Abstract
Background:
We measured the effectiveness of a city-wide school-located influenza vaccination (SLIV) program implemented in over 102 elementary schools in Oakland, California.
Methods:
We conducted a retrospective cohort study among Kaiser Permanente Northern California (KPNC) members of all ages residing in either the intervention or a multivariate-matched comparison site from September 2011 - August 2017. Outcomes included medically attended acute respiratory illness (MAARI), influenza hospitalization, and Oseltamivir prescriptions. We estimated difference-in-differences (DIDs) in 2014–15, 2015–16, and 2016–17 using generalized linear models and adjusted for race, ethnicity, age, sex, health plan, and language.
Results:
Pre-intervention member characteristics were similar between sites. The proportion of KPNC members vaccinated for influenza by KPNC or the SLIV program was 8–11% higher in the intervention site than the comparison site during the intervention period. Among school-aged children, SLIV was associated with lower Oseltamivir prescriptions per 1,000 (DIDs: −3.5 (95% CI −5.5, −1.5) in 2015–16; −4.0 (95% CI −6.5, −1.6) in 2016–17) but not with other outcomes. SLIV was associated with lower MAARI per 1,000 in adults 65 + years (2014–15: −13.2, 95% CI −23.2, −3.2; 2015–16: −21.5, 95% CI −31.1, −11.9; 2016–17: −13.0, 95% CI −23.2, −2.9). There were few significant associations with other outcomes among adults.
Conclusions:
A city-wide SLIV intervention was associated with higher influenza vaccination coverage, lower Oseltamivir prescriptions in school-aged children, and lower MAARI among people over 65 years, suggesting possible indirect effects of SLIV among older adults.
Keywords: Influenza, Influenza vaccination, School-located influenza vaccination
1. Introduction
In the United States, seasonal influenza has been responsible for 140,000 – 590,000 hospitalizations and 12,000 – 61,000 deaths annually since 2010 [1]. Children are responsible for the majority of influenza transmission, and mathematical models suggest that vaccinating 50% – 70% of school-aged children for influenza can produce herd immunity [2].
School-located influenza vaccination (SLIV) interventions may increase vaccine coverage among schoolchildren and reduce influenza transmission community-wide [3]. SLIV is associated with higher influenza vaccination coverage [4-13] and lower medically attended acute respiratory illness (MAARI) [14], influenza-like illness [4] and laboratory-confirmed influenza [14,15] in schoolchildren. Some studies have reported indirect effects of SLIV among non-school aged individuals, while others found none [7,8,14,16-20]. With the exception of one study [4], prior SLIV evaluations have measured health outcomes using observational designs that did not rigorously account for systematic differences between intervention and comparison sites prior to intervention [5-8,13-20].
We previously reported the findings from a matched cohort study of a city-wide SLIV program called Shoo the Flu that was implemented in a diverse, primarily low-income population in Oakland, California [21]. The initial evaluation found higher vaccination coverage and lower influenza hospitalization among non-elementary school aged individuals in the intervention site.
Here, we report the results of a retrospective cohort study to measure associations between Shoo the Flu program and additional outcomes, including MAARI and Oseltamivir prescriptions, among Kaiser Permanente Northern California (KPNC) members residing within either the intervention or a matched comparison area. Using data from 2011 to 2017, we estimated associations with SLIV among school-aged individuals and assessed potential indirect effects in other age groups.
2. Methods
2.1. SLIV intervention
This study evaluated the Shoo the Flu intervention (www.shootheflu.org), which has delivered free influenza vaccinations to schools in Oakland, California since 2014. The intervention was delivered to children in all public and charter elementary schools in Oakland Unified School District (OUSD, the “intervention district”) and offered to all other charter and private pre-schools and elementary schools in Oakland. OUSD has a diverse population of over 26,000 elementary school students, and > 70% are low-income. From 2014 to 2017, Shoo the Flu vaccinated 7,502 – 10,106 students annually (22 – 28% of eligible students) in 102–138 schools. Each influenza season, 23–24% of intervention participants reported KPNC health plan membership. Additional intervention details are in the Supplement 1.
