Abstract
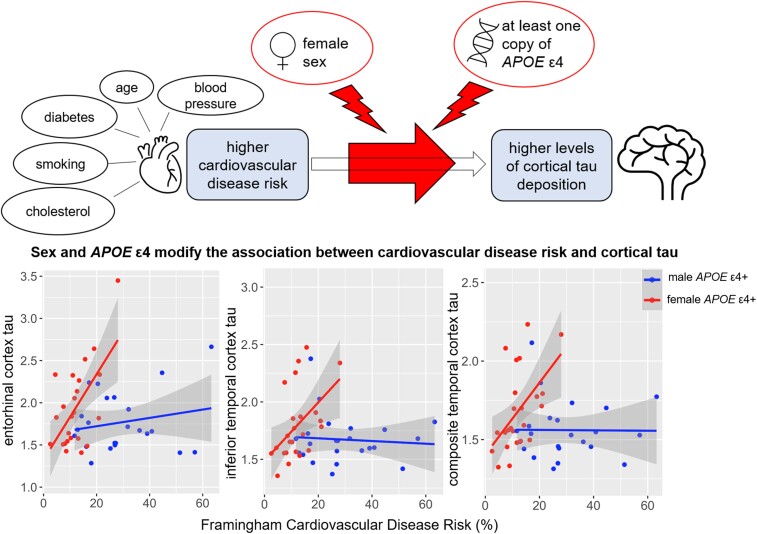
The interaction between APOE ɛ4 and vascular risk factors on cognitive function is stronger in women than in men. These effects may be mediated by the amount of tau pathology in the brain. Therefore, we examined whether APOE ɛ4 and sex modify cross-sectional associations between cardiovascular risk and tau deposition in cognitively normal older adults from the Alzheimer’s Disease Neuroimaging Initiative. We calculated the Framingham Heart Study cardiovascular disease risk score for 141 participants (74 women, 47 APOE ɛ4 carriers) with complete medical history data, processed tau-PET data and a Clinical Dementia Rating global score of 0.0 at the time of the tau-PET scan, implying no significant cognitive or functional impairment. We used linear regression models to examine the effects of sex, APOE ɛ4, cardiovascular risk and their interactions on tau deposition in the entorhinal cortex, inferior temporal cortex and a composite meta-region of interest of temporal lobe areas. We found a significant three-way interaction among sex, APOE ɛ4 status and cardiovascular disease risk on tau deposition in the entorhinal cortex (β = 0.04; 95% CI, 0.01–0.07; P = 0.008), inferior temporal cortex (β = 0.02; 95% CI, 0.0–0.05; P = 0.029) and meta-region (β = 0.02; 95% CI, 0.0–0.04; P = 0.042). After stratifying by APOE ɛ4 status to examine interactions between sex and cardiovascular disease risk on tau in APOE ɛ4 carriers and non-carriers, we found a significant two-way interaction between sex and cardiovascular disease risk on tau in the entorhinal cortex (β = 0.05; 95% CI, 0.02–0.08; P = 0.001), inferior temporal cortex (β = 0.03; 95% CI, 0.01–0.05; P =0.009) and meta-region (β = 0.02; 95% CI, 0.01–0.04; P = 0.008) only among APOE ɛ4 carriers. In analyses stratified by sex, higher cardiovascular risk scores were associated with higher levels of tau in the entorhinal cortex (β = 0.05; 95% CI, 0.02–0.08; P = 0.002), inferior temporal cortex (β = 0.02; 95% CI, 0.0–0.05; P = 0.023) and meta-region (β = 0.02; 95% CI, 0.01–0.04; P = 0.013) in female APOE ɛ4 carriers but not in male carriers. Our findings suggest that cognitively normal older women carrying at least one APOE ɛ4 allele, may be particularly vulnerable to the effects of cardiovascular disease risk on early tau deposition.
Keywords: Alzheimer’s disease, apolipoprotein E, cardiovascular disease risk, sex differences tau pathology
Tsiknia et al. report that among cognitively normal older adults, sex and APOE ɛ4 modify associations between cardiovascular disease risk and cortical tau deposition. Cognitively normal older women carrying at least one APOE ɛ4 allele, may be particularly vulnerable to the effects of cardiovascular disease risk on cortical tau deposition.
Graphical Abstract
Graphical Abstract.
Introduction
While it is clear that vascular risk factors1,2 and vascular neuropathology2 are associated with the clinical diagnosis of Alzheimer’s disease, some investigators have also proposed that vascular risk factors may be aetiologically relevant to Alzheimer’s disease neuropathology.3 Risk factors such as hypertension can contribute to brain hypoperfusion and blood–brain barrier damage, potentially facilitating amyloid and tau accumulation in the brain.4 Growing evidence from animal5 and human6–9 studies indicates that vascular risk burden is associated with increases in tau pathology, while a few studies report associations between vascular risk and amyloid accumulation only,10 or both amyloid and tau pathology.11 One group found that tau deposition mediated associations between cerebrovascular disease and cognitive impairment,12 and another study showed that tau burden mediated associations of cerebral blood flow and a marker of pericyte injury with cognitive scores.13
Studies have also shown that the impact of cardiovascular risk burden on cognitive decline and Alzheimer’s disease progression is stronger among carriers of the apolipoprotein ɛ4 (APOE ɛ4) allele.14–16APOE ɛ4, the major genetic risk factor for Alzheimer’s disease, cannot only promote Alzheimer’s disease pathogenesis directly by reducing amyloid clearance and increasing tau phosphorylation,17–20 but can also indirectly impact Alzheimer’s disease pathogenesis by increasing cardiovascular risk factors such as elevated plasma low-density lipoproteins, which are also risk factors for Alzheimer’s disease.21 A recent study of dementia free older adults demonstrated that higher vascular risk was associated with a stronger effect of APOE ɛ4 on memory decline in women but not men.22 Although these findings are consistent with evidence of a more robust APOE ɛ4 effect on clinical outcomes23 and tau pathology24–27 in women, the mechanism for a stronger APOE ɛ4 effect in women remains unknown.
Sex differences in the effect of cardiovascular burden on brain health could constitute a strong candidate mechanism. Our group previously found that among cognitively normal (CN) older adults aged between 56 and 75 years, women displayed stronger associations between higher pulse pressure, a surrogate marker of arterial stiffness, and white matter microstructural abnormalities, compared with men in the same age group.28 We also previously demonstrated that among a cohort of community-dwelling older adults with a mean age of 67 years, higher cardiovascular risk was associated with greater executive function and memory decline among women but not men, despite higher risk scores among men.29 Similarly, another group found that among older adults between 60 and 100 years of age, higher systolic blood pressure was correlated with worse cognitive performance among hypertensive women but not men.30 Given the strong link between tau pathology and clinical presentation of Alzheimer’s disease,31,32 the exacerbated effects of cardiovascular risk on cognition in women could be related to tau pathology. Therefore, we examined the role of sex and APOE ɛ4 status in modifying the effect of cardiovascular disease risk on cortical tau in CN older adults.
Materials and methods
Participants
All data used in preparation of this manuscript were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (accessed from ida.loni.usc.edu in April 2021). ADNI is a study which aims to measure the progression of MCI and early Alzheimer’s disease using multi-modal imaging methods. ADNI inclusion criteria have been described previously (refer to http://adni.loni.usc.edu/). Notably, individuals with Hachinski ischaemic scores >4 at screening are excluded from ADNI. The Hachinski Ischaemic score is a widely used questionnaire designed to distinguish individuals with vascular dementia from individuals with pure Alzheimer’s disease and mixed dementia (i.e. dementia caused by both Alzheimer’s disease and vascular pathology).33 Additional inclusion criteria for our study included fully processed regional Flortaucipir PET (FTP-PET) for measurement of tau deposition, clinical and medical history data required for cardiovascular disease risk calculations, as well as a Clinical Dementia Rating global score (CDR-GS) equal to 0.0 at the time of FTP-PET scan.
