Abstract
Mucormycosis is a deadly opportunistic disease caused by a group of fungus named mucormycetes. Fungal spores are normally present in the environment and the immune system of the body prevents them from causing disease in a healthy immunocompetent individual. But when the defense mechanism of the body is compromised such as in the patients of diabetes mellites, neustropenia, organ transplantation recipients, and other immune-compromised states, these fungal spores invade our defense mechanism easily causing a severe systemic infection with approximately 45–80% of case fatality. In the present scenario, during the COVID-19 pandemic, patients are on immunosuppressive drugs, glucocorticoids, thus are at high risk of mucormycosis. Patients with diabetes mellitus are further getting a high chance of infection. Usually, the spores gain entry through our respiratory tract affecting the lungs and paranasal sinuses. Besides, they can also enter through damage into the skin or through the gastrointestinal route. This review article presents the current statistics, the causes of this infection in the human body, and its diagnosis with available recent therapies through recent databases collected from several clinics and agencies. The diagnosis and identification of the infection were made possible through various latest medical techniques such as computed tomography scans, direct microscopic observations, MALDI-TOF mass spectrometry, serology, molecular assay, and histopathology. Mucormycosis is so uncommon, no randomized controlled treatment studies have been conducted. The newer triazoles, posaconazole (POSA) and isavuconazole (ISAV) (the active component of the prodrug isavuconazonium sulfate) may be beneficial in patients who are refractory to or intolerant of Liposomal Amphotericin B. but due to lack of early diagnosis and aggressive surgical debridement or excision, the mortality rate remains high. In the course of COVID-19 treatments, there must be more vigilance and alertness are required from clinicians to evaluate these invasive fungal infections.
Introduction
Mucormycosis is a fungal infection generally caused by the filamentous molds, and it belongs to the mucorals and entomophthorales order (Chakrabarti et al. 2006). Mucorales can be found in a variety of habitats including dirt, rotting plant matter, bread, and dust (Lazar et al. 2014). Mucorales infections can be contracted by inhalation of spores, absorption of infected meals, or inoculation of damaged surfaces or injuries (Lelievre et al. 2014). Mucormycosis is most common in highly immunocompromised hosts in developing countries (Nasa et al. 2017; Wang et al. 2018). In developed economies, mucormycosis is commonly seen in patients with a bad prognosis regulated diabetes mellitus (DM). Furthermore, people suffered from trauma are also prone to mucormycosis (Petrikkos et al. 2012a, b). Mucormycosis has a strong proclivity for invading blood vessels, causing necrosis, thrombosis, and tissue infarction (Moreira et al. 2016a, b). Some belonging genera of Mucorales are Rhizopus, Lichtheimia (formerly Absidia), Mucor, Cunninghamella, Rhizomucor, Apophysomyces spp., and Saksenaea (Petrikkos et al. 2012a, b; Riley et al. 2016). However, Cunninghamella, Rhizomucor, and Saksenaea are predicted to show similar classes of the genera (Jeong et al. 2019). Although, some species display significant variation due to their origin and geographical features. For instance, Apophysomyces reside in a subtropical and tropical environment (Corzo-León et al. 2018), where it can cause principle cutaneous contamination (Bonifaz et al. 2014), widespread soft tissue necrosis necrotizing fasciitis (Rodríguez et al. 2018).
Absorption or inhalation of sporangiospores or inoculation of conidia by puncture trauma or wounds are the initial steps in the pathogenesis of mucormycosis (Lelievre et al. 2014; Petrikkos et al. 2012a, b). Mucormycosis nosocomial outbreaks have been attributed to surgical instruments, infected bandages, and breathing systems, but they are exceedingly rare (Gamarra et al. 2018). Polymorphonuclear phagocytes (PMNs) and mononuclear destroy hyphae and fungal spores in healthy young people using oxidative killing mechanisms and nonoxidative killing mechanisms (Kontoyiannis and Lewis 2006). Deficiencies in phagocytic behavior encourage the organism’s survival or development (e.g., defects in phagocyte function or neutropenia). Acidosis and hyperglycemia, in particular, hinder phagocytic killing and chemotaxis. The enzyme ketone reductase is also generated by Rhizopus, which allows it to expand in acidic and glucose-rich conditions like ketoacidosis (Ibrahim et al. 2012). Mucorales have a natural susceptibility which are killed by human phagocytes, that can elaborate their virulency factor (Chamilos et al. 2008). In the pathogenesis of mucormycosis, iron metabolism is crucial (Petrikkos and Tsioutis 2018). It was clinically observed that patients with iron overload are more prone to the mucormycosis (Ribes et al. 2000). On the other hand, deferoxamine stimulates in vitro fungal development by acting as a Mucorales siderophore (Boelaert et al. 1993). Furthermore, improved serum iron supply in people with acidosis, which is partly attributable to transferrin's decreased affinity for free iron at pH below 7.4, may increase mucormycosis susceptibility (Artis et al. 1983). Mucormycosis has a penchant to attack blood vessels, causing thrombosis and tissue necrosis as a result (Danion et al. 2015). Angioinvasion may be aided by the endothelial cell association with fungal spores (Spellberg et al. 2005a, b). Interaction with endothelial cell receptors in the host can also facilitate endothelial cell damage and fungal dissemination. There are some excellent discussions of pathogenesis that can be found elsewhere (Skiada et al. 2018).
Besides this, COVID-19 has already claimed the lives of over a million people around the world. Supportive care is critical in the management of COVID-19 in the absence of a viable vaccination or antiviral medication. Only glucocorticoids and remdesivir are often effective in COVID-19. Glucocorticoids are affordable and widely available, and they have been found to lower mortality in COVID-19 hypoxemic individuals (Sterne et al. 2020). In contrast, glucocorticoids can raise the risk of subsequent infections. Furthermore, the virus’s immunological dysregulation, as well as the use of immunomodulatory medicines like tocilizumab, may enhance the likelihood of infection in COVID-19 patients (Kumar et al. 2021).
Epidemiology and incidence
Mucormycosis becomes more popular in the last two decades around the world, especially in Belgium, France, India, and Switzerland (Ambrosioni et al. 2010; Lelievre et al. 2014; Saegeman et al. 2010). Mucormycosis is seen in immune compromised patients in the community (Jeong et al. 2019). From 2001 to 2010, the National Hospital Discharge database in France reported 35,876 invasive fungal infections (IFIs), with mucormycosis accounting for 1.5% of IFIs (Bitar et al. 2014). Mucormycosis incidence rose from 0.7 per million in 1997 to 1 per million in 2006 in France while nineteen cases of mucormycosis were diagnosed in a single-center sample in Spain during 2007 to 2015 (incidence 3.2 per 100,000) compared to 1.2 cases per 100,000 from 1988 to 2006 (Guinea et al. 2017). Similarly, three cases were also treated in a tertiary hospital in Switzerland, Geneva, between 1989 and 2003, compared to 16 cases between 2003 and 2008 (Ambrosioni et al. 2010). The increment in treatment were the consequences of rise in immunocompromised victims as well as the use of caspofungin and voriconazole (VORI) (Ambrosioni et al. 2010).
The appearance of mucormycosis rose from 1.7/1000 in 2001 to 6.2/1000 in 2004, according to a prospective monitoring survey of 25 transplant institutes in the United States, although the events of other opportunistic molds remained steady (Park et al. 2011). In comparison, IFIs were found in 121 of 3228 (3.7%) hematopoietic stem cell transplant (HSCT) beneficiaries in an inspection of 11 Italian transplant centers (Pagano et al. 2007). The diagnostics feasibility, use of prophylactic azoles, immunocompromised victims (particularly organ transplant recipients) leads to the significant rise in cases of mucormycosis (Lamoth et al. 2017; Saegeman et al. 2010). Specially, patient with prophylactic POSA53 has been seen to be more prone to mucormycosis.
In a retrospective study conducted at Duke University during 2009–2013, 24 episodes of IFIs were discovered in patients with HemeM or solid organ transplant recipients (SOTRs) receiving POSA (n 148) or VORI prophylaxis (n 14 16) (Lamoth et al. 2017). The most common breakthrough IFI was mucormycosis, which accounted for 9 of the 24 (37.5%) episodes (Lamoth et al. 2017). Despite the abovementioned patterns, mucormycosis remains uncommon.
Between 2006 and 2015, a study of the Intermountain Health sector, a vast US chain of hospitals and clinics, found 3374 IFIs in 3154 topics (Webb et al. 2018). Mucorales are found in 1.1% of IFIs (0.3 cases a 100,000 per year on average). On the other hand, HemeM (19.4%), DM (36.1%), immunosuppressive therapy, and HSCT (11.1%) were among the underlying diseases (61.1%) (Webb et al. 2018). From January 2005 to June 2014, a survey of more than 560 hospitals in the United States (US) serving 104 million patients found 555 mucormycosis-related hospitalizations among more than 47 million inpatient experiences (prevalence of 0.12 per 10,000 discharges) (Kontoyiannis et al. 2016). Table 1
Table 1.
Different forms of clinical observations and their possible symptoms (Petrikkos and Drogari-Apiranthitou 2011)
| Medical structures | Indicators and clinical epitome |
|---|---|
| Pulmonary |
•Dyspnea •Chest pain •Hemoptysis |
| Rhinocerebral, rhino-orbito-cerebral |
•Headache •Facial pain •loss of vision •Lethargy •Brownish •Black eschar on palate •Blood-stained nasal discharge •Chemosis •Ophthalmoplegia •Ptosis •Periorbital cellulitis •Dysfunction of cranial nerves Proptosis |
| Gastrointestinal |
•Non specific oDiarrhea oAbdominal pain oMelena oHematemesis •Depend on site involved |
| Cutaneous |
•Resemble ecthyma gangrenosum •Painful lesions •Necrotizing fasciitis •Cotton-like growth |
| Focal |
•Mediastinitis •Endocarditis •Osteomyelitis •Peritonitis •Otitis external •Pyelonephritis •Corneal infection |
| Disseminated |
•Stroke •Pneumonia •Subarachnoid hemorrhage •Cellulitis •Brain abscess •Gangrene |
From 2003 to 2010, the nationwide inpatient sample in the US found 5346 reports of mucormycosis amongst more than 319 million hospitalizations (incidences of less than 0.01% of all hospitalizations in the US) (Zilberberg et al. 2014). Six percent of mucormycosis patients have no known risk factors. From 2004 to 2012, 74 cases of mucormycosis were discovered in 15 tertiary hospitals across Australia (Kennedy et al. 2016). Eight patients (10.8%) had previously been stable, in which trauma is a factor in seven of this immune-competent patients (Kennedy et al. 2016). Mucormycosis affects only a small percentage of infants (Elgarten et al. 2018). In just 24 h, two international registries were established. Between 2005 and 2014, two foreign registrations in up to twenty-four countries reported only 63 cases in children (aged 19 years) (Pana et al. 2016). In conclusion, mucormycosis is still uncommon, but certain factors (discussed later) significantly raise the risk. A 10-year study from Southern India (Tamilnadu) showed an annual incidence of 18. over 4 cases per year during 2005–2015 (Manesh et al. 2019). Another study from Tamilnadu reported 9.5 cases per year during 2015–2019. A multi-center study across India reported 465 cases from 12 centers 21 months; the study reported an annual incidence of 22 cases per year and an average of 38.8 cases for each participating center (A. Patel et al. 2020). Though invasive aspergillosis is given importance among invasive mold infections in intensive care units (ICUs), a multi-center study in Indian ICUs reported mucormycosis in a considerable (14%) number of patients (Chakrabarti et al. 2019). Sindhu et al. reported mucormycosis at 12% in ICU patients at a single center from North India. Without population-based estimates, it is difficult to determine the exact incidence and prevalence of mucormycosis in the Indian population. The computational-model-based method estimated a prevalence of 14 cases per 100,000 individuals in India (Koffi et al. 2021). The cumulative burden ranged between 137,807 and 208,177 cases, with a mean of 171,504 (SD: 12,365.6; 95% CI: 195,777–147,688) and mean attributable mortality at 65,500 (38.2%) deaths per year (Koffi et al. 2021; Prakash and Chakrabarti 2019). The data indicates that the estimated prevalence of mucormycosis in India is nearly 70 times higher than the global data, which were estimated to be at 0.02 to 9.5 cases (with a median of 0.2 cases) per 100,000 persons (Prakash and Chakrabarti 2019).
Three and two instances were reported from the US and India, respectively, out of the eight cases documented so far (including the index case). Brazil, Italy, and the UK each reported one instance (Hanley et al. 2020a, b; Do Monte et al. 2020; Pasero et al. 2020a, b). The median (range) age was 57.5 (22–86), and seven of the participants were men. Diabetes mellitus (n = 4.50%) was the most common predisposing factor, as diabetes was previously undetected in one case. Three (37.5%) of the participants had no typical risk indicators for mucormycosis (Hanley et al. 2020a, b; Do Monte et al. 2020; Pasero et al. 2020a, b). In seven cases, COVID-19 caused acute respiratory distress syndrome. In five of the cases, the serum creatinine level was elevated, while in the other three, the details were unavailable. Two of the participants had symptoms that suggested mucormycosis (rhino-orbital mucormycosis), while the rest acquired mucormycosis after receiving COVID-19 medication (typically between 10 and 14 days in the hospital) (Mekonnen et al. 2021a, b; Werthman-Ehrenreich 2021a, b). In two of the individuals, the diagnosis was made after death (Hanley et al. 2020a, b; Do Monte et al. 2020). Mucormycosis was seen in the rhino-orbitocerebral (n = 3), pulmonary (n = 3), stomach (n = 1), and disseminated (n = 1) areas. Except for the index case, everyone died. Fifteen hospitalized patients with COVID-19 infection acquired bloodstream candida infections in one cluster from New Delhi, India. Ten of them had a Candida Auris infection, and six of them died (60%) (Chowdhary et al. 2020a, b). Invasive fungal infections were found in 26.7% of 135 individuals with COVID-19 infection, mainly candida. Patients with invasive fungal illnesses had a greater mortality rate, which could be lowered greatly with the right treatment.
An increased incidence of the invasive fungal disease has been linked to corticosteroid medication and a history of chronic lung disease (White et al. 2020). Similarly, significant incidences have been reported in Pakistan (23/147, 15.6%) and Italy (30/108, 27.7%), with the authors claiming that the development of invasive fungal infections modifies the disease’s natural history (Arastehfar et al. 2020; Nasir et al. 2020).
