Abstract
Background and Purpose:
Limited data exist detailing the role of salvage reirradiation following local-regional recurrence (LR) in previously irradiated pediatric patients with rhabdomyosarcoma (RMS).
Materials and Methods:
We evaluated outcomes and prognostic factors in a multi-institutional cohort of 23 patients with LR-only (N=19) or LR with distant failure (N=4) RMS managed with (N=12) or without (N=11) re-irradiation who were treated from 1996 to 2012.
Results:
At a median follow-up of 4.6 years from LR, 7 (30%) patients were alive and 5 (22%) had no evidence of disease. Median OS and PFS from LR were 19.3 and 16.9 months, respectively. LFFS and DFFS at 3 years from relapse were 54% and 56%, respectively. Salvage re-irradiation occurred in 12 (52%) patients, with 9 (75%) receiving resection before re-irradiation. Patients classified as low-risk at diagnosis with favorable primary tumor location had improved 3-year PFS 80% (95% CI 51.6–100%) vs. 47.1% (95% CI 27.3–81.2%), p=0.066], and OS 80% [(95% CI 22.4–100%) vs. 47.1% (95% CI 27.3–81.3%), p=0.051] following LR. Median LFFS and OS in unirradiated vs. re-irradiated patients was 12.4 vs. 19.6 (p=0.1) and 18.8 vs. 26.1 months (p=0.46). No patients experienced ≥ grade 4 acute toxicity from re-irradiation. LR failure was a component of cancer-related death in 60% vs. 40% of the unirradiated and re-irradiated group (p= 0.02).
Conclusion:
Salvage re-irradiation appears tolerable with acceptable morbidity and may reduce the risk of subsequent LR as a component of death in patients with LR RMS.
Keywords: Rhabdomyosarcoma, Recurrent, Re-irradiation, Relapse, Salvage, Pediatrics, Radiation
INTRODUCTION
Rhabdomyosarcoma (RMS) is the most common soft-tissue sarcoma in children. Outcomes have improved over the past few decades for most patients; however, tumor progression is expected in approximately 20%, 40%, and 85% of patients with low- [1], intermediate- [1], or high-risk [2] disease, respectively. Tumor progression leads to high mortality rates ranging from 50–95% depending on clinical and treatment-related factors.[3–5] Patients with unfavorable primary tumor site, unresectable tumors, time to relapse < 18 months from diagnosis, prior RT, concurrent distant-failure, translocation-positive disease, and progression during initial therapy are at highest risk of poor outcome after salvage treatment. [6] [5, 7]
The value of additional local therapy at the time of local tumor recurrence, with or without distance recurrence, has not been determined, although improved overall survival has been documented in select patients that undergo resection. [8] Radiation therapy (RT) is used frequently for palliation at recurrence but has not been used systematically in the relapse setting for patients with local recurrence. Re-irradiation may be an efficient means to mitigate local failures and an adjunct or alternative to aggressive surgical resection with reduced morbidity.
The Children’s Oncology Group ARST0121 protocol was the only prospective clinical trial to date for patients with relapsed RMS which allowed re-irradiation. [9] In the ARST0121 trial, surgical resection of residual disease at sites of prior RT was encouraged. Re-irradiation was recommended for any site with residual disease (i.e., positive margin, suspected microscopic or gross residual) if tissue radiation dose tolerance had not been reached with the prior course and sufficient time had passed between courses. For cases in which tissue tolerance had been reached, and surgical resection was planned but positive margins or residual disease was considered likely, intraoperative RT or brachytherapy were encouraged as alternatives. Only a few patients received re-irradiation in this trial; therefore, prospective data comparing the durability of primary site local control with and without radiotherapy are lacking. With the goal of understanding the role of re-irradiation as a component of salvage therapy in children, adolescents, and young adults with locoregional (LR) recurrent RMS, we reviewed a multi-institutional cohort treated with or without re-irradiation. Our review focused on the morbidity and potential benefit of a second course of irradiation.
MATERIALS AND METHODS
We reviewed the electronic medical records of consecutively treated RMS patients with LR RMS with or without distant recurrences at St. Jude Children’s Research Hospital and Emory University Winship Cancer Institute from 2000 to 2015 after obtaining permission from the institutional review board at each participating institution. In the St. Jude cohort, 50 patients with group 3 rhabdomyosarcoma were treated from 2000–2011 as a part of a prospective protocol (RTSARC, NCT00186992). Five failed with local only disease while eleven progressed at a distant site. One of the eleven patients had a component of local recurrence and was included in the cohort. In the Emory cohort, across 110 children with nonmetastatic RMS treated from 2000 to 2015, there were 28 failures of which, 10 had a component of local failure at first recurrence among the initially non-metastatic patients. The remaining 18 experienced distant only recurrence.
Patient and initial and recurrent disease characteristics were abstracted from the medical record. Initial and salvage therapy and management characteristics were reviewed and stored in a counting process dataset. Clinical group and stage information was categorized based on the Intergroup Rhabdomyosarcoma Study Group (IRSG) staging system; the Oberlin score and risk stratification were recorded.
Treatment timing and primary site management, including surgical resection extent, chemotherapy, immunotherapy, stem cell transplant, and RT dose were variably applied across multiple trials during the period of this study. Patients were grouped according to the use of RT during salvage therapy (unirradiated vs. reirradiated). Overlap with prior radiation treatment fields was confirmed by either direct examination of the initial and retreatment plans or portal images. When the initial treatment plans or images were unavailable, overlap was assumed if specifically documented in the clinical chart by the attending radiation oncologist supervising retreatment. Radiotherapy plans were analyzed, and follow-up radiology records were reviewed at the time of primary treatment and subsequent relapse to define failure as in-field (>20% overlap), out-of-field (<20% overlap), or mixed as compared to the initial treatment plan. Extent of resection was described according to those with radiographic residual or unresected disease at the primary site vs gross total resection with margins positive or negative.
