Abstract
COVID-19 has affected the progress made in the prevention and treatment of tuberculosis (TB); hence, the mortality of tuberculosis has risen. Different strategies-based novel TB vaccine candidates have been developed. This study identifies strategies to overcome the limitations of Bacille Calmette-Guérin (BCG) in preventing latent infection and reactivation of TB. The latency antigen Rv0572c was selected based on the mechanism of interaction between Mycobacterium tuberculosis and its host. The rRv0572c protein was used to stimulate whole blood samples derived from patients with clinically diagnosed active TB (ATBs) or latent TB infections (LTBIs) and healthy control (HCs) donors, confirming that this protein can be recognized by T cells in patients with TB, especially LTBIs. C57BL/6 mice were used to investigate the immunogenicity of the rRv0572c protein emulsified in the liposome adjuvant dimethyldioctadecylammonium [DDA], monophosphoryl lipid A [MPLA], trehalose-6, 6′-dibehenate [TDB] (DMT). The results demonstrated that rRv0572c/DMT could boost BCG-primed mice to induce antigen-specific CD4+ T cell production and generate functional T cells dominated by antigen-specific CD8+ T cells. The rRv0572c/DMT vaccine could also trigger limited Th2 humoral immune responses. These findings suggest that rRv0572c/DMT is a potential subunit vaccine candidate that can be used as a booster vaccine for BCG.
Keywords: Tuberculosis, Subunit vaccine, DosR protein, Adjuvant, Liposome
1. Introduction
Tuberculosis (TB) is a chronic and persistent respiratory infectious disease caused by Mycobacterium tuberculosis. In 2020, there were 1.3 million deaths among HIV-negative individuals, and 21,400 deaths among HIV-positive individuals [1]. Additionally, an estimated 9.9 million individuals fell ill with TB in 2020 [1]. As the only vaccine currently licensed for TB, Bacille Calmette-Guérin (BCG) confers sufficient protective immunity to children but shows highly variable efficacy against adult pulmonary TB, ranging from 0% to 80% [2]. Moreover, immunocompromised individuals, such as patients with human immunodeficiency virus (HIV) co-infection, cannot benefit from BCG; it may even accelerate their death [3]. Importantly, with nearly 2 billion cases of latent TB infections (LTBIs) worldwide [4], BCG vaccination fails to induce ideal immune responses to latency antigens [5]. Recently, several novel TB vaccines have been developed either in clinical trials or in preclinical studies, which are mainly designed as prophylactic vaccines and used for pre-exposure administration. In most cases, these vaccines are based on target antigens recognized by the immune system during the early stage of TB infection [4], which cannot effectively prevent the establishment of dormancy and resuscitation of M. tuberculosis.
Cell trafficking between infection sites and draining lymph nodes (LN) is critical in immune dynamics. Lymphocytes conduct their immune functions independently and in collaboration with each other. Proinflammatory signals (IFN- and TNF) are secreted by certain immune cells, such as macrophages and some CD4+ T cells. Aggregation of inflammatory cells causes inflammation, gradually resulting in granuloma formation. After evading elimination by macrophages, M. tuberculosis replicates slowly in the hypoxic environment of the granuloma, resulting in the establishment of dormancy and evasion of the host innate immune responses [6]. The long-term presence of M. tuberculosis in the dormant phase is a key factor in the protracted course of TB [7]. In vitro models have attempted to simulate these features by subjecting M. tuberculosis to hypoxia [8,9] and nutrient deprivation [10] to explore the changes in M. tuberculosis gene expression during dormancy. Until now, 48 dormancy antigens have been reported to be associated with LTBIs; these antigens are encoded by the M. tuberculosis dormancy regulon (DosR). Genomics research has revealed eight functional categories of DosR genes [11]. Among them, hypothetical proteins are relatively unexplored but important. Kwon et al. reported that a novel vaccine prepared with a latent hypothetical nitroreductase rRv3131 and formulated in well-defined adjuvant GLA-SE has the potential to eliminate M. tuberculosis [12]. However, related research is scarce regarding another hypothetical protein encoded by Rv0572c within DosR.
The occurrence and progression of M. tuberculosis infection are mainly associated with cell-mediated immunity of the host. CD4+ Th1 responses play a critical role in the resistance to M. tuberculosis infection by secreting cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) [13,14]. The BCG vaccine can induce strong CD4+ Th1 responses, which confer effective protection against primary TB in children. However, recent studies have demonstrated that induction of CD8+ T cell response is critical in preventing the reactivation of LTBI [15,16]. Therefore, BCG fails to effectively control the prevalence of adult TB and LTBI. In addition, humoral responses contribute to the control of TB. Niki et al. have revealed that induction of humoral immunity should be recognized as an option for TB vaccine development because IgA antibody titers against several M. tuberculosis antigens show protection against M. tuberculosis [17]. The liposome adjuvant DMT, containing dimethyldioctadecylammonium (DDA), monophosphoryl lipid A (MPLA), and trehalose-6, 6′-dibehenate (TDB), has been found to enhance the number of antigen-specific CD8+ T cells while eliciting limited humoral responses [18].
This study sought to develop a subunit vaccine that would be effective against latent and active TB. To achieve this, the latency-associated hypothetical protein Rv0572c was screened, and its antigenicity was confirmed based on immune recognition by T cells derived from patients with LTBI and active TB (ATB). The T cell response was analyzed using recombinant ESAT6-CFP10-whole blood IFN-γ release assay (rEC-WBIA). Moreover, the recombinant protein Rv0572c was produced in E. coli, purified, emulsified in the liposome adjuvant DMT, and its immunogenicity was compared with that of BCG in a C57BL/6 mouse model.
2. Materials and methods
2.1. Cloning and purification
The gene Rv0572c was amplified by polymerase chain reaction (PCR) from the genome of the M. tuberculosis standard H37Rv strain (kindly provided by the Affiliated Heping Hospital of Changzhi Medical College, Changzhi, China) using the following primers: Rv0572c, 5′-TTCGGATCCGGTGAGCACGCCATCAAGCG-3' (forward) and 5′-ATCTCGAGTAGGTCATCGGATTGAGGTGATCGA-3' (reverse). The PCR product was first digested with restriction enzymes BamH I and Xho I (Thermo Scientific, Waltham, Massachusetts, USA) and then inserted into the corresponding sites of the prokaryotic expression vector pPROEX. The recombinant plasmid was verified by restriction enzyme digestion, and DNA sequencing was used to confirm the accuracy of the inserted fragment. The plasmid was transformed into the Escherichia coli BL21 (DE3) strain, and then the target protein was induced by incubation with 1 mM isopropyl thio-β-d-galactoside (IPTG) (Mdbio, Qingdao, China) at 37 °C for 4 h. Subsequently, the medium was removed by centrifugation, and the bacteria was resuspended in binding buffer. After processing, sonication, and centrifugation the supernatant was collected and recombinant protein with an N-terminal His-tag was purified using Ni-NTA-metal ion affinity chromatography (Sangon, Shanghai, China) under denaturation conditions according to the manufacturer's protocol. The specificity of the purified protein was identified by western blotting. Briefly, the purified protein was separated in a 15% SDS-PAGE gel, transferred onto a NC membrane (Biosharp, Hefei, China) and probed using mouse anti-His mAb (Tiangen, Beijing, China) and goat anti-mouse IgG antibody HRP conjugate (Proteintech, Wuhan, China). The recombinant protein was dialyzed against sterilized phosphate buffered saline (PBS) with a urea concentration gradient ranging from 0 to 8 M for 36 h, at 6 h intervals. The protein was lyophilized and diluted in PBS using pyrogen-free reagents and stored at −20 °C in aliquots. Ni-NTA agarose gel 6FF (Henghuibio, Beijing, China) was used as an affinity chromatography filler to adsorb endotoxin. Residual endotoxin contamination in the solution was verified to be below 0.1 EU/mL. Protein concentration was determined using a BCA Protein Assay kit (Cwbiotech, Beijing, China).
