Abstract
Background
Immunotherapy is a novel hotspot for the treatment of pancreatic adenocarcinoma (PAAD). However, potential biomarkers which could identify the inflamed tumor microenvironment (TME) are urgently required.
Methods
In the present study, we measured the levels of B7-H3, B7-H4, and major tumor-infiltrating immune cells (TIICs) using bioinformatics analyses and immunohistochemistry (IHC) staining on PAAD samples represented in the tissue microarray (TMA) format. Statistical analysis and figures exhibition were performed using R 4.1.0, SPSS 26.0, and GraphPad Prism 6.0.
Results
B7-H3 and B7-H4 were up-regulated in PAAD compared with para-tumor tissues, and their expression exhibited no tight correlation in PAAD tissues. B7-H3 and B7-H4 were lowly expressed in well-differentiated PAAD tissues and correlated with poorly differentiated grades. Besides, single B7-H3 or B7-H4 expression exhibited limited prognostic value, but co-deficiency of B7-H3 and B7-H4 predicted a better prognosis in PAAD. Moreover, co-deficiency of B7-H3 and B7-H4 indicated immuno-hot tumors with high CD8 + T cell infiltration.
Conclusions
Overall, combined B7-H3 and B7-H4 expression is a promising stratification strategy to assess prognosis and immunogenicity in PAAD, which could be used as a novel classifier in clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-09294-w.
Keywords: B7-H3, B7-H4, prognosis, Immune cell infiltration, pancreatic cancer
Background
Pancreatic adenocarcinoma (PAAD) is one of the most fatal malignant tumors in the world, featured with dreadful invasiveness, powerful proliferative potential, and poor clinical outcome. The early diagnosis of PAAD is rare on account of the obscure symptoms, and the morbidity of PAAD has been significantly elevated over the last few decades. Although PAAD does not account for a high proportion of all patients, survival is lowest for cancers of the pancreas (10%) [1]. With the rapid progress of emerging therapeutic programs, immunotherapy is becoming a promising hotspot for the treatment of PAAD [2]. It has been revealed that the response for immunotherapy is low in PAAD due to its non-inflamed tumor microenvironment (TME) [3–5]. Growing evidence indicates that tumor progression and therapeutic response were critically affected by host immune response, which depends on the abundance of tumor-infiltrating immune cells (TIICs) in TME [6, 7]. Thus, potential biomarkers which could identify the abundance of TIICs in TME of PAAD are urgently required in clinical practice.
In recent years, the roles of co-stimulatory B7 family molecules in regulating tumor immunity have been widely concerned, specially programmed cell death ligand 1 (PD-L1), also named as B7-H1 [8]. PD-L1 expression is usually correlated with inflamed TME phenotype and predicts a high response rate to immunotherapy in the clinic [9, 10]. In addition to B7-H1, B7-H3 and B7-H4 are becoming promising hotspots [11]. According to previous reports, B7-H3 and B7-H4 are significantly up-regulated in PAAD tissues compared with non-tumor or normal pancreas tissues [12, 13]. Besides, co-expressed or mutually-exclusive patterns of B7 molecules predict inflamed or non-inflamed TME in multiple human cancers [14, 15]. However, the correlation between B7-H3 and B7-H4 expression and TIICs abundance as well as the predictive value of combined B7-H3 and B7-H4 in assessing prognosis has not been investigated yet.
In this research, we first analyzed the expression of B7-H3 and B7-H4 as well as their associations between clinic-pathological features in PAAD. Besides, the prognostic values and immuno-correlations of B7-H3, B7-H4, and combined expression were also evaluated. As result, we found that B7-H3 and B7-H4 were upregulated in PAAD tissues and correlated with advanced differentiated grades. Moreover, co-deficiency of B7-H3 and B7-H4 in PAAD predicted better clinical outcomes and identifies high CD8 + T cell infiltration. Overall, co-deficiency of B7-H3 and B7-H4 is a promising prognostic and immunogenic biomarker in PAAD.
Methods
Acquisition of TCGA data
Normalized RNA-sequencing (RNA-seq) data and corresponding clinical information of PAAD samples in the Cancer Genome Atlas (TCGA) database were downloaded from the UCSC Xena website (https://xenabrowser.net/datapages/). Patients with missing or insufficient data were excluded from this research. Finally, a total of 178 tumor samples were retained for further analysis.
Analyses of the GEPIA and CPTAC databases
GEPIA (http://gepia.cancer-pku.cn/) was an interactive website based on the TCGA and GTEx databases and used for RNA expression analyses [16]. In the present study, the GEPIA website was used to explore the expression levels of B7-H3 and B7-H4 in PAAD and adjacent pancreas tissues. In addition, to further compare the differential expressions of B7-H3 and B7-H4 at protein levels, the proteome data of the CPTAC dataset (http://ualcan.path.uab.edu/analysis-prot.html) were also used for differential analyses of B7-H3 and B7-H4 [17].
Immune infiltration analysis
Tumor Immune Estimation Resource (TIMER) database is an online tool for systematic analysis of immune cell infiltration across diverse cancer types from TCGA [18]. We evaluated the correlation of B7-H3 & B7-H4 expressions with the infiltration of main types of immune cells, including B cells, CD8 + T cells, CD4 + T cells, neutrophils, macrophages, and dendritic cells (DCs).
The relative abundance of more types of infiltrating immune cells was analyzed using the xCell algorithm (https://xcell.ucsf.edu/), an emerging tool to estimate the abundance of 64 immune and stromal cell types based on gene expression profiles [19]. Pre-calculated infiltrating data of TIICs corresponding to TCGA-PAAD samples were downloaded from the xCell website.
Clinical samples
The PAAD tissue microarray (TMA, Cat. no HPanA150CS04) was purchased from Outdo BioTech (Shanghai, China). A total of 120 PAAD and 30 paired para-tumor tissues were included in the TMA. Detailed clinic-pathological characteristics of these patients were also provided by Outdo BioTech. Ethical approval for the use of the TMA was granted by the Clinical Research Ethics Committee (Outdo BioTech).
