Abstract
Background
Breast cancer is the most common form of cancer and the second leading cause of death amongst women in Europe. Amongst five invasive cancers per 1000 women detected in screening, 2.7 were < 15 mm in diameter; and others reported that over one third of excised breast lesions were clinically occult. The challenge is to accurately locate small non‐palpable lesions intraoperatively for optimal therapeutic outcome. A secondary important goal is to remove the smallest amount possible of healthy glandular tissue for optimal cosmesis. Currently the most widely adopted approach (80% in one survey) in guided breast‐conserving surgery for excising non‐palpable breast lesions is wire‐guided localization (WGL). With the clinical setting shifting towards earlier non‐palpable breast lesions being detected through screening, we investigated whether the current standard in assisting surgical excision of these lesions, WGL, yields the best therapeutic outcome for women with breast cancer.
Objectives
To assess the therapeutic outcomes of any new form of guided surgical intervention for non‐palpable breast lesions against wire‐guided localization, the current gold standard.
Search methods
We searched the Cochrane Breast Cancer Group's (CBCG) Specialized Register, MEDLINE (via PubMed), the Cochrane Central Register of Controlled Trials (CENTRAL), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal from the earliest available date up to 30 March 2015. We also handsearched recent conference proceedings and sought information from experts in the field.
Selection criteria
Two review authors, BC and RJ, independently screened by title and abstract the studies we had identified through the search strategy; when this was inconclusive, they examined the full‐text article for inclusion. We resolved any discrepancies regarding eligibility by discussion with a third review author, RA.
Data collection and analysis
Three review authors, BC, JW, and RJ, independently extracted data using a standardized data sheet. We performed all analyses using Review Manager (RevMan) or the R meta package, and in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. We reported results via a graphical assessment using forest plots showing the study estimates. We considered and discussed additional subgroup and sensitivity analyses.
Main results
We identified 11 randomized controlled trials (RCTs) that met the inclusion criteria of this Cochrane review and included eight trials in the meta‐analyses. Six RCTs compared radioguided occult lesion localization (ROLL) versus WGL, and two RCTs compared radioactive iodine (125I) seed localization (RSL) versus WGL. Of the three remaining trials, one RCT compared cryo‐assisted techniques (CAL) versus WGL, one compared intraoperative ultrasound‐guided lumpectomy (IOUS) versus WGL, and one compared modified ROLL technique in combination with methylene dye (RCML) versus WGL. Of the trials we included in the meta‐analysis, there were a total of 1273 participants with non‐palpable breast lesions (627 participants (WGL); 443 participants (ROLL); and 203 participants (RSL)). The participant population varied considerably between included trials, which included participants with both non‐palpable benign and malignant lesions, and varied in defining clear margins. The included trials did not report any long‐term outcomes.
In general, the outcomes of WGL, ROLL and RSL were comparable.
ROLL demonstrated favourable results in successful localization (risk ratio (RR) 0.60, 95% confidence interval (CI) 0.16 to 2.28; 869 participants; six trials), positive excision margins (RR 0.74, 95% CI 0.42 to 1.29; 517 participants; five trials), and re‐operation rates (RR 0.51, 95% CI 0.21 to 1.23; 583 participants; four trials) versus WGL, but none were statistically significant. WGL was significantly superior to RSL in successfully localizing non‐palpable lesions (RR 3.85, 95% CI 1.21 to 12.19; 402 participants; two trials). However, for successful excision, ROLL and RSL have comparable outcomes versus WGL (ROLL versus WGL: RR 1.00, 95% CI 0.99 to 1.01; 871 participants; six trials; RSL versus WGL: RR 1.00, 95% CI 0.99 to 1.01; 402 participants; two trials). These findings were similar in that RSL demonstrated favourable results over WGL in positive tumour margins (RR 0.67, 95% CI 0.43 to 1.06; 366 participants; two trials), and re‐operation rates (RR 0.80, 95% CI 0.48 to 1.32; 305 participants; one trial) but neither reached statistical significance. In contrast, WGL had fewer postoperative complications to both ROLL (RR 1.18, 95% CI 0.71 to 1.98; 642 participants; four trials) and RSL (RR 1.51, 95% CI 0.75 to 3.03; 305 participants; one trial), although this was also not statistically significant.
The overall quality of evidence was good. The main risk of bias amongst included studies consisted of incomplete data sets, selective reporting, and allocation concealment. Interpretation and applicability of this meta‐analysis was hindered by the mixed indication of diagnostic versus therapeutic purposes when undertaking WGL, ROLL, or RSL, leading to a high level of mixed pathology in numerous trials. Other limitations include underpowered studies, lack of data in standardized format for meta‐analysis, lack of complete data amongst the trials, and absence of long‐term data.
Authors' conclusions
Owing to a lack of trials in certain localization techniques, we could only draw conclusions about ROLL and RSL versus WGL. There is no clear evidence to support one guided technique for surgically excising a non‐palpable breast lesion over another. Results from this Cochrane review support the continued use of WGL as a safe and tested technique that allows for flexibility in selected cases when faced with extensive microcalcification. ROLL and RSL could be offered to patients as a comparable replacement for WGL as they are equally reliable. Other techniques such as IOUS, RCML, and CAL are of academic interest, but recommendation for routine use in the clinical environment and oncological outcomes require further validation. The results of this Cochrane review also stress the need for more fully powered RCTs to evaluate the best technique according to the comprehensive criteria described, with a more consistent and standardized approach in outcome reporting.
Plain language summary
Different localization techniques during surgery of non‐palpable breast lumps
Review question We reviewed the evidence on new localization techniques against the gold standard (wire‐guided) for the surgical removal of non‐palpable breast lumps.
Background Breast cancer screening has brought a shift towards earlier detection of non‐palpable breast lumps (i.e. lumps that cannot be felt by palpation by a doctor). Surgical removal of non‐palpable lumps can be challenging as it involves locating and removing the entire lump while removing the smallest amount of healthy tissue possible and maintaining optimal breast appearance. The commonly used technique in guiding the surgical removal of non‐palpable breast lumps is wire‐guided localization (WGL; inserting a wire to the centre of the lump). We wanted to examine whether WGL was better or worse than other newer alternatives. Study characteristics The evidence is current to 30 March 2015. Eleven trials met the inclusion criteria of this Cochrane review but we included only eight in our analyses. Six studies compared WGL to radio‐guided occult lesion localization (ROLL; it uses a radioactive tracer injected into the lump) and two studies compared WGL to radioactive seed localization (RSL; it involves implanting an Iodine seed in the centre of the tumour). We included a total of 1273 participants with non‐palpable breast lumps (627 participants (WGL), 443 participants (ROLL), 203 participants (RSL)). There was considerable variation in the participants' tumours in the studies. The included studies did not report any long‐term outcomes.
Key results People who had WGL and ROLL treatment gave similar results in being able to successfully localize and remove the lump as planned, and also similar postoperative complication rates. ROLL resulted in slightly fewer positive tumour margins (that is, the when the tumour is removed, some surrounding tissue is removed and cancer cells extend out into the margins) compared to WGL, and ROLL also had lower re‐intervention rates (that is, less likely to require further surgery) over WGL, but neither differences were statistically significant.
WGL was superior to RSL in successfully locating the lump, but both techniques seemed equally effective in successfully removing the lump. Similarly, RSL provided fewer positive tumour margins compared to WGL (though not statistically significant). However only one study reported on re‐intervention rates where the rates were comparable for RSL and WGL.
The studies either did not report or inconsistently reported information on the operation time, length of hospital stay, recurrence, breast appearance, and participant preference when using these different techniques.
Quality of the evidence The overall quality of the evidence was good. There was no clear evidence to support one guided technique for surgically removing a non‐palpable breast lesion over another. The results from this Cochrane review support the continuing use of WGL as a safe and tested technique. ROLL and RSL could be offered to participants as a comparable replacement for WGL. This Cochrane review highlights the need for more fully‐powered trials (that is, trials large enough to detect intervention differences) to evaluate the best localization techniques.
Background
Description of the condition
Breast cancer is the most common form of cancer and second leading cause of death amongst women in Europe (Ferlay 2007). In the developed world, it is estimated that one in 11 women will develop breast cancer, with some figures quoting one in eight women in the UK and one in nine women for the USA (Ferlay 2007). Early detection of breast malignancies decreases mortality and morbidity (Philips 2008). This has led to the development of wide‐spread mammogram screening and subsequently a rapid increase in early detected tumours that tend to be small and non‐palpable (Wilson 1993). Amongst the five invasive cancers per 1000 women, detected in screening, 2.7 (54%) were less than 15 mm in diameter; with others reporting that more than one third of excised breast lesions were clinically occult (UK NSC Breast Cancer Screening Recommendation 2012; Rahusen 2002). In women with small malignant lesions, current literature suggests breast conserving therapy, including lumpectomy or wide‐local excision together with adjuvant radiotherapy to the tumour bed, as generally suitable and yielding comparable results to mastectomy (Fisher 2002; Schwartz 2006). However, the challenge remains in accurately and precisely locating small non‐palpable lesions intraoperatively, for optimal therapeutic outcome.
Description of the intervention
Currently the most widely adopted approach (80% in one survey) in guided breast‐conserving surgery for excising non‐palpable breast lesions is wire‐guided localization (WGL) (Van Esser 2008). A wire is inserted to localize the lesion to be excised, commonly under stereotactic or ultrasonographic guidance and alternatively magnetic resonance imaging (MRI) or computerized tomography (CT).
Key disadvantages of WGL include: 1. the presence of a foreign body at pathological assessment; 2. wire transection; 3. wire migration; 4. patient distress and discomfort; 5. injury associated with barbs; 6. pneumothorax; and 7. interference with the surgical approach (De Cicco 2002). This has led to the development of other guidance techniques, including: 1. radioguided occult lesion localization (ROLL); 2. intraoperative ultrasound (IOUS) guided resection; 3. cryoprobe‐assisted localization (CAL); 4. carbon marking; 5. methylene blue dye marking; and 6. near‐infrared fluorescence optical imaging (Hirsch 1989; Luini 1998; Rahusen 2002; Rose 2003; Tafra 2006; Tromberg 2008). Amongst these, the ROLL technique, which was developed in 1998 at the European Institute of Oncology, Milan, has been demonstrated as a feasible technique and is increasingly popular in many countries (Audisio 2005; Rovera 2008). ROLL utilizes a non‐specific radioisotope, commonly technetium‐99m (99mTc), injected intratumorally under stereotactic or ultrasonographic (US) guidance preoperatively, with the lesion detected intraoperatively by a handheld gamma probe. The technique is highly feasible across many settings and variations of the technique have since evolved, including the use of a radioactive iodine (125I) seed (RSL) (Gray 2001; Hughes 2008; Nadeem 2005). The procedures of ROLL, a cryoprobe, carbon and methylene blue dye marking are relatively similar. Their differences are mainly in the agent used for marking the lesion.
In the UK, all breast lesions are investigated with the triple test of clinical examination, imaging by mammogram or ultrasound, or both, and cytology or histology involving fine‐needle aspiration or core biopsy, or both. Hence both clinicians and patients are aware of the preliminary pathology diagnosis prior to surgery, which may then be confirmed from the excised specimen. However, elsewhere the last step may be missed and an open surgical biopsy performed for suspicious breast lesions (Ernst 2002). For the purpose of this Cochrane review, we will adopt a pragmatic approach by including studies aimed at completely excising the breast lesion for therapeutic or therapeutic combined with diagnostic purposes but not solely diagnostic purposes.
Why it is important to do this review
Recently there have been three systematic reviews in this research area. Van der Ploeg 2008 and Rovera 2008 both reviewed trials investigating ROLL versus WGL, while Jakub 2010 reviewed radioactive seed localization (RSL) versus WGL. Their findings were similar in that they concluded that ROLL and RSL are reliable and safe alternatives to WGL; and are at least equivalent in accuracy, obtaining negative margins, with comparable re‐intervention rates and operation times. However, none of these reviews evaluated all the forms of guided surgical techniques together, and they included non‐randomized trials. Pleijhuis 2009 provides the most comprehensive overview of current modalities and future directions in breast‐conserving therapy for early breast cancer. The review describes a number of studies evaluating most techniques of guided surgery. Unfortunately, this is not a structured review.
With the clinical setting witnessing a shift towards earlier non‐palpable breast lesions being detected through screening, we investigated whether the current gold standard localizing technique, WGL, yields the best therapeutic outcome. The lack of evidence to support one specific guidance technique for excising non‐palpable breast lesions calls for a systematic review of the literature and a meta‐analysis to synthesize the results of a number of smaller studies investigating the many forms of guided surgery, if appropriate.