Risk factors for mucormycosis
The progression of mucormycosis has been linked to a number of factors, including the following: poorly regulated DM1, HemeM with neutropenia, HSCT48, SOTRs, immunosuppression or chemotherapy, rheumatic or autoimmune disorders, human immunodeficiency virus infection, peritoneal dialysis, iron overload states, malnutrition, trauma, burns, and prior receipt of VORI (Dimaka et al. 2014; Husain et al. 2017; Kennedy et al. 2016; Kontoyiannis et al. 2005; Lanternier et al. 2012a, b; Moreira et al. 2016a, b; Pana et al. 2016). Mucormycosis appears to protect immunocompetent people (Radner et al. 1995), although infections have been identified after soft tissue damage, or local cutaneous including rhino-orbital, cutaneous, and disseminated infections (Blauwkamp et al. 2019; Tribble et al. 2018). The following risk factors were established in a latest meta-statistics of 600 publications published between 2000 and 2017, which included 851 cases of mucormycosis from around the country: natural disasters (11%), burns (11%), SOTR (14%), no underlying condition (18%), neutropenia (20%), diabetic ketoacidosis (20%), HemeM (32%), trauma (33%), and DM (40%) (Jeong et al. 2019). Figure 1
Fig. 1.
Risk factors for mucormycosis
In Asia, the most common risk factor for mucormycosis is diabetes mellitus (DM), while in North America and Europe, HemeM and organ transplants are far more popular (Jeong et al. 2019). While COVID-19-associated pulmonary aspergillosis (CAPA) has got a lot of attention (Arastehfar et al. 2020; Koehler et al. 2021). The presence of risk factors, consistent radiography, and evidence of Aspergillus in tissue culture or microscopy are all used to diagnose CAPA (Koehler et al. 2021). Invasive mold infections have similar risk factors, clinical symptoms, and radiological findings. As a result, CAM diagnosis is considerably more difficult. Mucormycosis may be underdiagnosed due to a lack of clinical suspicion and difficulty isolating the pathogenic fungi. Furthermore, biomarkers for invasive aspergillosis, such as beta-d-glucan and galactomannan are not accessible for mucormycosis.
To our knowledge, this is the first instance of suspected pulmonary mucormycosis arising following COVID-19 treatment that has been effectively handled. Severity has been linked to diabetes mellitus (Apicella et al. 2020). Furthermore, diabetes patients who are inadequately controlled may develop overt or covert renal impairment. Multiple risk factors or concomitant conditions, combined with increased immunosuppression caused by glucocorticoids, raise the net state of immune suppression in severe COVID-19 patients, predisposing them to invasive mold infections. Table 2
Table 2.
Risk factors associated with patients in Indian geographical regions
| Factors | Manesh et al. 2019 (Manesh et al. 2019) | Prakash et al. 2019 (Prakash et al. 2019) | Patel et al. 2020 (A. K. Patel et al. 2020) | Priya et al. 2020 (Priya et al. 2020) |
|---|---|---|---|---|
| Duration (year) | 10 | 3 | 2 | 4 |
| Origin/location in India | South India | North and South India | West India (Gujrat) | South India |
| Case rises | 184 | 388 | 27 | 38 |
| Neutropenia | - | 18 | - | - |
| Chronic alcoholism | - | 28 | - | - |
| Pulmonary disease | - | 21 | 2 | - |
| Skin brach | 20 | 31 | 6 | 8 |
| Hematological and solid organ malignancy | 14 | 23 | 1 | 2 |
| HSCT | 4 | 1 | - | - |
| DM | 120 | 172 | 15 | 29 |
| CKD | 1 | 27 | 1 | 2 |
| HIV | - | 3 | - | - |
| Steroid therapy | - | 30 | 6 | - |
Poorly controlled diabetes mellitus
Mucormycosis is a well-known impediment of largely unregulated DM (Jeong et al. 2019; A. Patel et al. 2020), and it is linked to innate immunity defects like phagocytosis, chemotaxis, and killing by PMNs, as well as macrophages/monocytes (Geerlings and Hoepelman 1999). DM is the proximate cause of mucormycosis in 27–52% cases (Kennedy et al. 2016; Kontoyiannis et al. 2016; Webb et al. 2018). The rhinocerebral type of mucormycosis is more common in diabetics with ketoacidosis (Roden et al. 2005). The sinuses are the most prominent initial source of involvement, and they may even extend to the orbit, brain, and bone. In diabetes-associated pulmonary, mucormycosis, or disseminated infections are rare, (Rapidis 2009) unlike in transplant patients or recipients with HemeM (Kontoyiannis et al. 2016; Webb et al. 2018).
Hematological malignancies with neutropenia
Chemotherapy-induced innate host defense neutropenia, deficiencies, mucociliary dysfunction, and phagocytic dysfunction all raise the chances of contamination (Park et al. 2011). In victims with neutropenia and HemeM, mucormycosis is a severe but life-threatening complication (Kontoyiannis et al. 2005; Lanternier et al. 2012a, b; Pana et al. 2016). Mucormycosis is a fungal infection that can affect HSCT recipients, especially those who have graft versus host complication (Kontoyiennis et al. 2010; Marr et al. 2002).
Between 1985 and 1999, 27 (0.6%) of 5589 patients who received HSCT at the Fred Hutchinson Cancer Research Center in Seattle experienced confirmed or suspected mucormycosis. Seven hundred sixty-five cases of mucormycosis were included in a later sample of 1248 allogeneic HSCT recipients transplanted between 1998 and 2002 at that hospital (incidence 0.4%) (Garcia-Vidal et al. 2008). The mean prevalence of mucormycosis was 0.48% in a report of 16,200 HSCT recipients from 23 transplant centers in the US from March 2001 to September 2015 (Kontoyiennis et al. 2010). However, a systematic study of HSCT and SOTRs at Johns Hopkins Hospital (Baltimore, MD) found that mucormycosis was responsible for 8.5% of invasive mold infections between 2000 and 2009 (Kontoyiennis et al. 2010).
The Centre for International Blood and Marrow Transplant Research gathered registry data from almost sixty-six transplant centers around the world and found up to 72 cases of mucormycosis during the first year of postlogeneic HSCT (incidence 6.0/1000), which was close to previous years (Riches et al. 2016). IFIs were found in 121 of 3228 (3.7%) HSCT patients investigated by 11 Italian transplant centers, but only one case of mucormycosis was diagnosed (Pagano et al. 2007).
Organ transplant recipients
Mucormycosis is a relatively uncommon impediment in SOTRs (Husain et al. 2017). Based on statistics from 23 US centers that make up the Transplant-Associated Infection Surveillance Network, the average annual incidence of mucormycosis among SOTRs was 0.07% from 2001 to 2006 (Park et al. 2011). University of Pittsburgh’s researcher identified ten cases of mucormycosis among SOTRs and analyzed 106 cases previously published between 1970 and 2002 (Almyroudis et al. 2006). Just 22 IMIs (6.9%) were mucormycosis, according to the Prospective Antifungal Therapy Alliance list, which tracked 333 IMIs among SOTRs transplanted at 25 centers between 2004 and 2008 (Husain et al. 2017). From 1995 to 2012, only one case of mucormycosis (Lichtheimia) was found among 362 heart transplant patients in only one center (Rabin et al. 2015). Song and colleagues looked at 174 cases of mucormycosis in renal transplant patients from 123 papers written between 1970 and 2015 (Song et al. 2017). As a whole, 42.5% of people died (Song et al. 2017). Between 1998 and 2009, Brazilian researchers found only one case of mucormycosis among 908 renal transplant recipients who were monitored until July 2015 (Guimarães et al. 2016). Numerous case reports and limited sequence of mucormycosis in SOTRs have been reported, including liver, kidney (Clark et al. 2018), heart, and lung recipients (Bhaskaran et al. 2013).
Immunocompetent host
In India, 3–26% of mucormycosis cases have been recorded from the immunocompetent host, compared to 18–19% globally (Jeong et al. 2019; Roden et al. 2005). Cutaneous or isolated renal mucormycosis were common in the Indian patients. However, trauma is a risk factor in 7.5–22% of mucormycosis cases in India. The majority of the patients present with cutaneous mucormycosis after trauma, burns, and nosocomial infections at the surgery or injection site (Manesh et al. 2019). Another study from North India reported that 9% of the mucormycosis cases are nosocomial in origin (A. Chakrabarti et al. 2009).
Contaminated intramuscular injections and surgery, adhesive tapes, and endobronchial tubes were sources of infection in nosocomial mucormycosis (C. Kumar et al. 2017). Isolated renal mucormycosis in an immunocompetent host is an emerging entity in India. The pathogenesis of the disease is still not known (Devana et al. 2019). Other predisposing factors associated with mucormycosis in India are chronic kidney disease (CKD), steroid therapy, pulmonary tuberculosis, and chronic obstructive pulmonary disease (COPD) (Patel et al. 2020; Prakash et al. 2019).
CKD is a new risk factor for mucormycosis in India (Prakash and Chakrabarti 2021). Studies reported that mucormycosis patients had CKD in 9–32% of cases (Chakrabarti et al. 2019; A. Patel et al. 2020). Similarly, a study from Turkey reported that 18% of the patients with mucormycosis had chronic renal insufficiency (Kursun et al. 2015). Pulmonary tuberculosis and COPD were seen in 7–46% of patients with mucormycosis (Jeong et al. 2019; Patel et al. 2020). A few cases of breakthrough mucormycosis after voriconazole treatment were reported in India (Sharma et al. 2017). Other risk factors reported in India included intravenous drug use, autoimmune disease, HIV infection, immunosuppressant drugs, malnutrition, and ICU stay.
Diagnosis
Clinical diagnosis
A high index of suspicion, identification of host conditions, and rapid evaluation of clinical symptoms are all needed for the diagnosis of mucormycosis. Diplopia in a diabetic patient or pleuritic pain in a neutropenic patient may be signs of infection, prompting the use of imaging techniques and the eventual collection of specimens for histology, microbiology, and advanced molecular research. As stated earlier, rhinocerebral, pulmonary, soft tissue, and disseminated infection are the most frequent clinical manifestations of Mucorales infectious disease (Petrikkos et al. 2012a, b). Mucormycosis is characterized by tissue necrosis, however, the appearance and syndrome-based methods of diagnosis lack sensitivity and accuracy. Other fungi, such Aspergillus or Fusarium, may cause similar complications. Furthermore, in tuberculosis-endemic countries, the two infections can coexist, as in the case of a diabetic patient (Aggarwal et al. 2015). Nonetheless, certain characteristics should boost the fear of intrusive pulmonary mucormycosis. Table 3
Table 3.
Antifungal drugs used to treat mucormycosis: a summary of recommendations
| Antifungal agent | Dose | Duration | Source |
|---|---|---|---|
| Posaconazole |
• Posaconazole IV/tablet: 1 × 300 mg from day 2, 2 × 300 mg day 1 • Oral suspension: 2 × 400 mg/day or 4 × 200 mg/day |
6 months | (Greenberg et al. 2006; Graves et al. 2016; A. Skiada et al. 2011) |
| Amphotericin B (ABCL, AMB, LAMB) |
CNS participation: 1 mg/100 g per day LAMB No CNS participation: 0.5 mg/100 g per day LAMB |
Usually 6–12 weeks | (Shoham et al. 2010; Walsh et al. 2012) |
| Combination |
• Isavuconazole or posaconazole and LAMB • LAMB + echinocandin |
(Kyvernitakis et al. 2016; Rodriguez et al. 2018; Vujanovic et al. 2017) | |
| Isavuconazole | IV or PO: 3 × 200 mg day 1, 1 × 200 mg/day from day 3 | 3 months (oral/iv/both) | (DiPippo et al. 2019; Maertens et al. 2016; Mellinghoff et al. 2018) |
A history of previous voriconazole prophylaxis or the occurrence of breakthrough fungal infection in an immunocompromised patient receiving antifungal agents functional against Aspergillus (Chamilos et al. 2005). Corzo-Leon and colleagues suggested an algorithm for detecting rhinocerebral mucormycosis in diabetic patients. A cranial nerve palsy, sinus pain, diplopia, periorbital swelling, proptosis, and palate ulcers orbital apex syndrome are among the signs and symptoms that can be called “red flags” (Corzo-León et al. 2018).
Mucormycosis is said to be associated with numerous nodules and pleural effusion on radiography (Chamilos et al. 2005). The reverse halo symbol (RHS) is another CT scan finding that seems to suggest the existence of mucormycosis. The RHS was seen in 15/16 patients (94%) within a week of the disease in a new review of consecutive thoracic CT scans of leukemic patients with neutropenia. Other radiologic observations, like numerous nodules, appeared later. The investigators suggested that the appearance of the RHS on CT was a good indication of pulmonary mucormycosis in neutropenic leukemic patients with pulmonary infection. The CT scans of 24 patients with lung mucormycosis were similar to the CT scans of 96 patients with suspected lung aspergillosis in another report. The RHS was more prevalent in mucormycosis patients (54%) than in aspergillosis patients (6%, P.001), while certain airway-invasive characteristics, such as clusters of centrilobular nodules, bronchial wall thickening, and peribronchial consolidations were more frequent in aspergillosis patients (Jung et al. 2015). Although these results aren't definitive, they can be seen as an initial point for more intensive medical laboratory studies. The positron emission tomography-computed tomography (PET/CT) with [18F]-fluorodeoxyglucose (FDG) is yet another emerging imaging strategy that may ultimately help in the management and diagnosis of mucormycosis (Liu et al. 2013). Endobronchial ultrasound-guided fine-needle aspiration is indeed a helpful screening technique (Haas et al. 2017).
Culture and microscopic examination
Direct and histopathology microscopy and different clinical culture specimens are diagnosing mucormycosis’s cornerstones. Mediclinic samples are examined under direct microscopy, specifically using optical brighteners such as Calcofluor and Blankophor (Frater et al. 2001; Lass-Flörl 2009). Clinical samples that are white allow for a quick preliminary analysis of mucormycosis (Lass-Flörl et al. 2007). Mucorales hyphae have a variable diameter (6–25 m), are nonseptate or pauci-septate (Monheit et al. 1984), and have an irregular, ribbon-like shape.
The branching angle varies, with wide-angle (90°) bifurcations being common. On hematoxylin and eosin parts, fungal elements are easily visible. Gomori’s silver staining is often used to illuminate fungal hyphae, allowing for a more detailed analysis of anatomy (Lass-Flörl 2009). Inflammation, whether neutrophilic or granulomatous, dominates tissue histopathologic results in a few cases. Inflammation appears to be absent especially in immunocompromised patients (Spellberg et al. 2005a, b). The presence of prominent infarcts and angioinvasion characterizes the invasive disease. A perineural invasion can be present when the nerve structures are involved. As compared to nonneutropenic patients, neutropenic patients have a more severe angioinvasion (Frater et al. 2001).
