Abstract
Background and objectives:
Some surgeons remain hesitant to perform immediate breast reconstruction (IBR) in patients with higher risk cancers due to concerns about cancer recurrence and/or detection. Our objective was to determine the rate of ipsilateral local-regional recurrence for stage II/III patients who underwent IBR.
Methods:
The National Cancer Database special study mechanism was used to create a stratified sample of women diagnosed with stage II/III breast cancer from 1,217 facilities. Demographic, tumor, and recurrence data for women who underwent mastectomy with or without IBR were abstracted, including location of recurrence and method of detection. Estimates of 5-year local-regional recurrence rates were calculated and factors associated with recurrence were identified with multivariable Cox regression.
Results:
13% (692/5,318) of stage II/III patients underwent IBR after mastectomy. Patients undergoing IBR were younger (p<0.001), with fewer comorbid conditions (p<0.001), and with lower tumor burden in the breast (p=0.001) and the lymph nodes (p=0.01). The 5-year rate of ipsilateral local-regional recurrence was 3.6% with no significant difference between patients with or without IBR (3.0% vs. 3.7%, p=0.4). Most recurrences were detected by the patient (45%) or on physician exam (24%). Reconstruction was not associated with recurrence on multivariable analysis (HR=0.83, p=0.52).
Conclusions:
Women with stage II/III breast cancer selected for IBR had similar rates of ipsilateral local-regional recurrence compared to those undergoing mastectomy alone. Offering IBR after mastectomy in a patient-centered manner to select patients with stage II/III breast cancer is an acceptable consideration.
MicroAbstract:
We studied 5,318 patients with stage II/III breast cancer who underwent mastectomy with and without immediate reconstruction. Patients selected for reconstruction were younger, with lower tumor burden in the breast and axilla. No difference in rates of ipsilateral local recurrence were observed between patients with and without immediate reconstruction. Offering immediate reconstruction to select high risk patients remains appropriate.
Introduction
Immediate breast reconstruction (IBR) after mastectomy for breast cancer confers several well-documented benefits to patients. IBR1 expediently restores the breast mound so that the patient does not undergo the complex and often distressing experience accompanying dramatic changes in body image after mastectomy.[1–3] IBR may also confer the best esthetic result. Preservation of the breast skin envelope allows for a more natural reconstruction of the breast mound which contributes to patient satisfaction with postoperative breast appearance.[4] Overall, IBR has been demonstrated in several studies of patient reported outcomes to improve satisfaction and well-being in a variety of domains, including body perception, sexual function and pain.[2,3,5–7]
Concerns have been raised that IBR could negatively contribute to cancer recurrence through several mechanisms. First, there is the concern that immediate breast reconstruction could lead to recurrence through local cellular and biochemical effects such as exacerbation of tissue hypoxia[8–10], maintenance of an “immunologic refuge” for cancer cells in the remaining dermis,[11] or preservation of breast tissue adherent to the hypodermis.[12,13] Second, there is concern that the physical presence of an implant or autologous tissue would mask detection of a chest wall recurrence.[14,15] Importantly, even patients who desire reconstruction may still harbor fears that reconstruction will lead to an increased risk of recurrence and/or mask recurrence.[16] Despite theoretical and in situ-based concerns of immediate reconstruction causing recurrence, existing studies have not found clinical differences in overall survival or cancer recurrence rates for patients undergoing IBR after mastectomy versus mastectomy alone.[17–21] Likewise, cancer recurrence after IBR, whether in the chest wall or subcutaneous tissue, has been found to occur at similar rates with similar detectability compared to cancer recurrence after mastectomy alone.[14,22,23] However, these results are from older studies that have largely focused on in-situ pathology or stage I and early stage II cancers that have inherently lower baseline risk of cancer recurrence.[24] There is limited data evaluating both the risks of recurrence and the appropriate detection of recurrence for patients with higher stage II/III cancers undergoing IBR.[25,26]
The objective of our study was to evaluate the rates of ipsilateral local-regional recurrence of breast cancer after IBR in stage II/III patients in a modern, nation-wide cohort. We also sought to determine whether the method of detection of recurrence in this cohort differed based on IBR status.
Materials and Methods
Data Source
The National Cancer Database (NCDB) is a hospital-based cancer registry estimated to capture 70% of all malignancies diagnosed in the United States.[27,28] A Commission on Cancer special study mechanism was used to obtain a stage-stratified sample of stage II/III breast cancer patients diagnosed 2006-2007 (n=11,366) identified from 1,217 facilities in the National Cancer Database (NCDB). Diagnosis years of 2006-2007 were selected as they were the most contemporary years that would provide 5 years of recurrence data at the time the study was designed. Medical records for 10 patients per institution were abstracted by trained cancer registrars at each site for the present study. The analysis of de-identified data was exempted from the University of Wisconsin Institutional Review Board.
