Abstract
Background
Several studies have suggested that COVID-19 is a systemic disease that can affect several organs, including the brain. In the brain, specifically, viral infection can cause dyshomeostasis of some trace elements that promote complex biochemical reactions in specialized neurological functions.
Objective
Understand the neurovirulence of SARS-CoV-2 and the relationship between trace elements and neurological disorders after infection, and provide new insights on the drug development for the treatment of SARS-CoV-2 infections.
Methods
The main databases were used to search studies published up September 2021, focusing on the role of trace elements during viral infection and on the correct functioning of the brain.
Results
The imbalance of important trace elements can accelerate SARS-CoV-2 neurovirulence and increase the neurotoxicity since many neurological processes can be associated with the homeostasis of metal and metalloproteins. Some studies involving animals and humans have suggested the synapse as a vulnerable region of the brain to neurological disorders after viral infection. Considering the combined evidence, some mechanisms have been suggested to understand the relationship between neurological disorders and imbalance of trace elements in the brain after viral infection.
Conclusion
Trace elements play important roles in viral infections, such as helping to activate immune cells, produce antibodies, and inhibit virus replication. However, the relationship between trace elements and virus infections is complex since the specific functions of several elements remain largely undefined. Therefore, there is still a lot to be explored to understand the biochemical mechanisms involved between trace elements and viral infections, especially in the brain.
Keywords: Viral infection, Trace elements, Neurological disorders, Immunity system, Antiviral
Graphical Abstract
Nomenclature
- ACE2
angiotensin-converting enzyme 2
- APP
amyloid precursor protein
- ARDS
acute respiratory distress syndrome
- As
arsenic
- ASD
autism spectrum disorder
- AβP
beta-amyloid peptide
- BBB
blood-brain barrier
- BBE
brainstem encephalitis
- CAR
carnosine
- Cd
cadmium
- CNS
central nervous system
- COVID-19
coronavirus disease
- COX-2
prostaglandin endoperoxide synthase 2
- Cu
copper
- FDA
Food and Drug Administration
- Fe
iron
- GABA
g-aminobutyric acid
- GBS
Guillain Barré syndrome
- GPx
glutathione peroxidase
- GSH
glutathione
- GSSH
glutathione dissulfite
- Hg
human T cell leukemia virus type 1
- HTLV-1
human T cell leukemia virus type 1
- Ig
immunoglobulin
- IL-1β
interleukin 1 beta
- LA-ICP-MS
laser ablation-inductively coupled plasma-mass spectrometry
- Li
Lithium
- MAGT1
magnesium transporter 1
- MD
Menkes disease
- MERS-CoV
middle east respiratory syndrome-associated coronavirus
- Mg
magnesium
- MHRA
Medicines and Healthcare products Regulatory Agency
- Mn
manganese
- Mpro
main protease of SARS-CoV-2
- MT
metallothioneins
- ND
neurodegenerative diseases
- NF-kB
nuclear factor kappa-B
- NF-kB
nuclear factor kappa-B
- NF-α
tumor necrosis factor – alpha
- Ni
nickel
- NKCs
natural killer cells
- NMDA
N-methyl-D-aspartate
- Pb
lead
- PrP
prion protein
- PrPC
cellular prion protein
- RdRp
RNA-dependent RNA polymerase
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SARS-CoV-2
severe acute respiratory syndrome-associated coronavirus-2
- SCC
sodium copper chlorophyllin
- Se
selenium
- SOD
superoxide dismutase
- SP-ICP-MS
single particle-ICP-MS
- SV-ICP-MS
single virus ICP-MS
- TIBC
total iron-binding capacity
- TrxRs
thioredoxin reductase
- WD
Wilson’s disease
- ZFP
zinc-finger proteins
- Zn
zinc
- ZnT
Zn transporter
1. Introduction
Coronavirus disease (COVID-19) is currently one of the main causes of death worldwide, resulting millions of deaths since the end of 2019 [1], [2], [3]. COVID-19 is caused by the SARS-CoV-2 (severe acute respiratory syndrome-coronavirus-2) virus that originated in Wuhan, China, and spread rapidly around the world [4]. Studies in animal and human have reported that the viral responses of innate and adaptive immune machinery depend on the host’s metabolism, which includes age, sex, smoking habits, co-existing medical conditions, and especially nutritional status. In this sense, the balance between immune activation and micronutrients is crucial to combat viral infection. Therefore, several trace elements have been identified as essential to immunomodulatory effects, since many components of innate immunity can be influenced by elements such as zinc (Zn), selenium (Se), iron (Fe), copper (Cu), manganese (Mn), among others [2], [5]. Trace elements can act as cofactors for many enzymes, such as, superoxide dismutase (SOD), RNA polymerase, and glutathione peroxidase (GPx), and can mediate vital biochemical functions, modifying oxidant tissue injury mediated by oxidants and eliminating reactive oxygen species (ROS) in response to infection [2], [6], [7]. Furthermore, the deregulation of element homeostasis during infection can play an essential role in virus survival since metals are cofactors of important metalloproteins responsible for virus attachment to the host. For example, Zn, Fe, and Cu are some of the most common metals that bind to proteins associated with viral infections, participating in genome maturation (RNA or DNA), activation, and catalytic mechanisms, as well as, initial integration processes, and the protection of newly synthesized DNA [2].
Initially, it was believed that COVID-19 infection only affected the respiratory tract, however the appearance of neurological, hematological, and gastrointestinal, symptoms attested its more systemic character [4], [8], [9], [10], [11]. For example, it was reported that nearly 40% of critically ill COVID-19 patients presented strokes, cognitive dysfunction, depression, psychosis, and delirium, suggesting that this virus may predispose to several neurological disorders [4], [12]. In the case of neurological dysfunction, some syndromes can vary depending on which part of the brain is infected. For example, exacerbation of pre-existing cognitive, motor and non-motor symptoms has been frequently observed, indicating viral neurotropism [12]. In addition, migration defects in the ventral cerebellum, olfactory bulbs hypoplasia and delayed-onset neuronal dropout in the hippocampus were reported as neurological dysfunction after viral infection [13]. In the brain, specifically, viral infection can cause dyshomeostasis of some trace elements that promote complex biochemical reactions in specialized neurological functions, such as: (i) neurotransmitter synthesis; (ii) neural information processing; (iii) redox processes; (iv) oxygen storage; (v) myelination; and (vi) electron transport [2], [5], [7], [14], [15], [16], [17], [18], [19], [20]. Insufficient immunity, high viral load, increased age, history of neurotrophic viral infection, glucocorticosteroids administration, and increased hospitalization have been reported as factors in the spread of SARS-CoV-2 to the central nervous system. Neural proliferation can occur in the cells of amygdala, basal ganglia, thalamus, hypothalamus, cortex and in the brain stem. Clinically, the neuropsychiatric manifestations of COVID-19 can be either acute or chronic. In addition to anxiety, depression and delirium, other neuropsychiatric manifestations with current evidence have been reported, such as acute psychosis and manic disorders, confusional states, acute cerebrovascular events, encephalitis, and encephalopathies [4], [12].
In order to understand the neurovirulence of SARS-CoV-2 and the relationship between trace elements and neurological disorders after infection, this review summarizes studies on the impact of excessive and deficient conditions of the trace elements Fe, Cu, Mn, Zn, and Se, and the respective consequences on neuronal genomic stability and its maintenance, aiding in the development of drugs for the treatment of SARS-CoV-2 infections.
2. Complex interaction between trace elements and viral infection
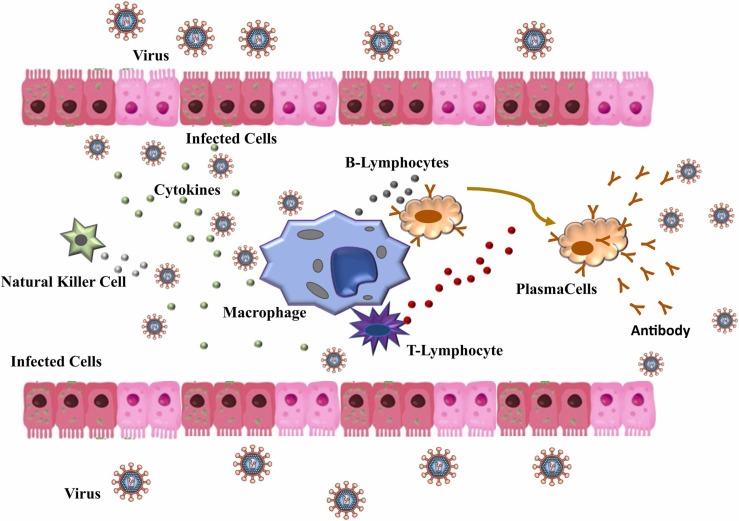
It is well known that several changes occur in the biological system during an infection caused by a virus, such as increased vascular permeability and vasodilation [2]. This alteration allows the arrival of immune system cells to the infected tissues [21], [22]. The immune system is primarily responsible for maintaining the physiological integrity of the body, eliminating foreign material, such as virus [21]. Several defense cells modulate the immune system ( Fig. 1). Due to their phagocytic, cytotoxic, and secretory activities, neutrophils and macrophages represent the first-line defenses (innate response) against the virus, being they the main physical and biochemical barriers of the body [11], [23], [24]. When any virus enters in the body cell via ACE2 (angiotensin-converting enzyme 2), it is phagocyted and digested by macrophages [3], [22]. The effector cells of the immune system, as for example B-lymphocytes, produce specific antibodies in response to the antigen [3], [25]. Immunoglobulin (IgA, IgM and IgG) are the main antibodies produced and comprise the second line of defense (adaptative response) of the immune system [26], [27]. In addition, other molecules, such as cytokines, play a key role in cell communication during the infection process, indicating infected cells [28], [29]. Although the effects of micronutrients on neutrophil functions are not clear, micronutrients such as Fe, Zn, Cu, Se, among others can influence various components of innate immunity [2], [5], [30], [31], [32]. For example, the contributions of trace elements to the immune system are observed both to regulate the number and function of innate immune cells (natural killer cells (NKCs), macrophages, monocytes, and neutrophils) and to assist the production of pro-inflammatory and anti-inflammatory cytokines in responses to inflammation (adaptive immunity) [10], [33], [34], [35], [36], [37]. In this sense, many studies have reported changes in metals levels in the host’s biological system during viral infections [2], [5], [6], [17], [30], [32], [38], [39], [40], [41]. The following sections explore the consequences of some micronutrient deficiencies and the potential effects of their supplementations against COVID-19 infection.
Fig. 1.
Immune system scheme during a viral infection involving cytokines, macrophages, natural killer (NK) cells, and B and T cells that constitute the first line of defense of the immune system (innate response). Antibodies are produced to fight the infection, therefore constituting the second line of defense of the immune system (adaptive response).