Histopathological examination of tissue samples is not always capable of distinguishing Mucorales hyphae with Aspergillus or morphological characteristics related to fungi. Tissue identification, on the other hand, is an important diagnostic tool because it distinguishes between the existence of fungi as a pathogen in a tissue and the presence of a culture contaminant. Mucorales grow quickly on most fungal culture media, like Potato dextrose and sabouraud agar incubated at 25–30 °C (3 to 7 days) (Chakrabarti et al. 2006; Ribes et al. 2000). A microaerophilic condition increases culture yield for certain varieties (Lass-Flörl and Mayr 2009). Surprisingly, even though fungal hyphae are visible in the histopathologic examination, only 50% of fungal cultures are positive. Since hyphae are brittle, they could be harmed as a result of tissue manipulation.
Antifungal susceptibility testing and species identification
Characterization of organisms is critical for having a deeper epidemiological knowledge of mucormycosis and could aid epidemic investigations. Mucorales fungi are easily distinguishable from Aspergillus fungi when grown in culture. When assessed by individuals with expertise in fungal detection, Alvarez et al. (2009) found that morphological characteristics alone could provide high accuracy. However, morphological specimen classification is challenging and may be due to speciation failures (Frater et al. 2001).
ID32C kit (bio Merieux, Marcy lÉtoile, France) and API 50CH (bioMerieux) (Ramani et al. 1998) have been successfully used for the recognition of R. pusillus, and Lichtheimiacorymbifera as well as Mucor species. Both tests failed to differentiate M. circinelloides and M. rouxii. L. ramosa is detected using ID32C and positive melezitose assimilation (Schwarz et al. 2007). MALDI-TOF mass spectrometry (matrix-assisted laser desorption/ionization time-of-flight) is a promising instrument, but it has not yet been validated for all Mucorales (Schrödl et al. 2012). M. circinelloides has high MICs for posaconazole, as well as Cunninghamella and Rhizopus for amphotericin B. (Vitale et al. 2012) The MIC against amphotericin B has also increased in certain apophysomyces isolates (Alvarez et al. 2009; Bonifaz et al. 2014). The role of such data in patient care is uncertain, requiring further investigations.
Serology
A reasonable test such as enzyme-linked immunosorbent assays (Sandven and Eduard 1992), immunoblots (Wysong and Waldorf 1987), and immunodiffusion were evaluated based on the degree of their effectiveness. In three hematological patients who developed invasive mucormycosis, an enzyme-linked immunospot (ELISpot) assay has been used to identify Mucorales-specific T cells. Additionally, Mucorales-specific T cells were used in the infected patients to recover over the disease (Potenza et al. 2011). Although, there will be further studies towards the use of these special T cells as surrogate diagnostic markers.
Molecular assays
Conventional PCR, RFLP, and DNA sequencing of identified gene regions (Machouart et al. 2006; Nagao et al. 2005; Shirley and Scott 2016) and melt curve analysis of PCR products are all examples of molecular-based assays (Kasai et al. 2008). Many of the essays mentioned above can be used to detect or identify Mucorales. The internal transcribed spacer or the 18S rRNA genes are the focus of the majority of molecular assays (Lass-Flörl and Mayr 2009). Several studies have been conducted using paraffin-embedded or fresh tissue samples, formalin-fixed, with varying results. The studies performed for sensitivity (70–100%) and specificity (not measured to 100%) shows varied results, but a lower number of patients examined being a critical shortcoming. Since the efficacy of these in-house assays has not been extensively tested and clinically evaluated, they cannot be put forward as a single, stand-alone in clinical routine diagnostics, this approach is used. Molecular diagnosis from blood and serum has yielded positive clinical results in 39% of cases (Guinea et al. 2017; Ino et al. 2017; Millon et al. 2016). When opposed to culture, early diagnosis and overall confirmed culture-proven cases were achieved using molecular-based diagnosis from serum. At this time, molecular-based diagnostic assays may put forth as useful supplements to traditional diagnostic procedures (Yang et al. 2016).
Advancement in the diagnosis
Histopathology and culture are used to diagnose mucormycosis (Hamilos et al. 2011). Mucorales are prone to vascular invasion and tissue damage in the organs they infect (Ribes et al. 2000). Tissue infarction occurs when blood vessels become thrombosed. Consequently, a black eschar may form. Gram stain is ineffective on Mucorales. Mucorales have long (10–20 µm in diameter) nonseptate ribbon-like hyphae with branches at right angles in tissue specimens (Frater et al. 2001).
Mucormycotic infections may cause neutrophilic, granulomatous, or nonspecific inflammatory changes, as well as angioinvasion or infarcts in some cases (Frater et al. 2001). Fine needle aspiration biopsy may be used to confirm the diagnosis when focal pulmonary nodules or masses are present (Haas et al. 2017; Sharma et al. 2017). Even if histopathology reveals the characteristic organism, cultures can be negative. Grinding tissue specimens for culture also results in hyphae damage due to the scarcity of septations which prevents growth in culture. In mucormycosis, serological tests for D-glucan and Aspergillus galactomannan are negative (Pyrgos et al. 2008). For certain organisms (e.g., Rhizopus, Mucor, Rhizomucor, Lichtheimia), quantitative PCR in serum or tissue is available and may be superior to culture (Hata et al. 2008; Rickerts et al. 2006; Shigemura et al. 2016). Matrix-assisted laser desorption/ionization-time of flight tends to be another tool with high precision for separating mould from cultures (Cao et al. 2018; Gholinejad-Ghadi et al. 2018). Next-generation sequencing can detect IMIs in blood samples which could lead to earlier detection of these infections (Blauwkamp et al. 2019).
Clinical features of mucormycosis
Mucormycosis is divided into six types based on anatomic localization (see Table 4). Roden et al. (2005) investigated the common sites of the infection in the 929 cases of the mucormycosis. The investigated locations were lungs (24%); sinuses (39%); soft tissue infection and skin (SSTI) (19%) and disseminated (23%). In the study, it was observed that 15 patients having cancer were chosen from a group of 154 patients in which 6 patients have rhino-orbital-cerebral mucormycosis (ROCM), while 92 have pulmonary disease. In contrary, 222 patients out of 337 with DM were found to be affected by sinus disease while 145 having ROCM. In a study conducted by European Confederation of Medical Mycology (ECMM) registered 230 cases of mucormycosis between 2005 and 2007 in European countries. The profound cases were of ROCM (27%), lungs (30%), dissemination (15%), and SSTI (26%) (Skiada et al. 2011). During 2005–2007, France was also reports the mucormycosis cases of ROCM (25%), lungs (28%), dissemination (18%), and SSTI (20%) (Lanternier et al. 2012a, b). Between 2004 and 2012, underlying diseases in an Australian cohort of 74 patients with mucormycosis included HemeM (49%), corticosteroids (53%), DM (29%), chemotherapy (43%), autoimmune disease/rheumatological (12%), and no implicit disease (11%) (Kennedy et al. 2016). Trauma was the cause of seven of the eight patients that had no previous underlying illness.
Table 4.
Forms of mucormycosis
| S. no | Infection site | Percentage of cases | Source |
|---|---|---|---|
| 1 | Disseminated | 15–23% | (Riley et al. 2016) |
| 2 | Rhino-orbital-cerebral mucormycosis (ROCM) | 25–39% | (Dimaka et al. 2014) |
| 3 | Gastrointestinal | 2–11% | (Bernardo et al. 2016) |
| 4 | Cutaneous/soft tissue | 19–26% | (Li et al. 2013) |
| 5 | Pulmonary | 24–30% | (Lamoth et al. 2017) |
| 6 | Other sites (joints/bone, peritoneum, heart) | Rare | (Moreira et al. 2016a, b) |
ROCM
Mucormycosis is a fungal infection that can spread from the sinuses to the oral mucosa, palate, brain and bone, orbit (Li et al. 2013). ROCM is the name given to this clinical condition. Patients with poorly treated diabetes mellitus are more likely to develop ROCM. But it may also happen in SOTRs and other immunocompromised hosts (Lanternier et al. 2012a, b). In patients with poorly regulated diabetes, dental procedures can be a risk factor for ROCM (Prabhu et al. 2018). Dental infections can lead to destructive infections of the mandible or maxilla (Aras et al. 2012; Prabhu et al. 2018). Headache, fever, nasal inflammation, facial pain, palatine mucosa eschar, or dark nasal and periorbital swelling are some of the signs and symptoms of the problem (Augustine et al. 2017).
Cellulitis, chemosis, proptosis, and blurred vision are all signs of orbital invasion (Mattingly and Ramakrishnan 2016). Intracranial progression through direct extension or angioinvasion can happen quickly (within days). A high mortality rate is linked to brain involvement (Vogt et al. 2017). Rhizopus oryzae was found to be responsible for 85% of ROCM in a French analysis, compared to just 17% of non-rhinocerebral mucormycosis (p < 0.001) (Lanternier et al. 2012a, b). For a good outcome from ROCM, aggressive and rapid care is needed, including resection/surgical debridement as well as medical therapy (Guinea et al. 2017). LFAB at a high dose should be given at the same time. ISAV (Ananda-Rajah and Kontoyiannis 2015) and amphotericin B (Greenberg et al. 2006) are FDA-approved mucormycosis treatments, while POSA has been used off-label as a step-down therapy or salvage (Manesh et al. 2016).
Pulmonary involvement
Involvement of the lungs (Nam et al. 2015), perilesional ground-glass opacities (GGOs) (Hammer et al. 2018), centrilobular nodules, “reverse halo sign” (RHS), air-crescent sign (Dykhuizen et al. 1994) obar consolidation or peribronchial (Jung et al. 2015), upper lobe predominance (Jamadar et al. 1995), pleural effusions (Dykhuizen et al. 1994), and bronchopleural fistula (Hammer et al. 2018) are the affected locations through invasion of active environment. Necrotizing pneumonia may occur when pulmonary vessels are invaded (Lee et al. 2016). Aspergillus spp. causes offensive ulcerative tracheobronchitis at the lung transplant anastomotic site recipients, but Rhizopus tracheobronchitis has also been reported (Grossi et al. 2000). All but one patient in each group had HemeM. By concomitant sinusitis, logistical regression analysis and VORI prophylaxis were importantly associated with PulM. Further, pleural effusions and CT findings of 10 nodules were importantly connected with PulMs (Dykhuizen et al. 1994).
Many researchers noticed that RHS is much more widespread in PulM than IPA (Jung et al. 2015). Central GGO emphasis surrounded by a crescent or stable ring of consolidation is referred to as the RHS (Stanzani et al. 2012). Patients with suspected or confirmed fungal pneumonia, including PulM (n ¼ 37), IPA (n ¼ 132), and fusariosis (n ¼ 20), were analyzed by researchers from the MD Anderson Cancer Center in Houston, TX. RHS was found in 7/37 (19%) of PulM patients and 1/132 (0.8%) of IPA patients. A retroactive review from 2003 to 2012 at a single cancer center found 16 cases of PulM in 752 subsequent cases of acute myeloblastic or lymphoblastic leukemia. RHS with a capacity of 94% (15 of 16) was seen in 186 CT scans taken during the first week of disease. Other CT observations such as pleural and nodules effusion were uncommon during the first week (12 and 6%, respectively), but occurred in 55 and 64%, respectively, after the first week. The CT characteristics of 24 patients with PulM (proven or probable) and 96 patients with IPA were matched by South Korean researchers (proven or probable) (Jung et al. 2015). RHS was included in 54% of PulM subjects but just 6% of IPA subjects (p < 0.001). On the other hand, airway signals were much more frequent in IPA than in PulM (i.e., clusters of centrilobular nodules) (Jung et al. 2015).
The development or reversal of lung lesions can be tracked using serial chest CT scans (Choo et al. 2014; Nam et al. 2015; Wahba et al. 2008). From 1997 to 2016, consolidation of GGOs, nodules/mass lesions, or a halo was the most frequent initial lesions on CT in a cohort of 20 immunocompromised patients with PulM at a single center (90%) (Nam et al. 2015). RHS, air crescents, and central necrosis were often seen on follow-up CT scans in 15 patients, and these morphological characteristics were associated with histological observations of pulmonary hemorrhage, arterial thromboses, and lung tissue infarction. Invasive tracheobronchitis caused by Mucorales or other molds has been reported in manually ventilated patients in ICUs, with a 93.5% overall mortality rate (Lin et al. 2017). DM in 58% and chronic lung disease in 39% were found in cohort. Between 2000 and 2012, MD Anderson researchers observed 75 subsequent patients with PulM and hematological diseases (Lewis et al. 2014). Within 4 weeks, 28 people (37.3%) died. Acute Physiology and Chronic Health Evaluation (APACHE II) ranking, extreme lymphopenia, and high serum lactate dehydrogenase level were all independent risk factors for accelerated development and death (Lewis et al. 2014). In 36 patients with presumed IFIs and HemeM, pulmonary CT angiography was performed. The findings were associated with arterial artery interruption and were found in 5/5 proven cases and 5/7 possible IFIs (Stanzani et al. 2012). However, the test’s usefulness is still debatable. Overall mortality rates for PulM vary from 50 to 70%, but rates for extrathoracic dissemination reach 90% (Lee et al. 2016). Surgical resection (along with medical therapy) can be lifesaving in the case of localized PulM (Mills et al. 2018).
Cutaneous involvement
Involvement of the cutaneous mucormycosis can manifest as a localized or disseminated disease in immunocompromised individuals. Traumatic injury (Jeong et al. 2019), surgery (Tilak et al. 2009), fires (Ledgard et al. 2008), natural hazards (Austin et al. 2014), war (Tribble et al. 2018), insect or animal bites (Lechevalier et al. 2008), and also the inoculation of infected soil, trees, grasses, thorns, or water (Lewis et al. 2014) cause cutaneous mucormycosis in immunocompetent hosts (Losee et al. 2002). Mucormycosis is a fungal infection that spreads quickly across the subcutaneous tissues, skin, bone, and fascia. A total of 16 cases of posttraumatic mucormycosis (PTM) and 85 cases of nontraumatic mucormycosis were identified by French researchers. PTM patients ranged from nontraumatic mucormycosis patients in many ways, such as cutaneous localization, rarity of underlying disease, short time before diagnosis, better 90-day survival rate, and the species involved.