Objectives
To assess the therapeutic outcomes of any new form of guided surgical intervention for non‐palpable breast lesions against wire‐guided localization (WGL), the current gold standard.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) comparing WGL against another form of guided surgery for women with non‐palpable breast lesions for therapeutic and diagnostic purposes.
In the absence of RCTs that meet the inclusion criteria of this review, we will consider well‐designed cohort or case control studies for inclusion and we will provide a narrative description of the evidence available. In this Cochrane review, RCTs met the inclusion criteria and therefore we excluded non‐randomized studies.
Types of participants
Female participants of any age with a diagnosis of a breast lesion. The lesion should have been non‐palpable on clinical examination and the participant deemed fit for surgical intervention.
Types of interventions
Surgical intervention assisted by WGL versus any technique of guidance aimed at the complete excision of the breast lesion for therapeutic or therapeutic combined with diagnostic purposes but not solely for diagnostic purposes. Guidance techniques included, but were not limited to:
radioguided occult lesion localization (ROLL);
radioactive seed localization (RSL);
intraoperative ultrasound (IOUS);
cryoprobe‐assisted localization (CAL); and
haematoma‐directed ultrasound‐guided (HUG) localization.
Types of outcome measures
Primary outcomes
Successful localization of the lesion: using the planned technique to image and localize the lesion preoperatively;
successful excision of the lesion: excision of the lesion as planned;
positive excision margins: cancer in mural edge;
re‐operation rate: the need for further excision of primary lesion, not including axilla only interventions.
Secondary outcomes
Operation time;
length of hospital stay;
postoperative complications;
recurrence;
cosmesis;
patient preference (including as a result of pain).
Search methods for identification of studies
We used the methods described in the Cochrane Breast Cancer Group (CBCG) module (Cochrane Breast Cancer Group). We also consulted the CBCG Trials Search Co‐ordinator.
We only included articles and trials published in English.
Electronic searches
We searched the following databases up to 30 March 2015:
The CBCG Specialized Register. The CBCG's module provides details of the search strategies used by the CBCG for the identification of studies and the procedures used to code references (www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). We selected trials coded with the key words 'high risk', 'surgery', 'wire guided localisation', 'radioguided occult lesion localization', 'radioactive seed localization', 'intraoperative ultrasound', 'cryoprobe‐assisted localization', and 'haematoma‐directed ultrasound‐guided' and considered them for inclusion in the review;
MEDLINE (via PubMed) (from earliest available to 30 March 2015) (Appendix 1);
the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, current issue) (Appendix 2);
the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/Default.aspx), for all prospectively registered and ongoing trials (Appendix 3); and
ClinicalTrials.gov (https://clinicaltrials.gov/) for additional unpublished and ongoing studies (Appendix 4).
Searching other resources
Bibliography searching
We identified further studies from the reference lists of relevant trials and reviews. When possible, we obtained the full‐text article for each reference we identified as a potentially eligible trial.
Data collection and analysis
Selection of studies
Two review authors, BC and RJ, independently screened each study identified through the search strategy by title and abstract; when this was inconclusive, they examined the full‐text article for further assessment. We resolved any discrepancies regarding eligibility by discussion involving a third review author (RA). We recorded the excluded studies and the reasons for exclusion in the 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors, BC and RJ, independently appraised all suitable studies using a standardized data extraction sheet, and summarized these details in the 'Characteristics of included studies' table. We extracted data extracted from included trials on the following: trial design, trial methods, trial population size, patient baseline characteristics, specimen pathology, therapeutic or combined diagnostic and therapeutic intervention, details of intervention, and outcomes. We did not need to seek additional information from the trial authors because all primary outcomes were reported, or the trial design did not capture it.
Assessment of risk of bias in included studies
Two review authors, BC and JW, independently assessed the methodological quality of included trials. We assessed the included RCTs using the CBCG's ‘Quality Assessment Form’ (Schulz 1995), available on the CBCG website. We used the ‘Risk of bias' assessment form to determine the specific domain of risk, and used the 'Risk of bias' table for recording judgements on the type of risk (low, high, or unclear), as per the Cochrane 'Risk of bias' assessment tool (Higgins 2011).
Potential bias arose from the different diagnostic protocols practised as mentioned above, and gave rise to heterogeneous pathologies; this made it difficult for us to reconcile the results on re‐intervention rates, recurrence, and excision margins.
We only included RCTs in this Cochrane review, and therefore we did not need to perform 'Risk of bias' assessments of non‐randomized studies (as planned in the published Cochrane protocol (Chan 2011)).
Measures of treatment effect
We calculated a pooled estimate for the size of the effect, as specified below, and 95% confidence intervals (CIs) combining all trials. In an effort to determine the likely beneficial therapeutic outcomes between various treatment approaches, we measured dichotomous data using the risk ratio (RR) for successful localization, excision of lesion, positive margins, and re‐intervention rate.
We did not estimate mean differences (MD) and standardized mean difference (SMD) values, as the included trials inconsistently reported data and often SD values were unavailable. Therefore, we reported the findings in the review text and in the review tables.
We did not calculate time‐to‐event data (for example, for the outcome recurrence). There were no data available for the outcome recurrence.
Unit of analysis issues
We did not include cluster‐RCTs in this Cochrane review; hence, we assessed outcomes at the individual level.
Cross‐over trials met the inclusion criteria of this review. However, we did not analyse the data from these trials due to the variability in the reporting of data. In the event of future update of this Cochrane review, we will incorporate cross‐over trials providing sufficient data in compliance with the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 16, section 16.4.5; Higgins 2011). Otherwise, we will forego such an analysis.
We did not identify trial that included multiple treatment groups for inclusion in this review, nor studies that used repeated observations (no study reported recurrence, one of the secondary outcomes in this Cochrane review).
We did not identify any participants with multiple occurrences of the event (that is, excision of a non‐palpable breast lesion) to consider effect measures for counts and rates. If this occurs in future updates of this Cochrane review, we will analyse the data using the rate ratio, and not dichotomous data, in order to capture each individual event (Higgins 2011).
Dealing with missing data
Due to the potential high risk of attrition bias in Gray 2001, we sought additional information from the trial authors. We have discussed this further in the 'Incomplete outcome data (attrition bias)' section.
Assessment of heterogeneity
We assessed clinical heterogeneity in the included RCTs by examining variability in the participants, interventions, and outcomes. We also examined methodological heterogeneity, which is variability in study design and risk of bias (Higgins 2011). Then we quantified statistical heterogeneity using the I² statistic which describes "the percentage of variability in effect estimates that is due to heterogeneity rather than sampling error" (Chapter 9, section 9.5.2; Higgins 2011). An I² statistic value of greater than 50% indicates substantial heterogeneity (Higgins 2011). We conducted a test of statistical heterogeneity to detect any differences of effects between the included trials. We performed this by: 1. by reviewing the confidence intervals (CIs) for each of the individual included trials in order to assess overlap. We ascertained this using a forest plot (Bruce 2008); and 2. by formally conducting a statistical test for heterogeneity using the Cochrane Q (Chi² test for heterogeneity) and I² statistic values (Bruce 2008). This further assessed whether the study estimates of effects are due to chance alone.
Where we identified heterogeneity, we conducted a meta‐analysis and we discussed the possible reasons for heterogeneity in the ‘Discussion’ section.
Assessment of reporting biases
In order to address the issue of reporting bias, we used risk assessment forms to determine the ‘level’ of risk (either high, low, uncertain) and ‘type’ of risk (selection, attrition, performance, other). We then entered this information into Review Manager (RevMan) software (RevMan 5.3). We considered performing tests for funnel plot asymmetry but as we included fewer than 10 trials per comparison in the meta‐analysis, we did not conduct funnel plots. This is because the power of the tests is too low to determine real symmetry from chance in such a small numbers of included studies (Egger 1997). We reported the results using 'Risk of bias' graphs and summaries in RevMan (RevMan 5.3). In the event of future update of this Cochrane review, we will perform plot asymmetry when we include more than 10 studies. Furthermore, we will assess the presence of publication bias in the 'Discussion' section.
Data synthesis
We used a random‐effects model to synthesize measures of treatment effect. We preferred the random‐effects model to a fixed‐effect model in order to address a possible clinical heterogeneity between the included trials. However, in case of only two trials to be pooled, we chose to use a fixed‐effect model. For only a very small number of trials, a random‐effects model would provide a poor estimate of the width of the distribution of treatment effects (Chapter 9, section 9.5.4; Higgins 2011).
Continuous data
The study results did not yield continuous data suitable for a meta‐analysis, so we did not perform this. In future updates of this Cochrane review, if sufficient data are available, we will pool the mean difference (MD) or standardized mean difference (SMD), depending on the scale, using a random‐effects or fixed‐effect model, respectively, using the inverse‐variance method.
Dichotomous data
We synthesized the risk ratio (RR) by the random‐effects or fixed‐effect model, respectively, using the Mantel‐Haenszel method.
We performed all analyses using RevMan (RevMan 5.3) or the R meta package (R; R package 'meta'), and in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Systematic Review Workshop (2010). In the case of double‐zero trials, we used the R meta package (R package 'meta') instead of RevMan (RevMan 5.3) in order to incorporate these trials in the overall effect measure. We reported the results via a graphical assessment using forest plots showing the study estimates, as we have noted above. We also reported any additional subgroup and sensitivity analyses.
We assessed the quality of the evidence using the GRADE components of risk of bias assessments, indirectness and inconsistency (Schünemann 2011).
Subgroup analysis and investigation of heterogeneity
We considered performing a separate subgroup analysis, but our search of the literature did not reveal evidence in support of clinical characteristics to be identified a priori. In this Cochrane review, in an effort to better understand this, we conducted a post‐hoc evaluation of the characteristics table. Additionally, owing to the low overall number of included trials, we did not consider performing a meta‐regression analysis.
Sensitivity analysis
We considered doing a sensitivity analysis in order to determine how sensitive (i.e. robust) the results of the meta‐analyses are to the included RCTs, given differences in size, quality, and other methodological variations of the included trials.
For the comparison of RSL versus WGL, we did not conduct a sensitivity analysis as there were only two trials in the comparison. For the comparison of ROLL versus WGL, we did not perform a sensitivity analysis because the 'Risk of bias' table did not reveal a difference in risk between trials for the comparison where heterogeneity was present. Therefore, it was not justified (Higgins 2011).
Results
Description of studies
Results of the search
See: Figure 1.
1.
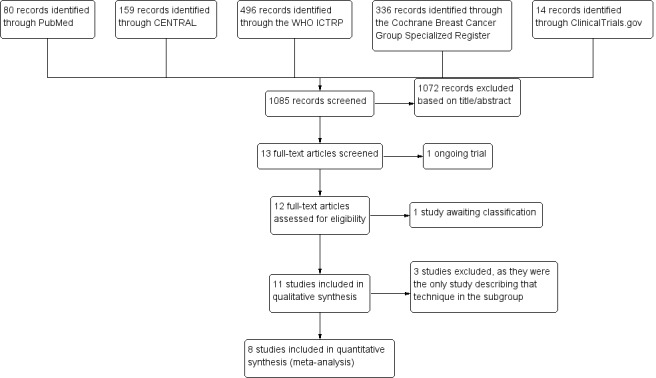
Study flow diagram.
Through our database searches, we retrieved 1085 records, of which we excluded 1072 records after we screened these articles by title and abstract. We excluded a further two articles as they were: 1. still recruiting until 2017 (Langhans 2017); and 2) awaiting classification (Parvez 2014). Eleven RCTs met the inclusion criteria of this Cochrane review for qualitative synthesis (Gray 2001; Lovrics 2011a; Mariscal Martinez 2009; Medina‐Franco 2008; Moreno 2008; Ocal 2011; Postma 2012; Rampaul 2004; Rahusen 2002; Tafra 2006; Tang 2011), and we included eight in the meta‐analysis (Gray 2001; Lovrics 2011a; Mariscal Martinez 2009; Medina‐Franco 2008; Moreno 2008; Ocal 2011; Postma 2012; Rampaul 2004). We excluded the remaining three trials from meta‐analysis as there was only one study in each respective technique subgroup (Rahusen 2002; Tafra 2006; Tang 2011).
Included studies
See: Characteristics of included studies (Table 1; Table 2).