2.2. Patient participants
Population-based experiment was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Cancer Hospital of the Anhui University of Science and Technology (No. T20171108-25). Written informed consent was obtained from all the participants. Patients with ATBs (n = 12) (median age 42.7, range 23–67; male/female: 7/5) or LTBIs (n = 13) (median age 35.9, range 20–65; male/female: 8/5), and healthy control donors (HCs) (n = 10) (median age 39.2, range 19–67; male/female: 5/5) were enrolled by the Affiliated Cancer Hospital of the Anhui University of Science and Technology. All donors were selected through history taking, questionnaire investigations, and general checkups. ATBs and LTBIs were classified based on the tuberculin skin test (TST) and diagnostic standards. To avoid any effects of BCG vaccination on the diagnostic results, all TB infections were further confirmed by IFN-γ release assays (IGRAs) using a diagnostic kit for T cells infected with M. tuberculosis (TB-IGRA) (Wantai, Beijing, China), which has higher sensitivity and specificity than TST [19]. Healthy controls had no history of prior M. tuberculosis contact or exposure and showed negative results in both TST and TB-IGRA. All participants were seronegative for HIV infection (Listed in Supplementary File 1).
2.3. Whole blood IFN-γ assay
Peripheral blood was collected from each donor in heparinized tubes and seeded (500 μL/well) in a sterile 24-well plate. 20 μL rRv0572c (5 μg/mL) and the same amount of rEC were used as stimulators in each well, followed by incubation at 37 °C for 18–24 h. As a comparison, we selected the most widely studied latent protein, rRv0081, rRv2032, and rRv2628 (kindly provided by the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China), and performed human peripheral blood stimulation at the same time. Phytohemagglutinin (PHA) (Solarbio, Beijing, China) was used as a positive control, and PBS served as a negative control. The supernatant (200 μL) from each well was collected and stored at −20 °C for further analysis. The IFN-γ concentration was determined using a human IFN-γ enzyme-linked immunosorbent assay (ELISA) kit (MultiSciences, Hangzhou, China) with a detection limit of 4 pg/mL. The assay was set arbitrarily at 100 pg/mL for the PHA-WBIA. The antigen-specific concentration of IFN-γ in each sample was calculated by subtracting the value of the PBS control from the value of the sample stimulated with the target antigen.
2.4. Experimental animals
Mouse experiments were performed in accordance with the guidelines of the Chinese Council on Animal Care and approved by the Institutional Animal Care and Use Committee at the Anhui University of Science and Technology (No. 20180313-019). Specific pathogen-free female C57BL/6 mice at 6–8 weeks of age (Cavion Experimental Animal Co. Ltd, Changzhou, China) were housed in separate cages and fed with standard laboratory chow. Mice were divided into five groups namely PBS, DMT, rRv0572c/DMT, BCG, and rRv0572c/DMT, with 6 mice in each group.
2.5. Preparation of adjuvants and vaccines
Adjuvant DMT (100 μL/dose) comprised 250 μg of DDA (Sigma-Aldrich, St. Louis, Missouri, USA), 25 μg of MPLA (Avanti Polar Lipids, Alabaster, Alabama, USA), and 50 μg of TDB (Avanti Polar Lipids), initially dissolved in chloroform/methanol (9:1, v/v). The organic solvent was then completely removed using a gentle stream of N2 and the resulting lipid membrane was hydrated with 1 mL of 10 mM sterile Tris-buffer (pH 7.4) at 60 °C for 1 h, mixed uniformly at 10-min intervals, collected, and labeled as DMT. Finally, the protein subunit vaccine was prepared by emulsifying 20 μg of rRv0572c in 100 μL adjuvant DMT. High-performance liquid chromatography (HPLC) was conducted to evaluate the adjuvant content, while dynamic light scattering (DLS) was conducted for particle size analysis, and SDS-PAGE for protein analysis.
2.6. Immunization protocol
Mice were immunized with 200 μL of rRv0572c/DMT mixture, injected subcutaneously (s.c.), three times at 3-week intervals. To confirm the immune-enhancing activity of rRv0572c/DMT mixture in BCG-primed mice, a second group of mice were vaccinated with BCG (approximately 1.2 × 106 CFUs in 200 μL PBS, injected s.c. at the proximal end of the tail) once before immunization with rRv0572c/DMT mixture (injected twice at 3-week intervals). The control groups were injected with BCG alone (positive control), PBS alone (negative control), or DMT alone (adjuvant control). BCG and PBS were inoculated during the first vaccination, and DMT was injected s.c. three times at 3-week intervals (Fig. S1).
2.7. Antibody titers
Three weeks after the final immunization, the rRv0572c-specific endpoint titers for IgG1, IgG2a, and total IgG antibodies in the sera from vaccinated mice were detected using ELISA. Briefly, microtiter plates were pre-coated with 100 μL rRv0572c (5 μg/mL) protein or bovine serum albumin (BSA) (irrelevant control) in carbonate/bicarbonate buffer (pH 9.6) overnight at 4 °C and blocked with 1% BSA in PBST (PBS plus 0.1% Tween 20) at 37 °C for 1 h. Serial dilutions of serum samples were added to microtiter plates, followed by addition of horseradish peroxidase-conjugated goat anti-mouse IgG (Biosharp), IgG1 (Proteintech), or IgG2a (Proteintech). 3, 3′, 5, 5′-tetramethylbenzidine (TMB) substrate (Solarbio) was used for visualization, and the enzymatic reaction was terminated using 1 N H2SO4. Plates were read at 450 nm using a microtiter plate ELISA reader, with a detection limit of 450 nm set at 0.05. Antibody titers in each mouse were obtained and expressed as reciprocal endpoint titers by comparison with the PBS control when the value of P/N ≥ 2.1. The results are shown as the mean (±SEM) log10 endpoint titers per group and as the ratio of IgG2a/IgG1 (n = 6).
2.8. Th1 cytokines secreted by splenocytes
The spleens were aseptically removed from all immunized mice (n = 6/group). Splenocytes were prepared in RPMI-1640 medium (Sangon) supplemented with 10% FBS (Gibco, Grand Island, New York State, USA), counted, seeded in 24-well microtiter plates (2.5 × 106 cells/well), and then incubated with rRv0572c protein (10 μg/mL) at 37 °C for 24 h to detect IL-2, and for 72 h to detect IFN-γ and TNF-α. RPMI-1640 medium was used as a negative control, while purified protein derivative (PPD; 10 μg/mL) served as a positive control. Culture supernatants from six separate wells were harvested and stored at −20 °C for further assays. Detection was carried out using mouse IFN-γ, TNF-α, and IL-2 ELISA kits (MultiSciences) according to the manufacturer's instructions with detection limits of 0.83 pg/mL, 1.63 pg/mL, and 0.54 pg/mL, separately. The results are shown as mean ± SD (pg/mL) per group (n = 6).
2.9. Flow cytometry
Splenocytes from six individual mice per group were counted and seeded (3.5 × 106 cells) in polystyrene tubes, stimulated with rRv0572c protein (10 μg/mL) in 250 μL complete RPMI. Subsequently, 1 μL brefeldin A (BFA) (0.75 mg/mL)/Monesin mixture (0.35 mg/mL) (MultiSciences) was added into each well and then incubated at 37 °C for 4–6 h. PPD (10 μg/mL) was a positive control, and complete RPMI1640 medium served as a negative control. 1 μL phorbol-12-myristate-13-acetate (PMA) (12.5 μg/mL)/Ionomycin mixture (0.25 mg/mL) (MultiSciences) was used to monitor cell responses. After incubation, the cell surface markers were stained using 5 μL anti-mouse CD3ε FITC (0.5 μg/test) (MultiSciences), 5 μL anti-mouse CD4 PerCP-Cy5.5 (0.06 μg/test) (MultiSciences), and 5 μL anti-mouse CD8 APC-Cy7 (0.25 μg/test) (MultiSciences) mAbs in the dark at 15 °C–25 °C for 15 min. For intracellular cytokine staining (ICS), cells were fixed and permeabilized using the fixation (FIX) & permeabilization (PERM) Medium A (MultiSciences) following the manufacturer's protocol. The intracellular cytokines were stained with 5 μL anti-mouse IFN-γ PE (0.125 μg/test) (MultiSciences) and 5 μL anti-mouse IL-4 APC (0.5 μg/test) (MultiSciences) mAbs as previously described or overnight at 4 °C, washed with PERM buffer, and then resuspended in FACS buffer. The absolute number of PPD- or Rv0572c-specific CD4+ and CD8+ T cell subsets secreting single cytokines was determined using the LSRII multicolor flow cytometer (BD Biosciences, Franklin lakes, New Jersey, USA). The results were analyzed using FlowJo software (Version 7.6.1; Tree Star, Inc, Ashland, Oregon, USA) and are shown as the mean ± SEM per group (n = 6).
