Research has suggested that even 3 doses of severe acute respiratory syndrome coronavirus 2 mRNA-based vaccines might be unable to elicit a sufficient immune response in immunocompromised kidney transplant recipients.1 , 2 Although the antibody response mounted by the Pfizer and AstraZeneca vaccines in immunocompetent subjects seems sufficient to neutralize the Delta variant,3 the effectiveness appears 3- to 5-fold lower than that observed against the Alpha variant.4 , 5 In addition, standard vaccination schemes are beset by low immunogenicity in immunocompromised subjects, who remain prone to develop severe coronavirus disease 2019 (COVID-19).3 The purpose of this study is to describe the kinetics of the neutralizing antibody response against the Delta strain before and after a fourth dose of the mRNA-1273 (Moderna) vaccine in kidney transplant recipients who had experienced a weak antibody response after 3 previous doses. We also assessed the correlation between this neutralizing activity and levels of IgG against the receptor-binding domain (RBD) of the spike protein.
Results
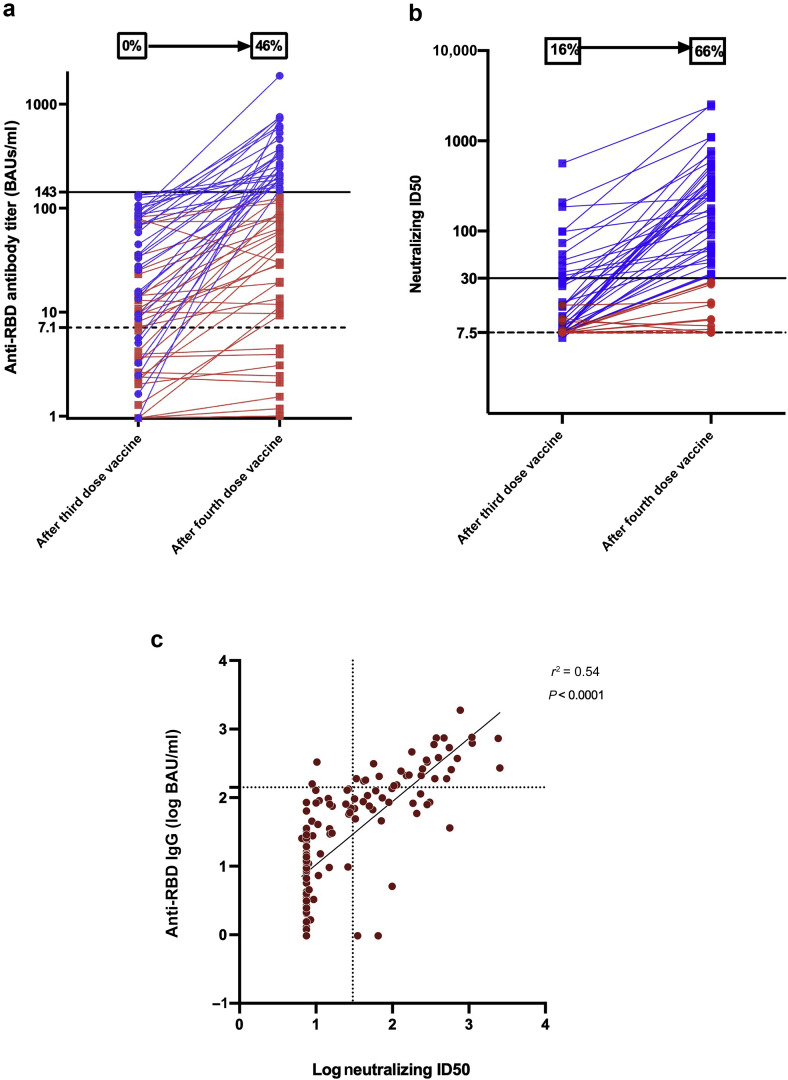
Sixty-seven kidney transplant recipients (median age 56.6 years; interquartile range [IQR] 47–64.6 years; n = 41 [61.2%] men) showed a weak humoral response after 3 doses of the mRNA-1273 vaccine and received a fourth dose (Supplementary Methods). None of these patients had a history of COVID-19 and displayed anti–nucleocapsid antibodies. The median interval from transplantation to the fourth dose was 6.1 years (IQR 2.2–11.4 years). As for immunosuppressive therapy, 97% of the study patients were being treated with calcineurin inhibitors, 82% with mycophenolate mofetil, 76% with steroids, and 18% with mammalian target of rapamycin inhibitors (Table 1 ). The median interval between the third and fourth doses was 68 days (IQR 63–82 days). After the fourth dose, the median anti-RBD titer increased significantly (P < 0.0001) from 13 binding antibody units (BAUs)/ml (IQR 2.6–66.3 BAUs/ml) to 112.5 BAUs/ml (IQR 13.5–260 BAUs/ml) (Figure 1 a). In parallel, median inhibitory dilution 50 (ID50) titers increased significantly (P = 0.0001) from <7.5 (IQR <7.5–15.1) to 47.1 (IQR <7.5–284.2). Although only 16% of patients (n = 11) harbored neutralizing antibodies against the Delta strain before the fourth injection, this percentage raised to 66% (n = 44) afterward (Figure 1b). Patients with undetectable neutralizing antibodies after the third dose were less likely to have their neutralizing capacity increased after the fourth dose (median fold change 1.1 [IQR 1–7.5] vs. 6.3 [IQR 2–15.9]; P = 0.01). Of the 9 patients who were seronegative after the third dose, only 1 displayed a strong anti-RBD IgG response (>143 BAUs/ml) and 2 were able to neutralize the Delta variant after