Abstract
The outbreak of e-cigarette or vaping product use-associated lung injury (EVALI) has been cause for concern to the medical community, particularly given that this novel illness has coincided with the COVID-19 pandemic, another cause of severe pulmonary illness. Though cannabis e-cigarettes tainted with vitamin E acetate were primarily associated with EVALI, acute lung injuries stemming from cannabis inhalation were reported in the literature prior to 2019, and it has been suggested that cannabis components or additives other than vitamin E acetate may be responsible. Despite these concerning issues, novel cannabis vaporizer ingredients continue to arise, such as Δ8-tetrahydrocannabinol, Δ10-tetrahydrocannabinol, hexahydrocannabinol, and cannabichromene. In order to address cannabis e-cigarette safety and vaping in an effective manner, we provide a comprehensive knowledge of latest products, delivery modes, and ingredients. This perspective highlights the types of cannabis vaping modalities common to the United States cannabis market, with special attention to cartridge type cannabis e-cigarettes toxicology and their involvement in the EVALI outbreak in particular acute lung injurious responses. Novel ingredient chemistry, origins, and legal statuses are reviewed, as well as the toxicology of known cannabis e-cigarette aerosol components.
Keywords: Cannabis, THC, vaping, e-cigarettes, lung, inflammation, EVALI
Introduction
Vaping cannabis and tobacco has rapidly expanded over the past decade, and though concern by the medical community has existed from the outset,1, 2 the perceived innocuity of cannabis due to this plant’s extensively reported medical uses painted cannabis vaping as a useful harm reduction strategy for medical cannabis users.3–5 However, the 68 deaths and 2,807 hospitalizations recorded by the Centers for Disease Control (CDC) until February 2020 due to the outbreak of EVALI6 were the wakeup call needed to realize that, despite its public image of harmlessness, cannabis vaping can indeed cause injury and mortality. Despite the fact that tobacco and cannabis are frequently co-used, EVALI was associated largely with cannabis vaping, as 82% of patients reported the use of e-cigarettes containing Δ9-tetrahydrocannabinol (Δ9-THC), 33% reported using only Δ9-THC e-cigarettes, and 14% reported using only electronic nicotine delivery systems (ENDS).7 Extensive media coverage during the outbreak, which peaked in September 2019,7 put cannabis vaping into focus, and states with legalized recreational and medical cannabis markets responded by banning potentially harmful ingredients.8–10 Researchers also responded by studying the in vitro and in vivo impacts of cannabis vaporizer adulterants on the respiratory system,11–13 but for the major and often only ingredients in cannabis vaporizers, cannabinoids and terpenes,14, 15 a dearth of toxicological data exists on how they may negatively impact the aerodigestive tract.
23.7% of US 12th graders reported lifetime use of any cannabis vaping in 2019, and 3.5% reporting near-daily use.16 One study indicates that this practice is associated with increased bronchitis and allergic rhinitis symptoms, such as wheeze or shortness of breath in young adults, even after adjusting for ENDS use and smoking.17 Stay-at-home orders during the COVID-19 pandemic have led to a significant increase in cannabis sales in US states with legal recreational markets.18 Given these concerning trends, toxicological research must stay on top of this practice in order to prevent not only another EVALI outbreak, but long term pulmonary illness amongst the increasingly growing population of young users. This review will outline the existing research deficits in the field of cannabis vaping, with a focus on the cannabis e-cigarettes (CECs), the vaping modality associated with EVALI. Types and compositions of CECs are reviewed, as well as the known inhalation toxicology of cannabinoids, terpenes, and volatile organic compounds.
