Abstract
Context.
Patients with advanced cancer often involve family caregivers in health-related decision-making from diagnosis to end-of-life; however, few interventions have been developed to enhance caregiver decision support skills.
Objectives.
Assess the feasibility, acceptability, and potential efficacy of individual intervention components of CASCADE (CAre Supporters Coached to be Adept DEcision Partners), an early telehealth, palliative care coach-led decision support training intervention for caregivers.
Methods.
Pilot factorial trial using the multiphase optimization strategy (October 2019-October 2020). Family caregivers and their care recipients with newly-diagnosed advanced cancer (n = 46 dyads) were randomized to1 of 8 experimental conditions that included a combination of one of the following three CASCADE components: 1) effective decision support psychoeducation; 2) decision support communication training; and 3) Ottawa Decision Guide training. Feasibility was assessed by completion of sessions and questionnaires (predefined as ≥80%). Acceptability was determined through postintervention interviews and participants’ ratings of their likelihood to recommend. Measures of effective decision support and caregiver and patient distress were collected at Twelve and Twenty four weeks.
Results.
Caregiver participants completed 78% of intervention sessions and 81% of questionnaires; patients completed 80% of questionnaires. Across conditions, average caregiver ratings for recommending the program to others was 9.9 on a scale from 1-Not at all likely to 10-Extremely likely. Individual CASCADE components were observed to have potential benefit for effective decision support and caregiver distress.
Conclusion.
We successfully piloted a factorial trial design to examine components of a novel intervention to enhance the decision support skills of advanced cancer family caregivers. A fully-powered factorial trial is warranted.
Keywords: Palliative care, family caregiving, cancer, multiphase optimization strategy
Introduction
From the time individuals are diagnosed with an advanced metastatic cancer to the end of life, they have numerous choices to make.1–5 These include decisions about treatment, surgery, devices, care transitions, location of care, self-care and treatment adherence, accessing palliative and hospice care, and life-sustaining treatments.2,6–10 Most research on serious illness decision-making has been guided by the 2-actor paradigm of shared decision-making that focuses on the provider and the patient.11 However, emerging research suggests this view is too narrow in scope. In a national sample of over 5,200 newly-diagnosed cancer patients, 49.4% reported that they shared decisions equally with family members, and 22.1% reported having elicited at least some input on decisions.12 Family caregivers assist persons with advanced cancer in the decision-making process in a number of ways, such as seeking information about the cancer and treatment options; having discussions with patients about their goals and values; posing “what if” scenarios about potential future health states; and identifying treatment and disease decision points (e.g., seeking emergency care).13,14 Given these different critical roles, patients making healthcare decisions with caregivers who are unprepared may experience inadequate family decision support leading to heightened distress and receipt of care/treatments inconsistent with their values and preferences.10–12,15,16 This in turn may increase distress for family caregivers.17–21
Hence, there is a critical need to train cancer family caregivers to be supportive of patient decision-making early in the advanced cancer trajectory. However, few early palliative care interventions exist that enhance caregivers’ decision support skills.20,22 Providing effective decision support to patients is one among several skills targeted within our prior evidence-based model of early concurrent oncology palliative care for family caregivers that demonstrated efficacy in improving caregiver depressive symptoms and stress burden.23–25 Decision support training content that was included in our prior work for family caregivers included brief content on providing effective social support, communication, and Ottawa Decision Guide training; however, we do not know from our prior intervention work which of these components and component interactions (if any) are effective in optimizing patient and caregiver decision-making outcomes (outcomes that were not the specific focus of our prior work).
In traditional intervention and development testing approaches, an intervention is typically comprised of a “bundle” of components that are “packaged” together and tested versus usual care or another comparator. However, this “bundled package” approach has limitations as the results cannot help one discern which components of a multicomponent intervention, including their levels and dosage amounts, are efficacious.26,27 Results also cannot inform one about component interactions and whether certain components might be too costly or consume too much time and resources given their return on value. Within the Multiphase Optimization Strategy (MOST) framework of intervention development and testing, an optimization trial (which includes factorial trial designs) are performed to identify the most effective and efficient components of a “packaged” intervention that can then be tested in traditional 2-arm randomized controlled trial. In-depth resources on MOST and factorial trial design can be found elsewhere.26–28
In light of this and based on intervention content from our prior work,23 we pilot tested a factorial trial design approach26,27 aimed at ascertaining the effect of individual components of a novel multicomponent intervention, called CASCADE (CAre Supporters Coached to be Adept DEcision partners). CASCADE is designed to enhance the decision support skills of family caregivers of persons with newly-diagnosed advanced cancer. As this was our group’s first attempt at a factorial trial approach, our primary aim was to determine the feasibility of this factorial trial approach and the acceptability of CASCADE intervention components (each with 2 levels), including psychoeducation on effective decision support (1 vs. 3 sessions), decision support communication training (1 session vs. none), and Ottawa Decision Guide training (1 session vs. none). We also aimed to explore the potential efficacy of these individual CASCADE components on caregiver and patient outcomes over Twenty four weeks.
Methods
Trial Design and Oversight
This was a pilot, single-site, single-blind randomized 23 (2 × 2 × 2) factorial trial following the MOST framework.26 We followed the Consolidated Standards for Reporting Trials (CONSORT) reporting guidelines for trial conduct and reporting.29–31 Dyads of family caregivers and their care recipients with newly-diagnosed advanced cancer were randomized to 1 of 8 experimental conditions that included a combination of 1 of the following three CASCADE components: 1) effective decision support psychoeducation; 2) decision support communication training; and 3) Ottawa Decision Guide training (Table 1). Patients were enrolled for data collection only and did not receive any intervention components. The study protocol and data safety plan were approved by the University of Alabama at Birmingham (UAB) Institutional Review Board. The trial is registered in ClinicalTrials.gov (NCT03947606). Participants provided verbal consent by telephone and were mailed a copy of the consent afterwards.
Table 1.
Study Design
| Condition | Social Support Effectiveness Psychoeducation (1 Session vs. 3 Sessions) | Decision Support Communication Training (Yes vs. No) | Ottawa Decision Guide Training (Yes vs. No) |
|---|---|---|---|
| 1 | 1 session | 1 session | 1 session |
| 2 | 1 session | 1 session | No sessions |
| 3 | 1 session | No sessions | 1 session |
| 4 | 1 session | No sessions | No sessions |
| 5 | 3 sessions | 1 session | 1 session |
| 6 | 3 sessions | 1 session | No sessions |
| 7 | 3 sessions | No sessions | 1 session |
| 8 | 3 sessions | No sessions | No sessions |
Note: This factorial design should not be considered an 8-arm trial in which 7 conditions are compared to a control condition. Rather, the purpose is to examine the main effects of each component and interactions between components. In this full factorial design, the component levels are balanced with respect to each other, hence it is possible to estimate the main component effects within the same model and using data from all participants.
