Abstract
OBJECTIVE
To improve visualization of upper tract urothelial carcinomas. Previous studies using the novel pH low insertion peptide (pHLIP) variant 3 (Var3) conjugated to indocyanine green (ICG) have demonstrated high sensitivity and specificity for imaging of bladder urothelial carcinoma. Here, we describe a novel approach for the imaging of upper tract urothelial carcinomas using ICG-Var3 pHLIP.
METHODS
Twelve ex-vivo upper urinary tract specimens were irrigated with ICG-Var 3 pHLIP for 15 minutes and then examined using a white light laparoscopic camera followed by near infrared fluorescent (NIRF) imaging using a Stryker 1588 AIM imaging system. Standard histopathologic evaluation was performed and findings were correlated with white light and ICG-Var3 NIRF imaging. One patient who underwent radical nephrectomy for renal cell carcinoma was used as a negative control.
RESULTS
Nineteen lesions were identified on histopathologic evaluation in 10 patients, including 82% high-grade urothelial carcinoma and 18% low-grade urothelial carcinoma. Nineteen (100%) malignant lesions were identified using NIRF imaging, while 15 (78.9%) lesions were identified using conventional white light examination. The sensitivity of ICG-Var3 pHLIP NIRF imaging was 100% compared to 78.9% white light examination. Both modalities are 100% specific. Benign collecting systems and ureters did not show uptake of the pHLIP construct.
CONCLUSION
In this feasibility study, the ICG-Var3 pHLIP imaging agent demonstrated superior diagnostic performance compared to conventional white light examination. While additional studies are required for validation and in-vivo translation, pHLIP-based imaging represents a promising tool to improve the evaluation and management of upper tract urothelial carcinoma. UROLOGY 139: 134–140, 2020.
Upper tract urothelial cell carcinoma (UTUC) accounts for 5%–10% of all urothelial neoplasms and has increased in incidence over the last 4 decades.1,2 Although, enhanced cystoscopic techniques have become increasingly adopted for the endoscopic diagnosis and management of lower tract urothelial carcinoma,3–5 there are limited data for the use of such approaches in upper tract urothelial carcinoma.6–11
The pH low insertion peptides (pHLIP) belong to a family of water soluble membrane peptides that have been shown to target the acidic microenvironment of malignant cells based on the Warburg effect.12 pHLIPs target low pH at the surface of cancer cells, where it is the lowest and independent of tumor perfusion, thus providing high specificity and sensitivity in tumor targeting.13 In preclinical trials, pHLIP has been used for imaging and pH specific drug delivery.14–21 Among investigated pHLIPs variant 3 (Var3) demonstrated the best tumor targeting.14,19,20 Currently 18F-Var3 pHLIP is underway to clinical translation for imaging of acidity.20 In a previous study using ICG-Var3 pHLIP, we demonstrated targeting of bladder urothelial carcinoma with high sensitivity (97%) and specificity (100%).21 Here, we report on a feasibility study for the use of ICG-Var3 pHLIP-based imaging of upper tract urothelial carcinoma in ex-vivo specimens.
MATERIALS AND METHODS
Study Cohort
Following Institutional Review Board approval, 12 consecutive patients undergoing surgical treatment of upper urinary tract malignancy (N = 11 UTUC; N = 1 RCC) at the Miriam Hospital (Providence, RI) were prospectively enrolled and consented for participation in this study. All surgeries were performed by a single urologic oncologist (D.G.).
Specimen Processing and Imaging Evaluation
ICG-Var3 was prepared as described previously.15 For each case, 100 mL of solution containing 0.40 μmol of ICG-Var3 in Phosphate Buffered Saline (PBS), pH 7.4, supplemented with 10 mM D-glucose was freshly prepared. After excision of the upper urinary tract specimen, an open-ended ureteral catheter was inserted into the ureter. Samples were washed with 100 mL of normal saline for 15 minutes. Upper tracts were then slowly irrigated with the freshly prepared 100 mL of ICG-Var3 pHLIP solution for 15 minutes, followed by another wash with 100 mL of normal saline. The washed upper tract specimens were incised longitudinally. Ex-vivo evaluation of the upper urinary tracts was performed using Stryker 1588 AIM imaging system using 10 mm and 5 mm laparoscopes under white light followed by near infrared fluorescent (NIRF) imaging by a single investigator trained in NIRF imaging (B.G.). White light examination preceded NIRF imaging in every case. In 2 cases the DaVinci Si Surgical System Firefly (Intuitive Surgical, Sunnyvale, CA) was used. Areas labeled by ICG-Var3 pHLIP were marked by ink and entirely submitted for standard histopathologic evaluation by a single genitourinary pathologist (A.A.).