the fourth dose. Additionally, 81% of patients with a weak immune response after the third dose displayed a strong anti-RBD IgG response after the fourth injection. Notably, 63.8% of them were able to neutralize the Delta variant after the fourth dose. Interestingly, patients under combined therapy with tacrolimus, mycophenolate mofetil, and steroids displayed lower neutralizing ID50 titers (median ID50 titers 25.6 vs. 140.6; P = 0.01). Neutralizing ID50 titers against the Delta variant were positively correlated with anti-RBD titers (Pearson r 2 = 0.54; P < 0.0001; Figure 1c). Once the anti-RBD titer exceeded 143 BAUs/ml after the fourth dose, the specificity, sensitivity, positive predictive value, and negative predictive value for detecting neutralizing activity were 92.9%, 74.3%, 93.6%, and 72.2%, respectively. Neither major adverse events nor graft rejections were observed after the fourth dose. One patient, who lacked neutralizing activity after the fourth injection (ID50 titer <7.5), developed symptomatic COVID-19 caused by the Delta variant 19 days after receiving the fourth dose.
Table 1.
General characteristics of the study patients according to the neutralizing ID50 titers after the fourth dose
| Characteristic | Entire cohort (N = 67) | Neutralizing ID50 below 30 (n = 28) | Neutralizing ID50 above 30 (n = 39) | P |
|---|---|---|---|---|
| Age, yr | 56.6 (47–64.6) | 53.1 (45–64.6) | 57.6 (50.6–64.6) | 0.29 |
| Male sex | 41 (61.2) | 12 (42.9) | 29 (74.4) | 0.01 |
| BMI, kg/m2 | 25.6 (22.3–29.6) | 24.3 (22.2–28.9) | 26.6 (22.7–29) | 0.48 |
| Comorbidities | ||||
| Cardiovascular disease | 19 (28.4) | 8 (28.5) | 11 (28.2) | 0.59 |
| Diabetes | 27 (39.3) | 10 (35.7) | 17 (43.6) | 0.61 |
| Hypertension | 53 (79.1) | 21 (75) | 32 (82) | 0.56 |
| Time from kidney transplantation, yr | 6.1 (2.2–11.4) | 5.1 (1.8–11.1) | 7.5 (2.5–11.3) | 0.32 |
| First transplantation | 54 (80.6) | 23 (82.1) | 31 (79.5) | 1 |
| Deceased donor | 55 (82.1) | 22 (78.6) | 33 (84.6) | 0.54 |
| CNI | 0.2 | |||
| Tacrolimus | 51 (76.1) | 24 (85.7) | 27 (69.2) | |
| Cyclosporine | 14 (20.9) | 3 (10.7) | 11 (28.2) | |
| No CNI | 2 (3) | 1 (3.6) | 1 (2.6) | |
| MMF/MPA | 55 (82) | 24 (85.7) | 31 (79.5) | 0.74 |
| mTOR inhibitors | 12 (17.9) | 4 (14.3) | 8 (20.5) | 0.74 |
| Steroids | 51 (76.1) | 24 (85.7) | 27 (69.2) | 0.15 |
| Tacrolimus + MMF/MPA + steroids | 35 (52.2) | 19 (67.9) | 16 (41) | 0.05 |
| Serum creatinine (μmol/l) | 132 (106.2–162.5) | 131.3 (100.2–157.8) | 132 (115.3–162.6) | 0.61 |
| History of graft rejection | 15 (22.4) | 12 (42.8) | 3 (7.7) | 0.002 |
BMI, body mass index; CNI, calcineurin inhibitor; ID50, inhibitory dilution 50; MMF, mycophenolate mofetil; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin.
Data are expressed as median (interquartile range) or n (%).
Figure 1.