2.10. Cytokine mRNA expression in lung tissues
The lungs were aseptically removed from immunized mice, and total RNA was extracted using TRIzol (Sangon). The revertaid first strand cDNA synthesis kit (Thermo Scientific) was used for cDNA synthesis. Primers used for reverse transcription were based on the sequences of IFN-γ, TNF-α, IL-10, and inducible nitric oxide synthase (iNOS). The primers used were IFN-γ, 5′-AGTGGCATAGATGTGGAA-3' (forward) and 5′-CTGATGGCCTGATTGTC-3' (reverse); TNF-α, 5′-GGCGGTGCCTATGTCTC-3' (forward) and 5′-TCCACTTGGTGGTTTGTGA-3' (reverse); IL-10, 5′-GGTTGCCAAGCCTTATCGGA-3' (forward) and 5′-ACCTGCTCCACTGCCTTGCT-3' (reverse); iNOS, 5′-GAGCGAGTTGTGGATTGTC-3' (forward) and 5′-AGGGCTTGGCTGAGTGA-3' (reverse). Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using a SYBR Green kit (Tiangen) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. The primers for GAPDH were 5′-GACCAGGTTGTCTCCTGCGACTTC-3' (forward) and 5′-GGTGGGTGGTCCAGGGTTTCTTAC-3' (reverse). PBS served as the negative control. The 2ΔΔCT was used to calculate the differences in mRNA expression between groups. The results are shown as the fold increase in cytokine expression per group (n = 6) compared with PBS. At the same time, anti-IFN-γ (Proteintech), anti-TNF-α (Proteintech), anti-iNOS (Proteintech), and anti-IL-10 (Proteintech) were used for western blotting, with β-actin (Antgene, Wuhan, China) as a loading control.
2.11. Statistical analysis
Nonparametric Mann-Whitney U test and one-way ANOVA analysis were performed using GraphPad Prism (Version 7.0; GraphPad Software, Inc, San Diego, California, USA). Statistical significance was set at P < 0.05.
3. Results
3.1. Purification and identification of recombinant protein
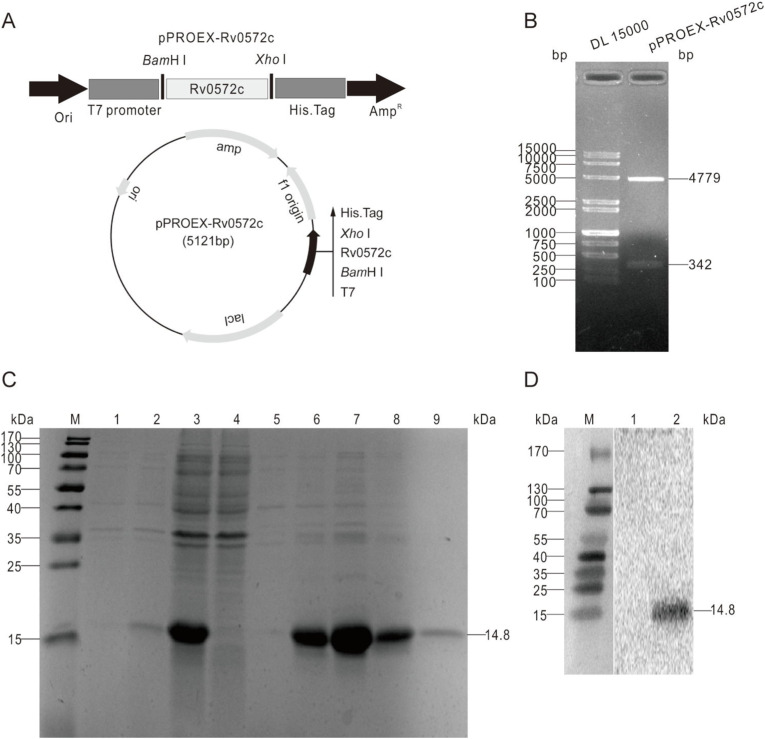
The encoding sequence of Rv0572c (342 bp) was amplified using PCR from the chromosomal DNA of the M. tuberculosis standard H37Rv strain and cloned into the corresponding sites of the prokaryotic expression vector pPROEX predigested with BamH I and Xho I. The successful construction of the recombinant plasmid pPROEX-Rv0572c (Fig. 1 A) was confirmed by restriction enzyme digestion (Fig. 1B). The subunit protein rRv0572c with its N-terminal His-tag was efficiently expressed as an inclusion body in the pPROEX-Rv0572c-transformed E. coli BL21 (DE3) strain, and the purification was performed under denaturing conditions. The purified protein was revealed using SDS-PAGE analysis as a single major band with a molecular weight of 14.8 kDa (Fig. 1C, lanes 6 and 7). The major expected band was identified as His-tagged Rv0572c by western blotting using a mouse anti-His mAb (Fig. 1D).
Fig. 1.
Production, purification, and identification of the recombinant protein rRv0572c. (A) The gene encoding Rv0572c was inserted into pPROEX, to construct prokaryotic expression recombinant plasmid pPROEX-Rv0572c. (B) The recombinant plasmid was confirmed by restriction enzyme digestion with BamH I and Xho I. (C) Expression and purification process of the protein rRv0572c were monitored and confirmed via 15% SDS-PAGE analysis. Lane M, protein marker (kDa); lane 1, recombinant E. coli BL21 (DE3) strain without the induction of IPTG; lane 2, recombinant E. coli BL21 (DE3) strain induced with IPTG for 4 h; lane 3, supernatant of recombinant bacteria induced by IPTG after sonication; lane 4, effluence after binding to the Ni-NTA column; lane 5, effluence after washing with 8 M urea-binding solution; lane 6–9, eluted with elution buffer containing 100 mM imidazole. (D) Identification of purified protein rRv0572c by western blotting with anti-His mouse mAb. Lane M, protein marker (kDa); lane 1, pPROEX empty vector; lane 2, purified rRv0572c probed with specific antibodies.
3.2. Rv0572c-specific IFN-γ levels in human whole blood detected by WBIA
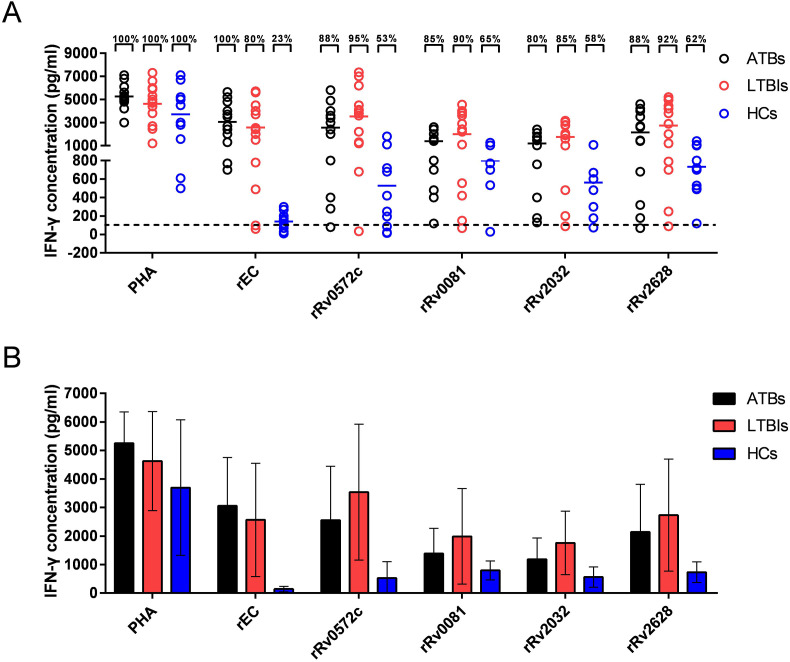
To confirm the immunogenicity of the recombinant protein rRv0572c, 12 cases of ATBs, 13 cases of LTBIs, and HCs were screened based on TST and the established diagnostic standard, then their peripheral blood was analyzed via WBIA. There were no significant differences in the levels of IFN-γ detected following PHA stimulation (positive control) among the three groups (Fig. 2 A and B). The level of IFN-γ induced by rEC in ATBs was higher than that in LTBIs (Fig. 2A and B). After stimulation with rRv0572c protein, a higher level of IFN-γ was observed in LTBIs (95%) than in ATBs (88%), whereas the frequency recognized by T cells in the HCs was only 53% relative to the positive (PHA) control (Fig. 2A and B). rRv0081, rRv2032, and rRv2628 elicited higher antigen-specific IFN-γ in ATBs and LTBIs, compared with HCs, but not as far as rRv0572c (Fig. 2A and B). In addition, when stimulated by rRv0572c, the antigen-specific IFN-γ elicited by ATBs and LTBIs showed significant difference, which was not achieved by rRv0081, rRv2032 and rRv2628 (Fig. 2A and B).