Toxicity of salvage therapy were graded retrospectively according to Common Toxicity Criteria for Adverse Events version 4 and attributed to surgery, chemotherapy, immunotherapy, stem cell transplant, or RT. [10] The timing, dose, sequencing, and resultant toxicities were reviewed and summarized by using frequency and count statistics. The occurrence of secondary malignancies and their management were described. Relapse was defined as local, regional, distant, or mixed according to diagnostic imaging review. Subsequent cancer related events were captured to preclude underreporting of the presence of LF at death. Mixed failurewas defined as both in-field and out-of-field failure. Death was attributed to either local or distant progression or to treatment type. Overall survival (OS), local failure–free survival (LFFS), regional failure—free survival (RFFS), and distant failure–free survival (DFFS) were defined relative to the date of first relapse.
Continuous data were summarized by using measures of central tendency. Count data were summarized by using frequencies and compared by using the chi-square or Fisher’s exact test. All time-to-event data are summarized by using the Kaplan-Meier estimator and compared across strata by using the log rank test. All data were managed by using Excel 2013. All analyses were completed in either SAS v9.3 or R Studio v1.0.143.
RESULTS:
This study included 23 patients with RMS who experienced local tumor recurrence (LR) with or without distant failure after prior RT and who were treated at participating institutions between 1996 and 2012. The median age at diagnosis and recurrence was 7 years (range, 1.8 y-19 y) and 8.5 years (range, 3–20y) respectively. The median follow-up following recurrence was 4.7 years (range, 1.0 y - 9.3 y). Seventy-four percent of patients were male. Histology was embryonal in 70% of tumors and alveolar in 30%. The most common primary sites were parameningeal (35%) and genitourinary (26%). Most patients (69.5%) were classified as intermediate-risk at the time of initial treatment and had either IRSG Stage 3 (52%) or Group III (78%) disease. Full patient demographic, tumor, and treatment information can be found in Table I and/or Supplementary Materials, Figure 1. A Consort diagram of patient treatments can be found in Figure 1. Treatment course timeline by individual patient is represented in Supplementary Materials, Figure 1.
Table 1:
Patient and Tumor Characteristics at Diagnosis and Failure
| Characteristic | All N (%) / Median (Range) | No Re-RT N (%) / Median (Range) | Re-RT N (%) / Median (Range) |
|---|---|---|---|
| Age, years | 7 (1.8–19) | 6 (1.8–18) | 9.9 (2–19) |
| Sex | |||
| Male | 17 (74) | 9 (39) | 8 (35) |
| Female | 6 (26) | 2 (9) | 4 (17) |
| Histology | |||
| Alveolar | 7 (30) | 1 (4) | 6 (26) |
| Embryonal | 16 (70) | 10 (43) | 6 (26) |
| Primary Site | |||
| Head and Neck Other | 2 (9) | 0 | 2 (9) |
| Parameningeal | 8 (35) | 4 (17) | 4 (17) |
| Bladder/Prostate | 6 (26) | 3 (13) | 3 (13) |
| Vagina | 1 (4) | 0 | 1 (4) |
| Extremity | 3 (13) | 1 (4) | 2 (9) |
| Pelvis, unknown | 1 (4) | 1 (4) | 0 |
| Orbital | 2 (9) | 2 (9) | 0 |
| Initial Regional Nodal Involvement | |||
| Yes | 7 (30) | 4 (17) | 3 (13) |
| No | 16 (70) | 7 (30) | 9 (39) |
| Initial Risk Stratification | |||
| Low | 4 (17.5) | 2 (18.2) | 2 (16.7) |
| Intermediate | 16 (69.5) | 8 (72.7) | 8 (66.7) |
| High | 3 (13) | 1 (9) | 2 (16.7) |
| Initial Oberlin Score | |||
| 1 | 2 (8.7) | 1 (9) | 1 (8) |
| 3 | 1 (4) | 0 | 1 (8) |
| Initial IRSG Stage | |||
| 1 | 7 (30) | 3 (13) | 4 (17) |
| 2 | 1 (4) | 1(4) | 0 |
| 3 | 12 (52) | 6 (26) | 6 (26) |
| 4 | 3 (13) | 1 (4) | 2 (9) |
| Initial IRSG Group | |||
| I | 1 (4) | 0 | 1 (4) |
| II | 1 (4) | 0 | 1 (4) |
| III | 18 (78) | 10 (43) | 8 (35) |
| IV | 3 (13) | 1 (4) | 2 (9) |
| Translocation Status | |||
| Absent | 6 (26) | 3 (13) | 3 (13) |
| Present (PAX3 t(2:13) or PAX7 t(1:13)) | 7 (30) | 2 (9) | 5 (22) |
| None Available | 10 (43) | 6 (26) | 4 (17) |
| Favorable Site | |||
| Yes | 5 (22) | 2 (9) | 3 (13) |
| No | 18 (78) | 9 (39) | 9 (39) |
| Time to 1st Failure from 1st RT course (months) | 12 (10–22) | 12 (9.4–15.5) | 12.2 (11–36) |
| 1st Failure Type | |||
| Local Only | 13 (56.5) | 8 (72.7) | 5 (41.6) |
| Local + Regional | 7 (30.4) | 3 (27.3) | 4 (33.3) |
| Local + Distant | 2 (8.7) | 0 (0) | 2 (16.7) |
| Local + Regional + Distant | 1 (4.3) | 0 (0) | 1 (8.3) |
No Re-RT = unirradiated at relapse, Re-RT = re-irradiated at relapse, M = median, IRSG = International Rhabdomyosarcoma Study Group.
Figure 1: Consort Diagram.