2.2. Study setting and population
KPNC is an integrated healthcare system that delivers care at 46 medical clinics and 21 hospitals operated by KPNC to approximately 4 million members. Members comprise at least 30% of the population and are representative of the race, ethnicity, and socioeconomic distribution of Northern California, although very low-income individuals are under-represented. Health care visits, diagnoses, prescriptions, immunizations, and laboratory results are captured in KPNC’s electronic medical record. Vaccines are offered free of charge to members, and the date, injection site, and vaccine brand, lot, and dose of each vaccination at KPNC are recorded. Whether to test patients for influenza A or B using polymerase chain reaction is at the discretion of KPNC clinicians.
All KPNC members who resided in the catchment areas of the intervention and comparison districts from September 1, 2011 - August 31, 2017 and had no more than a one-month gap in KPNC membership for each influenza season during the study period were included in this study.
2.3. Vaccines
In 2014–15 and 2015–16, the intervention provided the live attenuated influenza vaccine (LAIV) to students [22,23]. Students with LAIV contraindications were offered the trivalent inactivated injectable influenza vaccine (IIV3), as were staff and teachers. Because LAIV effectiveness in children was low in 2014–15 and 2015–16 [24-26], the intervention offered IIV4 to all participants following the Advisory Committee on Immunization Practices’ recommendation to use IIV for all children in 2016–17 [25].
2.4. Study design
We conducted a retrospective cohort study of KPNC health plan members who lived in the catchment areas of the intervention district and a comparison district (West Contra Costa Unified School District [WCCUSD]). We identified the comparison school district using a genetic multivariate matching algorithm [27] to pairmatch public elementary schools in the intervention district and each candidate comparison district using pre-intervention school-level characteristics (additional details in Supplement 2); detailed methods are available elsewhere [21]. We selected WCCUSD as the comparison site because it had the smallest average generalized Mahalanobis distance between paired schools [27].
2.5. Program data
KPNC electronic medical records did not include records for vaccinations administered at locations other than KPNC. We therefore estimated the number of vaccinations delivered by the program to KPNC members using data from the Shoo the Flu program, which tracks vaccination counts using the number of vaccination consent forms collected from the parents each year. Consent forms included information about insurance provider (e.g., KPNC or other provider), allowing us to estimate the number of children vaccinated by Shoo the Flu who were KPNC members.
2.6. Population and school district data
We obtained demographic information about the general population in study sites from the U.S. 2010 Census using zip codes that overlapped with the intervention and comparison school districts. We also obtained data about school district populations from the California Department of Education for these zip codes for 2013.
2.7. Outcomes
Outcomes included medically attended acute respiratory illness (MAARI) (see Supplement Table 1 for ICD-9 and ICD-10 codes), laboratory-confirmed influenza among tested individuals, influenza hospitalization, and filled Oseltamivir prescriptions. Individuals hospitalized with any of the following ICD-9-CM codes for otitis media and sinusitis (381–383, 461x), upper respiratory tract illness (79x, 460, 462–463, 465, 487.1), and lower respiratory tract illness (464x, 466x, 480x–487.0, 490x–496x, 510x–513x, 515x–516x, 518x, and 786.1) were classified as having an influenza hospitalization. We defined laboratory-confirmed influenza as a positive result from RT-PCR influenza diagnostic test.
2.8. Definition of influenza season
We defined influenza season based on the percentage of medical visits for influenza-like illness in California using data from the California Department of Public Health. Influenza season started after two consecutive weeks in which the percentage of medical visits for influenza-like illness was greater than or equal to 2%; it ended after two consecutive weeks with a percentage under 2%.
2.9. Statistical analysis
This study’s pre-analysis plan and replication scripts are available at https://osf.io/rtsf2/.
We defined the cumulative incidence as the proportion of individuals with at least one outcome event in each season. We restricted analyses to influenza season, when the intervention would be expected to affect influenza-related outcomes. Our primary pre-specified parameter was mean difference-in-differences (DIDs) that compared the difference in cumulative incidence during influenza season to that in three seasons prior to the intervention (2011–2014) in each study group (the “pre-intervention DID”). Pre-intervention monthly incidences of each outcome were consistent with the equal trends assumption (Supplement Fig. 2). DIDs remove time-invariant confounding but may be subject to time-dependent confounding [28]. To account for this, we conducted a post-hoc alternative “pre-season DID” analysis that compared the incidence in each influenza season to that in the period immediately preceding each season (May – September). While the pre-season DID does not account for pre-intervention differences, it is less subject to time-dependent confounding than the pre-intervention DID.
