Abstract
Background.
The first consensus Merkel cell carcinoma (MCC) staging system was published in 2010. New information on the clinical course prompts review of MCC staging.
Methods.
9,387 MCC cases from the National Cancer Data Base Participant User File (NCDB PUF) with follow-up and staging data (1998–2012) were analyzed. Prognostic differences based on clinical and pathological staging were evaluated. Survival estimates were compared by disease extent.
Results.
Sixty-five percent of cases presented with local disease, while 26% and 8% presented with nodal and distant disease. Disease extent at presentation was predictive of five-year overall survival (OS) with estimates of 51%, 35%, and 14% for local, nodal, and distant disease. Tumor burden at the regional nodal basin was predictive of five-year OS with estimates of 40% and 27% for clinically occult and clinically detected nodal disease. For local disease, we confirm improved prognosis when the regional nodal basin was negative by pathological compared to clinical staging. We identified 336 cases with clinically detected nodal disease and unknown primary tumor and showed improved prognosis over cases presenting with concurrent primary tumor (OS estimates of 42% versus 27%).
Conclusion.
Analysis of a national dataset of MCC cases validates the predictive value of disease extent at presentation. Separation of clinical and pathological stage groups and regrouping of unknown primary tumors are supported by the analysis. The revised staging system provides more accurate prognostication and has been formally accepted by the AJCC staging committee for inclusion in the 8th edition.
Introduction
Prior to 2010, there were five competing staging systems for Merkel cell carcinoma (MCC).1 Then, the first consensus MCC staging system for the American Joint Committee on Cancer (AJCC) was established, based on a study of 2,856 cases from the National Cancer Database (NCDB).2 Extent of disease, tumor size, and tumor burden in the regional nodal basin correlated with worsening survival and defined TNM criteria. In patients with local disease, those with pathologically-proven negative nodes appeared to have a better prognosis than those with clinically negative nodes that may have harbored occult metastases.2 To reflect this difference, staging for local disease is currently defined by whether or not a patient who is clinically node negative has undergone pathological nodal staging, frequently with sentinel lymph node biopsy (SLNB). Patients with a primary MCC and pathologically-proven negative lymph nodes are designated as stage IA or IIA whereas those with clinically negative nodes (not histologically verified) are either stage IB or IIB. In this system, stage is determined by the extent of evaluation (clinical staging) rather than the extent of disease (pathological staging).
New knowledge of the biology and clinical course has prompted review of the staging system. Importantly, many studies have shown that even the smallest primary MCC has at least a 10–20% risk of occult nodal metastases.3–5 SLNB has been increasingly used to stage patients with localized MCC, resulting in a decline in individuals currently staged as IB or IIB.3–8 National Comprehensive Cancer Network (NCCN) guidelines recommend that SLNB be performed for all clinically node negative patients whenever feasible.9 Additionally, several studies have demonstrated a significant survival advantage among MCC metastatic to a lymph node in the absence of a primary tumor (“unknown primaries”) over those with a known primary tumor and concurrent nodal metastasis.5,10–13
Here, we perform an analysis of prognostic factors using the largest national MCC cohort reported to date to validate and refine the current staging system. Moreover, we sought to determine whether the data support the separation of clinical and pathological staging systems to be consistent with all other AJCC staging systems.14 We evaluated outcomes associated with extent of nodal metastasis (in the context of known primary tumors as well as “unknown primaries”) to inform revisions.
Methods
We used the NCDB Participant User File (PUF) as the primary data source and this captures approximately 70% of incident US cancer cases, diagnosed at one of approximately 1500 American College of Surgeons Commission on Cancer (ACS CoC) accredited cancer programs.15,16 Using the International Classification of Diseases for Oncology (3rd edition) code 8247, 14,414 cases of MCC from 1,264 hospitals were identified (1998–2012). Figure 1 shows the algorithm for cohort derivation. Patients without follow-up data were excluded (n = 1,321). Among those with follow-up data, patients without any component of TNM category (TxNxMx) were excluded (n=2,928).
Figure 1.