1. Interventions in included RCTs.
| Trial | Period | Country | Participants | Sample size | Intervention | |
| Postma 2012 | December 2007 to Apr 2011 |
Netherlands Multicentre |
Confirmed occult breast cancer (core needle biopsy proven), requiring BCS and SNB. Women ≥ 18 years. Exclusion: pregnancy, breastfeeding, multifocal disease, in situ carcinoma only, and participants with breast implants. |
162 | ROLL | Intratumoural injection of 120 MBq 99mTc‐nanocolloid in max 0.5 cc and air bubble. Scintigraphy used 1 to 3 hrs preoperatively, and patent blue injected peri‐tumourally immediately preoperatively. |
| 152 | WGL | Guide wire inserted intratumourally. Scintigraphy used 1 to 3 hrs preoperatively, and patent blue injected peri‐tumourally immediately preoperatively. | ||||
| Ocal 2011 | June 2007 to May 2009 |
Turkey | Non‐palpable breast lesions requiring excisional biopsy. | 56 | ROLL | Intratumoural/peritumoural injection of 7.4 MBq/10.5 MBq 99mTc‐labelled colloidal human serum albumin. 4 or 24 hrs preoperatively respectively. |
| 52 | WGL | Single hooked wire inserted intratumourally. Immediately preoperatively. | ||||
| Mariscal Martinez 2009 | May 2004 to April 2007 |
Spain | Non‐palpable breast cancer, confirmed with FNA/core biopsy, for BCS + SNB. Exclusion: pregnant, previous excisional biopsy or non‐oncological breast surgery or neoadjuvant therapy, locally advanced or extensive multifocal carcinoma. |
66 | ROLL | 74 MBq 99mTc‐colloid, if > 3.5 cm microcalcification radiotracer dose was distributed in various points. 3 to 16 hrs preoperatively. |
| 68 | WGL | Double helix and a locking dual hook‐end design, if > 3.5 cm microcalcification a bracketing wire was used. 0.5 hrs preoperatively. | ||||
| Moreno 2008 | 2008 | Brazil | Suspicious breast opacity or microcalcification cluster requiring diagnostic excision. | 61 | ROLL | 5.55 MBq 99mTc‐macro albumin aggregate in 0.2 mL of saline, with 0.1 mL of water‐soluble non‐ionic iodinated contrast medium to confirm tracer position. On day of surgery, under local anaesthetic. |
| 59 | WGL | Malleable needle with spear at distal extremity to locate lesion. | ||||
| Medina‐Franco 2008 | April 2005 to December 2006 |
Mexico | Women > 18 years with a non‐palpable breast lesion requiring pathological diagnosis. Exclusion: albumin allergy, diffuse microcalcification or multicentric lesions, and therapeutic resections |
50 | ROLL | Average 3.7 MBq 0.2 to 0.3 mL 99mTc‐labelled human serum albumin particles (10 to 150 μm) 1 to 4 hrs preoperatively. Performed under local anaesthetic ± sedation. |
| 50 | WGL | Single hooked wire; no bracketing technique used in any case. | ||||
| Rampaul 2004 | 2004 | England | Impalpable breast lesion requiring either therapeutic or diagnostic excision. | 48 | ROLL | 99mTc‐labelled macro albumin aggregate + 0.2 mL water‐soluble non‐ionic iodinated contrast medium, to check accuracy of injection. |
| 47 | WGL | Wire tip position confirmed with balloting tip of an oversheath needle. | ||||
| Lovrics 2011a | June 2004 to January 2010 |
Canada Multicentre |
Women ≥ 18 years, nonpalpable breast tumour, histologically confirmed invasive or in situ breast carcinoma for BCS (lumpectomy or partial mastectomy) Exclusion: pregnancy, lactation, multicentric breast cancer, locally advanced disease, lobular carcinoma in situ only |
152 | RSL | I125 titanium seed (5x80mm) positioned via 18‐gauge spinal needle. |
| 153 | WGL | Standard localization with hooked wire. | ||||
| Gray 2001 | November 1999 to February 2001 |
USA | Women, nonpalpable breast lesions. Exclusion: carcinoma (only in first 30 recruits) |
51 | RSL | Titanium seed 0.29 mCi 125I (45 x 80 mm) passed through 18‐gauge needle < 5 days of operation. |
| 46 | WGL | Performed with standard techniques. | ||||
BCS: breast conserving surgery ROLL: radioguided occult lesion localization RSL: radioactive seed localization SNB: sentinel node biopsy WGL: wire guided localization
2. Baseline characteristics of included trials.
| Trial | Intervention | Sample size | Intervention cross‐over | Mean age (year) a | Benign | DCIS component | Invasive cancer | Localization | |||
| Ultrasound | Stereotactic | ||||||||||
| Postma 2012 | ROLL | 162 | 4 | 60.5 ± 7.7 | 0 | 90 | 162 | P = 0.42 | 150 | 13 | — |
| WGL | 152 | 1 | 61.1 ± 9.7 | 0 | 78 | 152 | — | 146 | 7 | — | |
| Ocal 2011 | ROLL | 56 | 0 | 45 (25 to 61) | 44 | 6 | 6 | P = 0.505 | 31 | 25 | P = 0.13 |
| WGL | 52 | 0 | 47 (34 to 72) | 38 | 7 | 7 | — | 21 | 31 | — | |
| Mariscal Martinez 2009 | ROLL | 66 | 0 | 57.5 ± 10.5 | 0 | 10 | 56 | — | 45 | 21 | P = 0.102 |
| WGL | 68 | 0 | 56.4 ± 11.7 | 0 | 14 | 54 | — | 37 | 31 | — | |
| Moreno 2008 | ROLL | 61 | 0 | 50.7 (32 to 76) | 51 | 8 | 2 | P = 0.047 | 24 | 27 | P = 0.13 |
| WGL | 59 | 0 | 49.9 (35 to 77) | 43 | 10 | 6 | — | 28 | 31 | — | |
| Medina‐Franco 2008 | ROLL | 50 | 0 | 55.8 ± 9.3 | — | — | 9 | P = 0.79 | 9 | 41 | P = 0.43 |
| WGL | 50 | 0 | 55.3 ± 10.24 | — | — | 8 | — | 11 | 39 | — | |
| Rampaul 2004 | ROLL | 48 | 2 | — | 9 | 13 | 26 | — | 24 | 24 | — |
| WGL | 47 | 0 | — | 8 | 12 | 27 | — | 21 | 26 | — | |
| Lovrics 2011a | RSL | 152 | 18 | 60.9 ± 9.5 | 0 | 30 | 124 | — | 107 | 45 | — |
| WGL | 153 | 0 | 59.9 ± 10.4 | 0 | 24 | 129 | — | 106 | 47 | — | |
| Gray 2001 | RSL | 51 | 0 | — | 16 | 4 | 31 | — | — | — | — |
| WGL | 46 | 0 | — | 20 | 5 | 21 | — | — | — | — | |
DCIS: ductal carcinoma in situ ROLL: radioguided occult lesion localization RSL: radioactive seed localization WGL: wire guided localization
aResults expressed either in ± SD or (range).
We identified eight randomized controlled trials (RCTs) for inclusion in meta‐analysis; six compared ROLL versus WGL (Postma 2012, Mariscal Martinez 2009, Ocal 2011, Moreno 2008, Medina‐Franco 2008, Rampaul 2004) and two compared RSL versus WGL (Gray 2001; Lovrics 2011a). We did not include any non‐randomized studies in this Cochrane review.
Characteristics of participants
The eight trials included a total of 1273 participants with non‐palpable breast lesions (627 participants (WGL), 443 participants (ROLL), and 203 participants (RSL)). The participant populations varied considerably amongst the included trials, which included participants with both non‐palpable benign and malignant lesions, and varied in defining clear margins.
There were major differences in preoperative diagnosis across the included trials. Three trials had a confirmed occult breast cancer diagnosis from either core needle or fine needle aspiration (FNA) (Lovrics 2011a; Mariscal Martinez 2009; Postma 2012). In Postma 2012, 40 of the 314 participants had positive margins where 26 participants (13 from each group) underwent further wide‐local excision, six out of 162 participants undergoing ROLL and two of 152 participants undergoing WGL received mastectomy, and the remaining six participants with focally involved margins received radiotherapy boost. In Mariscal Martinez 2009, a significant number of participants ultimately received a mastectomy (six of 66 participants (ROLL), six of 68 participants (WGL)). Of the six participants in the ROLL group, one was a conversion during the initial operation; all were included in the final analysis. In Lovrics 2011a, six of 152 participants having RSL and nine of 153 participants having WGL underwent mastectomy.
In contrast, five trials included participants with a non‐palpable breast lesion for either diagnostic or therapeutic purposes, leading to mixed pathology (Gray 2001; Medina‐Franco 2008; Moreno 2008; Ocal 2011; Rampaul 2004). In Ocal 2011 12 of the 56 participants undergoing ROLL and 14 of 52 participants with WGL had a cancer diagnosis. Amongst the participants with cancer, one of the 12 ROLL participants and six of the 14 WGL participants involved margins. One participant from each group underwent a mastectomy due to participant preference, three of the WGL participants required a mastectomy due to extensive ductal carcinoma in situ (DCIS), and the remaining two participants of the WGL group underwent re‐excision. A further three participants with clear margins requested a mastectomy (one ROLL and two WGL). In Moreno 2008, the significant difference was ROLL performed under local anaesthetic. There was also a significant mix in pathology, with a cancer diagnosis only in 10 of the 61 ROLL participants and 16 of the 59 WGL participants. Medina‐Franco 2008 observed a low volume of neoplastic diagnosis in nine of the 50 ROLL participants and eight of the 50 WGL participants; all of whom underwent breast‐conserving treatment. However in Rampaul 2004 the main pathology was neoplastic; in 39 of the 48 ROLL participants and 39 of the 47 WGL participants. In Gray 2001 intention for localization was again mixed, with a cancer diagnosis in 35 of 51 participants undergoing ROLL and 26 of 46 participants undergoing WGL.
Characteristics of interventions
There were only slight variations in technique in WGL, ROLL and RSL amongst the included trials, mainly regarding dosage and timing between localization and surgery (Table 1).
Outcomes
Primary outcomes
All eight included RCTs reported successful localization of the lesion without complication, with additional data from four trials describing intraoperative re‐excision rates (Lovrics 2011a; Medina‐Franco 2008; Ocal 2011; Rampaul 2004). Regarding treatment cross‐over, in Rampaul 2004 two participants having ROLL showed intraduct isotope dissemination: they were converted to WGL and excluded from the analysis. Three WGL participants had inadequate initial marking, but trial authors did not further state whether crossover occurred. Postma 2012 had four participants in ROLL who received WGL, and one participant in WGL received ROLL owing to logistics or technical reasons; analysis were based on intention‐to‐treat. Lovrics 2011a had 18 RSL participants who received WGL: six due to unavailability of seed, 12 due to technical failure of seed placement, and in three a wire was used in addition to the seed to bracket larger lesions; analyses were a comprehensive mix of per‐protocol‐treatment and intention‐to‐treat;
all eight included RCTs reported successful excision of the lesion;
all but one included trial reported positive excision margins (Rampaul 2004). Margin definitions varied, which made the literature difficult to interpret. Positive margins ranged from disease‐free > 10 mm (Mariscal Martinez 2009); ≥ 10 mm for invasive cancer, ≥ 5 mm for DCIS, and ≥ 1 mm for benign disease (Moreno 2008); ≥ 1 mm for invasive cancer and ≥ 5 mm for DCIS (Ocal 2011); < 1 mm from inked margin (Gray 2001); to at the inked margin (Lovrics 2011a; Postma 2012). Neither Medina‐Franco 2008 nor Rampaul 2004 made any description of margin definition. We have adopted a pragmatic approach and accepted what the trial authors defined as clear margins when making comparisons;
five trials reported re‐operation rate (Lovrics 2011a; Mariscal Martinez 2009; Medina‐Franco 2008; Ocal 2011, Postma 2012).
Secondary outcomes
All eight included RCTs reported operation time. However, the trials varied in whether they reported imaging time separately from imaging time, and the form of imaging used: either stereotactic or ultrasound guided;
only two trials reported length of hospital stay (Medina‐Franco 2008; Moreno 2008);
five trials reported postoperative complications (Lovrics 2011a; Medina‐Franco 2008; Moreno 2008; Ocal 2011; Postma 2012) and these included haematoma, seroma, and infection.
none of the eight included RCTs captured recurrence;
three trials reported cosmesis was reported (Medina‐Franco 2008; Moreno 2008; Postma 2012). This was participant‐reported, but all three trials used a different reporting tool;
four trials reported participant preference according to their pain perception (Lovrics 2011a; Moreno 2008; Postma 2012; Rampaul 2004).
The included RCTs did not report any long‐term outcomes.