3. Trace elements homeostasis and viral infection
3.1. Zinc role in the immune system and viral infection
As previously mentioned, some trace elements are involved in immune responses acting as immunoregulators for distinct viral infections [42], [43]. Zn is an essential element that is associated to structural or regulatory functions of proteins, being Zn a cofactor of hundreds of enzymes, thus, modulating their functions which are related to several biological and physiological processes [44], [45], [46], [47]. Zn is the second most abundant transition metal in the whole human body, and this micronutrient is found as free Zn2+ and bound form in cytosolic organelles, cytoplasm, nucleus and biomembrane proteins [5], [48], [49], [50], [51]. Zn has direct and indirect antiviral properties and its functions during distinct viral infections have been extensively studied [52], [53], [54], [55]. As Zn is a cofactor in several metalloenzymes, the integrity of immune barriers is preserved by Zn, subsequently improving the cytotoxic activity of NKCs, thus, maintaining cellular functions, as for example, the differentiation of innate immune cells [48], [56], [57], [58]. In terms of SARS-CoV-2, both free Zn2+ and Zn-coordinating compounds can effectively act to inhibit virus activity [50], [59], [60], [61]. In fact, through a SARS-CoV-2 infection, the elongation phase of RNA synthesis, which changes the activity of RdRp (RNA-dependent RNA polymerase), is inhibited by Zn due to its effect on mold binding, thus, affecting SARS-CoV-2 replication [5], [48], [49], [62], [63]. In this sense, Zn2+ can inhibit both RdRp activity and adequate proteolytic processing of polyproteins replicase during coronaviruses infection [2], [5], [46]. In addition, zinc-finger proteins (ZFP) and other Zn-ligating complexes can bind to the catalytic dyad from 3 C-like protease, thus, limiting proteinase activity by Zn-binding to the catalytic residues of the SARS-CoV-2 proteins [59], [64]. In case of Zn deficiency, it can lead to a form of immunodeficiency that, together with the production of inflammatory cytokines and oxidative stress, can be observed during symptoms of severe COVID-19 [49], [61], [65]. Zn dysregulation has been widely associated to disturbances in immune system functions, thus increasing the risk and morbidity of distinct pathologies, including viral infections [5], [48], [49]. Zn deficiency affects the development of acquired immunity by preventing both the outgrowth and certain functions of T lymphocytes such as activation, Th1 cytokine production, and B lymphocyte help. Furthermore, B lymphocyte development and antibody production, particularly immunoglobulin G, are compromised. The distinction between immature T-cells in the thymus and the function of mature peripheral T-cells, also, can be affected by the Zn deficit, since this element indirectly reduces the levels of active serum thymulin, a Zn-dependent nonapeptide hormone. Furthermore, Zn deficiency has been related with reduced NKC activity and decreased cytokine production [43], [49].
In addition to some adverse effects on the body already mentioned, viral infection and imbalance of essential element can predispose to neurodegenerative disorders [4], [66]. For example, Zn deficiency as consequence of a viral process may contribute to susceptibility to Autism Spectrum Disorder (ASD), since both environmental factors and Zn deficit, are crucial for neurodevelopment and degenerative process of the brain [66]. In this context, Zn has distinct functions involved in the antioxidant process, modulating the inflammatory response, and increasing the antiviral mechanism. Although more confirmatory evidence is needed, it is believed that Zn can be considered for adjuvant protective therapy against viral infections [5], [48], [50], [59], [60], [61]. Therefore, Zn supplementation may be of great importance in the treatment of COVID-19, regulating basic cell functions, such as cell division and cell activation, thus, playing an important role in both innate and adaptative immunity against viral infections, including SARS-CoV-2 [5], [61].
3.2. Iron role in the immune system and viral infection
Iron is the most abundant transition metal found in the human body and is involved in several vital functions in humans and pathogens, thus, playing a fundamental role in the innate immunity and host immune defense system against distinct viral infections [15], [49]. In fact, Fe modulates key cellular processes including DNA synthesis/replication to generation of ATP (adenosine triphosphate) and cell proliferation, as well as this essential element participates of oxygen binding reactions and electron transport [67], [68]. In the same way as other trace elements, Fe is present in the body as ionic forms, for example, Fe2+ and Fe3+, as well as bound to several molecules, such as enzymes and other proteins, which participate in fundamental processes at cellular level in many living organisms [61], [67], [68], [69]. Regarding the ionic forms of Fe, they can lead to ROS (reactive oxygen species) production via Fenton reaction, as a result of the interchange through redox reaction Fe2+ → Fe3+ + e− [67], [68]. As previously commented, after a viral invasion, an imbalance in the content of the essential elements, including Fe, is expected. In this scenario, Fe content might influence viral replication and damage to host cells since a viral infection results in Fe competition between the virus and host cells [60], [61], [67]. In fact, Zn and Cu status influences the function of macrophages, which play a crucial role in Fe homeostasis and their level in macrophages is vital in the production of pro-inflammatory cytokines, since the entry of SARS-CoV-2 and infection in host cells of almost all tissue and organs that are modulated by ACE2 [60], [67], [70]. In this context of inflammation, Fe homeostasis and its level in the organism plays a crucial role in the immune response during viral infections including SARS-CoV-2, as already pointed out in the recent years by some studies in the literature [67], [69], [71], [72], [73], [74], [75], [76], [77]. In addition to ionic forms, Fe-containing species, such as ferritin, transferrin, hemoglobin, lactoferrin, among others, have been associated with adverse outcomes in patients with COVID-19 and other serious effects in the body, including acute organ injuries due to a hyperinflammation process due to SARS-CoV-2 infection [67], [69], [76], [78]. In fact, under normal condition, ferritin acts by storing Fe in a biologically available form that is fundamental for cellular processes already mentioned, including cell survival/ferroptosis, however high levels of ferritin can be related to worse prognosis and COVID-19 acute phase response [77], [79]. In this context, the ferritin content can be increased by SARS-CoV-2, thus, resulting in the formation of ROS and additional tissue injury [67], [74]. Dahan et al. reported elevated ferritin levels in patients with severity of COVID-19 as a result of the autoimmune and inflammation status. According to the authors, ferritin status is strictly affected by cytokine production as a consequence of the unexpected functioning of the host’s immune system [77]. In this scenario, in addition to an Fe overload state, an elevated serum ferritin status can be considered a marker of adverse conditions, including inflammation and autoimmune conditions, which have been termed as hyperferritinemic syndrome [77], [79]. In addition to the high concentration of ferritin, Lv et al. also highlighted other Fe species which can also be associated to adverse effects due to a hyperinflammation condition of SARS-CoV-2 infection [67]. According to the results, distinct Fe parameters, e.g., serum iron, ferritin, and total iron-binding capacity (TIBC), could be related to COVID-19 severity. Moreover, the elevated serum Fe (ferritin) levels combined with high concentrations of cytokine are also associated to disease severity, as well as to the possibility of developing acute respiratory distress syndrome (ARDS), coagulopathy, and acute injuries in different organs, such as kidney, liver, and hearth in patients with COVID-19. Then, the authors suggested that Fe metabolism status may be indicative of risk factors for COVID-19 prognosis [67].
Furthermore, since a viral infection such as SARS-CoV-2 can result in excessive host immune-mediated release of pro-inflammatory cytokines, it can be expected that COVID-19 can lead to neurological manifestations [80], [81], [82], [83], [84]. In fact, it is hypothesized that neurological complications can be associated with COVID-19 through Fe status, since viral proteins can dissociate hemoglobin, thus, releasing Fe and heme to generate subsequent “ROS escape” or “ROS attack” that can impair multiple organs, including neural ones [81].
3.3. Copper role in the immune system and viral infection
Another essential element for adequate immunoregulatory activity is Cu, which is considered the third most abundant transition metal in the body [15], [85]. In human cells, Cu is stored mainly in mitochondria and its intracellular transport is mediated by Cu-transporter [86]. In circulatory blood, Cu is transported linked to proteins such as ceruloplasmin, albumin and alpha-2 macroglobulins, thus, ensuring its distribution and homeostasis on the several tissues from human body [86]. Copper is a cofactor for more than 30 enzymes involved in redox reactions including SOD which acts in defense against oxidative stress promoting the degradation of ROS, such as superoxide [86]. In addition to maintaining an intracellular antioxidant balance, Cu is related to the human immune system, acting on the function of neutrophils, monocytes, macrophages as well as stimulating T cells hematopoiesis and improving the activity of NKCs [87], [88]. For example, macrophages accumulate a high level of Cu in phagolysomes and exploit its redox activity to produce ROS which combined with antimicrobial proteins and acidity, generate a hostile and lethal environment for phatogens into the immune cells [87]. In this sense, Cu has a promising role to prevent and treat the viral activity of SARS-CoV-2 which is an enveloped virus, non-segmented, spherical, with a single RNA strand and a spike glycoprotein covered by a phospholipid bilayer [89]. This morphological structure of the novel COVID-19 has mediated its trapping and inactivation through copper-based materials such as laponite-Cu2+ nanocoating on filter membranes, CuS impregnated or copper film coating on fiber masks, nanohybrid formulation and surfaces of Cu-Ag [90], [91], [92], [93]. These materials trap the novel coronavirus through bound to the spike protein and inactivate it, promoting irreversible damage to the virus genome and morphology including disruption of envelope and dispersal of spike surface [16]. In a study in vitro, Rodriguez et al. demonstrated that Cu gluconate mitigated SARS-CoV-2 infection in Vero E6 cells, however, this benefit effect was quenched by albumin, indicating its low efficacy by parenteral administration [9]. Clark & Taylor-Robinson suggested sodium copper chlorophyllin (SCC) as an agent antiviral and potential immunomodulator in COVID-19 infection [94]. SCC may contribute to the innate immune response by two mechanisms: first, inhibiting ribonucleic acid synthesis of SARS-CoV-2 in human lung epithelial tissues; second, ensuring leukocyte homeostasis, restoring and maintaining adequate levels of CD4+ and CD8+ T lymphocytes in peripheral blood and blocking the expression of the pro-inflammatory cytokine IL-6. In this regard, SCC could reverse the lymphopenia observed during COVID-19 infection [94]. In addition, the copper-mediated immunity allied to the innate immune response, is decisive to mitigate the effects of novel coronavirus infection which has shown potential for triggering other diseases such circulatory, hepatic (e.g. Wilson’s and Menkes diseases) and neurological (e.g. Multiple Sclerosis, Alzheimer’s and Parkinson’s Disease). However, so far, data or knowledge is limited on the effects of therapeutic Cu supplementation about the susceptibility, severity, survival, and fatal outcome of COVID-19 [95], [96], although, Cu supplementation has been attributed to the high rate of recovered COVID-19 patients (ca.88%) from Yadgir district in India [95], [96].