Models adjusted for available potential confounders with at least 5% prevalence in each analysis; these included race, ethnicity, sex, mediCAL, subsidized KPNC health plan (proxy for low socioeconomic status), and primary language spoken. Enrollment in mediCAL or a subsidized KPNC health plan varied by season; other variables were static. To minimize empirical positivity violations from sparse data [29], we fit models only if the number of outcome events per variable was ≥ 10 and only fit adjusted models if the number of observations within age, site, and outcome strata was ≥ 30 [30]. We estimated 95% confidence intervals using robust standard errors [31] to account for clustering at the household level. Additional minor deviations from the plan are noted in Supplement 3, and additional analysis details are in Supplement 4.
We stratified estimates by pre-specified age groups (0–4, 5–12, 13–17, 18–64, and 65 + years). Estimates among children 5–12 years represent “total effects” (including intervention participants and non-participants) and estimates in other age groups represent “indirect effects” among non-participants (Supplement Fig. 1) [32]. Per our pre-analysis plan, we also stratified estimates by individual vaccination status.
We performed a sensitivity analysis using alternative influenza seasons with influenza-like-illness thresholds of 2.5% and 3% and the CDC influenza season definition (Week 40 to Week 20 of the following year).
We pre-specified two negative control analyses to detect residual confounding or selection bias [33,34]. First, we repeated our primary analysis with outcomes we did not expect SLIV to affect (medically attended diarrhea and medically attended gastrointestinal illness) [33,34]. We conducted a negative control time period analysis restricting to weeks outside influenza season.
2.10. Ethical statement
This study was approved by the Committee for the Protection of Human Subjects at the University of California, Berkeley (Protocol # 2017–03-9741) and the KPNC Institutional Review Board (Protocol #CN-16–2825).
3. Results
3.1. Pre-intervention characteristics
The analyses included 175,628 to 269,266 individuals and 9,436,202 to 11,500,570 person-weeks of observations per calendar year from 2011 to 2017 (Supplement Table 2). During the influenza season, the number of person-weeks ranged from 3,069,633 to 6,801,780 per year.
Pre-intervention characteristics were similar among individuals in the intervention and comparison sites (Table 1). In the intervention site vs. the comparison site, there were fewer Asian (14.2% vs. 19.3%) and Hispanic (30.5% vs. 43.0%) individuals and more Black / African American (28.2% vs. 20.2%) and White individuals (35.2% vs. 29.5%). The proportion of individuals enrolled in MediCAL was lower in the intervention site vs. the comparison site (4.4% vs. 6.7%).
Table 1.
Pre-intervention characteristics of the study population in each site.
| Intervention % (95% CI) |
Comparison % (95% CI) |
|
|---|---|---|
| Age (years) | ||
| Under 5 | 5.2 (5.1, 5.4) | 5.5 (5.4, 5.7) |
| 5–14 | 10.6 (10.4, 10.8) | 13.5 (13.3, 13.8) |
| 15–17 | 3.1 (3.0, 3.2) | 4.2 (4.0, 4.3) |
| 18–64 | 66.0 (65.6, 66.4) | 62.2 (61.7, 62.6) |
| 65 and over | 15.0 (14.7, 15.4) | 14.6 (14.1, 15.0 |
| Sex | ||
| Female | 53.8 (53.5, 54.0) | 52.8 (52.5, 53.1) |
| Male | 46.2 (45.9, 46.5) | 47.2 (46.9, 47.5) |
| Race | ||
| Asian | 14.2 (13.7, 14.6) | 19.3 (18.9, 19.8) |
| Black / African American | 28.2 (27.7, 28.8) | 20.2 (19.5, 20.8 |
| Hawaiian / Pacific Islander | 0.5 (0.4, 0.5) | 0.9 (0.8, 1.0 |
| Native American | 0.4 (0.3, 0.4) | 0.4 (0.4, 0.5) |
| Multiracial | 1.2 (1.1, 1.2) | 1.1 (1.0, 1.2 |
| White | 35.2 (34.7, 35.7) | 29.5 (29.0, 30.1) |
| Race not recorded | 20.4 (19.9, 20.8 | 28.5 (28.0, 29.1) |
| Hispanic Ethnicity a | 30.5 (29.9, 31.2 | 43.0 (42.2, 43.7) |
| MediCAL enrollee | 4.4 (4.2, 4.6) | 6.7 (6.4, 7.0) |
| Subsidized KPNC insurance | 1.0 (0.9, 1.1 | 1.5 (1.4, 1.7 |
| Primary language is not English b | 12.0 (11.7, 12.4) | 14.1 (13.7, 14.6) |
Includes data for 101,761 Kaiser Permanente Northern California patients between September 1, 2013 and August 31, 2014.