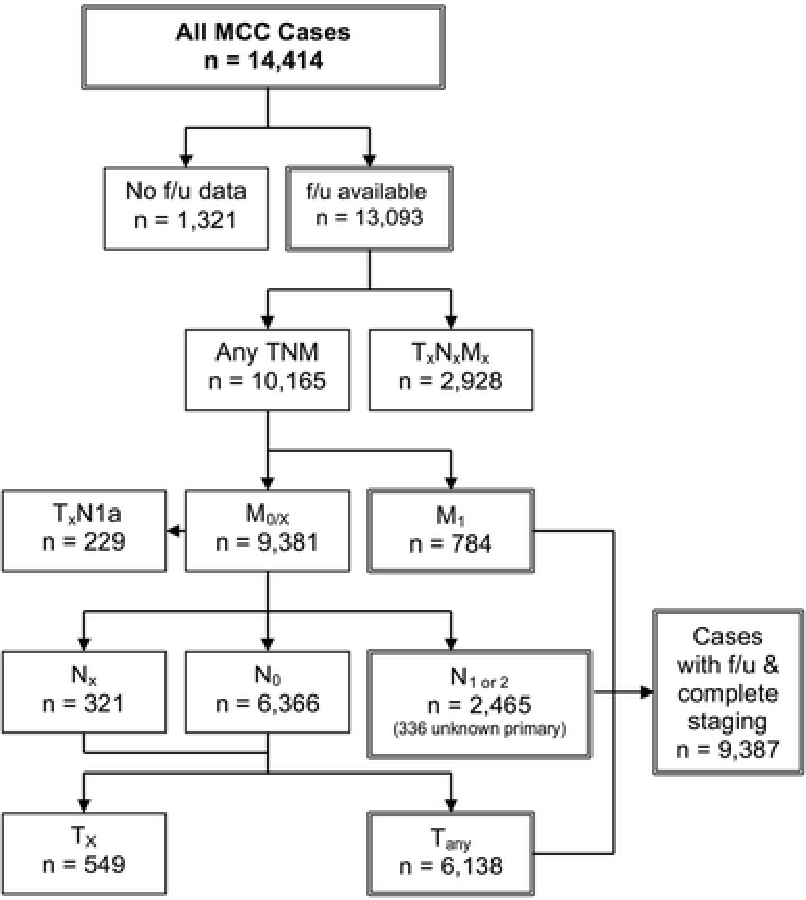
Flow diagram for inclusion/exclusion criteria of Merkel cell carcinoma (MCC) cases in the National Cancer Database (NCDB) that were analyzed for survival rates. 14,414 MCC cases were prospectively captured in the NCDB during 1998 to 2012. Of those, 13,093 cases had follow-up data available and were used to stratify TNM categories. Cases without any TNM classification (TxNxMx, n=2,928) were excluded. Cases with a recorded stage that did not represent a valid clinical entity were excluded (e.g. cases where the primary tumor was not assessed but with concurrent occult nodal metastasis noted (TxN1a, n=229) or without nodal metastases (TxN0, n=549) are likely to represent coding errors). Derivation of T, N, and M categories used for analysis is detailed in the Methods section. 9,387 cases with staging information and follow-up data were used for survival analysis.
For patients presenting with distant metastatic disease (M1, n=784), primary tumor (T) and nodal (N) status were not relevant for staging and these cases were excluded from subsequent T and N analyses. We combined M0 (n=9,188) and Mx (n=193) to inform T and N classification. We specifically defined nodal status as Nx (unspecified nodal status, n=321), N0 (no nodal disease, n=6,366), or N1/2 (clinically occult nodal disease, clinically detected nodal disease, and in-transit disease, n=2,465). Clinically occult nodal disease, previously designated “microscopic nodal disease,” is defined as occult regional nodal metastasis identified by SLNB or lymphadenectomy. Clinically detected nodal disease, previously designated “macroscopic nodal disease,” is defined as clinically palpable or radiologically detected regional nodal metastasis.2 For patients without nodal disease (Nx and N0, n=6,687), those with any T category were further analyzed (n=6,138). When the true stage was unclear (e.g. the recorded stage did not represent a valid clinical entity), these patients were excluded. We established a final cohort of 9,387 patients at 1,182 hospitals. Median follow-up time was 28.2 months. For this analysis, when both pathological and clinical staging information was available for tumor size and nodal status, pathological staging was used in analyses unless otherwise specified.
Cox proportional hazards regression analysis was used to generate overall survival estimates stratified by extent of disease and categories of local and nodal disease. We also compared prognosis in those who were staged as node negative by both clinical and pathological staging. All analyses were performed using Stata release 13 (StataCorp LP, College Station, TX).
Results
Patient demographics including sex, age, body site, and race are shown in Table 1. The majority of patients were Caucasian (96.4%) and male (62.1%); the median age was 76 years. As expected, the extent of disease at the time of diagnosis correlated with prognosis. Five-year overall survival (OS) estimates for local disease (n=6,138), regional metastatic disease (n=2,465), and distant metastatic disease (n=784) were 50.6%, 35.4%, and 13.5%, respectively (Figure 2A).