In tropical and subtropical regions, apophysomyces and saksenaea have become the most common species causing localized cutaneous mucormycosis (Page et al. 2008). Many of the isolates were apophysomyces trapezi form in a cluster of 13 cases of PTM in Joplin after a tornado. In a survey of 230 cases of mucormycosis conducted by the ECMM registry, 39 patients had PTM and 35 (85%) were immunocompetent, with surgical or other trauma serving as the predisposing factor (Skiada et al. 2011). The need for aggressive and immediate surgical debridement, as well as medical treatment, is critical (Lelievre et al. 2014; Losee et al. 2002).
Gastrointestinal tract involvement
The introduction of gastrointestinal tract by 2–11% in patient with mucormycosis develop gastrointestinal (GI) involvement which is fatal (Antony et al. 2015; Nandwani et al. 2015). In a study of 31 cases of gastrointestinal mucormycosis, 13 (42%) the stomach and 16 (52%) of the cases included the intestine. Hepatic presence is quite uncommon in the present scenario (Bernardo et al. 2016; Tuysuz et al. 2014). However, as per the suggestions, hospital therapy and surgical resection combined with aggressive surgery can be curative in this case (Tuysuz et al. 2014).
Therapy
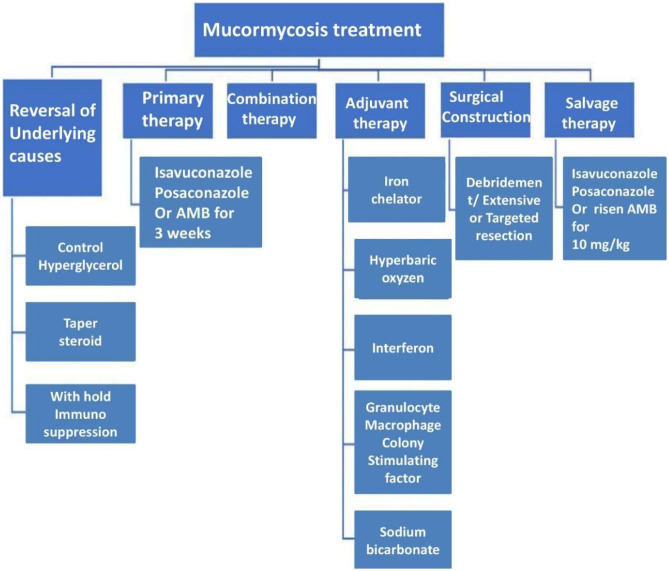
Mucormycosis is an uncommon phenomenon causing a deadliest opportunity in the affected patients. Several research groups from the global organization are working on this problem. Many suggestions and therapy treatments are provided by the researchers. Several researchers’ advice that randomized controlled trials (RCTs) have not been possible due to the rarity of mucormycosis. However, LFAB can be employed as first-line treatment to cure the mucormycosis disease (Rüping et al. 2009; Anna Skiada et al. 2013). Triazole with Mucorales activity was approved by the FDA to treat adult mucormycosis while, POSA, having Mucorales activity, was used as salvage and induction therapy (Jenks et al. 2018; Schwarz et al. 2019). Subsequently, surgical debridement serves an essential supporting role, especially when there is the participation of the soft tissue or rhino cerebral (Arendrup et al. 2014; Anna Skiada et al. 2013). The importance of reversing underlying risk factors such as glucocortico steroid discontinuation/taper, neutropenia resolution, deferoxamine discontinuation, diabetes regulation, and immunosuppressant reduction cannot be overstated (Arendrup et al. 2014; Tissot et al. 2017).
Many antifungals are immune to Mucorales. The most active agents include LFAB (Arendrup et al. 2014; Schwarz et al. 2019) and the newer triazoles, POSA and ISAV (Marty et al. 2016; Schwarz et al. 2019). On the other hand, the echinocandins and VORI had deficient activity against Mucorales (Schwarz et al. 2019).
Mucormycosis has been treated with LFAB for a long time (Arendrup et al. 2014; Anna Skiada et al. 2013). ISA and POSA have primarily been used as salvage therapy in patients who have developed resistance to or intolerance to LFAB (Schwarz et al. 2019). Mucorales resistance study in vitro may aid in treatment selection, but the clinical utility of such testing is unknown due to a lack of evidence linking susceptibility testing to results. Since mucormycosis can spread quickly, LFAB should be started as soon as the disease is suspected (Arendrup et al. 2014). A 6-day delay in AmB-based therapy was linked to a twofold increased risk of death at 12 weeks after diagnosis between 1989 and 2006. 70 patients with HemeM and mucormycosis took part in a single-center study. Although no RCTs have been conducted. Many studies indicate that LFAB outperforms AmB deoxycholate in terms of efficacy and protection. For mucormycosis, high-dose LFAB (5–10 mg/kg/day) for a minimum of 6–8 weeks is assumed as first-line therapy. In one study of SOTRs, patients treated with LFAB had a mortality rate of 15.4% (4/26) compared to 59.6% (28/47) for patients treated with AmB deoxycholate (Sun et al. 2010). The use of LFAB (rather than AmB deoxycholate) was related to decreased mortality, in multivariate analysis (Sun et al. 2010). Shoham et al. confirmed 28 cases of mucormycosis treated with LFAB from five medical centers in the US (Shoham et al. 2010). Immunosuppressive or HemeM treatment was found in 54% of the cases. The most common sites of involvement were the lungs (50%), sinuses (29%), and skin (14%) (18%). In 32% of cases, full remissions (CRs) or partial remissions (PRs) were obtained. The overall survival rate was 39%. In another study, concurrent surgery was done in 46% of the cases with a 31% survival rate. Mucormycosis was found to be the cause of death in 15 patients (36.6% mortality). Even though several therapies were used, although, the use of LFAB was connected to survival and a better response (Rüping et al. 2009). The ideal period of therapy has yet to be established, but recommendations supported by the ESCMID and ECMM suggest continuing antifungal care till the disease has now been fully resolved by clinical and imaging evaluation as the risk has been permanently corrected (Arendrup et al. 2014).
In vitro, both ISAV and POSA (Bagshaw et al. 2018) have an action against several molds and fungi, including Mucorales (Jenks et al. 2018; Perfect et al. 2018) although this amount of activity for both agents varies depending on the organisms and Mucorales genus (Denis et al. 2018). In refractory cases of mucormycosis, both POSA and ISAV have shown to be useful as adjunctive therapy or salvage (Graves et al. 2016; Natesan and Chandrasekar 2016). According to van Burik et al., 91 patients with mucormycosis were treated with POSA as salvage therapy, with 60% achieving PR or CR after 12 weeks and 21% remaining stable. PR and CR rates of 75 and 65%, respectively, were found in a study of 96 reported case reports of POSA care for mucormycosis (Vehreschild et al. 2013). Full responses were achieved with POSA in 8/12 (67%) of patients in a small study (Manesh et al. 2016). Following initial treatment with LFAB, sequential therapy with POSA to complete therapy was effective (Epstein et al. 2016; Tobón et al. 2016). For patients with mucormycosis who have failed LFAB172 or POSA, ISAV has shown to be a successful recovery therapy (Denis et al. 2018; Natesan and Chandrasekar 2016). In the VITAL study, patients with disseminated mucormycosis were given ISAV. which was an open-label, nonrandomized study (Marty et al. 2016). First-line therapy (n ¼ 21), antifungal agent(s) (n ¼ 5), refractory disorder (n ¼ 11), and intolerance to previous is among the indications for using ISAV. progression (5%), PR (14%), CR (5%), and stable (30%) were the most common responses (51%). In the Fungi Scope Registry, these 37 mucormycosis patients were paired with 33 LFAB-treated controls.
At care day 84, both arms had similar all-cause mortality (43% with ISAV; 50% with AmB). The FDA has approved ISAV for the treatment of invasive aspergillosis and invasive mucormycosis in adults in the United States (Perfect et al. 2018). The European Medicines Agency has approved ISAV for the treatment of mucormycosis when AmB is ineffective. ISAV is given as a loading dose (372 mg isavuconazonium sulfate [equivalent to 200 mg ISAV] every 8 h for six doses) and a maintenance dose (372 mg isavuconazonium sulfate [equivalent to 200 mg ISAV] every 8 h for six doses). Table 5
Table 5.
Conditional behavior of mucormycosis under different pathogenic treatments and their forms (Petrikkos and Drogari 2011)
| Mucormycosis activators | Treatment | Symptoms (in the form of frequency) |
|---|---|---|
|
Uncontrolled diabetes mellitus Diabetic ketoacidosis |
Fe usage by zygomycetes for growth/ Impairment of neutrophil activation (functional neutropenia) |
•Rhinocerebral •Pulmonary •Sino-orbital •Cutaneous |
| Hematological malignancies Hematopoietic stem cell transplantation (HSCT) | Prolonged neutropenia |
•Pulmonary •Cutaneous Sinus •Sino-orbital |
| Prolonged treatment with corticosteroids autoimmune disease | Defects in neutrophils and macrophages, hypocomplementemia, cortico- steroid induced diabetes |
•Renal •Cutaneous •Gastrointestinal •Disseminated •Rhino-cerebral |
| Intravenous illicit drug use/HIV infection | Injection of spores contained in drugs |
•Cerebral •Cutaneous •Heart •Disseminated •Renal •Rhinocerebral |
| Solid organ transplantation (SOT)/graft versus host disease (GVHD) | Cellular immune suppression, corticosteroid-induced diabetes |
•Sinus •Pulmonary •Rhinocerebral •Cutaneous disseminated |
| Iron/aluminum chelation therapy with deferoxamine (DFO), iron overload | Fe usage by Zygomycetes for growth Fe-DFO action as siderophore |
•Disseminated •Pulmonary •Cerebral •Gastrointestinal •Rhinocerebral •Cutaneous |
| Prolonged use of broad-spectrum antifungal agents (itraconazole, voriconazole and caspofungin) | •Sever infection | •Sino-pulmonary |
| Soft tissue or Skin breakdown trauma/burn/insect bite/surgical wound | Direct cutaneous inoculation with high number of spores |
•Cutaneous •Pulmonary •Rhinocerebral •Gastrointestinal •Sino-orbital |
| Miscellaneous Neonatal prematurity Malnourishment Prolonged use of broad-spectrum antimicrobial agents |
•Replacement of normal bacterial biota •Ingestion of spores |
•Pulmonary •Sino-orbital •Rhinocerebral •Gastrointestinal •Cutaneous |
In a retroactive survey of 100 leukemia patients who received ISAV as a single individual for prophylaxis, 13 patients (including four cases of mucormycosis) experienced breakthrough fungemia infection (BFI), which resulted in prolonged neutropenia and leukemia relapse. Therapeutic medication surveillance of triazoles like VORI and POSA was being proposed to control therapy for IMIs (Patterson et al. 2016), but considering ISAV’s excellent bioavailability. It is uncertain whether regular monitoring of ISAV levels is required (Natesan and Chandrasekar 2016). In a small sample, after 14 days of treatment, the values were 2.86 mg/L and 4.4 mg/L, respectively. In a small sample, seven cancer patients had levels of 2.86 mg/L after 14 days of therapy and 4.4 mg/L after 42 days of therapy. The patients were diagnosed from existing mucormycosis. Moreover, high dose of the medicine was associated with negative impact (Furfaro et al. 2019).
Despite this, no conclusive correlation between ISAV threshold levels and effectiveness or toxicity has been established yet. In India, current guidelines indicate 0.5–1 mg/kg/day intravenous methylprednisolone for 3 days in moderate instances and 1–2 mg/kg/day in severe instances are very helpful (Mahajan et al. 2020). Dexamethasone (6 mg per day for a maximum of 10 days) is recommended by the National Institute of Health in patients who are ventilated or require supplementary oxygen, but not in milder instances (Beigel et al. 2020). COVID-19 has pathophysiologic characteristics that may allow secondary fungal infections, such as a proclivity for causing extensive lung illness and subsequent alveolo-interstitial pathology, which may increase the likelihood of invasive fungal infections. Second, COVID-19’s immunological dysregulation, which includes lower numbers of T lymphocytes, CD4 + T cells, and CD8 + T cells, may disrupt the immune system. Therefore, remediation of such phenomenon is still challenging to prevent the occurrence of disease. Figure 2
Fig. 2.
Different treatments of mucormycosis
Combination antifungal therapy
The mucormycosis therapy was provided in patients in conjunction with several antifungal agents for diagnosing the intractable cases (Tacke et al. 2014); however, RCTs are inadequate (Candoni et al. 2015).
Pagano et al. in their study found 32 patients with evidence-based mucormycosis treated with such a variation of LFAB and POSA from two main European registries between 2007 and 2012. During a 3-month observation, 11 (34%) of patients had CRs, 5 (16%) 9 (28.1%) died of progressive mucormycosis, while the rest were healthy. Combination therapy was not shown to be superior to monotherapy in a retrospective review of patients with mucormycosis complicating HemeM. The effectiveness of combined antifungal therapy for mucormycosis is unknown due to lack of evidence. Hence, there is a need of experimental setup for observing the alterations in the given therapy.
Surgical therapy
Resection or surgical debridement plays a vital role as an adjunctive treatment in mucormycosis patients (Riley et al. 2016). An extensive study was carried out to check the survival rate over the 929 victims of mucormycosis. The study reported that survival rates were 57% for surgery, 61% for AmB, and 70% for AmB plus surgery (Roden et al. 2005). In another study, 178 cases of mucormycosis from an Indian tertiary care center; 74% of patients had ailing regulated diabetes were recorded (Chakrabarti et al. 2006). Although, the combination of AmB and surgical debridement resulted in a substantially higher survival rate (79.6%) than AmB alone (51.7% survival, p < 0.005). Several researchers have worked on the treatment of mucormycosis. In a study, 230 cases of mucormycosis were observed from the European countries to identify the factors responsible for better survival rate during 2005 to 20,017. The adjunct factors were LFAB treatment (p ¼ 0.006), surgery (p < 0.001), and trauma (p ¼ 0.02) (A. Skiada et al. 2011). However, the overall survival was higher (70.2%) with surgical debridement plus POSA and AmB in a cohort of 174 renal transplant recipients with mucormycosis than with antifungal therapy (32.4%), no therapy (0%) or surgery alone (36.4%) (Song et al. 2017). Overall mortality was 52% in 90 cases of SOTRs among ROCM (Sun et al. 2010). Surgery and LFAB were both linked to improved survival rate (Song et al. 2017). Consequently, SSTI, rhinocerebral mucormycosis, and the cases of pulmonary disease were seen to be effectively diagnosed by surgical therapy (Chretien et al. 2016; Anna Skiada et al. 2013; Sun et al. 2010). In addition to it, surgical resection combined with medical therapy can be curative when PulM is localized (Coffey et al. 1992).
