Studies in adult patients with kidney diseases have shown an attenuated immune response to natural coronavirus disease 2019 (COVID-19) and COVID-19 vaccination.1 The pivotal trial of BNT162b2 demonstrated a far higher neutralizing antibody titer in adolescents than in adults.2 Yet, the immunogenicity of COVID-19 vaccines in adolescent patients receiving kidney replacement therapy or those who are on immunosuppression remains unknown.
One of our aims in the Covid-19 Vaccination in Adolescents and Children study (ClinicalTrials.gov trial registration number NCT04800133) was to determine the reactogenicity and immunogenicity of the mRNA COVID-19 vaccine BNT162b2 (tozinameran, Fosun-BioNTech) in adolescent patients with kidney diseases and those who are immunocompromised and to compare their immune responses against healthy adolescents naive to COVID-19. The study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW21-157). Twenty patients aged 12 to 18 years were enrolled from 2 tertiary pediatric nephrology units in Hong Kong, and their characteristics are summarized in Table 1 . At diagnosis, 11 patients had glomerulopathy, 5 patients had hereditary nephropathy such as Alport syndrome and cystic kidney diseases, 3 patients had congenital anomalies of the kidney and urinary tract, and 1 patient had ischemic nephropathy due to perinatal asphyxia. Treatment-wise, 6 participants were on dialysis, with 4 on peritoneal dialysis and 2 on hemodialysis. Six patients received a kidney transplant from deceased donors, and 8 patients with glomerulopathy did not require kidney replacement therapy and were on immunosuppressive therapy only. All other patients were on immunosuppressive therapy except for 4 of the 6 participants on dialysis.
Table 1.
Participant profile
| Characteristic | Any kidney diseases | Dialysis | Post-kidney transplant | Immunosuppression only |
|---|---|---|---|---|
| Number of participants | 20 | 6 | 6 | 8 |
| Age, yr, median (range) | 16 (12–18) | 16.5 (15–18) | 17 (15–18) | 14.5 (12–18) |
| Sex: male:female |
9:11 |
2:4 |
4:2 |
3:5 |
|
Type ofkidneydisease, n | ||||
| Glomerulonephropathy | 11 | 2 | 1 | 8 |
| Hereditary nephropathy | 5 | 3 | 2 | 0 |
| Congenital anomalies of the kidney and urinary tract | 3 | 1 | 2 | 0 |
| Ischemic nephropathy |
1 |
0 |
1 |
0 |
|
Current immunosuppression, n | ||||
| Mycophenolate mofetil | 10 | 1 | 4 | 5 |
| Prednisolone | 14 | 2 | 6 | 6 |
| Azathioprine | 2 | 0 | 1 | 1 |
| Cyclosporine A | 1 | 0 | 1 | 0 |
| Tacrolimus | 7 | 0 | 5 | 2 |
| Everolimus | 1 | 0 | 1 | 0 |
| Hydroxychloroquine | 4 | 1 | 0 | 3 |
| None |
4 |
4 |
0 |
0 |
|
Immunosuppression within 1 yr, n (%) | ||||
| Rituximab |
2 (10) |
0 (0) |
0 (0) |
2 (25) |
|
Kidneyfunction, median (range) | ||||
| Estimated glomerular filtration rate, ml/min per 1.73 m2 a | 74.5 (25–138) | NA | 61.5 (29–77) | 102.5 (25–138) |
| Urine protein-to-creatinine ratio, SI unit | 26 (0–108) | NA | 23 (0–74) | 28 (0–108) |
| End-stage kidney disease vintage, yr |
3.75 (1–18) |
2.5 (1–5) |
7 (3–18) |
NA |
|
Blood count and comorbidities | ||||
| Hypertension, n (%) | 13 (65) | 5 (83) | 4 (67) | 4 (50) |
| Absolute lymphocyte count, ×106/ml, median (range) | 1.99 (1.57–2.97) | 1.76 (1.65–2.29) | 2.25 (1.67–2.97) | 2.18 (1.57–2.45) |
NA, not applicable.
Estimated glomerular filtration rate is estimated using the modified Schwartz equation for participants younger than 18 yr and the Chronic Kidney Disease Epidemiology Collaboration formula for those older than 18 yr.
For safety, adverse reactions such as injection site pain and fatigue were solicited with an electronic diary 7 days after each dose. Adverse reactions solicited were mostly mild (grade 1) or moderate (grade 2). One participant with hereditary nephropathy on chronic dialysis was admitted for conservative management of diarrhea 1 day after dose 1, but his condition resolved spontaneously, and he was discharged within 2 days. No disease relapse or graft rejection episodes were recorded in our participants.