Histopathologic Evaluation
Following imaging, specimens were sectioned and submitted after 24-hour fixation in 10% phosphate-buffered formalin according to the standard institutional grossing manual. Samples were processed for routine histology into paraffin embedded blocks. Five micrometer thick tissue sections were obtained and stained for Hematoxylin & Eosin (H&E). Evaluation of pathology was performed by a genitourinary pathologist and a standard report was prepared based on the American Joint Committee on Cancer Staging Manual, seventh edition, 2010. Correlation between presence of ICG-Var3 NIRF stained lesions, standard gross white light evaluation, and routine histopathologic evaluation was assessed.
Statistical Analysis
Clinicopathologic features were summarized in frequency tables. Fisher’s Exact chi-square analysis was used to correlate the difference between white light and NIRF to presence of malignancy. Statistical analyses were performed using IBM SPSS Statistics for Windows Version 20 (IBM (IBM Corp, Armonk, NY), with P values <.05 considered statistically significant.
RESULTS
Twelve patients were included in the study, of whom 11 underwent radical nephroureterectomy (N = 10) or distal ureterectomy (N = 1) for UTUC, while 1 control patient underwent radical nephrectomy for Renal Cell Carcinoma (RCC) (Table 1, Supplementary Table 1a). A total of 19 urothelial carcinoma lesions were identified by standard histopathologic evaluation, including 8 (42.1%) invasive UTUC and 11 (57.9%) noninvasive UTUC (Table 1, Supplementary Table 1b). Fifteen (78.9%) lesions were high-grade and 4 (21.1%) were low-grade.
Table 1.
Demographic and pathology of lesions in 10 cases examined by white light and ICG-Var3 pHLIP NIRF imaging
| Case | Sex/Age (y) | Pathologic Stage | Pathologic Diagnosis | Grade | Location | Depth | Size | Lesion Number | White Light Diagnosis | NIRF Imaging |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/67 | pT1NxMx | Invasive high-grade papillary urothelial carcinoma | HGI | Proximal ureter | Lamina propria | 4.2 cm | 1 | + | + |
| 2 | M/68 | pT2NxMx | Invasive high-grade papillary urothelial carcinoma | HGI | ||||||
| Ureteropelvic junction | Muscularis propria | 3.0 cm | 2 | + | + | |||||
| 3 | F/75 | mpTa | Noninvasive high-grade papillary urothelial carcinoma | HGN | Distal ureter and bladder cuff | − | 1.0 cm | 3 | + | + |
| Noninvasive high-grade papillary urothelial carcinoma | HGN | Bladder cuff | − | 0.9 cm | 4 | + | + | |||
| Noninvasive high-grade papillary urothelial carcinoma | HGN | Bladder cuff | − | 0.3 cm | 5 | − | + | |||
| 4 | F/75 | pT3pN1pMx | High-grade urothelial carcinoma and small cell carcinoma | HGI | Mid ureter | Periureteric adipose tissue | 2.0 cm | 6 | + | + |
| High-grade urothelial carcinoma and small cell carcinoma | HGI | Mid ureter | Periureteric adipose tissue | 1.0 cm | 7 | + | + | |||
| 5 | F/87 | pT3NxMx | Invasive high-grade papillary urothelial carcinoma | HGI | Renal pelvis | Renal cortex | 4.5 cm | 8 | + | + |