Excluded studies
We excluded three trials from the meta‐analysis; these met the inclusion criteria of this Cochrane review but we excluded them from the meta‐analysis as they were the only trial in their subgroup. We excluded intraoperative ultrasound‐guided lumpectomy (IOUS), modified ROLL technique in combination with methylene dye (RCML), and cryo‐assisted localization (CAL) from the meta‐analysis as they were the only RCTs in their localization technique subgroup (Rahusen 2002; Tafra 2006; Tang 2011).
Amongst the three excluded RCTs:
Rahusen 2002 investigated IOUS versus WGL in participants with a diagnosis of non‐palpable breast cancer. The trial authors argued that a dynamic intraoperative visualization offers better assessment of margins and maximises preservation of healthy glandular tissue. The trial recruited 49 participants (26 participants (IOUS), 23 participants (WGL)) and they found mean tumour diameter, specimen weight, and operating time were comparable. However, participants from the IOUS group had only 11% positive margins versus 65% for WGL (P = 0.007).
Tang 2011 investigated a modified ROLL technique in combination with methylene dye (RCML) versus WGL for non‐palpable breast lesions without a preoperative histological diagnosis. The trial authors hypothesised that this provided the advantage of making the lesion visible to the eye during excision. The trial recruited 157 participants (79 participants (RCML), 78 participants (WGL)) and they found RCML superior to WGL regarding positive margins (19% versus 40% P = 0.038), mean specimen weight (39 g versus 45 g, P < 0.001), mean incision length (36 mm versus 45 mm, P < 0.001), and mean operating time (14.7 mins versus 16 mins, P = 0.001).
Tafra 2006 investigated CAL versus WGL. Under ultrasound guidance the 2.7 mm Visica cryoprobe Treatment System (TM) (Sanarus Medical, USA) was inserted into the centre of the breast lesion. Upon setting to HI freeze, the lesion was immobilized to ‐160°C and established an ice margin of ≥ 8 mm around the lesion. The cryoprobe would then be set to LO freeze to maintain the cryo‐status. With the lesion now palpable, surgeons would then excise the lesion either via a separate incision or from the probe insertion point. The trial recruited 310 participants (206 participants (CAL), 104 participants (WGL)) and they found CAL and WGL comparable regarding positive margins and re‐excision rates. However, the trial found that CAL was superior in ease of lumpectomy, specimen quality, cosmesis, patient satisfaction, and operative time.
Risk of bias in included studies
See: Figure 2.
2.
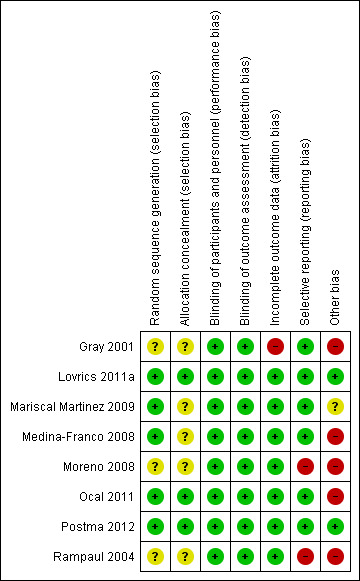
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.
Allocation
Five RCTs described the random sequence generation well using computer‐generated, random number table, at time of clinic visit, independent trial centre by phone, or by an independent computer consultant (Lovrics 2011a; Mariscal Martinez 2009; Medina‐Franco 2008; Ocal 2011; Postma 2012). Although the remaining studies (Gray 2001; Moreno 2008; Rampaul 2004) failed to mention specific randomization methods, the use of randomization was referenced.
Only three trials clearly stated concealment (Lovrics 2011a; Ocal 2011; Postma 2012).
Blinding
Any comparison in localization techniques with WGL as the gold standard makes it very difficult to blind the patient, personnel, or assessor. However, the outcome is unlikely to be influenced by lack of blinding, and therefore we judged this as low risk as a potential confounder.
Incomplete outcome data
Seven of the eight included trials reported excluded participants, with reasons stated (Lovrics 2011a; Mariscal Martinez 2009; Medina‐Franco 2008; Moreno 2008; Ocal 2011; Postma 2012; Rampaul 2004). However, Gray 2001 withdrew four participants because the lesion was no longer visible and excluded five participants because of missing data. In an effort to better understand the possible the extent of this bias, we contacted the original trial author. It was revealed that some data points were collected on these five participants and not reported; however, given that the amount of missing data was approximately 5%, we did not conduct a sensitivity analysis.
Furthermore, since missing data can also impact the overall findings and analysis, we addressed this issue as we have noted in the relevant section above ‘Dealing with missing data' and in the 'Discussion' section. We interpreted these findings and presented them in accordance with the guidelines set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and Oxman 1992.
Selective reporting
Seven of the eight trials reported marginal status as a main outcome, although there was also a wide discrepancy in details of further intervention and intraoperative re‐excision reported (Gray 2001; Lovrics 2011a; Mariscal Martinez 2009; Medina‐Franco 2008; Moreno 2008; Ocal 2011; Postma 2012). Rampaul 2004, however, did not describe marginal status and had significant intraoperative re‐excision rates in both groups (18 of the 46 ROLL and 13 of the 47 WGL). Whereas, Moreno 2008 did not report definitive intervention for the three positive margins with a neoplastic diagnosis.
Other potential sources of bias
Only three trials calculated sample size. Lovrics 2011a anticipated a 15% difference in positive margin (2‐sided α‐level of 5%, power 80%). Mariscal Martinez 2009 expected 20% difference in between techniques (2‐sided α‐level of 10%, power 80%). Postma 2012 expected 15% difference in tumour‐free margins (2‐sided α‐level of 5%, power 80%). Including the other underpowered studies for this meta‐analysis increases the chance of type II error, especially when analysing secondary outcomes. Additionally, of the eight trials included in the meta‐analysis, only three trials stated funding sources or conflicts of interest (Lovrics 2011a; Moreno 2008; Postma 2012). Contact was attempted with three authors (Medina‐Franco 2008; Mariscal Martinez 2009; Rampaul 2004) and a response from Medina‐Franco 2008 confirmed no conflicts of interest. Contact details were unavailable for Rampaul 2004 and contact was unsuccessful for Mariscal Martinez 2009.
Also discussed earlier, other sources of bias included mixed pathology (Gray 2001; Medina‐Franco 2008; Moreno 2008; Ocal 2011; Rampaul 2004) and undefined margins (Moreno 2008; Medina‐Franco 2008), which may have compromised the results. Observer variation was possible in all studies, as they were unblinded. Additionally, only one radiologist was used in Mariscal Martinez 2009 possibly further precluding interobserver variability.
Effects of interventions
See: Characteristics of included studies; Table 2; Table 3; and Table 4.
3. Primary outcomes of included trials.
| Trial | Intervention | Localization complication | Successful retrieval | Positive margins | Re‐operations | Intraoperative re‐excision | ||||
| Postma 2012 | ROLL | 3 | 162/162 | — | 22/162 | P = 0.644 | 19/162 | P = 0.582 | — | — |
| WGL | 1 | 152/152 | — | 18/152 | 15/152 | — | — | — | ||
| Ocal 2011 | ROLL | 0 | 56/56 | P = 0.503 | 1/12 | P = 0.05 | 2/56 | — | 3/56 | — |
| WGL | 1 dislodged | 51/52 | — | 6/14 | 8/52 | — | 4/52 | — | ||
| Mariscal Martinez 2009 | ROLL | 0 | 66/66 | — | 7/66 | P = 0.357 | 5/66 | — | — | — |
| WGL | 6 repositioning | 68/68 | — | 12/68 | — | 12/68 | — | — | — | |
| Moreno 2008 | ROLL | 0 | 61/61 | — | 1/10 | P = 0.67 | — | — | — | — |
| WGL | 2 dislodged | 59/59 | — | 2/16 | — | — | — | — | — | |
| Medina‐Franco 2008 | ROLL | 0 | 50/50 | — | 1/9 | P = 0.04 | 1/9 | P = 0.04 | 2/50 | — |
| WGL | 1 dislodged | 50/50 | — | 3/8 | — | 3/8 | — | 0/50 | — | |
| Rampaul 2004 | ROLL | 2 intraduct dissemination ‐ WGL | 46/48 | P = 0.242 | — | — | — | — | 18/46 | P = 0.276 |
| WGL | 0 | 44/47 | — | — | — | — | — | 13/47 | — | |
| Lovrics 2011a | RSL | 13 migration, displaced, failure to deploy | 152/152 | — | 29/152 | P = 0.609 | 23/152 | P = 0.389 | 69/152 | P = 0.644 |
| WGL | 3 migration, dislodged | 153/153 | — | 33/153 | — | 29/153 | — | 73/153 | — | |
| Gray 2001 | RSL | 0 | 51/51 | — | 9/35 | P = 0.02 | — | — | — | — |
| WGL | 0 | 46/46 | — | 15/26 | — | — | — | — | — | |
ROLL: radioguided occult lesion localization RSL: radioactive seed localization WGL: wire guided localization
4. Secondary outcomes of included trials.
| Trial | Intervention | Imaging time (min)a | Operation time (min)a | Length of hospital stay (hour) | Postoperative complications | Cosmesis | Participant painb | ||||||||
| Postma 2012 | ROLL | 5 (1 to 45) | — | 18 (7 to 50) | P = 0.133 | — | — | 25 | Not SF | — | — | 8# | P = 0.554 | 3 | P = 0.578 |
| WGL | 7 (1 to 60) | — | 16 (6 to 80) | — | — | — | 20 | — | — | — | 8# | — | 4 | — | |
| Ocal 2011 | ROLL | 15 | P = 0.001 | 31 (15 to 105) | P = 0.001 | — | — | 1 | P = 0.213 | — | — | — | — | — | — |
| WGL | 23 | — | 43 (20 to 120) | — | — | — | 1 | — | — | — | — | — | — | — | |
| Mariscal Martinez 2009 | ROLL | 14.4 ± 5.5 | P < 0.001 | 32.7 ± 12.7 | P = 0.657 | — | — | — | — | — | — | — | — | — | — |
| WGL | 20.9 ± 10.2 | — | 36.5 ± 13.7 | — | — | — | — | — | — | — | — | — | — | — | |
| Moreno 2008 | ROLL | — | — | 26.06 | P = 0.719 | 3.06 | P < 0.001 | 2 | P = 0.27 | 57 Excellent | 4 Good | 0 Poor | P < 0.001 | 1.62 | P = 0.021 |
| WGL | — | — | 37.2 | — | 18.7 | — | 1 | — | 49 Excellent | 10 Good | 0 Poor | — | 2.20 | — | |
| Medina‐Franco 2008 | ROLL | US: 8 (5 to 10) Stereotactic: 15 (10 to 21) |
P < 0.001 | 29 ± 12.8 | P = 0.23 | Same | — | 1 | — | 38 Excellent | 12 Good | — | P = 0.05 | — | — |
| WGL | US: 17 (11 to 20) Stereotactic: 23 (20 to 25) |
— | 33 ± 15.2 | — | Same | — | 1 | — | 26 Excellent | 24 Good | — | — | — | — | |
| Rampaul 2004 | ROLL | 16 | P = 0.058 | 31 | P = 0.147 | — | — | — | — | — | — | — | — | 2.7 | P = 0.012 |
| WGL | 23 | — | 35 | — | — | — | — | — | — | — | — | — | 3.6 | — | |
| Lovrics 2011a | RSL | 16.1 ± 14.1 | P = 0.916 | 19.4 ± 7.5 | P < 0.001 | — | — | 18 | P = 0.235 | — | — | — | — | Less | P = 0.038 |
| WGL | 15.8 ± 13.6 | — | 22.2 ± 8.9 | — | — | — | 12 | — | — | — | — | — | More | — | |
| Gray 2001 | RSL | 14 (4 to 27) | P = 0.49 | 5.4 (2 to 15) | P = 0.28 | — | — | — | — | — | — | — | — | — | — |
| WGL | 13.1 (3 to 25) | — | 6.1 (3 to 18) | — | — | — | — | — | — | — | — | — | — | — | |
DCIS: ductal carcinoma in situ ROLL: radioguided occult lesion localization RSL: radioactive seed localization WGL: wire guided localization
aResults expressed either in ± SD or (range).
bMean on a 0 to 10 scale.
Six trials compared ROLL versus WGL (Mariscal Martinez 2009; Medina‐Franco 2008; Moreno 2008; Ocal 2011; Postma 2012;Rampaul 2004) and we reported them using the random‐effects model.
Two trials compared RSL versus WGL (Gray 2001; Lovrics 2011a) and we reported them using the fixed‐effect model due to the limited number of trials eligible for analysis.