Study inclusion/exclusion criteria
For this study, we included patients that underwent unilateral or bilateral mastectomy with or without reconstruction. Of the 11,366 randomly selected stage-stratified patients included in the parent study, 171 were excluded as they did not have definitive surgery and 26 were excluded for cancer recurrence prior to definitive surgery. 6,186 patients underwent mastectomy. We then excluded patients who underwent any neoadjuvant treatment (n=846) because only post-chemotherapy pathologic stage was available in these patients. We excluded 22 additional patients with inflammatory breast cancer (n=22) (Fig. 1).
Figure 1.
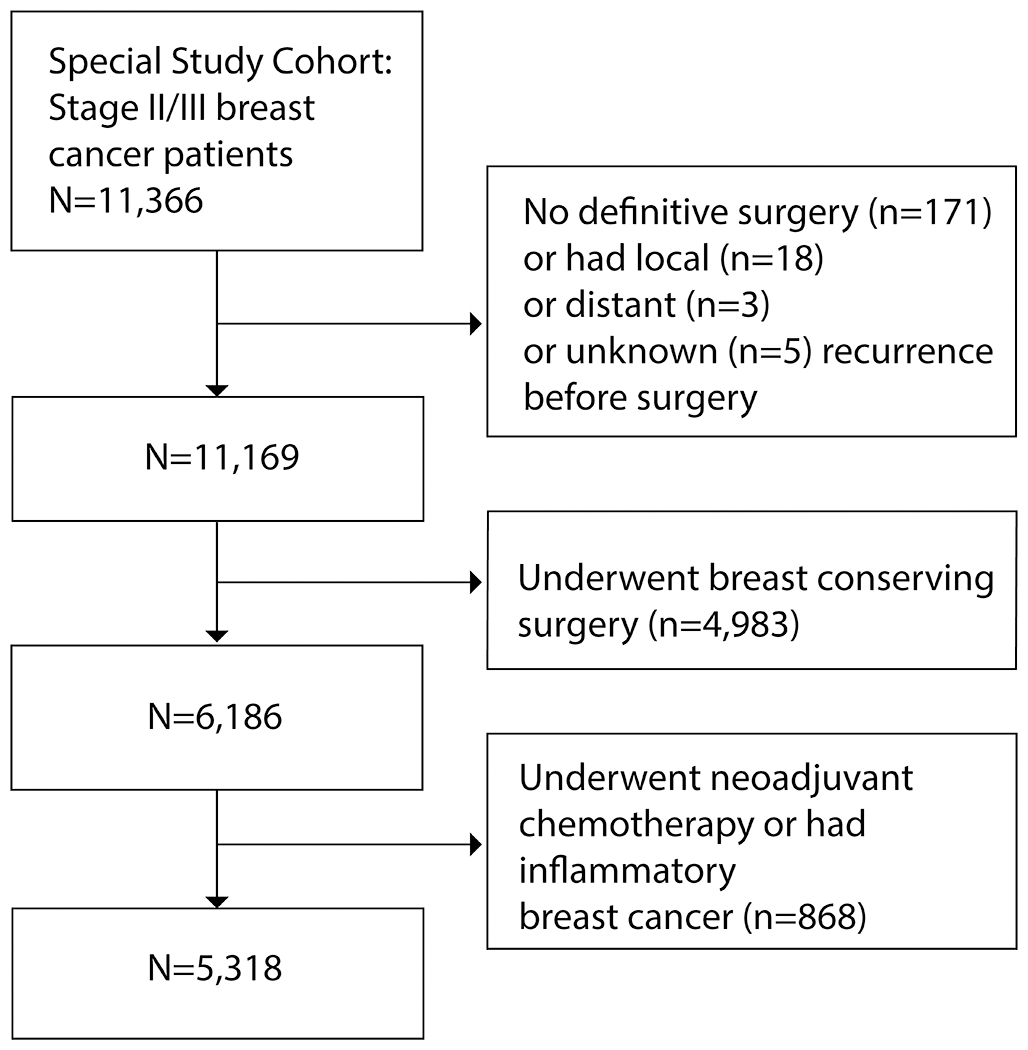
Flowchart of study inclusion/exclusion
Data Collection
NCDB specific surgical codes were used to identify patients who underwent mastectomy with and without IBR. The outcome variable of interest was the receipt of IBR (yes/no). The NCDB captures IBR after mastectomy, i.e. reconstruction planned as part of the initial course of treatment. Delayed reconstruction is not captured by the NCDB. Available data included typical data elements reported by the NCDB including patient demographics (age, race, Charlson-Deyo comorbidity index[29,30], insurance status), tumor characteristics (grade, number of positive lymph nodes, tumor size, hormone receptor status, Her2neu receptor status), treatment characteristics (receipt of radiation, chemotherapy, hormone therapy, surgery type), and mortality.