Cannabis vaping use modes
Before this discussion may begin, it is important to understand what exactly is a CEC and how the terminology surrounding vaping for cannabis is used, as at least three distinct practices may be referred to as “cannabis vaping.” Of the three vaping modalities, cannabis flower vaping (Figure 1a) was the earliest to surface, first documented in the literature in 200119 two years before the patent for the ENDS device was issued.20 Cannabis flower vaporizers are handheld or tabletop devices that pass hot air over milled cannabis to produce an aerosol.21, 22 2.9% of US 16–19 year-olds reported past 30-day flower vaping.23 Dabbing, another popular method for cannabis consumption first reported in 2014,24 consists of flash vaporization of cannabis wax/shatter/oil on a heated surface connected to a water pipe (Figure 1b).15, 25 4.2% of 16–19 year-olds reported past 30-day consumption of cannabis products (wax/shatter/oil)23 that are typically consumed by dabbing.26, 27 A third type of cannabis vaping is by use of the CEC (Figure 1c), which were first reported in 2011.2 Mimicking ENDS, CECs use a resistively-heated coil to vaporize cannabis oil contained in a small cartridge.14, 15 While cannabis flower vaping and dabbing often require extensive paraphernalia and connoisseur-level knowledge, CECs are portable, disposable, easily concealed and easy to use, making them readily accessible by novices including youth.28–30 4.2% of 16–19 year-olds reported past 30-day cannabis oil consumption.23
Figure 1.

Cannabis vaping modalities: a) is a tabletop flower vaporizer equipped with a balloon which traps the aerosol for inhalation, b) is a small “dab rig” which consists of a water pipe with a ceramic “nail” which is heated with a blow torch prior to administration of cannabis extract, and c) displays two types of CECs, a pod type (left) and cartridge type (right).
Cannabis extract types and physical properties
Cannabis extracts can vary greatly based on the methodology employed and the intended consumption mode. Of the three vaping modalities, dabbing and CEC vaping exclusively use some form of cannabis extract/oil. Figure 2 displays the most common extraction and processing methods used for producing cannabis extracts used for CEC vaping and dabbing. CEC compositions are described further below in Table 2.
Figure 2.
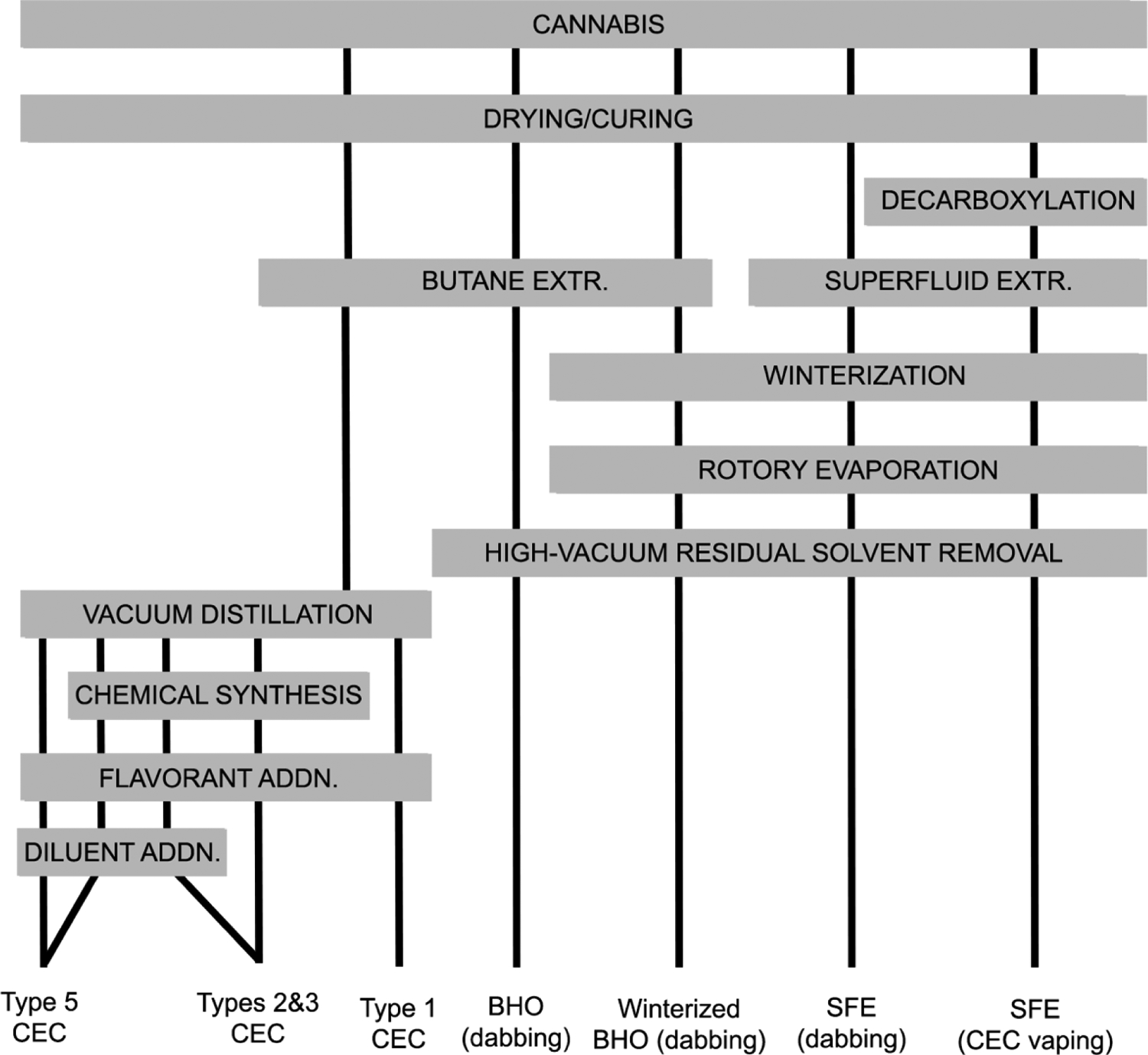
Common cannabis extraction and processing methods for vaping and dabbing.
Table 2.
Classes of CECs by active ingredients, additives, legal statuses, and presence in the scientific literature.
| 1 | Δ9-THC, (70 −90%) | Terpenes/ flavorants (10–30%) | Legal in states with recreational and medical cannabis programs. | Oregon Liquor Control Commission (2020), Meehan-Atrash et al. (2021) |
| 2 | Δ8-THC, Δ10-THC, HHC, (60 −90%) | Terpenes/ flavorants (10–30%), cutting agents/viscosity modifiers (NA) | Federally legal under 2018 Farm Bill. Regulated or banned in some states. | US Cannabis Council (2021) |