Primary outcomes
1. Successful localization of the lesion: using the planned technique to localize the lesion preoperatively
Amongst the six trials that compared ROLL versus WGL, there was a degree of heterogeneity (I² statistic = 23%). The risk of complications in localizing non‐palpable breast lesions following ROLL is lower than WGL but not statistically significant (RR 0.60, 95% CI 0.16 to 2.28; 869 participants; six trials; Analysis 1.1;Figure 3).
1.1. Analysis.
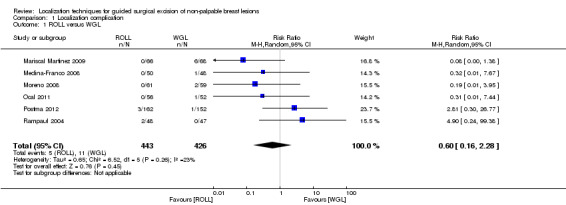
Comparison 1 Localization complication, Outcome 1 ROLL versus WGL.
3.
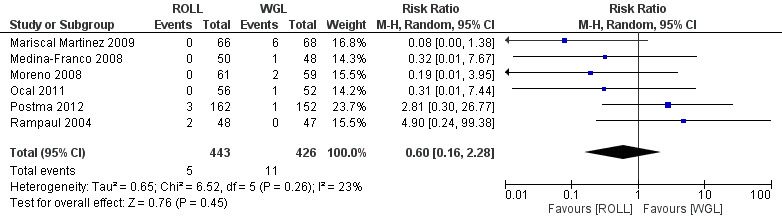
Forest plot of comparison: 1 Localization complication, outcome: 1.1 ROLL versus WGL.
One of the two trials, Gray 2001, that compared localization complications between RSL and WGL revealed a double‐zero study. We used the R meta package (R package 'meta') instead of RevMan (RevMan 5.3) because the R meta package allows to include double‐zero studies when synthesizing risk ratios. In the two trials that compared RSL versus WGL, we did not detect any heterogeneity (I² statistic = 0%). WGL was superior to RSL in complications when localizing non‐palpable breast lesions (RR 3.85, 95% CI 1.21 to 12.19; 402 participants; two trials; Figure 4).
4.
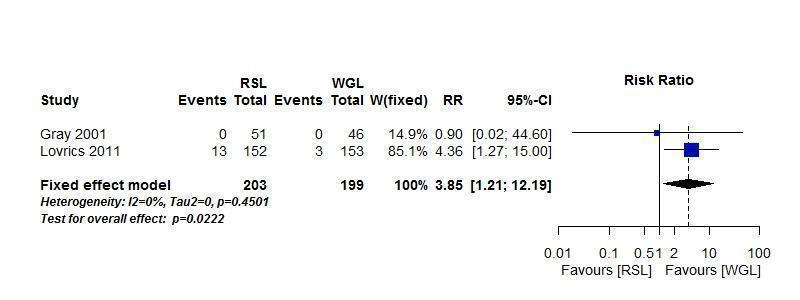
Forest plot of comparison: 1 RSL versus WGL, outcome: 1.2 Localization complications.
2. Successful excision of the lesion
Amongst the six trials that compared ROLL versus WGL, there was no heterogeneity (I² statistic = 0%). ROLL and WGL demonstrated equal effectiveness in excision of non‐palpable breast lesions (RR 1.00, 95% CI 0.99 to 1.01; 871 participants; six trials; Analysis 2.1).
2.1. Analysis.
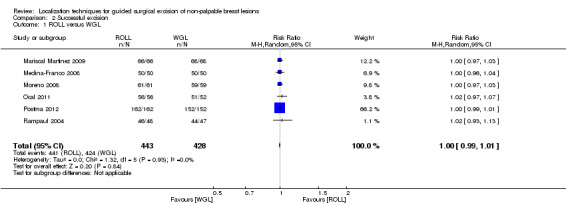
Comparison 2 Successful excision, Outcome 1 ROLL versus WGL.
In RSL versus WGL studies, there was no heterogeneity (I² statistic = 0%), RSL and WGL demonstrated equal effectiveness in excision of non‐palpable breast lesions (RR 1.00, 95% CI 0.99 to 1.01; 402 participants; two trials; Analysis 2.2).
2.2. Analysis.
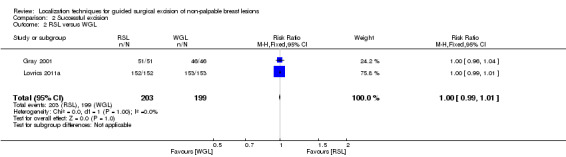
Comparison 2 Successful excision, Outcome 2 RSL versus WGL.
3. Positive excision margins
Amongst five of the six trials that compared ROLL versus WGL and reported on positive excision margins, there was a degree of heterogeneity (I² statistic = 17%). The risk of a positive tumour margin following ROLL is lower than WGL but this is not statistically significant (RR 0.74, 95% CI 0.42 to 1.29; 517 participants; five trials; Analysis 3.1).
3.1. Analysis.
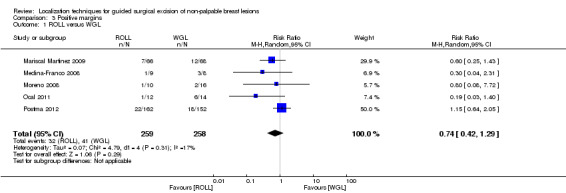
Comparison 3 Positive margins, Outcome 1 ROLL versus WGL.
In trials comparing RSL versus WGL, there was a moderate but insignificant heterogeneity (I² statistic = 57%). The risk of a positive tumour margin following RSL is lower than WGL but again not statistically significant (RR 0.67, 95% CI 0.43 to 1.06; 366 participants; two trials; Analysis 3.2; Figure 5).
3.2. Analysis.
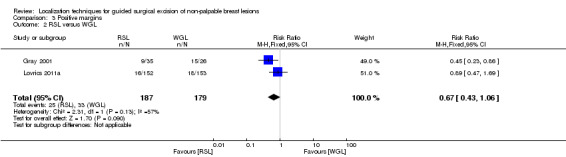
Comparison 3 Positive margins, Outcome 2 RSL versus WGL.
5.
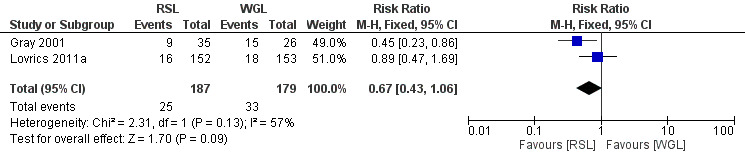
Forest plot of comparison: 3 Positive margins, outcome: 3.2 RSL versus WGL.
4. Re‐operation rate
Among four out of six trials that compared ROLL versus WGL and reported on re‐operation rate, there was moderate but insignificant heterogeneity (I² statistic = 57%). The risk of undergoing further re‐operation following ROLL is lower than WGL but not statistically significant (RR 0.51, 95% CI 0.21 to 1.23; 583 participants; four trials; Analysis 4.1; Figure 6).
4.1. Analysis.
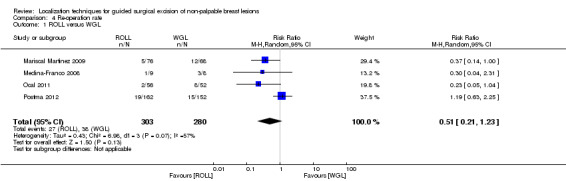
Comparison 4 Re‐operation rate, Outcome 1 ROLL versus WGL.
6.
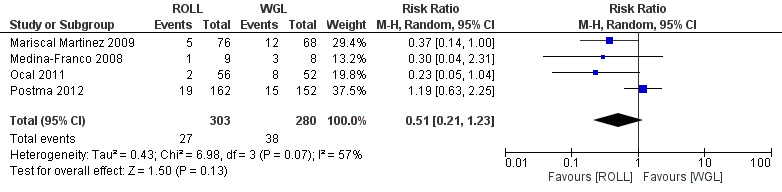
Forest plot of comparison: 4 Re‐operation rate, outcome: 4.1 ROLL versus WGL.
There were an insufficient number of trials that reported this outcome to allow a meta‐analysis of the RSL versus WGL group. Only one trial reported equal likelihood of RSL and WGL participants requiring re‐operations (RR 0.80, 95% CI 0.48 to 1.32; 305 participants; one trial; Analysis 4.2).
4.2. Analysis.
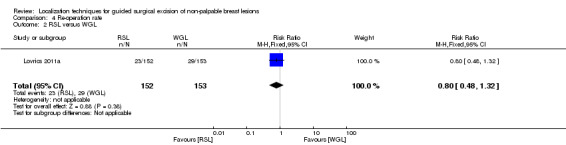
Comparison 4 Re‐operation rate, Outcome 2 RSL versus WGL.
Secondary outcomes
1. Operation time
All four of the six trials that compared ROLL versus WGL and both trials that compared RSL versus WGL reported on operation time, but there was variation in their statistical analyses so that a meaningful meta‐analysis was problematic (Table 4).
2. Length of hospital stay
Only one included trial reported length of stay, with ROLL significantly shorter than WGL (3.06 versus 18.7 hours; P < 0.001; Table 4).
3. Postoperative complications
Four of the six trials that compared ROLL versus WGL reported on postoperative complications. There was no heterogeneity (I² statistic = 0%). With the REM (RR 1.18, 95% CI 0.71 to 1.98; 642 participants; four trials), the risk of postoperative complications in WGL is lower than ROLL but not statistically significant (Analysis 5.1).
5.1. Analysis.
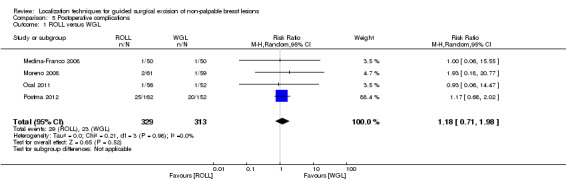
Comparison 5 Postoperative complications, Outcome 1 ROLL versus WGL.
There was inconsistency in the data format reported amongst trials, which did not allow for a meaningful meta‐analysis between RSL versus WGL. One trial reported equal likelihood of RSL and WGL participants requiring re‐operations (RR 1.51, 95% CI 0.75 to 3.03; 305 participants; one trial; Analysis 5.2).
5.2. Analysis.
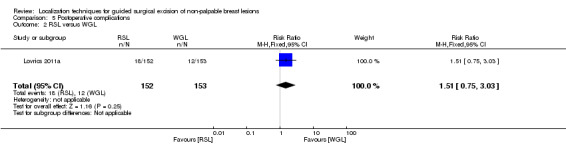
Comparison 5 Postoperative complications, Outcome 2 RSL versus WGL.
4. Recurrence
None of the included RCTs reported on this outcome.
5. Cosmesis
Three trials that compared ROLL versus WGL captured cosmesis data, but trials varied widely in the scoring tool they used and the data format they reported. While Medina‐Franco 2008 using a four‐point scoring system reported 38 versus 26 excellent scores (P = 0.05), and Moreno 2008 using a three grade scoring system reported 57 versus 49 excellent scores (P < 0.001), both favoured ROLL over WGL. Postma 2012 subsequently found them comparable using a Likert scale 8 versus 8 (P = 0.554) (Table 4).
6. Patient preference (including as a result of pain)
Three trials used a numeric pain rating score (one (no pain) to 10 (worst pain)) and found pain comparable between ROLL versus WGL. While Medina‐Franco 2008 (2.7 versus 3.6, P = 0.012) and Moreno 2008 (1.62 versus 2.20, P = 0.021) both favoured ROLL over WGL, Postma 2012 subsequently found them comparable (3 versus 4, P = 0.578) (Table 4).
Discussion
Summary of main results
In general, the outcomes of WGL, ROLL, and RSL were comparable. WGL was significantly superior to RSL in successfully localizing non‐palpable lesions (RR 3.85, 95% CI 1.21 to 12.19). However, ROLL (RR 1.00, 95% CI 0.99 to 1.01) and RSL (RR 1.00, 95% CI 0.99 to 1.01) have comparable outcomes with WGL in terms of successful excision rates. Furthermore, ROLL demonstrated favourable results in successful localization (RR 0.60, 95% CI 0.16 to 2.28), positive tumour margins (RR 0.74, 95% CI 0.42 to 1.29), and re‐operation rates (RR 0.51, 95% CI 0.21 to 1.23) versus WGL, but none of these results were statistically significant. These findings were similar in RSL that demonstrated favourable results in positive tumour margins (RR 0.67, 95% CI 0.43 to 1.06), and re‐operation rates (RR 0.80, 95% CI 0.48 to 1.32) over WGL, but neither reached statistical significance. In contrast, WGL had fewer postoperative complications to both ROLL (RR 1.18, 95% CI 0.71 to 1.98) and RSL (RR 1.51, 95% CI 0.75 to 3.03), although this was also not statistically significant.