3.4. Selenium role in the immune system and viral infection
Selenium also plays a fundamental role in human health regarding by acting in a wide range of protective functions, including antioxidant, anti-inflammatory and immunomodulatory [97], [98]. Se is biologically active as selenoproteins whose coding involves 25 genes from human genome [98], [99]. For example, GPxs and thioredoxin reductase (TrxRs) have an important antioxidant role acting in the inactivation of ROS and RNS (reactive nitrogen species). GPx catalyzes the glutathione (GSH) – glutathione dissulfite (GSSH) redox system, promoting the ROS reduction and subsequently regulating the nuclear factor kappa-B (NF-kB) which controls the production of various pro-inflammatory cytokines and chemokines [100]. Se may also contribute to innate and adaptive immunity including antibody production, NKC activity, T-lymphocyte and B-lymphocyte [100], [101]. In this sense, Se has a recognized role in the prophylaxis and therapy of some viral diseases caused by human immunodeficiency virus (HIV), human T cell leukemia virus type 1 (HTLV-1), Ebola virus, coxsackievirus, influenza and hantavirus, as well as diabetic retinopathy, asthma, and tuberculosis [102], [103]. Considering SARS-CoV-2, studies have been reported demonstrating a positive relationship between the high levels of Se in hair and serum from surviving COVID-19 patients in Germany and in different provinces of China [97], [104]. Recently, Hackler et al. also found the highest serum Se levels in another group of patients surviving COVID-19 in Germany [105]. These findings on the effects of Se can be attribute to the antioxidant defense of GPx and TrxR maintaining ROS homeostasis and preventing a cytokine storm due to NF-kB by the new coronavirus [100], [106]. Furthermore, GPx and the synthetic counterpart called ebselen have been shown to be potent inhibitor of the main protease of SARS-CoV-2 (Mpro) that plays a key role in viral replication in the host [107], [108]. Moreover, ACE2 can also be inhibited by Se in the form of selenoneine [109]. Sodium selenite has shown a protective function by inactivating the new coronavirus through the oxidation of thiol groups of the protein dissulfite isomerase and avoiding its penetration into healthy host cells [110]. Although the essential level of intake is very low, Se deficiency is prevalent in a large part of the population, especially in some European countries, sub-Saharan Africa, and several provinces in China [97], [110]. Most studies have demonstrated that Se deficit leads to increase host-susceptibility to viral infections, as well as serious diseases and fatal outcomes, since a Se deficiency promotes the weakening of the antioxidant defense and immune response, favoring mutation, replication and increased in viral virulence [106], [109]. Furthermore, SARS-CoV-2 can significantly suppress the expression of ferroptosis-associated GPx4, DNA synthesis-related TrxR3 and endoplasmic reticulum-resident selenoproteins as SelF, SelK, SelM and SelS [111]. In South Korea, Se deficit has been associated with ca. 42% of critically ill patients with COVID-19 [112]. On the other hand, Se supplementation has well-known beneficial effects on viral infection in different types of hosts [101], [103]. For example, supplying Se can restore GPx and TrxR levels alleviating oxidative stress and the inflammatory response in respiratory system tissues, as well as enhancing innate immunity, increasing CD4+ T lymphocytes proliferation and activity of natural killer cells [97], [103], [106]. Indeed, a high daily intake and maintenance of the optimal Se level can contribute to protection and resistance against COVID-19 infection [112], [113], [114]. It is important to highlight that interference of SARS-CoV-2 in the Se metabolism can induce diseases such as coagulopathy and/or increase the severity of others such as neurological diseases [106], [115]. In addition to the disturbance of Se homeostasis related to GPx and selenoproteins, SARS-CoV-2 has been shown to have the potential to disrupt Rho GTpases associated with synaptic irregularity exhibited in patients with Alzheimer’s disease [96].
3.5. Role of other elements in immune system and viral infection
In addition to the essential elements already described, other elements can present distinct functions in immune system against viral infection. Although, Mn is also a cofactor of SOD like Zn and Cu, it has shown a less prominent contribution to the immune system [2], [86]. In fact, recent findings on the human proteome provide evidence of a low interaction (ca. 0.3%) between Mn metalloproteins and proteins of SAR-CoV-2 [48], [96], [101]. In contrast, nickel (Ni) has demonstrated a significant effect in modulating the immune response which it can improve the T and B lymphocyte cells proliferation and increase the natural killer cells activity [2], [5]. Furthermore, like Cu, the new coronavirus demonstrated high sensitivity on Ni-composite surfaces which has been attributed to ROS production induced by the high redox activity of metals [5]. Lithium (Li) has been shown to have high antiviral potential against several viruses, including members of the coronavirus family as coronavirus bronchitis virus [116]. The dose-dependent effect of lithium prevented the entry of coronaviruses in Vero cells and inhibited their replication blocking transcription and translation of the viral protein [5], [116]. Lithium can improve severe inflammation by inhibiting pro-inflammatory factors of the immune system for example COX-2 (prostaglandin endoperoxide synthase 2), IL-1β (interleukin 1 beta) and TNF-α (tumor necrosis factor – alpha) [116], [117]. Lithium prophylaxis and therapy against COVID-19 infection requires more data, however, Li-based drugs have been used for decades to treat serious mental illnesses as schizophrenia [15]. Magnesium (Mg) plays a crucial role for cell-mediated and humoral adaptive immunity [2], [48]. Deficiency of Mg is linked to a defect in the magnesium transporter 1 (MAGT1) and promotes a drop in the serum levels IgG and IgM, NKCs and T CD8+ cells [2], [118], [119]. Prolonged severe acute inflammation in lung tissues has been related to Mg deficit due to susceptibility to production of cytokines, free radicals (ROS and RNS) and acute-phase proteins [48]. The efficacy of Mg supplementation to alleviate the severity of complications induced by several cardiovascular, neurological and respiratory disease has been reported and therefore can be considered for COVID-19 [120], [121]. However, for this, it is important to consider the predicted high probability of interaction between Mg-binding proteins with SAR-CoV-2 ones, resulting in possible imbalances in the homeostasis of these metalloproteins in human cells [96]. Regarding non-essential elements like arsenic (As), cadmium (Cd), mercury (Hg) and lead (Pb) have been associated as damage promoters to human innate and adaptive immunity that depend on the level of exposure [122]. Therefore, exposure to toxic element can be considered as a risk factor for increasing susceptibility and severity of viral diseases focused on the respiratory system including COVID-19 [2], [122].
4. Trace elements distribution in the brain and its imbalance after coronavirus infection: an implication for neurological system
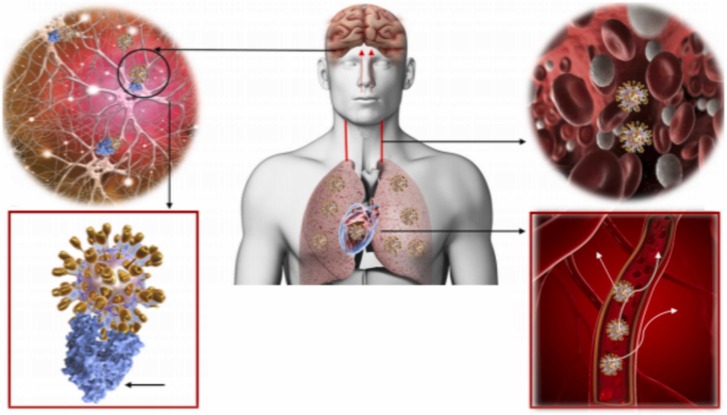
The brain is composed by two important components: (i) neurons and (ii) glial cells [15]. Neurons are specialized cells that can receive and transmit chemical or electrical signals, while glia are cells that provide support for neurons, playing a role in processing information complementary to neurons [15], [123], [124]. Neurons are characterized by not regenerating and are maintained during an individual’s entire lifetime [15]. Chronic dysfunction of neuronal cells (a characteristic of neurological disorders) can be caused by irreversible damage and cell death [14]. A wide range of viruses can cause immediate or delayed neurological manifestations in humans and animals [13], [14], [18], [125]. For example, studies involving patients infected with two different coronaviruses that caused epidemic infections in the past, SARS-CoV, and Middle East respiratory syndrome-associated coronavirus (MERS-CoV) have developed neurological symptoms, from two to three weeks after the appearance of typical symptoms, such as neuropathy, myopathy, bickerstaff, brainstem encephalitis (BBE), and Guillain Barré syndrome (GBS) [125], [126]. The SARS-CoV-2 infection in the brain begins with the spike protein S1 binding to the host receptor ACE2 ( Fig. 2). The human brain expresses ACE2 at a high level, which can allow the virus to invade the central nervous system (CNS) [127]. Moriguchi et al. reported the first confirmed cases of encephalitis and meningitis caused by SARS-CoV-2 [128]. It has been suggested that these signs of neurological damage may be caused by severe hypoxemia and hypoxia, an inflammatory process triggered by SARS-CoV-2 infection, or by virus infiltration and spread in the brain [129]. In addition, although neurodegenerative diseases have not been described in association with COVID-19, there is strong evidence of protein unfolding after viral infection, suggesting accumulation of amyloidogenic prion protein (PrP) in the brain which is a hypothesis of neurodegenerative disorder.
Fig. 2.
The SARS-COV-2 spreads throughout the body via the bloodstream. Brain infection can occur via circulation and/or an upper nasal transcranial route that enables the COVID-19 to reach the brain. COVID-19 docks on the ACE2 via spike protein. Lungs, heart, intestines, and brain express ACE2 receptors and are possible targets for COVID-19.
Image adapted of Baig et al. [122].
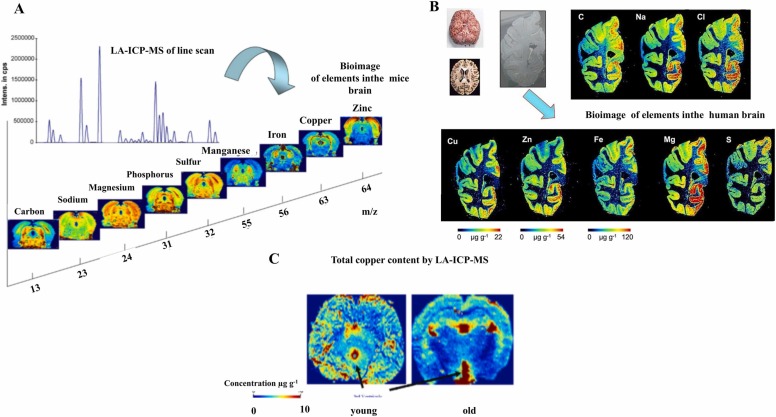
The imbalance of metals, such as Cu, Zn, Mn among others can accelerate SARS-CoV-2 neurovirulence and increase the neurotoxicity. Laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) images of trace elements in mice brain show the distribution of trace elements in different sections of brain, evidencing the important role of trace elements in the neural cell homeostasis ( Fig. 3)[125], [126], [127]. Fig. 3-A and Fig. 3-B show elemental distribution in mice and human brains, respectively, highlighting the different distribution of analytes (metals and non-metals) in substructures of interest, as for example, the hippocampus, corpus callosum, and the substantia nigra. Fig. 3-C illustrates Cu distribution in young (2-month-old) and old (14-month-old) mice, showing significant differences in elements distribution with age, suggesting a high risk of neuronal dysfunction after COVID-19 infection in the elderly due to micronutrients deficit.