Participants
From October 2019 to October 2020, study staff screened adult oncology outpatient clinic schedules of a large tertiary academic medical center in the South-eastern United States. Patient participants were eligible if they were: 1) ≥18 years old; 2) diagnosed within past 60–90 days with an advanced cancer, defined as metastatic, recurrent, or progressed stage III/IV cancer, including brain, lung, breast, gynecologic, head and neck, gastrointestinal, genitourinary, melanomas, or hematologic malignancies; 3) English-speaking; 4) and able to complete baseline measures. Patient exclusion criteria included: documented active severe mental illness (e.g. schizophrenia, bipolar disorder, or major depressive disorder), dementia, active suicidal ideation, uncorrected hearing loss, or active substance abuse. Eligible patient participants were approached either in-person or by telephone by trained community-based recruiters prior to their outpatient oncology visit. Patients were required to have a willing family caregiver to participate in the study. Family caregivers were eligible if they were: 1) ≥18 years old, 2) identified by themselves or the patient as “a relative, friend, or partner that has a close relationship with you and who assists you with your medical decisions and who may or may not live in the same residence as you and who is not paid for their help,” 3) caring for a patient with advanced stage cancer as detailed above (and who was also willing to participate), 4) English-speaking; and 5) able to complete baseline measures. Caregiver and patient participants each received $40, $50, and $60 per completion of baseline, Twelve and Twenty four week questionnaires, respectively. Caregivers received $40 for completing acceptability interviews. To adequately ascertain study feasibility and intervention components acceptability within the constraints of available funds and resources, a sample size of 40 dyads was pre-specified.
Randomization and Blinding
After caregiver and patient participants completed informed consent and baseline measures, caregivers were randomized by the project manager (S.E.) to 1 of the 8 experimental conditions with block lengths of 8. Randomization was concealed and performed using a computer-generated randomization scheme overseen by the study statistician (A.A.). Participants, clinicians, and supportive care coaches were not blinded. The principal investigator, co-investigators, and data collectors were blinded. Data were unblinded after analysis.
Intervention and Fidelity Monitoring
CASCADE is theoretically based on Social Support Effectiveness Theory32 and the Ottawa Decision Support Framework (Fig. 1).33,34 Caregiver decision partnering training is designed to modify family caregiver skills, including their ability to: 1) provide effective social support through psychoeducation on key social support principles that will optimize decision support to patients; 2) elicit patient decisional needs, including patient values, preferences, and coping through better decision support communication, enhancing the quantity of decision-making conversations; and 3) provide structured decision support using an evidence-based tool (i.e., the Ottawa Decision Guide) to help patients clarify choices and guide deliberation, increasing patient’s decision-making self-efficacy. Modification of these skills and improvement in patient mediating outcomes is hypothesized to lead to higher positive decision influence by the caregiver from the patient’s perspective and lower patient and caregiver distress and specifically as it relates to co-decision-making being a potentially stressful context.
Fig. 1.
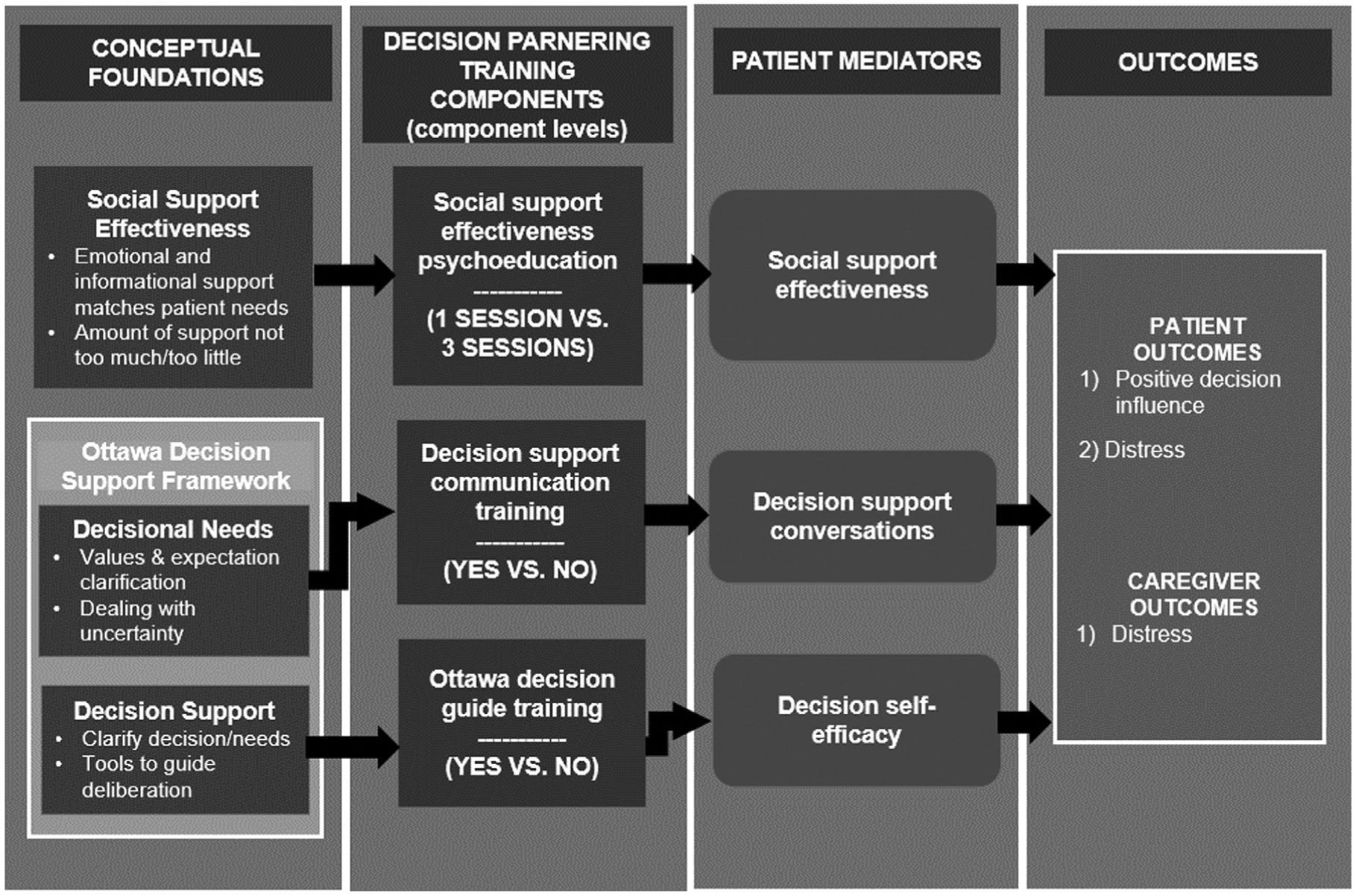
Conceptual framework guiding CASCADE.