| 3 | CBD, CBC, CBL, CBT, etc. (60 −90%) | Terpenes/ flavorants (10–30%) | Federally legal under 2018 Farm Bill. Regulated in some states. | NA |
| 4 | Δ9-THC, CBD. (10 −60%) | Terpenes/ flavorants (10–30%), cutting agents/viscosity modifiers (20–60%) | MCT oil, VEA, and polyethylene glycol banned in CO; squalene, squalane, MCT oil, VEA, PG banned in OR; VEA banned in WA and requires labelling for non-cannabis additives. | Peace et al. (2016a), Muthumalage et al. (2020b), Duffy et al. (2020) |
| 5 | CBD (1 −3%) | Flavorants (NA), PG, GL (<90%) | Federally legal under 2018 Farm Bill. Regulated in some states. | Peace et al. (2016b) |
Cannabinoids are biosynthesized as acids and contain an aryl carboxyl moiety at the 2-position of the resorcinol ring.31 Extracts that have not undergone decarboxylation (heating to 100+ °C32) contain acid cannabinoids such as Δ9-tetrahydrocannabinolic acid (Δ9-THCA) and cannabidiolic acid (CBDA), and are solids or thick, sappy oils at room temperature, making them readily amenable for dabbing as they are easily handled with small spoons/spatulas for administration onto the dabbing nail.33 Vacuum distilled cannabis extract amended with terpenes/flavorants and superfluid extract (SFE) are the most amenable extracts/oils for CEC vaping given that these contain decarboxylated, or neutral Δ9-THC, which is a viscous liquid at room temperature.33 In the case of distillate cannabis oil, added terpenes serve not only to reduce viscosity further, but as added flavors.33 A sufficiently low viscosity of the vape liquid/oil is necessary for the CEC vaporizer to function given these devices reliance on wicking to deliver the vape liquid/oil to the atomizer from the cartridge reservoir.33
It is known that the aerosolization temperature in an ENDS is controlled by the boiling point of the propylene glycol (PG)/glycerol (GL) system.34 The normal boiling points of Δ9-THC and CBD (Table 1) were recently predicted via equations of state calculated by measuring vapor pressures using porous layered open tubular cryoadsorption.35 The diversity of ingredients and co-extracted components make it difficult to determine an exact aerosolization temperature, however, recent analysis of gas phase degradation products of a simplified system that recreates distillate cannabis oil has shown that CEC vaping of Δ9-THC added with increasing levels of β-myrcene (an abundant cannabis terpene) is correlated with reaction products associated with lowered aerosolization temperatures.14 A recent analysis that measured temperatures occurring at the atomizer using a thermocouple when vaping 1:1 Δ9-THC:vitamin E acetate (VEA) indicates that reaction temperatures in this system increase with each sequential puff, and may exceed 400 °C.36 Further work to characterize cannabis extract/oil boiling points will aid the description of chemical degradation and oxidation reactions that occur during aerosolization.
