usual care |
| Jensen (2012) [61] | Telephone consultation | Telephone calls | Individual |
1 week Fixed Interactions: 1 |
Nursing | Usual care |
| Kelleher (2021) [62] | Coping skills training | Telephone calls | Individual |
5 weeks Fixed Interactions: 5 |
Health-related Research Assistant | Usual care |
| Kim (2020) [63] |
Comprehensive Health Enhancement Support System + Mentor |
In-Person; General Internet Portal; Telephone calls |
Individual |
24 weeks Fixed Interactions: 10 |
Cancer Information Specialist Mentor |
Internet access with list of websites; Comprehensive Health Enhancement Support System—Information and Support (CHESS-IS) |
| Koller (2018) [64] | Education, skill building, and coaching to support self-management |
Telephone calls; In-person |
Individual |
6 weeks Not reported |
Nursing | Usual care |
| Lahart (2016) [65] | Physical activity counseling |
Telephone calls; In-Person; Other |
Individual |
24 weeks Not reported |
Not specified | Usual care |
| Lee (2019) [66] | Exercise |
Telephone calls; Mobile Application; Other |
Individual |
12 weeks Not reported |
Medical Physician | Pedometer |
| Ligibel (2012) [67] | Exercise | Telephone calls | Individual |
16 weeks Variable 10–11 interactions |
Social Work | Usual care |
| Meneses (2020) [68] | Education and support |
Telephone calls; In person; |
Individual |
52 weeks Fixed Interactions: 11 |
Nursing | Delayed support |
| Meneses (2018) [69] | Education |
Telephone calls; Other |
Individual |
3 weeks Fixed Interactions: 9 |
Not specified | Wait list |
| Meneses (2009) [70] | Psychoeducation and support |
Telephone calls; In-person |
Individual |
28 weeks Fixed Interactions: 8 |
Nursing | Wait list |
| Meneses (2007) [71] | Psychoeducation and support |
Telephone calls; In-person |
Individual |
24 weeks Fixed Interactions: 9 |
Nursing | Wait list |
| Morey (2009) [72] | Counseling and education | Telephone calls | Individual |
52 weeks Fixed Interactions: 15 |
Social Work | Wait list |
| Pinto (2005) [73] | Physical activity counseling |
Telephone calls; Activity Tracker; In-person |
Individual |
12 weeks Fixed Interactions: 15 |
Health-related Research Assistant | Contact control |
| Pinto (2013) [74] | Advice and counseling |
Telephone calls; In-Person; Other |
Individual |
12 weeks Fixed Interactions: 8 |
Not specified | Advice plus contact control |
| Pinto (2013) [75] | Physical activity |
Telephone calls; In-Person; |
Individual |
12 weeks Fixed Interactions: 12 |
Health-related Research Assistant | Symptom surveys |
| Raphaelis (2020) [76] | Self-management support |
Telephone calls; In-Person |
Family Dyad or Group |
Not specified Variable (based on pain intensity; maximum of 4 interactions) |
Nursing | Usual care |
| Sajid (2016) [77] | Home-based walking and exercise |
Telephone calls; Activity Tracker; In-person |
Individual |
6 weeks Fixed Interactions: 6 |
Exercise Physiology |
Wii-fit; Usual care |
| Scura (2004) [78] | Support and education |
Telephone calls; Other |
Individual |
52 weeks Fixed Interactions: 25 |
Nursing; Health-related Research Assistant |
Education |
| Silvers (2014) [79] | Nutrition education and behavior change techniques |
Telephone calls; In-person |
Individual |
26 weeks Fixed Interactions: 19 |
Not specified | Standard care |
| Sui (2020) [80] | WeChat app-based education and rehabilitation |
Mobile App; In-person |
Individual |
52 weeks Fixed Interactions: 130 |
Nursing | One education session with telephone follow-up as needed |
| Syrjala (2018) [81] | Education, resources and Cognitive Behavioral Therapy |
General Internet Portal; Telephone calls |
Individual |
24 weeks Variable |
Psychology |
Education and resources; Wait list |
| Temur (2019) [82] | Self-management for lymphedema using exercises and lymphatic drainage |
Telephone calls; In-person |
Individual |
24 weeks Fixed Interactions: 6 |
Health-related Research Assistant | Usual care |
| Vallerand (2018) [83] | Counseling | Telephone calls | Individual |
12 weeks Fixed Interactions: 12 |
Not specified | Written guidelines and goal |
| Waller (2021) [84] | Trimodal prehabilitation to promote physical activity, nutrition, and psychological well-being |
Mobile Application; Activity Tracker; Telephone calls |
Individual |
Two weeks Variable |
Physical Therapy | Usual care |
| Wang (2019) [85] | Support and monitoring |
Telephone calls; In-person |
Individual |
12 weeks Fixed Interactions: 6 |
Nursing | Education pamphlet |
| Wenzel (2015) [86] | Psychosocial counseling | Telephone calls | Individual |
9 weeks Fixed Interactions: 6 interactions |
Not specified | Usual care |
| Yanez (2015) [87] | Cognitive behavioral stress management treatment |
Video Conferencing; General Internet Portal |
Group |
10 weeks Fixed Interactions: 10 |
Occupational Therapy | Attention control of health promotion |
| Yates (2005) [88] | Psychoeducation |
Telephone calls; In-person |
Individual |
3 weeks Fixed Interactions: 3 |
Nursing | General cancer education |
| Zhang (2015) [89] | Biofeedback exercise + support group |
Telephone calls; In-person |
Hybrid |
12 weeks Fixed Interactions: 7 |
Psychology; Nursing |
Exercise + telephone support; Usual care |
| Zhou (2020) [90] | WeChat app-based education and rehabilitation |
Mobile application; In-person |
Individual |
26 weeks Fixed Interactions: 182 |
Medical Physician; Nursing; Health-related Research Assistant |
Usual care |
For most studies, the interventions were delivered through one-on-one provider to patient care (n = 59, 88%). Only three studies (4%) used interventions delivered to families or family dyads, three studies (4%) used groups of unrelated people, and three studies used a blend of group and individual interactions (4%). Duration of the interventions ranged from one to 52 weeks, with a mean of 16.5 weeks (SD = 13.1). Most interventions (n = 53, 78%) described a fixed number of interactions between the participant and provider, compared with a variable number of sessions (n = 10, 15%; n = 5 unreported, 7%) based on participant need.
Most interventions (n = 39, 57%) were delivered by practitioners in one discipline as opposed to those that involved practitioners from multiple disciplines (n = 12, 18%; not reported by 17, 25%). The most frequent discipline involved in intervention administration was nursing professionals (n = 24, 35%), followed by health-related research or graduate assistants (n = 10, 15%), physical therapy practitioners (n = 9, 13%), social workers (n = 7, 10%), or psychologists (n = 7, 10%) (see Table 2 for other discipline interventionists).
Functional disability outcome measures
The studies examined a variety of outcome measures related to disability and functional aspects of health-related quality of life (see Table 3). Less than half of the studies (n = 32, 47%) had a primary outcome measure assessing disability. We extracted data from 55 distinct outcome measures that assessed disability. In many instances, studies reported on specific subscales within the 55 outcome measures rather than composite scores. For example, Scura and colleagues [78] used two subscales of the Functional Assessment of Cancer Therapy—General (FACT-G) as independent outcome measures. If subscales were considered independent measures, the total number of unique outcome measures would be expanded to 84. Outcome tools were primarily self-report (n = 40, 73%) except for those that measured community mobility or community ambulation (n = 15, 27%). Community mobility was measured using performance-based measures of disability such as the Six Minute Walk Test. The most commonly reported outcome measures were the Functional Assessment of Cancer Therapy—General (n = 13, 19%), Medical Outcomes Study Short-Form (SF-36)—Physical Functioning Subscale (n = 13, 19%), Functional Assessment of Chronic Illness Therapy—Fatigue Scale (FACIT-F) (n = 8, 12%), and European Organisation for Research and Treatment Quality of Life (EORTC QLQC-30)—Physical Functioning Subscale (n = 6, 9%).