Guided by this theoretical basis, we identified decision support training content embedded in our prior work,23 that addressed key conceptual targets including social support effectiveness in the context of providing decision support, communication training to help caregivers have decision-making conversations with patients, and Ottawa Decision Guide training to provide families a structured tool to help systematically make decisions (Table 1).
For the social support effectiveness component, a “basic” (1 session) and an “advanced” (3 session) version were developed. The 1-session version addressed the role of families in patient decision-making in serious illness and basic principles of effective social support when helping with health-related decisions. The 3-session version addressed this same content plus tips for making decisions about cancer treatment, roles of values in deciding with and for others, and supporting advance directive completion and being a durable power of attorney for healthcare. The decision support communication training component (1 session vs. none) was a single session that addressed effective listening skills and strategies to effectively express oneself in the context of decision making. The Ottawa Decision Guide training component (1 session vs. none) was a single session that reviewed the 4 steps of the Ottawa Guide and how the tool can be used in partnership with patients. Further details of the Ottawa Guide are available on the publisher’s website (decisionaid.ohri.ca) and in published work.35
Family caregivers were partnered with a trained palliative care coach who scheduled and facilitated a series of one to five weekly 20–30 minute phone sessions (depending on their assigned condition) and one monthly follow-up call. On average, sessions lasted 30 minutes. Prior to sessions, caregiver participants were mailed a condition-specific CASCADE Family Toolkit that provided educational content and space for written reflection and that served as a medical organizer (e.g., calendar, pen/pencil case, sheets to record patient medical information).
A number of fidelity strategies were employed to ensure reliability of intervention delivery and ensure participants received their assigned intervention condition.36 Palliative care coaches, 1 registered nurse (B. H.) and 1 experienced lay navigator (C.D.), underwent approximately 24 hours of structured orientation and training overseen by the principal investigator (J.N.D.-O.) and study staff. Training included independent reading, skills practice, and role play. Coaches were guided by condition-specific scripts and standardized charting templates to ensure session objectives were consistently addressed. All sessions were audio-recorded and independently reviewed by study team members (R.A.T., R.D.W., R.D.R.) for intervention fidelity using a checklist. There was no protocol nonad-herence, defined as 3 consecutive ratings less than 80% over the course of the study.
Outcomes
The pre-specified primary outcomes for this pilot were the feasibility of performing a factorial trial design and participant acceptability of CASCADE intervention components. Like traditional clinical trial approaches to examining intervention feasibility and acceptability, factorial trials track rates of enrollment, retention, data and intervention completion rates, fidelity, and participants’ satisfaction with the intervention. In factorial trials, special attention is paid to these characteristics within each of the experimental conditions, which contain different combinations of the intervention components.
Feasibility was measured by tracking completion rates of intervention sessions and data collection at all timepoints both overall and by condition. The threshold for feasibility was predefined as ≥80% for overall data collection and session completion rates. Acceptability was assessed through a semi-structured interview conducted after caregiver participants completed the intervention. To assess acceptability, a brief semi-structured interview was conducted immediately following intervention completion by one of the study co-investigators with qualitative expertise (E.R.C.). Participants answered questions about overall impressions of the program and practical aspects of participation including what recommendations they had for improvement. As an indicator of overall acceptability, caregiver participants were also asked how likely they would be to recommend the program overall to someone else in a similar circumstance, from 1 (Not at all likely) to 10 (Extremely likely). For each CASCADE component/component level, participants rated on scales of 1 to 10 how relevant (from 1 [Not at all relevant]) to 10 [Extremely relevant]), how helpful (from 1 [Not at all helpful] to 10 [Extremely helpful]), and how satisfied (from 1 [Not at all satisfied] to 10 [Extremely satisfied]) they were with the individual sessions.
While the pilot was not powered to determine efficacy, secondary outcomes included examining the preliminary efficacy of individual CASCADE components on patient-reported positive decision influence and patient- and caregiver reported distress measured over Twenty four weeks. Positive decision influence was measured by an adapted version of the Rini Decision Influence Scale9 (α =.86, score range: 30–150 higher scores=more positive/effective decision influence). Items assess the degree to which patients felt positively supported in their health-related decision-making by a close other (e.g., help with understanding choices, respect thoughts and feelings about decisions) on a 5-point scale from “Never” to “Very often.” Patient and caregiver distress was measured using the 14-item Hospital Anxiety and Depression Scale (α =.82; score range: 0–42; higher scores=higher distress) with subscales measuring anxiety and depressive symptoms.37
Analysis
Descriptive statistics were used to tabulate sociodemographic characteristics, rates of intervention and questionnaire completion, and acceptability ratings. Acceptability interviews were analyzed using thematic analysis,38,39 consistent with our prior work.14,40 For examining preliminary efficacy, linear mixed-effect models with random effects for participant and week were fitted to the repeated measurements (baseline, Twelve-, and 2 Twenty four -weeks) of each caregiver or patient outcome variables. The models included fixed effects for time-point, binary indicators for each component (Social support effectiveness psychoeducation [1 session vs. 3 sessions], decision support communication training [1 session vs. none], and Ottawa Decision Guide training [1 session vs. none]), and interaction terms between time-point and each component indicator. Interaction parameters estimated the mean difference in change from baseline between levels of a component at each post-intervention time-point, i.e., the component’s effect at each follow-up time-point. Linear contrasts were then used to average component effects across the 2 post-intervention time-points to obtain a single time-averaged estimate of effect for each component and its 95% Confidence Interval (CI). Next, the baseline standard deviation of the outcome was used to rescale each time-averaged component effect and its 95% CI into a standardized measure of effect size, Cohen’s d [ref] (small~.2, medium~.5, large~.8).41 No between-component interaction effects were included due to the small sample size. The modeling approach allowed using all available data-points for each outcome and is robust to bias from missing data as long the missing data is missing at random. Analyses were conducted using SAS/STAT software version 9.4 (SAS Institute Inc., 2016).