Table 1.
Physical properties of some common cannabis extract components at standard temperature and pressure.
Cannabis e-cigarette compositions
Type 1: Δ9-THC; terpenes/ flavorants
Type 1 CECs are highly available in states with recreational and/or medical cannabis programs, but may be diverted to black markets in illegal states by producers or third parties.46 Due to the highly restricted legal status of drug-type cannabis (i.e. cannabis containing >0.3% Δ9-THC), academic researchers are not able to access products for sale in dispensaries in states with recreational and/or medicinal cannabis programs, even those with licenses issued by the Drug Enforcement Agency (DEA). Specialized channels through the Department of Justice exist to allow access to products seized by law enforcement for research, though this is a rare occurrence, and does not necessarily guarantee access to the aforementioned dispensary products.
Type 2: Δ8-THC, Δ10-THC, HHC; terpenes/ flavorants; cutting agents/viscosity modifiers
Hemp-derived cannabidiol (CBD) for vaping in CECs rapidly proliferated after the 2018 Agriculture Improvement Act (the “Farm Bill”) effectively legalized hemp and its derivatives at the federal level.47 After concerns that hemp producers were synthesizing Δ9-THC from hemp-derived CBD (Figure 3), the DEA published an interim final rule in August, 2020 to clarify that the legal definition of “tetrahydrocannabinols” does not include any compound derived from hemp, and that “marijuana extract” is limited to any formulation containing >0.3% Δ9-THC.48 This theoretically left open the possibility of selling hemp-derived cannabinoids other than Δ9-THC, an opportunity that was capitalized on to market Δ8-tetrahydrocannabinol (Δ8-THC). Δ8-THC is a Δ9-THC thermal isomerization artefact that occurs in situ on storage and heating49 and is approximately half as psychoactive as Δ9-THC.50 Δ8-THC for ingestion and vaping currently is sold through the existing hemp marketing network, both online and brick-and-mortar, that operates independently of