Clinical assessment
We used the participants’ Clinical Dementia Rating scale (CDR) score to determine clinical status at the time of FTP-PET scan.34 This was done to avoid potential sex-related bias from using ADNI-determined diagnoses, which rely on verbal memory tests in which women frequently outperform men despite similar levels of Alzheimer’s disease pathology.35–38 We obtained CDR-GS measured either at the same study visit as the FTP-PET scan or at the study visit closest to the FTP-PET scan (average time difference between CDR and FTP-PET measurement was 52 days). Participants with a CDR-GS equal to 0.0 were included.
Cardiovascular disease risk assessment
We quantified cardiovascular disease risk using the Framingham Heart Study cardiovascular disease (FHS-CVD) risk algorithm—a well-validated tool that has been used in both research and primary care.39,40 The FHS-CVD risk score is a sex-specific measure of cardiovascular disease risk accounting for age, systolic blood pressure, anti-hypertensive treatment, total cholesterol, high density lipoprotein cholesterol, self-reported history of diabetes and current smoking status.39 The risk score represents the probability of cardiovascular events (such as coronary death, myocardial infarction, etc.) occurring within 10 years of risk assessment. We chose the FHS-CVD risk measure obtained closest to the FTP-PET scan with the restriction that the period of time between FHS-CVD assessment and FTP-PET scan could not be more than 10-years. This criterion was applied to ensure this time lag did not exceed the 10-year predictive window of the cardiovascular risk assessment.
APOE genotyping
APOE genotype data were available for all participants and was obtained from the LONI database. Details regarding blood sample collection and genotyping procedures for ADNI can be found in adni.loni.usc.edu. Homozygotes and heterozygotes for the ɛ4 allele (including ɛ2/ɛ4, ɛ3/ɛ4 and ɛ4/ɛ4 genotypes) were combined into a single group and categorized as APOE ɛ4 carriers, while participants with ɛ2/ɛ3 or ɛ3/ɛ3 genotypes were categorized as APOE ɛ4 non-carriers. There were no ɛ2 homozygotes in our study sample.
Brain imaging acquisition and processing
We downloaded Aβ and tau-PET data (from ida.loni.usc.edu, accessed in April 2021) which Landau and colleagues had previously processed at the University of California, Berkeley. ADNI brain imaging acquisition and processing procedures have been described in detail elsewhere http://adni.loni.usc.edu/methods/documents. Briefly, structural MRI scans are performed on 3T scanners using either a 3D MPRAGE or IR-SPGR T1-weighted sequence with sagittal slices and spatial resolution of 1.1 × 1.1 × 1.2 mm3. Structural MRI scans are then skull-stripped, segmented and parcellated using the FreeSurfer (version 5.3.0; http://surfer.nmr.mgh.harvard.edu). Aβ PET images are acquired 50–70 min post Florbetapir injection in a series of four 5-min frames and tau-PET images are acquired 75–105 min after FTP injection in a series of six 5-min frames. After the raw PET data are assessed for quality, each of the acquired frames is extracted and co-registered to the first frame to account for subject motion. The motion-corrected dynamic image set is then averaged and smoothed to a uniform isotropic resolution of 8 mm full width at half maximum and then co-registered with the subject’s processed structural MRI. Standardized uptake value ratios (SUVRs) of FTP uptake are computed for each FreeSurfer-derived region by referencing to the mean cerebellar grey matter deposition.41 To determine Aβ positivity we used the SUVR of a cortical summary region, which is intensity normalized by a whole-cerebellum FreeSurfer region. An SUVR cut-off of 1.11 was used to determine Aβ positivity.42,43 Lastly, regional FTP data are corrected for partial-volume effects using the Geometric Transfer Matrix approach.44,45 To examine regional tau burden, we used a bilateral volume-weighted composite of the entorhinal cortex (EC) and the inferior temporal cortex (ITC), given that these regions are cortical sites of early Alzheimer’s disease-related tau deposition.46–48 Additionally, we examined tau deposition in a larger and likely more stable composite region of interest (meta-ROI) of tau deposition, consisting of a volume-weighted average of the bilateral amygdala, fusiform gyrus, middle temporal cortex, EC and ITC.
Statistical analyses
All statistical analyses were computed with the R (version 4.0.3). Differences in demographic and clinical variables by sex and APOE ɛ4 status were assessed using Welch’s independent t-tests for continuous variables and Fisher’s exact tests for categorical variables. Next, we applied a series of linear regression models to examine the modifying role of sex and APOE ɛ4 on the association between FHS-CVD risk score and tau deposition covarying for the time lag between FHS-CVD risk measurement and FTP-PET scan. First, we used a linear regression model testing for a three-way interaction between sex, APOE ɛ4 status and FHS-CVD risk on tau. In the case of a significant three-way interaction, we stratified our sample by APOE ɛ4 status, and used a linear regression model testing for a two-way interaction between sex and FHS-CVD risk on tau in APOE ɛ4 carriers and APOE ɛ4 non-carriers separately. Finally, in the case of a significant two-way interaction between sex and FHS-CVD risk on tau, we further stratified by sex to examine the main effect of cardiovascular risk in men and women separately. Analyses were repeated with models additionally adjusted for age at FTP-PET scan, Aβ status and years of education to assess potential confounding effects.
Data availability
Data used in preparation of this manuscript were obtained from the LONI database in April 2021 (ida.loni.usc.edu).
Results
Sex differences in demographic characteristics
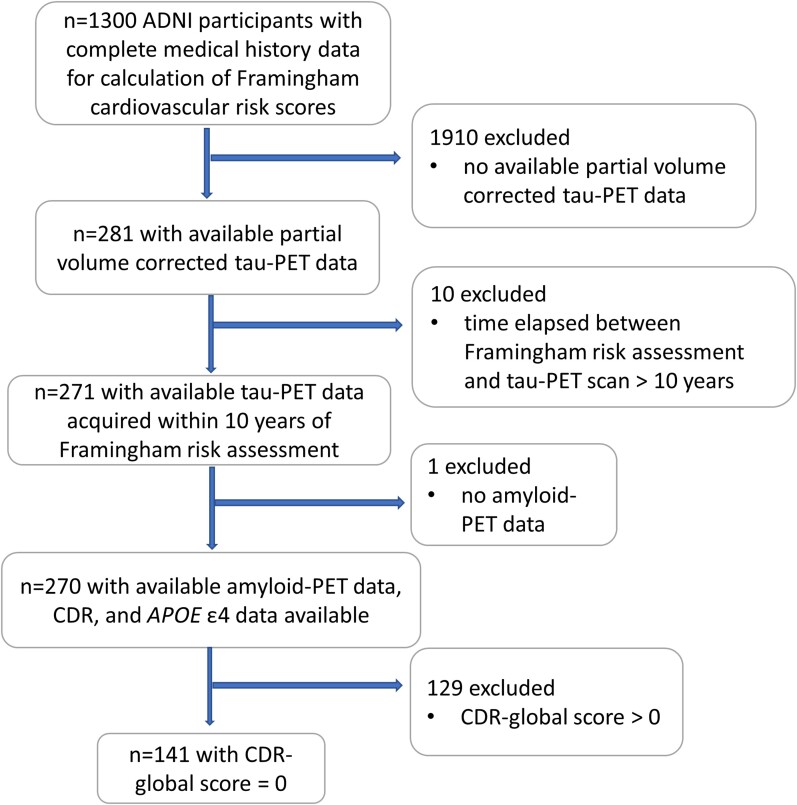
Of the sample of 141 participants who met our inclusion criteria (Fig. 1), 52% were women and 33% carried at least one APOE ɛ4 allele. Participants’ age at the time of cardiovascular risk assessment ranged between 55 and 90 years [mean (SD) = 72.1 (6.45)] and time lag between cardiovascular risk assessment and FTP-PET scan ranged between 2 and 10 years [mean (SD) = 4.94 (1.55)]. Women in our sample had fewer years of education (P < 0.05), lower FHS-CVD risk scores (P < 0.0001) and higher total and high density lipoprotein (HDL) cholesterol (P < 0.001 and P < 0.0001, respectively) than men. Among APOE ɛ4 carriers, women had lower FHS-CVD risk scores (P < 0.0001), were younger at the time of FHS-CVD risk assessment (P < 0.05) and had higher total and HDL cholesterol (P < 0.05 and P < 0.01, respectively) than men. Among APOE ɛ4 non-carriers, women had fewer years of education (P < 0.01), higher HDL cholesterol (P < 0.001) and lower FHS-CVD risk scores (P < 0.0001) than men. Details regarding demographic and clinical variables of our sample can be found under Table 1.