Results
Study Participants
From October 2019 to October 2020, a total of 46 family caregivers and their care recipients were randomized (Fig. 2). 83 caregivers were approached, 59 consented, and 46 were randomized (enrollment rate: 55.4%). Caregivers had a mean age of 58.8 (12.9) and were mostly female (33 [71.7%]), married or living with a partner (29 [67.4%]), Protestant (39 [84.8%]), and the spouse/partner of the patient (28 [60.9%]) (Table 2). Nearly one-third of the sample was Black/African American (14 [30.4%]). Most caregivers had been providing support every day (42 [91.3%]) for over 8 hours per day (23 [50.0%]) but for less than a year (33 [71.7%]). Patients had a mean age of 64.8 (12.8), were nearly split between females (25 [54.3%]) and males (21 [45.7]) and had the same racial proportions as caregivers (Table 3). Patients had a wide variety of advanced metastatic cancers and most had received anticancer treatments within 3 months of enrollment including chemotherapy (43 [93.5%]) and surgery (24 [52.2%]).
Fig. 2.
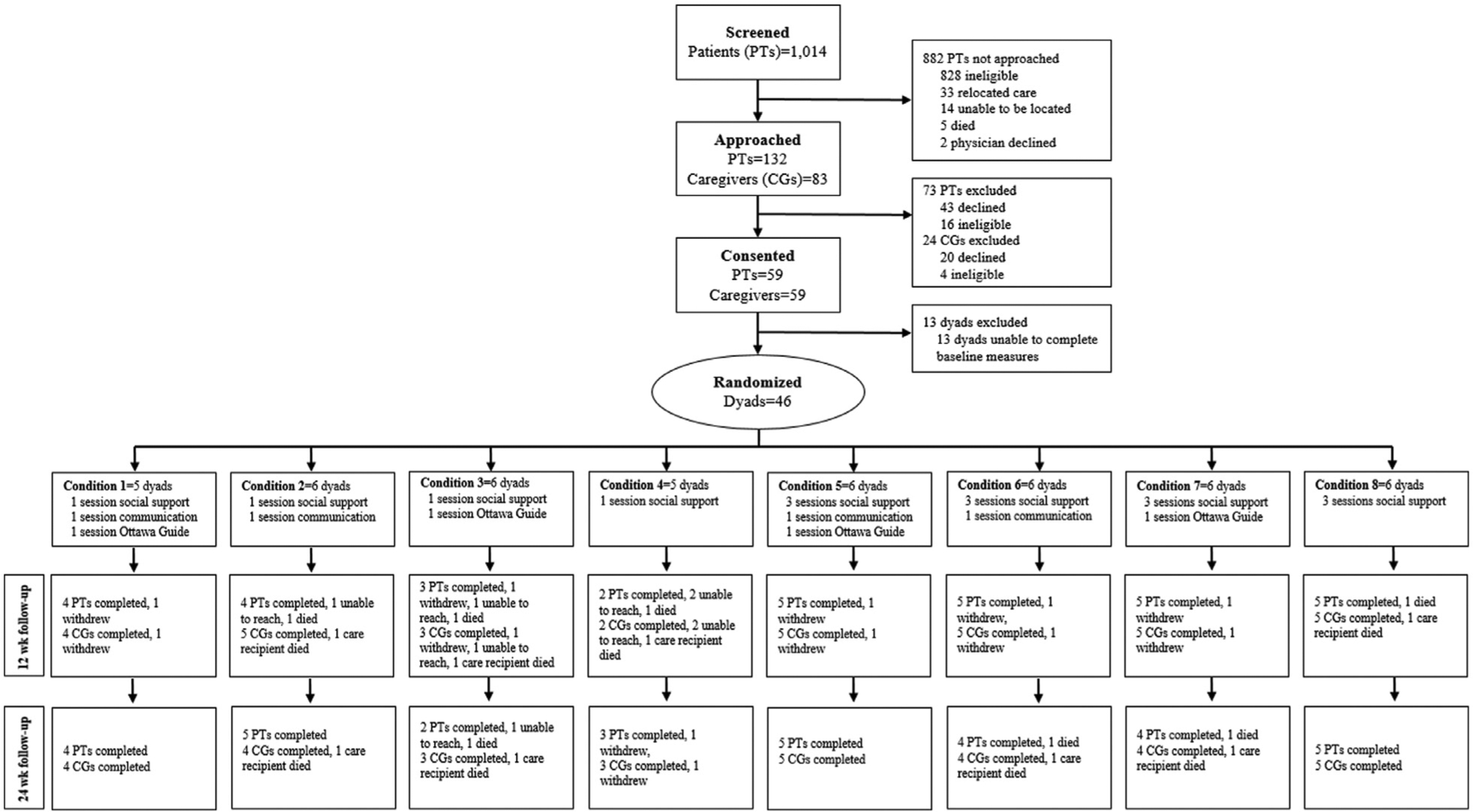
Participant screening, enrollment, allocation, and data collection.
Table 2.
Demographic Characteristics of Family Caregivers (n = 46)
| Characteristic | n | % or Standard Deviation |
|---|---|---|
| Age, mean (SD), yr | 58.8 | 12.9 |
| Gender, n (%) | ||
| Female | 33 | 71.7 |
| Male | 13 | 28.3 |
| Race | ||
| White | 31 | 67.4 |
| African-American/Black | 14 | 30.4 |
| Other | 1 | 2.2 |
| Hispanic | 1 | 2.2 |
| Marital Status | ||
| Married or living with partner | 29 | 63.0 |
| Never married | 8 | 17.4 |
| Divorced or separated | 7 | 15.2 |
| Widowed | 2 | 4.3 |
| Education | ||
| Some high school | 1 | 2.2 |
| High school or GED | 10 | 21.7 |
| Some College or Technical School | 15 | 32.6 |
| College graduate or above | 20 | 43.5 |
| Employment Status | ||
| Full or part time or student | 22 | 47.8 |
| Retired or homemaker | 19 | 41.3 |
| Not employed | 5 | 10.8 |
| Religious Affiliation, n, % | ||
| Protestant | 39 | 84.8 |
| Catholic | 4 | 8.7 |
| None | 2 | 4.3 |
| Other | 1 | 2.2 |
| Relationship to patient (The patient is my…) n (%) | ||
| Spouse/partner | 28 | 60.9 |
| Parent | 10 | 21.7 |
| Other relative or friend | 8 | 17.4 |
| Yrs serving as a caregiver, n, % | ||
| <1 yr | 33 | 71.7 |
| 1 – 4 yrs | 8 | 17.3 |
| ≥5 yrs | 5 | 10.9 |
| Days/Week Providing Care, N (%) | ||
| 1 – 4 days/week | 4 | 8.7 |
| Every day | 42 | 91.3 |
| Hours per day providing care, n, % | ||
| 1 – 4 hours/day | 14 | 30.4 |
| 5 – 8 hours/day | 9 | 19.5 |
| >8 hours/day | 23 | 50.0 |
| Measurement scores at baseline | ||
| Total Distress (HADS, score range: 0 – 42, higher scores=higher distress) | 11.6 | 7.0 |
| Anxiety (HADS, score range: 0 – 21, higher scores=higher anxiety symptoms) | 6.5 | 4.4 |
| Depressive symptoms (HADS, score range: 0 – 42, higher scores=higher anxiety) | 5.1 | 3.5 |
Table 3.