Table 3.
Summary of functional outcome measures and effect size ranges
| Outcome tool | Number of articles including outcome tool | Number of articles with available effect sizes (d) | Range of effect sizes |
|---|---|---|---|
| Functional Assessment of Cancer Therapy—General (FACT-G) | 13 [25, 27, 28, 38, 42, 44, 47, 62, 86–88] | 7 [25, 27, 38, 42, 47, 62, 88] | − 0.67 to 0.8 |
| FACT-G – Physical Well-Being Subscale | 2 [47, 78] | 1 [47] | − 0.22 to − 0.16 |
| FACT-G – Social Well-Being Subscale | 1 [47] | 1 [47] | − 0.10 to 0.22 |
| FACT-G – Emotional Well-Being Subscale | 1 [47] | 1 [47] | 0.13–0.29 |
| FACT-G – Functional Well-Being Subscale | 3 [47, 49, 78] | 2 [47, 49] | 0.18–0.3 |
| Medical Outcomes Study Short-Form (SF-36) – Total Score | 6 [45, 55, 56, 59, 68, 83] | 3 [45, 55, 83] | 0.63–15.66 |
| SF- 36—Physical Functioning Subscale | 13 [32, 33, 36, 46, 48, 68, 69, 71, 75, 81] | 8 [32, 33, 36, 46, 48, 71, 81] | − 0.67 to 4.62 |
| SF- 36 – Role Limitations Physical Subscale | 3 [32, 46, 71] | 3 [32, 46, 71] | 0.08–4.34 |
| SF- 36 – Role Limitations Emotional Subscale | 2 [32, 46] | 2 [32, 46] | − 0.03 to 0.43 |
| SF- 36 – Mental Well-Being Subscale | 3 [32, 36, 46] | 3 [32, 36, 46] | − 2.04 to 0.89 |
| SF- 36 – Vitality Subscale | 3 [32, 45, 46] | 3 [32, 45, 46] | 0.63–4.39 |
| SF- 36 – Physical Component Summary | 1 [51] | 1 [51] | 0.16–0.54 |
| SF- 36 – General Health Subscale | 2 [32, 46] | 2 [32, 46] | 0.79–1.4 |
| SF- 36 – Bodily Pain Subscale | 2 [32, 46] | 2 [32, 46] | − 0.11 to 1.36 |
| SF- 36 – Social Functioning Subscale | 2 [32, 46] | 2 [32, 46] | 0.27–0.7 |
| SF- 36 – Reported Health Transition Subscale | 1 [46] | 1 [46] | 0.75 |
| Short Form – 6D | 1 [55] | 1 [55] | − 0.01 |
| Functional Assessment of Chronic Illness Therapy – Fatigue Scale | 8 [34, 38, 39, 51, 56, 58, 67, 75] | 3 [38, 51, 67] | 0.25–1.87 |
| European Organisation for Research and Treatment Quality of Life (EORTC QLQC-30) – Total Score | 4 [23, 26, 76, 79] | 3 [23, 26, 79] | − 0.05 to 2.54 |
| EORTC QLQC-30 – Physical Functioning Subscale | 6 [43, 50, 53, 60, 67, 82] | 4 [43, 53, 60, 67] | 0.06–1.02 |
| EORTC QLQC-30 – Global Quality of Life Subscale | 5 [43, 53, 60, 67, 80] | 5 [43, 53, 60, 67, 80] | − 3.58 to 3.47 |
| EORTC-QLQ-C30—Emotional Function Subscale | 5 [43, 50, 53, 60, 82] | 3 [43, 53, 60] | 0.0–0.77 |
| EORTC-QLQ-C30—Cognitive Function Subscale | 5 [43, 50, 53, 60, 82] | 3 [43, 53, 60] | − 0.31 to 0.86 |
| EORTC-QLQ-C30—Social Function Subscale | 5 [43, 50, 53, 60, 82] | 3 [43, 53, 60] | 0.26–0.76 |
| EORTC-QLQ-C30 – Role Function Subscale | 4 [26, 50, 53, 82] | 2 [26, 53] | − 0.07 to 0.9 |
| Functional Assessment of Cancer Therapy—Breast (FACT-B) | 5 [44, 51, 65, 90, 91] | 4 [44, 51, 65, 90] | − 0.05 to 0.92 |
| FACT-B – Physical Well-Being Subscale | 3 [59, 65, 90] | 2 [65, 90] | − 0.22 to − 0.03 |
| FACT-B – Social Well-Being Subscale | 1 [90] | 1 [90] | 0.97 |
| FACT-B – Emotional Well-Being Subscale | 1 [90] | 1 [90] | 0.37 |
| FACT-B – Functional Well-Being Subscale | 3 [59, 65, 90] | 2 [65, 90] | − 0.2 to 1.25 |
| FACT-B4 | 1 [58] | 0 | – |
| Six Minute Walk Test | 5 [33, 43, 52, 67, 84] | 4 [33, 43, 52, 67] | 0.32–0.6 |
| Brief Pain Inventory – Pain Interference Subscale | 3 [53, 64, 76] | 1 [64] | − 0.76 to 0.42 |
| Activity Measure for Post-Acute Care Mobility Short Form | 3 [38–40] | 1 [38] | 1.05 |
| EuroQol- 5 Dimension | 3 [39, 40, 79] | 1 [79] | 1.7 |
| Moderate Intensity Physical Activity | 3 [43, 56, 60] | 3 [43, 56, 60] | − 0.19 to 0.22 |
| Daily Steps | 3 [41, 43, 77] | 1 [43] | 0.38 |
| Seven -Day Physical Activity Recall (min/week) | 2 [67, 74] | 1 [67] | 0.31 |
| Stand-up and sit-down chair test | 2 [45, 46] | 2 [45, 46] | 0.91–1.25 |
| Arm Lifting Test | 2 [45, 46] | 2 [45, 46] | − 2.43 to 0.65 |
| Trial Outcome Index | 2 [65, 86] | 1 [65] | − 0.04 |
| The World Health Organization Quality of Life (WHOQOL) | 2 [57, 91] | 0 | – |
| QOL-BC—Social Well-Being Subscale | 2 [70, 71] | 1 [70] | − 0.27 |
| Functional Assessment of Cancer Therapy-Cognition (FACT-Cog) – Quality of Life Subscale | 2 [47, 49] | 2 [47, 49] | 0.12–0.43 |
| PEG Scale Assessing Pain Intensity and Interference | 2 [39, 40] | 0 | – |
| Moderate to Vigorous Physical Activity | 2 [41, 54] | 1 [54] | 0.39 |
| Disabilities of the Arm, Shoulder and Hand Questionnaire | 2 [58, 82] | 0 | – |
| European Organisation for Research and Treatment Quality of Life – Breast Cancer (EORTC QLQ-BR23) – Sexual Function Subscale | 1 [82] | 0 | – |
| EORTC QLQ-BR23 – Sexual Enjoyment Subscale | 1 [82] | 0 | – |
| Quality of Life –Breast Cancer Survivors– Physical Health Subscale | 1 [64] | 1 [64] | 0.6 |
| Piper Fatigue Scale-revised | 1 [53] | 0 | – |
| Multidisciplinary Fatigue Inventory | 1 [29] | 1 [29] | 0.29 |
| Functional Assessment of Cancer Therapy – Colorectal | 1 [74] | 0 | – |
| Functional Assessment of Cancer Therapy – Cervix | 1 [86] | 0 | – |
| Functional Assessment of Cancer Therapy-Endocrine Subscale | 1 [48] | 1 [48] | 0.22–1.48 |
| Functional Assessment of Cancer Therapy – Anemia | 1 [47] | 1 [47] | 0.08–0.22 |
| Functional Assessment of Cancer Therapy – Prostate | 1 [57] | 0 | – |
| Expanded Prostate Cancer Index Composite | 1 [57] | 0 | – |
| Cantril’s Ladder of Life | 1 [30] | 1 [30] | 0.45 |