Registrars performed additional abstraction for data relevant to this study, including local-regional and distant breast events, and method of event detection. Local-regional recurrence was defined as chest wall recurrence after mastectomy and/or regional lymph node recurrence (ipsilateral supraclavicular lymph nodes, ipsilateral axillary lymph nodes). Regarding method of identification of cancer recurrence, patient detected was defined as the patient detecting a sign or symptom prompting a non-routine doctor visit leading to the discovery of cancer recurrence; physician detected was defined as physician detection during a scheduled, routine visit; asymptomatic imaging detected was defined as detection during routine surveillance imaging in absence of new signs/symptoms; and incidental detection was defined as an incidental finding on unrelated other imaging. “Other” detection was defined as “unable to determine” the method of detection.
Patients were followed for a maximum of 5 years from the time of initial surgery for recurrence and survival outcomes. The primary outcome for this analysis was ipsilateral local-regional recurrence.
Statistical analysis
Descriptive statistics were generated for the cohort with inclusion of missing observations. Chi squared analysis was used to compare characteristics in the IBR versus mastectomy alone groups. Five-year ipsilateral local-regional recurrence estimates were obtained using the Kaplan-Meier method. Patients were censored at the time of distant recurrence or death if either event occurred before an ipsilateral local-regional recurrence. We summarized the location of recurrence (chest wall, lymph nodes) and the method of detection (patient, physician or imaging detected). Log-rank tests were used to assess differences in time to ipsilateral local-regional recurrence by receipt of IBR. Multivariable Cox regression analysis was used to assess the relationship between IBR and time to ipsilateral local-regional recurrence within 5 years while controlling for patient and tumor factors known to be associated with recurrence. Patients with missing observations were excluded from the multivariable analysis.
Stata software (version 15) was used for all statistical analysis with p< 0.05 considered statistically significant.
Results
We included 5,318 patients in our study (Fig. 1). Consistent with the sampling framework, most patients were stage II (64%) and the remaining stage III (36%). 13% of individuals (n=692) underwent immediate breast reconstruction. Of those, 32% received implant-based reconstruction, 36% tissue-based reconstruction, 10% combined tissue and implant, and 22% did not have reconstruction type otherwise specified in the data. 64 patients in the cohort underwent bilateral mastectomy with removal of uninvolved contralateral breast. 9 of these women had immediate breast reconstruction.
Patient and tumor characteristics are summarized in Table 1. Patients undergoing IBR were overall younger (p<0.001), more likely to be privately insured (p<0.001), and had fewer comorbidities (p<0.001). Patients selected for IBR also differed based on tumor characteristics. Overall, the IBR group had smaller tumors (p=0.001) and fewer positive lymph nodes (p=0.018).
Table 1.