Immunohistochemistry
Immunohistochemistry (IHC) staining was performed on the TMA of PAAD tissues. The primary antibodies used in the research were as follows: anti-B7-H3 (1:8000 dilution, Cat. no ab219648, Abcam, Cambridge, UK), anti-B7-H4 (1:50 dilution, Cat. no ab252438, Abcam, Cambridge, UK), and anti-CD8 (Ready-to-use, Cat. no PA067, Abcarta, Suzhou, China). Antibody staining was visualized using diaminobenzidine (DAB) and hematoxylin counterstain, and stained TMA was scanned using Aperio Digital Pathology Slide Scanners.
Semi-quantitative assessment
A total of 104 TMA points were retained for further analysis after the exfoliated points were removed. All stained points were independently assessed by two senior pathologists. For semi-quantitative evaluation of B7-H3 and B7-H4 staining, the percentage of positively stained tumor cells was scored as 0–4: 0 (< 1%), 1 (1–5%), 2 (6–25%), 3 (26–50%) and 4 (> 50%). The staining intensity was scored as 0–3: 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). The immune-reactivity score (IRS) equals the percentages of positive cells multiplied with staining intensity. For semi-quantitative evaluation of CD8 staining, the infiltration level of CD8 + immune cells was evaluated by estimating the percentage of cells with strong intensity of membrane staining in the stroma cells [20].
Statistical analysis
Statistical analysis and figures exhibition were performed using R 4.1.0, SPSS 26.0, and GraphPad Prism 6.0. Most of the data were analyzed by Student’s t-test, Mann–Whitney test, and one-way ANOVA. Kaplan–Meier survival plots were generated with survival curves compared by log-rank test. The Chi-square test was used to assess differences in clinic-pathological features between groups with different risks. For all analyses, differences were deemed statistically significant when P-value was less than or equal 0.05.
Results
B7-H3 and B7-H4 are up-regulated in PAAD compared with para-tumor tissues
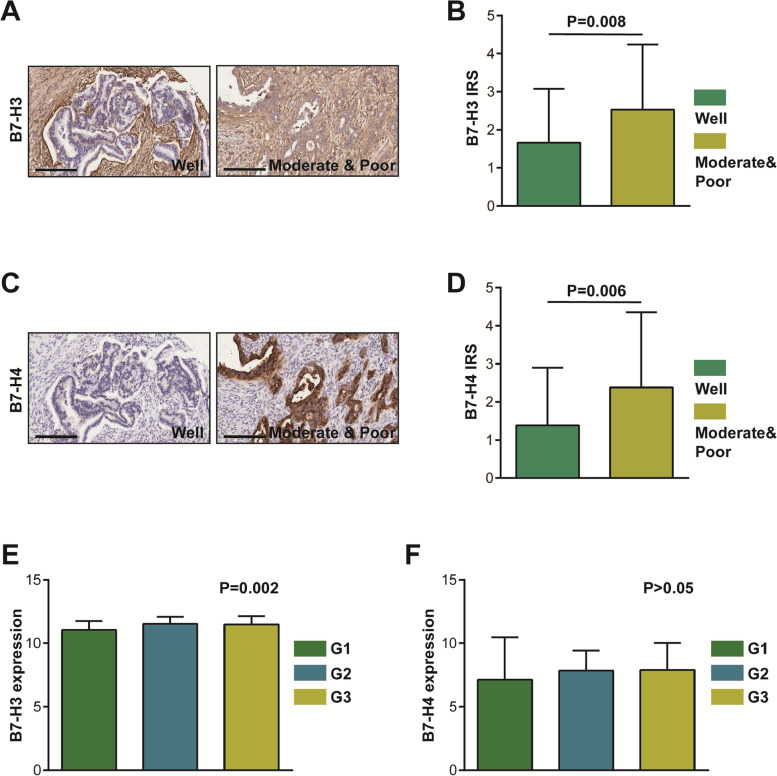
As described previously, several research groups reported that B7-H3 and B7-H4 are up-regulated in multiple cancers [21, 22]. In the GEPIA and CPTAC databases, B7-H3 was upregulated in PAAD tissues, while B7-H4 showed no difference between tumor and para-tumor tissues (Figure S1A-D). We also assessed B7-H3 and B7-H4 expression based on IHC staining. As shown in Fig. 1A, the immuno-reactivity of B7-H3 was mostly localized to the cytomembrane of tumor cells and tumor stroma. After the semi-quantitative analysis, we found that the IRS of B7-H3 in PAAD tissues was significantly higher than para-cancerous tissues (Fig. 1B). Similar to B7-H3, the immuno-reactivity of B7-H4 was also localized to the cytomembrane of tumor cells and but not stroma (Fig. 1C). Besides, the expression of B7-H4 was notably up-regulated in PAAD tissues compared with para-cancerous tissues (Fig. 1D). We also evaluated the correlation between B7-H3 and B7-H4 expression, and the results showed that the protein expression of B7-H3 and B7-H4 had no obvious correlation (Fig. 1E). However, in the TCGA database, B7-H3 mRNA was positively correlated with B7-H4 mRNA (Fig. 1F). Overall, these data suggest that the expression of B7-H3 and B7-H4 proteins are up-regulated in PAAD tissues and have no notable correlation.
Fig. 1.
Expression levels of B7-H3 and B7-H4 PAAD tissues. A Representative microphotographs revealing low B7-H3 expression in para-tumor tissues and low, medium, and high B7-H3 expression in tumor tissues using IHC staining. Brown, B7-H3. Blue, haematoxylin. Bar = 200 μm. B7-H3 was mostly localized to the cytomembrane of tumor cells and tumor stroma. B The semi-quantitative analysis of B7-H3 in tumor and para-tumor tissues. B7-H3 was significantly up-regulated in tumor tissues compared with para-tumor tissues. C Representative microphotographs revealing low B7-H4 expression in para-tumor tissues and low, medium, and high B7-H4 expression in tumor tissues using IHC staining. Brown, B7-H4. Blue, haematoxylin. Bar = 200 μm. B7-H4 was mostly localized to the cytomembrane of tumor cells but not tumor stroma D The semi-quantitative analysis of B7-H4 in tumor and para-tumor tissues. B7-H4 was significantly up-regulated in tumor tissues compared with para-tumor tissues. E Correlation between B7-H3 and B7-H4 expression in the TMA cohort. No obvious correlation was found between B7-H3 and B7-H4 expression. F Correlation between B7-H3 and B7-H4 mRNA expression in the TCGA database. B7-H3 was positively correlated with B7-H4 expression
B7-H3 and B7-H4 are lowly expressed in well-differentiated PAAD tissues
Next, the associations between clinic-pathological features and B7 molecules expression were evaluated in the current patients’ cohort. As shown in Table 1, the expression levels of B7-H3 and B7-H4 were not associated with gender, age, T stage, N stage, M stage, and clinical stage. However, these two B7 molecules were significantly associated with differentiation (Table 1). We next compared the expression levels of B7-H3 and B7-H4 in well-differentiated and moderate & poor-differentiated groups, and the results exhibited that B7-H3 and B7-H4 were notably downregulated in well-differentiated PAAD tissues (Fig. 2A-D). Besides, in the TCGA database, B7-H3 was significantly correlated with advanced differentiated grades (Fig. 2E). Although B7-H4 tended to be upregulated with advanced differentiated grades, the difference was not statistically significant (Fig. 2F). Overall, deficiency of B7-H3 and/or B7-H4 identifies well-differentiated tumors in PAAD.