| Functional Performance Inventory | 1 [31] | 0 | – |
| Community Healthy Activities Model Program for Seniors | 1 [33] | 1 [33] | 0.1 |
| Sickness Impact Profile – 8 | 1 [23] | 0 | – |
| Symptoms Impact Profile | 1 [24] | 1 [24] | − 0.31 |
| AMPAC Activity Short Form | 1 [38] | 1 [38] | 0.13 |
| UCLA Prostate Cancer Index—Sexual Bother Subscale | 1 [36] | 1 [36] | 0.03 |
| UCLA Prostate Cancer Index—Urinary Incontinence Subscale | 1 [89] | 0 | –- |
| Total Physical Activity | 1 [65] | 1 [65] | − 0.07 |
| Gait Speed | 1 [66] | 1 [66] | − 0.27 |
| 2-Min Walk Test | 1 [66] | 1 [66] | 0.19 |
| 30-Second Chair Stand Test | 1 [66] | 1 [66] | 0.47 |
| International Physical Activity Questionnaire | 1 [65] | 1 [65] | − 0.07 |
| Late-Life Function & Disability Instrument – Basic Lower Extremity Function Subscale | 1 [72] | 1 [72] | 3.72 |
| Late-Life Function & Disability Instrument – Advanced Lower Extremity Function Subscale | 1 [72] | 1 [72] | 3.63 |
| Swallowing Function: Maximum interincisal opening and Mandible Function Impairment | 1 [85] | 1 [85] | − 1.40 to 1.26 |
| Godin Leisure Time Exercise Questionnaire | 1 [83] | 1 [83] | 2.91–4.22 |
| Champs Survey (Physical Activity and Lifestyle Behavior Subscales) | 1 [37] | 0 | – |
| PROMIS® Global Health (Physical and Mental Health Subscales) | 1 [41] | 0 | – |
| PROMIS® Depression Scale | 1 [87] | 0 | – |
| Short Physical Performance Battery | 1 [77] | 0 | – |
| International Index of Erectile Function Scale | 1 [78] | 0 | – |
| 3-Min Step Test | 1 [58] | 0 | – |
| One Mile Walk Test | 1 [73] | 0 | – |
| Tread walk Test | 1 [74] | 0 | – |
| Sit to Stand Test | 1 [43] | 0 | – |
Estimated effect sizes
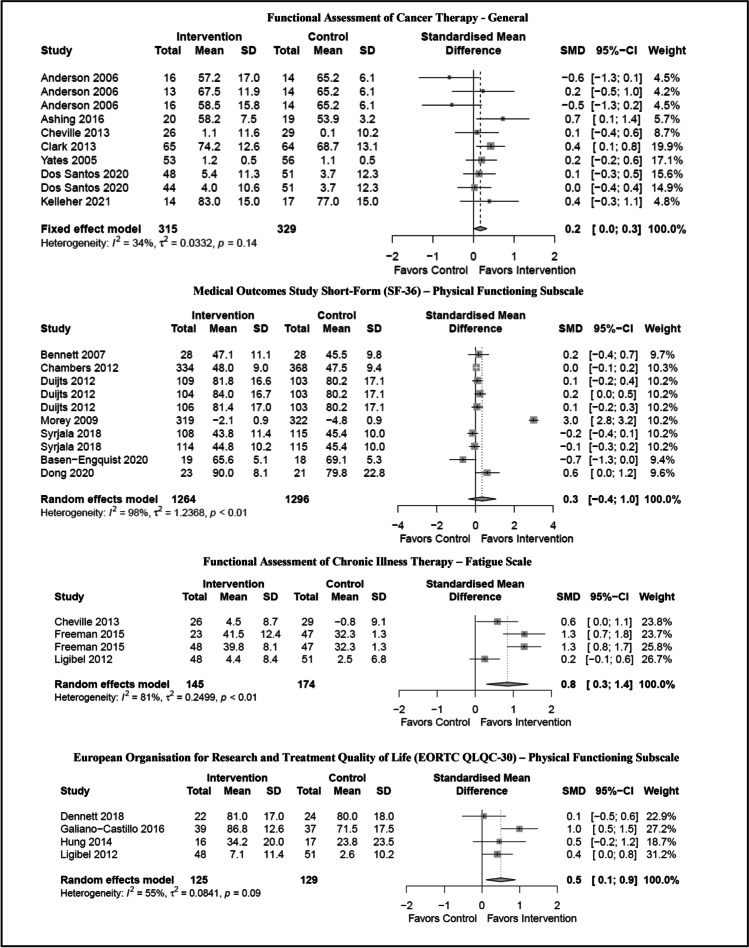
On average, the studies reported on 3.1 functional outcomes (range: 1–11 outcomes) for a total of 253 outcomes across 68 studies. Data to calculate between-group effect sizes was available for 154 (54%) of available outcomes (see Table 3). Effect sizes ranged from − 3.58 (clinically meaningful outcomes favoring control intervention) to 15.66 (highly clinically meaningful outcomes favoring the experimental intervention). Given the multitude of outcome measures, we further analyzed the four most used outcome measures to examine influence of telehealth-based cancer rehabilitation interventions on disability (see Fig. 2 for associated forest plots).
Fig. 2.
Forest plots of most commonly reported functional outcome measures with available data increase font of each section of figure
FACT-G
Of the thirteen articles contained the FACT-G, only seven articles contributed to the analysis. Two articles [25, 47] were comparative effectiveness studies as represented by two or more contrasts in Fig. 2. Heterogeneity across studies was not detected (I2 = 34%; p = 0.14); however, the interventions varied in content and delivery. The ten interventions represented had a small clinically meaningful effect on overall health-related quality of life (SMD: 0.2, CI: 0.0–0.3).
SF-36—physical functioning
Thirteen articles contained the SF-36 Physical Functioning subscale. Eight articles, representing ten distinct interventions, contributed to the pooled outcome. Heterogeneity across studies was detected (I2 = 98%; p < 0.001). Non-significant clinical reductions in perceived physical well-being favored the intervention; however, these were not significant (SMD: 0.3, CI: − 0.4 to 1.0).
FACIT-F
Of the eight articles used the FACIT-F, only three articles contributed to the pooled outcome. The three articles represented four distinct interventions. Heterogeneity across studies was detected (I2 = 81%; p < 0.001). The pooled outcome revealed a significant decrease in interference of fatigue on health-related quality of life (SMD: 0.8, CI: 0.3–1.4).
EORTC QLQC-30—physical functioning subscale
Of the six articles contained the EORTC QLQC-30—Physical Functioning Subscale, only four articles contributed to the pooled outcome. Heterogeneity across studies was detected (I2 = 55%; p < 0.09). The pooled outcome revealed a significant improvement perceived physical well-being (SMD: 0.5, CI: 0.1–0.9) among the available data.
Discussion
This systematic review assesses the current state of the science and effectiveness of telehealth-based cancer rehabilitation interventions influencing disability. Included studies were randomized controlled trials with at least one synchronous intervention delivery component and incorporated a measure of disability as a primary or exploratory outcome. Given the available data, telehealth-based cancer rehabilitation interventions appear to have a small positive effect on quality of life as measured by the FACT-G and FACIT-F measurement system, which includes an assessment of a person’s perceived ability to function in daily life. The effect on physical functioning is less clear, with no effect seen when measured by the SF-36 and a small effect seen when measured by the EORTC QLQC-30. It is possible that the effects are underestimated because disability and health-related quality of life measures were not the primary outcome for the majority of the studies. In other words, a study intervention with a primary purpose to reduce a specific symptom (e.g., pain, cognitive deficits, balance) may not generalize to reductions in disability or health-related quality of life.