Demographic Characteristics of Patients (n = 46)
| Characteristic | n | % or Standard Deviation |
|---|---|---|
| Age, mean, SD | 64.8 | 12.8 |
| Gender, n, % | ||
| Female | 25 | 54.3 |
| Male | 21 | 45.7 |
| Race | ||
| White | 31 | 67.4 |
| African-American/Black | 14 | 30.4 |
| Other | 1 | 2.2 |
| Hispanic | 1 | 2.2 |
| Marital status | ||
| Married | 31 | 67.4 |
| Never married | 5 | 10.9 |
| Divorced | 4 | 8.7 |
| Widowed | 6 | 13 |
| Education | ||
| Some high school | 2 | 4.3 |
| High school or GED | 12 | 26.1 |
| Some College or Technical School | 18 | 39.1 |
| College graduate or above | 14 | 30.4 |
| Employment Status | ||
| Full or part time or student | 7 | 15.2 |
| Retired or homemaker | 23 | 50 |
| Not employed | 16 | 30.4 |
| Religious Affiliation, n, % | ||
| Protestant | 43 | 93.5 |
| Catholic | 2 | 4.3 |
| None | 1 | 2.2 |
| Primary cancer site of patient | ||
| Breast | 12 | 26.1 |
| Colon/rectal | 8 | 17.4 |
| Lung | 6 | 13.0 |
| Head and Neck | 5 | 10.9 |
| Leukemia/Non-Hodgkin’s Lymphoma | 4 | 8.7 |
| Pancreatic | 4 | 8.7 |
| Other (liver, bladder, kidney, brain) | 7 | 15.2 |
| Anticancer treatments at enrollment | ||
| Surgery | 24 | 52.2 |
| Chemotherapy | 43 | 93.5 |
| Radiotherapy | 12 | 26.1 |
| Immunotherapy | 4 | 8.7 |
Feasibility
Out of 138 possible data collection time points for caregiver participants, 112 were completed (81.2%); similarly, patient participants completed 111 out of 138 possible data collection time points (80.4%). For intervention sessions, 105 of 134 (78%) possible sessions were completed by caregiver participants (Table 4). Pilot testing this factorial trial design also allowed us to assess variances in participation by condition (i.e., the different combinations of CASCADE intervention components). While not statistically powered to examine such differences, we did observe the lowest session completion rate (25%) in condition 4 (which had just one session of basic decision support psychoeducation); it is relevant to highlight this condition had the highest mean baseline caregiver distress score (Mean HADS Total Score =16.2) while yet having the fewest number of sessions of all the conditions (1 session).
Table 4.
Caregiver Participation Rates and Acceptability Scores Across Experimental Conditions
| Condition | Mean Baseline Caregiver Distress Score (HADSa) | n | Total # of Weekly Sessions Per Condition | Attrition | Sessions Completed (Completed/Assigned) | Average Likelihood of Recommending Program to Others (1 [Not at All Likely) to 10 [Extremely Likely] | |
|---|---|---|---|---|---|---|---|
| Withdrawals | Deathsb | ||||||
| 1 | 13.0 | 5 | 3 | 1 | 0 | 11/15 (73%) | 9.5 |
| 2 | 10.0 | 6 | 2 | 0 | 1 | 10/10 (100%) | 9.7 |
| 3 | 12.2 | 6 | 2 | 1 | 1 | 8/10 (80%) | 9.8 |
| 4 | 16.2 | 5 | 1 | 1 | 1 | 1/4 (25%) | 10.0 |
| 5 | 12.3 | 6 | 5 | 1 | 0 | 26/30 (87%) | 10.0 |
| 6 | 11.9 | 6 | 4 | 1 | 0 | 15/24 (63%) | 10.0 |
| 7 | 7.7 | 6 | 4 | 1 | 0 | 20/24 (83%) | 10.0 |
| 8 | 10.8 | 6 | 3 | 0 | 1 | 14/17 (82%) | 10.0 |
| Total | 11.6 | 46 | n/a | 6 | 4 | 105/134 (78%) | 9.9 |
Hospital and Anxiety Depression Scale (Score range: 0 – 42; higher scores = higher distress).
For patients who died prior to caregivers completing assigned sessions, remaining sessions not counted in denominator of possible session completions.
Acceptability
Over all conditions, average caregiver ratings for recommending the program to other individuals in a similar situation was 9.9 on a scale from 1-Not at all likely to 10-Extremely likely (Table 4). Participants also highly rated the relevance, helpfulness, and satisfaction with each of the individual CASCADE components (Table 5), with scores ranging from 7.7 to 9.5. Based on thematic analysis of responses to how the program impacted participants’ thoughts and behaviors, many commented on how the program stimulated self-reflection and introspection about how they supported their care recipient’s decision-making and invoked a desire to change how they communicated with them (including participants who did not receive the communication skills training). As one participant stated: “It made me stop to ask my husband more questions of what he was feeling….you get so caught up in what you’re going through that you forget to stop and think about—should I be asking him?” While all participants were asked about future changes to the program to improve to, most did not have specific recommendations. A few felt that CASCADE had come “kind of late in [their] cancer journey” and that some of the topics covered by the program related to things that they had already had to “figure out on [their own]” before becoming part of the study.” They simply “wish [they] had had it sooner”, because for them, “the timing [was] just so key.” Some participants expressed a desire to have more follow up after the sessions ended. One participant stated that she felt that “it would’ve been nice” because “it could be a follow-up on how are you doing, how has it helped in the long run.”
Table 5.