Table 1.
Association between B7-H3 & B7-H4 expression and clinic-pathological parameters in PAAD
| Clinic-pathological parameters | Cases | B7-H3 expression | χ2 value | P value | B7-H4 expression | χ2 value | P value | ||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||||
| Gender | |||||||||
| Female | 46 | 24 | 22 | 0.156 | 0.693 | 24 | 22 | 0.698 | 0.404 |
| Male | 58 | 28 | 30 | 35 | 23 | ||||
| Age | |||||||||
| ≤ 60 | 47 | 25 | 22 | 0.349 | 0.554 | 22 | 25 | 3.439 | 0.064 |
| > 60 | 57 | 27 | 30 | 37 | 20 | ||||
| Differentiation | |||||||||
| Well | 65 | 39 | 26 | 5.455 | 0.020 | 42 | 23 | 6.292 | 0.012 |
| Moderate & poor | 34 | 12 | 22 | 13 | 21 | ||||
| T stage | |||||||||
| T1-2 | 29 | 17 | 12 | 0.829 | 0.363 | 19 | 10 | 1.466 | 0.226 |
| T3-4 | 56 | 27 | 29 | 29 | 27 | ||||
| N stage | |||||||||
| N0 | 48 | 25 | 23 | 0.157 | 0.692 | 28 | 20 | 0.009 | 0.925 |
| N1 | 54 | 26 | 28 | 31 | 23 | ||||
| M stage | |||||||||
| M0 | 98 | 51 | 47 | / | 0.205* | 58 | 40 | / | 0.083* |
| M1 | 6 | 1 | 5 | 1 | 5 | ||||
| Clinical stage | |||||||||
| 1-2A | 46 | 25 | 21 | 0.624 | 0.430 | 27 | 19 | 0.130 | 0.719 |
| 2B-4 | 58 | 27 | 31 | 32 | 26 | ||||
Note: *P value was calculated by Fisher test
Fig. 2.
Expression levels of B7-H3 and B7-H4 in variously differentiated PAAD tissues. A Representative microphotographs revealing low B7-H3 expression in well-differentiated tissues and high B7-H3 expression in moderate and poor-differentiated tissues using IHC staining. Brown, B7-H3. Blue, haematoxylin. Bar = 200 μm. B The semi-quantitative analysis of B7-H3 in variously differentiated PAAD tissues. B7-H3 was significantly up-regulated in moderate and poor-differentiated tissues compared with well-differentiated tissues. C Representative microphotographs revealing low B7-H4 expression in well-differentiated tissues and high B7-H4 expression in moderate and poor-differentiated tissues using IHC staining. Brown, B7-H4. Blue, haematoxylin. Bar = 200 μm. D The semi-quantitative of B7-H4 in variously differentiated PAAD tissues. B7-H4 was significantly up-regulated in moderate and poor-differentiated tissues compared with well-differentiated tissues. E B7-H3 mRNA expression was various in differently differentiated PAAD tissues in the TCGA database. F B7-H4 mRNA expression showed no changes in differently differentiated PAAD tissues in the TCGA database
Correlations between B7-H3 & B7-H4 and infiltration of main types of immune cells
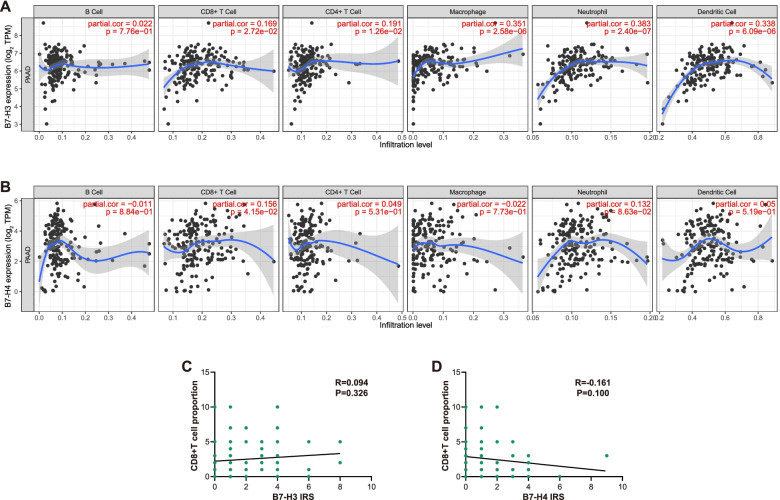
Given B7-H3 & B7-H4 were correlated with TIICs in other cancers [23, 24], we also assessed the correlations between B7-H3 & B7-H4 and infiltration of main types of immune cells. B7-H3 was positively correlated with CD8 + T cells, CD4 + T cells, neutrophils, macrophages, and DCs, while B7-H4 was only positively correlated with CD8 + T cells (Fig. 3A-B). To validate the results, we performed IHC staining using anti-CD8 antibody. However, neither B7-H3 nor B7-H4 was correlated with CD8 + T cell infiltration (Fig. 3C-D). Thus, the correlations between B7-H3 & B7-H4 and immune cells infiltration are contradictory and need to be further confirmed.
Fig. 3.