| Invasive high-grade papillary urothelial carcinoma | HGI | Calyces | Sinus fat | 0.9 cm | 9 | + | + | |||
| 6 | M/71 | n/a | Ureteritis cystica, no tumor | n/a | − | − | − | 10 | − | − |
| Ureteritis cystica, no tumor | n/a | − | − | − | 11 | − | − | |||
| Ureteritis cystica, no tumor | n/a | − | − | − | 12 | − | − | |||
| Ureteritis cystica, no tumor | n/a | − | − | − | 13 | − | − | |||
| Ureteritis cystica, no tumor | n/a | − | − | − | 14 | − | − | |||
| Ureteritis cystica, no tumor | n/a | − | − | − | 15 | − | − | |||
| 7 | F/61 | pT3N0Mx | Invasive high-grade papillary urothelial carcinoma | HGI | Distal ureter | Periureteric adipose tissue | 1.9 cm | 16 | + | + |
| 8 | M/46 | mpTaNxMx | Noninvasive low-grade papillary urothelial carcinoma | LGN | Distal ureter | − | 0.9 cm | 17 | + | + |
| Noninvasive low-grade papillary urothelial carcinoma | LGN | Distal ureter | − | 0.4 cm | 18 | + | + | |||
| Noninvasive low-grade papillary urothelial carcinoma | LGN | Distal ureter | − | <0.1 cm | 19 | − | + | |||
| 9 | M/82 | pTaN0Mx | Noninvasive high-grade papillary urothelial carcinoma | HGN | Mid ureter | − | 4.9 cm | 20 | + | + |
| Noninvasive high-grade papillary urothelial carcinoma | HGN | Distal ureter | − | <0.1 cm | 21 | − | + | |||
| Noninvasive high-grade papillary urothelial carcinoma | HGN | Distal ureter | − | <0.1 cm | 22 | − | + | |||
| 10 | M/58 | pT1N0Mx | Invasive high-grade papillary urothelial carcinoma with squamous differentiation | HGI | Distal ureter | Lamina propria | 5.2 cm | 23 | + | + |
| 11 | M/81 | pTaNxMx | Noninvasive low-grade papillary urothelial carcinoma | LGN | Mid ureter | − | 2.7 cm | 24 | + | + |
| 12 | M/86 | pTaNxMx | Noninvasive high-grade papillary urothelial carcinoma | HGN | ||||||
| Ureteropelvic junction | − | 4.7 cm | 25 | + | + |
HGI, high-grade invasive; HGN, high-grade noninvasive; n/a, not applicable tumor grade; LGN, low-grade noninvasive.
Mean patient age is 69.
ICG-Var3 NIRF ex-vivo imaging identified 19 lesions, while conventional white light examination identified 15 lesions (Table 2a and 2b).
Table 2a.
Operator characteristics for determination of sensitivity and specificity of ICG-Var 3 pHLIP
| Histopathologic Diagnosis | |||
|---|---|---|---|
| Cancer | No Cancer | ||
| NIRF ICG-Var3 pHLIP | Signal | 19 | 0 |
| No signal | 0 | 6 | |
Table 2b.
Operator characteristics for determination of sensitivity and specificity of white light examination
| Histopathologic Diagnosis | |||
|---|---|---|---|
| Cancer | No Cancer | ||
| White light | Seen | 15 | 0 |
| Not seen | 4 | 6 | |
Figure 1 demonstrates representative images of ICG-Var3 pHLIP targeting of high-grade invasive (Fig. 1A–C), high-grade noninvasive (Fig. 1D–F), and low-grade noninvasive (Fig. 1G–I) lesions. Three lesions were low-grade noninvasive UTUC. Of the 9 invasive high-grade lesions, 2 were presented with features of small cell carcinoma, and 1 with squamous differentiation.
Figure 1.