the traditional recreational and medical cannabis markets.51 Even before this DEA’s interim final rule, Duffy et al. (2020) reported that several EVALI-associated CEC oils contained unnatural levels of Δ8-THC, and it was speculated this may be manufacturing artefact, deliberately purified from drug-type cannabis, or manufactured from hemp using a synthetic process.52
Figure 3.
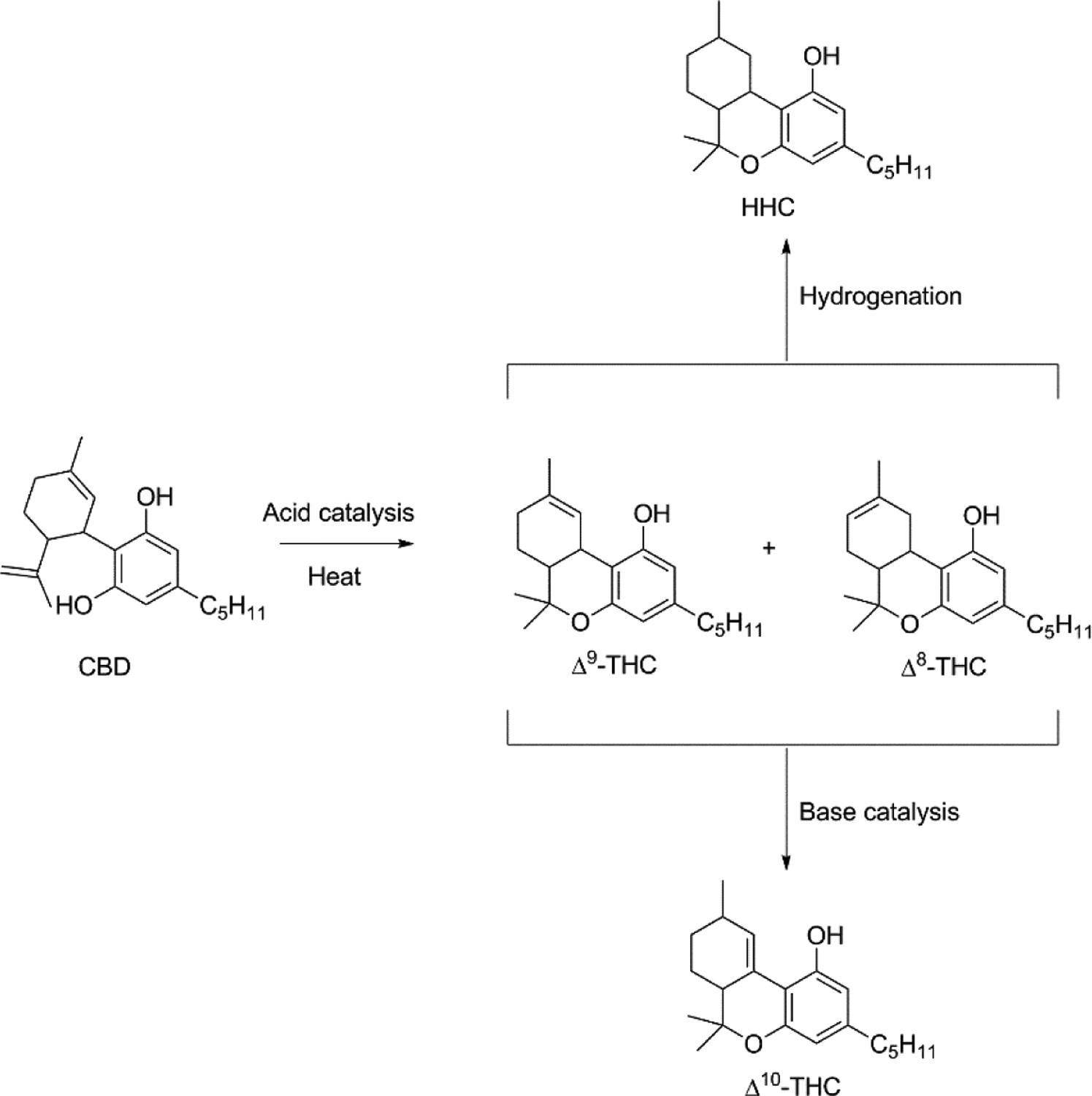
Synthetic pathways used for manufacturing alternative cannabinoids for CECs.
CBD conversion to tetrahydrocannabinols by acid catalysis (Figure 3) was reported in the literature as early as 1940 by Adams et al.,53 and at least one patent has been issued for this process.54 The ring closing reaction to Δ9-THC is accompanied by the double bond isomerization to Δ8-THC which also occurs under acid catalysis,55 and generation of the latter may be favored by longer reaction times. The use of CBD as a substrate for the synthesis of other cannabinoids has been recently reviewed56 and additional products that may result from these processes have been described.57, 58 Crude online guides exist for this synthesis,59 and it has been suggested that some Δ8-THC CEC manufacturers do not include a post-reaction purification step, potentially resulting in traces of sulfuric acid, hydrochloric acid, trifluoroacetic acid or p-toluenesulfonic acid.60 One analysis by the US Cannabis Council found that a majority of CEC products tested contained Δ9-THC concentrations far in excess of the 0.3% Δ9-THC required to not meet the DEA’s definition “marijuana extract,” and were also contaminated with residual solvents and heavy metals.51 Several states have blocked the sale of Δ8-THC,61 and members of the medical community have expressed alarm over the lack of safety data and regulations that has allowed the sale of products with packaging and flavors that may entice consumption by minors.62 These vaporizers may be flavored with terpenes to recreate the scent of cannabis, but may also include non-cannabis flavors including menthol, fruit, etc.