Fig. 3.
Bioimaging of brain by LA-ICP-MS at µm scale. A) Mass spectrum of a line scan (top left) and images of selected metals and non-metals measured by LA-ICP-MS in routine mode. B) Metal distributions in the human brain measured by LA-ICP-MS. C) Comparison of copper images of mice brain sections from 2- to 14-month-old mice measured by LA-ICP-MS.
(a) Image adapted of Becker et al. [125] under permission of Elsevier. (b) Image adapted of Becker [127] under permission of Wiley. (c) Images adapted of Becker et al. [125] under permission of Elsevier.
In fact, after Fe, Zn is the second most abundant transition metal in the brain, followed by Cu [128]. Zn is found at the highest levels in the amygdala, hippocampus, cerebral cortex, and thalamus [12], [120], [129]. General brain activity is modulated by secreted Zn2+, which binds to N-methyl-D-aspartate (NMDA) type glutamate receptors, g-aminobutyric acid (GABA) receptors, and glycine receptors [129], [130], [131]. In addition, secreted Zn is essential for information processing, synaptic plasticity, learning, and memory [15], [132]. Zn imbalance in the brain results delayed mental, immune dysfunction, and learning disabilities in children, whereas in adults, Zn deficiency produces learning, taste, and odor disorders [15]. Cu dysregulation caused by mutations of Cu transporters has been linked to neurodegenerative diseases (ND), including Wilson’s disease (WD) and Menkes disease (MD) [133], [134]. Recent studies suggest that intracellular Cu2+ accumulates in the synaptic vesicles and is then released into synaptic clefts during neuronal excitation, like Zn [131], [132], [134], [135], [136]. Released Cu2+ has modulating effects on neuronal information processes, as it supposedly binds to NMDA-type glutamate and other receptors and modulates neuronal excitability [137], [138]. Fe can exist in two different forms: ferrous iron (Fe2+) and ferric iron (Fe3+) [15]. When Fe2+ ions enter the circulation system, they are oxidized to Fe3+ by ferroxidases, such as hephaestin or ceruloplasmin [139], [140]. The Fe3+ ion binds to transferrin (an iron-binding protein that binds two Fe3+ ions), crosses the blood-brain barrier (BBB) via transferrin receptors, and enters neurons or glial cells [140], [141]. Then, Fe3+ is reduced to bioactive Fe2+ by ferrireductase, and transferred to neuronal enzymes, which require Fe2+ as a cofactor [15], [140], [142]. Therefore, Fe levels as well as the ratio between Fe2+ and Fe3+ are strictly regulated in normal brains [140], [143].
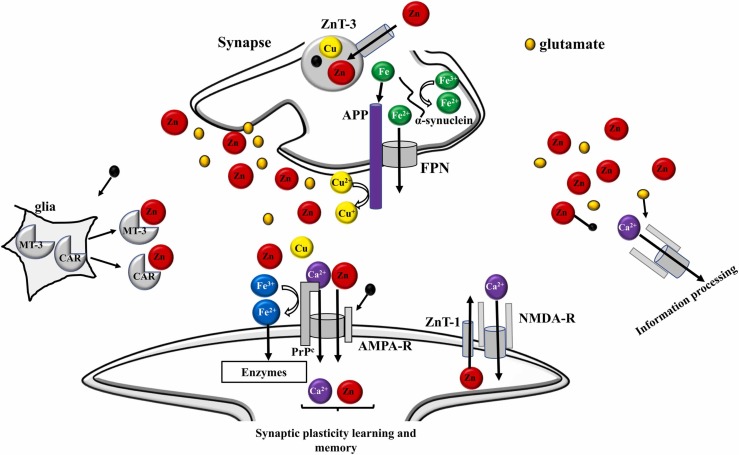
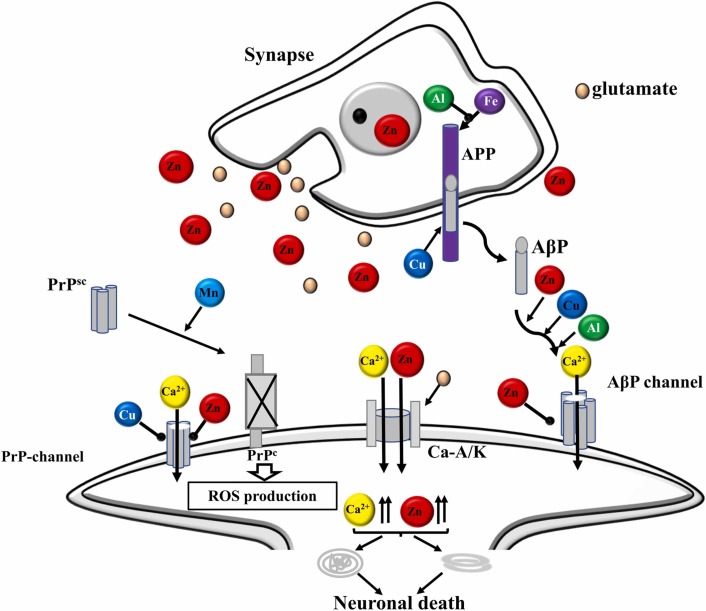
Some studies involving animals have suggested the synapse as a vulnerable region of the brain to neurological disorders after viral infection [132], [144], [145]. Synapse is an important region of the brain where memory formation takes place [144]. Memory formation can be derived from the interaction between metals and neurotransmitters that are co-released from the synapse, at presynaptic terminals [145]. In this region, metals bind to receptors in postsynaptic domains (PSDs) [132]. In this sense, it is plausible that neurotransmitters and metals may be concentrated in synaptic clefts, and their levels may be much higher than the level of extracellular fluid [15], [132], [144], [145]. Many neurological processes must certainly be associated with homeostasis of metal and metalloproteins [119], [133]. Considering this combined evidence, a possible mechanism can be suggested involving the metal’s synaptic effects under normal and abnormal conditions, such as neurological disorders ( Fig. 4) [15], [132], [138], [145]. Under typical physiological conditions, Zn can be released with glutamate and can bind to NMDA type glutamate receptors or other receptors, thus, inhibiting general brain excitability [137], [138]. Fig. 4 shows a general scheme pointing out the relationship between trace elements and amyloidogenic proteins at the synapse under normal conditions. In Fig. 4, it is observed that under normal conditions, Zn has a neuromodulating function in the synapse, transmitting the space-time information on neuronal activity. This fact can allow ‘‘lateral inhibition’’ based on signaling contrast and may be based on synaptic plasticity [15], [132], [144], [145]. Synaptic Zn enters postsynaptic neurons through NMDA channels and Ca2+ channels, regulating the functions of several channels and receptors [137], [138]. Recent evidence suggests that the Zn transporter (ZnT-1), which increases the flow of Zn to the extracellular compartment, is in the postsynaptic membranes [137]. The Zn transporter binds to NMDA-type glutamate receptors and regulates their activity. In comparison, the cellular prion protein (PrPC), an analog of ZIP (Zn transporters), is on the postsynaptic membranes that bind to the AMPA-type glutamate receptor, which facilitates the Zn flux. Therefore, it is likely that Zn synaptic levels are controlled by ZnT-1 and PrPC [132], [138], [145]. Metallothioneins (MT) secreted by neurons or glia can also regulate Zn homeostasis at synapses. Another contributor to Zn homeostasis is carnosine (CAR), an endogenous antioxidant and anti-crosslinking peptide, which is synthesized in glial cells [131], [132], [146], [147]. Cu is also secreted in the synaptic clefts after neuronal excitation [15], [145], [147]. PrPC binds to Cu in its N-terminal domain and regulates synaptic levels of Cu [15]. For PrPC, it is also possible to provide Cu to amyloid precursor protein (APP) or to NMDA-type glutamate receptor, thus, influencing the production of beta-amyloid peptide (AβP) or the neuronal excitability [138], [148]. APP also regulates Cu levels by reducing Cu2+ to Cu+, and both APP and PrPC attenuate Cu-induced toxicity [145]. Furthermore, APP controls Fe homeostasis by binding to ferroportin and promoting Fe flux. On the other hand, both PrPC and α-synuclein have ferrireductase activity that regulates the Fe2+/Fe3+ ratio in synapses, thereby, controlling the neurotransmitter synthesis [15], [145], [146]. However, when homeostasis of metals is interrupted, degradation of neuronal synapses occurs, contributing to the pathogenesis of neurological disorders [15], [19], [149], [150]. A mechanism of metal imbalance in the synapse, contributing to neurological disorders has also been suggested ( Fig. 5) [15].
Fig. 4.
General scheme of the synapse under normal conditions, showing a relationship between trace elements and amyloidogenic proteins. High concentrations of Zn are secreted from synaptic vesicles along with glutamate. Zn binds to the NMDA–type glutamate receptor (NMDA–R) and regulates its excitability. Secreted Zn can then spread into nearby neurons and regulate the information, and finally, contribute to memory formation. ZnT-1 (zinc transporter 1), AMPA-R (AMPA-type glutamate receptor), NMDA-R (NMDA-type glutamate receptor), and MT-3 (metallothionein 3).
Fig. 5.
Disruption of metal homeostasis and neurological disorder after viral infection. In the case of viral infection in the brain, an imbalance in metal homeostasis can occur. This imbalance of metals in the brain can accelerate AβP oligomerization and increase neurotoxicity. AβP oligomers can form amyloid channels in synaptic membranes, producing Ca dyshomeostasis and initiating synaptotoxicity and neurotoxicity, causing neurological disorders.
In case of neurotoxicity, AβP oligomerization can form amyloid channels on synaptic membranes, producing Ca2+ dyshomeostasis and initiating synaptotoxicity and neurotoxicity, resulting in neurological disorders [131], [151], [152], [153]. In contrast, micronutrients supplementation, can inhibit amyloid channels and have neuroprotective properties [15], [48], [84], [133], [154]. However, excess of micronutrients from overexcitation during ischemia also disrupts metal homeostasis at the synapse, starting neurodegeneration and contributing to the pathogenesis of vascular dementia [15].