Figure 1.
Subject exclusion based on eligibility criteria and arrival at the final study sample. ADNI = Alzheimer’s Disease Neuroimaging Initiative; CDR = Clinical Dementia Rating.
Table 1.
Demographic and clinical sample characteristics
| Variables | Total cohort (n = 141) | APOE ɛ4 carriers (n = 47) | APOE ɛ4 non-carriers (n = 94) | |||
|---|---|---|---|---|---|---|
| Women (n = 74) | Men (n = 67) | Women (n = 24) | Men (n = 23) | Women (n = 50) | Men (n = 44) | |
| Aβ positive, n (%) | 28 (37.8) | 23 (34.3) | 14 (58.3) | 12 (52.2) | 14 (28) | 11 (25) |
| Education, years, mean (SD) | 16.3 (2.44) | 17.3 (2.47) | 16.5 (2.38) | 16.7 (2.85) | 16.1 (2.48) | 17.6 (2.21) |
| White, n (%) | 70 (94.6) | 63 (94) | 23 (95.8) | 22 (95.7) | 47 (94) | 41 (93.2) |
| African American or Black, n (%) | 3 (4.1) | 2 (3) | 1 (4.2) | 1 (4.3) | 2 (4) | 1 (2.3) |
| Asian, n (%) | 0 | 2 (3) | 0 | 0 | 0 | 2 (4.5) |
| More than one race, n (%) | 1 (1.4) | 0 | 0 | 0 | 1 (2) | 0 |
| FHS-CVD risk score, %, mean (SD) | 12.1 (5.76) | 26.7 (11.3) | 12.1 (5.89) | 29.6 (14.3) | 12.1 (5.76) | 25.1 (6.25) |
| Age, years, mean (SD) | 71.7 (6.08) | 72.6 (0.86) | 69.0 (6.04) | 73.4 (7.96) | 73.0 (5.7) | 72.1 (6.25) |
| Systolic blood pressure, mm Hg, mean (SD) | 134 (14.1) | 136 (14.8) | 136 (14.9) | 136 (13.9) | 134 (13.8) | 136 (15.4) |
| Total cholesterol, mg/dL, | 197 (38.6) | 181 (29.6) | 207 (38.6) | 181 (35.5) | 192 (38) | 182 (26.5) |
| mean (SD) | ||||||
| HDL cholesterol, mg/dL, | 64.6 (15.5) | 53.5 (11.0) | 67.1 (15.4) | 53.3 (12.0) | 63.4 (15.6) | 53.6 (10.6) |
| mean (SD) | ||||||
| Taking anti-hypertensive | 15 (20.3) | 11 (16.4) | 6 (25) | 7 (30.4) | 9 (18) | 4 (9.1) |
| medication, n (%) | ||||||
| Diabetic, n (%) | 6 (8.1) | 8 (11.9) | 2 (8.3) | 4 (17.4) | 4 (8) | 4 (9.1) |
| Smokers, n (%) | 1 (1.4) | 3 (4.5) | 0 | 1 (4.3) | 1 (2) | 2 (4.5) |
| Entorhinal cortex tau-PET SUVR, mean (SD) | 1.82 (0.398) | 1.65 (0.303) | 1.94 (0.492) | 1.77 (0.351) | 1.76 (0.334) | 1.58 (0.255) |
| Inferior temporal cortex tau-PET SUVR, mean (SD) | 1.73 (0.282) | 1.60 (0.172) | 1.8 (0.309) | 1.67 (0.212) | 1.70 (0.265) | 1.56 (0.134) |
| Meta-ROI temporal lobe tau-PET SUVR, mean (SD) | 1.62 (0.229) | 1.50 (0.150) | 1.68 (0.257) | 1.56 (0.185) | 1.59 (0.210) | 1.47 (0.119) |
| Time between FHS-CVD Assessment and tau-PET scan, years, mean (SD) | 4.77 (1.55) | 5.13 (1.55) | 4.79 (1.82) | 5.00 (1.21) | 4.76 (1.42) | 5.2 (1.71) |
Mean and SD are provided for continuous variables and n and % are provided for categorical variables. Aβ = amyloid-β; FHS-CVD = Framingham Heart Study cardiovascular disease; HDL = high density lipoprotein; SUVR = standardized uptake value ratio
Interaction effect of sex, APOE ɛ4 and FHS-CVD risk on tau
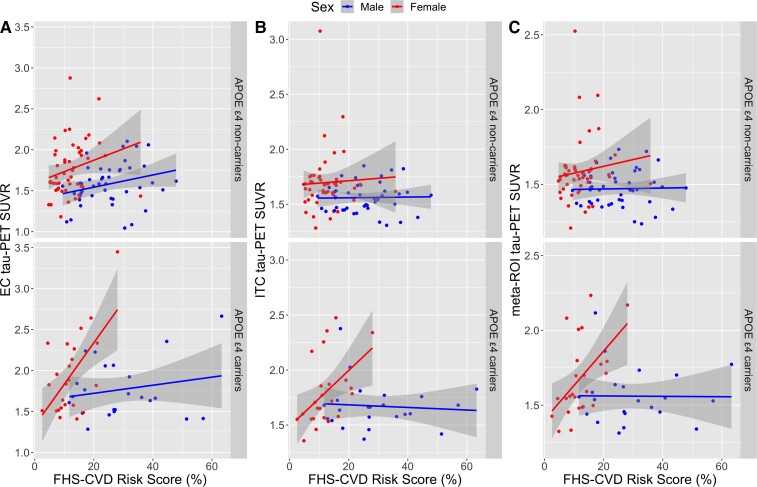
There was a significant three-way interaction among sex, APOE ɛ4 status and FHS-CVD risk on tau deposition in the EC (β = 0.04; 95% CI, 0.01–0.07; P = 0.008), ITC (β = 0.02; 95% CI, 0.0–0.05; P = 0.029) and meta-ROI (β = 0.02; 95% CI, 0.0–0.04; P = 0.042) (Table 2). To aid our interpretation of the three-way interaction between sex, APOE ɛ4 and FHS-CVD risk on tau deposition, we stratified by APOE ɛ4 status to examine two-way interactions between sex and FHS-CVD risk on tau in APOE ɛ4 carriers and non-carriers separately. We found a significant two-way interaction between sex and FHS-CVD risk on tau in the EC (β = 0.05; 95% CI, 0.02–0.08; P = 0.001) (Fig. 2A), ITC (β = 0.03; 95% CI, 0.01–0.05; P = 0.009) (Fig. 2B) and meta-ROI (β = 0.02; 95% CI, 0.01–0.04; P = 0.008) (Fig. 2C) only among APOE ɛ4 carriers (Table 3). Since two-way interactions between sex and FHS-CVD risk were significant only among APOE ɛ4 carriers, we further stratified by sex to examine main effects of FHS-CVD risk on tau in male and female APOE ɛ4 carriers separately. We found that higher FHS-CVD risk was associated with higher levels of tau in the EC (β = 0.05; 95% CI, 0.02–0.08; P = 0.002), ITC (β = 0.02; 95% CI, 0.0–0.05; P = 0.023) and meta-ROI (β = 0.02; 95% CI, 0.01–0.04; P = 0.013) in female APOE ɛ4 carriers but not male carriers (Table 4).