Table 1.
Demographics of 14,414 patients with Merkel cell carcinoma in the National Cancer Data Base (1998 – 2012)
| No. |
Percent |
|
|---|---|---|
| Sex | ||
| Male | 8,945 | 62.1 |
| Female | 5,469 | 37.9 |
| Age, y (median = 76) | ||
| <40 | 69 | 0.5 |
| 40–49 | 362 | 2.5 |
| 50–59 | 1,298 | 9.0 |
| 60–69 | 2,678 | 18.6 |
| 70–79 | 4,766 | 33.1 |
| 80–89 | 4,323 | 30.0 |
| ≥ 90 | 918 | 6.4 |
| Body site | ||
| Head and neck | 6,144 | 42.6 |
| Trunk | 1,575 | 10.9 |
| Upper limb and shoulder | 3,397 | 23.6 |
| Lower limb and hip | 2,211 | 15.3 |
| Skin, Other | 1,087 | 7.5 |
| Race | ||
| Caucasian* | 13,891 | 96.4 |
| Black | 171 | 1.2 |
| American Indian, Aleutian, or Eskimo | 31 | 0.2 |
| Asian, Pacific Islander | 109 | 0.8 |
| Other/unknown | 212 | 1.5 |
Of those classified as Caucasian, 2.2% (n = 305) are of Spanish/Hispanic ethnicity.
Figure 2.
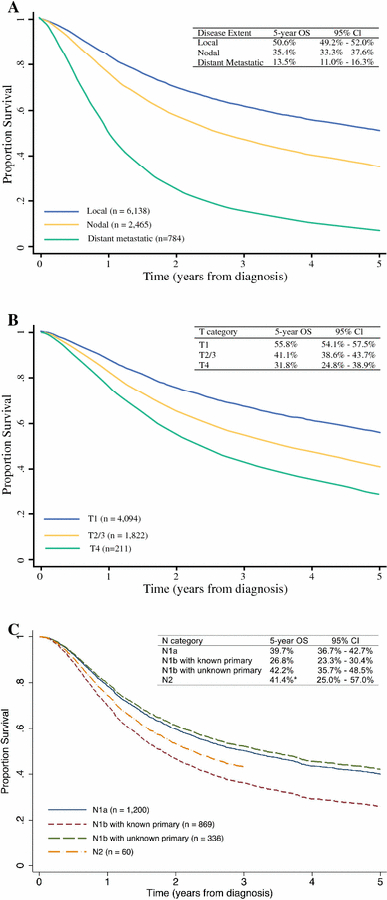
Five-year overall survival (OS) in Merkel cell carcinoma (MCC). (a) Survival curves of 9,387 MCC patients stratified by local, nodal, and distant metastatic disease. (b) Survival curves of 6,127 MCC patients presenting with local disease only stratified by primary tumor size using T categories (T1: primary tumor ≤ 2 cm, T2/3: primary tumor > 2 cm, T4: primary tumor invades fascia, muscle, cartilage, or bone). Eleven patients presenting with in situ tumor (Tis) were excluded from analysis given the small sample size. (c) Survival curves of 2,465 MCC patients presenting with nodal metastases stratified by occult nodal disease (N1a), clinically detected nodal disease with known primary tumor (N1b with known primary), clinically detected nodal disease with unknown primary tumor (N1b with unknown primary), and in-transit metastasis (N2). *The N2 category had a small sample size of 60 and survival represents three-year OS instead of five-year OS.
Staging for localized MCC
Sixty-five percent (n =6,138) presented with local disease only. This cohort includes those with clinically or pathologically negative regional lymph nodes. Five-year survival rates for T1 (primary tumor ≤ 2 cm, n=4,094), T2/T3 (T2: primary tumor > 2 cm but ≤ 5 cm, n=1511; T3: primary tumor > 5 cm, n = 311; T2/T3 n=1822), and T4 (primary tumor invades fascia, muscle, cartilage, or bone, n=211) are shown in Figure 2B. It is important to consider that T category cannot be used in isolation for prognostication and that TNM staging should be used for such evaluation.