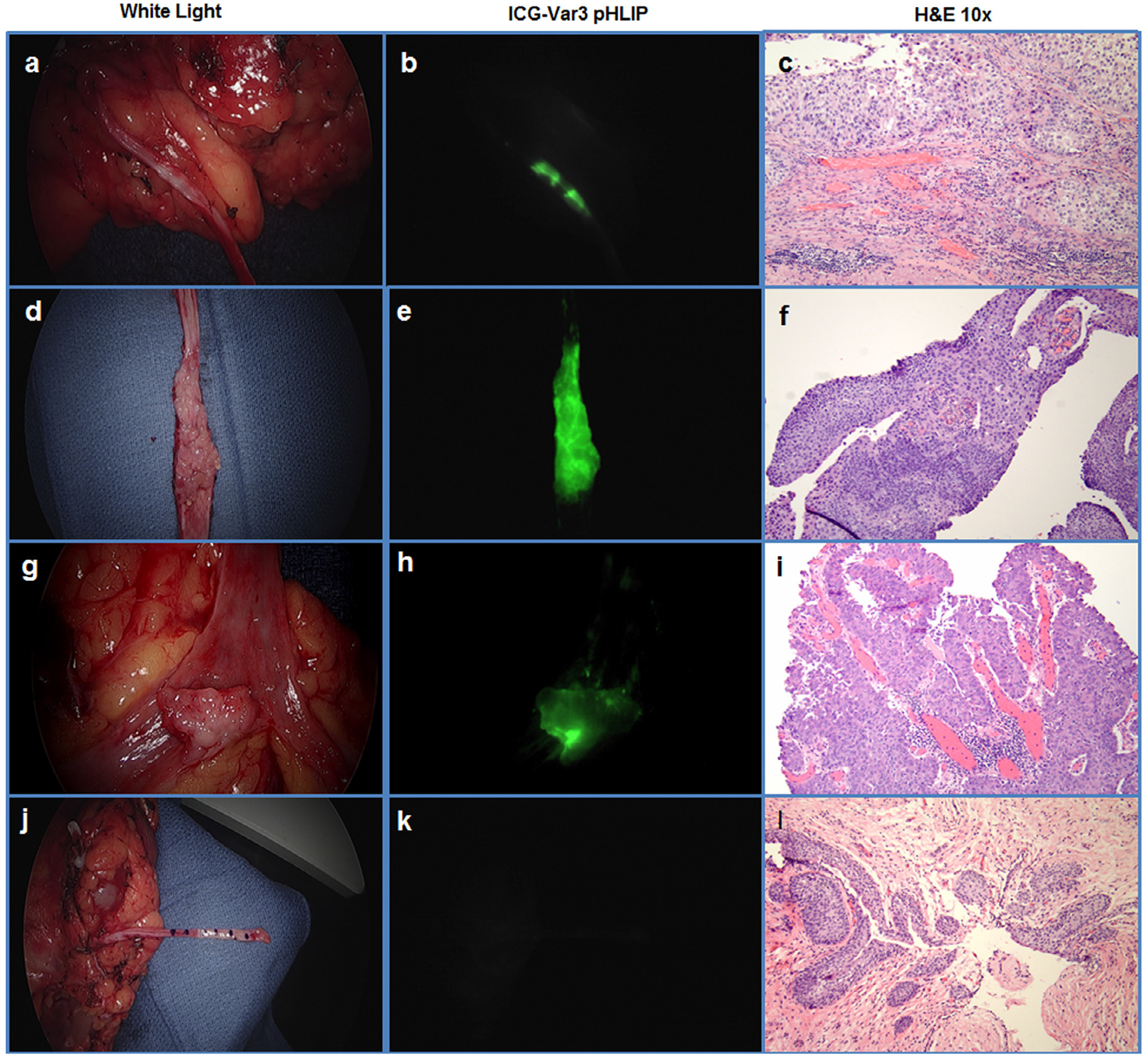
Ex vivo human upper tract urothelial carcinoma. (A-C) Case #5, high-grade UTUC, pT3NxMx; (D-F) case #9, high-grade noninvasive papillary UTUC, pTaN0Mx; (G-I) case # 8, noninvasive low-grade UTUC, pTaNxMx; (J-L) case #6, control––no tumor seen in ureteritis cystica. White light (A, D, G, J), ICG-Var3 NIRF (B, E, H, K), and (H) and (E) (C, F, I, L) images are presented.
Compared to white light examination, NIRF pHLIP-based imaging demonstrated superior sensitivity in visualizing UTUC (100% vs 78.9%; P = .001) and equal specificity (100% vs 100%) (Table 3).
Table 3.