Correlations between B7-H3 & B7-H4 and infiltration of main types of immune cells. A, B Correlations between B7-H3 & B7-H4 expression levels and the infiltration of six immune cells. B7-H3 was positively correlated with CD8 + T cells, CD4 + T cells, neutrophils, macrophages, and DCs, while B7-H4 was only positively correlated with CD8 + T cells. C, D Correlations between B7-H3 & B7-H4 expression levels and the infiltration of CD8 + T cell. Neither B7-H3 nor B7-H4 was correlated with CD8 + T cell infiltration
Co-deficiency of B7-H3 and B7-H4 predicts a better prognosis
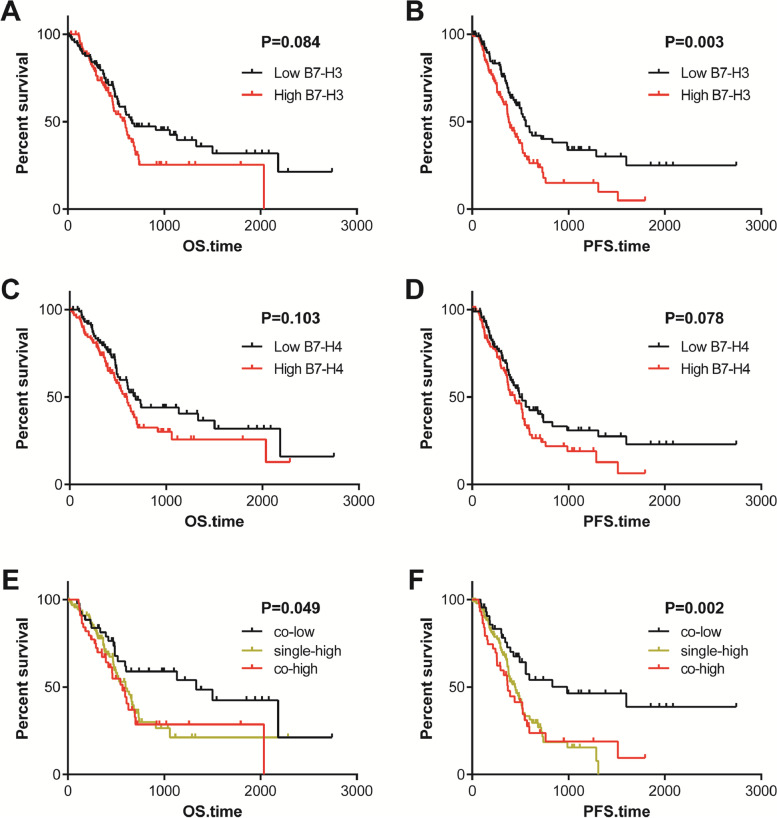
We further definite the prognostic values of these two B7 molecules in patients with PAAD. Patients in the TCGA cohort were divided into low (n = 89) and high (n = 89) groups at the cut-off value of the median expression. The Kaplan–Meier curves exhibited B7-H3 and B7-H4 could not effectively predict overall survival (OS) in patients with PAAD (Fig. 4A, C). In term of progression-free survival (PFS), patients with high B7-H3 expression had a significantly worse prognosis than those with low expression (Fig. 4B). However, B7-H4 could not effectively predict PFS in PAAD patients (Fig. 4D). Furthermore, combined B7-H3 and B7-H4 expression was a promising prognostic biomarker. Co-deficiency of B7-H3 and B7-H4 predicted better prognosis in terms of both OS and PFS (Fig. 4E-F) in PAAD. Taken together, these results indicated that co-deficiency of B7-H3 and B7-H4 was a favorable prognostic factor in PAAD patients.
Fig. 4.
Survival plots of B7-H3 & B7-H4 in PAAD patients. A, B Prognostic value of B7-H3 expression in PAAD patients in term of OS and PFS. High B7-H3 expression was associated with a poor PFS but not associated with OS. C, D Prognostic value of B7-H4 expression in PAAD patients in term of OS and PFS. B7-H4 expression was not associated with OS and PFS. E, F Prognostic value of combined B7-H3 & B7-H4 expression in PAAD patients in term of OS and PFS. Co-deficiency of B7-H3 and B7-H4 was associated with a better prognosis
Co-deficiency of B7-H3 and B7-H4 indicates high CD8 + T cell infiltration
Given co-expressed or mutually-exclusive patterns of B7 molecules predict inflamed or non-inflamed TME in multiple human cancers [14, 15], we next assess whether co-deficiency of B7-H3 and B7-H4 predicted specific TME features. The xCell tool was used to estimate the abundance of 64 immune and stromal cell types in the TCGA database, and the abundance of these cells in the co-low, single-high and co-high groups were next compared. A subset of non-tumor cells was different in the three groups, and total CD8 + T cells and CD8 + Tcm cells were increased in the co-low groups (Table 2, Fig. 5A-B). As expected, the infiltrating abundance of CD8 + T cell was highest in the co-low group among these three groups (Fig. 5C-D). Overall, co-deficiency of B7-H3 and B7-H4 predicts high CD8 + T cell infiltration, which may explain the better prognosis in the co-low group of PAAD patients.
Table 2.