Table 2.
Three-way interaction effect of APOE ɛ4, sex and cardiovascular risk on tau deposition
| Predictor variables | EC tau-PET SUVR | ITC tau-PET SUVR | Meta-ROI tau-PET SUVR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | CI | P-value | Estimate | CI | P-value | Estimate | CI | P-value | |
| FHS-CVD risk | 0.01 | −0.0 to 0.02 | 0.126 | 0.0 | −0.01 to 0.01 | 0.939 | 0.0 | −0.01 to 0.01 | 0.927 |
| APOE (ɛ4+) | 0.28 | −0.14 to 0.70 | 0.187 | 0.15 | −0.15 to 0.45 | 0.317 | 0.10 | −0.15 to 0.34 | 0.425 |
| Sex (female) | 0.23 | −0.12 to 0.58 | 0.195 | 0.12 | −0.13 to 0.37 | 0.358 | 0.07 | −0.14 to 0.27 | 0.525 |
| Time lag | 0.03 | −0.0 to 0.07 | 0.059 | −0.0 | −0.03 to 0.02 | 0.913 | −0.00 | −0.02 to 0.02 | 0.920 |
| APOE (ɛ4+) × FHS-CVD risk | −0.00 | −0.02 to 0.01 | 0.563 | −0.0 | −0.01 to 0.01 | 0.781 | −0.00 | −0.01 to 0.01 | 0.927 |
| Sex (female) × FHS-CVD risk | 0.01 | −0.01 to 0.02 | 0.539 | 0.00 | −0.01 to 0.02 | 0.783 | 0.00 | −0.01 to 0.02 | 0.477 |
| APOE (ɛ4+) × sex (female) | −0.57 | −1.12to−0.01 | 0.048* | −0.33 | −0.73 to 0.06 | 0.099 | −0.23 | −0.55 to 0.10 | 0.175 |
| APOE (ɛ4+) × sex (female) × FHS-CVD risk | 0.04 | 0.01–0.07 | 0.008** | 0.02 | 0.00–0.05 | 0.029* | 0.02 | 0.00–0.04 | 0.042* |
Results from linear regression analysis revealed a significant three-way interaction effect of APOE ɛ4, sex and FHS-CVD risk on tau deposition in the EC, ITC and meta-ROI after adjusting for the time between FHS-CVD risk assessment and tau-PET scan. Significant P-values are presented in bold, where *P <0.05, **P < 0.01, ***P < 0.005. CI = confidence interval; EC = entorhinal cortex; FHS-CVD risk = Framingham Heart Study cardiovascular disease risk; ITC = inferior temporal cortex; SUVR = standardized uptake value ratio.
Figure 2.
Sex and APOE ɛ4 modify associations between cardiovascular disease risk and tau deposition. Scatter plots depicting sex differences in associations between FHS-CVD risk score and tau deposition in the (A) entorhinal cortex, (B) inferior temporal cortex and (C) a composite temporal lobe meta-ROI, among APOE ɛ4 non-carriers (top) and APOE ɛ4 carriers (bottom). Shaded regions represent 95% confidence intervals. We found significant interactions between sex and FHS-CVD risk on tau deposition in the EC (β = 0.05; 95% CI, 0.02–0.08; P = 0.001), ITC (β = 0.03; 95% CI, 0.01–0.05; P = 0.009) and meta-ROI (β = 0.02; 95% CI, 0.01–0.04; P = 0.008) among APOE ɛ4 carriers (bottom) but not among non-carriers (top).
Table 3.
Two-way interaction effect of sex and cardiovascular disease risk on cortical tau deposition among APOE ɛ4 carriers but not non-carriers
| Predictor variables | EC tau-PET SUVR | ITC tau-PET SUVR | Meta-ROI tau-PET SUVR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | CI | P-value | Estimate | CI | P-value | Estimate | CI | P-value | |
| APOE ɛ4 carriers | |||||||||
| FHS-CVD risk | 0.00 | −0.01 to 0.01 | 0.553 | −0.00 | −0.01 to 0.01 | 0.753 | −0.00 | −0.01 to 0.01 | 0.968 |
| Sex (female) | −0.36 | −0.88 to 0.16 | 0.169 | −0.22 | −0.57 to 0.13 | 0.21 | −0.16 | −0.45 to 0.13 | 0.275 |
| Time lag | 0.06 | −0.02 to 0.13 | 0.132 | 0.00 | −0.05 to 0.05 | 0.965 | 0.00 | −0.04 to 0.04 | 0.995 |
| Sex (female) × FHS-CVD risk | 0.05 | 0.02–0.08 | 0.001*** | 0.03 | 0.01–0.05 | 0.009** | 0.02 | 0.01–0.04 | 0.008** |
| APOE ɛ4 non-carriers | |||||||||
| FHS-CVD risk | 0.01 | −0.00 to 0.02 | 0.105 | 0.00 | −0.01 to 0.01 | 0.942 | 0.00 | −0.01 to 0.01 | 0.925 |
| Sex (female) | 0.22 | −0.10 to 0.54 | 0.176 | 0.12 | −0.12 to 0.36 | 0.341 | 0.07 | −0.13 to 0.26 | 0.503 |
| Time lag | 0.02 | −0.02 to 0.06 | 0.236 | −0.00 | −0.03 to 0.03 | 0.863 | −0.00 | −0.02 to 0.02 | 0.894 |
| Sex (female) × FHS-CVD risk | 0.01 | −0.01 to 0.02 | 0.490 | 0.00 | −0.01 to 0.01 | 0.771 | 0.00 | −0.01 to 0.01 | 0.418 |
Results from linear regression analysis on APOE ɛ4 stratified groups revealed a significant interaction effect of sex and FHS-CVD risk on tau deposition in the EC, ITC and meta-ROI only among APOE ɛ4 carriers, after adjusting for the time between FHS-CVD risk assessment and tau-PET scan. Significant P-values are presented in bold, where *P < 0.05, **P < 0.01, ***P < 0.005. CI = confidence interval; EC = entorhinal cortex; FHS-CVD risk = Framingham Heart Study cardiovascular disease risk; ITC = inferior temporal cortex; SUVR = standardized uptake value ratio.
Table 4.
Main effect of cardiovascular disease risk on cortical tau deposition in female APOE ɛ4 carriers but not male carriers
| Predictor variables | EC tau-PET SUVR | ITC tau-PET SUVR | Meta-ROI tau-PET SUVR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | CI | P-value | Estimate | CI | P-value | Estimate | CI | P-value | |
| Female APOE ɛ4 carriers | |||||||||
| FHS-CVD risk | 0.05 | 0.02–0.08 | 0.002*** | 0.02 | 0.00–0.05 | 0.023* | 0.02 | 0.01–0.04 | 0.013* |
| Time lag | 0.03 | −0.07 to 0.13 | 0.511 | −0.02 | −0.09 to 0.04 | 0.475 | −0.02 | −0.08 to 0.03 | 0.349 |
| Male APOE ɛ4 carriers | |||||||||
| FHS-CVD risk | 0.00 | −0.01 to 0.01 | 0.768 | −0.00 | −0.01 to 0.00 | 0.371 | −0.00 | −0.01 to 0.00 | 0.494 |
| Time lag | 0.12 | −0.01 to 0.25 | 0.064 | 0.07 | −0.02 to 0.15 | 0.107 | 0.07 | −0.00 to 0.13 | 0.062 |
Results from linear regression analysis on sex-stratified groups of APOE ɛ4 carriers revealed a significant main effect of FHS-CVD risk on tau deposition in the EC, ITC and meta-ROI only among female APOE ɛ4 carriers, after adjusting for the time between FHS-CVD risk assessment and tau-PET scan. Significant P-values are presented in bold, where *P < 0.05, **P < 0.01, ***P < 0.005. CI = confidence interval; EC = entorhinal cortex; FHS-CVD risk = Framingham Heart Study cardiovascular disease risk; ITC = inferior temporal cortex; SUVR = standardized uptake value ratio.