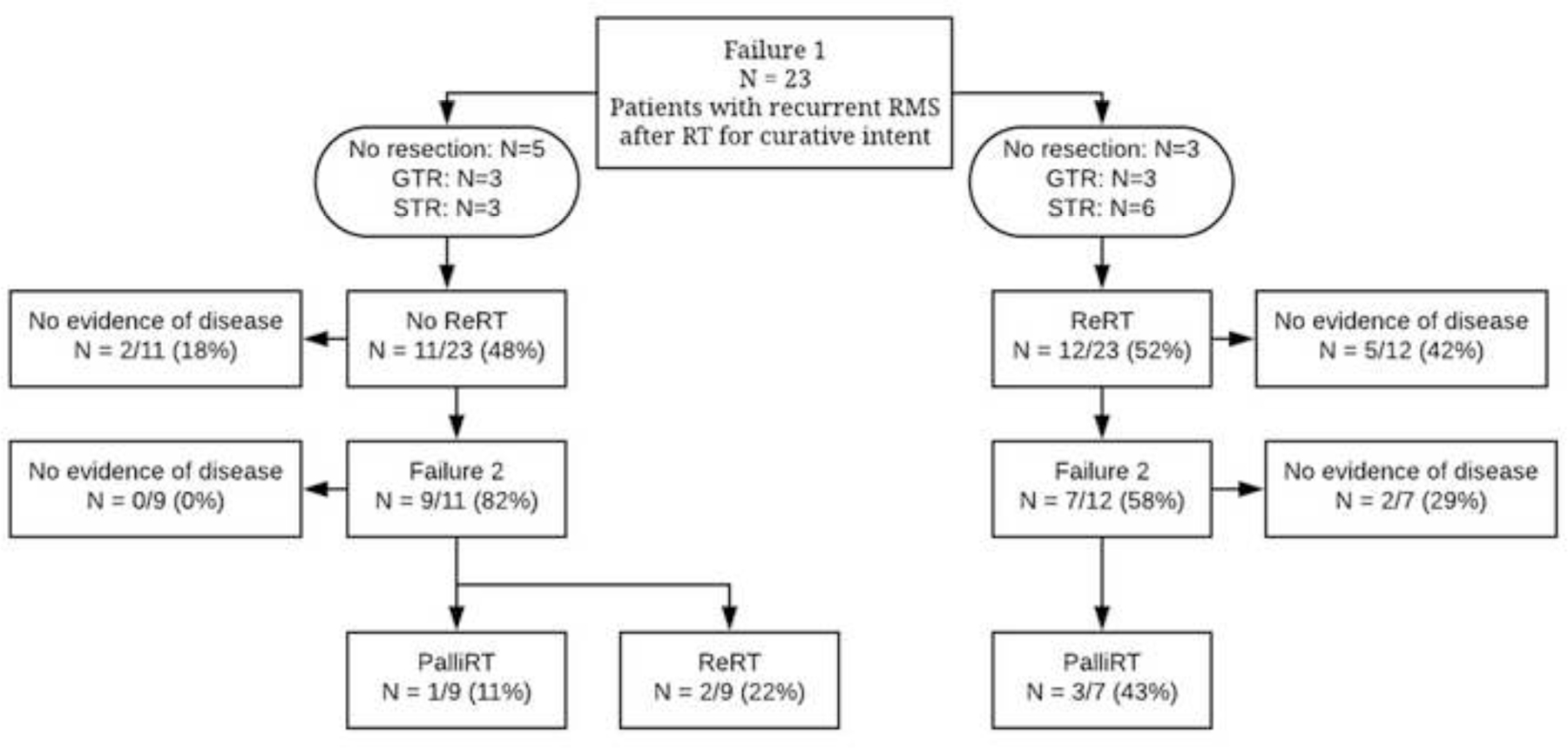
Patients were initially treated according to the institutional or cooperative group protocol at the time of presentation and recurrence. Initial treatment regimens followed six different clinical trial guidelines cited in Supplementary Table 1 and reviewed in Supplementary Figure 1. Sixty-five percent of these patients did not have tumor resection during their initial treatment course; 2 patients underwent gross total resection prior to first recurrence. Initial RT courses were characterized by median dose of 50.4 Gy (range, 24–54 Gy), the use of external beam radiation therapy in 21 patients (92%) and brachytherapy in 2 (8%). A full list of initial treatment characteristics is presented in Table 2.
Table 2:
Primary and Re-treatment Characteristics
| Characteristic | All N (%) /M (Range) | No Re-RT N (%) /M (Range) | Re-RT N (%) /M (Range) |
|---|---|---|---|
| Initial Treatment Per Protocol | |||
| D9803 | 5 (22) | 1 (4) | 4 (17) |
| ARST-0531 | 5 (22) | 3 (13) | 2 (9) |
| ARST-08P1 | 2 (9) | 0 | 2 (9) |
| ARST-0331 | 3 (13) | 1 (4) | 2 (9) |
| IRS-IV | 1 (4) | 1 (4) | 0 |
| ARST-0431 | 2 (9) | 1 (4) | 1 (4) |
| Non-Protocol Treatment Plan | 5 (22) | 4 (17) | 1 (4) |
| Initial Surgical Extent | |||
| No resection | 15 (65) | 10 (43) | 5 (22) |
| Subtotal resection, gross residual disease | 3 (13) | 1 (4) | 2 (9) |
| Subtotal resection, microscopic residual disease | 1 (4) | 0 | 1 (4) |
| Gross total resection | 1 (4) | 0 | 1 (4) |
| Gross total resection with DPE | 3 (13) | 0 | 3 (13) |
| Initial Surgery Type | |||
| Wide Local Excision | 5 (22) | 1 (4) | 4 (17) |
| Composite Resection | 3 (13) | 0 | 3 (13) |
| Initial RT | |||
| Dose (Gy) | 50.4 (24–54) | 50.4 (36–50.4) | 50.4 (24–50.4) |
| Salvage Chemo | |||
| Yes | 23 (100) | 11 (48) | 12 (52) |
| Re-RT | |||
| Yes | 12 (52) | 0 (0) | 12 (52) |
| Dose (Gy) | - | - | 41.4 (36–59.4) |
| Salvage Surgery | |||
| Unresected or Subtotal resection | 8 (35) | 3 (13) | 5 (22) |
| Gross total resection | 4 (17) | 1 (4) | 3 (13) |
No Re-RT = unirradiated at relapse, Re-RT = re-irradiated at relapse, M = median, DPE = delayed primary excision, RT = radiotherapy, Gy = Gray.