CECs containing Δ10-THC are also available, and at least one online source suggests this synthesis uses Δ9-THC as a starting material,63 though the cited chemical literature indicates that both Δ9-THC and Δ8-THC may be isomerized to Δ10-THC under strongly basic conditions (Figure 3).64 Hexahydrocannabinol (HHC), a hydrogenated tetrahydrocannabinol, also has limited commercial availability but may continue to expand.65
Type 3: CBD, CBC, CBL, and CBT, terpenes/ flavorants
Advances in hemp extraction technologies have allowed the proliferation of Type 3 CECs which are non-psychoactive. These are often advertised as CBD vaporizers, though other cannabinoids are included for which additional health claims are made, such as cannabichromene (CBC), cannabicylol (CBL), cannabicitran (CBT), etc. While Δ9-THC and Δ8-THC are viscous oils at room temperature38 allowing them to be formulated as the sole cannabinoid in a CEC, pure CBD is a solid39 and requires the addition of other compounds to lower its melting temperature and prevent crystallization in the cartridge. These may also be formulated as hemp extracts with sufficiently high contents of terpenes and other plant lipids/waxes to prevent CBD crystallization, and may also include viscosity modifiers such as PG.
Type 4: Δ9-THC, CBD; terpenes/ flavorants; cutting agents/viscosity modifiers
The existence of Type 4 CECs was brought to the public’s attention primarily due to the EVALI outbreak. Initial chemical analyses of vaporizer cartridges from affected individuals by the New York State Department of Health showed EVALI was largely associated with counterfeit or black market CECs containing elevated levels of VEA, which was subsequently confirmed by The Food and Drug Administration.66 Subsequent publications identified a variety of adulterants including squalene, phytol, medium chain triglyceride oil, etc.52, 67 Identification of VEA in all the bronchoalveolar lavage (BAL) fluids of a convenience sample of 29 EVALI patients prompted the CDC to identify this substance as a potential causative agent, though the organization was not able to rule the potential for other contributing factors.68 It has been speculated that the rapid decrease is EVALI patients is due to the discontinuation of VEA specifically as a cutting agent,7 and though some compounds have been banned from use in certain states9, 10, 69 (Table 2), it is not known how common cutting agents continue to be in legal, medical, or black market CECs.
Type 5: CBD; flavorants; PG, GL
Type 5 CECs are a combination of cannabis and ENDS formulation techniques. Propylene glycol (PG) and glycerol (GL) are the two most commonly used solvents in ENDS,70 and CBD is moderately soluble in the least polar of the two, PG. Such products were first reported by Peace et al. (2016)71 and have more recently been used in toxicological studies (vide infra). While Types 1–4 are often formulated using terpenes as their primary flavorant source in a manner that seeks to replicate the flavor/scent of cannabis flower, Type 5 CEC liquids may be formulated with any combination of the diverse flavorant molecules used in ENDS including, but not limited to, menthol, tobacco, fruit, beverages, candy, and caramel.
Pulmonary toxicology of CEC aerosol components: cannabinoids
Inhaled cannabis smoke, a well-studied consumption modality, produces a measurable Δ9-THC content in plasma within seconds and peaks in 9–10 minutes.72, 73 Δ9-THC plasma concentrations after the first inhalation of a 3.55% Δ9-THC cannabis cigarette have been reported at 18±12 ng/mL,73 and ~150 ng/mL after consuming a cigarette with ~34 mg Δ9-THC. Lipophilic cannabinoids are protein bound in plasma and efficiently penetrate vascularized tissues, such as lung, liver, and muscle. leading a rapid concentration decrease in plasma.72 Δ9-THC is metabolized by hepatic cytochrome P450 enzymes to the active 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-Δ9-THC) with a concentration maximum in tandem with its parent molecule.72, 73 This metabolite more easily penetrates the blood-brain-barrier than its parent and is thought to contribute significantly to its impacts on the central nervous system.72 A further oxidized metabolite, 11-nor-carboxy-Δ9-tetrahydrocannabinol (Δ9-THCCOOH), peaks within an hour of smoking and has a long time course of detection in plasma.73 Bioavailability of Δ9-THC has been recently determined to be 14% in a naturalistic population pharmacokinetic study of regular cannabis smokers, and given the known high bioavailability of components of inhaled aerosols, it is estimated that chemical degradation due to combustion and sidestream smoke losses account for the majority of the transfer losses. The bioavailability of cannabinoids in CEC aerosols has not been studied in humans, but a recent chemical analysis of CEC aerosol components determined a Δ9-THC yield of 50–90% and 3–5 mg/puff depending on device parameters, suggesting that this format may afford aerosols of higher Δ9-THC concentrations.14 Both cannabis and tobacco smoking has been shown to induce CYP1A1 and CYP1A2 leading to more efficient clearance of drugs, such as theophylline and chlorpromazine, though it is postulated that polycyclic aromatic hydrocarbons present in cannabis smoke tar induce these enzymes by activating the aromatic hydrocarbon receptor in a similar manner to that seen in tobacco smoke.74 However, the interactions of the above two metabolites in aerosol is not known.