Demographics of patients undergoing mastectomy for stage II/III cancer by receipt of immediate breast reconstruction
| Overall (n=5,318) | Mastectomy alone (n=4,626) | Immediate breast reconstruction (n=692) | P value | |
|---|---|---|---|---|
| Demographics | ||||
|
| ||||
| Age | <0.001 | |||
| ≤ 50 | 29.0% (1542) | 24.9% (1152) | 56.4% (390) | |
| > 50 to ≤ 70 | 43.8% (2330) | 44.3% (2048) | 40.7% (282) | |
| > 70 | 27.2% (1446) | 30.8% (1426) | 2.9% (20) | |
| Race | 0.26 | |||
| White | 85.2% (4532) | 84.9% (3928) | 87.3% (604) | |
| Black | 10.8% (576) | 11.1% (512) | 9.2% (64) | |
| Other | 4.0% (210) | 4.0% (186) | 3.5% (24) | |
| Insurance | <0.001 | |||
| Private/managed care | 48.8% (2598) | 44.1% (2042) | 80.3% (556) | |
| Not insured | 2.8% (147) | 2.9% (134) | 1.9% (13) | |
| Medicaid | 8.2% (435) | 8.4% (390) | 6.5% (45) | |
| Medicare or other government | 38.6% (2052) | 42.8% (1979) | 10.6% (73) | |
| Unknown | 1.6% (86) | 1.8% (81) | 0.7% (5) | |
| Charlson-Deyo Comorbidity Index | <0.001 | |||
| 0 | 81.7% (4346) | 80.7% (3732) | 88.7% (614) | |
| 1+ | 14.8% (789) | 15.5% (720) | 10.0% (69) | |
| Unknown | 3.5% (183) | 3.8% (174) | 1.3% (9) | |
|
| ||||
| Tumor characteristics | ||||
|
| ||||
| Stage | 0.001 | |||
| 2 | 64.0% (3405) | 63.2% (2922) | 69.8% (483) | |
| 3 | 36.0%(1913) | 36.8% (1704) | 30.2% (209) | |
| Tumor size | 0.001 | |||
| <2cm | 22.5% (1194) | 22.0% (1017) | 25.6% (177) | |
| 2-5cm | 60.2% (3202) | 60.0% (2770) | 62.4% (432) | |
| >5cm or diffuse/infiltrating | 16.1% (857) | 16.8% (781) | 11.0% (76) | |
| Unknown | 1.2% (65) | 1.2% (58) | 1.0% (7) | |
| Positive nodes | 0.018 | |||
| 0 | 29.9% (1591) | 30.1% (1393) | 28.6% (198) | |
| 1-3 | 39.8% (2118) | 39.2% (1811) | 44.4% (307) | |
| ≥4 | 28.6% (1522) | 28.9% (1340) | 26.3% (182) | |
| Unknown | 1.7% (87) | 1.8% (82) | 0.7% (5) | |
| Tumor Grade | 0.62 | |||
| 1 | 11.4% (606) | 11.5% (532) | 10.7% (74) | |
| 2 | 41.0% (2179) | 40.7% (1881) | 43.1% (298) | |
| 3 | 42.0% (2234) | 42.1% (1949) | 41.2% (285) | |
| Unknown | 5.6% (299) | 5.7% (264) | 5.1% (35) | |
| Receptor subtypes | 0.006 | |||
| ER+ or PR+, /Her2neu− | 58.2% (3095) | 58.0% (2,683) | 59.5% (412) | |
| ER− and PR− /Her2neu− | 14.1% (747) | 14.4% (668) | 11.4% (79) | |
| ER+ or PR+ /Her2neu+ | 12.4% (658) | 12.0% (551) | 15.5% (107) | |
| ER− and PR− /Her2neu+ | 7.1% (380) | 7.1% (329) | 7.4% (51) | |
| ER/PR and/or Her2neu | 8.2% (438) | 8.5% (395) | 6.2% (43) | |
| Unknown | ||||
|
| ||||
| Treatment received | ||||
|
| ||||
| Adjuvant chemo | <0.001 | |||
| None | 30.9% (1645) | 33.4% (1545) | 14.4% (100) | |
| Received | 66.5% (3536) | 64.2% (2972) | 81.5% (564) | |
| Unknown | 2.6% (137) | 2.4% (109) | 4.1% (28) | |
| Endocrine therapy | 0.073 | |||
| None | 41.6% (2211) | 42.0% (1954) | 38.4% (266) | |
| Received | 58.4% (3107) | 58.0% (2681) | 61.6% (426) | |
| Radiation therapy | 0.82 | |||
| None | 62.0% (3296) | 61.9% (2865) | 62.3% (431) | |
| Received | 35.8% (1907) | 36.0% (1663) | 35.2% (244) | |
| Unknown | 2.2% (115) | 2.1% (98) | 2.5% (17) | |
ER, estrogen receptor; PR, progesterone receptor; Her2neu, human epidermal growth factor receptor 2
Table 2 lists the rates of ipsilateral local-regional recurrence, the location of recurrence, and the method of recurrence detection. Overall rates of ipsilateral local-regional recurrence at 5 years were 3.6%, with no difference based on receipt of IBR (3.0% with IBR and 3.7% for mastectomy alone, p=0.41). Overall, the majority of local-regional recurrence occurred in the chest wall alone (56.8%), followed by the lymph nodes alone (37.4%), and the chest wall and lymph nodes combined (5.8%). The location of recurrence did not significantly differ based on IBR (p=0.21). Most recurrences were detected by the patient (45.3%) or on physician exam (23.7%).
Table 2.