Staging for regional metastatic MCC
Twenty-six percent (n=2,465) presented with regional nodal metastases. The five-year OS was 35.4% (Figure 2A). We compared survival rates of occult regional metastatic disease (N1a) to clinically detected regional metastatic disease (N1b) and in-transit disease (N2). Though SLNB provides the most accurate assessment of the presence of occult nodal metastases, this is not explicitly captured in the NCDB. Thus, as a proxy for occult nodal disease, we identified 1,200 patients with a primary tumor (T1-T4) without distant metastases (M0/x) who had either a pathologically-proven occult nodal metastasis (N1a, n=140) or who were clinically node negative but had pathologically positive lymph node(s) (cN0/x and pN1, n=1,060). Their estimated five-year OS was 39.7% (Figure 2C). For clinically detected regional nodal disease, we identified 869 patients with a primary tumor (T1-T4) without distant metastases (M0/x) who had clinically detected nodal disease regardless of associated pathological nodal stage (cN1, n=734) or a pathologically verified clinically detected nodal metastasis (N1b, n=135). The estimated five-year OS for this group was 26.8% (Figure 2C). Patients with in-transit metastases (N2) comprised a small group (n=60) and had an estimated 3-year OS of 41.4% (Figure 2C).
We then analyzed survival for “unknown primaries.” We identified 336 cases, defined as those without a primary tumor (T0, n=70) or primary tumor cannot be assessed (Tx, n=266) without distant metastases (M0/Mx) who had a clinically positive lymph node (cN1) or who had a pathological stage denoting a clinically detected regional nodal metastasis (N1b). While Tx is defined as a primary tumor that cannot be assessed, we presume that in this context, these represent “unknown primaries.” The combined cohort of 336 patients represents 4% of all cases, consistent with the proportion of “unknown primaries” in previous reports.17 Five-year OS estimates “unknown primaries” were 42.2% compared to 26.8% for those with metastatic MCC with a known primary tumor (Figure 2C).
To evaluate prognosis associated with pathological staging of the regional nodal basin compared to clinical nodal evaluation, we compared survival in patients with tumors ≤ 2 cm who were clinically node negative (T1 cN0 pNx, n=1,272) with those who were pathologically node negative (T1 cN0 pN0, n=1502). Cases where the N category was unknown (Nx) or missing were omitted from sub-analysis. Patients with T1 tumors who were pathologically node negative had a better five-year OS (62.8%, n=1,502) (pathological stage I, Figure 3B) than those who were clinically node negative (45%, n=1,272) (clinical stage I, Figure 3A). Similarly, in patients with tumors ≥ 2 cm (T2/T3) as well as tumors that invaded the fascia, muscle, cartilage, or bone (T4), those who