In sum, telehealth-based cancer rehabilitation does have an impact that merits further evaluation and study; however, the descriptive results indicate that future research will need to include more diverse patient populations, create more consensus around appropriate measure selection, and focus on implementation science methods to address challenges to translating evidence into practice.
Patient populations and study characteristics
Studies included in this systematic review were not evenly distributed across cancer type, timing of treatment/intervention, and location. Included studies were heavily weighted toward individuals with breast cancer (n = 30, 60%) and post-primary treatment interventions (n = 34, 50%). This is somewhat unsurprising as breast cancer is the most frequently diagnosed new cancer in women in the USA [92] and one of the most common diagnoses in more than 25 countries globally [93]. In addition, the field of cancer survivorship was initially conceptualized as beginning post-treatment completion [94]. Thus, this skew toward post-treatment intervention is consistent with the larger body of literature in cancer and rehabilitation and prevalence estimates. However, it presents three challenges. First, findings may have limited utility in determining implications for other cancer populations. Second, the implications for delivery outside the post-treatment phase also remain somewhat unclear and exposes the relatively smaller bodies of research examining effectiveness in the pre-treatment phase, during treatment, and end-of-life care. Lastly, study locations were primarily located in the USA and Northern Europe, not only limiting generalizability to many indigenous peoples and non-Western countries but also potentially reflecting lack of access to telehealth-based cancer rehabilitation services in low-income countries.
Race and ethnicity of study participants presented another area of imbalance in the included studies. Of the studies that reported ethnicity (n = 38, 56%), participants were almost exclusively White and non-Hispanic. The high number of White participants, as well as the large number of studies that did not report ethnicity (n = 30, 44%), may limit the ability to generalize findings to other race and ethnicity groups. Similar to findings in this review, this has been a persistent challenge in the larger world of clinical trials [95], including in rehabilitation medicine [96]. This lack of inclusion for historically or socially marginalized groups may undermine personalized medicine efforts and contribute to the persistence of health inequities [97]. The results described in our analysis indicate that there are challenges to translation in clinical care settings beyond individuals with breast cancer and individuals outside of economically developed nations and socially marginalized populations.
There are consequences associated with ongoing lack of inclusion of historically or socially marginalized groups in cancer rehabilitation research and clinical practice. Lack of representative study samples may prevent researchers and clinicians from delivering high-quality patient-centric care [96]. Furthermore, lack of examination of social determinants of health on access, delivery, or effectiveness of telehealth-based cancer rehabilitation may perpetuate healthcare disparities and sub-optimal outcomes among vulnerable populations. Without evidence that acknowledges the influence of telehealth-based cancer rehabilitation services among marginalized groups, we are at risk of constraining the growth or applicability of the cancer rehabilitation field among the broader cancer community and inefficiently utilizing costly healthcare and research resources [96]. Thus, future research and clinical practice using telehealth-based cancer rehabilitation interventions must leverage research designs and clinical practice delivery that support inclusion of historically or socially marginalized groups.
Outcomes and measurement
When evaluating the effectiveness of interventions, one significant challenge was attributed to the number of measures utilized across the included studies. In this review, we found a wide range of patient-reported and performance-based measures of disability, specifically, 55 different measures (or 84 if you consider the sub-scales of larger patient-reported outcome measures). Similar to previous studies [98, 99], this has led to difficulty in determining outcome magnitude and direction across this body of research [100]. Furthermore, the measures most frequently used in this study are typically free for research, but may not be financially feasible in clinical practice [101]. This places additional financial burden on clinics that who have to pay per patient to use the measure as well as cover costly and time-consuming data entry solutions.
For research and systematic reviews to inform clinical practice, guidelines, and policy, the outcomes chosen need to be prioritized in terms of meaningfulness to the patients served. Second, the outcomes chosen in clinical research should be standardized to decrease potential bias, poor selection of measures with lacking psychometrics, or gathering data that may not be relevant across studies which prevent further synthesis and knowledge gained. It is challenging to integrate this research directly into clinical practice as well as for the clinical practice to be prepared to gather relevant data. There is some recent work that seeks to harmonize outcome measures to place multiple measures onto one metric and, therefore, allow meta-analysis or comparative effectiveness research to occur [102]. Until harmonization efforts are underway, standardized, and easy to use and understand in a busy clinical practice, in clinic- or telehealth-based cancer rehabilitation, we strongly encourage a consensus and collaboration to determine measures that have clinical utility and research utility.
Intervention development and intervention considerations
Technology and patient preferences
The significant role of “old” or “low-tech” technology of the telephone (n = 40, 59%) stands out in contrast to rapid upscaling of video technology during the COVID-19 pandemic. Although our inclusion of articles spanned through April 2021, our dataset contained only 11 articles published in 2020 or 2021 and most of them were accepted for publication prior to March 2020. It is likely that our inclusion criterion regarding the use of an RCT design limited our ability to capture and describe studies that incorporated video technology and newer telehealth platforms that were introduced in the COVID-19 pandemic era.
During the COVID-19 pandemic, less restrictive use of telehealth has been permitted within the USA [103], including reimbursement for services provided by telephone. Globally, utilization of telehealth-based services tended to use different types of telehealth services based on the health condition or severity of complaint [104]. Nevertheless, if telehealth-based cancer rehabilitation via telephone delivery is deemed a non-reimbursed service post-pandemic, it limits use of an easily accessible mode of delivery. There is little research comparing patient preferences for phone versus other telehealth delivery methods; however, three recent studies [105–107] in oncology supportive services and medical consultation have examined patient preferences and satisfaction. These studies found that a plurality of patients preferred telephone delivered care over face-to-face and/or video delivered care. Interestingly, one study in Canada found comprehensive functional assessments could also be delivered primarily via telephone and supplemented with one or two in-person visits [107]. Qualitative methods examining patient preferences and satisfaction with telehealth-based cancer rehabilitation services can help shape modes of delivery in future and existing interventions across global healthcare systems.
Professional disciplines
In our results, over one-third (n = 24, 35%) of study interventions were delivered by nursing professionals. Our classification of nursing professionals included nursing assistants, nurses, and nurse practitioners. The billing implications of this finding are unclear given the wide range of legal independent practice ability and billing requirements within this category. Moreover, varying scope of practice issues (i.e., implementing an intervention versus setting and advancing the plan of care) between rehabilitation clinicians also render this finding challenging to interpret and implement. Given the breadth of interventionists and missing data, we were unable to identify if differences in discipline delivery influenced improvement in disability. Future research can elucidate differences in outcomes based on discipline.
Intervention characteristics
Interventions varied in duration and time and format of delivery. The mean intervention duration was 16.5 weeks. In conjunction with our findings, most studies were delivered post-treatment. In addition, interventions varied in intensity and format (i.e., one-on-one services to group therapy). Therefore, it may be possible that intervention intensity also impacts duration. Moreover, real-world application of these findings may not be feasible in regional and/or global clinical settings. For example, the duration may not accurately reflect the shorter timetables necessary for telehealth-based cancer rehabilitation interventions prior to initiating primary treatment or in the context of advanced disease or end of life.
Limitations
To our knowledge, this is the first systematic review to broadly address the effectiveness of telehealth-based cancer rehabilitation in cancer survivors. Given this, we chose a wide lens through which to select eligible studies to provide the broadest perspective on randomized controlled trials in this area of practice. That said, there are limitations. First, inclusion of only randomized controlled trials may have led us focus on efficacy trials in academic settings and miss pragmatic trials in clinical settings, including those stimulated by the COVID-19 pandemic. Although there were eleven articles published between 2020 and 2021 [32, 37, 41, 46, 50, 62, 71, 76, 80, 84, 90], no articles took place during or referenced the COVID-19 pandemic. Future research should consider differences among pre-, during-, and post-pandemic telehealth-based cancer rehabilitation intervention research in terms of populations reached, functional measures used and associated outcomes, as well as technology platforms utilized. This information could be used to influence health system access and broader policy related to telehealth reimbursement and utilization.