5. Trace elements role in available treatment for COVID-19
Although a variety of vaccines have been developed and are being applied to prevent severe cases of COVID-19 infection, the protective efficacy of these vaccine decreases when new variants of the virus emerge [155]. In this sense, the search for treatment against SARS-CoV-2 is a reality. Some treatments have been suggested for this infection from another viral infection. For example, some drugs used to treat HIV (e.g., lopinavir/ritonavir), and Ebola virus, and MERS diseases (e.g., remdesivir) have been tested for the treatment of SARS-CoV-2, suggesting similarity in the viral life cycle [2]. Recently, the Medicines and Healthcare products Regulatory Agency (MHRA), in the United Kingdom, approved Lagevrio (molnupiravir) as first oral antiviral for treatment of COVID-19. Molnupiravir showed to be a safe and effective drug against severity of COVID-19. In addition, Food and Drug Administration (FDA) has approved and authorized the emergency use of monoclonal antibodies for the treatment of mild or moderate COVID-19 in patients at high risk of progression to severe COVID-19 and/or hospitalization. Monoclonal antibodies are laboratory-made molecules that act as substitute antibodies [156]. They can help the immune system recognize and respond more effectively to the virus, making it more difficult for the virus to reproduce and cause harm. Considering this fact and all the points mentioned above about the role of trace elements in the immune system, it is reasonable to suggest the importance of micronutrients supplementation (Zn, Cu, Fu, Mg among others) for the success of these treatments against SARS-Cov-2 replication, since such micronutrients can normalize impaired immune functions through the modulation of neutrophil activity, the blastogenic response to T cell mitogens, and the upregulation of B cells, producing more antibodies [2]. However, more studies are needed to ensure this evidence.
6. Outlook and summary
Considering all the points discussed in this text, it is clear that trace elements play important roles in viral infections, such as helping in the activation of immune cells, producing antibodies, and inhibiting virus replication. However, the relationship between trace elements and virus infections is still complex. Therefore, there is still a lot to be explored to understand the biochemical mechanisms involved between trace elements and viral infections, especially in the brain. The inclusion of viruses in the etiology and diagnosis of neurological disorders has a positive impact on the management and treatment of disabling and potentially lethal complications.
In this sense, the search for complementary processes, including the exploration of strategies based on metallomics to assist health professionals in better understanding and treating diseases, is welcome and of great importance, mainly aiming at the development of novel control and intervention therapies, such as specific antiviral drug and more efficient vaccines against different variants of the virus. In this sense, some analytical alternatives can be applied to help the medical, biological, toxicological team, in such assessments, which involve the application of ICP-MS based-techniques such as inductively coupled plasma mass spectrometry, laser ablation mass spectrometry, among others [157]. Additionally, recently, based on the concept of the single particle-ICP-MS (SP-ICP-MS), Degueldre reported the concept of single virus ICP-MS (SV-ICP-MS) that brings the possibility of characterizing smallest biological systems, as virus, through the evaluation some trace elements, allowing the characterization of the species using molar ratios and quantification of their number concentration [158]. As these ratios vary considering the nature of each virus, their identification through SV-ICP-MS might be possible, therefore, helping to identify new variants of SARS-CoV-2 and the development of new treatment against viral infection.
Authors statement
All authors contributed equally to this manuscript.
Conflict of Interest
The authors have no conflict of interest, and no funding was received for this article.
Acknowledgments
The author MAZA thanks to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2017/50085-3, 2018/25207-0, 2019/00063-9, and 2019/24445-8), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 303231/2020-3), for financial support. The author CALJ thanks to Universidade Federal do Piauí (UFPI, Edital nº 07/2020/PROPESQI/PRPG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 200275/2020-8), for financial support.
References
- 1.Xiao N., Nie M., Pang H., Wang B., Hu J., Meng X., Li K., Ran X., Long Q., Deng H., Chen N., Li S., Tang N., Huang A., Hu Z. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021;12:1618. doi: 10.1038/s41467-021-21907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jesus J.R., de Araújo Andrade T. Understanding the relationship between viral infections and trace elements from a metallomics perspective: implications for COVID-19. Metallomics. 2020;12:1912–1930. doi: 10.1039/d0mt00220h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., Chng M.H.Y., Lin M., Tan N., Linster M., Chia W.N., Chen M.I.C., Wang L.F., Ooi E.E., Kalimuddin S., Tambyah P.A., Low J.G.H., Tan Y.J., Bertoletti A. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 4.Sfera A., Osorio C., Maguire G., Rahman L., Afzaal J., Cummings M., Maldonado J.C. COVID-19, ferrosenescence and neurodegeneration, a mini-review. Prog. Neuro Psychopharmacol. Biol. Psychiatry. 2021;109 doi: 10.1016/j.pnpbp.2020.110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharmalingam K., Birdi A., Tomo S., Sreenivasulu K., Charan J., Yadav D., Purohit P., Sharma P. Trace elements as immunoregulators in SARS-CoV-2 and other viral infections. Indian J. Clin. Biochem. 2021;36:416–426. doi: 10.1007/s12291-021-00961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishida T. Antiviral activities of Cu2+ ions in viral prevention, replication, RNA degradation, and for antiviral efficacies of lytic virus, ROS-mediated virus, copper chelation. World Sci. 2018;99:148–168. [Google Scholar]
- 7.Osuna-Padilla I.A., Briceño O., Aguilar-Vargas A., Rodríguez-Moguel N.C., Villazon-De la Rosa A., Pinto-Cardoso S., Flores-Murrieta F.J., Perichart-Perera O., Tolentino-Dolores M., Vargas-Infante Y., Reyes-Terán G. Zinc and selenium indicators and their relation to immunologic and metabolic parameters in male patients with human immunodeficiency virus. Nutrition. 2020;70 doi: 10.1016/j.nut.2019.110585. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez K., Saunier F., Rigaill J., Audoux E., Botelho-Nevers E., Prier A., Dickerscheit Y., Pillet S., Pozzetto B., Bourlet T., Verhoeven P.O. Evaluation of in vitro activity of copper gluconate against SARS-CoV-2 using confocal microscopy-based high content screening. J. Trace Elem. Med. Biol. 2021;68 doi: 10.1016/j.jtemb.2021.126818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons-Weiler J., McFarland G., la Joie E. Impact of catch-up vaccination on aluminum exposure due to new laws and post social distancing. J. Trace Elem. Med. Biol. 2020;62 doi: 10.1016/j.jtemb.2020.126649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taheri S., Asadi S., Nilashi M., Ali Abumalloh R., Ghabban N.M.A., Mohd Yusuf S.Y., Supriyanto E., Samad S. A literature review on beneficial role of vitamins and trace elements: evidence from published clinical studies. J. Trace Elem. Med. Biol. 2021;67 doi: 10.1016/j.jtemb.2021.126789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Jiang Y., Zhang Y., Li N., Yin Q., Liu L., Lv X., Liu Y., Li A., Fang B., Li J., Ye H., Yang G., Cui X., Liu Y., Qu Y., Li C., Li J., Li D., Gai Z., Wang S., Zhan F., Liang M. Abnormal upregulation of cardiovascular disease biomarker PLA2G7 induced by proinflammatory macrophages in COVID-19 patients. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-85848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee D., Viswanath B. Neuropsychiatric manifestations of COVID-19 and possible pathogenic mechanisms: insights from other coronaviruses. Asian J. Psychiatry. 2020;54 doi: 10.1016/j.ajp.2020.102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S., Basu A. Viral infection and neural stem/progenitor cell’s fate: Implications in brain development and neurological disorders. Neurochem. Int. 2011;59:357–366. doi: 10.1016/j.neuint.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Wouk J., Rechenchoski D.Z., Rodrigues B.C.D., Ribelato E.V., Faccin-Galhardi L.C. Viral infections and their relationship to neurological disorders. Arch. Virol. 2021;166:733–753. doi: 10.1007/s00705-021-04959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesus J.R., Arruda M. Unravelling neurological disorders through metallomics-based approaches. Metallomics. 2020;12:1878–1896. doi: 10.1039/D0MT00234H. [DOI] [PubMed] [Google Scholar]
- 16.Raha S., Mallick R., Basak S., Duttaroy A.K. Is copper beneficial for COVID-19 patients? Med. Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin Z., Peng R., Baravik I.K., Liu X. Fighting COVID-19: integrated micro- and nanosystems for viral infection diagnostics. Matter. 2020;3:628–651. doi: 10.1016/j.matt.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood H. New insights into the neurological effects of COVID-19. Nat. Rev. Neurol. 2020;16:403. doi: 10.1038/s41582-020-0386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.J.R. de Jesus, Application of the ionomic strategy to evaluate difference in metal ion concentration between patients with bipolar disorder and other psychiatric disorder, 2019, 39–58. https://doi.org/10.1007/978-3-030-29473-1_3.