Differences of immune cells levels estimated by xCell algorithm
| Immune cells | Average | F value | P value | ||
|---|---|---|---|---|---|
| co-low | single-high | co-high | |||
| aDC | 0.080 | 0.108 | 0.097 | 2.582 | 0.079 |
| Adipocytes | 0.108 | 0.076 | 0.031 | 3.456 | 0.034 |
| Astrocytes | 0.040 | 0.087 | 0.106 | 13.338 | 0.000 |
| B cells | 0.108 | 0.111 | 0.062 | 0.538 | 0.585 |
| Basophils | 0.038 | 0.012 | 0.007 | 6.142 | 0.003 |
| CD4 + memory T cells | 0.137 | 0.125 | 0.107 | 1.028 | 0.360 |
| CD4 + naïve T cells | 0.060 | 0.064 | 0.045 | 0.577 | 0.563 |
| CD4 + T cells | 0.004 | 0.002 | 0.000 | 0.622 | 0.538 |
| CD4 + Tcm | 0.006 | 0.004 | 0.003 | 2.194 | 0.115 |
| CD4 + Tem | 0.014 | 0.011 | 0.011 | 0.850 | 0.429 |
| CD8 + naïve T cells | 0.008 | 0.009 | 0.007 | 1.943 | 0.146 |
| CD8 + T cells | 0.034 | 0.015 | 0.011 | 10.264 | 0.000 |
| CD8 + Tcm | 0.022 | 0.014 | 0.010 | 3.071 | 0.049 |
| CD8 + Tem | 0.003 | 0.002 | 0.000 | 2.196 | 0.114 |
| cDC | 0.026 | 0.022 | 0.019 | 0.701 | 0.498 |
| Chondrocytes | 0.126 | 0.161 | 0.163 | 4.418 | 0.013 |
| Class switched memory B cells | 0.034 | 0.025 | 0.020 | 1.493 | 0.228 |
| CLP | 0.048 | 0.042 | 0.044 | 1.146 | 0.320 |
| CMP | 0.000 | 0.000 | 0.000 | 1.071 | 0.345 |
| DC | 0.060 | 0.071 | 0.056 | 1.160 | 0.316 |
| Endothelial cells | 0.142 | 0.143 | 0.145 | 0.010 | 0.990 |
| Eosinophils | 0.030 | 0.036 | 0.038 | 3.502 | 0.032 |
| Epithelial cells | 0.726 | 0.891 | 0.918 | 7.364 | 0.001 |
| Erythrocytes | 0.000 | 0.000 | 0.000 | 0.943 | 0.391 |
| Fibroblasts | 0.121 | 0.138 | 0.137 | 0.559 | 0.573 |
| GMP | 0.004 | 0.002 | 0.000 | 2.493 | 0.086 |
| Hepatocytes | 0.085 | 0.090 | 0.098 | 0.516 | 0.598 |
| HSC | 0.196 | 0.221 | 0.215 | 1.009 | 0.367 |
| iDC | 0.119 | 0.131 | 0.114 | 0.591 | 0.555 |
| Keratinocytes | 0.092 | 0.137 | 0.142 | 5.336 | 0.006 |
| Ly Endothelial cells | 0.154 | 0.157 | 0.154 | 0.016 | 0.984 |
| Macrophages | 0.054 | 0.074 | 0.072 | 2.933 | 0.056 |
| Macrophages M1 | 0.046 | 0.065 | 0.065 | 5.435 | 0.005 |
| Macrophages M2 | 0.015 | 0.017 | 0.013 | 1.055 | 0.350 |
| Mast cells | 0.032 | 0.029 | 0.024 | 3.281 | 0.040 |
| Megakaryocytes | 0.014 | 0.014 | 0.012 | 1.286 | 0.279 |
| Melanocytes | 0.013 | 0.012 | 0.011 | 0.447 | 0.640 |
| Memory B cells | 0.015 | 0.016 | 0.009 | 0.305 | 0.738 |
| MEP | 0.035 | 0.030 | 0.027 | 1.792 | 0.170 |
| Mesangial cells | 0.081 | 0.093 | 0.106 | 8.969 | 0.000 |
| Monocytes | 0.021 | 0.033 | 0.021 | 2.337 | 0.100 |
| MPP | 0.000 | 0.000 | 0.000 | 0.056 | 0.945 |
| MSC | 0.140 | 0.265 | 0.310 | 28.875 | 0.000 |
| Mv Endothelial cells | 0.052 | 0.057 | 0.059 | 0.483 | 0.618 |
| Myocytes | 0.006 | 0.005 | 0.004 | 1.011 | 0.366 |
| Naïve B cells | 0.010 | 0.013 | 0.007 | 0.398 | 0.672 |
| Neurons | 0.066 | 0.015 | 0.014 | 17.406 | 0.000 |
| Neutrophils | 0.001 | 0.001 | 0.001 | 0.094 | 0.910 |
| NK cells | 0.000 | 0.000 | 0.000 | 0.458 | 0.633 |
| NKT | 0.024 | 0.022 | 0.026 | 0.959 | 0.385 |
| Osteoblast | 0.077 | 0.036 | 0.034 | 10.543 | 0.000 |
| pDC | 0.072 | 0.058 | 0.046 | 1.981 | 0.141 |
| Pericytes | 0.074 | 0.099 | 0.115 | 3.779 | 0.025 |
| Plasma cells | 0.011 | 0.008 | 0.005 | 6.987 | 0.001 |
| Platelets | 0.001 | 0.000 | 0.000 | 7.379 | 0.001 |
| Preadipocytes | 0.009 | 0.002 | 0.001 | 3.916 | 0.022 |
| Pro B cells | 0.002 | 0.002 | 0.001 | 0.319 | 0.727 |
| Sebocytes | 0.408 | 0.533 | 0.534 | 4.005 | 0.020 |
| Skeletal muscle | 0.000 | 0.000 | 0.000 | 2.332 | 0.100 |
| Smooth muscle | 0.386 | 0.332 | 0.333 | 12.517 | 0.000 |
| Tgd cells | 0.000 | 0.000 | 0.000 | 0.263 | 0.769 |
| Th1 cells | 0.058 | 0.046 | 0.047 | 1.671 | 0.191 |
| Th2 cells | 0.009 | 0.010 | 0.009 | 0.041 | 0.960 |
| Tregs | 0.016 | 0.011 | 0.009 | 3.210 | 0.043 |
Abbreviations: aDC Activated dendritic cells, CD4 + Tcm, CD4 + central memory T-cells, CD4 + Tem CD4 + effector memory T-cells, CD8 + Tcm CD8 + central memory T-cells, CD8 + Tem CD8 + effector memory T-cells, cDC Xonventional dendritic cells, CLP Common lymphoid progenitors, CMP Common myeloid progenitors, DC Dendritic cells, GMP Granulocyte–macrophage progenitors, HSC Hematopoietic stem cells, iDC Immature dendritic cells, ly endothelial cells Lymphatic endothelial cells, MEP Megakaryocyte–erythroid progenitors, MPP Multipotent rogenitors, MSC Mesenchymal stem cells, mv endothelial cells Microvascular endothelial cells, NKT Natural killer T-cells, pDC Plasmacytoid dendritic cells, Tgd cells Gamma delta T-cells, Th1 cells Type 1 T-helper cells, Th2 cells Type 2 T-helper cells, Tregs Regulatory T-cells
Fig. 5.