were pathologically node negative (pathological stage IIA and IIB, Figure 3B) fared better than those who were clinically node negative (clinical stage IIA and IIB, Figure 3A). In patients with local disease, 49% did not undergo pathological nodal staging. In bivariate analysis, increasing age but not extent of comorbidity was found to be associated with lack of pathological staging. The average age was 72.9 years for those with pathological staging versus 77.6 years for those with clinical staging only (p < 0.001). The average Charlson-Deyo comorbidity score was 0.26 for both groups (p = 0.949). Importantly, over time, the proportion of patients undergoing pathological nodal staging increased from 29% in 1998 to 63% in 2011.
Figure 3.
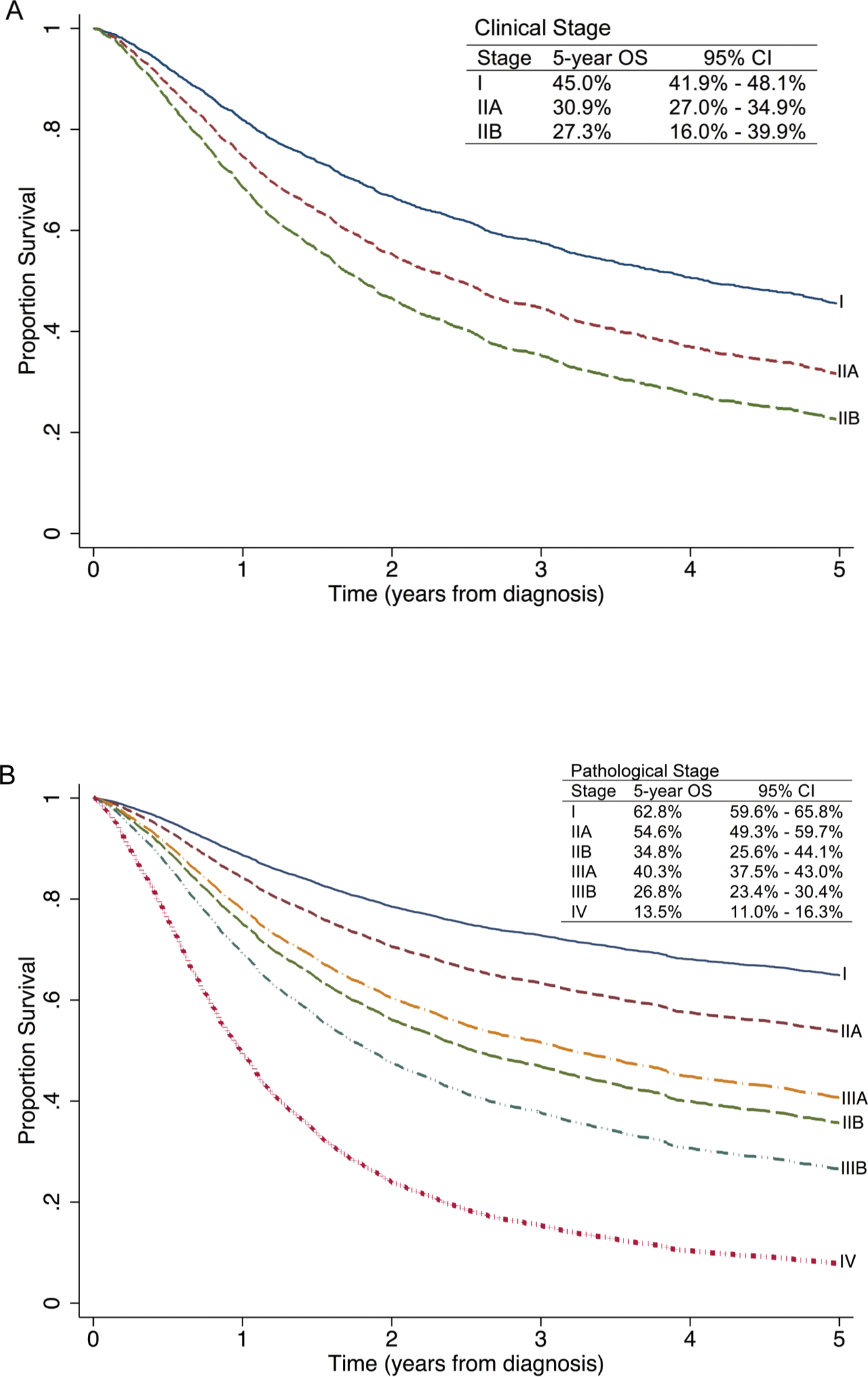
Five-year overall survival for new MCC clinical (a) and pathological (b) staging shows distinct prognostication. (a) Survival curves of 2,013 patients with clinical staging for local disease when pathological staging data was unavailable. 1,272 patients presented with clinical stage I (T1 N0 M0), 675 presented with clinical stage IIA (T2/3 N0 M0), and 66 patients presented with clinical stage IIB (T4 N0 M0). (b) Survival curves of 5,371 patients with pathological staging, including 1,502 patients with pathological stage I (T1 N0 M0), 493 patients with pathological stage IIA (T2/3 N0 M0)*, 127 patients with pathological stage IIB (T4 N0 M0), 1,536 patients with pathological stage IIIA (T1–4 N1a M0 and T0 N1b M0), 929 patients with pathological stage IIIB (T1–4 N1b M0 and T0–4 N2 M0), and 784 patients with pathological stage IV (Tany Nany M1).
*5-year OS for T2N0M0 (n = 414) is 56.0% with 95% confidence interval of 50.1–61.5%; 5-year OS for T3N0M0 (n=79) is 47.3% with 95% confidence interval of 34.3–59.3%.
Revision of the MCC staging system
Using the current findings to inform more precise prognostication, revised TNM categories and staging groups are shown in Table 2, noting that clinical staging is expanded and separated from pathological staging. Differences between the AJCC 7th and 8th edition staging systems are summarized in Table 3. Stage I and II are determined solely on primary tumor characteristics and eliminates the previous distinction between stage IA (or IIA) and stage IB (or IIB) based on pathological confirmation of node negative disease. For local disease, the T classification is validated to accurately stratify prognostic groups. Local disease is divided into stage I (T1 N0 M0), stage IIA (T2/3 N0 M0), and stage IIB (T4 N0 M0), noting a new definition of stage IIB to reflect what was previously IIC. Five-year OS estimates for clinical (Figure 3A) and pathological (Figure 3B) staging of local disease were 45.0% and 62.8% for stage I, 30.9% and 54.6% for stage IIA, and 27.3% and 34.8% for stage IIB, respectively.
Table 2.