Second, creating an operational definition of cancer rehabilitation was a challenge that introduced heterogeneity into the sample. As such, our review is broad and expansive and may include interventions that the authors may not have identified as rehabilitation. Co-authors also represented a broad range of professional disciplines and expertise to capture all interventions that may be considered as cancer rehabilitation.
For this study, we chose not to analyze active ingredients or components of the included interventions as our primary focus was to characterize delivery and evaluate the effect on disability. Future work will identify and examine which active ingredients or intervention content influence function and disability via telehealth-based services the most. Following this, future research may also consider stakeholder input on preferred or prioritized types of intervention to be delivered via telehealth to ensure value of future intervention.
We chose to include studies with some concerns and high risk of bias in order to comprehensively characterize the state of the science in this area. Thus, inclusion of this evidence helps to broaden our understanding of potential implications for future intervention development, implementation, and policy applications. However, given the large amount of missing data (i.e., raw means and standard deviations) and moderate to high risk of bias associated with many included studies, the certainty, or the test of accuracy, of our findings may have been impacted. The variation in interventions and outcome measures included in this systematic review preclude meta-analysis. No inquiries were made to the authors of the original articles for clarification or missing data which could affect the validity of the effectiveness analysis. While our analyses indicated small positive effects on disability, heterogeneity across population and intervention characteristics prevent us from drawing firm conclusions.
Conclusion
To summarize, in the rapidly changing context of science, practice, and policy, telehealth-based cancer rehabilitation demonstrates a positive impact that merits further evaluation and study. Based on the results of this review, telehealth-based cancer rehabilitation interventions show promise to improve disability for cancer patients. In light of the cited limitations, how should the field move forward? Widening the patient populations studied building the evidence base for interventions prior to primary cancer treatment, and across multiple stages of the cancer trajectory, including end of life are necessary. Patient preferences, satisfaction, and outcomes with all telehealth modalities including low-tech options such as telephones should be explored. In addition, determining which therapies are most efficacious in telehealth modalities and which patients will benefit from specific therapies is of utmost importance. Consensus around appropriate measures is needed to be able to build the body of evidence. Lastly, assessing feasibility and translation potential must be addressed via implementation science methods. It is essential to conduct pragmatic trials, in order to know how to best meet the needs of cancer survivors. For future intervention research, it will be imperative to recruit and involve representative study samples related to race/ethnicity, socioeconomic status, and technology literacy to enhance generalizability of study findings to real-world settings. The questions are there; it is our mission to build the evidence.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge the members of the Outcomes and Research Task Force of the Cancer Rehabilitation Networking Group of the American Congress of Rehabilitation Medicine for their support of this work. Portions of these data were presented at the 2021 American Congress of Rehabilitation Medicine Virtual Conference on September 28, 2021 in a symposia presentation.
Author contribution
The following authors contributed to study conception and design: RB, KDL, JJ, LP, KCW, MP, TM, GC, JKS, AV, AL, TK. The following authors contributed to data screening: RB, KDL, JJ, LP, KCW, MP, TM, GC, RE, JKS, AV, AMF, MF, RK, JB, TK. The following authors contributed to data extraction: RB, KDL, JJ, LP, KCW, MP, TM, GC, RE, JS, AV, AMF, MF, TK, JB, RE. The following authors contributed to data analysis and interpretation: RB, KDL, MP, LP, JJ, JB, AB, GB, SK. All authors contributed to manuscript writing and approval.
Funding
Dr. Flores reports funding from the National Cancer Institute (3UM1CA233035-01S1).
Data availability
The de-identified transcriptions and dataset analyzed in the current study are available upon request to the corresponding author.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Drs. Pergolotti and Covington, and Tiffany Kendig report personal fees from Select Medical, ReVital Cancer Rehab outside the submitted work. Julie K. Silver has participated in research funded by the Binational Scientific Foundation and is a venture partner for Third Culture Capital outside of the work submitted. Aneesha Virani receives salary for full-time employment at Northside Hospital for work outside of submitted work. Aneesha Virani reports participating in research funded by the American Speech & Hearing Foundation for work outside of this project. Grace Campbell reports funding from the National Institute of Disability, Independent Living, and Rehabilitation Research. Kathleen D. Lyons reports participating in research funded by the National Cancer Institute for work outside of this project. All other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.O’Young B, Gosney J, Ahn C. The concept and epidemiology of disability. Phys Med Rehabil Clin N Am. 2019;30(4):697–707. doi: 10.1016/j.pmr.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Neo J, Fettes L, Gao W, Higginson IJ, Maddocks M. Disability in activities of daily living among adults with cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2017;61(Supplement C):94–106. doi: 10.1016/j.ctrv.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Alfano CM, Kent EE, Padgett LS, Grimes M, de Moor JS. Making cancer rehabilitation services work for cancer patients: recommendations for research and practice to improve employment outcomes. PM R. 2017;9(9, Supplement 2):S398–S406. doi: 10.1016/j.pmrj.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starmer HM. Factors influencing adherence to treatment for head and neck cancer. In: Doyle PC, editor. Clinical care and rehabilitation in head and neck cancer. Cham: Springer International Publishing; 2019. p. 413–22. 10.1007/978-3-030-04702-3_24.
- 5.Chasen MR. Cancer nutrition and rehabilitation – its time has come! Curr Oncol. 2008;15(3):6. 10.3747/co.v15i3.244. [DOI] [PMC free article] [PubMed]
- 6.Cheville AL, Morrow M, Smith SR, Basford JR. Integrating function-directed treatments into palliative care. PM R. 2017;9(9, Supplement 2):S335–S46. doi: 10.1016/j.pmrj.2017.07.073. [DOI] [PubMed] [Google Scholar]
- 7.Khan F, Amatya B, Ng L, Demetrios M, Zhang NY, Turner-Stokes L. Multidisciplinary rehabilitation for follow-up of women treated for breast cancer. Cochrane Database Syst Rev. 2012;12:CD009553. doi: 10.1002/14651858.CD009553.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan F, Amatya B, Ng L, Drummond K, Galea M. Multidisciplinary rehabilitation after primary brain tumour treatment. Cochrane Database Syst Rev. 2015;8:CD009509. doi: 10.1002/14651858.CD009509.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott DA, Mills M, Black A, et al. Multidimensional rehabilitation programmes for adult cancer survivors. Cochrane Database Syst Rev. 2013;3:CD007730. doi: 10.1002/14651858.CD007730.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mewes JC, Steuten LMG, IJzerman MJ, van Harten WH. Effectiveness of multidimensional cancer survivor rehabilitation and cost-effectiveness of cancer rehabilitation in general: a systematic review. Oncologist. 2012;17(12):1581–93. 10.1634/theoncologist.2012-0151. [DOI] [PMC free article] [PubMed]
- 11.Pergolotti M, Deal AM, Lavery J, Reeve BB, Muss HB. The prevalence of potentially modifiable functional deficits and the subsequent use of occupational and physical therapy by older adults with cancer. J Geriatr Oncol. 2015;6(3):194–201. doi: 10.1016/j.jgo.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 Suppl 1):S27–37. doi: 10.1097/PHM.0b013e31820be3be. [DOI] [PubMed] [Google Scholar]
- 13.Stubblefield MD. The underutilization of rehabilitation to treat physical impairments in breast cancer survivors. PM R. 2017;9(9, Supplement 2):S317–S23. doi: 10.1016/j.pmrj.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Cheville AL, Mustian K, Winters-Stone K, Zucker DS, Gamble GL, Alfano CM. Cancer rehabilitation: an overview of current need, delivery models, and levels of care. Phys Med Rehabil Clin N Am. 2017;28(1):1–17. doi: 10.1016/j.pmr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Cheville AL, Rhudy L, Basford JR, Griffin JM, Flores AM. How receptive are patients with late stage cancer to rehabilitation services and what are the sources of their resistance? Arch Phys Med Rehabil. 2017;98(2):203–210. doi: 10.1016/j.apmr.2016.08.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfano CM, Leach CR, Smith TG, et al. Equitably improving outcomes for cancer survivors and supporting caregivers: a blueprint for care delivery, research, education, and policy. CA Cancer J Clin. 2019;69(1):35–49. doi: 10.3322/caac.21548. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Telemedicine: Opportunities and developments in member states. Global Observatory for eHealth series - Volume 2. Geneva, Switzerland. 2010. Accessed 6 Dec 2021. https://www.who.int/goe/publications/goe_telemedicine_2010.pdf.