The three-way interaction between APOE ɛ4, sex and FHS-CVD risk on EC tau deposition (β = 0.04; 95% CI, 0.01–0.07; P = 0.012) remained significant after additionally adjusting for age, Aβ status and education, though this effect was attenuated in the ITC (β = 0.02; 95% CI, −0.01–0.04; P = 0.058) and meta-ROI (β = 0.01; 95% CI, −0.01–0.02; P = 0.093) (Table 5). Notably, Aβ positive participants had higher levels of tau across all three regions, though the main effect of Aβ status on tau was more robust in ITC (β = 0.18; 95% CI, 0.10–0.26; P < 0.001) and meta-ROI (β = 0.15; 95% CI, 0.09–0.22; P < 0.001) compared with the EC (β = 0.13; 95% CI, 0.01–0.26; P = 0.030). APOE ɛ4 stratified analyses revealed a significant two-way interaction effect of sex and FHS-CVD risk on EC (β = 0.05; 95% CI, 0.02–0.08; P = 0.004), ITC (β = 0.02; 95% CI, 0.00–0.04; P = 0.049) and meta-ROI (β = 0.02; 95% CI, 0.00–0.03; P = 0.045) tau deposition among APOE ɛ4 carriers but not non-carriers, after additionally adjusting for age, Aβ status and education (Table 6). Aβ status had a consistent main effect on tau deposition in the ITC and meta-ROI among both APOE ɛ4 carriers and non-carriers. Finally, in sex-stratified analyses of APOE ɛ4 carriers we found that higher FHS-CVD risk scores were associated with higher levels of tau in the EC (β = 0.06; 95% CI, 0.03–0.09; P = 0.002) and meta-ROI (β = 0.02; 95% CI, 0.00–0.04; P = 0.028) among female but not male carriers (Table 7). The effect of FHS-CVD risk on ITC tau in female carriers was no longer significant (β = 0.02; 95% CI, −0.00 to 0.04; P = 0.052) after adjusting for age, education and Aβ status. Lastly, we observed no effect of age and education on tau deposition in any of the models.
Table 5.
Three-way interaction effect of APOE ɛ4, sex and cardiovascular risk on tau deposition, after additionally adjusting for age, Aβ status and education
| Predictor variables | EC tau-PET SUVR | ITC tau-PET SUVR | Meta-ROI tau-PET SUVR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | CI | P-value | Estimate | CI | P-value | Estimate | CI | P-value | |
| FHS-CVD risk | 0.01 | −0.01 to 0.02 | 0.239 | 0.00 | −0.01 to 0.01 | 0.793 | 0.00 | −0.00 to 0.01 | 0.652 |
| APOE (ɛ4+) | 0.19 | −0.24 to 0.61 | 0.390 | 0.01 | −0.28 to 0.30 | 0.945 | −0.03 | −0.27 to 0.20 | 0.773 |
| Sex (female) | 0.25 | −0.11 to 0.61 | 0.166 | 0.11 | −0.14 to 0.35 | 0.392 | 0.05 | −0.15 to 0.24 | 0.641 |
| Age | 0.00 | −0.01 to 0.01 | 0.532 | 0.00 | −0.01 to 0.01 | 0.643 | 0.00 | −0.00 to 0.01 | 0.210 |
| Time lag | 0.02 | −0.01 to 0.06 | 0.224 | −0.01 | −0.04 to 0.01 | 0.389 | −0.01 | −0.03 to 0.01 | 0.223 |
| Aβ status (Aβ+) | 0.13 | 0.01–0.26 | 0.030* | 0.18 | 0.10–0.26 | <0.001**** | 0.15 | 0.09–0.22 | <0.001**** |
| Education | 0.01 | −0.01 to 0.04 | 0.226 | 0.00 | −0.01 to 0.02 | 0.600 | 0.00 | −0.01 to 0.02 | 0.588 |
| APOE (ɛ4+) × FHS-CVD risk | −0.00 | −0.02 to 0.01 | 0.809 | 0.00 | −0.01 to 0.02 | 0.687 | 0.00 | −0.00 to 0.01 | 0.455 |
| Sex (female) × FHS-CVD risk | 0.00 | −0.01 to 0.02 | 0.681 | 0.00 | −0.01 to 0.01 | 0.911 | 0.00 | −0.01 to 0.01 | 0.554 |
| APOE (ɛ4+) × sex (female) | −0.50 | −1.06 to 0.07 | 0.084 | −0.23 | −0.61 to 0.16 | 0.242 | −0.11 | −0.42 to 0.20 | 0.475 |
| APOE (ɛ4+) × sex (female) × FHS-CVD risk | 0.04 | 0.01–0.07 | 0.012* | 0.02 | −0.00 to 0.04 | 0.058 | 0.01 | −0.00 to 0.03 | 0.093 |
Results from linear regression analysis revealed a significant three-way interaction effect of APOE ɛ4, sex and FHS-CVD risk on tau deposition in the EC, but not the ITC or meta-ROI after adjusting the time between FHS-CVD risk assessment and tau-PET scan, age, Aβ status and education. Significant P-values are presented in bold, where *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001. Aβ = amyloid-β; CI = confidence interval; EC = entorhinal cortex; FHS-CVD risk = Framingham Heart Study cardiovascular disease risk; ITC = inferior temporal cortex; SUVR = standardized uptake value ratio.
Table 6.
Two-way interaction effect of sex and cardiovascular disease risk on cortical tau deposition among APOE ɛ4 carriers but not non-carriers, after additionally adjusting for age, Aβ status and education
| Predictor variables | EC tau-PET SUVR | ITC tau-PET SUVR | Meta-ROI tau-PET SUVR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | CI | P-value | Estimate | CI | P-value | Estimate | CI | P-value | |
| APOE ɛ4 carriers | |||||||||
| FHS-CVD risk | 0.01 | −0.01 to 0.02 | 0.239 | 0.00 | −0.01 to 0.01 | 0.793 | 0.00 | −0.00 to 0.01 | 0.652 |
| Sex (female) | −0.28 | −0.81 to 0.25 | 0.294 | −0.10 | −0.42 to 0.23 | 0.541 | −0.05 | −0.32 to 0.22 | 0.714 |
| Time lag | 0.05 | −0.03 to 0.13 | 0.200 | −0.02 | −0.06 to 0.03 | 0.490 | −0.02 | −0.06 to 0.02 | 0.402 |
| Age | −0.00 | −0.02 to 0.01 | 0.612 | 0.00 | −0.00 to 0.01 | 0.845 | 0.00 | −0.01 to 0.01 | 0.575 |
| Aβ status (Aβ+) | 0.19 | −0.05 to 0.43 | 0.116 | 0.25 | 0.10–0.39 | 0.002*** | 0.21 | 0.08–0.33 | 0.001*** |
| Education | 0.02 | −0.02 to 0.06 | 0.318 | −0.01 | −0.04 to 0.01 | 0.297 | −0.01 | −0.03 to 0.01 | 0.358 |
| Sex (female × FHS-CVD risk | 0.05 | 0.02–0.08 | 0.004*** | 0.02 | 0.00–0.04 | 0.049* | 0.02 | 0.00–0.03 | 0.045* |
| APOE ɛ4 non-carriers | |||||||||
| FHS-CVD risk | 0.00 | −0.01 to 0.01 | 0.499 | −0.00 | −0.01 to 0.01 | 0.992 | −0.00 | −0.01 to 0.01 | 0.729 |
| Sex (female) | 0.18 | −0.16 to 0.52 | 0.304 | 0.15 | −0.10 to 0.39 | 0.242 | 0.07 | −0.12 to 0.27 | 0.452 |
| Time lag | 0.00 | −0.04 to 0.05 | 0.887 | −0.01 | −0.04 to 0.02 | 0.561 | −0.01 | −0.04 to 0.01 | 0.351 |
| Age | 0.01 | −0.00 to 0.02 | 0.103 | 0.00 | −0.01 to 0.01 | 0.821 | 0.00 | −0.00 to 0.01 | 0.339 |
| Aβ status (Aβ+) | 0.08 | −0.06 to 0.23 | 0.268 | 0.16 | 0.05–0.26 | 0.004*** | 0.14 | 0.05–0.22 | 0.002*** |
| Education | 0.00 | −0.02 to 0.03 | 0.739 | 0.01 | −0.01 to 0.03 | 0.170 | 0.01 | −0.01 to 0.03 | 0.185 |
| Sex (female) × FHS-CVD risk | 0.00 | −0.01 to 0.02 | 0.617 | 0.00 | −0.01 to 0.01 | 0.998 | 0.00 | −0.01 to 0.01 | 0.615 |
Results from linear regression analysis on APOE ɛ4 stratified groups revealed a significant interaction effect of sex and FHS-CVD risk on tau deposition in the EC, ITC and meta-ROI only among APOE ɛ4 carriers, after adjusting for the time between FHS-CVD risk assessment and tau-PET scan, age, Aβ status and education. Significant P-values are presented in bold, where *P < 0.05, **P < 0.01, ***P < 0.005, P < 0.001.Aβ = amyloid-β; CI = confidence interval; EC = entorhinal cortex; FHS-CVD risk = Framingham Heart Study cardiovascular disease risk; ITC = inferior temporal cortex; SUVR = standardized uptake value ratio.