Cannabinoids have been shown to modulate lung immune response in vitro and in vivo. Cannabinoid receptors 1 and 2 (CB1R and CB2R) are both present in human lungs, though CB1Rs show higher expression than CB2Rs.75 Expression of both receptors with higher levels of CB2R is observed in both resident alveolar macrophages (AMs) and monocyte-derived macrophages, though CB2Rs in the latter are non-functional.76 Δ9-THC (a CB1R and CB2R partial agonist) was shown to suppress chemotaxis and negatively impact bronchial epithelial cell energetics in a CB2R-dependent manner in vitro,77, 78 and CB1R agonism by CP55,940 in a murine model induced the expression of inflammatory cytokines and oxidized phosphatidylcholines, which was postulated to result in lung surfactant disruption.79 Δ9-THC has been shown to downregulate nitric oxide production80 and tumor necrosis factor-α secretion,81 and impair phagocytic activity in murine macrophages.82 In human cannabis smokers, but not tobacco smokers, AMs capability to ingest and kill Staphylococcus aureus is impaired, suggesting a reduced host defense response in the presence of Δ9-THC.83 Incidentally, an impaired host defense response was offered as an explanation for the 6 day delay in reaction to BHO dabbing that caused an acute lung injury as described in a case report by McMahon et al. (2016).84
Conversely, cannabinoids have been shown to possess anti-inflammatory capabilities in lipopolysaccharide-induced (LPS) murine models of lung inflammation.85 Ribeiro et al. (2012) showed CBD attenuation of LPS-induced inflammation was reversed by prior adenosine-2A receptor antagonism, suggestive of CB2-A2A receptor heteromers which warrant further investigation.86 Muthumalage & Rahman (2019) also showed that CBD vaporized in PG attenuates the LPS-induced inflammatory response in bronchial epithelial cells, but is pro-inflammatory in absence of LPS, and acts to override the anti-inflammatory effect of dexamethasone (steroid) when used in combination.87 Notwithstanding, Leigh & Goniewicz (2020a) and Leigh & Goniewicz (2020b) demonstrate that CBD vaporized with PG is more cytotoxic to bronchial epithelial cells than PG vaporized alone, and leads to the release of higher levels of inflammatory biomarkers than PG alone.88, 89
Muthumalage et al. (2020) represents the only available literature that studied the toxicological impact of CECs other than Type 5.11 In this case, EVALI-associated “counterfeit” CEC cartridges generated increased levels of reactive oxygen species in bronchial epithelial cells as compared to control at levels similar to cells exposed to VEA though lower than MCT oil, but significantly increased levels of inflammatory biomarkers interleukin-6 and −8 as compared to control, MCT oil, or VEA. EVALI CECs, MCT oil, and VEA all significantly induced epithelial barrier dysfunction. Both the EVALI CECs and MCT oil induced LLM formation in vitro, though curiously, VEA did not induce LLM significantly as compared to control. In vivo, aerosol exposure to the EVALI CEC aerosols increased total cell counts in mouse BAL fluids, with a more than fivefold increase in neutrophils, and nearly sevenfold increase in T-helper cells. Murine lung homogenates also showed lower levels of surfactant-associated proteins after EVALI CEC exposure and increased levels of leukotrienes. Together, these results indicate that EVALI CECs may induce cytotoxicity, barrier dysfunction, and inflammation associated with acute lung injuries, but also add to the complexity associated with the toxicological impacts of inhaling an aerosol with multiple and unknown components.