Of the 23 patients included in our study, 19 had local only failure (10 of 11 in non-reirradiated and 9 of 12 in the reirradiated cohort), while 4 experienced local failure with a component of distant recurrence. Twelve patients (52%) experienced a second recurrence after salvage therapy, and 2 patients experienced a third recurrence after two rounds of salvage therapy.
Initial failure was classified as in-field relative to prior RT in 17 patients (74%), out-of-field in 4 patients (17%), and mixed in 2 patients. Of the 12 patients who received re-irradiation, 4 (25%) experienced failures that were out-of-field to prior RT, and 1 experienced a mixed failure.
Re-irradiation was used in 12 patients (52%) at the time of first recurrence, 11 patients (48%) were not re-irradiated. The median dose was 41.4 Gy (range, 36 – 59.4 Gy). Salvage surgery preceded re-irradiation in 9 of 12 (75%) patients. Gross total resection was achieved in 3 (25%) patients who subsequently underwent re-irradiation while 6 (50%) received subtotal resection with radiographic evidence of residual disease.
Salvage resection was utilized in 6 of the 11 (55%) patients in the cohort that did not undergo repeat radiotherapy. Resection was complete and subtotal in 3 (27%) and 3 (27%) patients respectively. Full details of salvage treatment characteristics, salvage treatment patterns of failure, and radiation-related toxicities are presented in Supplementary Table 1. Three patients (27%) who did not undergo reirradiation at initial relapse received palliative irradiation at subsequent progression to painful local or metastatic sites, as represented in Consort diagram, Figure 1.
Overall toxicities for re-irradiation were minimal for most patients (Supplementary Table 2). The most common acute symptom was grade II mucositis, which occurred in 5 patients with head and neck region tumors. No patient experienced acute toxicity ≥ 4. Grade III toxicity occurred late in 2 patients. One patient experienced dysphagia requiring a percutaneous endoscopic gastrostomy tube after three courses of radiation therapy to a head and neck site. In another patient, a non–life-threatening in-field secondary malignancy (non-rhabdomyosarcoma soft tissue sarcoma, undifferentiated) developed 7 years after initial therapy and after 2 courses of definitive RT. One patient experienced a late grade V toxicity — a secondary malignancy (sinonasal carcinoma) within the RT field that led to death 12 years after initial therapy. This patient was treated with 2 courses of definitive RT to the orbit followed by stem cell transplant.
After a median follow-up period of 4.6 years from local recurrence, 7 of 23 patients (30%) were alive, and 5 (22%) had no evidence of disease. Median OS and PFS times from LR were 19.3 and 16.9 months, respectively. Kaplan Meier curves of OS, LFFS, and DFFS can be seen in Figure 2. Patients with metastatic disease at first failure had a non-significant detriment in median overall survival (12.8 vs. 20.5 months, p=0.12). Following LR, patients with favorable initial site or low risk disease exhibited a trend toward improved 3 year PFS relative to those with those with either an unfavorable site or initial intermediate/high risk disease 80% (95% CI 51.6–100%) vs. 47.1% (95% CI 27.3–81.2%), p =0.066, and OS 80% (95% CI 22.4–100%) vs. 47.1% (95%CI 27.3–81.3%), p=0.051 (Figure 3).
Figure 2. Time to Event Analysis.
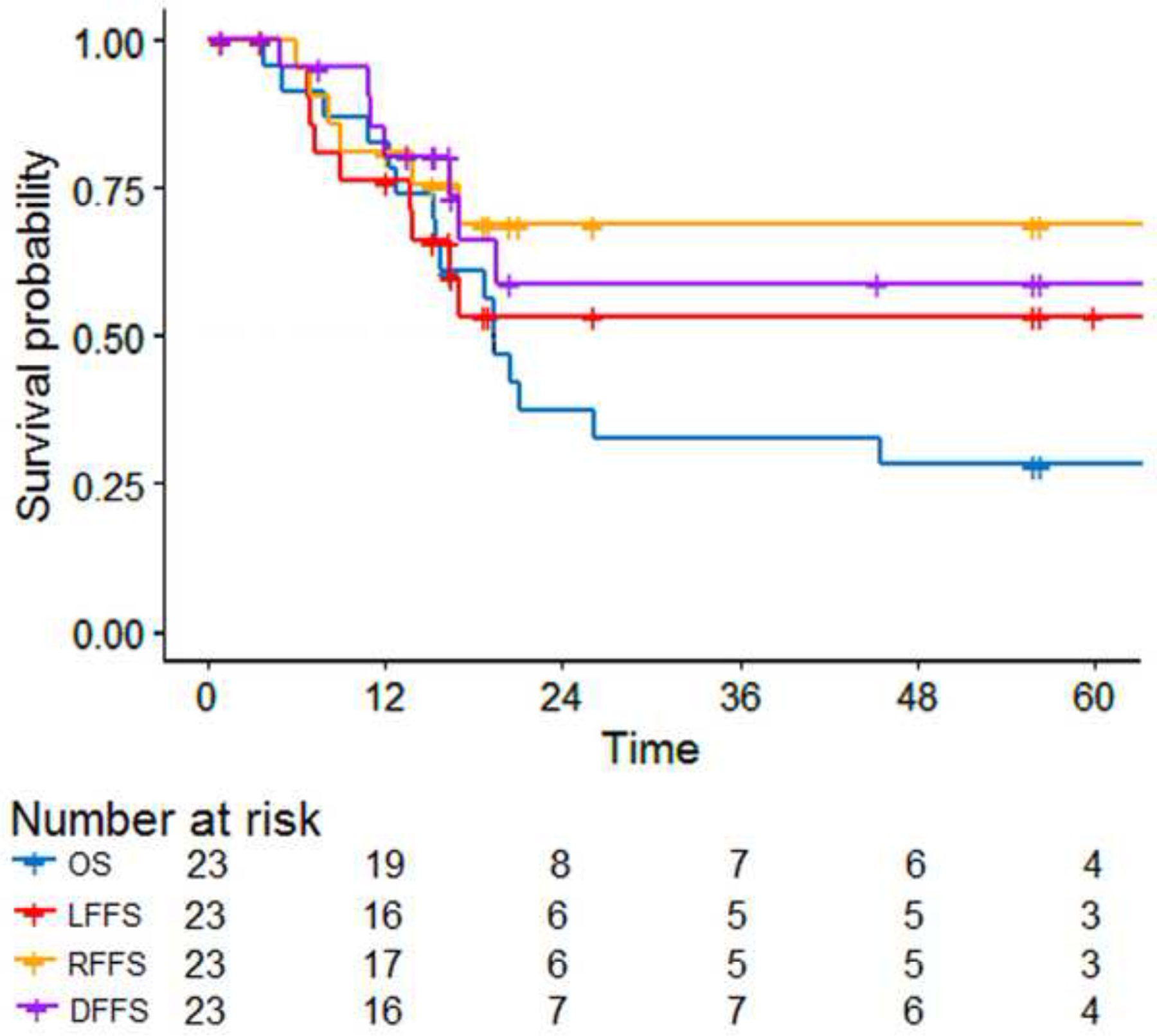
Time = Months, LFFS = Local Failure Free Survival, RFFS = Regional Failure Free Survival, DFFS = Distant Failure Free Survival, OS = Overall Survival.