- 20.Ishida T. Antiviral activities of Cu2+ ions in viral prevention, replication, RNA degradation, and for antiviral efficacies of lytic virus, ROS-mediated virus, copper chelation. World Sci. News. 2018;99:148–168. [Google Scholar]
- 21.Paludan S.R., Pradeu T., Masters S.L., Mogensen T.H. Constitutive immune mechanisms: mediators of host defence and immune regulation. Nat. Rev. Immunol. 2020;21:137–150. doi: 10.1038/s41577-020-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera A., Siracusa M.C., Yap G.S., Gause W.C. Innate cell communication kick-starts pathogen-specific immunity. Nat. Immunol. 2016;17:356–363. doi: 10.1038/ni.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvestre M.C., dos Reis V.M.S., Sato M.N. Innate immunity and effector and regulatory mechanisms involved in allergic contact dermatitis. An. Bras. Dermatol. 2018;93:242–250. doi: 10.1590/abd1806-4841.20186340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., López-Camacho C., Slon-Campos J., Zhao Y., Stuart D.I., Paesen G.C., Grimes J.M., Antson A.A., Bayfield O.W., Hawkins D.E.D.P., Ker D.-S., Wang B., Turtle L., Subramaniam K., Thomson P., Zhang P., Dold C., Ratcliff J., Simmonds P., de Silva T., Sopp P., Wellington D., Rajapaksa U., Chen Y.-L., Salio M., Napolitani G., Paes W., Borrow P., Kessler B.M., Fry J.W., Schwabe N.F., Semple M.G., Baillie J.K., Moore S.C., Openshaw P.J.M., Ansari M.A., Dunachie S., Barnes E., Frater J., Kerr G., Goulder P., Lockett T., Levin R., Zhang Y., Jing R., Ho L.-P., Cornall R.J., Conlon C.P., Klenerman P., Screaton G.R., Mongkolsapaya J., McMichael A., Knight J.C., Ogg G., Dong T. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ezhilan M., Suresh I., Nesakumar N. SARS-CoV, MERS-CoV and SARS-CoV-2: a diagnostic challenge. Meas. J. Int. Meas. Confed. 2021;168 doi: 10.1016/j.measurement.2020.108335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M., Liu W., Bauch T., Graviss E.A., Arduino R.C., Kimata J.T., Chen M., Wang J. Clearance of HIV infection by selective elimination of host cells capable of producing HIV. Nat. Commun. 2020;11:4051. doi: 10.1038/s41467-020-17753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan H., Du X., Zhang J., Zheng H., Lu X., Wu Q., Li H., Wang H., Shi Y., Gao G., Zhou Z., Tan D.X., Li X. Selective inhibition of Ebola entry with selective estrogen receptor modulators by disrupting the endolysosomal calcium. Sci. Rep. 2017;7:41226. doi: 10.1038/srep41226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahin M., Karayakar F., Koksal A.R., Yetim A., İyisoy M.S., Şen İ., Alkım H., Alkım C., Colak T. Changes in liver tissue trace element concentrations during hepatitis B viral infection treatment. Biol. Trace Elem. Res. 2019;188:245–250. doi: 10.1007/s12011-018-1414-y. [DOI] [PubMed] [Google Scholar]
- 31.Liu W., Zhang S., Nekhai S., Liu S. Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr. Clin. Microbiol. Rep. 2020;7:13–19. doi: 10.1007/s40588-020-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chasapis C.T. Interactions between metal binding viral proteins and human targets as revealed by network-based bioinformatics. J. Inorg. Biochem. 2018;186:157–161. doi: 10.1016/j.jinorgbio.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Sivasubramanian S., Shenoy J., Kamath S.P., Kulkarni V., Srinivasan M., Shantharam Baliga B. Micronutrients status among human immunodeficiency virus-infected children in Southern India. HIV AIDS Rev. 2020;9:56–60. doi: 10.5114/hivar.2020.93062. [DOI] [Google Scholar]
- 34.Shah K.K., Verma R., Oleske J.M., Scolpino A., Bogden J.D. Essential trace elements and progression and management of HIV infection. Nutr. Res. 2019;71:21–29. doi: 10.1016/j.nutres.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Ansari R.A., Rabiu K.M. Oxidative cascade prognosis, antioxidants & selected trace elements in COVID-19. Open J. Appl. Sci. 2020;10:688–700. doi: 10.4236/ojapps.2020.1011048. [DOI] [Google Scholar]
- 36.W.M. Saod, N.T. Darwish, T.A. Zaidan, A.W.A. Alfalujie, Trace elements in sera of patients with hepatitis B: determination and analysis, in: AIP Conference Proceedings, American Institute of Physics Inc., 2018. 10.1063/1.5030883. [DOI]
- 37.Kassu A., Yabutani T., Mahmud Z.H., Mohammad A., Nguyen N., Huong B.T.M., Hailemariam G., Diro E., Ayele B., Wondmikun Y., Motonaka J., Ota F. Alterations in serum levels of trace elements in tuberculosis and HIV infections. Eur. J. Clin. Nutr. 2006;60:580–586. doi: 10.1038/sj.ejcn.1602352. [DOI] [PubMed] [Google Scholar]
- 38.Raciti L., Calabrò R.S. Can volcanic trace elements facilitate Covid-19 diffusion? A hypothesis stemming from the Mount Etna area, Sicily. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molin Y., Frisk P., Hjelm E., Blomberg J., Friman G., Ilbäck N.G. Arsenic trioxide influences viral replication in target organs of coxsackievirus B3-infected mice. Microbes Infect. 2010;12:1027–1034. doi: 10.1016/j.micinf.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Liu W., Zhang S., Nekhai S., Liu S. Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr. Clin. Microbiol. Rep. 2020;7:13–19. doi: 10.1007/s40588-020-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsatsakis A., Petrakis D., Nikolouzakis T.K., Docea A.O., Calina D., Vinceti M., Goumenou M., Kostoff R.N., Mamoulakis C., Aschner M., Hernández A.F. COVID-19, an opportunity to reevaluate the correlation between long-term effects of anthropogenic pollutants on viral epidemic/pandemic events and prevalence. Food Chem. Toxicol. 2020;141 doi: 10.1016/j.fct.2020.111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maywald M., Wessels I., Rink L. Zinc signals and immunity. Int. J. Mol. Sci. 2017;18:2222. doi: 10.3390/ijms18102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered. Am. J. Clin. Nutr. 1998;68:447–463. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 45.Read S.A., Parnell G., Booth D., Douglas M.W., George J., Ahlenstiel G. The antiviral role of zinc and metallothioneins in hepatitis C infection. J. Viral Hepat. 2018;25:491–501. doi: 10.1111/jvh.12845. [DOI] [PubMed] [Google Scholar]
- 46.Rahman M.T., Karim M.M. Metallothionein: a potential link in the regulation of zinc in nutritional immunity. Biol. Trace Elem. Res. 2018;182:1–13. doi: 10.1007/s12011-017-1061-8. [DOI] [PubMed] [Google Scholar]
- 47.Read S.A., O’Connor K.S., Suppiah V., Ahlenstiel C.L.E., Obeid S., Cook K.M., Cunningham A., Douglas M.W., Hogg P.J., Booth D., George J., Ahlenstiel G. Zinc is a potent and specific inhibitor of IFN-λ3 signalling. Nat. Commun. 2017;8:15245. doi: 10.1038/ncomms15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gasmi A., Tippairote T., Mujawdiya P.K., Peana M., Menzel A., Dadar M., Gasmi Benahmed A., Bjørklund G. Micronutrients as immunomodulatory tools for COVID-19 management. Clin. Immunol. 2020;220 doi: 10.1016/j.clim.2020.108545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jesus J.R., Aragão A.Z.B., Arruda M.A.Z., Ramos C.H.I. Optimization of a methodology for quantification and removal of zinc gives insights into the effect of this metal on the stability and function of the zinc-binding Co-chaperone Ydj1. Front. Chem. 2019;7:416. doi: 10.3389/fchem.2019.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma P., Prasanna K.Reddy, Kumar B. Trace element zinc, a nature’s gift to fight unprecedented global pandemic COVID-19. Biol. Trace Elem. Res. 2021;199:3213–3221. doi: 10.1007/s12011-020-02462-8/Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han Y., Sanford L., Simpson D.M., Dowell R.D., Palmer A.E. Remodeling of Zn2+ homeostasis upon differentiation of mammary epithelial cells. Metallomics. 2020;12:346–362. doi: 10.1039/c9mt00301k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kar M., Khan N.A., Panwar A., Bais S.S., Basak S., Goel R., Sopory S., Medigeshi G.R. Zinc chelation specifically inhibits early stages of dengue virus replication by activation of NF-κB and induction of antiviral response in epithelial cells. Front. Immunol. 2019;10:2347. doi: 10.3389/fimmu.2019.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y., Song Z., Bai J., Liu X., Nauwynck H., Jiang P. ZAP, a CCCH-type zinc finger protein, inhibits porcine reproductive and respiratory syndrome virus replication and interacts with viral Nsp9. J. Virol. 2019;93 doi: 10.1128/jvi.00001-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang B., Goraya M.U., Chen N., Xu L., Hong Y., Zhu M., Chen J.L. Zinc finger CCCH-type antiviral protein 1 restricts the viral replication by positively regulating type I interferon response. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin R.J., Huang C.H., Liu P.C., Lin I.C., Huang Y.L., Chen A.Y., Chiu H.P., Shih S.R., Lin L.H., Lien S.P., Yen L.C., Liao C.L. Zinc finger protein ZFP36L1 inhibits influenza A virus through translational repression by targeting HA, M and NS RNA transcripts. Nucleic Acids Res. 2020;48:7371–7384. doi: 10.1093/nar/gkaa458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu D., Lewis E.D., Pae M., Meydani S.N. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front. Immunol. 2019;10(9):3160. doi: 10.3389/fimmu.2018.03160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao H., Dai W., Zhao L., Min J., Wang F. The role of zinc and zinc homeostasis in macrophage function. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/6872621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chasapis C.T., Ntoupa P.S.A., Spiliopoulou C.A., Stefanidou M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020;94:1443–1460. doi: 10.1007/s00204-020-02702-9. [DOI] [PubMed] [Google Scholar]
- 59.Pormohammad A., Monych N.K., Turner R.J., Pormohammad A. Zinc and SARS-CoV-2: a molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes. Int. J. Mol. Med. 2021;47:326–334. doi: 10.3892/ijmm.2020.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asl S.H., Nikfarjam S., Zolbanin N.M., Nassiri R., Jafafri R. Immunopharmacological perspective on zinc in SARS-CoV-2 infection. Int. Immunopharmacol. 2021;96 doi: 10.1016/j.intimp.2021.107630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joachimiak M.P. Zinc against covid-19? Symptom surveillance and deficiency risk groups. PLoS Negl. Trop. Dis. 2021;15:1–17. doi: 10.1371/journal.pntd.0008895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.te Velthuis A.J.W., van den Worml S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahman M.T., Syed, Idid Z. Can Zn be a critical element in COVID-19 treatment? Biol. Trace Elem. Res. 2021;199:550–558. doi: 10.1007/s12011-020-02194-9/Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee C.C., Kuo C.J., Ko T.P., Hsu M.F., Tsui Y.C., Chang S.C., Yang S., Chen S.J., Chen H.C., Hsu M.C., Shih S.R., Liang P.H., Wang A.H.J. Structural basis of inhibition specificities of 3C and 3C-like proteases by zinc-coordinating and peptidomimetic compounds. J. Biol. Chem. 2009;284:7646–7655. doi: 10.1074/jbc.M807947200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoon S.H., Choi J., Lee W.J., Do J.T. Genetic and epigenetic etiology underlying autism spectrum disorder. J. Clin. Med. 2020;9:966. doi: 10.3390/jcm9040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lv Y., Chen L., Liang X., Liu X., Gao M., Wang Q., Wei Q., Liu L. Association between iron status and the risk of adverse outcomes in COVID-19. Clin. Nutr. 2021;40:3462–3469. doi: 10.1016/j.clnu.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lawaczeck R. COVID-19 and body iron: a survey on phenomenological and genetic correlations. ACS Chem. Neurosci. 2020;11:3996–4000. doi: 10.1021/acschemneuro.0c00572. [DOI] [PubMed] [Google Scholar]