Rates of ipsilateral local-regional recurrence, location of recurrence and method of recurrence detection by receipt of immediate breast reconstruction
| Overall (n=5,318) | Mastectomy alone (n=4,626) | Immediate breast reconstruction (n=692) | P value |
|
|---|---|---|---|---|
| Ipsilateral Local-Regional Recurrence at 5-years | 3.6% (190) | 3.7% (169) | 3.0% (21) | 0.41 |
| Location of recurrence | 0.21 | |||
| Chest wall | 56.8% (108) | 57.4% (97) | 52.4% (11) | |
| Lymph nodes | 37.4% (71) | 37.9% (64) | 33.3% (7) | |
| Lymph nodes + chest wall | 5.8% (11) | 4.7% (8) | 14.3% (3) | |
| Method of recurrence detection | 1.0 | |||
| Patient | 45.3% (86) | 44.9% (76) | 47.6% (10) | |
| Physician exam | 23.7% (45) | 23.7% (40) | 23.8% (5) | |
| Asymptomatic imaging | 16.3% (31) | 16.6% (28) | 14.3% (3) | |
| Incidental imaging/Other | 14.7% (28) | 14.8% (25) | 14.3% (3) |
IBR was not associated with recurrence in both unadjusted (HR= 0.74, p value= 0.25) and adjusted analysis (HR=0.83, p value=0.52). On multivariable Cox regression, higher grade, larger tumors, and more positive lymph nodes were associated with higher rates of recurrence (Table 3). In addition, compared with ER+ or PR +tumors, ER− and PR− tumors (regardless of Her2neu status) were associated with higher rates of recurrence.
Table 3.
Multivariable Cox regression analysis for ipsilateral local-regional recurrence in women with stage II/III breast cancer undergoing mastectomy (n=4,757)
| Hazard Ratio (HR) | 95% Confidence Ratio (CI) | P value | |
|---|---|---|---|
| Immediate breast reconstruction | 0.83 | 0.47-1.47 | 0.52 |
| Age | |||
| ≤ 50 | Reference | - | 0.19 |
| > 50 to ≤ 60 | 1.12 | 0.71-1.75 | |
| >60 to ≤ 70 | 0.64 | 0.37-1.12 | |
| > 70 | 1.12 | 0.67-1.86 | |
| Race | |||
| White | Reference | - | 0.29 |
| Black | 1.41 | 0.89-2.23 | |
| Other | 1.29 | 0.60-2.79 | |
| Charlson-Deyo Comorbidity Index | |||
| 0 | Reference | - | 0.88 |
| 1+ | 1.03 | 0.66-1.62 | |
| Tumor size | |||
| <2cm | Reference | - | 0.006 |
| 2-5cm | 1.35 | 0.84-2.19 | |
| >5cm or diffuse/infiltrating | 2.29 | 1.32-3.94 | |
| Positive nodes | |||
| 0 | Reference | - | 0.001 |
| 1-3 positive | 1.36 | 0.87-2.12 | |
| ≥4 positive | 2.20 | 1.38-3.49 | |
| Grade | |||
| 1 | Reference | - | 0.003 |
| 2 | 1.09 | 0.52-2.27 | |
| 3 | 2.28 | 1.10-4.69 | |
| Receptor Subtype | |||
| ER+ or PR+, /Her2neu− | Reference | - | <0.001 |
| ER− and PR− /Her2neu− | 2.57 | 1.65-4.00 | |
| ER+ or PR+ /Her2neu+ | 1.15 | 0.65-2.02 | |
| ER− and PR−/Her2neu+ | 2.25 | 1.31-3.87 | |
| Adjuvant Chemotherapy | |||
| Not received | Reference | 0.001 | |
| Received | 0.48 | ||
| Adjuvant Radiation therapy | |||
| Not received | Reference | 0.17 | |
| Received | 0.76 |
ER, estrogen receptor; PR, progesterone receptor; Her2neu, human epidermal growth factor receptor 2
Discussion
This is the first large, multi-institutional analysis demonstrating that IBR is not associated with increased rates of ipsilateral local-regional recurrence in higher risk patients with stage II/III breast cancer. Further, we did not observe a difference in where or how recurrence was detected for patients who did and did not receive IBR. The findings from our multi-center study advance the understanding of the relationship between IBR and recurrence, as previous studies of local-regional recurrence after IBR in stage II/III patients have been mostly small, single center studies.[14,19,25] For stage II/III patients and their clinicians considering IBR, our study provides additional information to consider during the decision making process for IBR.