Overall completeness and applicability of evidence
Owing to a lack of trials in various localization techniques, such as IOUS, HUG, and RCML, we can only draw our analysis over ROLL and RSL versus WGL. Furthermore, in the absence of consistency of reported patient‐centred outcomes amongst trials (i.e. careful assessment of cosmetic results, patients' satisfaction with the localization received in the x‐ray department and in surgical theatre) there is no clear evidence to support one guided technique for surgically excising non‐palpable breast lesions over another. ROLL supporters claim a much higher flexibility of this technique, which allows approaching all breast quadrants through cosmetic incisions. This differs from WGL, which inevitably has to rely on the track of the wire as inserted by the radiologist. However, we have not been able to retrieve evidence on cosmesis and patients’ preference, since this information is lacking.
Quality of the evidence
The overall quality of evidence was good. We have discussed the main sources of bias in the Risk of bias in included studies section, though it is worth again noting the possibility of attrition bias (Gray 2001). Although classified as high risk due to the fact that partial data was collected and not reported, this was not believed to impact the results, as the total amount of missing data was approximately 5%, and therefore not believed to impact the overall quality of the evidence. Additionally, five of the eight trials did not state funding sources or conflicts of interest (Gray 2001; Mariscal Martinez 2009; Medina‐Franco 2008;Ocal 2011; Rampaul 2004). However, we contacted three of the five primary trial authors and requested further details regarding funding sources (Mariscal Martinez 2009; Medina‐Franco 2008; Ocal 2011). Medina‐Franco 2008 confirmed that the trial was not funded nor were there any conflicts of interest or external industry support. Of the remaining studies where additional response or clarification was not provided, we cannot state with certainty whether there was a potential issue that influenced the findings; however it is unlikely as these were not industry sponsored trials.
Other limitations include a lack of data in standardized format for meta‐analysis, lack of complete data amongst the trials, and absence of long‐term data. Interpretation and applicability of this meta‐analysis is hindered by the mixed indication of diagnostic versus therapeutic purposes when undertaking WGL, ROLL, or RSL, which lead to a high level of mixed pathology in several trials.
Potential biases in the review process
We undertook this Cochrane review in line with the Cochrane Breast Cancer Group (CBCG) guidelines. We performed a thorough electronic database search and scanned bibliographies of retrieved articles. At least two review authors assessed all articles. The main limitation of this Cochrane review is the limitation of included trials to those published in the English language. Furthermore, with only three trials undertaking power calculations, together with the conservative statistical analysis we have adopted, this is likely to give a lack of power to detect the true effect from the interventions. The effect of underpowered studies is further amplified when attempting to draw conclusions from the subgroup analysis, such as excision margins and re‐intervention rate.
Agreements and disagreements with other studies or reviews
Three reviews in this subject have been discussed earlier, but they included non‐RCTs and did not undertake a meta‐analysis (Jakub 2010; Rovera 2008; Van der Ploeg 2008). A further three reviews has recently been published where a meta‐analysis have been undertaken. Lovrics 2011b analysed five RCTs involving ROLL and RSL together versus WGL, with additional analyses involving seven non‐randomized cohort studies. The combined ROLL and RSL group was superior to WGL, when analysing RCTs alone, for surgical margins (odds ratio (OR) 0.389, 95% CI 0.197 to 0.768, P = 0.007) and re‐operation rates (OR 0.347, 95% CI 0.126 to 0.954, P = 0.040). When including non‐randomized cohorts, the superiority of ROLL and RSL over WGL in surgical margins (OR 0.367, 95% CI 0.277 to 0.487, P < 0.001) and re‐operation rates (OR 0.347, 95% CI 0.250 to 0.481, P < 0.001) was more significant. There was however, no difference in operative times. Sajid 2012 analysed four RCTs involving ROLL versus WGL. There was a statistical difference in favour of ROLL over WGL in positive margins (OR 0.47, 95% CI 0.22 to 0.99, P < 0.05), localization duration (Mean Difference (MD) ‐6.09, 95% CI ‐6.81 to ‐5.37, P < 0.00001), surgery duration (MD ‐5.33, 95% CI ‐7.54 to ‐3.13, P < 0.00001), while no difference was demonstrated when comparing localization rate, complication rate, re‐operation rate, and weight and volume of excised breast tissue. Ahmed 2013 combined six RCTs involving ROLL versus WGL together with one RSL versus WGL for meta‐analysis. ROLL and RSL together were found to be superior over WGL in operative time (MD ‐2.95, 95% CI ‐4.43 to ‐1.47, P < 0.0001), however it was inferior to WGL in volume of excised breast tissue (MD 6.79, 95% CI 0.03 to 13.56, P = 0.05). No difference was found in re‐operation rates, successful sentinel lymph node biopsy, positive margins, or weight of specimen.
Current literature consistently demonstrates both newer ROLL and RSL techniques comparable to WGL, especially with regard to key markers: localization rate, complications, and re‐operation. The adoption of ROLL and RSL is also supported by earlier trials demonstrating fewer positive margins and shorter procedure times. But it is worth remembering that earlier studies tend to be of mixed pathology and not powered to investigate neoplastic marginal status. RSL has the additional benefit to solve scheduling conflicts by being able to be placed in situ several days prior surgery. Support for ROLL has been further dampened by a more recent high powered RCT comparing ROLL versus WGL, finding no difference in margin status, re‐operation rate or procedure duration, but a larger volume of excised breast tissue in ROLL (P = 0.017) (Postma 2012). Whilst there is further subjective evidence of patient comfort and technical preference from the surgeon and localizing radiologist to support ROLL or RSL over WGL, this should take into account the potential halo effect. WGL retains its role in clinical practice as a safe and tested technique, with the flexibility of being able to place several wires when faced with extensive microcalcification. It is also not time‐dependent, like a radiotracer that will diminish upon injection.
Whilst other techniques not included in our meta‐analysis, such as IOUS, RCML and CAL, may have limited evidence to support their use for oncological resections, their applications in certain scenarios maybe worth noting to aid future development of localization techniques. IOUS has the obvious advantage of being a completely non‐invasive technique. On the other hand, it requires either a radiologist in the operating room or the surgeon to possess adequate ultrasound skills. RCML is a variation of ROLL with the theoretical benefit of dual localization technique in the form of a visual guide. Whilst evidence supports its use compared to WGL, there has been no evidence demonstrating benefits over the main stream ROLL technique. CAL presents an interesting concept of converting the non‐palpable lesion to a palpable one with a commercial cryoprobe device. However, the device causes necrosis at the centre of the specimen disrupting pathologic assessment and relying on preoperative core biopsy results, although in the RCT conducted this was not an issue.
Authors' conclusions
Implications for practice.
Currently WGL is the most widely adopted approach for localizing non‐palpable breast lesions for surgical excision, with ROLL and RSL increasingly being adopted. However, results from the present review supports its continuing use as a safe and tested technique that allows for flexibility in selected cases. ROLL and RSL could be offered to patients as a comparable replacement for WGL as they are equally reliable. ROLL allows for simultaneous sentinel node identification (SNOLL), while RSL has the benefit of a longer and more flexible timing between seed insertion and surgery. The slow uptake of the latter radioisotope‐based localization techniques maybe a combination of difficulties in acquiring the radioisotope and suitable technical expertise.
Implications for research.
Given the popularity and long‐term data of WGL, it should be considered as the gold standard with new localization techniques randomized against it. The results of this review also stress the need for more fully powered RCTs to evaluate the best technique according to the comprehensive criteria described, especially regarding patient‐centred outcomes (e.g. cosmesis, pain, and satisfaction), in addition to more consistent and standardized approaches in outcome reporting. Other techniques such as IOUS, RCML and CAL are of academic interest, but recommendation for routine use in the clinical environment and oncological outcomes require further validation.
What's new
| Date | Event | Description |
|---|---|---|
| 1 February 2016 | Amended | Corrected labels for Analysis 2.1 and Analysis 2.2, and also the risk of bias text and table related to 'other sources of bias' |
Acknowledgements
We thank the staff at the CBCG editorial base for their assistance.
Appendices
Appendix 1. PubMed (MEDLINE)
1.((((((((((((randomised controlled trial*) OR randomized controlled trial*) OR controlled clinical trial*) OR random allocation) OR double‐blind method ) OR single‐blind method ) OR clinical trial*) OR randomised) OR randomized) OR placebo) OR randomly) OR crossover) OR cross‐over
2.((((lesion*) OR cancer*) OR neoplasm*) OR carcinoma*) AND breast
3.(((nonpalpable) OR non‐palpable) OR non palpable) OR occult
4.(((((((((((((((((localization) OR localization) OR wire guided localization) OR WGL) OR needle wire localization) OR NWL) OR radioguided occult lesion localization) OR roll) OR radioactive seed localization) OR RSL) OR intraoperative ultrasound) OR IOUS) OR cryo‐assisted localization) OR cryoprobe‐assisted lumpectomy) OR cryoprobe assisted localization) OR CAL) OR haematoma‐directed ultrasound‐guided) OR HUG
5.#1 AND #2 AND #3 AND #4
6.#1 AND #2 AND #3 AND #4 Limits: Humans, English
Appendix 2. CENTRAL
1.Breast 2.Breast lesion 3.Breast lesions 4.Breast cancer 5.Breast cancers 6.Breast neoplasm 7.Breast neoplasms 8.Breast carcinoma 9.Breast carcinomas 10.#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 11.Nonpalpable 12.Non‐palpable 13.Non palpable 14.Occult 15.#11 or #12 or #13 or #14 16.#10 and #15
17.Limit to trials
Appendix 3. WHO ICTRP Search Portal
Basic search
1. Localization techniques for guided surgical excision of non‐palpable breast lesions 2. non‐palpable breast lesion* OR breast cancer* AND wire guided localisation 3. non‐palpable breast lesion* OR breast cancer* AND WGL 4. non‐palpable breast lesion* OR breast cancer* AND radioguided occult lesion localization 5. non‐palpable breast lesion* OR breast cancer* AND ROLL 6. non‐palpable breast lesion* OR breast cancer* AND radioactive seed localization 7. non‐palpable breast lesion* OR breast cancer* AND RSL 8. non‐palpable breast lesion* OR breast cancer* AND intraoperative ultrasound 9. non‐palpable breast lesion* OR breast cancer* AND IOUS 10. non‐palpable breast lesion* OR breast cancer* AND cryoprobe‐assisted localization 11. non‐palpable breast lesion* OR breast cancer* AND CAL 12. non‐palpable breast lesion* OR breast cancer* AND haematoma‐directed ultrasound‐guided 13. non‐palpable breast lesion* OR breast cancer* AND HUG 14. non‐palpable breast lesion* OR breast cancer* AND cryoprobe‐assisted localisation 15. non‐palpable breast lesion* OR breast cancer* AND haematoma directed ultrasound guided 16. non‐palpable breast lesion* OR breast cancer* AND cryoprobe assisted localization
Advanced search
1. Title: Localization techniques for guided surgical excision of non‐palpable breast lesions Recruitment Status: ALL
2. Condition: non‐palpable breast lesion* OR non palpable breast lesion* OR breast cancer* OR breast neoplasm* OR breast carcinoma*
Intervention: wire guided localization OR wire guided localisation OR WGL OR radioguided occult lesion localization OR ROLL OR radioactive seed localization OR RSL OR intraoperative ultrasound OR IOUS OR cryoprobe‐assisted localization OR CAL OR haematoma‐directed ultrasound‐guided OR HUG OR radioguided occult lesion localisation OR cryoprobe‐assisted localisation OR haematoma directed ultrasound guided OR cryoprobe assisted localisation Recruitment Status: ALL
3. Condition: non‐palpable breast lesion% OR non palpable breast lesion%
Intervention: (surgical intervention% OR surger% OR wire guided localization OR wire guided localisation OR WGL) AND (radioguided occult lesion localization OR ROLL OR radioactive seed localization OR RSL OR intraoperative ultrasound OR IOUS OR cryoprobe‐assisted localization OR CAL OR haematoma‐directed ultrasound‐guided OR HUG OR radioguided occult lesion localisation OR cryoprobe‐assisted localisation OR haematoma directed ultrasound guided OR cryoprobe assisted localisation)
Recruitment Status: ALL
4. Condition: non‐palpable breast lesion% OR non palpable breast lesion%
Intervention: (surgical intervention% OR surger%) AND (wire guided localization OR wire guided localisation OR WGL OR radioguided occult lesion localization OR ROLL OR radioactive seed localization OR RSL OR intraoperative ultrasound OR IOUS OR cryoprobe‐assisted localization OR CAL OR haematoma‐directed ultrasound‐guided OR HUG OR radioguided occult lesion localisation OR cryoprobe‐assisted localisation OR haematoma directed ultrasound guided OR cryoprobe assisted localisation)
Recruitment Status: ALL
Appendix 4. ClinicalTrials.gov
Basic search