Figure 3. Kaplan-Meier Analyses of Outcomes Stratified by No Re-RT and Re-RT.
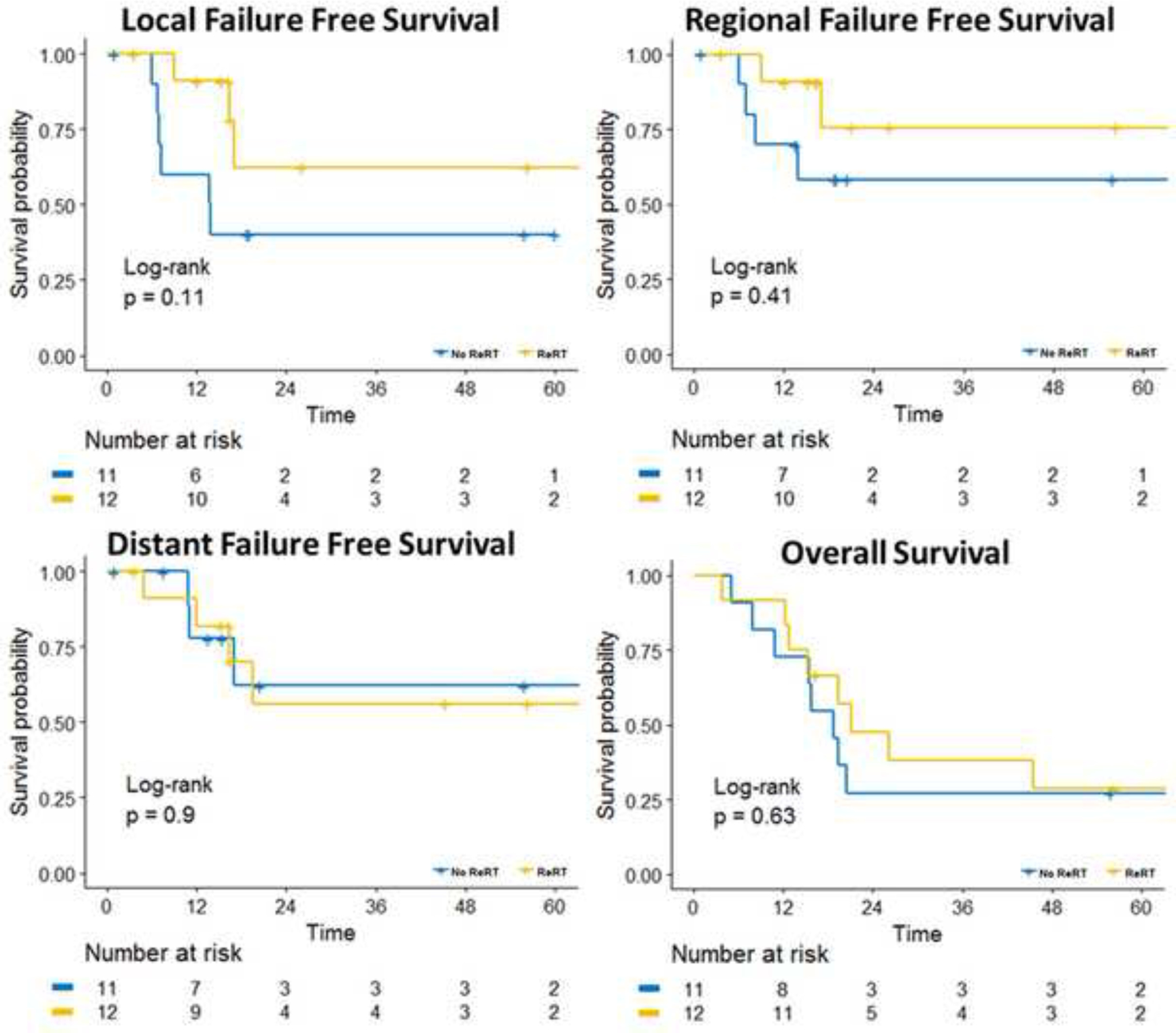
No Re-RT = unirradiated, Re-RT = re-irradiated, Time = Months, LR = Local-Regional.