Iron chelators
Deferasirox is a kind of iron-chelating agent that increases longevity in mice with diabetic ketoacidosis (Ibrahim et al. 2007). Deferasirox was seen to be very effective in patients against the LFAB treatment. Furthermore, Deferasirox was successful in one patient with ROCM (Reed et al. 2006). Six patients with invasive mucormycosis were diagnosed using deferiprone (an iron chelator) after receiving initial care with surgery, echinocandins, step-down therapy with POSA, and combination therapy with polyenes, in a retrospective study in Thailand (Chitasombat and Niparuck 2018). Three of the six patients died after 180 days. Twenty patients with confirmed or suspected mucormycosis were randomized to receive either LFAB plus placebo or LFAB plus deferasirox (Spellberg et al. 2012). At 90 days, the deferasirox party had a slightly higher mortality rate (82 vs. 22%, p ¼ 0.01). Although guidelines from the 6th European Conference on Leukemia Infections (ECIL-6) suggests the avoidance of deferasirox for the remediation of the mucormycosis (Schwarz et al. 2019; Tissot et al. 2017).
Salvage therapy
Salvage therapy is provided when patient does not respond to the normal first-line treatment with amphotericin B. The assessment of antifungal medication failure, necessitates caution (Dimaka et al. 2014). Patients with mucormycosis frequently have co-existing fungal and bacterial infections, as well as non-infectious diseases such as leukemic infiltration, medication toxicity, or organ failure, which can make measuring therapy response difficult. Furthermore, an initial paradoxical deterioration, reported in cancer patients with pulmonary mycoses (including mucormycosis) in the context of neutrophil recovery, could be misinterpreted as a treatment failure (Prakash et al. 2019). Switching to a different class of anti-fungal agents is the general principle of salvage therapy. However, Mucorales is only susceptible to two types of antifungal drugs. In this case, the options are to increase the LAMB dose or switch to posaconazole or isavuconazole. Table 6
Table 6.
Patient groups, intervention and their salvage treatment during mucormycosis (Vujanovic et al. 2017)
| Treatment | Patient group | Intervention |
|---|---|---|
| Fever driven or Empirical | • unresponsive to antibiotics, Febrile neutropenia, imaging–reverse halo, galactomannan negative; other atypical presentation or multiple nodules | Liposomal amphotericin B |
| Prophylaxis |
• graft versus host disease or Prolonged neutropenia • SOT adult, Prolonged neutropenia, heart, and lung • Immunocompromised due to a previous mucormycosis diagnosis |
Posaconazole DR intravenous or tablet 2 × 300 mg day 1, 1 × 300 mg from day 2 Isavuconazole intravenous or per oral 3 × 200 mg days 1–2, 1 × 200 mg/day from day 3, or 1 × 200 mg/day from day 1 In the same patient, surgical resection and the last medicine effective as a secondary prophylactic, switch from amphotericin B to posaconazole after 3–6 weeks if practicable |
| Salvage |
• Refractory disease • Toxicity to primary therapy |
Isavuconazole intravenous or per oral 3 × 200 mg days 1–2, 1 × 200 mg from day 3 Posaconazole DR tablet or intravenous 2 × 300 mg day 1, 1 × 300 mg from day 2 Amphotericin B, lipid formulations, 5–10 mg/kg (lower dose in patients with renal toxicity) |
Role of antifungal prophylaxis
It is still under investigation that whether anti-Mucorales antifungals should be used for primary or secondary prophylaxis in high-risk individuals. Presently, posaconazole is strongly suggested as a main prophylactic to prevent IFI (particularly mucormycosis) in the patients suffering from severe neutropenia (Lee et al. 2016). Monitoring serum posaconazole levels during prophylaxis is also displayed to document appropriate antifungal absorption and therapy compliance, as undetectable serum levels increase the risk of breakthrough infections; however, isavuconazole could be another option based on the results of a recent clinical trial (NCT03019939) (Chitasombat and Kontoyiannis 2016). The adoption of posaconazole and isavuconazole prophylaxis offers the low cost comparatively with other alternatives (Davari et al. 2003). Despite of the available solutions, the raise in breakthrough IFIs (bIFI) in patients using vuconazole prophylaxis is also raise the concern. Although, the choice of antifungal for secondary prophylaxis is a typical clinical question. Moreover, secondary prophylaxis reacts with immunocompetent patient to control the infectious disease. The treatment satisfactorily controls the rate of mucormycosis through the antifungal medication. (Almyroudis et al. 2006). Table 3 shows the list of prophylactic, empiric, and therapeutic interventions.
Clinician observations and medical advices for mucormycosis and COVID-19 disease
COVID-19 infection has a peculiar threat from mild to fatal pneumonia and associated secondary bacterial or fungal infections. Due to comorbidity and an immunocompromised state, the death rate is high which allowing mucormycosis to thrive (Salehi et al. 2020). A cluster of reports on rhino-orbital mucormycosis post-COVID-19 disease is available among a number of other opportunistic infections, including oropharyngeal candidiasis, pulmonary aspergillosis, and jiroveci pneumonia (Chowdhary et al. 2020a, b). According to a recent article, India accounts for 71% of all CAM cases, with an accurate number of 140 instances per million (John et al. 2019, Prakash and Chakrabarti 2019). This is attributable to a number of factors, including the largest population of the country and second-highest population of persons aged 20 to 79 with diabetes mellitus (IDF 2019). It was found that 50% of the CAM patients were diabetic, 18% had diabetic ketoacidosis, and 57% had diabetes mellitus that was uncontrolled. As per the reports from the clinical observations, mucormycosis can show in six different clinical presentations based on the location of fungal proliferation and dissemination. The pulmonary, rhinocerebral, gastrointestinal, cutaneous, and disseminated systems are among them (Spellberg et al. 2005a, b). The rhino cerebral form is the most prevalent, followed by the pulmonary and cutaneous types (Petrikkos et al. 2012a, b).
Sarkar et al. recently reported the occurrence of orbital mucormycosis in 10 patients with concurrent COVID-19 diseases. All of them had diabetes, with four of them having been diagnosed with DKA and the other five developing DKA while on COVID-19 corticosteroid medication. For mucormycosis, intravenous dexamethasone and liposomal amphotericin B were used. Ventilator and remdesivir were the useful practice to combat the situation in the patients. In a report, four patients–but lost their vision permanently, and just one patient recovered with good ocular and systemic outcomes (Sarkar et al., 2021). It means that the complete dependencies over the remedials were also not efficient to cater the wide cases of mucormycosis and COVID-19. The situation was differed case by case. Although, the patients had a great relief in respiration due to applied ventilators. COVID-19 does not appear to be a risk factor for mucormycosis.
According to the case reports in Table 7, associated short-term corticosteroid medication, together with concomitant illnesses, was the actual influencing factor for the initiation and advancement of mucor infection. To confirm the causes of mucormycosis, however, huge datasets are still needed. Table 7 presented a review of case reports that were available in online resources spanning the years 2020–2021.
Table 7.
Case reports on medical history of COVID 19 and associated mucormycosis disease
| Case no | Male/female | Phase of COVID-19 | Medical history | Therapy implemented | Predisposing aspect for mucormycosis | Histological examination | Symptom duration (days) | Clinical manifestation | Alive/dead | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male/60 | Severe | Diabetes | High-dose steroid for COVID 19. Methylprednisolone | Hyperglycemic steroid for COVID-19 | Non-septate hyphae | 12 |
•Oedema •Periorbital facial pain •Acute vision loss |
Dead | Mehta and Pandey 2020 |
| 2 | Male/22 | Severe | Pancreatitis | High-dose steroid for COVID Linezolid Meropenem | Steroid for COVID 19-treatment | Non-septate hyphae | 27 |
•Diagnosed as Rhino-orbito-cerebral mucormycosis •Disseminated to oLymph nodes oHeart oBrain oKidney |
Dead | Hanley et al. 2020a, b |
| 3 | Female/33 | Severe | Diabetes Asthma hypertension | No steroids Remdesivir Vancomycin | DKA Diabetic ketoacidosis | Non-septate hyphae | 2 |
•Diagnosed as Rhihino-orbital mucormycosis •Necrosis in oNasal oPalate oLeft eye ptosis oConfused mental status oOphthalmoplegia proptosis |
Dead | Werthman-Ehrenreich 2021a, b |
| 4 | Male/66 | Severe | Hypertension | Hydroxychloroquine Lopinavir–ritonavir | Lymphopenia | Non-septate hyphae | 21 |
•Diagnosed as Pulmonary mucormycosis in lungs •Necrotic empyema •Spontaneous pneumothorax |
Dead | Pasero et al. 2020a, b |
| 5 | Male/49 | Severe | Normal |
Dexamethasone Tocilizumab Remdesivir Ceftriaxone |
Steroid for COVID-19 treatment | Non-septate hyphae | 21 |
•Diagnosed as Pulmonary mucormycosis in lungs •Necrotic empyema •Spontaneous pneumothorax |
Dead | Placik et al. 2020 |
| 6 | Male/60 | Severe | Diabetes, asthma, hypertension, hyperlipidemia |
Dexamethasone Remdesivir Convalescent plasma therapy (single dose) |
Hyperglycemia, steroid for COVID-19 | Non-septate hyphae | 5 |
•Diagnosed as Pulmonary mucormycosis •Gastric ulcers •Acute diarrhea •Melena •Severe anemia •Fever |
Dead | Mekonnen et al. 2021a, b |
| 7 | Male/86 | Severe | Hypertension |
Hydrocortisone for COVID-19 Ceftriaxone Azithromycin Oseltamivir |
Steroid for COVID-19 treatment | Non-septate hyphae | 21 |
•Diagnosed as Pulmonary mucormycosis with Bronchopulmonary fistula •Pulmonary infiltrates •Parenchymal thickening of the whole left lung •Cavitary lesions •Pleural effusion •Opacity of the left maxillary sinus |
Dead | Do Monte et al. 2020 |
| 8 | Female/ 40 | Mild | None |
Remdesivir Levofloxacin Dexamethasone |
Short-term corticosteroid therapy | Non-septate hyphae | 8 |
•Diagnosed as Rhinoorbital mucormycosis •Opacifications of paranasal sinuses |
Dead | Veisi et al. 2021 |
| 9 | Male/38 | Mild |
Remdesivir Dexamethasone |
Short-term corticosteroid therapy | Confirmed with CT scan | 18 | •Diagnosed as rhino-orbital mucormycosis | Alive | Maini et al. 2021 | |
| 10 | Male/54 | Severe | Non-insulin-dependent diabetes mellitus (DM) |
Remdesivir Levofloxacin Dexamethasone |
Short-term corticosteroid therapy | Non-septate hyphae | 12 |
•Diagnosed as rhino-orbitocerebral mucormycosis •Unilateral opacifications of the left orbit •Paranasal sinuses |
Alive | Veisi et al. 2021 |
| 11 | Male/41 | Mild | Diabetes mellitus | Steroids and hydroxychloroquine | Developed diabetic ketoacidosis (DKA) | Confirmed with CT scan | 16 |
•Tissue necrosis from angioinvasion and subsequent thrombosis •Diagnosed as rhinocerebral mucormycosis |
Alive | Alekseyev et al. 2021 |
| 12 | Male/72 | Severe | Steroidinduced diabetic, hypothyroid | Ramdevpir Methyl prednisolone convalescent plasma (2 doses) | Impaired immune functioning | Non-septate hyphae | 9 |
•Diagnosed as sino-orbital mucormycosis •Pneumothorax Diagnosed as pulmonary mucormycosis |
Alive | Chennamchetty et al. 2021 |
| 13 | Male/68 | Severe | Heart transplant recipient Diabetes mellitus | Remdesivir Hydroxychloroquine Convalescent plasma infusion (single dose) Methylprednisolone Prednisone taper | Previously under immunosuppressive medication for transplantation | Non-septate hyphae | 13 |
•Purplish skin discoloration with fluctuant swelling was noted in the right axilla •Diagnosed as cutaneous mucormycosis |
Dead | Khatri et al. 2021 |
| 14 | Female/32 | Mild | Uncontrolled diabetes Left eye complete ptosis and left facial pain | Not mentioned | Immunosuppression due to COVID 19 | Confirmed with CT scan | 18 |
•Opacification of the left ethmoid •Maxillary •Frontal sinus |
Alive | Saldanha et al. 2021 |
Conclusion
Although the actual prevalence of mucormycosis is unknown, it is believed to be substantially greater in developing countries as compared to developed countries. The prevalence of Mucorales in the community and hospital environment, the enormous number of susceptible hosts, particularly diabetics, and the Indian population’s neglect for regular health check-ups are all plausible reasons for the high prevalence. Many people are unaware of their diabetic status until they develop mucormycosis. Chronic kidney disease, pulmonary tuberculosis, and critically ill patients are all significant mucormycosis risk factors. Due to delays in seeking medical assistance and diagnosing the condition, as well as difficulty in controlling the advanced stage of infection, the mortality rate linked with mucormycosis is rather high. Despite major advances in mucormycosis treatment in recent years, the disease's fatality rate remains high. Although, a lot of gaps in treatment remain due to delays in diagnosis, limited antifungal medicines, and difficult management alternatives. The advancement in the clinical trials advice to use the surgical procedures for the treatment of mucormycosis. This study provides the most recent consensus recommendations for the management of mucormycosis in different clinical scenarios. The current scenario of COVID-19 pandemics and the rapid rise in the incidence of mucormycosis paves the way to a better understanding and the possibilities of the invention of newer management techniques and treatment protocols to reduce the percentage morbidity and mortality associated with such disease.
Acknowledgements
The authors are deeply thankful to Kamal Kishore, Assistant Professor, Department of Civil Engineering, GLA University for their exceptional support to complete this review article. His enthusiasm, knowledge and exacting attention to detail have been an inspiration during drafting this review article.