Results of sensitivity and specificity tests for 2 visualization methods
| Measure | NIRF of ICG-Var3 pHLIP | White Light |
|---|---|---|
| Sensitivity | 1.000 | 78.947 |
| Specificity | 1.000 | 1.000 |
| Positive predictive value | 1.000 | 1.000 |
| Negative predictive value | 1.000 | 0.600 |
Without the aid of ICG-Var3 pHLIP (white light assessment only), only 15 lesions were grossly identified (78.9%). Of the lesions missed by white light assessment, 3 were high-grade noninvasive papillary UCC, and the fourth missed lesion was low-grade noninvasive UCC
In all cases, routine histopathologic evaluation demonstrated that benign urothelium, ureter, and the renal pelvis did not show any uptake of the pHLIP construct. Nonmalignant lesions like ureteritis cystica (6 locations) seen in the control patient were negative for ICG-Var3 pHLIP staining (Fig. 1J–L).
DISCUSSION
In this feasibility study, the ICG-Var3 pHLIP NIRF imaging agent demonstrated excellent performance for the diagnosis of upper tract urothelial carcinoma, with 100% specificity and sensitivity. It improved the diagnosis of UTUC by 21.2% compared to conventional white light examination, identifying lesions not seen using white light gross examination due to small size. Normal mucosa was not stained by ICG-Var3 pHLIP, nor were nonmalignant lesions such as ureteritis cystica.
Cross-sectional imaging and traditional white-light endoscopic visual have historically demonstrated poor performance for the diagnosis and staging of UTUC.22–24 Recognizing the need for better and more accurate diagnostic methods, new technologies have been proposed. Maruschke et al detailed various imaging techniques used for UTUC identification in a retrospective cohort of 113 patients, citing sensitivities of 87.7% for retrograde ureteropylography, 83.1% for CT scan, 57.4% for intravenous urograms, and 50% for MRI.25 Although prior studies have reported on the performance of diagnostic imaging modalities such as computed tomography, intravenous urograms, and magnetic resonance imaging, none of these imaging modalities are a substitute for the direct visualization of tumors that is required to perform endoscopic biopsy.
Novel and advanced ureteroscopic techniques are extensively reported in a review by Baard et al in which benefits of narrow band imaging (NBI), Image 1S, and photodynamic diagnosis (PDD) for augmenting ureteroscopy (URS), especially as aids in diagnosing carcinoma in Situ and sessile lesions that may otherwise be difficult to identify.7 Advances in augmented and enhanced URS are further reviewed by Knoedler and Raman, describing the few reported cases where NBI was used with great success, the high specificity of PDD and difficulties associated with administration of the necessary fluorochrome, and the emerging confocal imaging microscopy of UTUC.26 Nonetheless, novel imaging modalities for the diagnosis of UTUC require more study as pitfalls have been documented. NBI provides subjective improvements in the visualization, with few noticeable improvements in the image compared to white light URS27; the fluorochrome associated with PDD can have adverse effects in up to 25.8% of patients10; and confocal imaging microscopy has been reported with unreliable relationship to tumor grade and stage.28 Utility of these advanced imaging modalities in diagnosis and management of UTUC is unmistakable, as white light URS has been reported as falsely omitting up to 50% of lesions in patients undergoing URS for UTUC and up to 15% of high-grade lesions are misdiagnosed as low-grade.29,30
This is the first study to report the feasibility of enhanced visual diagnosis of upper tract urothelial carcinoma using a novel and specific targeting agent, as has become accepted for lower tract urothelial carcinoma.21 The pHLIP peptide is specific to cellular microenvironments with higher acidity, with high rates of glucose metabolism, and can detect 0.2–0.3 pH unit changes in vivo.13,14 Additionally, the ICG-Var3 pHLIP signal is easier to make out than that in NBI and PDD. In this regard, the pHLIP construct holds great promise to improve the evaluation and management of UTUC.
Since histopathologic diagnosis of UTUC is based on endoscopic identification of tumors, it follows that improved diagnostic tools will enhance current techniques. To this end, NIRF pHLIP-based imaging may facilitate endoscopic identification of target lesion or biopsy and treatment. pHLIP based targeting may have other applications in the management of UTUC-including enhancing the performance of urine cytologic evaluation, targeting lesions for endoscopic ablation, or allowing novel drug delivery mechanisms of pHLIP to be implemented in targeted treatments of urothelial carcinomas. However, these applications require further research.