- 69.Campione E., Lanna C., Cosio T., Rosa L., Conte M.P., Iacovelli F., Romeo A., Falconi M., del Vecchio C., Franchin E., Lia M.S., Minieri M., Chiaramonte C., Ciotti M., Nuccetelli M., Terrinoni A., Iannuzzi I., Coppeda L., Magrini A., Bernardini S., Sabatini S., Rosapepe F., Bartoletti P.L., Moricca N., di Lorenzo A., Andreoni M., Sarmati L., Miani A., Piscitelli P., Valenti P., Bianchi L. Lactoferrin against SARS-CoV-2: in vitro and in silico evidences. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.666600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ganz T., Nemeth E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015;15:500–510. doi: 10.1038/nri3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dorward D.A., Russell C.D., Um I.H., Elshani M., Armstrong S.D., Penrice-Randal R., Millar T., Lerpiniere C.E.B., Tagliavini G., Hartley C.S., Randle N.P., Gachanja N.N., Potey P.M.D., Dong X., Anderson A.M., Campbell V.L., Duguid A.J., al Qsous W., BouHaidar R., Kenneth Baillie J., Dhaliwal K., Wallace W.A., Bellamy C.O.C., Prost S., Smith C., Hiscox J.A., Harrison D.J., Lucas C.D. Tissue-specific immunopathology in fatal COVID-19. Am. J. Respir. Crit. Care Med. 2021;203:192–201. doi: 10.1164/rccm.202008-3265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dahan S., Segal G., Katz I., Hellou T., Tietel M., Bryk G., Amital H., Shoenfeld Y., Dagan A. Ferritin as a marker of severity in COVID-19 patients: a fatal correlation. Isr. Med. Assoc. J. 2020;22:494–500. [PubMed] [Google Scholar]
- 73.Chowdhury S.F., Anwar S. Management of hemoglobin disorders during the COVID-19 pandemic. Front. Med. 2020;7:306. doi: 10.3389/fmed.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perricone C., Bartoloni E., Bursi R., Cafaro G., Guidelli G.M., Shoenfeld Y., Gerli R. COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol. Res. 2020;68:213–224. doi: 10.1007/s12026-020-09145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amezcua J.M.G., Jain R., Kleinman G., Muh C.R., Guzzetta M., Folkerth R., Snuderl M., Placantonakis D.G., Galetta S.L., Hochman S., Zagzag D. COVID-19-induced neurovascular injury: a case series with emphasis on pathophysiology mechanisms. SN Compr. Clin. Med. 2020;2:2109–2125. doi: 10.1007/s42399-020-00598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestation of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wenzhong L., Hualan L. COVID-19: captures iron and generates reactive oxygen species to damage the human immune system. Autoimmunity. 2021;54:213–224. doi: 10.1080/08916934.2021.1913581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alshebri M.S., Alshouimi R.A., Alhumidi H.A., Alshaya A.I. Neurological complications of SARS-CoV, MERS-CoV, and COVID-19. SN Compr. Clin. Med. 2020;2:2037–2047. doi: 10.1007/s42399-020-00589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lawaczeck R. COVID-19 and body iron: a survey on phenomenological and genetic correlations. ACS Chem. Neurosci. 2020;11:3996–4000. doi: 10.1021/acschemneuro.0c00572. [DOI] [PubMed] [Google Scholar]
- 80.Andreou A., Trantza S., Filippou D., Filippou D., Sipsas N., Tsiodras S. COVID-19: the potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. In Vivo. 2020;34:1567–1588. doi: 10.21873/invivo.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lelièvre P., Sancey L., Coll J.L., Deniaud A., Busser B. The multifaceted roles of copper in cancer: a trace metal element with dysregulated metabolism, but also a target or a bullet for therapy. Cancers. 2020;12:1–25. doi: 10.3390/cancers12123594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Culbertson E.M., Culotta V.C. Copper in infectious disease: using both sides of the penny. Semin. Cell Dev. Biol. 2021;115:19–26. doi: 10.1016/j.semcdb.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Domingo J.L., Marquès M. The effects of some essential and toxic metals/metalloids in COVID-19: a review. Food Chem. Toxicol. 2021;152 doi: 10.1016/j.fct.2021.112161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arruda de Souza Monnerat J., Ribeiro de Souza P., Monteiro da Fonseca Cardoso L., Dario Mattos J., de Souza Rocha G., Frauches Medeiros R. Micronutrients and bioactive compounds in the immunological pathways related to SARS-CoV-2 (adults and elderly) Eur. J. Nutr. 2021;60:559–579. doi: 10.1007/s00394-020-02410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jung S., Byeon E.Y., Kim D.G., Lee D.G., Ryoo S., Lee S., Shin C.W., Jang H.W., Yang J.Y., Kim H.J., Lee S. Copper-coated polypropylene filter face mask with SARS-COV-2 antiviral ability. Polymers. 2021;13:1367. doi: 10.3390/polym13091367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi D., Choi M., Jeong H., Heo J., Kim T., Park S., Jin Y., Lee S., Hong J. Co-existing “spear-and-shield” air filter: anchoring proteinaceous pathogen and self-sterilized nanocoating for combating viral pandemic. Chem. Eng. J. 2021;426 doi: 10.1016/j.cej.2021.130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mosselhy D.A., Kareinen L., Kivistö I., Aaltonen K., Virtanen J., Ge Y., Sironen T. Copper-silver nanohybrids: Sars-cov-2 inhibitory surfaces. Nanomaterials. 2021;11:1820. doi: 10.3390/nano11071820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hewawaduge C., Senevirathne A., Jawalagatti V., Kim J.W., Lee J.H. Copper-impregnated three-layer mask efficiently inactivates SARS-CoV2. Environ. Res. 2021;196 doi: 10.1016/j.envres.2021.110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clark N.F., Taylor-Robinson A.W. COVID-19 therapy: could a copper derivative of chlorophyll a be used to treat lymphopenia associated with severe symptoms of SARS-CoV-2 infection? Front. Med. 2021;8 doi: 10.3389/fmed.2021.620175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chasapis C.T., Georgiopoulou A.K., Perlepes S.P., Bjørklund G., Peana M. A SARS-CoV-2 –human metalloproteome interaction map. J. Inorg. Biochem. 2021;219 doi: 10.1016/j.jinorgbio.2021.111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patil S., US V.R., Arakeri G., Patil S., Brennan P.A. Does Yadgir population have copper-mediated intrinsic immunity to resist COVID-19 challenge? Med. Hypotheses. 2021;146:1–8. doi: 10.1016/j.mehy.2020.110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Q., Zhao X., Ma J., Mu Y., Wang Y., Yang S., Wu Y., Wu F., Zhou Y. Selenium (Se) plays a key role in the biological effects of some viruses: implications for COVID-19. Environ. Res. 2021;196:1–26. doi: 10.1016/j.envres.2021.110984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Radomska D., Czarnomysy R., Radomski D., Bielawska A., Bielawski K. Selenium as a bioactive micronutrient in the human diet and its cancer chemopreventive activity. Nutrients. 2021;13:1649. doi: 10.3390/nu13051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cardoso B.R., Roberts B.R., Bush A.I., Hare D.J. Selenium, selenoproteins and neurodegenerative diseases. Metallomics. 2015;7:1213–1228. doi: 10.1039/c5mt00075k. [DOI] [PubMed] [Google Scholar]
- 95.Manzanares W., Moreira E., Hardy G. Pharmaconutrition revisited for critically ill patients with coronavirus disease 2019 (COVID-19): does selenium have a place? Nutrition. 2021;81:1–24. doi: 10.1016/j.nut.2020.110989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Calder P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health. 2020;3:74–92. doi: 10.1136/bmjnph-2020-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saghi E., Norouzy A., Nematy M., Jarahi L., Boostani R., Zemorshidi F., Vahidi Z., Rafatpanah H. Dietary intake and serum selenium levels influence the outcome of HTLV-1 infection. 2011;199:3242–3252. doi: 10.1007/s12011-020-02472-6/Published. [DOI] [PubMed] [Google Scholar]
- 98.Poles J., Karhu E., McGill M., McDaniel H.R., Lewis J.E. The effects of twenty-four nutrients and phytonutrients on immune system function and inflammation: a narrative review. J. Clin. Transl. Res. 2021;7:333–376. doi: 10.18053/jctres.07.202103.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hackler J., Heller R.A., Sun Q., Schwarzer M., Diegmann J., Bachmann M., Moghaddam A., Schomburg L. Relation of serum copper status to survival in covid‐19. Nutrients. 2021;13:1898. doi: 10.3390/nu13061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khatiwada S., Subedi A., Mechanistic Link A. Between selenium and coronavirus disease 2019 (COVID-19) Curr. Nutr. Rep. 2021;10:125–136. doi: 10.1007/s13668-021-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Amporndanai K., Meng X., Shang W., Jin Z., Rogers M., Zhao Y., Rao Z., Liu Z.J., Yang H., Zhang L., O’Neill P.M., Samar Hasnain S. Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives. Nat. Commun. 2021;12:3061. doi: 10.1038/s41467-021-23313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 104.Grober U., Holick M.F. The coronavirus disease (COVID-19) – a supportive approach with selected micronutrients. Int. J. Vitam. Nutr. Res. 2021:1–22. doi: 10.1026/a000002. [DOI] [PubMed] [Google Scholar]
- 105.Kieliszek M., Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19) Med. Hypotheses. 2020;143:1–10. doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y., Huang J., Sun Y., Stubbs D., He J., Li W., Wang F., Liu Z., Ruzicka J.A., Taylor E.W., Rayman M.P., Wan X., Zhang J. SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, endoplasmic reticulum stress and DNA synthesis. Food Chem. Toxicol. 2021;153 doi: 10.1016/j.fct.2021.112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Im J.H., Je Y.S., Baek J., Chung M.H., Kwon H.Y., Lee J.S. Nutritional status of patients with COVID-19. Int. J. Infect. Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang H.Y., Zhang A.R., bin Lu Q., Zhang X.A., Zhang Z.J., Guan X.G., le Che T., Yang Y., Li H., Liu W., Fang L.Q. Association between fatality rate of COVID-19 and selenium deficiency in China. BMC Infect. Dis. 2021;21:1–8. doi: 10.1186/s12879-021-06167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang J., Saad R., Taylor E.W., Rayman M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vavougios G.D., Ntoskas K.T., Doskas T.K. Impairment in selenocysteine synthesis as a candidate mechanism of inducible coagulopathy in COVID-19 patients. Med. Hypotheses. 2021;147 doi: 10.1016/j.mehy.2020.110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gómez-Bernal G. Lithium for the 2019 novel coronavirus. Med. Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dalamaga M., Karampela I., Mantzoros C.S. Commentary: could iron chelators prove to be useful as an adjunct to COVID-19 Treatment Regimens? Metab. Clin. Exp. 2020;108 doi: 10.1016/j.metabol.2020.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Minton K. Immunodeficiency: magnesium regulates antiviral immunity. Nat. Rev. Immunol. 2013;13:548–549. doi: 10.1038/nri3501. [DOI] [PubMed] [Google Scholar]