1. Localization techniques for guided surgical excision of non‐palpable breast lesions 2. non‐palpable breast lesion* AND wire guided localisation 3. non‐palpable breast lesion* AND WGL 4. non‐palpable breast lesion* AND radioguided occult lesion localization 5. non‐palpable breast lesion* AND ROLL 6. non‐palpable breast lesion* AND radioactive seed localization 7. non‐palpable breast lesion* AND RSL 8. non‐palpable breast lesion* AND intraoperative ultrasound 9. non‐palpable breast lesion* AND IOUS 10. non‐palpable breast lesion* AND cryoprobe‐assisted localization 11. non‐palpable breast lesion* AND CAL 12. non‐palpable breast lesion* AND haematoma‐directed ultrasound‐guided 13. non‐palpable breast lesion* AND HUG 14. non‐palpable breast lesion* AND cryoprobe‐assisted localisation 15. non‐palpable breast lesion* AND haematoma directed ultrasound guided 16. non‐palpable breast lesion* AND cryoprobe assisted localization
Advanced search
1. Title: Localization techniques for guided surgical excision of non‐palpable breast lesions Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies 2. Condition: non‐palpable breast lesion* OR breast cancer* OR breast neoplasm* OR breast carcinoma* Intervention: wire guided localisation OR radioguided occult lesion localization OR radioactive seed localization OR intraoperative ultrasound OR cryoprobe‐assisted localization OR haematoma‐directed ultrasound‐guided Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies 3. Condition: non‐palpable breast lesion* OR non palpable breast lesion* Intervention: surgical intervention* OR surger* OR wire guided localization OR wire guided localisation OR radioguided occult lesion localization OR radioactive seed localization OR intraoperative ultrasound Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies 4. Condition: non‐palpable breast lesion* OR non palpable breast lesion* Intervention: cryoprobe‐assisted localization OR haematoma‐directed ultrasound‐guided OR radioguided occult lesion localisation OR cryoprobe‐assisted localisation OR haematoma directed ultrasound guided OR cryoprobe assisted localisation Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies 5. Condition: non‐palpable breast lesion* OR non palpable breast lesion* Intervention: (surgical intervention* OR surger*) AND (wire guided localization OR wire guided localisation OR radioguided occult lesion localization OR radioactive seed localization OR radioguided occult lesion localisation OR intraoperative ultrasound) Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies 6. Condition: non‐palpable breast lesion* OR non palpable breast lesion* Intervention: (surgical intervention* OR surger*) AND (cryoprobe‐assisted localization OR haematoma‐directed ultrasound‐guided OR cryoprobe‐assisted localisation OR haematoma directed ultrasound guided OR cryoprobe assisted localisation) Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies 7. Condition: breast cancer* OR breast neoplasm* Intervention: (surgical intervention* OR surger* OR wire guided localization OR wire guided localisation) AND (radioguided occult lesion localization OR radioactive seed localization OR radioguided occult lesion localisation OR intraoperative ultrasound) Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies 8. Condition: breast cancer* OR breast neoplasm* Intervention: (surgical intervention* OR surger* OR wire guided localization OR wire guided localisation) AND (cryoprobe‐assisted localization OR haematoma‐directed ultrasound‐guided OR cryoprobe‐assisted localisation OR haematoma directed ultrasound guided) Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies 9. Condition: breast cancer* OR breast neoplasm* Intervention: (surgical intervention* OR surger*) AND (wire guided localization OR wire guided localisation OR radioguided occult lesion localization OR radioactive seed localization OR radioguided occult lesion localisation OR intraoperative ultrasound) Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies 10. Condition: breast cancer* OR breast neoplasm* Intervention: (surgical intervention* OR surger*) AND (cryoprobe‐assisted localization OR haematoma‐directed ultrasound‐guided OR cryoprobe‐assisted localisation OR haematoma directed ultrasound guide) Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies 11. Condition: breast cancer* OR breast neoplasm* OR breast lesion* Intervention: wire guided localization OR wire guided localisation OR radioguided occult lesion localization OR radioguided occult lesion localisation OR radioactive seed localization OR intraoperative ultrasound Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies 12. Condition: breast cancer* OR breast neoplasm* OR breast lesion* Intervention: cryoprobe‐assisted localization OR haematoma‐directed ultrasound‐guided OR OR cryoprobe‐assisted localisation OR haematoma directed ultrasound Recruitment Status: All studies Study Results: All studies Study Type: All studies Gender: All studies
Data and analyses
Comparison 1. Localization complication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ROLL versus WGL | 6 | 869 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.16, 2.28] |
Comparison 2. Successful excision.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ROLL versus WGL | 6 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |
| 2 RSL versus WGL | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.99, 1.01] |
Comparison 3. Positive margins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ROLL versus WGL | 5 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.29] |
| 2 RSL versus WGL | 2 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.43, 1.06] |
Comparison 4. Re‐operation rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ROLL versus WGL | 4 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.21, 1.23] |
| 2 RSL versus WGL | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.48, 1.32] |
Comparison 5. Postoperative complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ROLL versus WGL | 4 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.71, 1.98] |
| 2 RSL versus WGL | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.75, 3.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gray 2001.
| Methods | Prospective RCT | |
| Participants | 97 participants; 51 (intervention) 46 (control) | |
| Interventions | RSL (intervention) versus WGL (control); no crossover | |
| Outcomes | Successful localization; excision; margins | |
| Notes | Source of funding not stated; mixed pathology | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Five participants excluded due to missing data |
| Selective reporting (reporting bias) | Low risk | Only malignant lesions included in analysis for rate of involved margins |
| Other bias | High risk | Mixed Pathology |
Lovrics 2011a.
| Methods | Prospective multicentred RCT | |
| Participants | 305 participants; 152 (intervention), 153 (control), 18 crossover | |
| Interventions | RSL (intervention) versus WGL (control) | |
| Outcomes | Successful localization; excision; margins; re‐intervention rate | |
| Notes | Secondary outcomes: operation time; complications; patient preference | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized stratified |
| Allocation concealment (selection bias) | Low risk | Concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not reported |
| Selective reporting (reporting bias) | Low risk | Fifteen RSL and 13 WGL participants were withdrawn post‐randomization; 18 received WGL though random to RSL |
| Other bias | Low risk | Not reported |
Mariscal Martinez 2009.
| Methods | RCT prospective | |
| Participants | 134 participants; 66 ROLL; 68 WGL | |
| Interventions | ROLL + SLNB; versus WGL + SLNB; versus SLNB | |
| Outcomes | Successful localization; excision; margins; re‐intervention rate | |
| Notes | Secondary outcomes: operation time; complications; tumour weight/volume; histopathology; operation time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None (all data reported) |
| Selective reporting (reporting bias) | Low risk | None (all data reported) |
| Other bias | Unclear risk | Lack of inter‐observer variability |
Medina‐Franco 2008.
| Methods | Prospective RCT | |
| Participants | 100 participants; 50 ROLL; 50 WGL | |
| Interventions | ROLL (intervention) versus WGL (control) | |
| Outcomes | Successful localization; excision; margins; re‐intervention rate; intraoperative re‐resection | |
| Notes | Secondary outcomes: operation time; complications; LOS; cosmesis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT; at office |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | High risk | Mixed pathology; no definitions of margins |
Moreno 2008.
| Methods | RCT | |
| Participants | 120 participants; 61 ROLL; 59 WGL | |
| Interventions | ROLL (intervention) versus WGL (control); no crossover | |
| Outcomes | Successful localization; excision; margins | |
| Notes | Secondary outcomes: operation time; complications; cosmesis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | High risk | Re‐intervention rate not reported, though noted as an outcome |
| Other bias | High risk | Different criteria for histology |
Ocal 2011.
| Methods | RCT | |
| Participants | 108 enrolled; 56 ROLL; 52 WGL (115 total participants; 7 enrolled but not randomized) | |
| Interventions | ROLL (intervention) versus WGL (control); no crossover | |
| Outcomes | Successful localization; excision; margins; re‐intervention rate; intraoperative re‐resection | |
| Notes | Secondary outcomes: operation time; complications; LOS; histopathology (mixed pathology) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list |
| Allocation concealment (selection bias) | Low risk | Independent |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Seven enrolled but not randomized; no available data |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | High risk | Mixed pathology; sample size |
Postma 2012.
| Methods | RCT | |
| Participants | 333 enrolled; 168 ROLL; 165 WGL (of which there were 314 participants in total: 162 ROLL (163 lesions); 152 WGL (153 lesions)) | |
| Interventions | ROLL (intervention) versus WGL (control); no crossover | |
| Outcomes | Successful localization; excision; margins; re‐intervention rate; tumour volume | |
| Notes | Secondary outcomes: operation time; complications; LOS; cosmesis; patient preference | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally computerised randomization |
| Allocation concealment (selection bias) | Low risk | Independent telephone operator |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up and attrition in both groups (reported) |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | Funding addressed |
Rampaul 2004.
| Methods | RCT | |
| Participants | 95 enrolled; 48 ROLL; 47 WGL | |
| Interventions | ROLL (intervention) versus WGL (control); no crossover | |
| Outcomes | Successful localization; excision; intraoperative re‐resection | |
| Notes | Secondary outcomes: operation time; complications; patient preference; histopathology; tumour weight; adequacy of initial marking | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcome unlikely to be influenced (acceptable as per surgical design) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two ROLL subjects converted to WGL ‐ data not included |
| Selective reporting (reporting bias) | High risk | Data unavailable on all outcomes |
| Other bias | High risk | No ITT; No margin definition; Mixed path/lesions |
ITT: intention‐to‐treat analysis LOS: length of stay RCT: randomized control trial ROLL: radioguided occult lesion localization RSL: radioactive seed localization SLNB: sentinel lymph node biopsy WGL: wire guided localization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Rahusen 2002 | Only study of the technique |
| Tafra 2006 | Only study of the technique |
| Tang 2011 | Only study of the technique |
Characteristics of studies awaiting assessment [ordered by study ID]
Parvez 2014.
| Methods | Multicentre Computer generated, centrally concealed randomization Randomization was stratified by surgeon and blocked within surgeon. Frontal photographs were taken 1 and 3 years postsurgery. The study authors used the European Organization for Research and Treatment of Cancer Cosmetic Rating System to evaluate cosmesis outcomes by the patient and a panel of 5 raters. |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | RSL versus WGL |
| Outcomes | The cosmetic outcome of RSL and WGL in women with early‐stage breast cancer |
| Notes |
BCS: breast conserving surgery RSL: radioactive seed localization WGL: wire‐guided localization
Characteristics of ongoing studies [ordered by study ID]
Langhans 2017.
| Trial name or title | A Randomized Study of Localization of Nonpalpable Breast Lesions ‐ RSL vs WGL |
| Methods | Allocation: randomized Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | RSL versus WGL |
| Outcomes | Primary: re‐operation rate due to positive microscopic margins detected at the final pathological evaluation. Secondary: amount of excised breast tissue in relation to tumour size. Other: duration of the surgical procedure. |
| Starting date | January 2014 |
| Contact information | linnea.langhans@regionh.dk |
| Notes | Recruiting |
RSL: radioactive seed localization WGL: wire‐guided localization
Differences between protocol and review
1. We planned to examine randomized controlled trials (RCTs) comparing WGL against another form of guided surgery for non‐palpable breast lesions for therapeutic and diagnostic purposes; the guidance techniques we considered included:
radioguided occult lesion localization (ROLL);
radioactive seed localization (RSL);
intraoperative ultrasound (IOUS);
cryoprobe‐assisted localization (CAL);
haematoma‐directed ultrasound‐guided (HUG) localization.
However, only ROLL and RSL had a sufficient number of published trials for meta‐analysis.
2. We changed our third primary outcome from "negative excision margins" to "positive" to be consistent with the terminology in reported studies.
3. We elaborated on complications to be specifically "post‐operative" in our third secondary outcome. This was always our intention but it became apparent during the course of the review that it might not have been obvious.
4. We planned to investigate other outcomes of interest not featured in our main analysis, such as practicalities and cost, but there were too few data for meaningful analysis.
5. We did not determine risk differences (RD) because the RD is judged to be conservative in case of zero events. We considered the risk ratio (RR) to be the more appropriate measure of effect.
6. We revised the search strings to allow for optimal search results from the WHO ICTRP. We noted that we retrieved an exceedingly high number of irrelevant records when we had included "advanced function" search strings.