Table 7.
Main effect of cardiovascular disease risk on cortical tau deposition in female APOE ɛ4 carriers but not male carriers, after additionally adjusting for age, Aβ status and education
| Predictor variables | EC tau-PET SUVR | ITC tau-PET SUVR | Meta-ROI tau-PET SUVR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | CI | P-value | Estimate | CI | P-value | Estimate | CI | P-value | |
| Female APOE ɛ4 carriers | |||||||||
| FHS-CVD risk | 0.06 | 0.03–0.09 | 0.002*** | 0.02 | −0.00 to 0.04 | 0.052 | 0.02 | 0.00–0.03 | 0.028* |
| Time lag | 0.03 | −0.07 to 0.12 | 0.549 | −0.03 | −0.09 to 0.03 | 0.293 | −0.03 | −0.08 to 0.02 | 0.180 |
| Age | −0.02 | −0.06 to 0.02 | 0.279 | −0.00 | −0.03 to 0.02 | 0.811 | −0.00 | −0.02 to 0.02 | 0.734 |
| Aβ status (Aβ+) | 0.27 | −0.10 to 0.63 | 0.138 | 0.33 | 0.11–0.56 | 0.005** | 0.28 | 0.11–0.46 | 0.003*** |
| Education | 0.03 | −0.04 to 0.11 | 0.364 | 0.01 | −0.04 to 0.05 | 0.809 | 0.00 | −0.04 to 0.04 | 0.952 |
| Male APOE ɛ4 carriers | |||||||||
| FHS-CVD risk | 0.00 | −0.01 to 0.02 | 0.675 | −0.00 | −0.01 to 0.00 | 0.434 | −0.00 | −0.01 to 0.00 | 0.568 |
| Time lag | 0.11 | −0.08 to 0.30 | 0.227 | 0.04 | −0.06 to 0.15 | 0.409 | 0.04 | −0.05 to 0.13 | 0.389 |
| Age | −0.00 | −0.03 to 0.03 | 0.964 | 0.00 | −0.01 to 0.02 | 0.797 | 0.00 | −0.01 to 0.02 | 0.625 |
| Aβ status (Aβ+) | 0.07 | −0.30 to 0.43 | 0.703 | 0.10 | −0.11 to 0.30 | 0.340 | 0.08 | −0.10 to 0.26 | 0.357 |
| Education | 0.00 | −0.06 to 0.06 | 0.893 | −0.03 | −0.06 to 0.00 | 0.067 | −0.02 | −0.05 to 0.01 | 0.143 |
Results from linear regression analysis on sex-stratified groups of APOE ɛ4 carriers revealed a significant main effect of FHS-CVD risk on tau deposition in the EC and meta-ROI only among female APOE ɛ4 carriers, after adjusting for the time between FHS-CVD risk assessment and tau-PET scan, age, Aβ status and education. Significant P-values are presented in bold, where *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001. Aβ = amyloid-β; CI = confidence interval; EC = entorhinal cortex; FHS-CVD risk = Framingham Heart Study cardiovascular disease risk; ITC = inferior temporal cortex; SUVR = standardized uptake value ratio.
Discussion
In this study of CN older adults, we found that sex and APOE ɛ4 status modified the association between cardiovascular risk and tau deposition. After stratifying our sample by APOE ɛ4 status, we found that sex modified the association between cardiovascular risk and tau burden, among APOE ɛ4 carriers only. Finally, sex-stratified analyses showed that higher cardiovascular risk was associated with higher levels of EC, ITC and meta-ROI tau among female APOE ɛ4 carriers but not male carriers. Adjusting for additional covariates such as age, Aβ status and education attenuated interaction effects of sex, APOE ɛ4 and cardiovascular risk on tau deposition in the ITC and meta-ROI, but not the EC. The absence of a three-way interaction effect in the ITC and meta-ROI appears to be due to a more robust main effect of Aβ status on tau deposition in these regions among both APOE ɛ4 carriers and non-carriers. There was no such effect on tau deposition in the EC, which has been shown to be associated with APOE ɛ4, independently of Aβ burden.49 Furthermore, a recent study on CN older adults found that the interaction between higher cardiovascular risk and elevated Aβ burden was associated with higher levels of tau deposition in the ITC but not the EC.50 Despite the strong main effect of Aβ status on ITC and meta-ROI tau among APOE ɛ4 carriers and non-carriers alike, two-way interactions between female sex and cardiovascular risk on tau deposition were seen only among APOE ɛ4 carriers. Therefore, studies on larger samples should examine sex differences in the association between cardiovascular risk and tau deposition among groups stratified by both APOE ɛ4 and Aβ status.
Our findings are in line with other studies demonstrating stronger predictive relationships between vascular risk factors such as elevated plasma lipids and hypertension and incident Alzheimer’s disease in women compared with men,1,51 and are consistent with our prior study demonstrating that among community-dwelling older adults, higher FHS-CVD risk scores were associated with greater executive function and memory decline among women but not men, despite higher risk scores among men.29 Tau pathology is strongly correlated with clinical symptomatology,32 and recent evidence suggests that associations between cerebrovascular function and cognition are mediated by tau pathology.12,13 Therefore, the exacerbated effect of cardiovascular disease risk on cognition and clinical outcomes observed among women may be, at least partly, due to elevated tau deposition.
While our study was not designed to probe mechanisms of this effect, there are potential explanations, including the impact of hormonal changes in post-menopausal women: The abrupt decrease of oestrogen levels in post-menopausal women is associated with reduced glucose tolerance, as well as increased blood pressure, endothelial dysfunction and vascular inflammation.52 Oestrogen-binding on endothelial and vascular smooth muscle cells has been shown to prevent neointimal responses to acute vascular injury, resulting from atherosclerosis, hypertension and other vascular diseases.53,54 Therefore, the absence of the aforementioned vaso- and neuroprotective effects of oestrogen in post-menopausal women may contribute to a heightened inflammatory response to vascular risks, potentially leading to worse brain outcomes in women. Evidence of a more direct link between oestrogen and tau comes from animal studies showing that oestrogen depletion can lead to an increase in enzymatic activity (including protein kinase A and glycogen synthase kinase 3-β) involved in tau hyperphosphorylation.55
We found that sex differences in the association between cardiovascular risk and tau deposition were restricted to APOE ɛ4 carriers. This finding is consistent with a recent study showing that higher vascular risk and APOE ɛ4 interactively predicted memory decline in women but not men.22 Our results extend prior evidence of a stronger APOE ɛ4 effect on tau pathology in women,24–27 and introduce the role of cardiovascular disease risk as a potential factor contributing to this female APOE ɛ4-related susceptibility to tauopathy. Notably, APOE ɛ4 confers greater risk of developing MCI in women specifically between 55 and 70 years of age, compared with men.56 In this study, we found an effect of cardiovascular disease risk on tau deposition only among female APOE ɛ4 carriers, who were between 55 and 78 years of age. It is conceivable that the first two decades following menopause could be a critical time-window during which elevated cardiovascular vulnerability may predispose female APOE ɛ4 carriers to worse pathological and clinical outcomes.