Fig. 2.
IFN-γ response to the subunit protein in human subjects. Whole blood samples were obtained from ATBs (n = 12), LTBIs (n = 13), and HCs (n = 10), then stimulated with 20 μL of rRv0572c for 18–24 h. PHA served as a positive control with a cut-off value of 100 pg/mL rEC (ESAT6-CFP10), rRv0081, rRv2032, and rRv2628 for comparison with rRv0572c. A commercial ELISA kit was used to detect the concentration of IFN-γ in the supernatants. For (A) scatter diagram, each spot represents an individual sample, the dotted line represents the cut-off value for a positive response to proteins, and the median values are shown as horizontal lines. The percentages and numbers reflect the amount and dispersion of individual IFN-γ release values; 100% is shown when no IFN-γ value falls below the dotted line. For (B) histogram, the results are shown as the mean ± SD (pg/mL) in each group (n = 6).
3.3. Antibody responses induced by DMT-adjuvanted rRv0572c
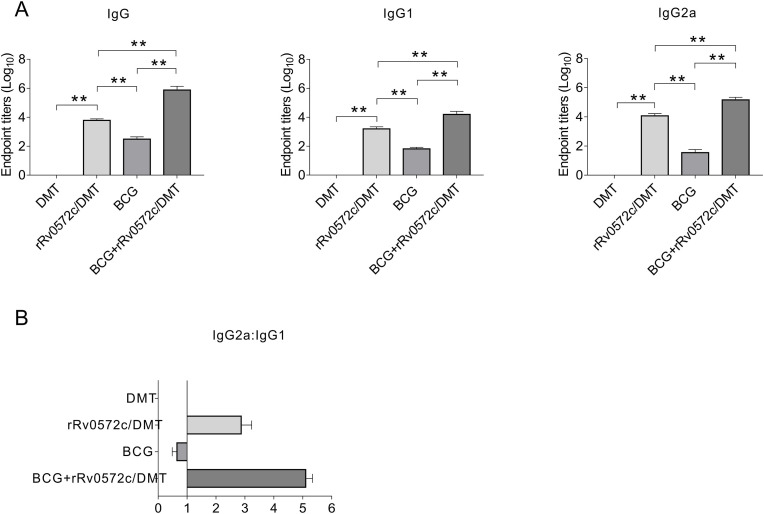
The immunogenicity of DMT-adjuvanted rRv0572c was evaluated in C57BL/6 mice. Rv0572c-specific antibodies, including IgG1, IgG2a, and total IgG, were detected using ELISA in the 9th week following vaccinations, as described in the Materials and methods. As expected, these antibodies were not induced in either the PBS control (data not shown) or DMT alone group (Fig. 3 A and B). Mice vaccinated with a combination of BCG and rRv0572c/DMT exhibited notably higher levels of Rv0572c-specific IgG1, IgG2a, and total IgG antibodies than the BCG alone or rRv0572c/DMT alone group (P < 0.01, Fig. 3A). In addition, the ratios of IgG2a/IgG1 in the rRv0572c/DMT group and the BCG + rRv0572c/DMT group (the ratios of IgG2a/IgG1 > 1) were much higher than those in the BCG group (the ratios of IgG2a/IgG1 < 1), indicating a switch of the IgG subclass toward a Th1-biased response (Fig. 3B). Notably, the ratio of IgG2a/IgG1 observed in the BCG + rRv0572c/DMT group was significantly higher than that in the rRv0572c/DMT group (Fig. 3B).
Fig. 3.
Rv0572c-specific IgG1, IgG2a, and total IgG antibodies in the immunized mice. Nine weeks after immunization, sera were collected from each C57BL/6 mouse in all groups (n = 6). The titers of rRv0572c-specific antibodies, total IgG, and subclasses in the sera were determined using ELISA. The results are shown as the mean (±SEM) (A) log10 endpoint titer and (B) ratio of IgG2a/IgG1 in the immunized mice. Comparable results were obtained from two independent experiments. *P < 0.05, **P < 0.01.
3.4. Rv0572c-specific Th1 cytokines induced by splenocytes
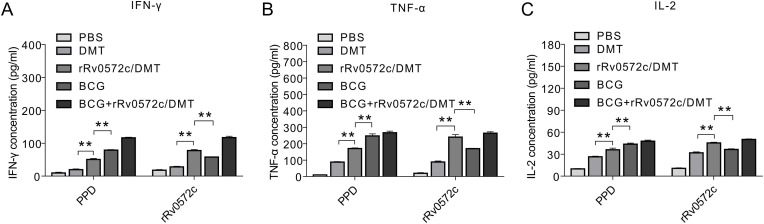
Splenocytes from the PBS control group produced the lowest levels of Th1 cytokines, including IFN-γ, TNF-α, and IL-2, when stimulated with either PPD or rRv0572c protein (Fig. 4 A–C). In contrast, the highest levels of Th1 cytokines were secreted in splenocytes from the BCG + rRv0572c/DMT group (Fig. 4A–C). As expected, the levels of the Th1 cytokines induced in the rRv0572c/DMT group were significantly higher than in mice vaccinated with DMT alone (P < 0.01, Fig. 4A–C). In addition, the mice immunized with BCG secreted higher levels of PPD-specific IFN-γ, TNF-α, and IL-2 than those induced by rRv0572c/DMT (P < 0.01, Fig. 4A–C). Higher Th1 cytokine response to rRv0572c restimulation was observed in mice vaccinated with rRv0572c/DMT rather than in the BCG-vaccinated group (P < 0.01, Fig. 4A–C).
Fig. 4.
Levels of Th1 cytokines produced by splenocytes of different immunized mice. Nine weeks after immunization, splenocytes were obtained from each C57BL/6 mouse of all groups. Cells (2.5 × 106 cells/well) were seeded in 24-well microtiter plates and incubated with rRv0572c (10 μg/mL), PPD (10 μg/mL, positive control), or media alone (negative control) at 37 °C for 24 h to detect (C) IL-2, and for 72 h to detect (A) IFN-γ and (B) TNF-α. The cytokine concentrations in the culture supernatant were detected using commercial ELISA kits. The results are shown as the mean ± SD (pg/mL) in each group (n = 6). This experiment was repeated twice with comparable results. *P < 0.05, **P < 0.01.