Includes data for 61,847 Kaiser Permanente Northern California with recorded Hispanic ethnicity.
Includes data for 100,492 Kaiser Permanente Northern California with recorded primary language.
Characteristics were similar overall between the study population, general population (all individuals residing in zip codes overlapping with the study site) and school district population (Supplement Table 3). The percentage of individuals in the study population who were Hispanic was lower than in the general population (31–43% vs. 39–54%). The percentage of the study population whose primary language spoken was not English was also lower (12–14%) than in the general population (40–47%) and school district population (38–43%).
3.2. Influenza vaccination
Among the study population, influenza vaccination coverage was lower in the intervention site than the comparison site across age groups and years. Prior to the intervention, vaccination coverage was also consistently lower in the intervention site than the comparison site (Supplement Table 4); this difference was larger during the intervention period for children 5–12 years (Supplement Fig. 3). Including all vaccinations (administered by both KPNC and the SLIV intervention), coverage was 8–11% higher in the intervention site than the comparison site during the intervention period (Fig. 1). Across all years, KPNC mostly administered IIV to elementary school aged children in the study (Supplement Fig. 4).
Fig. 1.
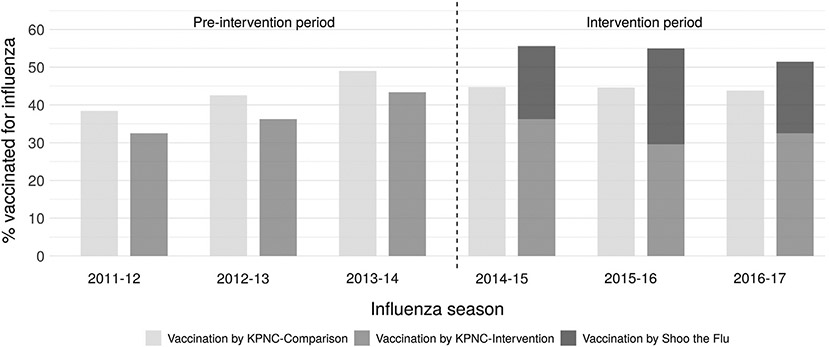
Percentage of elementary school aged study population vaccinated for influenza by Kaiser Permanente Northern California or the Shoo the Flu intervention. “Vaccination by KPNC – Comparison” includes influenza vaccinations delivered by KPNC in the comparison site. “Vaccination by KPNC – Intervention” includes influenza vaccinations delivered by KPNC in the intervention site. “Vaccination by Shoo the Flu” includes influenza vaccinations delivered by the Shoo the Flu intervention in the intervention site. The percentage vaccinated for influenza does not include vaccinations that were not delivered by KPNC or Shoo the Flu. The denominator is KPNC patients aged 5–12 years.
3.3. Cumulative incidence of influenza-related outcomes
The cumulative incidence of MAARI ranged from 0.14 to 0.58 per season by age and was highest among children 0–4 years (Supplement Fig. 5). Very few individuals were tested for influenza (<1% of individuals per season), and the proportion who tested positive varied substantially by age, season, and site. The incidence of influenza hospitalization was low in all age groups, with the highest incidence among adults ≥ 65 years (range: 0.053 to 0.097 per season). The incidence of filled Oseltamivir prescriptions ranged from 0.0007 to 0.0134 per season.
3.4. Associations among elementary school aged children
Overall, the intervention was not associated with MAARI in elementary school aged children except for in 2015–16, when it was associated with lower MAARI when accounting for pre-season differences between sites (Fig. 2, Supplement Fig. 6, Supplement Table 5). Among elementary school aged children, in 2016–17 the unadjusted pre-intervention DID in the cumulative incidence of filled Oseltamivir prescriptions per 1,000 was −3.5 (95% CI −5.5, −1.5) in 2015–16 and −4.0 (95% CI −6.5, −1.6) in 2016–17; there was no association in 2014–15. These associations were attenuated towards the null in the analysis accounting for pre-season differences. There was no association with influenza hospitalization elementary school aged children.
Fig. 2.