TNM criteria and stage groupings for new 8th edition American Joint Committee on Cancer Merkel cell carcinoma staging system
| Clinical Stage Groups (cTNM)* |
Pathological Stage Groups (pTNM)** |
||||||
|---|---|---|---|---|---|---|---|
| T | N | M | T | N | M | ||
| 0 | Tis | N0 | M0 | 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 | I | T1 | N0 | M0 |
| IIA | T2–3 | N0 | M0 | IIA | T2–3 | N0 | M0 |
| IIB | T4 | N0 | M0 | IIB | T4 | N0 | M0 |
| III | T0–4 | N1–3 | M0 | IIIA | T1–4 | N1a(sn) or N1a | M0 |
| T0 | N1b | M0 | |||||
| IIIB | T1–4 | N1b-3 | M0 | ||||
| IV | T0–4 | Any N | M1 | IV | T0–4 | Any N | M1 |
|
| |||||||
| T | N | M | |||||
|
| |||||||
| Tx, primary tumor cannot be assessed T0, no primary tumor Tis, in situ primary tumor T1, primary tumor ≤ 2 cm T2, primary tumor > 2 cm but ≤ 5 cm T3, primary tumor > 5 cm T4, primary tumor invades fascia, muscle, cartilage, or bone |
cNx, regional lymph nodes cannot be clinically assessed (e.g. previously removed for another reason, body habitus) cN0, no regional lymph node metastasis by clinical or radiological evaluation cN1, clinically detected regional nodal metastasis cN2, in-transit metastasis without lymph node metastasis cN3, in-transit metastasis with lymph node metastasis |
pNx, regional lymph nodes cannot be assessed (e.g. previously removed for another reason) or not removed for pathological evaluation pN0, no regional lymph node metastasis detected on pathological evaluation pN1a(sn), clinically occult nodal metastasis identified only by sentinel lymph node biopsy pN1a, clinically occult regional lymph node metastasis following lymph node dissection pN1b, clinically or radiologically detected regional lymph node metastasis, pathologically confirmed pN2, in-transit metastasis without lymph node metastasis pN3, in-transit metastasis with lymph node metastasis |
M0, no distant metastasis M1, distant metastasis - M1a, metastasis to distant skin, distant subcutaneous tissue, or distant lymph nodes - M1b, lung - M1c, all other distant sites |
||||
Clinical staging is defined by microstaging of the primary Merkel cell carcinoma (MCC) with clinical and/or radiological evaluation for metastasis
Pathological staging is defined by microstaging of the primary MCC and pathological nodal evaluation of the regional lymph node basin with sentinel lymph node biopsy or complete lymphadenectomy or pathologic confirmation of distant metastasis
Table 3.
Differences between 7th Edition (2010) and new 8th Edition American Joint Committee on Cancer Merkel cell carcinoma staging system
| 7th Edition Stage | 8th Edition Stage | Comments |
|---|---|---|
| Clinical and pathological staging are combined in local disease stages I and II | Separate clinical and pathological staging | |
| IA* and IB** | I | Eliminate IB as clinical and pathological staging are separate |
| IIA* and IIB** | IIA | Eliminate IIB as clinical and pathological staging are separate |
| IIC | IIB | Rename previous IIC as IIB |
| IIIA Included: - occult nodal disease |
IIIA Includes: - occult nodal disease (sn) - occult nodal disease - clinically detected nodal disease with unknown primary |
Distinguish occult nodal disease identified by sentinel lymph node biopsy without further lymphadenectomy, pN1a(sn), from those who underwent completion lymphadenectomy, pN1a Include clinically detected nodal disease with unknown primary for similar prognostication |
| IIIB Included: - clinically detected nodal disease with known primary - clinically detected nodal disease with unknown primary tumor - in-transit metastases |
IIIB Includes: - clinically detected nodal disease with known primary - in-transit metastases without nodal disease - in-transit metastases with nodal disease Omit: - clinically detected nodal disease with unknown primary |
Continue to combine clinically detected nodal disease with known primary and in-transit disease. Distinguish in-transit metastases without regional nodal disease (revised N2 category) from in-transit metastases with regional nodal disease (new N3 category) |
| IV | IV | Same |
Abbreviations: MCC, Merkel cell carcinoma; sn (sentinel node)
“A” distinction in the 7th Edition of MCC staging of local disease stages I and II designated a negative pathological lymph node evaluation
“B” distinction in the 7th Edition of MCC staging of local disease stages I and II designated that pathological nodal evaluation was not performed
For regional metastatic disease, the N classification was revised to reflect improved prognostication for “unknown primaries”. Prognosis of this group was similar to those with occult nodal metastases (42.2% vs 39.7% five-year OS), but was distinct from those with clinically detected nodal metastases and concurrent primary tumor (26.8% five-year OS). Thus, the stage group IIIA was revised to include both occult nodal disease (T1–4 N1a M0) and “unknown primaries” (T0 N1b M0). The five-year OS estimate for revised stage IIIA was 40.3% (Figure 3B). Stage IIIB now includes those with a known primary tumor and clinically detected regional metastatic disease (T1–4 N1b M0) or in-transit disease (T0–4 N2 M0). The five-year OS estimate for stage IIIB was 26.8% (Figure 3B). Calculation of the akaike information criterion (AIC) enabled statistical comparison between the 7th and 8th Edition of MCC staging. The 8th edition staging had a more favorable AIC score, indicating that it represents a better statistical model (data not shown).