Various infiltration of CD8 + T cell in co-low, single-high and co-high groups. A The infiltration of total CD8 + T cell estimated by xCell algorithm was various in co-low, single-high, and co-high groups. B The infiltration of total CD8 + Tcm cell estimated by xCell algorithm was various in co-low, single-high, and co-high groups. C Representative microphotographs revealing various infiltration of CD8 + T cells using IHC staining. Brown, CD8. Blue, haematoxylin. Bar = 200 μm. D The infiltration of CD8 + T cell estimated by IHC staining was various in co-low, single-high and co-high groups
Discussion
It has been proved that increased CD8 + T cell infiltration is one of the notable features of immuno-hot tumors, which indicates a better prognosis and high therapeutic response [25–27]. Thus, reliable biomarkers for the identification of immuno-hot tumors in PAAD are urgent in clinical practice. In the current research, we analyzed the expression patterns of B7-H3 and B7-H4 in PAAD and combined their expression as a novel stratification strategy. We found that B7-H3 and B7-H4 were highly expressed in PAAD tissues and higher in poorly differentiated tumors. Moreover, co-deficiency of B7-H3 and B7-H4 indicates a better prognosis and high CD8 + T cell infiltration.
B7-H3 is a negative regulator and inhibits T cell proliferation and cytokine production mediated by antibody to CD3 [28]. In cancers, B7-H3 acts as an inhibitory immune checkpoint that negatively regulates anti-tumor immunity. Overexpression of B7-H3 in tumor tissues is a poor prognostic biomarker in prostate cancer [29], upper tract urothelial carcinoma [30], small cell lung cancer [31], etc. Besides, inhibition of B7-H3 expression is a promising therapeutic strategy for human cancer. In previous research, B7-H3 targeted therapies have been mentioned, which shows promising applications, including monoclonal antibodies against B7-H3, specific antibody-dependent cell-mediated cytotoxicity, antibody drug conjugates, specific small-molecule inhibitor, and chimeric antigen receptor T-cell therapy [21]. B7-H3 expression shows no notable correlation with major TIICs, including CD3 + , CD8 + and CD20 + TIICs in small cell lung cancer [32], whereas B7-H3 expression is positively correlated with the abundance of CD45 + and CD8 + TIICs in non-small-cell lung cancer [33]. In PAAD, B7-H3 was overexpressed and promoted tumor progression [34]. In addition, tumor high B7-H3 expression was independently associated with poor survival [35]. In our research, B7-H3 was positively correlated with CD8 + T cells, CD4 + T cells, neutrophils, macrophages, and DCs estimated by TIMER algorithm, but B7-H3 expression was not correlated with the abundance of CD8 + TIICs in our validated cohort. The contradictory results need to be further confirmed.
Similar to B7-H3, B7-H4 is also an inhibitory immune checkpoint and predicts poor prognosis in multiple human cancers [36–38]. Besides, immunotherapy targeting B7-H4 is under pre-clinical investigation [22]. For example, pharmacologic inhibition of B7-H4 glycosylation restores anti-tumor immunity in immuno-cold breast cancer [39]. It has been reported that B7-H4 expression is inversely correlated with T cell infiltration in clear cell ovarian cancer [40] and breast cancer [24]. In PAAD, B7-H4 promoted cancer progression and inhibited apoptosis in PAAD cells [41]. In addition, a meta-analysis revealed that high expression of B7-H4 was an unfavorable prognostic factor for patients with PAAD [42]. In the current research, we assessed the expression of B7-H4 in tumor and para-tumor tissues in PAAD. However, the results revealed by the GEPIA and CPTAC databases showed no difference between tumor and para-tumor tissues, but IHC staining uncovered that B7-H4 was significantly overexpressed in PAAD tissues. Since B7-H4 was almost only expressed in tumor cells and not in the tumor stroma, we speculated that bulk-RNA sequencing could not distinguish cell subtypes, leading to the false low expression in tumor tissues.
Interestingly, growing numbers of studies have suggested that B7 molecules exhibit limited co-expression patterns [15, 32, 43]. B7-H3 and B7-H4 exhibited no tight correlation in PAAD in our research, but no obvious pattern of mutually exclusive expression was observed as well. Novel prognostic and/or immunogenic classifiers based on different expression patterns of B7 molecules have been preliminarily investigated. For example, B7-H4 is negatively correlated with PD-L1 and identifies immuno-cold tumors in glioma [15]. In our research, we found that co-deficiency of B7-H3 and B7-H4 indicates better prognosis and immuno-hot tumors with high CD8 + T cell infiltration, which could be applied as a novel classifier for prognostic and immunogenic assessment in PAAD.
Conclusion
To sum up, we analyze the expression patterns and prognostic values of B7-H3 and B7-H4 in PAAD. Single B7-H3 or B7-H4 expression exhibits limited prognostic value for assessment of clinical outcome in PAAD, while combined their expression is a promising stratification strategy to evaluate prognosis and immunogenicity in PAAD.
Supplementary Information
Additional file 1: Figure S1. Expression levels of B7-H3 and B7-H4 PAAD tissues based on public data.
Acknowledgements
Not applicable.
Abbreviations
- PAAD
Pancreatic adenocarcinoma
- TME
Tumor microenvironment
- TIICs
Tumor-infiltrating immune cells
- PD-L1
Programmed cell death ligand 1
- RNA-seq
RNA-sequencing
- TCGA
The Cancer Genome Atlas
- TMA
Tissue microarray
- IHC
Immunohistochemistry
- DAB
Diaminobenzidine
- OS
Overall survival
- PFS
Progression-free survival
- APC
Antigen-presenting cells
Authors’ contributions
Qiang Zhan conceived and designed the study. Shuping Si, Lei Wang, Hui Cao and Yuhua Xu performed the assays and bioinformatics analysis. Shuping Si prepared the figures and tables. Shuping Si and Lei Wang wrote the manuscript. Qiang Zhan revised the manuscript. All authors approved the final version of the manuscript.
Funding
This work was founded by the National Natural Science Foundation of China (No. 81773227) to Qiang Zhan and the High-end Medical Expert Team of the 2020 Taihu Talent Plan to Qiang Zhan.
Availability of data and material
All public data are available in corresponding websites and other necessary data are included in the article. In addition, the current research does not include in-house sequencing data.
Declarations
Ethics approval and consent to participate
Ethical approval for the use of the TMA was granted by the Clinical Research Ethics Committee (Outdo BioTech). Informed consent was obtained from all subjects and/or their legal guardian(s). In addition, all methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shuping Si, Email: pippup@163.com.