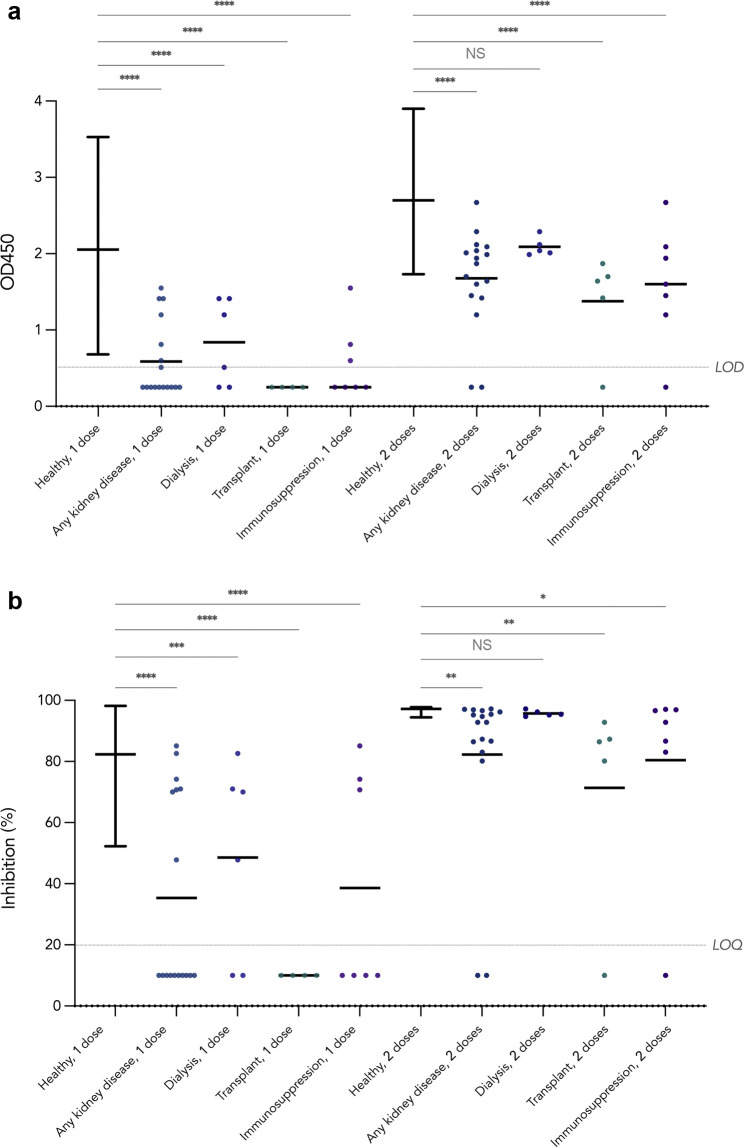
For immunogenicity, anti–severe acute respiratory syndrome coronavirus 2 spike receptor–binding domain (anti–S-RBD) IgG was evaluated using an in-house enzyme-linked immunosorbent assay and neutralizing antibodies were determined using the surrogate virus neutralization test after both doses (Supplementary Methods) in the World Health Organization severe acute respiratory syndrome coronavirus 2 reference laboratory in the School of Public Health of the University of Hong Kong.3 , 4 Participants had no history of COVID-19. After a single dose of BNT162b2, 10 of the 17 patients tested (59%) failed to seroconvert on anti–S-RBD IgG enzyme-linked immunosorbent assay, all of whom were on immunosuppressive therapy, as shown in Figure 1 . These 10 nonresponders included 4 kidney transplant recipients, 2 patients on dialysis, and 4 patients with glomerulopathy who were on immunosuppression alone. Neutralizing activity was positive in all who seroconverted. The mean anti–S-RBD IgG optical density 450 (OD450) values and neutralizing antibody inhibition percentages in patients with kidney diseases overall and each treatment subgroup were all significantly lower than those in 116 healthy adolescents aged 11 to 17 years determined using 1-way analysis of variance with the Dunnett post hoc test. After the second dose, only 2 of the 17 patients (12%) remained seronegative, and similarly, neutralizing activity was detected in all those who seroconverted; yet the mean anti–S-RBD IgG level and inhibition percentages remained lower than those in healthy adolescents, which were significant except in patients on dialysis in analysis of variance. The only 4 patients without immunosuppression in our study seroconverted after the first dose, but all had a lower anti–S-RBD IgG level than the mean in healthy adolescents. Interestingly, 2 patients who received rituximab 12 months before COVID-19 vaccination in our study for nephrotic syndrome and lupus nephritis were seronegative after dose 1, and only the patient with lupus nephritis seroconverted after dose 2 but with an IgG level below the range in healthy adolescents; these 2 patients were on mycophenolate mofetil and prednisolone, and in the patient with lupus nephritis, hydroxychloroquine as well. B cell analysis at 12 months after rituximab treatment before vaccination was available for the patient with nephrotic syndrome and revealed incompletely repleted B cells with CD19+ cell percentage at 5.67% (reference interval 7.73%–16.84%) and an absolute count of 124 cells/μL (reference interval 177–416 cells/μL) as well as a low CD19+ CD27+ memory B cell percentage of 0.12% among total lymphocytes, suggesting that incomplete B cell recovery contributed to vaccine failure.5 , 6 The other nonresponder to the second dose in our study was a transplant recipient, who was on triple maintenance immunosuppression with prednisolone, tacrolimus, and mycophenolate mofetil.