This study has a number of limitations. First and fore-most, it is a small, ex-vivo feasibility study; as such, results will need to be validated in larger cohorts, and more importantly, translated into in-vivo applications. This will require validation of NIRF-based imaging within ureteroscopic approaches to determine whether smaller scope diameter, tangential lesion viewing, and in-vivo irrigation limit lesion identification. In addition, the ability of pHLIP-based imaging to enhance staging and differentiation of low-grade vs high-grade lesions is a critical question of clinical importance. Finally, we compared NIRF imaging to conventional white light evaluation; although white light ureteroscopic examination remains the standard of care in the diagnosis of UTUC, the recent adoption of enhanced cystoscopic techniques, such as PDD and NBI, will require comparison with pHLIP if they are adapted for upper tract use. Despite these limitations, pHLIP-based NIRF imaging demonstrated very encouraging results for the identification of UTUC that require validation in ongoing studies.
Supplementary Material
Acknowledgment.
The authors are grateful to their partner, Stryker Endoscopy (San Jose, CA), for providing imaging system and technical assistance. The research reported in this publication was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM073857 to O.A.A. and Y.K.R., and in part by the Feibelman Family research grant awarded to DG.
Financial Disclosure:
O.A.A. and Y.K.R. are founders of pHLIP, Inc. They have shares in the company, but the company did not fund any part of the work reported in the paper, which was done in their academic laboratories.
Footnotes
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.urology.2019.01.008.
References
- 1.Soria F, Shariat SF, Lerner SP, et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol. 2017;35:379–387. [DOI] [PubMed] [Google Scholar]
- 2.Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int. 2011;107:1059–1064. [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Böhle A, Burger M, et al. EAU Guidelines on Non–Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2017;71:447–461. [DOI] [PubMed] [Google Scholar]
- 4.Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196:1021–1029. [DOI] [PubMed] [Google Scholar]
- 5.Spiess PE, Agarwal N, Bangs R, et al. Bladder cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1240–1267. [DOI] [PubMed] [Google Scholar]
- 6.Aragon-Ching JB. Challenges and advances in the diagnosis, biology, and treatment of urothelial upper tract and bladder carcinomas. Urol Oncol. 2017;35:462–464. [DOI] [PubMed] [Google Scholar]
- 7.Baard J, Freund JE, de la Rosette JJ, Laguna MP. New technologies for upper tract urothelial carcinoma management. Curr Opin Urol. 2017;27:170–175. [DOI] [PubMed] [Google Scholar]
- 8.Kata SG, Aboumarzouk OM, Zreik A, et al. Photodynamic diagnostic ureterorenoscopy: a valuable tool in the detection of upper urinary tract tumour. Photodiagnosis Photodyn Ther. 2016;13:255–260. [DOI] [PubMed] [Google Scholar]
- 9.Mathieu R, Bensalah K, Lucca I, et al. Upper urinary tract disease: what we know today and unmet needs. Transl Androl Urol. 2015;4:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osman E, Alnaib Z, Kumar N. Photodynamic diagnosis in upper urinary tract urothelial carcinoma: a systematic review. Arab J Urol. 2017;15:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetherell DR, Ling D, Ow D, et al. Advances in ureteroscopy. Transl Androl Urol. 2014;3:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyatt LC, Lewis JS, Andreev OA, Reshetnyak YK, Engelman DM. Applications of pHLIP technology for cancer imaging and therapy. Trends biotechnol. 