Lei Wang, Email: wldy0208@126.com.
Hui Cao, Email: caohui412@163.com.
Yuhua Xu, Email: 456441xyh@sina.com.
Qiang Zhan, Email: ryzhanqiang@163.com.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Foley K, Kim V, Jaffee E, Zheng L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016;381(1):244–251. doi: 10.1016/j.canlet.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilmi M, Bartholin L, Neuzillet C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J Gastroenterol. 2018;24(20):2137–2151. doi: 10.3748/wjg.v24.i20.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee K, Kumar S, Ross KA, Gautam S, Poelaert B, Nasser MW, Aithal A, Bhatia R, Wannemuehler MJ, Narasimhan B, et al. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett. 2018;417:35–46. doi: 10.1016/j.canlet.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schizas D, Charalampakis N, Kole C, Economopoulou P, Koustas E, Gkotsis E, Ziogas D, Psyrri A, Karamouzis MV. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat Rev. 2020;86:102016. doi: 10.1016/j.ctrv.2020.102016. [DOI] [PubMed] [Google Scholar]
- 6.Tahkola K, Mecklin JP, Wirta EV, Ahtiainen M, Helminen O, Bohm J, Kellokumpu I. High immune cell score predicts improved survival in pancreatic cancer. Virchows Arch. 2018;472(4):653–665. doi: 10.1007/s00428-018-2297-1. [DOI] [PubMed] [Google Scholar]
- 7.Stenzel PJ, Schindeldecker M, Tagscherer KE, Foersch S, Herpel E, Hohenfellner M, Hatiboglu G, Alt J, Thomas C, Haferkamp A, et al. Prognostic and Predictive Value of Tumor-infiltrating Leukocytes and of Immune Checkpoint Molecules PD1 and PDL1 in Clear Cell Renal Cell Carcinoma. Transl Oncol. 2020;13(2):336–345. doi: 10.1016/j.tranon.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung J, Suh WK. The CD28-B7 Family in Anti-Tumor Immunity: Emerging Concepts in Cancer Immunotherapy. Immune Netw. 2014;14(6):265–276. doi: 10.4110/in.2014.14.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YM, Yu JM, Liu ZY, Yang HJ, Tang J, Chen ZN. Programmed Death Ligand 1 Indicates Pre-Existing Adaptive Immune Response by Tumor-Infiltrating CD8(+) T Cells in Non-Small Cell Lung Cancer. Int J Mol Sci. 2019;20(20):5138. doi: 10.3390/ijms20205138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei XL, Liu QW, Liu FR, Yuan SS, Li XF, Li JN, Yang AL, Ling YH. The clinicopathological significance and predictive value for immunotherapy of programmed death ligand-1 expression in Epstein-Barr virus-associated gastric cancer. Oncoimmunology. 2021;10(1):1938381. doi: 10.1080/2162402X.2021.1938381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni L, Dong C. New B7 Family Checkpoints in Human Cancers. Mol Cancer Ther. 2017;16(7):1203–1211. doi: 10.1158/1535-7163.MCT-16-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamato I, Sho M, Nomi T, Akahori T, Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H, Nakajima Y. Clinical importance of B7–H3 expression in human pancreatic cancer. Br J Cancer. 2009;101(10):1709–1716. doi: 10.1038/sj.bjc.6605375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen L, Qian Y, Wu W, Weng T, Wang FXC, Hong B, Wu Z, Wang Q, Sang Y, Zhang H, et al. B7–H4 is a prognostic biomarker for poor survival in patients with pancreatic cancer. Hum Pathol. 2017;66:79–85. doi: 10.1016/j.humpath.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Cherif B, Triki H, Charfi S, Bouzidi L, Kridis WB, Khanfir A, Chaabane K, Sellami-Boudawara T, Rebai A. Immune checkpoint molecules B7–H6 and PD-L1 co-pattern the tumor inflammatory microenvironment in human breast cancer. Sci Rep. 2021;11(1):7550. doi: 10.1038/s41598-021-87216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, Li G, Ji C, Lu Q, Qi Y, Tang C, Xiong J, Hu J, Yasar FBA, Zhang Y, et al. Enhanced B7–H4 expression in gliomas with low PD-L1 expression identifies super-cold tumors. J Immunother Cancer. 2020;8(1):e000154. doi: 10.1136/jitc-2019-000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen F, Chandrashekar DS, Varambally S, Creighton CJ. Pan-cancer molecular subtypes revealed by mass-spectrometry-based proteomic characterization of more than 500 human cancers. Nat Commun. 2019;10(1):5679. doi: 10.1038/s41467-019-13528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017;77(21):e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;18(1):220. doi: 10.1186/s13059-017-1349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai Y, Ji W, Sun C, Xu R, Chen X, Deng Y, Pan J, Yang J, Zhu H, Mei J. Interferon-Induced Transmembrane Protein 3 Shapes an Inflamed Tumor Microenvironment and Identifies Immuno-Hot Tumors. Front Immunol. 2021;12(3162):704965. [DOI] [PMC free article] [PubMed]
- 21.Liu S, Liang J, Liu Z, Zhang C, Wang Y, Watson AH, Zhou C, Zhang F, Wu K, Zhang F, et al. The role of CD276 in Cancers. Front Oncol. 2021;11:654684. doi: 10.3389/fonc.2021.654684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JY, Wang WP. B7-H4, a promising target for immunotherapy. Cell Immunol. 2020;347:104008. [DOI] [PubMed]
- 23.Fauci JM, Straughn JM, Jr, Ferrone S, Buchsbaum DJ. A review of B7–H3 and B7–H4 immune molecules and their role in ovarian cancer. Gynecol Oncol. 2012;127(2):420–425. doi: 10.1016/j.ygyno.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Kim NI, Park MH, Kweon SS, Lee JS. B7–H3 and B7–H4 expression in breast cancer and their association with clinicopathological variables and T Cell infiltration. Pathobiology. 2020;87(3):179–192. doi: 10.1159/000505756. [DOI] [PubMed] [Google Scholar]