Although the mechanisms of the interaction between APOE ɛ4 and vascular risk and its exacerbated impact on brain health among women remains unknown, the influence of sex hormones may be relevant. Oestrogen has been shown to inhibit microglial activation in response to acute inflammatory stimuli, thereby reducing the production of reactive oxygen species and proinflammatory molecules such as matrix metalloproteinase 9.57 However, the presence of an APOE ɛ4 allele inhibits the anti-inflammatory effects of oestrogen on microglial and peripheral microphage activation,58 suggesting that female APOE ɛ4 carriers are less able to benefit from the anti-inflammatory properties of circulating oestrogen, compared with non-carriers. Conversely, testosterone, which has similar anti-inflammatory properties, has also been shown to interact with APOE ɛ4 to impact cognitive function and Alzheimer’s disease risk.59–61 Animal studies showed that APOE ɛ4 can reduce androgen receptor levels in the brain, suggesting that APOE ɛ4 carriers may be particularly vulnerable to the effects of lower testosterone.61 Sundermann et al.62 found that the association between lower testosterone and higher levels of cerebrospinal fluid phosphorylated tau were strongest among female APOE ɛ4 carriers. Consequently, the heightened vascular inflammation seen in post-menopausal women along with the heightened cerebrovascular impact of APOE ɛ4 in the absence of the neuroprotective effects of sex hormones may interact to exacerbate the effects of cardiovascular risk factors on Alzheimer’s disease tauopathy in female APOE ɛ4 carriers. Future experimental research is needed to probe this possible explanation for our findings.
Limitations
The comparatively higher mortality rates at younger ages due to cardiovascular disease in men may have resulted in a survival bias in our study. This survival bias along with women’s resilience against cardiovascular disease up until mid-life and heightened vascular vulnerability after menopause may lead to greater vascular and Alzheimer’s disease comorbidity among women. Given that participants with a Hachinski ischaemic score >4 are excluded from the ADNI study, our sample was composed of participants in relatively good cardiovascular health. Larger studies in samples that are more representative of cardiovascular health in the aging population are needed to investigate potential sex-disparities further. ADNI also does not provide details on women’s health such as age at menopause and exposure to female-specific cardiovascular risk factors such as pre-eclampsia, which could be critical to the interpretation of our findings. Furthermore, since our sample was predominantly composed of non-Hispanic white men and women, our results may not generalize to other racial and ethnic groups. Studies have shown that risk factors and cardiovascular disease manifestations can differ significantly between racial groups,63 highlighting the importance of studying racially and ethnically diverse samples. Another limitation of our study was its small sample size, which may have particularly affected the performance of tau deposition models in male and female APOE ɛ4 carriers (n = 23 and n = 24, respectively). Nevertheless, given the similar numbers of APOE ɛ4 carriers among men and women in the study, it is probably unlikely that the effect seen in women was driven by greater statistical power. Finally, the cross-sectional nature of the study along with the time lag between cardiovascular risk and tau-PET assessments limits our ability to draw conclusions about the temporality of associations between cardiovascular disease and tau deposition. Therefore, future longitudinal studies are needed to examine the extent to which sex and APOE ɛ4 impact the association of cardiovascular risk and accumulation of tau over the course of Alzheimer’s disease.
Conclusion
Our results suggest that female APOE ɛ4 carriers may be particularly vulnerable to the impact of cardiovascular risk on cortical tau deposition, despite having lower cardiovascular risk scores than men. Our findings are clinically relevant, given the modifiable nature of many cardiovascular risk factors such as smoking and high blood pressure, and may contribute to our understanding of the observed heightened susceptibility to tauopathy among female APOE ɛ4 carriers. If replicated in larger, population-based samples, these findings could have important implications for the treatment of even low levels of vascular risk in seemingly healthy CN older women who have a genetic risk for Alzheimer’s disease. Future studies exploring associations between cardiovascular risk and Alzheimer’s disease -related outcomes should consider stratifying by sex and APOE ɛ4.
Abbreviations
- ADNI =
Alzheimer’s Disease Neuroimaging Initiative
- Aβ =
amyloid-β
- CDR =
Clinical Dementia Rating
- CDR-GS =
Clinical Dementia Rating global score
- CN =
cognitively normal
- EC =
entorhinal cortex
- FHS-CVD risk =
Framingham Heart Study cardiovascular disease risk
- FTP =
Flortaucipir
- ITC =
inferior temporal cortex
- ROI =
region of interest
- SUVR =
standardized uptake value ratio
Funding
This work was supported by the National Institutes of Health (grant numbers 1R01AG066088-01, 1P30AG062429-01). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Competing interests
The authors report no competing interests.
References
- 1. Lee H, Kim K, Lee YC, et al. Associations between vascular risk factors and subsequent Alzheimer’s disease in older adults. Alzheimers Res Ther. 2020;12:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. White LR, Edland SD, Hemmy LS, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia aging studies. Neurology. 2016;17:1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012:367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He J-T, Zhao X, Xu L, Mao C-Y. Vascular risk factors and Alzheimer’s disease: Blood-brain barrier disruption. Metabolic syndromes, and molecular links. J Alzheimers Dis. 2020;73(1):39–58. [DOI] [PubMed] [Google Scholar]
- 5. Qiu L, Ng G, Tan EK, Liao P, Kandiah N, Zeng L. Chronic cerebral hypoperfusion enhances tau hyperphosphorylation and reduces autophagy in Alzheimer’s disease mice. Sci Rep. 2016;6:23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bos I, Vos SJB, Schindler SE, et al. Vascular risk factors are associated with longitudinal changes in cerebrospinal fluid tau markers and cognition in preclinical Alzheimer’s disease. Alzheimers Dement. 2019;15(9):1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arvanitakis Z, Capuano AW, Lamar M, et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology. 2018;91(6):e517–e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nation DA, Edmonds EC, Bangen KJ, et al. Pulse pressure in relation to tau-mediated neurodegeneration, cerebral amyloidosis, and progression to dementia in very old adults. JAMA Neurol. 2015;72(5):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hesse C, Rosengren L, Vanmechelen E, et al. Cerebrospinal fluid markers for Alzheimer’s disease evaluated after acute ischemic stroke. J Alzheimers Dis. 2000;2(3-4):199–206. [DOI] [PubMed] [Google Scholar]
- 10. Köbe T, Gonneaud J, Binette A. Association of vascular risk factors with β-amyloid peptide and tau burdens in cognitively unimpaired individuals and its interaction with vascular medication use. JAMA Netw Open. 2020;3:e1920780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: The HAAS. Neurobiol Aging. 2000;21(1):57–62. [DOI] [PubMed] [Google Scholar]
- 12. Kim HJ, Park S, Cho H, et al. Assessment of extent and role of tau in subcortical vascular cognitive impairment using 18F-AV1451 positron emission tomography imaging. JAMA Neurol. 2018;75(8):999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Albrecht D, Isenberg AL, Stradford J, et al. Associations between vascular function and tau PET are associated with global cognition and amyloid. J Neurosci. 2020;40(44):8573–8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helzner EP, Luchsinger JA, Scarmeas N, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66(3):343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mielke MM, Leoutsakos J-M, Tschanz JT, et al. Interaction between vascular factors and the APOE E4 allele in predicting rate of progression in Alzheimer’s dementia. J Alzheimers Dis. 2011;26(1):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falsetti L, Viticchi G, Buratti L, Grigioni F, Capucci A, Silvestrini M. Interactions between atrial fibrillation. Cardiovascular risk factors, and apoE genotype in promoting cognitive decline in patients with Alzheimer’s disease: A prospective cohort study. J Alzheimers Dis. 2018;62(2):713–725. [DOI] [PubMed] [Google Scholar]