Abbreviations
- DM
Diabetes mellitus
- PMN
Polymorphonuclear phagocytes
- COVID-19
Coronavirus disease 2019
- IFI
Invasive fungal infections
- VORI
Voriconazole
- HSCT
Hematopoietic stem cell transplant
- POSA
Posaconazole
- SOTR
Solid organ transplant recipients
- HemeM
Hematological malignancies
- ICU
Intensive care units
- COPD
Chronic obstructive pulmonary disease
- CKD
Chronic kidney disease
- RHS
Reverse halo symbol
- PET
Positron emission tomography
- CT
Computed tomography
- FDG
Fluorodeoxyglucose
- CAPA
Pulmonary aspergillosis
- MALDI-TOF
Matrix-assisted laser desorption/ionization time-of-flight
- MIC
Minimum inhibitory concentration
- ELISpot
Enzyme-linked immune spot
- PCR
Polymerase chain reaction
- RFLP
Restriction fragment length polymorphisms
- DNA
Deoxyribonucleic acid
- 18S rRNA
18S ribosomal RNA
- ROCM
Rhino-orbital-cerebral mucormycosis
- SSTI
Soft tissue infection and skin
- ECMM
European Confederation of Medical Mycology
- PTM
Posttraumatic mucormycosis
- RCT
Randomized controlled trials
- FDA
Food Development Authority
- ECIL
European Conference on Leukemia Infections
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ayushi Sharma, Email: ayushi.sharmabsc15@gmail.com.
Anjana Goel, Email: anjanagoel2000@gmail.com, Email: anjana.goel@gla.ac.in.
References
- Aggarwal D, Chander J, Janmeja AK et al (2015) Pulmonary tuberculosis and mucormycosis co-infection in a diabetic patient. Lung India. 32:53–55. 10.4103/0970-2113.148452 [DOI] [PMC free article] [PubMed]
- Alekseyev K, Didenko L, Chaudhry B (2021) Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases 12: 85–89. 10.14740/jmc3637 [DOI] [PMC free article] [PubMed]
- Almyroudis NG, Sutton DA, Linden P, Rinaldi MG, Fung J, Kusne S. Zygomycosis in solid organ transplant recipients in a tertiary transplant center and review of the literature. Am J Transplant. 2006;6:2365–2374. doi: 10.1111/j.1600-6143.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Alvarez E, Sutton DA, Cano J, et al. Spectrum of zygomycete species identified in clinically significant specimens in the United States. J Clin Microbiol. 2009;47:1650–1656. doi: 10.1128/JCM.00036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosioni J, Bouchuiguir-Wafa K, Garbino J. Emerging invasive zygomycosis in a tertiary care center: Epidemiology and associated risk factors. Int J Infect Dis. 2010;14:e100–e103. doi: 10.1016/j.ijid.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Ananda-Rajah MR, Kontoyiannis D. Isavuconazole: a new extended spectrum triazole for invasive mold diseases. Future Microbiol. 2015;10:693–708. doi: 10.2217/fmb.15.34. [DOI] [PubMed] [Google Scholar]
- Antony SJ, Parikh MS, Ramirez R, Applebaum B, Friedman G, Do J. Gastrointestinal mucormycosis resulting in a catastrophic outcome in an immunocompetent patient. Infect Dis Rep. 2015;7:60–65. doi: 10.4081/idr.2015.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aras MH, Kara MI, Erkiliç S, Ay S. Mandibular mucormycosis in immunocompromised patients: Report of 2 cases and review of the literature. J Oral Maxillofac Surg. 2012;70:1362–1368. doi: 10.1016/j.joms.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Arastehfar A, Carvalho A, van de Veerdonk FL, et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. J Fungi. 2020;6:1–17. doi: 10.3390/jof6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendrup MC, Boekhout T, Akova M, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20:76–98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- Artis WM, Patrusky E, Rastinejad F, Duncan RL. Fungistatic mechanism of human transferrin for Rhizopus oryzae and Trichophyton mentagrophytes: Alternative to simple iron deprivation. Infect Immun. 1983;41:1269–1278. doi: 10.1128/iai.41.3.1269-1278.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine HFM, White C, Bain J. Aggressive combined medical and surgical management of mucormycosis results in disease eradication in 2 pediatric patients. Plast Surg. 2017;25:211–217. doi: 10.1177/2292550317716119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CL, Finley PJ, Mikkelson DR, Tibbs B. Mucormycosis: a rare fungal infection in tornado victims. J Burn Care Res. 2014;35:164–171. doi: 10.1097/BCR.0b013e318299d4bb. [DOI] [PubMed] [Google Scholar]
- Bagshaw E, Enoch DA, Blackney M, Posthumus J, Kuessner D. Economic impact of treating invasive mold disease with isavuconazole compared with liposomal amphotericin B in the UK. Future Microbiol. 2018;13:1283–1293. doi: 10.2217/fmb-2018-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo RM, Gurung A, Jain D, Malinis MF. Therapeutic challenges of hepatic mucormycosis in hematologic malignancy: a case report and review of the literature. Am J Case Rep. 2016;17:484–489. doi: 10.12659/AJCR.898480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran A, Hosseini-Moghaddam SM, Rotstein C, Husain S. Mold infections in lung transplant recipients. Semin Respir Crit Care Med. 2013;34:371–379. doi: 10.1055/s-0033-1348475. [DOI] [PubMed] [Google Scholar]
- Bitar D, Lortholary O, Le Strat Y, et al. Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg Infect Dis. 2014;20:1149–1155. doi: 10.3201/eid2007.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwkamp TA, Thair S, Rosen MJ, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol. 2019;4:663–674. doi: 10.1038/s41564-018-0349-6. [DOI] [PubMed] [Google Scholar]
- Boelaert JR, De Locht M, Van Cutsem J, et al. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection: in vitro and in vivo animal studies. J Clin Invest. 1993;91:1979–1986. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifaz A, Stchigel AM, Guarro J, et al. Primary cutaneous mucormycosis produced by the new species Apophysomyces mexicanus. J Clin Microbiol. 2014;52:4428–4431. doi: 10.1128/JCM.02138-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candoni A, Aversa F, Busca A, et al (2015) Combination antifungal therapy for invasive mould diseases in haematologic patients. An update on clinical data. J Chemother 27:1–12. 10.1179/1973947814Y.0000000224 [DOI] [PubMed]
- Cao Y, Wang L, Ma P, Fan W, Gu B, Ju S. Accuracy of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of mycobacteria: a systematic review and meta-analysis. Sci Rep. 2018;8:1–9. doi: 10.1038/s41598-018-22642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A, Chatterjee SS, Das A, et al. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J. 2009;85:573–581. doi: 10.1136/pgmj.2008.076463. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A, Das A, Mandal J, et al. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med Mycol. 2006;44:335–342. doi: 10.1080/13693780500464930. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A, Kaur H, Savio J, et al. Epidemiology and clinical outcomes of invasive mould infections in Indian intensive care units (FISF study) J Crit Care. 2019;51:64–70. doi: 10.1016/j.jcrc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Chamilos G, Lewis RE, Lamaris G, Walsh TJ, Kontoyiannis DP. Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob Agents Chemother. 2008;52:722–724. doi: 10.1128/AAC.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamilos G, Marom EM, Lewis RE, Lionakis MS, Kontoyiannis DP. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis. 2005;41:60–66. doi: 10.1086/430710. [DOI] [PubMed] [Google Scholar]
- Chennamchetty VK, Adimulapu S, Kola BP, Rao MR (2021) Post-COVID pulmonary mucormycosis-a case report. IP Indian J Immunol Respir Med 6: 62–66. 10.18231/j.ijirm.2021.014
- Chitasombat MN, Kontoyiannis DP. Treatment of mucormycosis in transplant patients: Role of surgery and of old and new antifungal agents. Curr Opin Infect Dis. 2016;29:340–345. doi: 10.1097/QCO.0000000000000277. [DOI] [PubMed] [Google Scholar]
- Chitasombat MN, Niparuck P. Deferiprone as adjunctive treatment for patients with invasive mucormycosis: A retrospective case series. Infect Dis Rep. 2018;10:7765–7770. doi: 10.4081/idr.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo JY, Park CM, Lee HJ, Lee CH, Goo JM, Im JG. Sequential morphological changes in follow-up CT of pulmonary mucormycosis. Diagnostic Interv Radiol. 2014;20:42–46. doi: 10.5152/dir.2013.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Candida Auris Infect Coronavirus Dis. 2020;26:2694–2696. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerg Infect Dis. 2020;26:2694–2696. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien ML, Legouge C, Pagès PB, et al. Emergency and elective pulmonary surgical resection in haematological patients with invasive fungal infections: a report of 50 cases in a single centre. Clin Microbiol Infect. 2016;22:782–787. doi: 10.1016/j.cmi.2015.12.029. [DOI] [PubMed] [Google Scholar]
- Clark NM, Weigt SS, Fishbein MC, Kubak B, Belperio JA, Lynch JP. Fungal Infections Complicating Lung Transplantation. Semin Respir Crit Care Med. 2018;39:227–254. doi: 10.1055/s-0037-1617443. [DOI] [PubMed] [Google Scholar]
- Coffey MJ, Fantone J, Stirling MC, Lynch JP. Pseudoaneurysm of pulmonary artery in mucormycosis. Radiographic characteristics and management. Am Rev Respir Dis. 1992;145:1487–1490. doi: 10.1164/ajrccm/145.6.1487. [DOI] [PubMed] [Google Scholar]
- Corzo-León DE, Chora-Hernández LD, Rodríguez-Zulueta AP, Walsh TJ. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. Med Mycol. 2018;56:29–43. doi: 10.1093/mmy/myx017. [DOI] [PubMed] [Google Scholar]
- Danion F, Aguilar C, Catherinot E, et al. Mucormycosis: new developments into a persistently devastating infection. Semin Respir Crit Care Med. 2015;36:692–705. doi: 10.1055/s-0035-1562896. [DOI] [PubMed] [Google Scholar]
- Davari HR, Malekhossini SA, Salahi H Allah et al (2003) Outcome of mucormycosis in liver transplantation: four cases and a review of literature. Exp Clin Transplant 1:147–152. https://pubmed.ncbi.nlm.nih.gov/15859921/ [PubMed]
- Denis J, Ledoux MP, Nivoix Y, Herbrecht R. Isavuconazole: A new broad-spectrum azole. Part 1: In vitro activity. J Mycol Med. 2018;28:8–14. doi: 10.1016/j.mycmed.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Devana SK, Gupta VG, Mavuduru RS, et al. Isolated renal mucormycosis in immunocompetent hosts: Clinical spectrum and management approach. Am J Trop Med Hyg. 2019;100:791–797. doi: 10.4269/ajtmh.18-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPippo AJ, Rausch CR, Kontoyiannis DP (2019) Tolerability of isavuconazole after posaconazole toxicity in leukaemia patients. Mycoses 62:81–86. 10.1111/myc.12851 [DOI] [PubMed]
- Dimaka K, Mallis A, Naxakis SS, et al. Chronic rhinocerebral mucormycosis: a rare case report and review of the literature. Mycoses. 2014;57:699–702. doi: 10.1111/myc.12219. [DOI] [PubMed] [Google Scholar]
- Do Monte ES, Dos Santos MEL, Ribeiro IB, et al. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin Endosc. 2020;53:746–749. doi: 10.5946/CE.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen RS, Kerr KN, Soutar RL. Air crescent sign and fatal haemoptysis in pulmonary mucormycosis. Scand J Infect Dis. 1994;26:498–501. doi: 10.3109/00365549409008629. [DOI] [PubMed] [Google Scholar]
- Elgarten CW, Levy EM, Mattei P, Fisher BT, Olson TS, Freedman JL. Successful treatment of pulmonary mucormycosis in two pediatric hematopoietic stem cell transplant patients. Pediatr Transplant. 2018;22:1–5. doi: 10.1111/petr.13270. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Kupferman SB, Zabner R, et al. Early diagnosis and successful management of oral mucormycosis in a hematopoietic stem cell transplant recipient: case report and literature review. Support Care Cancer. 2016;24:3343–3346. doi: 10.1007/s00520-016-3170-x. [DOI] [PubMed] [Google Scholar]
- Frater JL, Hall GS, Procop GW. Histologic features of zygomycosis: Emphasis on perineural invasion and fungal morphology. Arch Pathol Lab Med. 2001;125:375–378. doi: 10.1043/0003-9985(2001)125<0375:HFOZ>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Furfaro E, Signori A, Di Grazia C, et al. Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J Antimicrob Chemother. 2019;74:2341–2346. doi: 10.1093/jac/dkz188. [DOI] [PubMed] [Google Scholar]
- Gamarra S, Chaves MS, Cabeza MS, et al. Mucormycosis outbreak due to Rhizopus microsporus after arthroscopic anterior cruciate ligament reconstruction surgery evaluated by RAPD and MALDI-TOF Mass spectrometry. J Mycol Med. 2018;28:617–622. doi: 10.1016/j.mycmed.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Garcia-Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041–1050. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings SE, Hoepelman AIM. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–265. doi: 10.1016/S0928-8244(99)00142-X. [DOI] [PubMed] [Google Scholar]
- Gholinejad-Ghadi N, Shokohi T, Seifi Z, et al. Identification of Mucorales in patients with proven invasive mucormycosis by polymerase chain reaction in tissue samples. Mycoses. 2018;61:909–915. doi: 10.1111/myc.12837. [DOI] [PubMed] [Google Scholar]
- Graves B, Morrissey CO, Wei A, et al. Isavuconazole as salvage therapy for mucormycosis. Med Mycol Case Rep. 2016;11:36–39. doi: 10.1016/j.mmcr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg RN, Mullane K, Van Burik JAH, et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother. 2006;50:126–133. doi: 10.1128/AAC.50.1.126-133.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi P, Farina C, Fiocchi R DGD (2000) Prevalence and outcome of invasive fungal infections in 1,963 thoracic organ transplant recipients: a multicenter retrospective study. Italian Study Group of Fungal Infections in Thoracic Organ Transplant Recipients. Transplantation 70:112–116. https://pubmed.ncbi.nlm.nih.gov/10919584/ [PubMed]
- Guimarães LFA, Halpern M, de Lemos AS, et al. Invasive fungal disease in renal transplant recipients at a Brazilian center: local epidemiology matters. Transplant Proc. 2016;48:2306–2309. doi: 10.1016/j.transproceed.2016.06.019. [DOI] [PubMed] [Google Scholar]
- Guinea J, Escribano P, Vena A, et al. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS ONE. 2017;12:1–10. doi: 10.1371/journal.pone.0179136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BM, Clayton JD, Elicker BM, Ordovas KG, Naeger DM. CT-guided percutaneous lung biopsies in patients with suspicion for infection may yield clinically useful information. Am J Roentgenol. 2017;208:459–463. doi: 10.2214/AJR.16.16255. [DOI] [PubMed] [Google Scholar]
- Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32:693–702. doi: 10.18093/0869-0189-2018-28-2-243-247. [DOI] [PubMed] [Google Scholar]