Relevance, Helpfulness, and Satisfaction of Individual CASCADE Components
| Component | Average Caregiver Baseline HADS Scores | How Relevant? | How Helpful? | How Satisfied? | ||
|---|---|---|---|---|---|---|
| Total | Anxiety | Depression | ||||
| Basic social support effectiveness psychoeducation | 12.7 (5.7) | 6.8 (4.1) | 5.9 (3.1) | 9.1 | 8.9 | 9.2 |
| Advanced social support effectiveness psychoeducation | 10.7 (7.9) | 6.3 (4.8) | 4.3 (3.7) | |||
| Part 1 | 8.1 | 8.7 | 9.5 | |||
| Part 2 | 8.8 | 8.7 | 9.3 | |||
| Part 3 | 9.0 | 9.2 | 9.5 | |||
| Decision support communication training | 11.8 (6.5) | 6.6 (4.5) | 4.9 (3.7) | 8.9 | 9.1 | 9.5 |
| No decision support communication training | 11.5 (7.5) | 6.6 (4.5) | 4.9 (3.7) | - | - | - |
| Ottawa decision guide training | 11.2 (4.9) | 5.9 (2.9) | 3.3 (3.3) | 7.7 | 7.7 | 8.6 |
| No Ottawa decision guide training | 12.1 (8.7) | 7.1 (5.6) | 4.9 (3.8) | - | - | - |
Preliminary Efficacy
In terms of caregiver distress and depressive symptoms, the results suggest that the most beneficial components were decision support communication training and Ottawa Decision Guide training (d ranging from −.49 to −.25) (Table 6). However, for caregiver anxiety, the only beneficial component was decision support communication training (d =−.26). In terms of patient distress, including anxiety, and depressive symptom subscales, the results suggest that the only beneficial component was Ottawa Decision Guide training (d ranging from −.22 to −.2). With regard to patient perceived positive decision influence, the component that resulted in the largest effect was decision support communication training (d =.62); however, the psychoeducation on effective decision support component also resulted in a relevant effect albeit of lower magnitude (d =.33).
Table 6.
Main Effects of Component/Component Levels on Caregiver and Patient Outcomes Over 24 Weeks
| Outcome | Psychoeducation on Effective Decision Support | Decision Support Communication Training | Ottawa Decision Guide Training | Cohen’s da (Magnitude of Differences in Change Over Time) | 95% CI |
|---|---|---|---|---|---|
| CAREGIVERS | |||||
| Total distress (higher scores=higher distress) | 3 sessions | - | - | 0.08 | (−0.39, 0.55) |
| - | 1 session | - | −0.30 | (−0.76, 0.17) | |
| - | - | 1 session | −0.25 | (−0.72, 0.21) | |
| Anxiety symptoms (higher scores=higher anxiety symptoms) | 3 sessions | - | - | −0.02 | (−0.48, 0.44) |
| - | 1 session | - | −0.26 | (−0.71, 0.20) | |
| - | - | 1 session | −0.01 | (−0.46, 0.45) | |
| Depressive symptoms (higher scores=higher depressive symptoms) | 3 sessions | - | - | 0.27 | (−0.25, 0.78) |
| - | 1 session | - | −0.29 | (−0.80, 0.23) | |
| - | - | 1 session | −0.49 | (−1.00, 0.03) | |
| PATIENTS (patient participants reported data only) | |||||
| Total distress (higher scores=higher distress) | 3 sessions | - | - | 0.34 | (−0.30, 0.98) |
| - | 1 session | - | 0.16 | (−0.48, 0.80) | |
| - | - | 1 session | −0.22 | (−0.86, 0.42) | |
| Anxiety symptoms (higher scores=higher anxiety symptoms) | 3 sessions | - | - | 0.34 | (−0.27, 0.95) |
| - | 1 session | - | 0.25 | (−0.35, 0.86) | |
| - | - | 1 session | −0.20 | (−0.80, 0.41) | |
| Depressive symptoms (higher scores=higher depressive symptoms) | 3 sessions | - | - | 0.27 | (−0.40, 0.94) |
| - | 1 session | - | 0.05 | (−0.61, 0.72) | |
| - | - | 1 session | −0.22 | (−0.89, 0.44) | |
| Positive decision influence (higher scores=more positive decision influence) | 3 sessions | - | - | 0.33 | (−0.52, 1.18) |
| - | 1 session | - | 0.62 | (−0.22, 1.46) | |
| - | - | 1 session | −0.59 | (−1.43, 0.24) | |
Interpretation guidance: If higher scores represent worse outcomes, then a negative effect size indicates more improvement for the listed component/component level; if higher scores represent better outcomes, then a positive effect size indicates more improvement for the listed component/component level.
Discussion
This is among the first demonstrations of how a factorial trial design can be performed in a palliative care behavioral intervention context, and specifically how this approach informs development of a novel decision support training intervention for family caregivers of individuals with newly-diagnosed advanced cancer. Pilot testing this design allowed us to refine procedures and protocols for effectively conducting trial procedures and managing 8 different intervention conditions with high feasibility and acceptability. The data and strategies learned and refined from this pilot will inform a fully powered study to build an optimized version of CASCADE.
The pilot had an enrollment rate of 55.4% and included a diverse sample, with African-American/Black participants comprising nearly a third of the sample (30.4%). Our overall enrollment rate was markedly higher than the average enrollment rate of 33% reported for cancer caregiver clinical trials in a recent systematic review.42 Our success in enrollment for a complex factorial trial design and in recruiting a diverse sample, particularly during COVID-19, could be attributed to several strategies developed during the implementation of the intervention. First, because of in-person clinic recruitment restrictions during COVID-19, we developed a mail and telephone recruitment approach and verbal consent process that allowed us to converse with potential participants in the familiar space of their homes and avoid approaching them in the often busy and time-constrained waiting room period prior to their oncology clinic appointments. Second, we developed plain language descriptions of the study and simple graphics to describe the study design, informing caregivers that they would be assigned randomly to 1 of 8 different versions of the CASCADE intervention and that everyone would receive some version of the program. Third, we employed a racially diverse recruitment team with extensive experience and expertise in recruiting minorities, potentially aiding in recruiting African American/Black participants. This is a notable strength given reviews of cancer caregiving trials that have underscored the lack of diversity in caregiving samples.22
Overall, completion rates for the intervention sessions were 78% (Table 4). While admittedly just short of our a priori benchmark of 80%, this session completion rate is higher than comparable family caregiver clinical trial populations.43 Unexpectedly, the condition with the lowest completion percentage had the lowest participation burden with only a single session on social support effectiveness, a component that had high relevance (9.1 out of 10), helpfulness (8.9 out of 10), and satisfaction scores (9.2 out of 10). Notably, the average baseline distress score for this condition (16.2) was the highest out of all 8 experimental conditions (average score: 11.6). As noted by others, distressed caregivers may have difficulty engaging in intervention activities due to time demands, scheduling conflicts, and having more urgent patient care concerns to prioritize.43 Strategies noted in the literature that we plan to integrate in a fully powered trial to promote intervention completion by distressed individuals (for whom the intervention may benefit the most) will include reinforcement of the potential benefit of the program to their circumstances, communicating endorsement of the program by their patient’s oncologist, addressing engagement barriers during initial contacts, and flexible and off-hour scheduling options (e.g., nights, weekends) with coaches.43 We also expect that in a fully powered study with a larger sample size, participant characteristics (including distress) will be more evenly randomized and distributed across conditions and thus feel confident that we can keep this condition (and the two other conditions that had completions rates <80%) going forward in a larger trial.