- 18.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Sterne JA, Savovic J, et al. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, McKenzie J, Boutron I, Welch V, editors. Cochrane Methods. Cochrane Database of Systematic Reviews. 2016; 10: p. 29–31. 10.1002/14651858.CD201601.
- 21.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.Abrahams HJG, Gielissen MFM, Donders RRT, et al. The efficacy of internet-based cognitive behavioral therapy for severely fatigued survivors of breast cancer compared with care as usual: a randomized controlled trial. Cancer. 2017;123(19):3825–3834. doi: 10.1002/cncr.30815. [DOI] [PubMed] [Google Scholar]
- 24.Allard NC. Day surgery for breast cancer: effects of a psychoeducational telephone intervention on functional status and emotional distress. Oncol Nurs Forum. 2007;34(1):133–141. doi: 10.1188/07.ONF.133-141. [DOI] [PubMed] [Google Scholar]
- 25.Anderson KO, Cohen MZ, Mendoza TR, Guo H, Harle MT, Cleeland CS. Brief cognitive-behavioral audiotape interventions for cancer-related pain: immediate but not long-term effectiveness. Cancer. 2006;107(1):207–214. doi: 10.1002/cncr.21964. [DOI] [PubMed] [Google Scholar]
- 26.Aranda S, Schofield P, Weih L, Milne D, Yates P, Faulkner R. Meeting the support and information needs of women with advanced breast cancer: a randomised controlled trial. Br J Cancer. 2006;95(6):667–673. doi: 10.1038/sj.bjc.6603320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashing KT, Miller AM. Assessing the utility of a telephonically delivered psychoeducational intervention to improve health-related quality of life in African American breast cancer survivors: a pilot trial. Psychooncology. 2016;25(2):236–238. doi: 10.1002/pon.3823. [DOI] [PubMed] [Google Scholar]
- 28.Ashing-Giwa KT. Enhancing physical well-being and overall quality of life among underserved Latina-American cervical cancer survivors: feasibility study. J Cancer Surviv. 2008;2(3):215–223. doi: 10.1007/s11764-008-0061-2. [DOI] [PubMed] [Google Scholar]
- 29.Badger TA, Segrin C, Hepworth JT, Pasvogel A, Weihs K, Lopez AM. Telephone-delivered health education and interpersonal counseling improve quality of life for Latinas with breast cancer and their supportive partners. Psychooncology. 2013;22(5):1035–1042. doi: 10.1002/pon.3101. [DOI] [PubMed] [Google Scholar]
- 30.Bailey DE, Jr, Mishel MH, Belyea M, Stewart JL, Mohler J. Uncertainty intervention for watchful waiting in prostate cancer. Cancer Nurs. 2004;27(5):339–346. doi: 10.1097/00002820-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Barsevick AM, Dudley W, Beck S, Sweeney C, Whitmer K, Nail L. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer. 2004;100(6):1302–1310. doi: 10.1002/cncr.20111. [DOI] [PubMed] [Google Scholar]
- 32.Basen-Engquist KM, Raber M, Carmack CL, et al. Feasibility and efficacy of a weight gain prevention intervention for breast cancer patients receiving neoadjuvant chemotherapy: a randomized controlled pilot study. Support Care Cancer. 2020;28(12):5821–5832. doi: 10.1007/s00520-020-05411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett JA, Lyons KS, Winters-Stone K, Nail LM, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors: a randomized controlled trial. Nurs Res. 2007;56(1):18–27. doi: 10.1097/00006199-200701000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Bruera E, Yennurajalingam S, Palmer JL, et al. Methylphenidate and/or a nursing telephone intervention for fatigue in patients with advanced cancer: a randomized, placebo-controlled, phase II trial. J Clin Oncol. 2013;31(19):2421–2427. doi: 10.1200/JCO.2012.45.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burns CL, Kularatna S, Ward EC, Hill AJ, Byrnes J, Kenny LM. Cost analysis of a speech pathology synchronous telepractice service for patients with head and neck cancer. Head Neck. 2017;39(12):2470–2480. doi: 10.1002/hed.24916. [DOI] [PubMed] [Google Scholar]
- 36.Chambers SK, Ferguson M, Gardiner RA, Aitken J, Occhipinti S. Intervening to improve psychological outcomes for men with prostate cancer. Psychooncology. 2013;22(5):1025–1034. doi: 10.1002/pon.3095. [DOI] [PubMed] [Google Scholar]
- 37.Chan JM, Van Blarigan EL, Langlais CS, et al. Feasibility and acceptability of a remotely delivered, web-based behavioral intervention for men with prostate cancer: four-arm randomized controlled pilot trial. J Med Internet Res. 2020;22(12):e19238. doi: 10.2196/19238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manag. 2013;45(5):811–821. doi: 10.1016/j.jpainsymman.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheville AL, Moynihan T, Basford JR, et al. The rationale, design, and methods of a randomized, controlled trial to evaluate the effectiveness of collaborative telecare in preserving function among patients with late stage cancer and hematologic conditions. Contemp Clin Trials. 2018;64:254–264. doi: 10.1016/j.cct.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheville AL, Moynihan T, Herrin J, Loprinzi C, Kroenke K. Effect of collaborative telerehabilitation on functional impairment and pain among patients with advanced-stage cancer: a randomized clinical trial. JAMA Oncol. 2019;5(5):644–652. doi: 10.1001/jamaoncol.2019.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow EJ, Doody DR, Di C, et al. Feasibility of a behavioral intervention using mobile health applications to reduce cardiovascular risk factors in cancer survivors: a pilot randomized controlled trial. J Cancer Surviv. 2020 doi: 10.1007/s11764-020-00949-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark MM, Rummans TA, Atherton PJ, et al. Randomized controlled trial of maintaining quality of life during radiotherapy for advanced cancer. Cancer. 2013;119(4):880–887. doi: 10.1002/cncr.27776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dennett AM, Shields N, Peiris CL, et al. Motivational interviewing added to oncology rehabilitation did not improve moderate-intensity physical activity in cancer survivors: a randomised trial. J Physiother. 2018;64(4):255–263. doi: 10.1016/j.jphys.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Djuric Z, Ellsworth JS, Weldon AL, et al. A diet and exercise intervention during chemotherapy for breast cancer. Open Obes J. 2011;3:87–97. doi: 10.2174/1876823701103010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong X, Yi X, Gao D, et al. The effects of the combined exercise intervention based on internet and social media software (CEIBISMS) on quality of life, muscle strength and cardiorespiratory capacity in Chinese postoperative breast cancer patients: a randomized controlled trial. Health Qual Life Outcomes. 2019;17(1):109. doi: 10.1186/s12955-019-1183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong X, Yi X, Ding M, et al. A longitudinal study of a multicomponent exercise intervention with remote guidance among breast cancer patients. Int J Environ Res Public Health. 2020;17(10). 10.3390/ijerph17103425. [DOI] [PMC free article] [PubMed]
- 47.Dos Santos M, Hardy-Léger I, Rigal O, et al. Cognitive rehabilitation program to improve cognition of cancer patients treated with chemotherapy: a 3-arm randomized trial. Cancer. 2020;126(24):5328–5336. doi: 10.1002/cncr.33186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duijts SF, van Beurden M, Oldenburg HS, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol. 2012;30(33):4124–4133. doi: 10.1200/JCO.2012.41.8525. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson RJ, Sigmon ST, Pritchard AJ, et al. A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction. Cancer. 2016;122(11):1782–1791. doi: 10.1002/cncr.29891. [DOI] [PubMed] [Google Scholar]