It is important to recognize that women with stage II/III breast cancer who undergo IBR are a select group. Of all the stage II/III patients undergoing mastectomy in this cohort, only 13% of patients underwent immediate breast reconstruction. This is lower than overall reported rates of IBR for breast cancer patients in the US, which range between 20–40% over a comparable time period.[31] We observed that those patients that underwent IBR were younger and had less advanced disease, suggesting a strong selection bias surrounding which patients with stage II and stage III breast cancer undergo immediate reconstruction. These tumor factors were also strongly associated with local-regional recurrence in our study. We also observed that women undergoing IBR were more likely to have private insurance. This may reflect improved access to reconstruction for private insurance (i.e. plastic surgeon willingness to accept insurance type). However, it may also reflect increased interest in reconstruction for younger patients who are more likely to have private insurance (compared with Medicare) Our findings are consistent with the demographics of patients undergoing IBR reported in other studies and reinforce the fact that women who undergo reconstruction are a select group.[18,26,32] However, our findings also suggest that IBR is not detrimental with regard to recurrence for those women with stage II and III breast cancer who are deemed by their surgical team to be good candidates for IBR.
While IBR in and of itself is not associated with increased local-regional recurrence in higher risk breast cancer patients, various other factors influence the decision-making for IBR. The appropriateness of IBR, while oncologically safe for select women, must remain an individualized decision. For example, when post-mastectomy radiation is under consideration, IBR may not be appropriate given the higher rate of complications with the reconstructed breast and poorer esthetic outcome when autologous tissue or implant is irradiated.[4,33,34] The various reconstructive options, their relationship to adjuvant therapy, and long-term reconstruction outcomes are additional important factors influencing the decision to pursue IBR.[31,32,34]
Limitations
Limitations of this study include the observational nature of the data with associated selection bias surrounding the patients with stage II/III breast cancer who undergo IBR. Our observed reconstruction rate overall was low, which may reflect the higher stage cancers (stage II/III) in this cohort. Further, we are only able to capture immediate breast reconstruction through this data source. While the group of stage II/III patients who are selected for and choose IBR have equivalent recurrence outcomes to their mastectomy alone counterparts, this does not indicate that all stage II/III patients would experience similar outcomes if IBR was uniformly performed. Consequently, the similar recurrence rates seen in our cohort between IBR and mastectomy alone groups cannot be extrapolated broadly in support of IBR for every stage II/III patient. Further, our recurrence data was abstracted from the chart rather than through prospective data collection. However, the risk of missingness in the data was minimized by robust data collection protocols as part of the CoC special study.
Conclusions
In this multi-center observational study, immediate breast reconstruction after mastectomy in selected stage II/III breast cancer patients was not associated with a risk of increased ipsilateral local-regional recurrence. However, the decision to consider IBR in higher risk patients must be individualized, accounting for individual patient and tumor factors. The current practice of offering IBR to select patients undergoing mastectomy, even those with more advanced cancer, remains appropriate.
Clinical Practice Points
Some surgeons are hesitant to perform immediate breast reconstruction in patients with higher risk cancers due to concerns that reconstruction can potentially cause cancer recurrence and mask detection. Similar concerns have been expressed by patients. Although immediate reconstruction has not been found to relate to increased recurrence or decreased ability to detect recurrence for women with early stage cancer, data on the recurrence risk associated with immediate reconstruction in women with higher risk cancer is limited. Our study of 5,318 stage II/III patients from a National Cancer Database special study found no difference in recurrence rates between women who are selected for immediate reconstruction compared to those that do not receive immediate reconstruction. Women who received immediate reconstruction were younger (p<0.001), with fewer comorbid conditions (p<0.001), and lower tumor burden in the breast (p=0.001) and lymph nodes (p=0.01). Regardless of reconstruction status, most recurrence was detected by the patient (45%) or on physician exam (24%). These findings support the current practice of offering immediate breast reconstruction to select stage II/III patients in a patient-centered manner.
Funding/Support:
This work was supported by the Patient Centered Outcomes Research Institute (PCORI) (Greenberg, Schumacher, Neuman, CE-1304-6543). This publication was further made possible by the National Institute of Health (NIH) funded University of Wisconsin Carbone Comprehensive Cancer Center Academic Oncologist Training Program (Neuman, NIH 5K12CA087718), Building Interdisciplinary Research Careers in Women’s Health Scholar Program (Neuman, NIH K12 HD055894), as well as the National Cancer Institute funded Surgical Oncology Research Training Program (Wiener, Stankowski-Drengler, T32 CA090217) and the Alliance for Clinical Trials in Oncology (U10CA180821). The data used in the study are derived from a de-identified National Cancer Database file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. Further, the contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of PCORI or NIH.