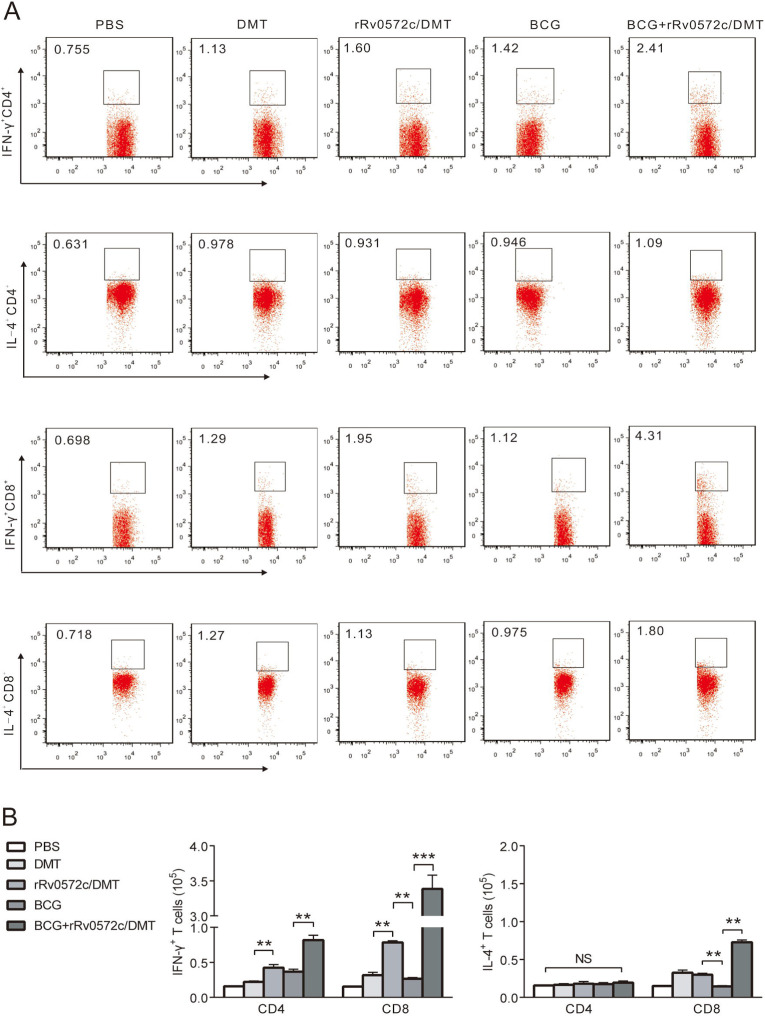
3.5. Functional T cell subsets response to PPD and rRv0572c
To further clarify the immune mechanism related to protection, functional IFN-γ and IL-4 secreted by T cell subsets in response to rRv0572c in the splenocytes of mice from different vaccinated groups were detected by multicolor flow cytometry (Fig. 5 ). Overall, the Rv0572c-specific T cells found in the splenocytes of mice were mainly IFN-γ+ CD4+ and CD8+ subtypes (Fig. 6 ). BCG vaccine evoked mainly IFN-γ+ CD4+ T cells (Fig. 6). Compared with Rv0572c-specific CD4+ T cells, more Rv0572c-specific CD8+ T cells were elicited in the splenocytes of mice vaccinated with DMT-adjuvanted rRv0572c or immunized with BCG-primer and rRv0572c/DMT-booster, and the values were significantly higher than in mice injected with adjuvant DMT alone (Fig. 6). Notably, Rv0572c-specific IFN-γ+ CD8+ T cells predominated in mice from the BCG+rRv0572c/DMT group (Fig. 6). Interestingly, more IFN-γ+ CD4+ T cells were found in the BCG+rRv0572c/DMT group than in the BCG group (Fig. 6). In addition, the splenocytes from mice in the three vaccinated groups also produced a small number of Rv0572c-specific IL-4+ CD8+ T cells (Fig. 6).
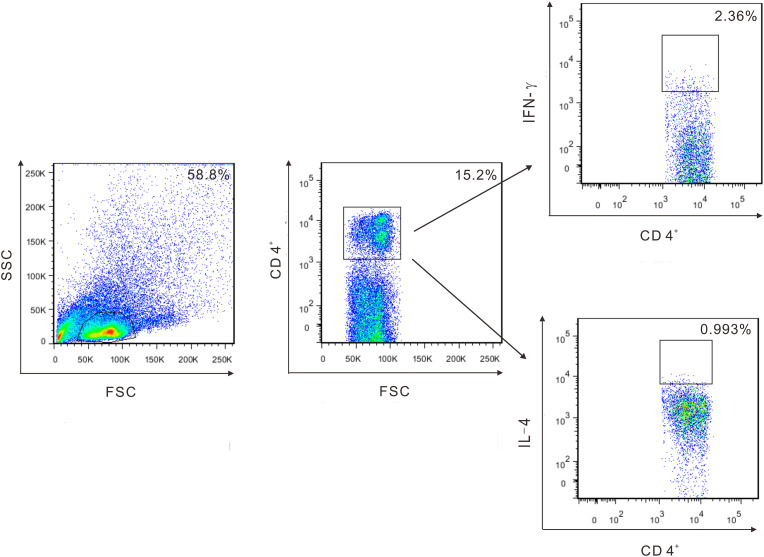
Fig. 5.
CD4 and CD8 antigen-specific T cell subsets were identified by ICS as IFN-γ+ CD4+, IL-4+ CD4+, IFN-γ+ CD8+, and IL-4+ CD8+. Figure shows gating strategy using CD4+ T cell subsets as an example.
Fig. 6.
Frequency of rRv0572c-specific IFN-γ-and IL-4-producing CD4+ or CD8+ T cells detected using multicolor flow cytometry. Nine weeks after the immunization, splenocytes were obtained from each mouse in the different vaccinated groups (n = 6). Cells (3.5 × 106 cells/tube) were stimulated with rRv0572c (10 μg/mL), PPD (10 μg/mL, positive control), or media alone (negative control), as described in the Materials and methods section. Multicolor flow cytometry was used to detect (A, B) the intracellular cytokine profiles for IFN-γ and IL-4 in individual cells by gating for CD4+ or CD8+ T cells. The absolute number of antigen-specific T cells expressing the cytokines are shown as mean (±SEM) in each group (n = 6). The experiment was repeated twice with comparable results. *P < 0.05, **P < 0.01, ***P < 0.001.
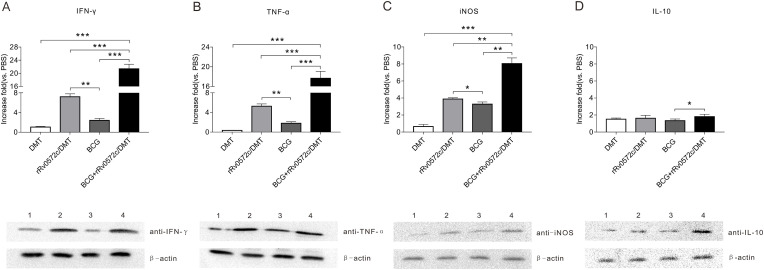
3.6. Rv0572c-specific cytokines mRNA expressed in lung tissues
There were differences in the levels of cytokines, including IFN-γ, TNF-α, and iNOS, in the lung tissues of mice receiving different vaccinations (Fig. 7 A–C). The PBS control group had the lowest levels of Rv0572c-specific cytokines among all groups (data not shown). Administration of the adjuvant DMT alone resulted in a low frequency of Rv0572c-specific cytokines in lung tissues (Fig. 7A–C). In contrast, the highest levels of Rv0572c-specific cytokines were observed in lung tissues from the BCG + rRv0572c/DMT group (Fig. 7A–C). The levels of cytokines including IFN-γ, TNF-α, and iNOS, evoked in responses to rRv0572c, in the lung tissues were significantly higher in the rRv0572c/DMT group than in the BCG control (P < 0.05, Fig. 7A–C). In addition, Rv0572c-specific IL-10 was expressed at a relatively low level in the lung tissues from different vaccination groups (P > 0.05, Fig. 7D). The result of western blotting is consistent with the statistical results.
Fig. 7.