(a) Anti–receptor-binding domain (RBD) antibody titers after the third and fourth doses of the mRNA-1273 (Moderna) vaccine in 67 kidney transplant recipients. A titer of >7.1 binding antibody units (BAUs)/ml was regarded as positive (dotted line), whereas a titer of <143 BAUs/ml was considered to reflect a weak humoral response (black line). The percentages of patients with titers above 143 BAUs/ml before and after the fourth dose are shown in the graph. Blue lines represent patients with a titer of >143 BAUs/ml, whereas red lines denote patients with a titer of <143 BAUs/ml after the fourth dose. (b) Neutralizing inhibitory dilution 50 (ID50) against the Delta variant after the third and fourth doses of the mRNA-1273 (Moderna) vaccine in 67 kidney transplant recipients. Neutralizing antibodies were expressed as the concentration capable of inhibiting 50% of the virus inoculum (ID50; limit of detection 7.5; dotted line). A neutralizing ID50 of >30 was considered as positive (black line). The percentages of patients showing neutralizing activity against the Delta variant before and after the fourth dose are shown in the graph. Blue lines represent patients with a neutralizing ID50 of >30, whereas red lines denote patients with a neutralizing ID50 of <30 after the fourth dose. (c) Scattergram and regression line showing a significant positive correlation between anti-RBD IgG antibody titers (log BAUs/ml) and neutralizing ID50 against the Delta strain (log ID50) before and after the fourth dose of the mRNA-1273 (Moderna) vaccine in 67 kidney transplant recipients (Pearson r2 = 0.54; P < 0.0001). The dotted line parallel to the y axis represents the cutoff for a positive neutralizing ID50 titer (30), whereas the dotted line parallel to the x axis represents the cutoff for a weak RDB IgG antibody titer (143 BAUs/ml).
Discussion
In this cohort of 67 kidney transplant recipients who showed a low immune response after 3 doses of the mRNA-1273 (Moderna) vaccine, a fourth dose significantly increased the neutralizing response against the Delta variant. Specifically, the proportion of patients harboring neutralizing antibodies against the Delta strain rose significantly from 16% to 66%. In our study, men displayed higher neutralizing ID50 titers than did women. Although this observation has no obvious pathophysiological explanations, it is in accordance with the results published by Masset et al. 6 after the third dose. Patients with a history of cellular or antibody-mediated rejection were less likely to display a neutralizing antibody response. This may be attributed to a higher burden of immunosuppression implemented in response to the rejection episodes. Only 2 published studies have focused on the effect of a fourth vaccine dose in patients who had undergone solid organ transplantation. In a small cohort of 18 solid organ transplant recipients, Alejo et al. 7 found that 8 patients with a negative or low response after the third dose developed high antibody titers after the fourth injection, a result in line with our current data.7 Moreover, 6 of the 10 patients who already had high positive titers after 3 doses showed additional boosting after the fourth injection. This report did not provide data on the neutralizing activity, which has been shown to be highly predictive of immune protection against symptomatic severe acute respiratory syndrome coronavirus 2 infection.8 In another cohort of 37 patients, Kamar et al. 9 assessed antibody response, neutralizing activity, and T-cell response; a slight improvement of humoral response was observed. In the work by Kamar et al.,9 86% of the study patients (32 of 37) had no immune response after 3 doses. Conversely, 87% of our study participants (58 of 67) showed a weak antibody response after 3 doses. This may at least in part explain the discrepancies between the 2 investigations.
This is, to our knowledge, the first study to assess the effect of a fourth dose vaccine by taking into account the neutralizing activity against the Delta variant. Previous research has focused on the response of solid organ transplant recipients after a third vaccine dose.1 , 6 Although improvements have been observed for both B- and T-cell responses, a significant proportion of nonresponders (30%–50%) was reported. In a double-blind, randomized, placebo-controlled trial of a third dose of mRNA-1273 vaccine, Hall et al. 2 found a higher neutralizing activity in the vaccine group versus the placebo group (median percent virus neutralization 71% vs. 25%, respectively). These observations suggest the existence of an “antigen dose effect,” indicating that stronger immune stimuli are required to improve vaccine immunogenicity. There have been previous reports of better immunogenicity after additional vaccine doses or higher vaccine dosing in immunocompromised patients.S1,S2 Although a consensus threshold for COVID-19 seroprotection has not been defined yet, higher antibody titers have been correlated with a reduced risk of symptomatic infections.S3,S4 These data, coupled with our findings, indicate that a fourth vaccine injection is beneficial in transplant recipients who show a weak response after 3 doses. It is nonetheless concerning that a fourth injection was still unable to elicit neutralizing antibodies against this variant in approximately one-third of our study sample. These patients should maintain strict sanitary protection measures and/or be considered as candidates for higher vaccine doses or prophylactic administration of monoclonal anti–severe acute respiratory syndrome coronavirus 2 antibodies. Although immunosuppressive modulation during vaccination could be another alternative to improve vaccine response, this approach should be weighed against the risk of developing acute rejection. No data are currently available on the cost-effectiveness of this strategy.
In summary, our data indicate that a fourth mRNA-1273 vaccine injection in kidney transplant recipients with a weak antibody response after 3 previous doses improves serum neutralization against the Delta variant.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary Material
References
- 1.Benotmane I., Gautier G., Perrin P., et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326:1063–1065. doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall V.G., Ferreira V.H., Ku T., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbaraman N. How do vaccinated people spread Delta? What the science says. https://www.nature.com/articles/d41586-021-02187-1 [DOI] [PubMed]
- 4.Planas D., Veyer D., Baidaliuk A., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 5.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masset C., Kerleau C., Garandeau C., et al. A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int. 2021;100:1132–1135. doi: 10.1016/j.kint.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alejo J.L., Mitchell J., Chiang T.P.Y., et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105:e280–e281. doi: 10.1097/TP.0000000000003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 9.Kamar N., Abravanel F., Marion O., et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA–based vaccine in recipients of a solid organ transplant. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.