- 50.Fjell M, Langius-Eklöf A, Nilsson M, Wengström Y, Sundberg K. Reduced symptom burden with the support of an interactive app during neoadjuvant chemotherapy for breast cancer - a randomized controlled trial. Breast. 2020;51:85–93. doi: 10.1016/j.breast.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freeman LW, White R, Ratcliff CG, et al. A randomized trial comparing live and telemedicine deliveries of an imagery-based behavioral intervention for breast cancer survivors: reducing symptoms and barriers to care. Psychooncology. 2015;24(8):910–918. doi: 10.1002/pon.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galiano-Castillo N, Arroyo-Morales M, Lozano-Lozano M, et al. Effect of an internet-based telehealth system on functional capacity and cognition in breast cancer survivors: a secondary analysis of a randomized controlled trial. Support Care Cancer. 2017;25(11):3551–3559. doi: 10.1007/s00520-017-3782-9. [DOI] [PubMed] [Google Scholar]
- 53.Galiano-Castillo N, Cantarero-Villanueva I, Fernandez-Lao C, et al. Telehealth system: a randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer. 2016;122(20):3166–3174. doi: 10.1002/cncr.30172. [DOI] [PubMed] [Google Scholar]
- 54.Gell NM, Grover KW, Savard L, Dittus K. Outcomes of a text message, Fitbit, and coaching intervention on physical activity maintenance among cancer survivors: a randomized control pilot trial. J Cancer Surviv. 2019 doi: 10.1007/s11764-019-00831-4. [DOI] [PubMed] [Google Scholar]
- 55.Gordon LG, Patrao T, Kularatna S, Hawkes AL. A telephone-delivered multiple health behaviour change intervention for colorectal cancer survivors: making the case for cost-effective healthcare. Eur J Cancer Care. 2015;24(6):854–861. doi: 10.1111/ecc.12345. [DOI] [PubMed] [Google Scholar]
- 56.Hawkes AL, Chambers SK, Pakenham KI, et al. Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. J Clin Oncol. 2013;31(18):2313–2321. doi: 10.1200/JCO.2012.45.5873. [DOI] [PubMed] [Google Scholar]
- 57.Hawkins RP, Pingree S, Van Bogaert D, et al. The impact of combining human and online supportive resources for prostate cancer patients. J Commun Support Oncol. 2017;15(6):e321-e9. 10.12788/jcso.0330. [DOI] [PMC free article] [PubMed]
- 58.Hayes SC, Rye S, Disipio T, et al. Exercise for health: A randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat. 2013;137(1):175–186. doi: 10.1007/s10549-012-2331-y. [DOI] [PubMed] [Google Scholar]
- 59.Hegel MT, Lyons KD, Hull JG, et al. Feasibility study of a randomized controlled trial of a telephone-delivered problem-solving-occupational therapy intervention to reduce participation restrictions in rural breast cancer survivors undergoing chemotherapy. Psychooncology. 2011;20(10):1092–1101. doi: 10.1002/pon.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hung YC, Bauer JD, Horsely P, Coll J, Bashford J, Isenring EA. Telephone-delivered nutrition and exercise counselling after auto-SCT: a pilot, randomised controlled trial. Bone Marrow Transplant. 2014;49(6):786–792. doi: 10.1038/bmt.2014.52. [DOI] [PubMed] [Google Scholar]
- 61.Jensen BT, Kristensen SA, Christensen SV, Borre M. Efficacy of tele-nursing consultations in rehabilitation after radical prostatectomy: a randomised controlled trial study. Int J Urol Nurs. 2011;5(3):123–130. doi: 10.1111/j.1749-771X.2011.01130.x. [DOI] [Google Scholar]
- 62.Kelleher SA, Fisher HM, Winger JG, et al. Feasibility, engagement, and acceptability of a behavioral pain management intervention for colorectal cancer survivors with pain and psychological distress: data from a pilot randomized controlled trial. Support Care Cancer. 2021 doi: 10.1007/s00520-021-06126-8. [DOI] [PubMed] [Google Scholar]
- 63.Kim SH, Choe YH, Han AR, et al. Design of a randomized controlled trial of a partnership-based, needs-tailored self-management support intervention for post-treatment breast cancer survivors. BMC Cancer. 2020;20(1):367. doi: 10.1186/s12885-020-06861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koller A, Gaertner J, De Geest S, Hasemann M, Becker G. Testing the implementation of a pain self-management support intervention for oncology patients in clinical practice: a randomized controlled pilot study (ANtiPain) Cancer Nurs. 2018;41(5):367–378. doi: 10.1097/ncc.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 65.Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. BMC Cancer. 2016;16(1). 10.1186/s12885-016-2258-5. [DOI] [PMC free article] [PubMed]
- 66.Lee BJ, Park YH, Lee JY, Kim SJ, Jang Y, Lee JI. Smartphone application versus pedometer to promote physical activity in prostate cancer patients. Telemed J E Health. 2019;25(12):1231–1236. doi: 10.1089/tmj.2018.0233. [DOI] [PubMed] [Google Scholar]
- 67.Ligibel JA, Meyerhardt J, Pierce JP, et al. Impact of a telephone-based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Res Treat. 2012;132(1):205–213. doi: 10.1007/s10549-011-1882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meneses K, Pisu M, Azuero A, Benz R, Su X, McNees P. A telephone-based education and support intervention for rural breast cancer survivors: a randomized controlled trial comparing two implementation strategies in rural Florida. J Cancer Surviv. 2020;14(4):494–503. doi: 10.1007/s11764-020-00866-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meneses K, Gisiger-Camata S, Benz R, et al. Telehealth intervention for Latina breast cancer survivors: a pilot. Womens Health (Lond) 2018;14:1745506518778721. doi: 10.1177/1745506518778721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meneses K, McNees P, Azuero A, Loerzel VW, Su X, Hassey LA. Preliminary evaluation of psychoeducational support interventions on quality of life in rural breast cancer survivors after primary treatment. Cancer Nurs. 2009;32(5):385–397. doi: 10.1097/NCC.0b013e3181a850e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meneses KD, McNees P, Loerzel VW, Su X, Zhang Y, Hassey LA. Transition from treatment to survivorship: effects of a psychoeducational intervention on quality of life in breast cancer survivors. Oncol Nurs Forum. 2007;34(5):1007–1016. doi: 10.1188/07.Onf.1007-1016. [DOI] [PubMed] [Google Scholar]
- 72.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301(18):1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23(15):3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 74.Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Health Psychol. 2013;32(6):616–626. doi: 10.1037/a0029886. [DOI] [PubMed] [Google Scholar]
- 75.Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22(1):54–64. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- 76.Raphaelis S, Frommlet F, Mayer H, Koller A. Implementation of a nurse-led self-management support intervention for patients with cancer-related pain: a cluster randomized phase-iv study with a stepped wedge design (EvANtiPain) BMC Cancer. 2020;20(1):559. doi: 10.1186/s12885-020-06729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sajid S, Dale W, Mustian K, et al. Novel physical activity interventions for older patients with prostate cancer on hormone therapy: a pilot randomized study. J Geriatr Oncol. 2016;7(2):71–80. doi: 10.1016/j.jgo.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scura KW, Budin W, Garfing E. Telephone social support and education for adaptation to prostate cancer: a pilot study. Oncol Nurs Forum. 2004;31(2):335–338. doi: 10.1188/04.Onf.335-338. [DOI] [PubMed] [Google Scholar]
- 79.Silvers MA, Savva J, Huggins CE, Truby H, Haines T. Potential benefits of early nutritional intervention in adults with upper gastrointestinal cancer: a pilot randomised trial. Support Care Cancer. 2014;22(11):3035–3044. doi: 10.1007/s00520-014-2311-3. [DOI] [PubMed] [Google Scholar]