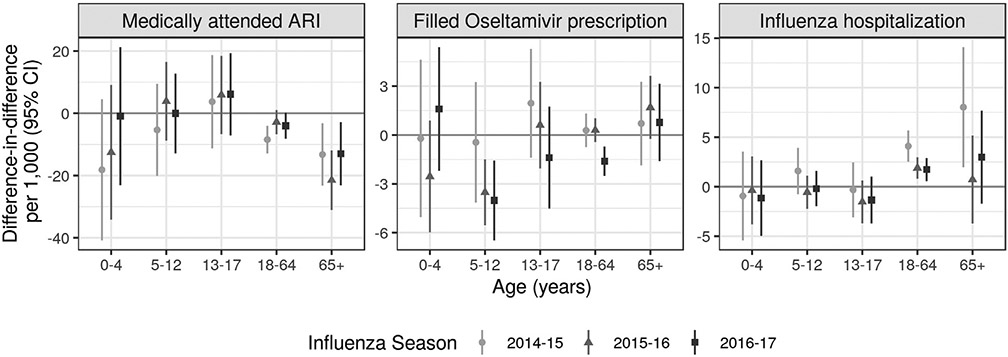
Difference-in-differences accounting for pre-intervention differences. Difference-in-difference in cumulative incidence of each outcome during each influenza season comparing the difference in mean outcome in each district in an intervention year compared to the three pre-intervention years (2011–2013). Difference-in-difference parameters remove any time-invariant differences between groups (measured or unmeasured). Parameters were estimated using a generalized linear model without covariate adjustment due to data sparsity. Standard errors accounted for clustering at the household level. Estimates in children 5–12 years measure total effects and estimates in other age groups measure indirect effects. Analyses were restricted to influenza season defined based the percentage of medical visits for influenza-like illness in California as reported by the California Department of Public Health. Influenza season started when there were at least 2 consecutive weeks in which the percentage of medical visits for influenza-like illness exceeded 2%, and the season ended when there were at least two consecutive weeks in which the percentage was less than or equal to 2%.
3.5. Associations among non-elementary school aged individuals
DIDs indicated no association between the intervention and laboratory-confirmed influenza in most program years and age groups among non-elementary school aged individuals (Supplement Table 5). In intervention vs. comparison sites, MAARI per 1,000 was lower among adults 65 years or older when accounting for pre-intervention differences (2014–15: −13.2, 95% CI −23.2, −3.2; 2015–16: −21.5, 95% CI −31.1, −11.9; 2016–17: −13.0, 95% CI −23.2, −2.9) (Fig. 2). When accounting for pre-season differences, DIDs indicated slightly stronger protective associations with MAARI for most age groups and seasons (Supplement Fig. 6). DIDs for filled Oseltamivir prescriptions per 1,000 were lower among adults 18–64 years in 2016–17 (−1.6, 95% CI −2.5, −0.7); results were similar when accounting for pre-season differences. DIDs indicated a higher incidence of influenza hospitalization among adults 18 years or older in 2014–15 and adults 18–64 years in 2015–16. Pre-season DIDs displayed a different pattern for adults: associations were null for adults 18 years or older except for adults 65 years or older in 2015–16, when the association was protective.
For all ages, the results of adjusted models were similar to those of unadjusted models (Supplement Table 6). Results stratified by vaccination status were similar overall to the primary analysis (Supplement Tables 7-8).
3.6. Negative control analyses
Analyses using non-influenza outcomes (medically attended diarrhea and medically attended gastrointestinal illness) produced primarily null associations (Supplement Table 9). Analyses restricted to weeks outside of influenza season produced almost exclusively null results (Supplement Table 10).
3.7. Sensitivity analyses using alternative influenza season definitions
Results were similar when using alternative influenza season definitions (Supplement Fig. 7). For outcomes with sufficient data to restrict to the peak week of influenza, results suggested no association with the intervention.
4. Discussion
In this three-year evaluation of a city-wide SLIV intervention, among elementary school aged children, SLIV was associated with lower Oseltamivir prescriptions but not with MAARI, laboratory-confirmed influenza, or influenza hospitalization. We found some evidence of indirect effects in non-elementary school aged individuals: SLIV was associated with lower MAARI among adults 65 years or older and lower Oseltamivir prescriptions among pre-school children in 2015–16.