Expression of Rv0572c-specific cytokines mRNA in the lung tissues of vaccinated mice. Nine weeks after immunization, the lungs were collected from each mouse in all groups (n = 6). RNA samples were exacted using TRIzol, and the expression of Rv0572c-specific mRNAs for (A) IFN-γ, (B) TNF-α, (C) iNOS, and (D) IL-10 in the lung tissues of different immunized mice was analyzed using qRT-PCR assay using a SYBR Green kit, as described in the Methods section. PBS was used as a negative control. The results are shown as the fold increase in cytokine expression in different immunized groups compared with PBS. The protein expression was shown below the statistical results. Lane 1, DMT group; lane 2, rRv0572c/DMT group; lane 3, BCG group; lane 4, BCG + rRv0572c/DMT group. This experiment was repeated twice with comparable results. *P < 0.05, **P < 0.01, ***P < 0.001.
4. Discussion
The BCG vaccine is a live attenuated vaccine derived from Mycobacterium bovis and was first used in 1921. Chemotherapy and BCG have been widely used to treat and prevent TB. However, a study has demonstrated that prior exposure to environmental mycobacteria may inhibit the activity of BCG, and BCG does not confer sufficient protection in areas with high TB prevalence [20]. Hence, TB remains a leading infectious disease worldwide. There are 14 TB vaccine candidates currently under clinical development, and many more in preclinical trials [4]. Most novel TB vaccines target the early secreted antigens [4]. However, as M. tuberculosis adapts to become persistent, it downregulates many genes characteristically expressed during the early stages of infection. Notably, M. tuberculosis can control phagosomal maturation, prevent phagosomal fusion with lysosomes, and reduce phagosome acidification [21,22]. Interestingly, M. tuberculosis, which survives in macrophages, remains active in granulomas induced by hypoxia and nutrient starvation [23]. Furthermore, resuscitation-promoting factor (rpf) secreted by M. tuberculosis during latency can stimulate the persistence and reactivation of M. tuberculosis [24]. HIV infection, renal failure, and immunosuppressive treatments are considered risk factors for reactivation [25]. It has been demonstrated that 10%–15% of M. tuberculosis in LTBIs will reactivate during their lifetime [26]. Thus, we selected antigens associated with latency from a potential antigenic reservoir to prevent the reactivation of M. tuberculosis during latency.
The secretion of IFN-γ in response to specific antigens has diagnostic significance. In this study, commonly used latency proteins, rRv0081, rRv2032, and rRv2628 were selected to measure the antigen-specific IFN-γ secretion ability of rRv0572c. Thereinto, transcriptional regulator Rv0081 was verified as one of the central hubs of dormancy [23]. A previous study demonstrated that Rv2032 could induce a strong immune response against M. tuberculosis, which shows the increase in the percentage of CD4+ and CD8+ memory T cells in TB infected individuals [27]. Pandey et al. reported that rRv2628 stimulation caused an increase in Th1/Th2 ratio in both contacts and ATBs, thereby stimulating a strong IFN-γ+ T cell response along with accentuation of memory T cells and other protective cytokines [28]. In our study, compared with Rv0081, Rv2032, and Rv2628, the highest level of IFN-γ secreted by T cells of LTBIs indicated that the latency-associated antigen Rv0572c is the most promising latency antigen when used to diagnose LTBIs.
The importance of adjuvants in TB vaccines has become increasingly prominent. Among the currently reported TB vaccines under development, ID93+GLA-SE, GamTBvac, H56:IC31, and M72/AS01E are vaccines comprised recombinant protein mixed in adjuvant [4]. In addition, Heterologous prime boosting is a powerful vaccination approach against M. tuberculosis. Of the 14 TB vaccines currently under development, GamTBvac is intended for use as a BCG booster vaccine to prevent TB, while both Ad5 Ag85A and MTBVAC were observed more immunogenicity in individuals previously vaccinated with BCG [4].
In this study, DMT-adjuvanted subunit vaccine was prepared and its immunogenicity was evaluated. Overall, the antibody endpoint titers reflected that the subunit vaccine elicited a Th1-biased immune response. Furthermore, the major proinflammatory cytokines, such as IFN-γ, TNF-α, and IL-2, have been widely recognized to confer Th1 protective responses against TB. Congruently, the levels of these three cytokines increased significantly in the rRv0572c/DMT and BCG + rRv0572c/DMT vaccinated groups compared to the controls.
Although functional T cells may be involved in immune protection against TB, effective biomarkers of functional T cells associated with vaccine-induced protection remain to be defined. A previous study has demonstrated that the frequencies of antigen-specific polyfunctional T cells that are triple-positive (IFN-γ+ IL-2+ TNF-α+), double-positive (IFN-γ+ or IL-2+), or IFN-γ single-positive reflect the immunogenicity of vaccine candidates expressing target antigens [29]. Our results demonstrated that the DMT-adjuvanted rRv0572c increased the numbers of CD4+ and CD8+ T cells producing IFN-γ or IL-4. IL-4 reflects the level of humoral immune response, and IFN-γ is the major cytokine involved in the cellular immune response. The BCG + rRv0572c/DMT group mainly induced a cellular immune response, accompanied by a limited humoral immune response. Notably, these immune responses were also observed in mice injected with the adjuvant DMT alone, but the overall level was less obvious.
Of all groups, the BCG + rRv0572c/DMT group showed the highest levels of iNOS. Following stimulation by iNOS, macrophages secrete nitric oxide (NO), which can kill intracellular M. tuberculosis, as previously reported [30]. The BCG vaccine only elicited low levels of the cytokine IL-10 and slightly higher expression levels were observed in the groups with DMT. Similar to IL-4, IL-10 can also reflect the level of humoral immune response. Therefore, DMT liposome-adjuvanted rRv0572c can enhance the Th1 immune response induced by BCG, with elicitation of a limited humoral immune response. One study has reported that the mRNA expression of antigen-specific cytokines in the lungs of mice immunized with BCG prime-pcD685A booster increased more significantly than BCG when adopting subcutaneous immunization [31]. Combined with our results, the antigen-specific expression of pulmonary cytokine mRNA can also be enhanced via subcutaneous immunization.
Previous studies in mouse models with T cell deficiency [32] or aerosol infections [33] have confirmed that T cells contribute to the immune response against TB. Furthermore, CD4+ T cells are essential for the control of M. tuberculosis infections [34]. However, some studies have revealed that CD8+ T cells play a substantial role in protection against TB in experimental animal models [15,35]. CD8+ cytotoxic T cells secrete perforin, granzyme, and granulysin, resulting in the lysis of M. tuberculosis infected host cells, and even directly kill the existing M. tuberculosis in macrophages. Moreover, CD8+ T cells can induce apoptosis in infected target cells by molecules such as Fas or tumor necrosis factor-R (TNF-R) family related cell-death receptors. Furthermore, activated CD8+ T cells can secrete Th1 cytokines, such as IFN-γ and TNF-α. In some cases, CD8+ T cells also secrete IL-2. As a traditional vaccine against TB, the BCG vaccine mainly elicits the immune response of CD4+ T cells, which limits the protection of M. tuberculosis infection.
In the development of a novel TB vaccine, the DMT liposome is a remarkable adjuvant as it mediates an antigen-specific CD8+ T cell immune response [18]. Recent data showed that DMT liposome adjuvant not only provided protection comparable to BCG to resist primary M. tuberculosis infection but also enhanced the ability of BCG to prevent the reactivation of LTBI [36]. As a cationic liposome, DDA can provide an antigen depot effect, eliciting a sustained immune response [37]. Extracellular degradation of RNA and DNA can be suppressed by DDA, facilitating nucleic acid entry into endosomal compartments and subsequent binding to Toll-like receptors (TLRs) located there. As the major pattern recognition receptor (PRR) of pathogen-associated molecular patterns (PAMPs), activated TLRs not only elicit innate immune responses [38] but also lead to dendritic cells (DCs) migration from the infected tissue to the draining lymph node where naïve T cells are stimulated to promote adaptive immunity [39,40]. In addition, MPLA is derived from the detoxified lipopolysaccharide (LPS) of Salmonella minnesota R595 and can be used as an immunostimulant for the TLR4 receptor to stimulate the production of IFN-γ and IL-2, inducing a Th1 immune response [41,42]. TDB binds to C-type lectin Mincle receptors, which can activate macrophages and DCs, and then combine with the FcεRI γ-chain/spleen tyrosine kinase/caspase recruitment domain family member 9 (FCRγ/Syk/Card9) signal peptide cascade [43,44], producing proinflammatory cytokines such as IFN-γ and iNOS [45]. Several studies have shown that CD4+ and CD8+ T cells mediate effector function by secreting the cytokines IFN-γ or TNF-α [46,47]. Furthermore, CD4+ T cells can augment IFN-γ cytokine release at the early stage of M. tuberculosis infection and CD8+ T cells play a critical role in the secretion of IFN-γ during the latent and chronic stages of TB.