The median LFFS and OS in unirradiated vs. re-irradiated patients was 12.4 vs. 19.6 (p=0.1) and 18.8 vs. 26.1 months (p=0.46), respectively. The 2 year LFFS and OS in unirradiated vs. re-irradiated patients was 63.6 vs. 42.0% (p=0.55) and 36.3 vs. 40% (p=0.81), respectively. The 3-year rate of DFFS was 62.2% vs. 56.1% for the re-irradiated and unirradiated patients, respectively (p=0.89). Kaplan Meier curves comparing LFFS, RFFS, DFFS, and OS in unirradiated and re-irradiated patients can be seen in Figure 3.
Patients with favorable site and group 3 disease, LR-only failure, and/or embryonal histology had improved 3-year LFFS with re-irradiation (62.3% (95%CI 35.4–100%) vs. 40% (95%CI 18.7–85.4%), p=0.11) (Figure 4).
Figure 4: Time-to-Event Analysis of Patients with Favorable Initial Site or Low Risk vs. Patients with Unfavorable Initial Site or Intermediate/High Risk.
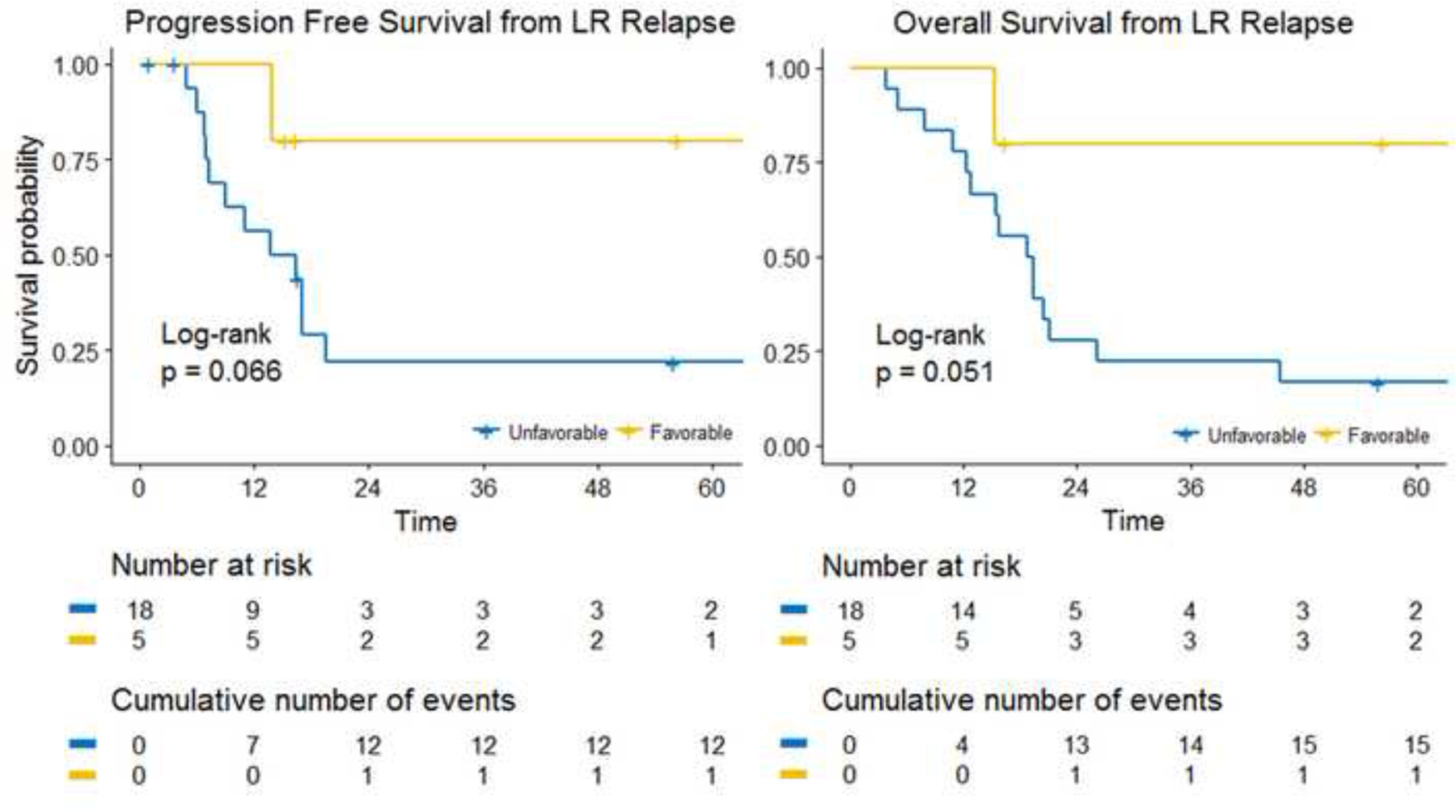
No Re-RT = unirradiated, Re-RT = re-irradiated, Time = Months, LR = Local-Regional.
Death was due to local progression in 10 patients (43%), distant progression in 2 patients (9%), chemotherapy induced cardiomyopathy in 2 patients, veno-occlusive disease of the liver in 1 patient after stem cell transplant, and RT-induced secondary malignancy in 1 patient. Local recurrence was a component of local progression–related death in 40% and 60% of patients who did and did not receive re-irradiation, respectively (p=0.02).
DISCUSSION:
Patients with relapsed RMS have a poor prognosis despite intensive, multimodal therapy. [1] Evidence to support additional local therapy including re-irradiation is lacking, although modern trials allow for its use in salvage regimens. [11, 12]
Overall survival in our population is similar to that reported by Haynes-Jordan et al. [8] In a similar cohort of 32 patients with locally recurrent RMS treated with aggressive salvage resection and chemotherapy plus or minus re-irradiation, 37% of patients managed with aggressive resection were alive, with a mean follow up of 4.9 years. Of patients managed without aggressive salvage resection, 8% survived. The breakdown of reirradiation and its effect on survival is not discussed in this study. In another study, De Corti et al. reported 5-year OS rates of 61.4% in patients with local-only relapse who received salvage surgery followed by salvage RT and 41.8% in those who received salvage surgery without RT (p≤0.0001). [13] Salvage RT conferred an OS benefit in patients managed without surgery as well, with a 37% 5-year OS rate in patients who received salvage RT alone and a 0% rate in patients who received only chemotherapy (p≤0.0001).