- 17. Uddin MS, Kabir MT, Al Mamun A, Abdel-Daim MM, Barreto GE, Ashraf GM. APOE and Alzheimer’s disease: Evidence mounts that targeting APOE4 may combat Alzheimer’s pathogenesis. Mol Neurobiol. 2019;56(4):2450–2465. [DOI] [PubMed] [Google Scholar]
- 18. Zlokovic BV. Cerebrovascular effects of apolipoprotein E: Implications for Alzheimer disease. JAMA Neurol. 2013;70(4):440–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baek MS, Cho H, Lee HS, Lee JH, Ryu YH, Lyoo CH. Effect of APOE ɛ4 genotype on amyloid-β and tau accumulation in Alzheimer’s disease. Alzheimers Res Ther. 2020;12(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahley RW. Apolipoprotein E: From cardiovascular disease to neurodegenerative disorders. J Mol Med. 2016;94(7):739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Makkar SR, Lipnicki DM, Crawford JD, et al. APOE ɛ4 and the influence of sex, age, vascular risk factors, and ethnicity on cognitive decline. J Gerontol A Biol Sci Med Sci. 2020;75(10):1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer’s disease. Ann Neurol. 2014;75(4):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mofrad RB, Tijms BM, Scheltens P, et al. Sex differences in CSF biomarkers vary by Alzheimer disease stage and APOE ɛ4 genotype. Neurology. 2020;95(17):e2378–e2388. [DOI] [PubMed] [Google Scholar]
- 25. Hohman TJ, Dumitrescu L, Barnes LL, et al. Sex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75(8):989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yan S, Zheng C, Paranjpe MD, et al. Association of sex and APOE ɛ4 with brain tau deposition and atrophy in older adults with Alzheimer’s disease. Theranostics. 2020;10(23):10563–10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu M, Paranjpe MD, Zhou X, et al. Sex modulates the ApoE ɛ4 effect on brain tau deposition measured by 18F-AV-1451 PET in individuals with mild cognitive impairment. Theranostics. 2019;9(17):4959–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reas ET, Laughlin GA, Hagler DJ, Lee RR, Dale AM, McEvoy LK. Age and sex differences in the associations of pulse pressure with white matter and subcortical microstructure. Hypertension. 2021;77(3):938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laughlin GA, McEvoy LK, von Mühlen D, et al. Sex differences in the association of Framingham cardiac risk score with cognitive decline in community-dwelling elders without clinical heart disease. Psychosom Med. 2011;73(8):683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seux ML, Thijs L, Forette F, et al. Correlates of cognitive status of old patients with isolated systolic hypertension: The Syst-Eur vascular dementia project. J Hypertens. 1998;16(7):963–969. [DOI] [PubMed] [Google Scholar]
- 31. Groot C, Doré V, Robertson J, et al. Mesial temporal tau is related to worse cognitive performance and greater neocortical tau load in amyloid-β-negative cognitively normal individuals. Neurobiol Aging. 2021;97:41–48. [DOI] [PubMed] [Google Scholar]
- 32. Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(5):1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hachinski V. Optimizing the hachinski ischemic scale. Arch Neurol. 2012;69(2):169. [DOI] [PubMed] [Google Scholar]
- 34. Morris JC. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–2414. [DOI] [PubMed] [Google Scholar]
- 35. Sundermann EE, Biegon A, Rubin LH, et al. Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology. 2016;86(15):1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sundermann EE, Biegon A, Rubin LH, et al. Does the female advantage in verbal memory contribute to underestimating AD pathology in women versus men? J Alzheimers Dis. 2017;56(3):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sundermann EE, Maki PM, Rubin LH, Lipton RB, Landau S, Biegon A. Female advantage in verbal memory. Neurology. 2016;87(18):1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sundermann EE, Maki P, Biegon A, et al. Sex-specific norms for verbal memory tests may improve diagnostic accuracy of amnestic MCI. Neurology. 2019;93(20):e1881–e1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117(6):743–753. [DOI] [PubMed] [Google Scholar]
- 40. Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: A special report from the American heart association and American college of cardiology. J Am Coll Cardiol. 2019;73(24):3153–3167. [DOI] [PubMed] [Google Scholar]
- 41. Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Landau SM, Mintun MA, Joshi AD, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72(4):578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Landau S, Jagust W. Florbetapir processing methods. Alzheimerrs. Disease Neuroimaging Initiative; 2015. [Google Scholar]
- 44. Baker SL, Maass A, Jagust WJ. Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Brief. 2017;15:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roussel OG, Ma Y, Evans AC. Correction for partial volume effects in PET: Principle and validation. J Nucl Med. 1998;39:904–911. [PubMed] [Google Scholar]
- 46. Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dani M, Brooks DJ, Edison P. Tau imaging in neurodegenerative diseases. Eur J Nucl Med Mol Imaging. 2016;43(6):1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 49. Therriault J, Benedet AL, Pascoal TA, et al. Association of apolipoprotein E ɛ4 with medial temporal tau independent of amyloid-β. JAMA Neurol. 2020;77(4):470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rabin JS, Yang H-S, Schultz AP, et al. Vascular risk and β-amyloid are synergistically associated with cortical tau. Ann Neurol. 2019;85(2):272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gilsanz P, Mayeda ER, Glymour MM, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017;89(18):1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rosano GMC, Vitale C, Marazzi G, Volterrani M. Menopause and cardiovascular disease: The evidence. Climacteric. 2007;10(Suppl 1):19–24. [DOI] [PubMed] [Google Scholar]
- 53. Brooks HL, Pollow DP, Hoyer PB. The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology. 2016;31(4):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oparil S. Hormones and vasoprotection. Hypertension. 1999;33(1):170–176. [DOI] [PubMed] [Google Scholar]
- 55. Grimm A, Mensah-Nyagan AG, Eckert A. Alzheimer, mitochondria and gender. Neurosci Biobehav Rev. 2016;67:89–101. [DOI] [PubMed] [Google Scholar]
- 56. Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol. 2017;74(10):1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Villa A, Vegeto E, Poletti A, Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev. 2016;37(4):372–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brown CM, Choi E, Xu Q, Vitek MP, Colton CA. The APOE4 genotype alters the response of microglia and macrophages to 17β-estradiol. Neurobiol Aging. 2008;29(12):1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hogervorst E, Lehmann DJ, Warden DR, McBroom J, Smith AD. Apolipoprotein E ɛ4 and testosterone interact in the risk of Alzheimer’s disease in men. Int J Geriatr Psychiatry. 2002;17:938–940. [DOI] [PubMed] [Google Scholar]
- 60. Pfankuch T, Rizk A, Olsen R, Poage C, Raber J. Role of circulating androgen levels in effects of apoE4 on cognitive function. Brain Res. 2005;1053(1-2):88–96. [DOI] [PubMed] [Google Scholar]
- 61. Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22(12):5204–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sundermann EE, Panizzon MS, Chen X, et al. Sex differences in Alzheimer’s-related tau biomarkers and a mediating effect of testosterone. Biol Sex Differ. 2020;11(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hackler E, Lew J, Gore MO, et al. Racial differences in cardiovascular biomarkers in the general population. J Am Heart Assoc. 2019;8(18):e012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in preparation of this manuscript were obtained from the LONI database in April 2021 (ida.loni.usc.edu).