This was our team’s first experience with this trial design and hence warranted a pilot to help us gain experience managing many more experimental conditions than is typical of a traditional 2-arm randomized trial.44 We were able to conduct the pilot with 8 different conditions with high fidelity to participants’ assigned conditions without any evident contamination. As further detailed elsewhere,27 strategies we developed from our experience and used to keep participants in their assigned condition included having separate interventionist scripts and charting templates for each of the 8 conditions. We also developed 8 different versions of the CASCADE Toolkit, so that participants were not exposed to material from other conditions. Finally, all sessions were audio-recorded and reviewed for fidelity by independent raters to assess adherence to assigned condition. In a future trial, we may also consider an additional exclusion criterion for patients regarding whether they are being considered for enrollment in hospice as we had several patient deaths with the study timeframe and several participants discussed wanting the intervention earlier.
Consistent with recent guidance for pilot behavioral intervention trials, which recruit small samples by design,45 a key limitation of this study is the lack of inferential conclusions that can be drawn from the preliminary efficacy data of the 3 CASCADE components. As expected from the small pilot sample and reflected in the width of the confidence intervals, the sample estimates had a large degree of uncertainty. While we reported our analytic approach and effect size estimates for each component/component level, we debated doing so as the confidence intervals are so wide as to essentially make these estimates wholly inconclusive. We did however want to provide readers with a sense of what factorial trial results look like and how they might be used to make decisions about assembling components of an optimized intervention. Readers are referred to more in depth resources on using factorial trial to make decisions about component selection in these types of trials.28 A fully powered factorial trial is the necessary next step to yield reliable estimates that allow us to draw conclusions about CASCADE component efficacy and ultimately component selection into the final “package” of the CASCADE intervention. Another limitation of this study was that we did not directly measure the decision support skills of caregivers, which would indicate a proximal mechanism of the interventions effect. While instruments do not exist that measure these skills, future work should consider more direct measurements of these skills acquisitions. Finally, the modeling approach allowed using all available data-points for each outcome, however, missing data were assumed to be missing at random. For future larger studies, a milder assumption would be that the data were missing conditionally at random (i.e., conditional on some covariates relevantly associated with the missingness). Analysis under this assumption can be conducted using maximum-likelihood modeling with covariate adjustment or multiple imputation for missing data.46,47 Data determined missing not at random would require that the missing data mechanism be modeled as part of the estimation process. For instance, because patient deaths can bias a longitudinal analysis by causing the estimated outcome means to show artificial improvements (as the distal time-point mean outcomes are estimated from healthier, surviving patients), an approach used in palliative care studies is joint-modeling for longitudinal and time-to-event data to obtain inferences on the longitudinal outcome corrected for non-ignorable missing data due to death.48
In summary, we successfully pilot tested a factorial trial approach to examining individual intervention components of CASCADE, a novel early telehealth, palliative care coach-led decision support training intervention for family caregivers of patients with newly diagnosed advanced cancer. Factorial trials using the MOST framework allow for the testing of individual interventions components in order to assemble an optimized intervention package. The MOST approach may provide palliative care intervention developers and clinical trialists with an efficient way to test “active ingredients” of serious illness interventions. For our own work, our next step is to conduct a fully powered factorial trial.
Key Message.
We pilot tested components of CASCADE, an early palliative care decision support training intervention for family caregivers of patients with advanced cancer. CASCADE components were acceptable and the trial design feasible, providing promising future directions for palliative care intervention development and testing. Pilot results will inform a fully-powered trial.
Disclosures and Acknowledgments
There are no relevant conflicts of interest to disclose. During this study, Dr. Dionne-Odom received grant support from the National Institute of Nursing Research (R00NR015903), the National Cancer Institute (R37CA252868), the National Palliative Care Research Center (no grant #), the Gordon and Betty Moore Foundation (no grant #), and the Cambia Health Foundation (no grant #). Dr. Wells was supported from the Agency for Healthcare Research and Quality (T32 HS013852) and the National Institute of Nursing Research (K99NR019854). Dr. Williams was supported, in part, by the National Cancer Institute (K08CA234225). Dr. Rosenberg was supported, in part, by the National Institutes of Health (R01CA222486, R01CA225629). The opinions presented here are those of the authors and do not necessarily represent those of the funders. The authors thank all of partnering oncologists, clinicians, and staff at the O’Neal Comprehensive Cancer Center at the University of Alabama at Birmingham for supporting this study. We also thank Julie Schach, James Mapson, Gjuana Cleveland, Tawny S. Martin, Diane Williams, Gail Averett, and Beth Ruf for assisting with recruitment and data collection and Charis Smith and Kayleigh Curry for study coordination. Most of all, we thank all family caregivers and patients for contributing their time to this study.
Funding
This work was supported by a pilot/exploratory award from the National Palliative Care Research Center (no grant number). The funder had no role in the study’s design; data collection, analysis, and interpretation; writing of the report; and decision to submit the study for publication.