During the preparation of this manuscript, the AJCC staging committee decided to add category pN1a(sn) to capture those MCC patients who have undergone nodal staging with SLNB but did not undergo a completion lymph node dissection. In addition, it was decided to distinguish those with in-transit metastases without regional nodal metastasis. Thus, the revised N2 category will designate in-transit metastases without nodal metastases and the new N3 category will denote in-transit metastases with metastases.
Discussion
We analyzed a large national cohort of MCC cases to validate and refine the current staging system, leading to the new (8th edition) AJCC staging manual. We demonstrate that pathological nodal staging more precisely predicts survival compared to clinical nodal staging. We validate increasing tumor size as predictive of survival with increasing T categories. Patients with “unknown primaries” have a distinctly better prognosis than those with clinically detected nodal disease and known primary tumor, and are included in substage group IIIA to yield more precise prognostic stratification.
Lemos et al., in an older study of a similar but only minimally overlapping NCBD cohort, showed that patients with local disease who were pathologically proven node negative had a better prognosis than those deemed node negative clinically.2 The obvious explanation for this difference is the heterogeneity of the clinically node negative group, which likely includes both node negative and occult node positive patients. This assumption is supported by studies that have demonstrated high rates of occult nodal disease in clinically node negative patients.3–5 This has led to widespread use of SLNB.9 However, current stage IB and IIB increasingly includes patients in whom SLNB is not performed based on age or co-comorbidity. As NCDB provides OS rather than disease specific survival (DSS) rates, prognosis based on the 7th edition stage groups IB and IIB is likely to become an increasingly inaccurate representation of MCC prognosis. In this study, we noted a decrease in pathological nodal staging with increasing age (72.9 vs. 77.6 years); however the extent of medical comorbidity, likely a better measure of physiologic age, was similar between groups with and without pathological nodal staging data.
Here, we separate and expand the clinical staging system from the pathological staging system to (1) to create consistency with all other AJCC staging systems; and (2) to eliminate upstaging based on the absence of pathological nodal staging.
Patients with occult nodal disease appear to have a better prognosis than those with clinically detected nodal disease,2,3 though others have found that sentinel node status did not correlate with recurrence or survival.18,19 Here, we found prognostic differences between clinically occult and clinically detected nodal disease. Our study demonstrates distinction between IIIA and IIIB disease and supports SLNB for staging. Given the improved prognostication, we recommend staging with SLNB for all reasonably healthy MCC patients, recognizing their generally advanced age.3–9
Several small studies have consistently found that patients with “unknown primaries” have a better prognosis than those with metastatic nodal MCC and a concurrent primary tumor.5,10–13 The survival benefit may be associated with improved cell-mediated immunity which clears the primary tumor and targets residual disease.1 While this subgroup only represents 5% of all MCC patients, it represents between 32–40% of those with clinically detected nodal disease.10–12,17 Data presented here support a better prognosis for this group; the proportion of cases is consistent with published reports, representing 4% of all cases and 28% of those with clinically detected nodal disease. It may be counterintuitive for “unknown primaries” to have a similar prognosis as occult nodal disease and known primary tumor. Our findings provide a strong rationale to include this cohort within substage group IIIA to reflect improved outcomes.
This study has several limitations. Data regarding DSS and recurrence rates are not available in the NCDB. Therefore, we use OS as an estimate of MCC-specific survival, recognizing that the difference between OS and DSS may be more pronounced in MCC given the elderly population affected by this disease. Another significant limitation is that SLN status is not specifically captured in the NCDB. As a proxy for clinically occult nodal disease (IIIA), we analyzed a cohort of patients who were staged with known occult nodal disease (N1a) combined with those who were staged as clinically negative but harbored pathologically positive nodal disease (cN0 pN1). OS of this substage is likely underestimated.