Our primary analysis found higher hospitalizations among adult KPNC members, while the post-hoc analysis, which may better account for time-dependent confounding, found null or protective associations. However, the prior study, which included all hospitalizations in the intervention and comparison sites, found that SLIV was associated with lower influenza hospitalizations in 2015–16 and 2016–17 [21]. One possible explanation for this discrepancy is that effects of SLIV on hospitalization may be stronger among very low-income and non-native English speaking households, which were under-represented in the KPNC population (Supplement Table 3). These households may be more likely to be inter-generational, resulting in higher contact rates between elementary children and adults 65 years or older; they may also have more comorbidities or barriers to healthcare that predispose them to more severe influenza outcomes. Prior estimates of indirect effects of SLIV on hospitalization have also had conflicting findings [4,7,8,13,16,17].
Some prior observational studies of SLIV programs have also reported indirect effects of SLIV on MAARI and related outcomes [14,18-20], while others have reported non-significant or null results [7,8,13,16,17]. These studies did not use analytic methods to account for differences between intervention and comparison sites prior to intervention or outside of influenza season. The one prior study that did so found no association between SLIV and hospitalization among the elderly [16]. The present study included three years of pre-intervention data and used a DID approach to adjust for pre-intervention differences; thus, our findings likely have higher internal validity than prior observational SLIV evaluations.
Vaccine effectiveness varied substantially during the study period. In 2014–15 all vaccine formulations had low effectiveness due to a poor strain match [24]. In 2015–16, the LAIV had poor vaccine effectiveness, but the IIV was moderately effective [26]. In 2016–17, only IIV was available and it was moderately effective [35]. These differences and varying vaccine coverage by the SLIV intervention each year [21] contribute to heterogeneous estimates across seasons.
SLIV may increase vaccination coverage among children who would not otherwise be vaccinated and/or shift vaccination location from doctor’s offices to schools. If SLIV merely shifts vaccination location, it would not be expected to reduce influenza. In this study, SLIV appeared to both increase vaccination and shift vaccination location among elementary school aged children. The proportion of 5–12 year-olds vaccinated by their medical provider was lower in the intervention site than the comparison site, but the proportion vaccinated by KPNC or SLIV combined was higher. This finding is consistent with our prior evaluation of Shoo the Flu [21] and evaluations of other SLIV interventions that also increased vaccination coverage [4-13].
This study is subject to several limitations. First, its observational design may be subject to unmeasured confounding. A small number of negative control analyses suggested that unmeasured confounding occurred. DID analyses controlled for time-invariant confounding [28], but unmeasured time-varying confounding may have occurred. Common confounders of influenza vaccine studies are age, calendar time, and health status [36]. Our analysis stratified by age and season, but health status was not available. Second, differences in sociodemographic characteristics between the study population and the general and student populations in the study sites limit the generalizability of our findings. Third, many outcomes were rare, precluding formal analyses for some outcomes and limiting statistical power, which may have contributed to null findings. Finally, we did not have complete individual vaccination information. It was not possible to link data from the California Immunization Registry with KPNC member data. This limitation underscores the need for more robust vaccine registries [37]. Notably, this study’s limitations apply to most prior SLIV evaluations, which primarily have been observational and leveraged existing data. We expect that among individuals not targeted by the SLIV intervention, influenza vaccinations outside KPNC were infrequent, so this limitation is unlikely to have meaningfully impacted study findings.
Overall, our findings bolster those of our prior evaluation of a city-wide SLIV intervention. This study supports our prior finding that SLIV both increased vaccination coverage and shifted vaccination location and provides additional evidence that SLIV was associated with lower Oseltamivir prescriptions in school aged children and lower MAARI in older adults.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Kristin Goddard, Karen Nunley, and Berwick Chan at Kaiser Permanente Northern California and Kate Holbrook at Alameda County Public Health Department for their contributions to data collection.
Funding
This work was supported by a grant from the Flu Lab (https://theflulab.org/) to the University of California, Berkeley (Grant number: 20142281; PI: AR).
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Art Reingold reports financial support was provided by Flu Lab. Nicola P Klein reports a relationship with Protein Science (Sanofi Pasteur), Sanofi Pasteur, GlaxoSmithKline, Merck & Co, and Pfizer that includes: funding grants.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.08.099.
References
- [1].Centers for Disease Control and Prevention. Burden of Influenza. 2020. Available at: https://www.cdc.gov/flu/about/burden/index.html. Accessed 27 July 2020.