2017;35:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson M, Moshnikova A, Engelman DM, Reshetnyak YK, Andreev OA. Probe for the measurement of cell surface pH in vivo and ex vivo. Proc Natl Acad Sci U S A. 2016;113:8177–8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adochite RC, Moshnikova A, Golijanin J, et al. Comparative study of tumor targeting and biodistribution of pH (Low) insertion peptides (pHLIP((R)) Peptides) conjugated with different fluorescent dyes. Mol Imaging Biol. 2016;18:686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng CJ, Bahal R, Babar IA, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozes AR, Wang Y, Zong X, et al. Therapeutic targeting using tumor specific peptides inhibits long non-coding RNA HOTAIR activity in ovarian and breast cancer. Sci Rep. 2017;7:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tapmeier TT, Moshnikova A, Beech J, et al. The pH low insertion peptide pHLIP Variant 3 as a novel marker of acidic malignant lesions. Proc Natl Acad Sci U S A. 2015;112:9710–9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Y, Liao R, Mahmood AA, Xu H, Zhou Q. pH-responsive pHLIP (pH low insertion peptide) nanoclusters of superparamagnetic iron oxide nanoparticles as a tumor- selective MRI contrast agent. Acta Biomater. 2017;55:194–203. [DOI] [PubMed] [Google Scholar]
- 19.Wyatt LC, Moshnikova A, Crawford T, Engelman DM, Andreev OA, Reshetnyak YK. Peptides of pHLIP family for targeted intracellular and extracellular delivery of cargo molecules to tumors. Proc Natl Acad Sci U S A. 2018;115:E2811–E2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demoin DW, Wyatt LC, Edwards KJ, et al. PET imaging of extracellular pH in tumors with (64)Cu- and (18)F-labeled pHLIP peptides: a structure-activity optimization study. Bioconjug Chem. 2016;27:2014–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golijanin J, Amin A, Moshnikova A, et al. Targeted imaging of urothelium carcinoma in human bladders by an ICG pHLIP peptide ex vivo. Proc Natl Acad Sci U S A. 2016;113:11829–11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grahn A, Melle-Hannah M, Malm C, et al. Diagnostic accuracy of computed tomography urography and visual assessment during ureterorenoscopy in upper tract urothelial carcinoma. BJU Int. 2017;119:289–297. [DOI] [PubMed] [Google Scholar]
- 23.Jeon SS, Sung HH, Jeon HG, et al. Endoscopic management of upper tract urothelial carcinoma: improved prediction of invasive cancer using a ureteroscopic scoring model. Surg Oncol. 2017;26: 252–256. [DOI] [PubMed] [Google Scholar]
- 24.Mammen S, Krishna S, Quon M, et al. Diagnostic accuracy of qualitative and quantitative computed tomography analysis for diagnosis of pathological grade and stage in upper tract urothelial cell carcinoma. J Comput Assist Tomogr. 2018;42:204–210. [DOI] [PubMed] [Google Scholar]
- 25.Maruschke M, Kram W, Zimpfer A, Kundt G, Hakenberg OW. Upper urinary tract tumors: which diagnostic methods are needed. Urol Int. 2017;98:304–311. [DOI] [PubMed] [Google Scholar]
- 26.Knoedler JJ, Raman JD. Advances in the management of upper tract urothelial carcinoma: improved endoscopic management through better diagnostics. Ther Adv Urol. 2018;10:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traxer O, Geavlete B, de Medina SG, Sibony M, Al-Qahtani SM. Narrow-band imaging digital flexible ureteroscopy in detection of upper urinary tract transitional-cell carcinoma: initial experience. J Endourol. 2011;25:19–23. [DOI] [PubMed] [Google Scholar]
- 28.Breda A, Territo A, Guttilla A, et al. Correlation between confocal laser endomicroscopy (Cellvizio((R))) and histological grading of upper tract urothelial carcinoma: a step forward for a better selection of patients suitable for conservative management. Eur Urol Focus. 2018;4:954–959. [DOI] [PubMed] [Google Scholar]
- 29.Yamany T, van Batavia J, Ahn J, Shapiro E, Gupta M. Ureterorenoscopy for upper tract urothelial carcinoma: how often are we missing lesions. Urology. 2015;85:311–315. [DOI] [PubMed] [Google Scholar]
- 30.Straub J, Strittmatter F, Karl A, Stief CG, Tritschler S. Ureterorenoscopic biopsy and urinary cytology according to the 2004 WHO classification underestimate tumor grading in upper urinary tract urothelial carcinoma. Urol Oncol. 2013;31:1166–1170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