Here, we present prognostic data in support of the new AJCC 8th edition MCC staging system. The revised system includes distinct clinical and pathological prognostic stage groups. Metastatic MCC with unknown primary tumor is grouped in substage IIIA to reflect improved prognosis compared to those with metastatic MCC and concurrent primary tumor. The revised staging system reflects a better understanding of the clinical behavior of MCC and yields more accurate prognostic information based on stage.
Synopsis:
This analysis of prognostic factors in MCC forms the basis for the new 8th Edition AJCC staging system. The revised staging system provides more accurate prognostication by separation of clinical and pathological stage groups and regrouping of unknown primary tumors.
Acknowledgements
The data used in the study are from the NCDB PUF. The ACS-CoC has not verified and are not responsible for the analytic/statistical methodology employed, or the conclusions drawn by the investigators.
Acknowledgement of research support:
MAH is supported by NIH T32CA009672–24
Footnotes
Disclosures:
KLH, MAH, TMJ, CKB, and SLW have no conflict of interest disclosures.
PN is a consultant for EMD Serono; travel, accommodations, other expenses from EMD Serono; research funding from Brisol Myers Squibb to PN’s institution
AJS has research funding from MELA Sciences; AJS has stock in Merck, Amgen, Teva, and Pfizer
References
- 1.Moshiri AS, Nghiem P. Milestones in the staging, classification, and biology of Merkel cell carcinoma. J Natl Compr Canc Netw 2014;12:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol 2010;63:751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyer JG, Storer BE, Paulson KG, et al. Relationships among primary tumor size, number of involved nodes, and survival for 8044 cases of Merkel cell carcinoma. J Am Acad Dermatol 2014;70:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz JL, Griffith KA, Lowe L, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol 2011;29:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith FO, Yue B, Marzban SS, et al. Both tumor depth and diameter are predictive of sentinel lymph node status and survival in Merkel cell carcinoma. Cancer 2015;121:3252–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kachare SD, Wong JH, Vohra NA, Zervos EE, Fitzgerald TL. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann Surg Oncol 2014;21:1624–1630. [DOI] [PubMed] [Google Scholar]
- 7.Tarantola TI, Vallow LA, Halyard MY, et al. Prognostic factors in Merkel cell carcinoma: Analysis of 240 cases. Journal of the American Academy of Dermatology 2013;68:425–432. [DOI] [PubMed] [Google Scholar]
- 8.Paulson KG, Iyer JG, Byrd DR, Nghiem P. Pathologic nodal evaluation is increasingly commonly performed for patients with Merkel cell carcinoma. J Am Acad Dermatol 2013;69:653–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bichakjian CK, Olencki T, Alam M, et al. Merkel cell carcinoma, version 1.2014. J Natl Compr Canc Netw 2014;12:410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarantola TI, Vallow LA, Halyard MY, et al. Unknown primary Merkel cell carcinoma: 23 new cases and a review. Journal of the American Academy of Dermatology 2013;68:433–440. [DOI] [PubMed] [Google Scholar]
- 11.Chen KT, Papavasiliou P, Edwards K, et al. A better prognosis for Merkel cell carcinoma of unknown primary origin. Am J Surg 2013;206:752–757. [DOI] [PubMed] [Google Scholar]
- 12.Foote M, Veness M, Zarate D, Poulsen M. Merkel cell carcinoma: the prognostic implications of an occult primary in stage IIIB (nodal) disease. J Am Acad Dermatol 2012;67:395–399. [DOI] [PubMed] [Google Scholar]
- 13.Deneve JL, Messina JL, Marzban SS, et al. Merkel cell carcinoma of unknown primary origin. Ann Surg Oncol 2012;19:2360–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edge SB BD, Compton CC, et al. AJCC cancer staging manual (ed 7). New York, NY: Springer; 2010. [Google Scholar]
- 15.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 16.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhtar S, Oza KK, Wright J. Merkel cell carcinoma: report of 10 cases and review of the literature. J Am Acad Dermatol 2000;43:755–767. [DOI] [PubMed] [Google Scholar]
- 18.Fields RC, Busam KJ, Chou JF, et al. Five hundred patients with Merkel cell carcinoma evaluated at a single institution. Ann Surg 2011;254:465–473; discussion 473–465. [DOI] [PubMed] [Google Scholar]
- 19.Howle JR, Veness MJ. Outcome of patients with microscopic and macroscopic metastatic nodal Merkel cell carcinoma: an Australian experience. Dermatol Surg 2014;40:46–51. [DOI] [PubMed] [Google Scholar]