- [2].Longini IM. A Theoretic Framework to Consider the Effect of Immunizing Schoolchildren Against Influenza: Implications for Research. Pediatrics 2012;129(Supplement 2):S63–7. [DOI] [PubMed] [Google Scholar]
- [3].Gaglani MJ. Editorial Commentary: School-Located Influenza Vaccination: Why Worth the Effort? Clin Infect Dis 2014;59(3):333–5. [DOI] [PubMed] [Google Scholar]
- [4].King JC, Stoddard JJ, Gaglani MJ, Moore KA, Magder L, McClure E, et al. Effectiveness of School-Based Influenza Vaccination. N Engl J Med 2006;355 (24):2523–32. [DOI] [PubMed] [Google Scholar]
- [5].Davis MM, King JC, Moag L, Cummings G, Magder LS. Countywide School-Based Influenza Immunization: Direct and Indirect Impact on Student Absenteeism. Pediatrics 2008;122(1):e260–5. [DOI] [PubMed] [Google Scholar]
- [6].Kjos SA, Irving SA, Meece JK, Belongia EA, Cowling BJ. Elementary School-Based Influenza Vaccination: Evaluating Impact on Respiratory Illness Absenteeism and Laboratory-Confirmed Influenza. PLoS ONE 2013;8(8):e72243. 10.1371/journal.pone.0072243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pebody RG, Green HK, Andrews N, et al. Uptake and impact of vaccinating school age children against influenza during a season with circulation of drifted influenza A and B strains, England, 2014/15. Eurosurveillance 2015; 20. [DOI] [PubMed] [Google Scholar]
- [8].Pebody RG, Green HK, Andrews N, et al. Uptake and impact of a new live attenuated influenza vaccine programme in England: early results of a pilot in primary school-age children, 2013/14 influenza season. Eurosurveillance 2014; 19:20823. [DOI] [PubMed] [Google Scholar]
- [9].Szilagyi PG, Schaffer S, Rand CM, Vincelli P, Eagan A, Goldstein NPN, et al. School-Located Influenza Vaccinations: A Randomized Trial. Pediatrics 2016;138(5):e20161746. 10.1542/peds.2016-1746. [DOI] [PubMed] [Google Scholar]
- [10].Szilagyi PG, Schaffer S, Rand CM, Goldstein NPN, Hightower AD, Younge M, et al. Impact of elementary school-located influenza vaccinations: A stepped wedge trial across a community. Vaccine 2018;36(20):2861–9. [DOI] [PubMed] [Google Scholar]
- [11].Szilagyi PG, Schaffer S, Rand CM, Goldstein NPN, Hightower AD, Younge M, et al. School-Located Influenza Vaccination: Do Vaccine Clinics at School Raise Vaccination Rates? J Sch Health 2019;89(12):1004–12. [DOI] [PubMed] [Google Scholar]
- [12].Humiston SG, Schaffer SJ, Szilagyi PG, Long CE, Chappel TR, Blumkin AK, et al. Seasonal Influenza Vaccination at School: A Randomized Controlled Trial. Am J Prev Med 2014;46(1):1–9. [DOI] [PubMed] [Google Scholar]
- [13].Pebody RG, Sinnathamby MA, Warburton F, et al. Uptake and impact of vaccinating primary school-age children against influenza: experiences of a live attenuated influenza vaccine programme, England, 2015/16. Eurosurveillance 2018; 23:1700496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Glezen WP, Gaglani M, Kozinetz C, Piedra P. Direct and Indirect Effectiveness of Influenza Vaccination Delivered to Children at School Preceding an Epidemic Caused by 3 New Influenza Virus Variants. J Infect Dis 2010;202(11):1626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pannaraj PS, Wang H-L, Rivas H, et al. School-Located Influenza Vaccination Decreases Laboratory-Confirmed Influenza and Improves School Attendance. Clin Infect Dis 2014; 59:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McBean M, Hull HF, O’Connor H. Possible Herd Immunity in the Elderly Following the Vaccination of School Children with Live, Attenuated Trivalent Influenza Vaccine: A Person-Level Analysis. Procedia Vaccinol 2011;4:59–70. [Google Scholar]
- [17].Hull HF, McBean AM, Caldwell D, O’Connor H. Assessing Herd Immunity in the Elderly Following the Vaccination of School Children with Live Attenuated Trivalent Influenza Vaccine (LAIV): A County-Level Analysis. Procedia Vaccinol 2010;2(1):92–100. [Google Scholar]