- 25.Craig SG, Humphries MP, Alderdice M, Bingham V, Richman SD, Loughrey MB, Coleman HG, Viratham-Pulsawatdi A, McCombe K, Murray GI, et al. Immune status is prognostic for poor survival in colorectal cancer patients and is associated with tumour hypoxia. Br J Cancer. 2020;123(8):1280–1288. doi: 10.1038/s41416-020-0985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren F, Zhao Q, Minghai Z, Shaogong Z, Liu B, Bukhari I, Zhang K, Wu W, Yuming F, Yu Y, et al. Immune infiltration profiling in gastric cancer and their clinical implications. Cancer Sci. 2021;112(9):3569–3584. doi: 10.1111/cas.15057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu R, Han Q, Zhang J. STAT3: A key signaling molecule for converting cold to hot tumors. Cancer Lett. 2020;489:29–40. doi: 10.1016/j.canlet.2020.05.035. [DOI] [PubMed] [Google Scholar]
- 28.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, et al. The B7 family member B7–H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4(9):899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 29.Nunes-Xavier CE, Kildal W, Kleppe A, Danielsen HE, Waehre H, Llarena R, Maelandsmo GM, Fodstad O, Pulido R, Lopez JI. Immune checkpoint B7–H3 protein expression is associated with poor outcome and androgen receptor status in prostate cancer. Prostate. 2021;81(12):838–848. doi: 10.1002/pros.24180. [DOI] [PubMed] [Google Scholar]
- 30.Koyama Y, Morikawa T, Miyama Y, Miyakawa J, Kawai T, Kume H, Sawabe M, Ushiku T. B7–H3 expression in upper tract urothelial carcinoma associates with adverse clinicopathological features and poor survival. Pathol Res Pract. 2020;216(12):153219. doi: 10.1016/j.prp.2020.153219. [DOI] [PubMed] [Google Scholar]
- 31.Qiu MJ, Xia Q, Chen YB, Fang XF, Li QT, Zhu LS, Jiang X, Xiong ZF, Yang SL. The expression of three negative Co-Stimulatory B7 family molecules in small cell lung cancer and their effect on prognosis. Front Oncol. 2021;11:600238. doi: 10.3389/fonc.2021.600238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvajal-Hausdorf D, Altan M, Velcheti V, Gettinger SN, Herbst RS, Rimm DL, Schalper KA. Expression and clinical significance of PD-L1, B7–H3, B7–H4 and TILs in human small cell lung Cancer (SCLC) J Immunother Cancer. 2019;7(1):65. doi: 10.1186/s40425-019-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yim J, Koh J, Kim S, Song SG, Ahn HK, Kim YA, Jeon YK, Chung DH. Effects of B7–H3 expression on tumour-infiltrating immune cells and clinicopathological characteristics in non-small-cell lung cancer. Eur J Cancer. 2020;133:74–85. doi: 10.1016/j.ejca.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X, Li DC, Zhu XG, Gan WJ, Li Z, Xiong F, Zhang ZX, Zhang GB, Zhang XG, Zhao H. B7–H3 overexpression in pancreatic cancer promotes tumor progression. Int J Mol Med. 2013;31(2):283–291. doi: 10.3892/ijmm.2012.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inamura K, Takazawa Y, Inoue Y, Yokouchi Y, Kobayashi M, Saiura A, Shibutani T, Ishikawa Y. Tumor B7–H3 (CD276) Expression and Survival in Pancreatic Cancer. J Clin Med. 2018;7(7):172. doi: 10.3390/jcm7070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu N, Shen W, Zhong Y, Bast RC, Jr, Jazaeri A, Sood AK, Liu J. Expression of B7–H4 and IDO1 is associated with drug resistance and poor prognosis in high-grade serous ovarian carcinomas. Hum Pathol. 2021;113:20–27. doi: 10.1016/j.humpath.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizuno T, Kamai T, Tsuzuki T, Nishihara D, Kijima T, Arai K, Yoshida KI. Elevated expression of B7 homolog 4 is associated with disease progression in upper urinary tract urothelial carcinoma. Cancer Immunol Immunother. 2021;2022(3):565–78. 10.1007/s00262-021-03011-5. [DOI] [PMC free article] [PubMed]
- 38.Ding S, Lv X, Liu Z, Zhan S, Xu Y, Zhang X, Liu C, Cao L. Overexpression of B7–H4 is associated with infiltrating immune cells and poor prognosis in metastatic colorectal cancer. Int Immunopharmacol. 2021;90:107144. doi: 10.1016/j.intimp.2020.107144. [DOI] [PubMed] [Google Scholar]
- 39.Song X, Zhou Z, Li H, Xue Y, Lu X, Bahar I, Kepp O, Hung MC, Kroemer G, Wan Y. Pharmacologic Suppression of B7–H4 Glycosylation Restores Antitumor Immunity in Immune-Cold Breast Cancers. Cancer Discov. 2020;10(12):1872–1893. doi: 10.1158/2159-8290.CD-20-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagnotti GM, Atkinson RM, Romeiser J, Akalin A, Korman MB, Shroyer KR. B7–H4 is Inversely Correlated With T-Cell Infiltration in Clear Cell but Not Serous or Endometrioid Ovarian Cancer. Appl Immunohistochem Mol Morphol. 2019;27(7):515–522. doi: 10.1097/PAI.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 41.Qian Y, Hong B, Shen L, Wu Z, Yao H, Zhang L. B7–H4 enhances oncogenicity and inhibits apoptosis in pancreatic cancer cells. Cell Tissue Res. 2013;353(1):139–151. doi: 10.1007/s00441-013-1640-8. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Tao L, Yuan C, Xiu D. The prognostic value of B7–H4 in pancreatic cancer: Systematic review and meta-analysis. Medicine (Baltimore) 2018;97(12):e0088. doi: 10.1097/MD.0000000000010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altan M, Pelekanou V, Schalper KA, Toki M, Gaule P, Syrigos K, Herbst RS, Rimm DL. B7–H3 Expression in NSCLC and Its Association with B7–H4, PD-L1 and Tumor-Infiltrating Lymphocytes. Clin Cancer Res. 2017;23(17):5202–5209. doi: 10.1158/1078-0432.CCR-16-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Expression levels of B7-H3 and B7-H4 PAAD tissues based on public data.
Data Availability Statement
All public data are available in corresponding websites and other necessary data are included in the article. In addition, the current research does not include in-house sequencing data.