Unlike the analysis from De Corti et al., our results did not demonstrate a statistically significant survival benefit of salvage RT. These differences in results are likely due to the contrasting patient population relative to our cohort —half of the patients in the study by De Corti et al. did not receive RT previously due to complete remission of their disease after initial therapy. Their patients’ tumors were likely more favorable given the omission of adjuvant radiotherapy in 40% of patients and exclusion of patients with concurrent distant failure. Our population is one of inherently higher risk, consisting solely of patients who received adjuvant RT initially and then experienced relapse. Our study also includes patients who experienced mixed local and distant relapse; De Corti et al. reports on locoregional relapse results only. Their report indicates that patients with initial favorable site without concurrent distant failure who experience local relapse may be salvaged to a reasonable outcome if the relapsed disease is resectable or can be irradiated. Given that all patients in our analysis were previously irradiated and an increased proportion had metastatic disease at recurrence compared to De Corti et al., the benefit of salvage RT may be more favorable in a better selected patient population.
Many pediatric oncologists are averse to referring patients for evaluation for salvage radiation due to toxicity concerns. One study reports that up to 80% of pediatric radiation oncologists think that this is a barrier to palliative RT referrals. [14] Few cases received a second course of irradiation on ARST0121, even though the toxicity of re-irradiation was minor in this cohort, with one case of radiation-induced odynophagia, mucositis, dysphagia, and dermatitis. [15] All retrospectively captured radiation-related adverse events were grade I-II except for mucositis and dysphagia, which was graded as CTCAE grade IV. [15]
Our study shows that re-irradiation for locoregionally recurrent RMS may be safely delivered with minimal acute toxicity. No patients in our series experienced greater-than-grade III acute toxicity, with most patients having grade II or less toxicity during re-irradiation.
However, salvage surgery series have reported grade III or greater acute complication rates of 15%. [8] Likewise, salvage chemotherapy series have reported grade III or greater acute complication rates of 30–60%. [9, 16–19] Of the 16 patients who died in our study, 3 (19%) had deaths attributable to salvage chemotherapy related complications. While our evaluation is retrospective and uncontrolled in the comparison of patients treated with and without repeat radiotherapy, repeat radiotherapy likely confers a small contribution to toxicity and, therefore, has a favorable therapeutic ratio.
Secondary malignancies are related to the type of chemotherapy, radiation dose and field design, and several patient-specific characteristics. [20] Due to an association between increased radiation dose and secondary malignancies, some have recommended dose reductions in the front-line setting. [21–23] A second course of radiotherapy drastically increases the patient integral dose, so this approach remains a potential source of late morbidity and mortality. Of 12 re-irradiated patients in our study, 2 experienced radiation-induced secondary malignancies. Given the generally unfavorable prognosis associated with relapsed RMS, we feel it is reasonable to consider re-irradiation for patients with locally relapsed disease not amenable to surgical resection. We encourage close surveillance for survivors.
We observe a trend towards improved local failure–free survival and a reduced proportion of patients with local progression as a component of failure at death in patients who received re-irradiation. Although only a small proportion of patients will survive relapse even with aggressive local therapy, re-irradiation likely offers some durable palliation and may improve quality of life in patients with certain unfavorable sites (parameningeal, genitourinary, spine, extremity). Early and effective palliative care improves quality of life and overall survival in patients with advanced cancers. [4,12,23] Up to 80–91% of pediatric patients treated with palliative RT have improved symptoms in the first 3 months after treatment, with 43% experiencing durable pain control at 6 months. [13,19,26] The positive effect of re-RT on patient symptoms, regardless of curative effect, could lead to reduced impact of local progression on death.
Although no consensus guideline exists for salvage chemotherapy, surgery, or radiation in relapsed RMS, a diverse list of salvage therapies has been evaluated. Limited data support local management with maximal safe resection followed by salvage RT in patients with a high risk of relapse or support salvage RT alone in unresectable tumors. [8, 13] Given the high rate of distant relapse, salvage chemotherapy should be considered in most cases. Survival rates across studies at 1 and 3 years with salvage chemotherapy are approximately 50% and 30%, respectively, with a relapse rate of 50–60% at one year and as such novel agents are needed. [9, 16–18, 24]
The goal of salvage therapy is to prolong PFS and OS. Radiation therapy and surgery are intended to improve local control. Although 40–50% of relapses in RMS are reported to be local-only in trials of salvage chemotherapy, LFFS and DFFS are seldom reported in outcome analyses. [9, 16–19] For patients with local-only failure, it is expected that improving local control will improve PFS. Our findings show a trend towards improvement in local relapse rates with re-irradiation and indicate that salvage radiotherapy may be best utilized to achieve local control in patients with local-only failure at relapse, favorable site or initial low risk disease. In patients who experience relapse with mixed or distant failure, or at unfavorable sites, palliative re-irradiation may be considered for the purposes of symptom control.
In summary, we recommend re-irradiation for salvage management of locally recurrent RMS when residual disease or positive margins occur after resection. Patients with favorable site and group 3 disease, LR-only failure, and embryonal histology may benefit most from re-irradiation. Radiotherapy at salvage may reduce the local morbidity of recurrence by reducing the risk of progressive local disease at death.
CONCLUSION:
Although distant failure remains common, salvage re-irradiation may reduce the risk of further local tumor progression and the morbidity of recurrent disease in pediatric patients with locoregional recurrent RMS. Acute toxicities of re-irradiation were minimal; secondary malignancies and chemotherapy-induced end organ failure resulted in late mortality.