- 80.Sui Y, Wang T, Wang X. The impact of WeChat app-based education and rehabilitation program on anxiety, depression, quality of life, loss of follow-up and survival in non-small cell lung cancer patients who underwent surgical resection. Eur J Oncol Nurs. 2020;45:101707. doi: 10.1016/j.ejon.2019.101707. [DOI] [PubMed] [Google Scholar]
- 81.Syrjala KL, Yi JC, Artherholt SB, et al. An online randomized controlled trial, with or without problem-solving treatment, for long-term cancer survivors after hematopoietic cell transplantation. J Cancer Surviv. 2018;12(4):560–570. doi: 10.1007/s11764-018-0693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Temur K, Kapucu S. The effectiveness of lymphedema self-management in the prevention of breast cancer-related lymphedema and quality of life: a randomized controlled trial. Eur J Oncol Nurs. 2019;40:22–35. doi: 10.1016/j.ejon.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 83.Vallerand JR, Rhodes RE, Walker GJ, Courneya KS. Feasibility and preliminary efficacy of an exercise telephone counseling intervention for hematologic cancer survivors: a phase II randomized controlled trial. J Cancer Surviv. 2018;12(3):357–370. doi: 10.1007/s11764-018-0675-y. [DOI] [PubMed] [Google Scholar]
- 84.Waller E, Sutton P, Rahman S, Allen J, Saxton J, Aziz O. Prehabilitation with wearables versus standard of care before major abdominal cancer surgery: a randomised controlled pilot study (trial registration: NCT04047524). 2021. Surg Endosc. 10.1007/s00464-021-08365-6. [DOI] [PMC free article] [PubMed]
- 85.Wang TJ, Su JH, Leung KW, Liang SY, Wu SF, Wang HM. Effects of a mouth-opening intervention with remote support on adherence, the maximum interincisal opening, and mandibular function of postoperative oral cancer patients: a randomized clinical trial. Eur J Oncol Nurs. 2019;40:111–119. doi: 10.1016/j.ejon.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 86.Wenzel L, Osann K, Hsieh S, Tucker JA, Monk BJ, Nelson EL. Psychosocial telephone counseling for survivors of cervical cancer: results of a randomized biobehavioral trial. J Clin Oncol. 2015;33(10):1171–1179. doi: 10.1200/jco.2014.57.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yanez B, McGinty HL, Mohr DC, et al. Feasibility, acceptability, and preliminary efficacy of a technology-assisted psychosocial intervention for racially diverse men with advanced prostate cancer. Cancer. 2015;121(24):4407–4415. doi: 10.1002/cncr.29658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yates P, Aranda S, Margraves M, et al. Randomized controlled trial of an educational intervention for managing fatigue in women receiving adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2005;23(25):6027–6036. doi: 10.1200/JCO.2005.01.271. [DOI] [PubMed] [Google Scholar]
- 89.Zhang AY, Bodner DR, Fu AZ, et al. Effects of patient centered interventions on persistent urinary incontinence after prostate cancer treatment: a randomized, controlled trial. J Urol. 2015;194(6):1675–1681. doi: 10.1016/j.juro.2015.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou K, Wang W, Zhao W, et al. Benefits of a WeChat-based multimodal nursing program on early rehabilitation in postoperative women with breast cancer: a clinical randomized controlled trial. Int J Nurs Stud. 2020;106:103565. doi: 10.1016/j.ijnurstu.2020.103565. [DOI] [PubMed] [Google Scholar]
- 91.Kim SC, Hawkins RP, Shah DV, Gustafson DH, Baker TB. Understanding how e-health interventions meet psychosocial needs of breast cancer patients: the pathways of influence on quality of life and cancer concerns. Psychooncology. 2020;29(10):1704–1712. doi: 10.1002/pon.5512. [DOI] [PubMed] [Google Scholar]
- 92.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 93.Ahmad A. Breast cancer statistics: Recent trends. In: Ahmad A, editor. Breast cancer metastasis and drug resistance: challenges and progress. Cham: Springer International Publishing; 2019. pp. 1–7. [Google Scholar]
- 94.Stout NL, Alfano CM, Belter CW, et al. A bibliometric analysis of the landscape of cancer rehabilitation research (1992–2016) J Natl Cancer Inst. 2018;110(8):815–824. doi: 10.1093/jnci/djy108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petkovic J, Jull J, Yoganathan M, et al. Reporting of health equity considerations in cluster and individually randomized trials. Trials. 2020;21(1):308. doi: 10.1186/s13063-020-4223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Silver JK, Flores LE, Mondriguez Gonzalez A, Frontera WR. An analysis of the inclusion of women, older individuals, and racial/ethnic minorities in rehabilitation clinical trials. Am J Phys Med Rehabil. 2021;100(6):546–554. doi: 10.1097/PHM.0000000000001750. [DOI] [PubMed] [Google Scholar]
- 97.Saini G, Gogineni K, Kittles RA, Aneja R. Undercutting efforts of precision medicine: roadblocks to minority representation in breast cancer clinical trials. Breast Cancer Res Treat. 2021;187(3):605–611. doi: 10.1007/s10549-021-06264-x. [DOI] [PubMed] [Google Scholar]
- 98.Williamson PR, Altman DG, Blazeby JM, et al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13(1):132. doi: 10.1186/1745-6215-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Macefield RC, Jacobs M, Korfage IJ, et al. Developing core outcomes sets: methods for identifying and including patient-reported outcomes (PROS) Trials. 2014;15(1):49. doi: 10.1186/1745-6215-15-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ribeiro IL, Moreira RFC, Ferrari AV, Alburquerque-Sendín F, Camargo PR, Salvini TF. Effectiveness of early rehabilitation on range of motion, muscle strength and arm function after breast cancer surgery: a systematic review of randomized controlled trials. Clin Rehabil. 2019;33(12):1876–1886. doi: 10.1177/0269215519873026. [DOI] [PubMed] [Google Scholar]
- 101.Ramsey I, Eckert M, Hutchinson AD, Marker J, Corsini N. Core outcome sets in cancer and their approaches to identifying and selecting patient-reported outcome measures: a systematic review. J Patient-Report Outcomes. 2020;4(1):77. doi: 10.1186/s41687-020-00244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: linking the BDI-II, CES-D, and PHQ-9 to PROMIS depression. Psychol Assess. 2014;26(2):513–527. doi: 10.1037/a0035768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fiani B, Siddiqi I, Lee SC, Dhillon L. Telerehabilitation: development, application, and need for increased usage in the COVID-19 era for patients with spinal pathology. Cureus. 2020;12(9):e10563-e. doi: 10.7759/cureus.10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Winburn AS, Brixey JJ, Langabeer J, Champagne-Langabeer T. A systematic review of prehospital telehealth utilization. J Telemed Telecare. 2017;24(7):473–481. doi: 10.1177/1357633X17713140. [DOI] [PubMed] [Google Scholar]
- 105.Myers Virtue S, Howrey HL, Duffy NM, Wallace M. Shifting psychosocial oncology care to telepsychology during the COVID-19 pandemic. J Psychosoc Oncol. 2021;39(3):416–427. doi: 10.1080/07347332.2021.1894526. [DOI] [PubMed] [Google Scholar]
- 106.Stewart R, Collins L, Kerr B, et al. A review of telephone consultations for head and neck cancer follow up: a patient satisfaction survey. J Laryngol Otol. 2021;135(9):815–819. doi: 10.1017/S0022215121001997. [DOI] [PubMed] [Google Scholar]
- 107.Lopez CJ, Edwards B, Langelier DM, Chang EK, Chafranskaia A, Jones JM. Delivering virtual cancer rehabilitation programming during the first 90 days of the COVID-19 pandemic: a multimethod study. Arch Phys Med Rehabil. 2021;102(7):1283–1293. doi: 10.1016/j.apmr.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The de-identified transcriptions and dataset analyzed in the current study are available upon request to the corresponding author.