- 114.Chaigne-Delalande B., Li F.Y., O’Connor G.M., Lukacs M.J., Jiang P., Zheng L., Shatzer A., Biancalana M., Pittaluga S., Matthews H.F., Jancel T.J., Bleesing J.J., Marsh R.A., Kuijpers T.W., Nichols K.E., Lucas C.L., Nagpal S., Mehmet H., Su H.C., Cohen J.I., Uzel G., Lenardo M.J. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341:186–191. doi: 10.1126/science.1240094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang C.F., Ding H., Jiao R.Q., Wu X.X., Kong L.D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur. J. Pharmacol. 2020;886:1–63. doi: 10.1016/j.ejphar.2020.173546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Story M.J. Essential sufficiency of zinc, ω-3 polyunsaturated fatty acids, vitamin D and magnesium for prevention and treatment of COVID-19, diabetes, cardiovascular diseases, lung diseases and cancer. Biochimie. 2021;187:94–109. doi: 10.1016/j.biochi.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Skalny A. v, Lima T.R.R., Ke T., Zhou J.C., Bornhorst J., Alekseenko S.I., Aaseth J., Anesti O., Sarigiannis D.A., Tsatsakis A., Aschner M., Tinkov A.A. Toxic metal exposure as a possible risk factor for COVID-19 and other respiratory infectious diseases. Food Chem. Toxicol. 2020;146 doi: 10.1016/j.fct.2020.111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wandt V.K., Winkelbeiner N., Bornhorst J., Witt B., Raschke S., Simon L., Ebert F., Kipp A.P., Schwerdtle T. A matter of concern – trace element dyshomeostasis and genomic stability in neurons. Redox Biol. 2021;41 doi: 10.1016/j.redox.2021.101877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carroll W.M. The global burden of neurological disorders. Lancet Neurol. 2019;18:418–419. doi: 10.1016/S1474-4422(19)30029-8. [DOI] [PubMed] [Google Scholar]
- 120.Li L., Mao S., Wang J., Ding X., Zen J.Y. Viral infection and neurological disorders—potential role of extracellular nucleotides in neuroinflammation. ExRNA. 2019;1:26. doi: 10.1186/s41544-019-0031-z. [DOI] [Google Scholar]
- 121.Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H., Ahn J.Y., Kim M.K., Choi J.P. Neurological complications during treatment of middle east respiratory syndrome. J. Clin. Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 123.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ogier M., Andéol G., Sagui E., Dal Bo G. How to detect and track chronic neurologic sequelae of COVID-19? Use of auditory brainstem responses and neuroimaging for long-term patient follow-up. Brain Behav. Immun. Health. 2020;5 doi: 10.1016/j.bbih.2020.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Becker J.S. Bioimaging of metals in brain tissue from micrometre to nanometre scale by laser ablation inductively coupled plasma mass spectrometry: state of the art and perspectives. Int. J. Mass Spectrom. 2010;289:65–75. doi: 10.1016/j.ijms.2009.10.011. [DOI] [Google Scholar]
- 126.Becker J.S., Matusch A., Palm C., Salber D., Morton K.A., Becker J.S. Bioimaging of metals in brain tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and metallomics. Metallomics. 2010;2:104–111. doi: 10.1039/b916722f. [DOI] [PubMed] [Google Scholar]
- 127.Becker J.S. Imaging of metals in biological tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS): state of the art and future developments. J. Mass Spectrom. 2013;48:255–268. doi: 10.1002/jms.3168. [DOI] [PubMed] [Google Scholar]
- 128.Grochowski C., Blicharska E., Krukow P., Jonak K., Maciejewski M., Szczepanek D., Jonak K., Flieger J., Maciejewski R. Analysis of trace elements in human brain: its aim, methods, and concentration levels. Front. Chem. 2019;7:115. doi: 10.3389/fchem.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stork C.J., Li Y. v. Zinc release from thapsigargin/IP3-sensitive stores in cultured cortical neurons. J. Mol. Signal. 2010;5:1–6. doi: 10.1186/1750-2187-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leal M.F.C., Catarino R.I.L., Pimenta A.M., Souto M.R.S. Roles of metal microelements in neurodegenerative diseases. Neurophysiology. 2020;52:80–88. doi: 10.1007/s11062-020-09854-5. [DOI] [Google Scholar]
- 131.Tanaka K., Kawahara M. Copper enhances zinc-induced neurotoxicity and the endoplasmic reticulum stress response in a neuronal model of vascular dementia. 2017;11:1–10. doi: 10.3389/fnins.2017.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kawahara M., Kato-Negishi M., Tanaka K. Cross talk between neurometals and amyloidogenic proteins at the synapse and the pathogenesis of neurodegenerative diseases. Metallomics. 2017;9:619–633. doi: 10.1039/c7mt00046d. [DOI] [PubMed] [Google Scholar]
- 133.Pfaender S., Grabrucker A.M. Characterization of biometal profiles in neurological disorders. Metallomics. 2014;6:960–977. doi: 10.1039/c4mt00008k. [DOI] [PubMed] [Google Scholar]
- 134.Witt B., Schaumlöffel D., Schwerdtle T. Subcellular localization of copper—cellular bioimaging with focus on neurological disorders. Int. J. Mol. Sci. 2020;21:10–12. doi: 10.3390/ijms21072341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lovell W.R.M.M.A., Robertson J.D., Teesdale W.J., Campbell J.L. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998;158:47–52. doi: 10.1177/1066896907302118. [DOI] [PubMed] [Google Scholar]
- 136.Costas-Rodríguez M., Colina-Vegas L., Solovyev N., de Wever O., Vanhaecke F. Cellular and sub-cellular Cu isotope fractionation in the human neuroblastoma SH-SY5Y cell line: proliferating versus neuron-like cells. Anal. Bioanal. Chem. 2019;411:4963–4971. doi: 10.1007/s00216-019-01871-6. [DOI] [PubMed] [Google Scholar]
- 137.Mellone M., Pelucchi S., Alberti L., Genazzani A.A., di Luca M., Gardoni F. Zinc transporter-1: a novel NMDA receptor-binding protein at the postsynaptic density. J. Neurochem. 2015;132:159–168. doi: 10.1111/jnc.12968. [DOI] [PubMed] [Google Scholar]
- 138.Kral R.F., Moutal A., Phillip M.B., Asraf H., Johnso J.W., Khanna R., Hershfinkel M., Aizenman E., Tzounopoulos T. Synaptic zinc inhibition of NMDA receptors depends on the association of GluN2A with the zinc transporter ZnT1. Sci. Adv. 2020;6:eabb1515. doi: 10.1126/sciadv.abb1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lal A. Iron in health and disease: an update. Indian J. Pediatr. 2020;87:58–65. doi: 10.1007/s12098-019-03054-8. [DOI] [PubMed] [Google Scholar]
- 140.Shindo M., Torimoto Y., Saito H., Motomura W., Ikuta K., Sato K., Fujimoto Y., Kohgo Y. Functional role of DMT1 in transferrin-independent iron uptake by human hepatocyte and hepatocellular carcinoma cell, HLF. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2006;35:152–162. doi: 10.1016/j.hepres.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 141.Carmona A., Roudeau S., Perrin L., Carcenac C., Vantelon D., Savasta M., Ortega R. Mapping chemical elements and iron oxidation states in the substantia nigra of 6-hydroxydopamine lesioned rats using correlative immunohistochemistry with proton and synchrotron micro-analysis. Front. Neurosci. 2019;13:1014. doi: 10.3389/fnins.2019.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hare D.J., Raven E.P., Roberts B.R., Bogeski M., Portbury S.D., McLean C.A., Masters C.L., Connor J.R., Bush A.I., Crouch P.J., Doble P.A. Laser ablation-inductively coupled plasma-mass spectrometry imaging of white and gray matter iron distribution in Alzheimer’s disease frontal cortex. NeuroImage. 2016;137:124–131. doi: 10.1016/j.neuroimage.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 143.Santa E.C., Madrid K.C., Arruda M.A.Z., Sussulini A. Journal of Trace Elements in Medicine and Biology Association between trace elements in serum from bipolar disorder and schizophrenia patients considering treatment effects. J. Trace Elem. Med. Biol. 2020;59 doi: 10.1016/j.jtemb.2020.126467. [DOI] [PubMed] [Google Scholar]
- 144.Bae J.R., Kim S.H. Synapses in neurodegenerative diseases. BMB Rep. 2017;50:237–246. doi: 10.5483/BMBRep.2017.50.5.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kawahara M., Kato-negishi M., Tanaka K. Amyloids: regulators of metal homeostasis in the synapse. Molecules. 2020;23:1441. doi: 10.3390/molecules25061441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.M. Kawahara, K. Tanaka, D. Mizuno, Disruption of metal homeostasis and the pathogenesis of prion diseases, prion - an overview, 2017. https://doi.org/10.5772/67327.
- 147.Kawahara M., Mizuno D., Koyama H., Konoha K. Disruption of zinc homeostasis and the pathogenesis of senile dementia, Metallomics Disruption of zinc homeostasis and the pathogenesis of senile dementia. 2014;6:209–219. doi: 10.1039/c3mt00257h. [DOI] [PubMed] [Google Scholar]
- 148.Inestrosa N.C., Cerpa W., Varela-Nallar L. Copper brain homeostasis: role of amyloid precursor protein and prion protein. IUBMB Life. 2005;57:645–650. doi: 10.1080/15216540500264620. [DOI] [PubMed] [Google Scholar]
- 149.Jesus J.R., Arruda M.A.Z. A feasible strategy based on high ultrasound frequency and mass spectrometry for discriminating individuals diagnosed with bipolar disorder and schizophrenia through ionomic profile. Rapid Commun. Mass Spectrom. 2020:1–10. doi: 10.1002/rcm.8798. [DOI] [PubMed] [Google Scholar]
- 150.Pessôa G.D.S., de Jesus J.R., Balbuena T.S., Arruda M.A.Z. Metallomics-based platforms for comparing the human blood serum profiles between bipolar disorder and schizophrenia patients. Rapid Commun. Mass Spectrom. 2020;34 doi: 10.1002/rcm.8698. [DOI] [PubMed] [Google Scholar]
- 151.Simoneau S., Rezaei H., Salès N., Kaiser-Schulz G., Lefebvre-Roque M., Vidal C., Fournier J.G., Comte J., Wopfner F., Grosclaude J., Schätzl H., Lasmézas C.I. In vitro and in vivo neurotoxicity of prion protein oligomers. PLoS Pathog. 2007;3:1175–1186. doi: 10.1371/journal.ppat.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rana M., Sharma A.K. Cu and Zn interactions with Aβ peptides: consequence of coordination on aggregation and formation of neurotoxic soluble Aβ oligomers. Metallomics. 2019;11:64–84. doi: 10.1039/c8mt00203g. [DOI] [PubMed] [Google Scholar]
- 153.Choi C.J., Kanthasamy A., Anantharam V., Kanthasamy A.G. Interaction of metals with prion protein: possible role of divalent cations in the pathogenesis of prion diseases. NeuroToxicology. 2006;27:777–787. doi: 10.1016/j.neuro.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 154.Kim Y., Connor J.R. The roles of iron and HFE genotype in neurological diseases. Mol. Asp. Med. 2020;75 doi: 10.1016/j.mam.2020.100867. [DOI] [PubMed] [Google Scholar]
- 155.World Health Organization (WHO). 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines〉, 2021. (Accessed 01 September 2021).
- 156.Food and Drug Administration (FDA). 〈https://www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19〉, 2021. (Accessed 01 September 2021).
- 157.Amais R.S., Donati G.L., Arruda M.A.Z. ICP-MS and Trace elements analysis as tools for better understanding medical conditions. Trends Anal. Chem. 2020;133 doi: 10.1016/j.trac.2020.116094. [DOI] [Google Scholar]
- 158.Degueldre C. Single virus inductively coupled plasma mass spectroscopy analysis: a comprehensive study. Talanta. 2021;228 doi: 10.1016/j.talanta.2021.122211. [DOI] [PubMed] [Google Scholar]