- Hammer MM, Madan R, Hatabu H. Pulmonary mucormycosis: radiologic features at presentation and over time. Am J Roentgenol. 2018;210:742–747. doi: 10.2214/AJR.17.18792. [DOI] [PubMed] [Google Scholar]
- Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. The Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata DJ, Buckwalter SP, Pritt BS, Roberts GD, Wengenack NL. Real-time PCR method for detection of zygomycetes. J Clin Microbiol. 2008;46:2353–2358. doi: 10.1128/JCM.01552-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S, Silveira FP, Azie N, Franks B, Horn D. Epidemiological features of invasive mold infections among solid organ transplant recipients: PATH Alliance® registry analysis. Med Mycol. 2017;55:269–277. doi: 10.1093/mmy/myw086. [DOI] [PubMed] [Google Scholar]
- Ibrahim AS, Gebermariam T, Fu Y, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649–2657. doi: 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54:1–7. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDF (2019). IDF Diabetes Atlas. https://diabetesatlas.org/en/resources/. (Accessed 4 Sept 2021)
- Ino K, Nakase K, Nakamura A, et al. Management of pulmonary mucormycosis based on a polymerase chain reaction (Pcr) diagnosis in patients with hematologic malignancies: a report of four cases. Intern Med. 2017;56:707–711. doi: 10.2169/internalmedicine.56.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamadar DA, Kazerooni EA, Daly BD, White CS, Gross BH. Pulmonary zygomycosis: Ct appearance. J Comput Assist Tomogr. 1995;19:733–738. doi: 10.1097/00004728-199509000-00008. [DOI] [PubMed] [Google Scholar]
- Jenks JD, Salzer HJF, Prattes J, Krause R, Buchheidt D, Hoenigl M. Spotlight on isavuconazole in the treatment of invasive aspergillosis and mucormycosis: design, development, and place in therapy. Drug Des Devel Ther. 2018;12:1033–1044. doi: 10.2147/DDDT.S145545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi. 2019;7:298–303. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Kim MY, Lee HJ, et al. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin Microbiol Infect. 2015;21:684.e11. doi: 10.1016/j.cmi.2015.03.019. [DOI] [PubMed] [Google Scholar]
- Kasai M, Harrington SM, Francesconi A, et al. Detection of a molecular biomarker for Zygomycetes by quantitative PCR assays of plasma, bronchoalveolar lavage, and lung tissue in a rabbit model of experimental pulmonary zygomycosis. J Clin Microbiol. 2008;46:3690–3702. doi: 10.1128/JCM.00917-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy K, Daveson K, Slavin M, et al. Mucormycosis in Australia: contemporary epidemiology and outcomes. Clin Microbiol Infect. 2016;22:775–781. doi: 10.1016/j.cmi.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Khatri A, Chang KM, Berlinrut I, Wallach F. Mucormycosis after coronavirus disease 2019 infection in a heart transplant recipient–case report and review of literature. J Med Mycol. 2021;31:101125–101130. doi: 10.1016/j.mycmed.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffi D, Bonouman IV, Toure AO, et al. Estimates of serious fungal infection burden in Côte d’Ivoire and country health profile. J Med Mycol. 2021;31:101086. doi: 10.1016/j.mycmed.2020.101086. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis DP, Lewis RE. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect Dis Clin North Am. 2006;20:581–607. doi: 10.1016/j.idc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis DP, Lionakis MS, Lewis RE, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: A case-control observational study of 27 recent cases. J Infect Dis. 2005;191:1350–1360. doi: 10.1086/428780. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis DP, Yang H, Song J, et al. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: a retrospective study. BMC Infect Dis. 2016;16:1–7. doi: 10.1186/s12879-016-2023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontoyiennis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the transplant- associated infection surveillance network (TRANSNET) database. Clin Infect Dis. 2010;50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- Kumar C, Jain P, Wadhwa N, Diwaker P, Khan NP. Nosocomial jejunal mucormycosis - An unusual cause of perforation peritonitis. Iran J Pathol. 2017;12:295–300. doi: 10.30699/ijp.2017.25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Adams A, Hererra M, et al. Predictors and outcomes of healthcare-associated infections in COVID-19 patients. Int J Infect Dis. 2021;104:287–292. doi: 10.1016/j.ijid.2020.11.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursun E, Turunc T, Demiroglu YZ, Alişkan HE, Arslan AH. Evaluation of 28 cases of mucormycosis. Mycoses. 2015;58(2):82–87. doi: 10.1111/myc.12278. [DOI] [PubMed] [Google Scholar]
- Kyvernitakis A, Torres HA, Jiang Y et al (2016) Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: a propensity score analysis. Clin Microbiol Infect 22:811–e1. 10.1016/j.cmi.2016.03.029 [DOI] [PubMed]
- Lamoth F, Chung SJ, Damonti L, Alexander BD. Changing epidemiology of invasive mold infections in patients receiving azole prophylaxis. Clin Infect Dis. 2017;64:1619–1621. doi: 10.1093/cid/cix130. [DOI] [PubMed] [Google Scholar]
- Lanternier F, Dannaoui E, Morizot G, et al. A global analysis of mucormycosis in France: the RetroZygo study (2005–2007) Clin Infect Dis. 2012;54:35–43. doi: 10.1093/cid/cir880. [DOI] [PubMed] [Google Scholar]
- Lanternier F, Sun HY, Ribaud P, Singh N, Kontoyiannis DP, Lortholary O. Mucormycosis in organ and stem cell transplant recipients. Clin Infect Dis. 2012;54:1629–1636. doi: 10.1093/cid/cis195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass-Flörl C. Zygomycosis: conventional laboratory diagnosis. Clin Microbiol Infect. 2009;15:60–65. doi: 10.1111/j.1469-0691.2009.02999.x. [DOI] [PubMed] [Google Scholar]
- Lass-Flörl C, Mayr A. Diagnosing invasive fungal diseases - limitations of microbiological diagnostic methods. Expert Opin Med Diagn. 2009;3:461–470. doi: 10.1517/17530050902878031. [DOI] [PubMed] [Google Scholar]
- Lass-Flörl C, Resch G, Nachbaur D, et al. The value of computed tomography-guided percutaneous lung biopsy for diagnosis of invasive fungal infection in immunocompromised patients. Clin Infect Dis. 2007;45:101–104. doi: 10.1086/521245. [DOI] [PubMed] [Google Scholar]
- Lazar S, Lukaszewicz J, Persad K, Reinhardt J (2014) Rhinocerebral Mucor circinelloides infection in immunocompromised patient following yogurt ingestion. Del Med J 86:245–248. https://pubmed.ncbi.nlm.nih.gov/25252436/ [PubMed]
- Lechevalier P, Hermoso DG, Carol A, et al. Molecular diagnosis of Saksenaea vasiformis cutaneous infection after scorpion sting in an immunocompetent adolescent. J Clin Microbiol. 2008;46:3169–3172. doi: 10.1128/JCM.00052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgard JP, Van Hal S, Greenwood JE. Primary cutaneous zygomycosis in a burns patient: a review. J Burn Care Res. 2008;29:286–290. doi: 10.1097/BCR.0b013e31816673b1. [DOI] [PubMed] [Google Scholar]
- Lee JH, Hyun JS, Kang DY, Lee HJ, Park SG. Rare complication of bronchoesophageal fistula due to pulmonary mucormycosis after induction chemotherapy for acute myeloid leukemia: A case report. J Med Case Rep. 2016;10:4–9. doi: 10.1186/s13256-016-0991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelievre L, Garcia-Hermoso D, Abdoul H, et al. Posttraumatic mucormycosis: a nationwide study in France and review of the literature. Med (united States) 2014;93:395–404. doi: 10.1097/MD.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RE, Georgiadou SP, Sampsonas F, Chamilos G, Kontoyiannis DP. Risk factors for early mortality in haematological malignancy patients with pulmonary mucormycosis. Mycoses. 2014;57:49–55. doi: 10.1111/myc.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HM, Hwang SK, Zhou C, Du J, Zhang JZ. Gangrenous cutaneous mucormycosis caused by Rhizopus oryzae: a case report and review of primary cutaneous mucormycosis in China over past 20 years. Mycopathologia. 2013;176:123–128. doi: 10.1007/s11046-013-9654-z. [DOI] [PubMed] [Google Scholar]
- Lin CY, Liu WL, Chang CC, et al. Invasive fungal tracheobronchitis in mechanically ventilated critically ill patients: underlying conditions, diagnosis, and outcomes. Ann Intensive Care. 2017;7:1–7. doi: 10.1186/s13613-016-0230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wu H, Huang F, Fan Z, Xu B. Utility of 18F-FDG PET/CT in diagnosis and management of mucormycosis. Clin Nucl Med. 2013;38:370–371. doi: 10.1097/rlu.0b013e3182867d13. [DOI] [PubMed] [Google Scholar]
- Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49:385–390. doi: 10.1097/00000637-200210000-00009. [DOI] [PubMed] [Google Scholar]
- Machouart M, Larché J, Burton K, et al. Genetic identification of the main opportunistic mucorales by PCR-restriction fragment length polymorphism. J Clin Microbiol. 2006;44:805–810. doi: 10.1128/JCM.44.3.805-810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens JA, Raad, II, Marr KA, et al (2016) Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 387:760–769. 10.1016/S0140-6736(15)01159-9 [DOI] [PubMed]
- Mahajan NN, Pednekar R, Patil SR, et al. Preparedness, administrative challenges for establishing obstetric services, and experience of delivering over 400 women at a tertiary care COVID-19 hospital in India. Int J Gynecol Obstet. 2020;151:188–196. doi: 10.1002/ijgo.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini A, Tomar G, Khanna D, Kini Y, Mehta H, Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int J Surg Case Rep. 2021;82:105957–105961. doi: 10.1016/j.ijscr.2021.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manesh A, John AO, Mathew B, et al. Posaconazole: an emerging therapeutic option for invasive rhino-orbito-cerebral mucormycosis. Mycoses. 2016;59:765–772. doi: 10.1111/myc.12529. [DOI] [PubMed] [Google Scholar]
- Manesh A, Rupali P, Sullivan MO, et al. Mucormycosis—a clinicoepidemiological review of cases over 10 years. Mycoses. 2019;2019:391–398. doi: 10.1111/myc.12897. [DOI] [PubMed] [Google Scholar]
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828–837. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- Mattingly JK, Ramakrishnan VR. Rhinocerebral mucormycosis of the optic nerve. Otolaryngol - Head Neck Surg (united States) 2016;155:888–889. doi: 10.1177/0194599816658024. [DOI] [PubMed] [Google Scholar]
- Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12:e10726–e10731. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonnen ZK, Ashraf DC, Jankowski T, et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthal Plast Reconstr Surg. 2021;37:E40–E42. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonnen ZK, Ashraf DC, Jankowski T, Grob SR, Vagefi MR, Kersten RC, Simko JP, Winn BJ. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthal Plast Reconstr Surg. 2021;37:e40–e80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellinghoff SC, Bassetti M, Dörfel D, et al (2018) Isavuconazole shortens the QTc interval. Mycoses. 61:256–60. 10.1111/myc.12731 [DOI] [PubMed]
- Millon L, Herbrecht R, Grenouillet F, et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF) Clin Microbiol Infect. 2016;22:810.e1–810.e8. doi: 10.1016/j.cmi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Mills SEA, Yeldandi AV, Odell DD. Surgical treatment of multifocal pulmonary mucormycosis. Ann Thorac Surg. 2018;106:e93–e95. doi: 10.1016/j.athoracsur.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monheit JE, Cowan DF, Moore DG (1984) Rapid detection of fungi in tissues using calcofluor white and fluorescence microscopy. Arch Pathol Lab Med 108:616–628. https://pubmed.ncbi.nlm.nih.gov/6204621/ [PubMed]
- Moreira J, Ridolfi F, Almeida-Paes R, Varon A, Lamas CC. Cutaneous mucormycosis in advanced HIV disease. Brazilian J Infect Dis. 2016;20:637–640. doi: 10.1016/j.bjid.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira J, Varon A, Galhardo MC, et al. The burden of mucormycosis in HIV-infected patients: A systematic review. J Infect. 2016;73:181–188. doi: 10.1016/j.jinf.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Nagao K, Ota T, Tanikawa A, et al. Genetic identification and detection of human pathogenic Rhizopus species, a major mucormycosis agent, by multiplex PCR based on internal transcribed spacer region of rRNA gene. J Dermatol Sci. 2005;39:23–31. doi: 10.1016/j.jdermsci.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Nam Y, Jung J, Park SS, et al. Disseminated mucormycosis with myocardial involvement in a renal transplant recipient. Transpl Infect Dis. 2015;17:890–896. doi: 10.1111/tid.12452. [DOI] [PubMed] [Google Scholar]
- Nandwani A, Jha PK, Duggal R, Kher V. Invasive gastric mucormycosis and cytomegalovirus infection in an ABO incompatible renal transplant recipient. Indian J Nephrol. 2015;25:373–376. doi: 10.4103/0971-4065.157428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasa M, Sharma Z, Lipi L, Sud R (2017) Gastric angioinvasive mucormycosis in immunocompetent adult, a rare occurrence. J Assoc Physicians India 65:103–104. https://pubmed.ncbi.nlm.nih.gov/29327534/ [PubMed]
- Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: An observational study from Pakistan. Mycoses. 2020;63:766–770. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan SK, Chandrasekar PH. Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: Current evidence, safety, efficacy, and clinical recommendations. Infect Drug Resist. 2016;9:291–300. doi: 10.2147/IDR.S102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: Results of the SEIFEM B-2004 study - Sorveglianza Epidemiologica Infezioni Fungine nelle Emopatie Maligne. Clin Infect Dis. 2007;45:1161–1170. doi: 10.1086/522189. [DOI] [PubMed] [Google Scholar]
- Page AV, Evans AJ, Snell L, Liles WC. Primary cutaneous mucormycosis in a lung transplant recipient: Case report and concise review of the literature. Transpl Infect Dis. 2008;10:419–425. doi: 10.1111/j.1399-3062.2008.00324.x. [DOI] [PubMed] [Google Scholar]
- Pana ZD, Seidel D, Skiada A, et al. Invasive mucormycosis in children: an epidemiologic study in European and non-European countries based on two registries. BMC Infect Dis. 2016;16:1–9. doi: 10.1186/s12879-016-2005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BJ, Pappas PG, Wannemuehler KA, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg Infect Dis. 2011;17:1855–1864. doi: 10.3201/eid1710.110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasero D, Sanna S, Liperi C, et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020;49:1055–1060. doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasero D, Sanna S, Liperi C, Piredda D, Branca GP, Casadio L, Simeo R, Buselli A, Rizzo D, Bussu F. A challenging complication following SARS-CoV-2 infection:a case of pulmonary mucormycosis. Infection. 2020;49:1055–1060. doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Kaur H, Xess I, et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26:944–949. doi: 10.1016/j.cmi.2019.11.021. [DOI] [PubMed] [Google Scholar]
- Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect JR, Cornely OA, Heep M, et al. Isavuconazole treatment for rare fungal diseases and for invasive aspergillosis in patients with renal impairment: challenges and lessons of the VITAL trial. Mycoses. 2018;61:420–429. doi: 10.1111/myc.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrikkos G, Drogari-Apiranthitou M (2011) Zygomycosis in immunocompromised non-haematological patients. Mediterr J Hematol Infect Dis 3:e2011012. 10.4084/MJHID.2011.012 [DOI] [PMC free article] [PubMed]
- Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54:23–34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54:S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- Petrikkos G, Tsioutis C. Recent advances in the pathogenesis of mucormycoses. Clin Ther. 2018;40:894–902. doi: 10.1016/j.clinthera.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Priya P, Ganesan V, Rajendran T, et al. (2020) Mucormycosis in a Tertiary Care Center in South India: A 4-Year Experience. Indian J Crit Care Med 24:168–171. 10.5005/jp-journals-10071-23387 [DOI] [PMC free article] [PubMed]
- Placik DA, Taylor WL, Wnuk NM. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol Case Rep. 2020;15:2378–2381. doi: 10.1016/j.radcr.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza L, Vallerini D, Barozzi P, et al. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood. 2011;118:5416–5419. doi: 10.1182/blood-2011-07-366526. [DOI] [PubMed] [Google Scholar]
- Prabhu S, Alqahtani M, Al Shehabi M. A fatal case of rhinocerebral mucormycosis of the jaw after dental extractions and review of literature. J Infect Public Health. 2018;11:301–303. doi: 10.1016/j.jiph.2017.09.026. [DOI] [PubMed] [Google Scholar]
- Prakash H, Chakrabarti A. Global Epidemiology of Mucormycosis J Fungi. 2019;5:26–30. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash H, Chakrabarti A. Epidemiology of Mucormycosis in India Microorganisms. 2021;9:1–12. doi: 10.3390/microorganisms9030523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash H, Ghosh AK, Rudramurthy SM, et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol. 2019;57:395–402. doi: 10.1093/mmy/myy060. [DOI] [PubMed] [Google Scholar]
- Pyrgos V, Shoham S, Walsh TJ. Pulmonary zygomycosis. Semin Respir Crit Care Med. 2008;29:111–120. doi: 10.1055/s-2008-1063850. [DOI] [PubMed] [Google Scholar]
- Rabin AS, Givertz MM, Couper GS, et al. Risk factors for invasive fungal disease in heart transplant recipients. J Hear Lung Transplant. 2015;34:227–232. doi: 10.1016/j.healun.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radner AB, Witt MD, Edwards JE. Acute Invasive Rhinocerebral Zygomycosis in an Otherwise Healthy Patient. Clin Infect Dis. 1995;20:163–166. doi: 10.1093/clinids/20.1.163. [DOI] [PubMed] [Google Scholar]
- Ramani R, Gromadzki S, Pincus DH, Salkin IF, Chaturvedi V. Efficacy of API 20C and ID 32C systems for identification of common and rare clinical yeast isolates. J Clin Microbiol. 1998;36:3396–3398. doi: 10.1128/jcm.36.11.3396-3398.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapidis AD. Orbitomaxillary mucormycosis (zygomycosis) and the surgical approach to treatment: Perspectives from a maxillofacial surgeon. Clin Microbiol Infect. 2009;15:98–102. doi: 10.1111/j.1469-0691.2009.02989.x. [DOI] [PubMed] [Google Scholar]
- Reed C, Ibrahim A, Edwards JE, Walot I, Spellberg B. Deferasirox, an iron-chelating agent, as salvage therapy for rhinocerebral mucormycosis. Antimicrob Agents Chemother. 2006;50:3968–3969. doi: 10.1128/AAC.01065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/CMR.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches ML, Trifilio S, Chen M, et al. Risk factors and impact of non-Aspergillus mold infections following allogeneic HCT: A CIBMTR infection and immune reconstitution analysis. Bone Marrow Transplant. 2016;51:277–282. doi: 10.1038/bmt.2015.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickerts V, Just-Nübling G, Konrad F, et al. Diagnosis of invasive aspergillosis and mucormycosis in immunocompromised patients by seminested PCR assay of tissue samples. Eur J Clin Microbiol Infect Dis. 2006;25:8–13. doi: 10.1007/s10096-005-0078-7. [DOI] [PubMed] [Google Scholar]
- Riley TT, Muzny CA, Swiatlo E, Legendre DP. Breaking the mold: a review of mucormycosis and current pharmacological treatment options. Ann Pharmacother. 2016;50:747–757. doi: 10.1177/1060028016655425. [DOI] [PubMed] [Google Scholar]
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- Rodríguez JY, Morales-López SE, Rodríguez GJ, et al. Necrotizing fasciitis caused by Apophysomyces variabilis in an immunocompetent patient. Med Mycol Case Rep. 2018;20:4–6. doi: 10.1016/j.mmcr.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüping MJGT, Heinz WJ, Kindo AJ, et al. Forty-one recent cases of invasive zygomycosis from a global clinical registry. J Antimicrob Chemother. 2009;65:296–302. doi: 10.1093/jac/dkp430. [DOI] [PubMed] [Google Scholar]
- Saegeman V, Maertens J, Meersseman W, Spriet I, Verbeken E, Lagrou K. Increasing incidence of Mucormycosis in university hospital, Belgium. Emerg Infect Dis. 2010;16:1456–1458. doi: 10.3201/eid1609.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha M, Reddy R, Vincent MJ. Paranasal mucormycosis in COVID-19 patient. Indian J Otolaryngol Head Neck Surg. 2021;22:1–4. doi: 10.1007/s12070-021-02574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M, Ahmadikia K, Badali H, Khodavaisy S. Opportunistic fungal infections in the epidemic area of COVID-19: A clinical and diagnostic perspective from Iran. Mycopathologia. 2020;185:607–611. doi: 10.1007/s11046-020-00472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandven P, Eduard W. Detection and quantitation of antibodies against Rhizopus by enzyme-linked immunosorbent assay. APMIS. 1992;100:981–987. doi: 10.1111/j.1699-0463.1992.tb04029.x. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Gokhale T, Choudhury SS, Deb AK. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69:1002–1004. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödl W, Heydel T, Schwartze VU, et al. Direct analysis and identification of pathogenic Lichtheimia species by matrix-assisted laser desorption ionization-time of flight analyzer-mediated mass spectrometry. J Clin Microbiol. 2012;50:419–427. doi: 10.1128/JCM.01070-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz P, Guedouar H, Laouiti F, Grenouillet F, Dannaoui E. Identification of mucorales by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J Fungi. 2019;5:1–11. doi: 10.3390/jof5030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz P, Lortholary O, Dromer F, Dannaoui E. Carbon assimilation profiles as a tool for identification of Zygomycetes. J Clin Microbiol. 2007;45:1433–1439. doi: 10.1128/JCM.02219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Gupta P, Gupta N, Lal A, Behera D, Rajwanshi A. Pulmonary infections in immunocompromised patients: the role of image-guided fine needle aspiration cytology. Cytopathology. 2017;28:46–54. doi: 10.1111/cyt.12359. [DOI] [PubMed] [Google Scholar]
- Shigemura T, Nishina S, Nakazawa H, Matsuda K, Yaguchi T, Nakazawa Y. Early detection of Rhizopus DNA in the serum of a patient with rhino-orbital-cerebral mucormycosis following allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2016;103:354–355. doi: 10.1007/s12185-016-1938-x. [DOI] [PubMed] [Google Scholar]
- Shirley M, Scott LJ. Isavuconazole: a review in invasive aspergillosis and mucormycosis. Drugs. 2016;6:1647–1657. doi: 10.1007/s40265-016-0652-6. [DOI] [PubMed] [Google Scholar]
- Shoham S, Magill SS, Merz WG, et al. Primary treatment of zygomycosis with liposomal amphotericin B: Analysis of 28 cases. Med Mycol. 2010;48:511–517. doi: 10.3109/13693780903311944. [DOI] [PubMed] [Google Scholar]
- Skiada A, Lanternier F, Groll AH et al (2013) Diagnosis and treatment of mucormycosis in patients with hematological malignancies: Guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica 98:492–504. 10.3324/haematol.2012.065110 [DOI] [PMC free article] [PubMed]
- Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56:S93–S101. doi: 10.1093/mmy/myx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiada A, Pagano L, Groll A, et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17:1859–1867. doi: 10.1111/j.1469-0691.2010.03456.x. [DOI] [PubMed] [Google Scholar]
- Song Y, Qiao J, Giovanni G, et al. Mucormycosis in renal transplant recipients: Review of 174 reported cases. BMC Infect Dis. 2017;17(1):1–6. doi: 10.1186/s12879-017-2381-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The deferasirox-AmBisome therapy for mucormycosis (Defeat Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67:715–722. doi: 10.1093/jac/dkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanzani M, Battista G, Sassi C, et al. Computed tomographic pulmonary angiography for diagnosis of invasive mold diseases in patients with hematological malignancies. Clin Infect Dis. 2012;54:610–616. doi: 10.1093/cid/cir861. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA - J Am Med Assoc. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HY, Forrest G, Gupta KL, et al. Rhino-orbital-cerebral zygomycosis in solid organ transplant recipients. Transplantation. 2010;90:85–92. doi: 10.1097/TP.0b013e3181dde8fc. [DOI] [PubMed] [Google Scholar]
- Tacke D, Koehler P, Markiefka B, Cornely OA. Our 2014 approach to mucormycosis. Mycoses. 2014;57:519–524. doi: 10.1111/myc.12203. [DOI] [PubMed] [Google Scholar]
- Tilak R, Raina P, Gupta SK, Tilak V, Prakash P, Gulati AK. Cutaneous zygomycosis: A possible postoperative complication in immunocompetent individuals. Indian J Dermatol Venereol Leprol. 2009;75:596–599. doi: 10.4103/0378-6323.57722. [DOI] [PubMed] [Google Scholar]
- Tissot F, Agrawal S, Pagano L, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102:433–444. doi: 10.3324/haematol.2016.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobón AM, Arango M, Fernández D, Restrepo A. Mucormycosis (zygomycosis) in a heart-kidney transplant recipient : recovery after posaconazole therapy. Clin Infect Dis. 2016;36:1488–1491. doi: 10.1086/375075. [DOI] [PubMed] [Google Scholar]
- Tribble DR, Krauss MR, Murray CK, et al. Epidemiology of trauma-related infections among a combat casualty cohort after initial hospitalization: the Trauma Infectious Disease Outcomes Study. Surg Infect (larchmt) 2018;19:494–503. doi: 10.1089/sur.2017.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuysuz G, Ozdemir N, Senyuz OF, et al. Successful management of hepatic mucormycosis in an acute lymphoblastic leukaemia patient: a case report and review of the literature. Mycoses. 2014;57:513–518. doi: 10.1111/myc.12184. [DOI] [PubMed] [Google Scholar]
- Vehreschild JJ, Birtel A, Vehreschild MJGT, et al. Mucormycosis treated with posaconazole: Review of 96 case reports. Crit Rev Microbiol. 2013;39:310–324. doi: 10.3109/1040841X.2012.711741. [DOI] [PubMed] [Google Scholar]
- Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Rezaei Kanavi M, Farjad R. Rhino- orbital mucormycosis during steroid therapy in COVID-19 patients: a case report. Eur J Ophthalmol. 2021;1:1–6. doi: 10.1177/11206721211009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale RG, De Hoog GS, Schwarz P, et al. Antifungal susceptibility and phylogeny of opportunistic members of the order Mucorales. J Clin Microbiol. 2012;50:66–75. doi: 10.1128/JCM.06133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt N, Heß K, Bialek R, et al. Epileptic seizures and rhinocerebral mucormycosis during blinatumomab treatment in a patient with biphenotypic acute leukemia. Ann Hematol. 2017;96:151–153. doi: 10.1007/s00277-016-2837-1. [DOI] [PubMed] [Google Scholar]
- Vujanovic M, Krietsch J, Raso MC et al (2017) Replication fork slowing and reversal upon DNA damage require PCNA polyubiquitination and ZRANB3 DNA translocase activity. Mol cell 67:882–890. 10.1016/j.molcel.2017.08.010 [DOI] [PMC free article] [PubMed]
- Wahba H, Truong MT, Lei X, Kontoyiannis DP, Marom EM. Reversed halo sign in invasive pulmonary fungal infections. Clin Infect Dis. 2008;46:1733–1737. doi: 10.1086/587991. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis) Clin Infect Dis. 2012;54(SUPPL. 1):55–60. doi: 10.1093/cid/cir868. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu M, Bao Y, et al. Cutaneous mucormycosis caused by Rhizopus microsporus in an immunocompetent patient: A case report and review of literature. Med (united States) 2018;97:4. doi: 10.1097/MD.0000000000011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BJ, Ferraro JP, Rea S, Kaufusi S, Goodman BE, Spalding J. Epidemiology and clinical features of invasive fungal infection in a US health care network. Open Forum Infect Dis. 2018;5:2–9. doi: 10.1093/ofid/ofy187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:264.e5–264.e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:e245–e264. doi: 10.1016/j.ajem.2020.09.032e265-264.e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PL, Dhillon R, Cordey A, et al. A national stretegy to diagnose COVID-19 associated invasive fungal disease in the ICU. SSRN. 2020;73:1–34. doi: 10.2139/ssrn.3644400. [DOI] [Google Scholar]
- Wysong DR, Waldorf AR. Electrophoretic and immunoblot analyses of Rhizopus arrhizus antigens. J Clin Microbiol. 1987;25:358–363. doi: 10.1128/jcm.25.2.358-363.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Lee JH, Kim YK, Ki CS, Huh HJ, Lee NY. Identification of mucorales from clinical specimens: A 4-year experience in a single institution. Ann Lab Med. 2016;36:60–63. doi: 10.3343/alm.2016.36.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg MD, Shorr AF, Huang H, Chaudhari P, Paly VF, Menzin J. Hospital days, hospitalization costs, and inpatient mortality among patients with mucormycosis: a retrospective analysis of US hospital discharge data. BMC Infect Dis. 2014;14:1–10. doi: 10.1186/1471-2334-14-310. [DOI] [PMC free article] [PubMed] [Google Scholar]