Supplementary Material
HIGHLIGHTS.
Salvage RT to sites of prior RT can be delivered with minimal acute toxicity.
Salvage RT may reduce the morbidity of progressive local recurrence at death.
Salvage RT may be best for favorable site, group 3 disease, LR-only failure, embryonal histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from john.lucas@stjude.org.
REFERENCES:
- 1.Crist WM, et al. , Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol, 2001. 19(12): p. 3091–102. [DOI] [PubMed] [Google Scholar]
- 2.Weigel BJ, et al. , Intensive Multiagent Therapy, Including Dose-Compressed Cycles of Ifosfamide/Etoposide and Vincristine/Doxorubicin/Cyclophosphamide, Irinotecan, and Radiation, in Patients With High-Risk Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J Clin Oncol, 2016. 34(2): p. 117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crist W, et al. , The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol, 1995. 13(3): p. 610–30. [DOI] [PubMed] [Google Scholar]
- 4.Klingebiel T, et al. , Treatment of children with relapsed soft tissue sarcoma: report of the German CESS/CWS REZ 91 trial. Med Pediatr Oncol, 1998. 30(5): p. 269–75. [DOI] [PubMed] [Google Scholar]
- 5.Raney RB Jr., et al. , Prognosis of children with soft tissue sarcoma who relapse after achieving a complete response. A report from the Intergroup Rhabdomyosarcoma Study I. Cancer, 1983. 52(1): p. 44–50. [DOI] [PubMed] [Google Scholar]
- 6.Pappo AS, et al. , Survival after relapse in children and adolescents with rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. J Clin Oncol, 1999. 17(11): p. 3487–93. [DOI] [PubMed] [Google Scholar]
- 7.Mazzoleni S, et al. , Outcomes and prognostic factors after recurrence in children and adolescents with nonmetastatic rhabdomyosarcoma. Cancer, 2005. 104(1): p. 183–90. [DOI] [PubMed] [Google Scholar]
- 8.Hayes-Jordan A, et al. , Outcome after surgical resection of recurrent rhabdomyosarcoma. J Pediatr Surg, 2006. 41(4): p. 633–8; discussion 633–8. [DOI] [PubMed] [Google Scholar]
- 9.Mascarenhas L, et al. , Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: a report from the Children’s Oncology Group. J Clin Oncol, 2010. 28(30): p. 4658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIH, Cancer Therapy Evaluation Program (CTEP): Common Terminology Criteria for Adverse Events (CTCAE) v4.0 https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. 2016.
- 11.Raney RB, et al. , Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup rhabdomyosarcoma study group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol, 2001. 23(4): p. 215–20. [DOI] [PubMed] [Google Scholar]
- 12.Wexler LH, Metastatic Rhabdomyosarcoma: Still Room for Improvement. J Clin Oncol, 2016. 34(2): p. 105–6. [DOI] [PubMed] [Google Scholar]
- 13.De Corti F, et al. , Does surgery have a role in the treatment of local relapses of non-metastatic rhabdomyosarcoma? Pediatr Blood Cancer, 2011. 57(7): p. 1261–5. [DOI] [PubMed] [Google Scholar]
- 14.Rao AD, et al. , Practice patterns of palliative radiation therapy in pediatric oncology patients in an international pediatric research consortium. Pediatr Blood Cancer, 2017. 64(11). [DOI] [PubMed] [Google Scholar]
- 15.Breitfeld P, A Groupwide Randomized Phase II Window Study of Two Different Schedules of Irinotecan and Pilot Assessment of Safety and Efficacy of Tirapazamine Combined with Multiagent Chemotherapy for First Relapse or Progressive Disease in Rhabdomyosarcoma and Related Tumors. ARST0121 Progress Report, Fall 2006.
- 16.Casanova M, et al. , Vinorelbine and low-dose cyclophosphamide in the treatment of pediatric sarcomas: pilot study for the upcoming European Rhabdomyosarcoma Protocol. Cancer, 2004. 101(7): p. 1664–71. [DOI] [PubMed] [Google Scholar]
- 17.Vassal G, et al. , Phase II trial of irinotecan in children with relapsed or refractory rhabdomyosarcoma: a joint study of the French Society of Pediatric Oncology and the United Kingdom Children’s Cancer Study Group. J Clin Oncol, 2007. 25(4): p. 356–61. [DOI] [PubMed] [Google Scholar]
- 18.Van Winkle P, et al. , Ifosfamide, carboplatin, and etoposide (ICE) reinduction chemotherapy in a large cohort of children and adolescents with recurrent/refractory sarcoma: the Children’s Cancer Group (CCG) experience. Pediatr Blood Cancer, 2005. 44(4): p. 338–47. [DOI] [PubMed] [Google Scholar]
- 19.Frascella E, et al. , Response of previously untreated metastatic rhabdomyosarcoma to combination chemotherapy with carboplatin, epirubicin and vincristine. Eur J Cancer, 1996. 32A(5): p. 821–5. [DOI] [PubMed] [Google Scholar]
- 20.Neglia JP, et al. , Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med, 1991. 325(19): p. 1330–6. [DOI] [PubMed] [Google Scholar]
- 21.Travis LB, et al. , Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA, 2003. 290(4): p. 465–75. [DOI] [PubMed] [Google Scholar]
- 22.Wong FL, et al. , Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk JAMA, 1997. 278(15): p. 1262–7. [DOI] [PubMed] [Google Scholar]
- 23.Kuttesch JF Jr., et al. , Second malignancies after Ewing’s sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol, 1996. 14(10): p. 2818–25. [DOI] [PubMed] [Google Scholar]
- 24.Bagatell R, et al. , Phase 1 trial of temsirolimus in combination with irinotecan and temozolomide in children, adolescents and young adults with relapsed or refractory solid tumors: a Children’s Oncology Group Study. Pediatr Blood Cancer, 2014. 61(5): p. 833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