Contributions of authors
Mr BKY Chan (BC) designed and wrote the protocol. BC undertook the literature search, study selection, extraction and analysis of the data, interpreted the results, and wrote the review.
Ms JA Wiseber (JW) supported writing the protocol. JW assessed study quality, extracted and analysed the data, interpreted the results, and helped write the review.
Mr RHS Jois (RJ) independently selected studies, extracted data, and assessed study quality.
Ms K Jensen (KJ) revised the meta‐analyses.
Professor RA Audisio (RA) supported the design and writing of the protocol. RA oversaw the literature search, study selection, data extraction and analysis, and assisted in interpreting the results and writing the review. RA is also the guarantor of this Cochrane review.
Sources of support
Internal sources
-
NHS, UK.
NHS Consultant Surgeon (RA) and Clinician (BC & HR).
-
University of Heidelberg, Germany.
KJ was supported in the form of a salary through the University of Heidelberg, Germany.
-
University of Liverpool, UK.
JW received a salary from the University of Liverpool.
External sources
No sources of support supplied
Declarations of interest
BC: None known. JW: None known. RJ: None known. KJ: None known. RA: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Gray 2001 {published data only}
- Gray RJ, Salud C, Nguyen K, Dauway E, Friedland J, Berman C, et al. Randomized prospective evaluation of a novel technique for biopsy or lumpectomy of nonpalpable breast lesions: radioactive seed versus wire localization. Annals of Surgical Oncology 2001;8(9):711‐5. [DOI] [PubMed] [Google Scholar]
Lovrics 2011a {published data only}
- Lovrics PJ, Goldsmith CH, Hodgson N, McCready D, Gohla G, Boylan C, et al. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Annals of Surgical Oncology 2011;18(12):3407‐14. [DOI] [PubMed] [Google Scholar]
Mariscal Martinez 2009 {published data only}
- Mariscal Martínez A, Solà M, Tudela AP, Julián JF, Fraile M, Vizcaya S, et al. Radioguided localization of nonpalpable breast cancer lesions: randomized comparison with wire localization in patients undergoing conservative surgery and sentinel node biopsy. American Journal of Roentgenology 2009;193(4):1001‐9. [DOI] [PubMed] [Google Scholar]
Medina‐Franco 2008 {published data only}
- Medina‐Franco H, Abarca‐Pérez L, García‐Alvarez MN, Ulloa‐Gómez JL, Romero‐Trejo C, Sepúlveda‐Méndez J. Radioguided occult lesion localization (ROLL) versus wire‐guided lumpectomy for non‐palpable breast lesions: a randomized prospective evaluation. Journal of Surgical Oncology 2008;97(2):108‐11. [DOI] [PubMed] [Google Scholar]
Moreno 2008 {published data only}
- Moreno M, Wiltgen JE, Bodanese B, Schmitt RL, Gutfilen B, Fonseca LM. Radioguided breast surgery for occult lesion localization ‐ correlation between two methods. Journal of Experimental & Clinical Cancer Research 2008;27:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ocal 2011 {published data only}
- Ocal K, Dag A, Turkmenoglu O, Gunay EC, Yucel E, Duce MN. Radioguided occult lesion localization versus wire‐guided localization for non‐palpable breast lesions: randomized controlled trial. Clinics (São Paulo) 2011;66(6):1003‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Postma 2012 {published data only}
- Postma EL, Verkooijen HM, Esser S, Hobbelink MG, Schelling GP, Koelemij R, et al. Efficacy of 'radioguided occult lesion localisation' (ROLL) versus 'wire‐guided localisation' (WGL) in breast conserving surgery for non‐palpable breast cancer: a randomised controlled multicentre trial. Breast Cancer Research and Treatment 2012;136(2):469‐78. [DOI] [PubMed] [Google Scholar]
Rampaul 2004 {published data only}
- Rampaul RS, Bagnall M, Burrell H, Pinder SE, Evans AJ, Macmillan RD. Randomized clinical trial comparing radioisotope occult lesion localization and wire‐guided excision for biopsy of occult breast lesions. The British Journal of Surgery 2004;91(12):1575‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Rahusen 2002 {published data only}
- Rahusen FD, Bremers AJ, Fabry HF, Amerongen AH, Boom RP, Meijer S. Ultrasound‐guided lumpectomy of nonpalpable breast cancer versus wire‐guided resection: a randomized clinical trial. Annals of Surgical Oncology 2002;9(10):994‐8. [DOI] [PubMed] [Google Scholar]
Tafra 2006 {published data only}
- Tafra L, Fine R, Whitworth P, Berry M, Woods J, Ekbom G, et al. Prospective randomized study comparing cryo‐assisted and needle‐wire localization of ultrasound‐visible breast tumors. American Journal of Surgery 2006;192(4):462‐70. [DOI] [PubMed] [Google Scholar]
Tang 2011 {published data only}
- Tang J, Xie XM, Wang X, Xie ZM, He JH, Wu YP, et al. Radiocolloid in combination with methylene dye localization, rather than wire localization, is a preferred procedure for excisional biopsy of nonpalpable breast lesions. Annals of Surgical Oncology 2011;18(1):109‐13. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Parvez 2014 {published data only}
References to ongoing studies
Langhans 2017 {unpublished data only}
- A Randomized Study of Localization of Nonpalpable Breast Lesions ‐ RSL vs WGL. Ongoing study January 2014.
Additional references
Ahmed 2013
- Ahmed M, Hemelrijck M, Douek M. Systematic review of radioguided versus wire‐guided localization in the treatment of non‐palpable breast cancers. Breast Cancer Research Treatment 2013;140(2):241‐52. [DOI] [PubMed] [Google Scholar]
Audisio 2005
- Audisio RA, Nadeem R, Harris O, Desmond S, Thind R, Chagla LS. Radioguided occult lesion localisation (ROLL) is available in the UK for impalpable breast lesions. Annals of the Royal College of Surgeons of England 2005;87(2):92‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruce 2008
- Bruce N, Pope D, Stanistreet D. Quantitative Methods for Health Research: a Practical Guide to Epidemiology and Statistics. 1st Edition. Chichester: Wiley‐Interscience, 2008. [Google Scholar]
Cochrane Breast Cancer Group
- Wilcken N, Ghersi D, Brunswick C, Clarke M, Dinh P, Ganz P, et al. Cochrane Breast Cancer Group. In: the Cochrane Library, 2009, Issue 3. Chichester: Wiley‐Blackwell. Updated quarterly. http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html (accessed 16 December 2015).
De Cicco 2002
- Cicco C, Pizzamiglio M, Trifirò G, Luini A, Ferrari M, Prisco G, et al. Radioguided occult lesion localisation (ROLL) and surgical biopsy in breast cancer. Technical aspects. The Quarterly Journal of Nuclear Medicine 2002;46(2):145‐51. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ernst 2002
- Ernst MF, Roukema JA. Diagnosis of non‐palpable breast cancer: a review. The Breast 2002;11(1):13‐22. [DOI] [PubMed] [Google Scholar]
Ferlay 2007
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Annals of Oncology 2007;18(3):581‐92. [DOI] [PubMed] [Google Scholar]
Fisher 2002
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty‐year follow‐up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. The New England Journal of Medicine 2002;347(16):1233‐41. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirsch 1989
- Hirsch JI, Banks WL Jr, Sullivan JS, Horsley JS 3rd. Effect of methylene blue on estrogen‐receptor activity. Radiology 1989;171(1):105‐7. [DOI] [PubMed] [Google Scholar]
Hughes 2008
- Hughes JH, Mason MC, Gray RJ, McLaughlin SA, Degnim AC, Fulmer JT, et al. A multi‐site validation trial of radioactive seed localization as an alternative to wire localization. The Breast Journal 2008;14(2):153‐7. [DOI] [PubMed] [Google Scholar]
Jakub 2010
- Jakub JW, Gray RJ, Degnim AC, Boughey JC, Gardner M, Cox CE. Current status of radioactive seed for localization of non palpable breast lesions. American Journal of Surgery 2010;199(4):522‐8. [DOI] [PubMed] [Google Scholar]
Lovrics 2011b
- Lovrics PJ, Cornacchi SD, Vora R, Goldsmith CH, Kahnamoui K. Systematic review of radioguided surgery for non‐palpable breast cancer. European Journal of Surgical Oncology 2011;37(5):388‐97. [DOI] [PubMed] [Google Scholar]
Luini 1998
- Luini A, Zurrida S, Galimberti V, Paganelli G. Radioguided surgery of occult breast lesions. European Journal of Cancer 1998;34(1):204‐5. [DOI] [PubMed] [Google Scholar]
Nadeem 2005
- Nadeem R, Chagla LS, Harris O, Desmond S, Thind R, Titterrell C, et al. Occult breast lesions: A comparison between radioguided occult lesion localisation (ROLL) vs. wire‐guided lumpectomy (WGL). The Breast 2005;14(4):283‐9. [DOI] [PubMed] [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. [DOI] [PubMed] [Google Scholar]
Philips 2008
- Philips N, Coldman A. Comparison of nonbreast cancer incidence, survival and mortality between breast screening program participants and nonparticipants. International Journal of Cancer 2008;122(1):197‐201. [DOI] [PubMed] [Google Scholar]
Pleijhuis 2009
- Pleijhuis RG, Graafland M, Vries J, Bart J, Jong JS, Dam GM. Obtaining adequate surgical margins in breast‐conserving therapy for patients with early‐stage breast cancer: current modalities and future directions. Annals of Surgical Oncology 2009;16(10):2717‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
R [Computer program]
- R Core Team. R: A Language and Environment for Statistical Computing. Version Version 3.2.2. Vienna: R Foundation for Statistical Computing, 14 August 2015.
R package 'meta' [Computer program]
- Schwarzer G. R: Package 'meta'. Version Version 4.3‐0 (02 July 2015). Vienna: R Foundation for Statistical Computing, 2015.
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2003
- Rose A, Collins JP, Neerhut P, Bishop CV, Mann GB. Carbon localisation of impalpable breast lesions. The Breast 2003;12(4):264‐9. [DOI] [PubMed] [Google Scholar]
Rovera 2008
- Rovera F, Frattini F, Marelli M, Corben AD, Vanoli C, Dionigi G, et al. Radio‐guided occult lesion localization versus wire‐guided localization in non‐palpable breast lesions. International Journal of Surgery 2008;6 Suppl 1:S101‐3. [DOI] [PubMed] [Google Scholar]
Sajid 2012
- Sajid MS, Parampalli U, Haider Z, Bonomi R. Comparison of radioguided occult lesion localization (ROLL) and wire localization for non‐palpable breast cancers: a meta‐analysis. Journal of Surgical Oncology 2012;105(8):852‐8. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schwartz 2006
- Schwartz GF, Veronesi U, Clough KB, Dixon JM, Fentiman IS, Heywang‐Köbrunner SH, et al. Consensus conference on breast conservation. Journal of the American College of Surgeons 2006;203(2):198‐207. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12: Interpretingresults and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Tromberg 2008
- Tromberg BJ, Pogue BW, Paulsen KD, Yodh AG, Boas DA, Cerussi AE. Assessing the future of diffuse optical imaging technologies for breast cancer management. Medical Physics 2008;35(6):2443‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
UK NSC Breast Cancer Screening Recommendation 2012
- The Independent UK Panel on Breast Cancer Screening. The Benefits and Harms of Breast Cancer Screening: An Independent Review. http://legacy.screening.nhs.uk/breastcancer (accessed 16 December 2015).
Van der Ploeg 2008
- Ploeg IM, Hobbelink M, Bosch MA, Mali WP, Borel Rinkes IH, Hillegersberg R. 'Radioguided occult lesion localisation' (ROLL) for non‐palpable breast lesions: a review of the relevant literature. European Journal of Surgical Oncology 2008;34(1):1‐5. [DOI] [PubMed] [Google Scholar]
Van Esser 2008
- Esser S, Hobbelink M, Ploeg IM, Mali WP, Diest PJ, Borel Rinkes IH, et al. Radio guided occult lesion localization (ROLL) for non‐palpable invasive breast cancer. Journal of Surgical Oncology 2008;98(7):526‐9. [DOI] [PubMed] [Google Scholar]
Wilson 1993
- Wilson M, Boggis CR, Mansel RE, Harland RN. Non‐invasive ultrasound localization of impalpable breast lesions. Clinical Radiology 1993;47(5):337‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chan 2011
- Chan BKY, Jois RHS, Wiseberg JA, Audisio RA. Localization techniques for guided surgical excision of non‐palpable breast lesions. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009206] [DOI] [PMC free article] [PubMed] [Google Scholar]