References
- 1.Bakitas M, Kryworuchko J, Matlock D, Volandes A. Palliative medicine and decision science: the critical need for a shared agenda to foster informed patient choice in serious illness. J Palliat Med 2011;14:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrillo LA, McMahan RD, Tang V, Dohan D, Sudore RL. Older adult and surrogate perspectives on serious, difficult, and important medical decisions. J Am Geriatr Soc 2018;66:1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Delivering high quality cancer care: Charting a new course for a system in crisis. Washington DC. [PubMed] [Google Scholar]
- 4.Institute of Medicine. Living well with chronic illness: A call for public health action. Washington DC. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine. Dying in America: Improving quality and honoring individual preferences near the end of life. Washington DC. [Google Scholar]
- 6.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure. Circulation 2012;125:1928–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krieger JL, Krok-Schoen JL, Dailey PM, et al. Distributed cognition in cancer treatment decision making: an application of the DECIDE decision-making styles typology. Qual Health Res 2017;27:1146–1159. [DOI] [PubMed] [Google Scholar]
- 8.Lee JE, Shin DW, Cho J, et al. Caregiver burden, patients’ self-perceived burden, and preference for palliative care among cancer patients and caregivers. Psychooncology 2015;24:1545–1551. [DOI] [PubMed] [Google Scholar]
- 9.Rini C, Jandorf L, Goldsmith RE, et al. Interpersonal influences on patients’ surgical decision making: the role of close others. J Behav Med 2011;34:396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laidsaar-Powell RC, Butow PN, Bu S, et al. Physician-patient-companion communication and decision-making: a systematic review of triadic medical consultations. Patient Educ Couns 2013;91:3–13. [DOI] [PubMed] [Google Scholar]
- 11.Lamore K, Montalescot L, Untas A. Treatment decision-making in chronic diseases: what are the family members’ roles, needs and attitudes? a systematic review. Patient Educ Couns 2017;100:2172–2181. [DOI] [PubMed] [Google Scholar]
- 12.Hobbs GS, Landrum MB, Arora NK, et al. The role of families in decisions regarding cancer treatments. Cancer 2015;121:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dionne-Odom J, Ejem D, Wells R, et al. How family caregivers of persons with advanced cancer assist with upstream healthcare decision-making: qualitative study. PLoS One. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dionne-Odom JN, Taylor R, Rocque G, et al. Adapting an early palliative care intervention to family caregivers of persons with advanced cancer in the rural deep south: a qualitative formative evaluation. J Pain Symptom Manag 2018;55:1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clayman ML, Roter D, Wissow LS, Bandeen-Roche K. Autonomy-related behaviors of patient companions and their effect on decision-making activity in geriatric primary care visits. Soc Sci Med 2005;60:1583–1591. [DOI] [PubMed] [Google Scholar]
- 16.Wen FH, Chou WC, Chen JS, Chang WC, Hsieh CH, Tang ST. Evolution and predictors of patient-caregiver concordance on states of life-sustaining treatment preferences over terminally Ill cancer patients’ last six months of life. J Palliat Med 2018;22:25–33. [DOI] [PubMed] [Google Scholar]
- 17.Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med 2011;154:336–346. [DOI] [PubMed] [Google Scholar]
- 18.Laryionava K, Pfeil TA, Dietrich M, et al. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care 2018;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butow PN, Price MA, Bell ML, et al. Caring for women with ovarian cancer in the last year of life: a longitudinal study of caregiver quality of life, distress and unmet needs. Gynecol Oncol 2014;132:690–697. [DOI] [PubMed] [Google Scholar]
- 20.Garvelink MM, Ngangue PA, Adekpedjou R, et al. A synthesis of knowledge about caregiver decision making finds gaps in support for those who care for aging loved ones. Health Aff (Millwood) 2016;35:619–626. [DOI] [PubMed] [Google Scholar]
- 21.Hauke D, Reiter-Theil S, Hoster E, Hiddemann W, Winkler EC. The role of relatives in decisions concerning life-prolonging treatment in patients with end-stage malignant disorders: informants, advocates or surrogate decision-makers? Ann Oncol 2011;22:2667–2674. [DOI] [PubMed] [Google Scholar]
- 22.Ferrell B, Wittenberg E. A review of family caregiving intervention trials in oncology. CA Cancer J Clin 2017;67:318–325. [DOI] [PubMed] [Google Scholar]
- 23.Dionne-Odom JN, Azuero A, Lyons KD, et al. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dionne-Odom JN, Lyons KD, Akyar I, Bakitas MA. Coaching family caregivers to become better problem solvers when caring for persons with advanced cancer. J Soc Work End Life Palliat Care 2016;12:63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dionne-Odom JN, Azuero A, Lyons KD, et al. Family caregiver depressive symptom and grief outcomes from the ENABLE III randomized controlled trial. J Pain Symptom Manag 2016;52:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins L Optimization of behavioral, biobehavioral, and biomedical interventions: The multiphase optimization strategy. New York, NY: Springer International Publishing; 2018. [Google Scholar]
- 27.Wells RD, Guastaferro K, Azuero A, et al. Applying the multiphase optimization strategy for the development of optimized interventions in palliative care. J Pain Symptom Manag 2020;62:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins LM, Trail JB, Kugler KC, et al. Evaluating individual intervention components: making decisions based on the results of a factorial screening experiment. Transl Behav Med 2014;4:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juszczak E, Altman DG, Hopewell S, Schulz K. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement. JAMA 2019;321:1610–1620. [DOI] [PubMed] [Google Scholar]
- 30.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery P, Grant S, Mayo-Wilson E, et al. Reporting randomised trials of social and psychological interventions: the CONSORT-SPI 2018 Extension. Trials 2018;19:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rini C, Schetter C. The effectiveness of social support attempts in intimate relationships. In: Sullivan K, Davila J, eds. Support processes in intimate relationships, New York: Oxford University Press; 2010. [Google Scholar]
- 33.Stacey D, Kryworuchko J, Belkora J, et al. Coaching and guidance with patient decision aids: a review of theoretical and empirical evidence. BMC Med Inform Decis Mak 2013;13(Suppl 2):S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Légaré F, O’Connor AM, Graham ID, Wells GA, Tremblay S. Impact of the ottawa decision support framework on the agreement and the difference between patients’ and physicians’ decisional conflict. Med Decis Making 2006;26:373–390. [DOI] [PubMed] [Google Scholar]
- 35.Feenstra B, Lawson ML, Harrison D, Boland L, Stacey D. Decision coaching using the Ottawa family decision guide with parents and their children: a field testing study. BMC Med Inform Decis Mak 2015;15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychol 2004;23:443–451. [DOI] [PubMed] [Google Scholar]
- 37.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale: an updated literature review. J Psychosom Res 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 38.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. [Google Scholar]
- 39.Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci 2013;15:398–405. [DOI] [PubMed] [Google Scholar]
- 40.Dionne-Odom JN, Ejem D, Wells R, et al. How family caregivers of persons with advanced cancer assist with upstream healthcare decision-making: a qualitative study. PLoS One 2019;14:e0212967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen J Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 42.Song L, Qan’ir Y, Guan T, et al. The challenges of enrollment and retention: a systematic review of psychosocial behavioral interventions for patients with cancer and their family caregivers [Online ahead of print]. J Pain Symptom Manage 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ingoldsby EM. Review of interventions to improve family engagement and retention in parent and child mental health programs. J Child Fam Stud 2010;19:629–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piper ME, Schlam TR, Fraser D, Oguss M, Cook JW. Implementing factorial experiments in real-world settings: lessons learned while engineering an optimized smoking cessation treatment. In: Kugler LMC KC, ed. Optimization of behavioral, biobehavioral, and biomedical interventions: advanced topics, New York, NY: Springer; 2018:23–46. [Google Scholar]
- 45.Freedland KE. Pilot trials in health-related behavioral intervention research: problems, solutions, and recommendations. Health Psychol 2020;39:851–862. [DOI] [PubMed] [Google Scholar]
- 46.Groenwold RH, Donders AR, Roes KC, Harrell FE, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol 2012;175:210–217. [DOI] [PubMed] [Google Scholar]
- 47.Allison P Missing data. The sage handbook of quantitative methods in psychology. New York: Sage Publications Ltd; 2009. [Google Scholar]
- 48.Rizopoulos D Joint models for longitudinal and time-to-event data: with applications in R. Boca Raton: CRC Press; 2012. [Google Scholar]