Figure 1.
Antibody response to BNT162b2. Antibody response was determined at 21 to 28 days after the first and second doses of BNT162b2 in 116 healthy adolescent participants in the Covid-19 Vaccination in Adolescents and Children study and 20 pediatric kidney disease patients. Lines on each dot plot are drawn at mean; error bars for the healthy subgroup show the range. Negative results are imputed as half the cutoff value. P values from 1-way analysis of variance with the Dunnett post hoc test are denoted by asterisks (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001) or NS (not significant; P ≥ 0.05). (a) Antispike receptor–binding domain IgG was determined by enzyme-linked immunosorbent assay. Results are shown as optical density 450 (OD450) values. LOD (lower limit of detection) is set at 0.5. (b) Neutralizing antibodies were determined using the surrogate virus neutralization test (GenScript). Results are presented as inhibition (%). LOQ (lower limit of quantification) is set at 20%.
Our results showed that adolescents with kidney diseases who are receiving immunosuppression or kidney replacement therapy could respond to 2 doses of BNT162b2 reasonably, but their antibody level was lower than that in healthy adolescents. As these patients are at an increased risk of mortality from COVID-19, it is important to protect them with a robust antibody response from vaccination.7 mRNA vaccines were found to be associated with myocarditis and pericarditis in adolescents; therefore, Hong Kong and the United Kingdom, among other places, previously recommended a single dose of BNT162b2 for adolescents.8 , 9 Our results support that immune response to a third dose of the COVID-19 vaccine should be studied in pediatric patients with kidney diseases on kidney replacement therapy or immunosuppression to better protect them from severe COVID-19.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by the Food and Health Bureau of the Hong Kong Government (COVID19F02).
Author Contributions
AL-TM, DL, EY-HC, SChi, FT-WH, W-ML, P-CT, MH-LL, SMC, JRD, and YLL were involved in the recruitment of participants. AL-TM, EY-HC, SChi, FT-WH, W-ML, P-CT, and MHLL provided clinical care. DL, WH-SW, S-MC, JRD, and YLL organized the trial. SChe and JSMP performed antibody testing. WH-SW provided statistical support. AL-TM, DL, WH-SW, SChe, JRD, JSMP, and YLL interpreted results. DL drafted the manuscript, critically revised by AL-TM, EY-HC, JRD, JSMP, and YLL. All authors reviewed and approved the final manuscript.
Footnotes
Supplementary Methods.
Supplementary Material
References
- 1.Ikizler T.A., Coates P.T., Rovin B.H., Ronco P. Immune response to SARS-CoV-2 infection and vaccination in patients receiving kidney replacement therapy. Kidney Int. 2021;99:1275–1279. doi: 10.1016/j.kint.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frenck R.W., Jr., Klein N.P., Kitchin N., et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perera R.A., Mok C.K., Tsang O.T., et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill. 2020;25:2000421. doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perera R., Ko R., Tsang O.T.Y., et al. Evaluation of a SARS-CoV-2 surrogate virus neutralization test for detection of antibody in human, canine, cat, and hamster sera. J Clin Microbiol. 2021;59:e02504–e02520. doi: 10.1128/JCM.02504-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colucci M., Carsetti R., Cascioli S., et al. B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol. 2016;27:1811–1822. doi: 10.1681/ASN.2015050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding Y., Zhou L., Xia Y., et al. Reference values for peripheral blood lymphocyte subsets of healthy children in China. J Allergy Clin Immunol. 2018;142:970–973.e8. doi: 10.1016/j.jaci.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Ng J.H., Hirsch J.S., Wanchoo R., et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall M., Ferguson I.D., Lewis P., et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann P., Pittet L.F., Finn A., et al. Should children be vaccinated against COVID-19? Arch Dis Child. 2021;107:e1. doi: 10.1136/archdischild-2021-323040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.