As M. tuberculosis is an intracellular pathogen that survives in macrophages, cellular immunity is predominant against M. tuberculosis infection. In addition, humoral immunity also participates in defense against M. tuberculosis [48]. An ideal adjuvant should induce both humoral and cellular immune responses. However, Th1 cellular immunity is responsible for protective immunity in TB [49] and Th2 cytokines, which reflect the humoral immune response and mediate delayed type hypersensitivity, accompanied by immunopathological injury [50]. Appropriately, the DMT liposome adjuvant showed sufficient cellular immunity and limited humoral immunity.
This study has certain limitations, such as the efficacy of the vaccine was not tested using an infection or challenge model. Nandakumar et al. reported a protein subunit vaccine using M. tuberculosis alanine-proline-rich antigen (Apa) as a candidate booster antigen for BCG-primed mouse model, thereby significantly improving the wanning immunity of BCG and reducing M. tuberculosis burdens post-challenge [51]. Ji et al. revealed that HG856A, created by inserting two copies of the ESAT6 gene into the AccI endonuclease restriction site of the Ag85A gene within the pVAX1 plasmid, boosting BCG-primed mice a more robust protection, with the lung and spleen bacillary loads being significantly decreased compared to the BCG group [52]. The above studies adopted the heterologous vaccine boosting strategy of BCG priming and achieved promising results. In addition to using the same immunization strategy, we adopted the antigen Rv0572c whose expression was upregulated during the latency period, and used a novel adjuvant DMT, which highlights the innovation of this study. The main emphasis here is on the stronger immunogenicity induced by rRv0572c mixed with adjuvant DMT as a booster vaccine for BCG-primed. To further explore the protective potential and therapeutic efficacy of the DMT-adjuvanted rRv0572c subunit vaccine, further experiments are warranted.
5. Conclusion
In this study, the subunit vaccine rRv0572c/DMT prepared with the DosR regulon hypothetical protein rRv0572c and combined with the liposome adjuvant DMT can enhance the immunogenicity of BCG, preventing the reactivation of M. tuberculosis during latency. Therefore, the subunit vaccine rRv0572c/DMT is a promising vaccine candidate for adult TB and should be evaluated in further preclinical trials.
Ethic approval
Population-based experiment was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Cancer Hospital of the Anhui University of Science and Technology (No. T20171108-25). Mouse experiments were performed in accordance with the guidelines of the Chinese Council on Animal Care and approved by the Institutional Animal Care and Use Committee at the Anhui University of Science and Technology (No. 20180313-019).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
CRediT authorship contribution statement
Lirong Mao: Project administration, Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Software, Writing – original draft, Visualization, Funding acquisition. Lifa Xu: Project administration, Conceptualization, Methodology, Formal analysis, Data curation, Resources, Validation, Writing – review & editing, Funding acquisition. Xiaochun Wang: Project administration, Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Validation, Writing – review & editing, Funding acquisition. Yingru Xing: Methodology, Investigation, Formal analysis, Data curation, Resources, Software. Jian Wang: Conceptualization, Methodology, Supervision. Yanpeng Zhang: Investigation, Formal analysis, Data curation, Visualization. Wei Yuan: Methodology, Investigation, Formal analysis, Data curation, Visualization, Software. Jianpeng Du: Investigation, Data curation, Visualization. Zilun Shi: Data curation, Visualization, Resources, Software. Jilei Ma: Conceptualization, Methodology. Jingyan Zhang: Conceptualization, Methodology, Resources. Xiaohan Zhang: Investigation, Data curation, Visualization. Xinping Wang: Investigation, Data curation, Visualization.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported by the University Natural Science Research Project of Anhui Province (grant numbers KJ2015A093 and KJ2016A211), the Graduate Student Innovation Fund grant number 2020CX2082, and the Scientific Research Project of Shanxi Provincial Health Commission grant number 2022152. We appreciate the generosity of the Affiliated Heping Hospital of Changzhi Medical College for providing the standard H37Rv strain and the First Affiliated Hospital of Zhengzhou University for providing rRv0081, rRv2032, and rRv2628. We thank the Affiliated Cancer Hospital of the Anhui University of Science and Technology for the technical assistance with flow cytometry and analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tube.2022.102186.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization . 2021. Global tuberculosis report 2021. 978-92-4-003702-1. [Google Scholar]
- 2.Foster M., Hill P.C., Setiabudiawan T.P., Koeken V.A.C.M., Alisjahbana B., van Crevel R. BCG-induced protection against Mycobacterium tuberculosis infection: evidence, mechanisms, and implications for next-generation vaccines. Immunol Rev. 2021;301:122–144. doi: 10.1111/imr.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelidou A., Diray-Arce J., Conti M.G., Smolen K.K., van Haren S.D., Dowling D.J., et al. BCG as a case study for precision vaccine development: lessons from vaccine heterogeneity, trained immunity, and immune ontogeny. Front Microbiol. 2020;11:332. doi: 10.3389/fmicb.2020.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 2020. Global tuberculosis report 2020. 978-92-4-001313-1. [Google Scholar]
- 5.Lin M.Y., Geluk A., Smith S.G., Stewart A.L., Friggen A.H., Franken K.L., et al. Lack of immune responses to Mycobacterium tuberculosis DosR regulon proteins following Mycobacterium bovis BCG vaccination. Infect Immun. 2007;75:3523–3530. doi: 10.1128/IAI.01999-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peddireddy V., Doddam S.N., Ahmed N. Mycobacterial dormancy systems and host responses in tuberculosis. Front Immunol. 2017;8:84. doi: 10.3389/fimmu.2017.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirofski L.A., Casadevall A. The state of latency in microbial pathogenesis. J Clin Invest. 2020;130:4525–4531. doi: 10.1172/JCI136221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson E.J.R., Abidi A.A., Arrieta-Ortiz M.L., Aguilar B., Yurkovich J.T., Kaur A., et al. Intricate genetic programs controlling dormancy in Mycobacterium tuberculosis. Cell Rep. 2020;31:107577. doi: 10.1016/j.celrep.2020.107577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prosser G., Brandenburg J., Reiling N., Barry C.E., Wilkinson R.J., Wilkinson K.A.Ⅲ. The bacillary and macrophage response to hypoxia in tuberculosis and the consequences for T cell antigen recognition. Microb Infect. 2017;19:177–192. doi: 10.1016/j.micinf.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betts J.C., Lukey P.T., Robb L.C., McAdam R.A., Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 11.Singh S., Saraav I., Sharma S. Immunogenic potential of latency associated antigens against Mycobacterium tuberculosis. Vaccine. 2014;32:712–716. doi: 10.1016/j.vaccine.2013.11.065. [DOI] [PubMed] [Google Scholar]
- 12.Kwon K.W., Kim W.S., Kim H., Han S.J., Hahn M.Y., Lee J.S., et al. Novel vaccine potential of Rv3131, a DosR regulon-encoded putative nitroreductase, against hypervirulent Mycobacterium tuberculosis strain K. Sci Rep. 2017;7:44151. doi: 10.1038/srep44151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper A.M. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Garra A., Redford P.S., McNab F.W., Bloom C.I., Wilkinson R.J., Berry M.P. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 15.Behar S.M., Woodworth J.S.M., Wu Y. Next generation: tuberculosis vaccines that elicit protective CD8+ T cells. Expert Rev Vaccines. 2007;6:441–456. doi: 10.1586/14760584.6.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G.B., Yang F., He X., Liu Z., Pi J., Zhu Y.Z., et al. Anti-tuberculosis (TB) chemotherapy dynamically rescues Th1 and CD8+ T effector levels in Han Chinese pulmonary TB patients. Microb Infect. 2020;22:119–126. doi: 10.1016/j.micinf.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Niki M., Suzukawa M., Akashi S., Nagai H., Ohta K., Inoue M., et al. Evaluation of humoral immunity to Mycobacterium tuberculosis-specific antigens for correlation with clinical status and effective vaccine development. J Immunol Res. 2015;2015:527395. doi: 10.1155/2015/527395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordly P., Agger E.M., Andersen P., Nielsen H.M., Foged C. Incorporation of the TLR4 agonist monophosphoryl lipid A into the bilayer of DDA/TDB liposomes: physico-chemical characterization and induction of CD8+ T-cell responses in vivo. Pharm Res. 2011;28:553–562. doi: 10.1007/s11095-010-0301-9. [DOI] [PubMed] [Google Scholar]