- [18].Tran CH, Sugimoto JD, Pulliam JRC, Ryan KA, Myers PD, Castleman JB, et al. School-Located Influenza Vaccination Reduces Community Risk for Influenza and Influenza-Like Illness Emergency Care Visits. PLoS ONE 2014;9(12):e114479. 10.1371/journal.pone.0114479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Piedra PA, Gaglani MJ, Kozinetz CA, Herschler G, Riggs M, Griffith M, et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine 2005;23(13):1540–8. [DOI] [PubMed] [Google Scholar]
- [20].Piedra PA, Gaglani MJ, Kozinetz CA, Herschler GB, Fewlass C, Harvey D, et al. Trivalent Live Attenuated Intranasal Influenza Vaccine Administered During the 2003–2004 Influenza Type A (H3N2) Outbreak Provided Immediate, Direct, and Indirect Protection in Children. Pediatrics 2007;120(3):e553–64. [DOI] [PubMed] [Google Scholar]
- [21].Benjamin-Chung J, Arnold BF, Kennedy CJ, Mishra K, Pokpongkiat N, Nguyen A, et al. Evaluation of a city-wide school-located influenza vaccination program in Oakland, California with respect to vaccination coverage, school absences, and laboratory-confirmed influenza: a matched cohort study. PLOS Med 2020;17(8):e1003238. 10.1371/journal.pmed.1003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grohskopf LA, Olsen SJ, Sokolow LZ, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) – United States, 2014–15 influenza season. Morb Mortal Wkly Rep 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- [23].Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 Influenza Season. Morb Mortal Wkly Rep 2015;64(30):818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zimmerman RK, Nowalk MP, Chung J, Jackson ML, Jackson LA, Petrie JG, et al. 2014–2015 Influenza Vaccine Effectiveness in the United States by Vaccine Type. Clin Infect Dis 2016;63(12):1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2016–17 Influenza Season. Morb Mortal Wkly Rep 2016;65:1–54. [Google Scholar]
- [26].Jackson ML, Chung JR, Jackson LA, Phillips CH, Benoit J, Monto AS, et al. Influenza Vaccine Effectiveness in the United States during the 2015–2016 Season. N Engl J Med 2017;377(6):534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Diamond A, Sekhon JS. Genetic Matching for Estimating Causal Effects: A General Multivariate Matching Method for Achieving Balance in Observational Studies. Rev Econ Stat 2013;95(3):932–45. [Google Scholar]
- [28].Wing C, Simon K, Bello-Gomez RA. Designing Difference in Difference Studies: Best Practices for Public Health Policy Research. Annu Rev Public Health 2018;39(1):453–69. [DOI] [PubMed] [Google Scholar]
- [29].Petersen ML, Porter KE, Gruber S, Wang Y, van der Laan MJ. Diagnosing and responding to violations in the positivity assumption. Stat Methods Med Res 2012;21(1):31–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49(12):1373–9. [DOI] [PubMed] [Google Scholar]
- [31].Freedman DA. On The So-Called “Huber Sandwich Estimator” and “Robust Standard Errors”. Am Stat 2006;60(4):299–302. [Google Scholar]
- [32].Benjamin-Chung J, Arnold BF, Berger D, et al. Spillover effects in epidemiology: parameters, study designs and methodological considerations. Int J Epidemiol 2018; 47:332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lipsitch M, Tchetgen ET, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiol Camb Mass 2010;21:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Arnold BF, Ercumen A, Benjamin-Chung J, Colford JM. Negative Controls to Detect Selection Bias and Measurement Bias in Epidemiologic Studies. Epidemiology 2016;27:637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Centers for Disease Control. Seasonal Influenza Vaccine Effectiveness, 2016-2017. 2019. Available at: https://www.cdc.gov/flu/vaccines-work/2016-2017.html. Accessed 14 June 2019.
- [36].Ainslie KEC, Haber M, Orenstein WA. Challenges in estimating influenza vaccine effectiveness. Expert Rev Vaccines 2019;18(6):615–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Benjamin-Chung J, Reingold A. Measuring the Success of the US COVID-19 Vaccine Campaign—It’s Time to Invest in and Strengthen Immunization Information Systems. Am J Public Health 2021;111(6):1078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