Disclosures:
Financial support for Ms. Dudley’s work was provided by a charitable contribution from the Peter and Myra Berk Cristall Breast Cancer Research Fund, which did not influence study design, collection, analysis and interpretation of data, the writing of the report, or the decision to submit the article for publication.
Footnotes
Data Sharing
The data that support the findings of this study will be available on request from the Alliance for Clinical Trials in Oncology Cancer Care Delivery Research Committee.
IBR: immediate breast reconstruction
References
- 1.Flanagan MR, Zabor EC, Romanoff A, et al. A Comparison of Patient-Reported Outcomes After Breast-Conserving Surgery and Mastectomy with Implant Breast Reconstruction. Ann Surg Oncol. 2019;26(10):3133–3140. doi: 10.1245/s10434-019-07548-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Hölzel D. Quality of life following breast-conserving therapy or mastectomy: results of a 5-year prospective study. Breast J. 2004;10(3):223–231. doi: 10.1111/j.1075-122X.2004.21323.x [DOI] [PubMed] [Google Scholar]
- 3.Aerts L, Christiaens MR, Enzlin P, Neven P, Amant F. Sexual functioning in women after mastectomy versus breast conserving therapy for early-stage breast cancer: a prospective controlled study. Breast Edinb Scotl. 2014;23(5):629–636. doi: 10.1016/j.breast.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 4.Chevray PM. Timing of breast reconstruction: immediate versus delayed. Cancer J Sudbury Mass. 2008;14(4):223–229. doi: 10.1097/PPO.0b013e3181824e37 [DOI] [PubMed] [Google Scholar]
- 5.Pusic AL, Matros E, Fine N, et al. Patient-Reported Outcomes 1 Year After Immediate Breast Reconstruction: Results of the Mastectomy Reconstruction Outcomes Consortium Study. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(22):2499–2506. doi: 10.1200/JCO.2016.69.9561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong T, McCarthy C, Min S, et al. Patient satisfaction and health-related quality of life after autologous tissue breast reconstruction: a prospective analysis of early postoperative outcomes. Cancer. 2012;118(6):1701–1709. doi: 10.1002/cncr.26417 [DOI] [PubMed] [Google Scholar]
- 7.Salhab M, Al Sarakbi W, Joseph A, Sheards S, Travers J, Mokbel K. Skin-sparing mastectomy and immediate breast reconstruction: patient satisfaction and clinical outcome. Int J Clin Oncol. 2006;11(1):51–54. doi: 10.1007/s10147-005-0538-1 [DOI] [PubMed] [Google Scholar]
- 8.Helczynska K, Larsson A-M, Holmquist Mengelbier L, et al. Hypoxia-inducible factor-2alpha correlates to distant recurrence and poor outcome in invasive breast cancer. Cancer Res. 2008;68(22):9212–9220. doi: 10.1158/0008-5472.CAN-08-1135 [DOI] [PubMed] [Google Scholar]
- 9.Vranken NPA, Weerwind PW, van Onna MA, Bouman EAC, van der Hulst RRWJ. Non-invasive tissue oximetry following unilateral DIEP-flap reconstruction: A pilot evaluation. JPRAS Open. 2017;12:59–65. doi: 10.1016/j.jpra.2017.01.008 [DOI] [Google Scholar]
- 10.Garner J, Davidson D, Eckert GJ, Barco CT, Park H, Park K. Reshapable polymeric hydrogel for controlled soft-tissue expansion: In vitro and In vivo evaluation. J Control Release Off J Control Release Soc. 2017;262:201–211. doi: 10.1016/j.jconrel.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graziano V, Scognamiglio MT, Zilli M, et al. Is the skin a sanctuary for breast cancer cells during treatment with anti-HER2 antibodies? Cancer Biol Ther. 2015;16(12):1704–1709. doi: 10.1080/15384047.2015.1108490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brett EA, Aitzetmüller MM, Sauter MA, Huemer GM, Machens H-G, Duscher D. Breast cancer recurrence after reconstruction: know thine enemy. Oncotarget. 2018;9(45):27895–27906. doi: 10.18632/oncotarget.25602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao D, Tsangaris TN, Kouprina N, et al. The Superficial Margin of the Skin-Sparing Mastectomy for Breast Carcinoma: Factors Predicting Involvement and Efficacy of Additional Margin Sampling. Ann Surg Oncol. 2008;15(5):1330–1340. doi: 10.1245/s10434-007-9795-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langstein HN, Cheng M-H, Singletary SE, et al. Breast cancer recurrence after immediate reconstruction: patterns and significance. Plast Reconstr Surg. 2003;111(2):712–720; discussion 721–722. doi: 10.1097/01.PRS.0000041441.42563.95 [DOI] [PubMed] [Google Scholar]