- 19.Diel R., Loddenkemper R., Nienhaus A. Predictive value of interferon-γ release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63–75. doi: 10.1378/chest.11-3157. [DOI] [PubMed] [Google Scholar]
- 20.Brandt L., Feino Cunha J., Weinreich Olsen A., Chilima B., Hirsch P., Appelberg R., et al. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun. 2002;70:672–678. doi: 10.1128/iai.70.2.672-678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundaramurthy V., Korf H., Singla A., Scherr N., Nguyen L., Ferrari G., et al. Survival of Mycobacterium tuberculosis and Mycobacterium bovis BCG in lysosomes in vivo. Microb Infect. 2017;19:515–526. doi: 10.1016/j.micinf.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Poirier V., Av-Gay Y. Mycobacterium tuberculosis modulators of the macrophage's cellular events. Microb Infect. 2012;14:1211–1219. doi: 10.1016/j.micinf.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Kundu M., Basu J. Applications of transcriptomics and proteomics for understanding dormancy and resuscitation in Mycobacterium tuberculosis. Front Microbiol. 2021;12:642487. doi: 10.3389/fmicb.2021.642487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arroyo L., Marín D., Franken K.L.M.C., Ottenhoff T.H.M., Barrera L.F. Potential of DosR and Rpf antigens from Mycobacterium tuberculosis to discriminate between latent and active tuberculosis in a tuberculosis endemic population of Medellin Colombia. BMC Infect Dis. 2018;18:26. doi: 10.1186/s12879-017-2929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin P.L., Flynn J.L. Understanding latent tuberculosis: a moving target. J Immunol. 2010;185:15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reviono R., Saptawati L., Redhono D., Suryawati B. Good agreement between an interferon gamma release assay and tuberculin skin tests in testing for latent tuberculosis infection among HIV-infected patients in Indonesia. J Kor Med Sci. 2019;34:e259. doi: 10.3346/jkms.2019.34.e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh S., Sharma M., Chaudhry A., Sharma S. Rv2626c and Rv2032 activate TH1 response and downregulate regulatory T cells in peripheral blood mononuclear cells of tuberculosis patients. Comp Immunol Microbiol Infect Dis. 2019;62:46–53. doi: 10.1016/j.cimid.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Pandey K., Singh S., Bhatt P., Medha, Sharma M., Chaudhry A., et al. DosR proteins of Mycobacterium tuberculosis upregulate effector T cells and down regulate T regulatory cells in TB patients and their healthy contacts. Microb Pathog. 2019;126:399–406. doi: 10.1016/j.micpath.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 29.Yu Q., Wang X.C., Fan X.L. A new adjuvant MTOM mediates Mycobacterium tuberculosis subunit vaccine to enhance Th1-type T cell immune responses and IL-2+ T cells. Front Immunol. 2017;8:585. doi: 10.3389/fimmu.2017.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang K., Wu Y.J., Xie H.P., Li M., Ming S.Q., Li L.Y., et al. Macrophage-mediated inflammatory response decreases mycobacterial survival in mouse MSCs by augmenting NO production. Sci Rep. 2016;6:27326. doi: 10.1038/srep27326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan K., Liang J., Teng X., Wang X., Zhang J., Yuan X., et al. Comparison of BCG prime-DNA booster and rBCG regimens for protection against tuberculosis. Hum Vaccines Immunother. 2014;10:391–398. doi: 10.4161/hv.26969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.North R.J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973;7:166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- 33.Orme I.M., Collins F.M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983;158:74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mogues T., Goodrich M.E., Ryan L., LaCourse R., North R.J. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prezzemolo T., Guggino G., La Manna M.P., Di Liberto D., Dieli F., Caccamo N. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front Immunol. 2014;5:180. doi: 10.3389/fimmu.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma J.L., Teng X.D., Wang X.C., Fan X.L., Wu Y.Q., Tian M.P., et al. A multistage subunit vaccine effectively protects mice against primary progressive tuberculosis, latency, and reactivation. EBioMedicine. 2017;22:143–154. doi: 10.1016/j.ebiom.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henriksen-Lacey M., Bramwell V.W., Christensen D., Agger E.M., Andersen P., Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Contr Release. 2010;142:180–186. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 38.Brubaker S.W., Bonham K.S., Zanoni I., Kagan J.C. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzgerald K.A., Kagan J.C. Toll-like receptors and the control of immunity. Cell. 2020;180:1044–1066. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palm N.W., Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 41.Peek L.J., Middaugh C.R., Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60:915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulrich J.T., Myers K.R. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharmaceut Biotechnol. 1995;6:495–524. [PubMed] [Google Scholar]
- 43.Ishikawa E., Ishikawa T., Morita Y.S., Toyonaga K., Yamada H., Takeuchi O., et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strasser D., Neumann K., Bergmann H., Marakalala M.J., Guler R., Rojowska A., et al. Syk kinase-coupled C-type lectin receptors engage protein kinase C-δ to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohanraj M., Sekar P., Liou H.H., Chang S.F., Lin W.W. The mycobacterial adjuvant analogue TDB attenuates neuroinflammation via Mincle-Independent PLC-γ1/PKC/ERK signaling and microglial polarization. Mol Neurobiol. 2019;56:1167–1187. doi: 10.1007/s12035-018-1135-4. [DOI] [PubMed] [Google Scholar]
- 46.Maurya S.K., Aqdas M., Das D.K., Singh S., Nadeem S., Kaur G., et al. A multiple T cell epitope comprising DNA vaccine boosts the protective efficacy of Bacillus Calmette–Guérin (BCG) against Mycobacterium tuberculosis. BMC Infect Dis. 2020;20:677. doi: 10.1186/s12879-020-05372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serbina N.V., Flynn J.L. CD8(+) T cells participate in the memory immune response to Mycobacterium tuberculosis. Infect Immun. 2001;69:4320–4328. doi: 10.1128/IAI.69.7.4320-4328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rijnink W.F., Ottenhoff T.H.M., Joosten S.A. B-cells and antibodies as contributors to effector immune responses in tuberculosis. Front Immunol. 2021;12:640168. doi: 10.3389/fimmu.2021.640168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Budak F., Uzaslan E.K., Cangür S., Göral G., Oral H.B. Increased pleural soluble Fas ligand (sFasL) levels in tuberculosis pleurisy and its relation with T-helper type 1 cytokines. Lung. 2008;186:337–343. doi: 10.1007/s00408-008-9107-5. [DOI] [PubMed] [Google Scholar]
- 50.Seah G.T., Scott G.M., Rook G.A. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181:385–389. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]
- 51.Nandakumar S., Kannanganat S., Dobos K.M., Lucas M., Spencer J.S., Amara R.R., et al. Boosting BCG-primed responses with a subunit Apa vaccine during the waning phase improves immunity and imparts protection against Mycobacterium tuberculosis. Sci Rep. 2016;6:25837. doi: 10.1038/srep25837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji P., Hu Z.D., Kang H., Yuan Q., Ma H., Wen H.L., et al. Boosting BCG-primed mice with chimeric DNA vaccine HG856A induces potent multifunctional T cell responses and enhanced protection against Mycobacterium tuberculosis. Immunol Res. 2016;64:64–72. doi: 10.1007/s12026-015-8674-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.