- 15.Taylor CW, Horgan K, Dodwell D. Oncological aspects of breast reconstruction. The Breast. 2005;14(2):118–130. doi: 10.1016/j.breast.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 16.Keith DJW, Walker MB, Walker LG, et al. Women who wish breast reconstruction: characteristics, fears, and hopes. Plast Reconstr Surg. 2003;111(3):1051–1056; discussion 1057–1059. doi: 10.1097/01.PRS.0000046247.56810.40 [DOI] [PubMed] [Google Scholar]
- 17.Carlson GW, Bostwick J, Styblo TM, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg. 1997;225(5):570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Zhu C, Gu Y. The Prognosis of Breast Cancer Patients after Mastectomy and Immediate Breast Reconstruction: A Meta-Analysis. PLoS ONE. 2015;10(5). doi: 10.1371/journal.pone.0125655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy RX, Wahhab S, Rovito PF, et al. Impact of immediate reconstruction on the local recurrence of breast cancer after mastectomy. Ann Plast Surg. 2003;50(4):333–338. doi: 10.1097/01.SAP.0000041488.88950.A2 [DOI] [PubMed] [Google Scholar]
- 20.Gieni M, Avram R, Dickson L, et al. Local breast cancer recurrence after mastectomy and immediate breast reconstruction for invasive cancer: a meta-analysis. Breast Edinb Scotl. 2012;21(3):230–236. doi: 10.1016/j.breast.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 21.Ha JH, Hong KY, Lee H-B, et al. Oncologic outcomes after immediate breast reconstruction following mastectomy: comparison of implant and flap using propensity score matching. BMC Cancer. 2020;20. doi: 10.1186/s12885-020-6568-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z-Y, Kim H-J, Lee J-W, et al. Breast Cancer Recurrence in the Nipple-Areola Complex After Nipple-Sparing Mastectomy With Immediate Breast Reconstruction for Invasive Breast Cancer. JAMA Surg. 2019;154(11):1030–1037. doi: 10.1001/jamasurg.2019.2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirzabeigi MN, Rhemtulla IA, Mcdonald ES, et al. Locoregional Cancer Recurrence after Breast Reconstruction: Detection, Management, and Secondary Reconstructive Strategies. Plast Reconstr Surg. 2019;143(5):1322–1330. doi: 10.1097/PRS.0000000000005522 [DOI] [PubMed] [Google Scholar]
- 24.Nedumpara T, Jonker L, Williams MR. Impact of immediate breast reconstruction on breast cancer recurrence and survival. The Breast. 2011;20(5):437–443. doi: 10.1016/j.breast.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 25.Downes KJ, Glatt BS, Kanchwala SK, et al. Skin-sparing mastectomy and immediate reconstruction is an acceptable treatment option for patients with high-risk breast carcinoma. Cancer. 2005;103(5):906–913. doi: 10.1002/cncr.20851 [DOI] [PubMed] [Google Scholar]
- 26.Lim W, Ko B-S, Kim H-J, et al. Oncological safety of skin sparing mastectomy followed by immediate reconstruction for locally advanced breast cancer. J Surg Oncol. 2010;102(1):39–42. doi: 10.1002/jso.21573 [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Database. American College of Surgeons. Accessed September 28, 2020. https://www.facs.org/quality-programs/cancer/ncdb
- 28.Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: An introduction to available quality assessment tools. J Surg Oncol. 2009;99(8):488–490. doi: 10.1002/jso.21173 [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 30.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 31.Albornoz CR, Bach PB, Mehrara BJ, et al. A Paradigm Shift in U.S. Breast Reconstruction: Increasing Implant Rates. Plast Reconstr Surg. 2013;131(1):15–23. doi: 10.1097/PRS.0b013e3182729cde [DOI] [PubMed] [Google Scholar]
- 32.Wilkins EG, Hamill JB, Kim HM, et al. Complications in Postmastectomy Breast Reconstruction One-year Outcomes of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study. Ann Surg. 2018;267(1):164–170. doi: 10.1097/SLA.0000000000002033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kronowitz SJ. Immediate versus delayed reconstruction. Clin Plast Surg. 2007;34(1):39–50; abstract vi. doi: 10.1016/j.cps.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 34.Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124(2):395–408. doi: 10.1097/PRS.0b013e3181aee987 [DOI] [PubMed] [Google Scholar]