Of the CECs available to consumers as displayed in Table 2, toxicological studies only exist for Types 4 and 5. Far more common in states with recreational and/or medical marijuana programs are Type 1 CECs, and Types 2 and 3 ostensibly share more similarities with these given that they contain cannabinoids at levels as high as 90 %.14, 15 Though Δ9-THC toxicology is relatively well understood, aerosols from Type 1 CECs contain approximately double the Δ9-THC content of cannabis smoke (7000–9000 ppm14 vs. 2400–4000 ppm90), and it is not known what effects elevated Δ9-THC concentrations have on the respiratory tract. Furthermore, no inhalation toxicology data exists for Δ8-THC, Δ10-THC, HHC, CBC, CBL, and CBT, and scant physicochemical characterization and commercial availability represent a significant hindrance to their evaluation. Inhalation models (mouse/cells) and human studies are lacking to understand the role of THC and CBD based products on lung and cardiovascular injuries.
Given the high use of novel vaporizer products among adolescents, the impacts of their use on development are a pressing concern. There are mixed findings with regards to early onset chronic cannabis use, and while a 2015 longitudinal study of youth cannabis users found that it may not be a predictor for later physical or mental health issues,91 a 2019 longitudinal study found evidence that increased cannabis potency increases risk for development of a cannabis use disorder.92 It is not yet known how exposure to novel cannabis vaporizer products may impact the development of adolescents who use them.
Pulmonary toxicology of CEC aerosol components: terpenes, volatile organic compounds, and metals
Toxicological impacts of terpene inhalation have been studied in the context of environmental exposure from indoor and outdoor ambient air, using primarily concentrations <100 ppm.93 Type 1–4 CECs may contain 10–20% terpenes by weight in the cannabis oil resulting in aerosols with 2500–5000 ppm total terpenes.14 Even at concentrations orders of magnitude lower than those seen in CECs, terpenes have been shown to induce sensory irritation, airflow limitation, and inflammatory responses in vivo.93 One analysis that assessed free radicals generated by flavored ENDS by electron paramagnetic resonance and lipid peroxidation in vitro found that the terpenes linalool, dipentene (i.e. racemic limonene), and citral were the most radical-generating molecules of all the flavorants identified.94 Terpenes easily auto-oxidize to form hydroperoxide species that are known to generate C-centered radicals which are potent sensitizers associated with allergic contact dermatitis.95–97 Though terpene auto-oxidation is a spontaneous process, the formation of terpene hydroperoxides at elevated temperatures and in physicochemical environments similar to nicotine or cannabis vaping has not been studied.
Wolkoff et al. (2008) demonstrated that airway irritation initiated by limonene-O3 reaction product aerosols is primarily due to gaseous products such as formaldehyde.98 While mice exposed to these aerosols displayed evidence of sensory irritation, mice exposed to the aerosols with prior removal of gaseous products with a denuder were indistinguishable from room air.98 Gaseous volatile organic compounds (VOCs) that are thermal degradation products of cannabis terpenes and Δ9-THC have been recently characterized.14, 15, 25 VOCs emitted by Δ9-THC share considerable overlap with those emitted by terpenes common in cannabis (Figure 4) due to the terpene backbone on Δ9-THC, which is responsible for 22±6% of its VOC emissions.33 Known carcinogens such as benzene, xylenes, isoprene, 1,3-butadiene etc., and potent respiratory tract irritants such as methyl vinyl ketone, methacrolein, butanal, etc. are present.14, 15, 25 Carbonyls present in CEC aerosols may produce significant harm when inhaled, as photochemical oxidation products of isoprene and 1,3-butadiene (methacrolein, methyl vinyl ketone, and formaldehyde) have been shown to cause cytotoxicity and inflammation in human lung cells in vitro.99 In addition to these known toxicants, many other molecules exist in high abundance that are not toxicologically characterized. For example, conjugated dienes are potent sensitizers for allergic contact dermatitis because they are metabolically oxidized to epoxides which in turn cause sensitization by covalently attaching to endogenous nucleophiles, such as cysteine residues,100 but their impact on lung tissue has not been studied. Gas phase epoxides and conjugated dienes have been detected, but other terpene epoxides shown to be sensitizers101, 102 may also exist in the particle phase.
Figure 4.
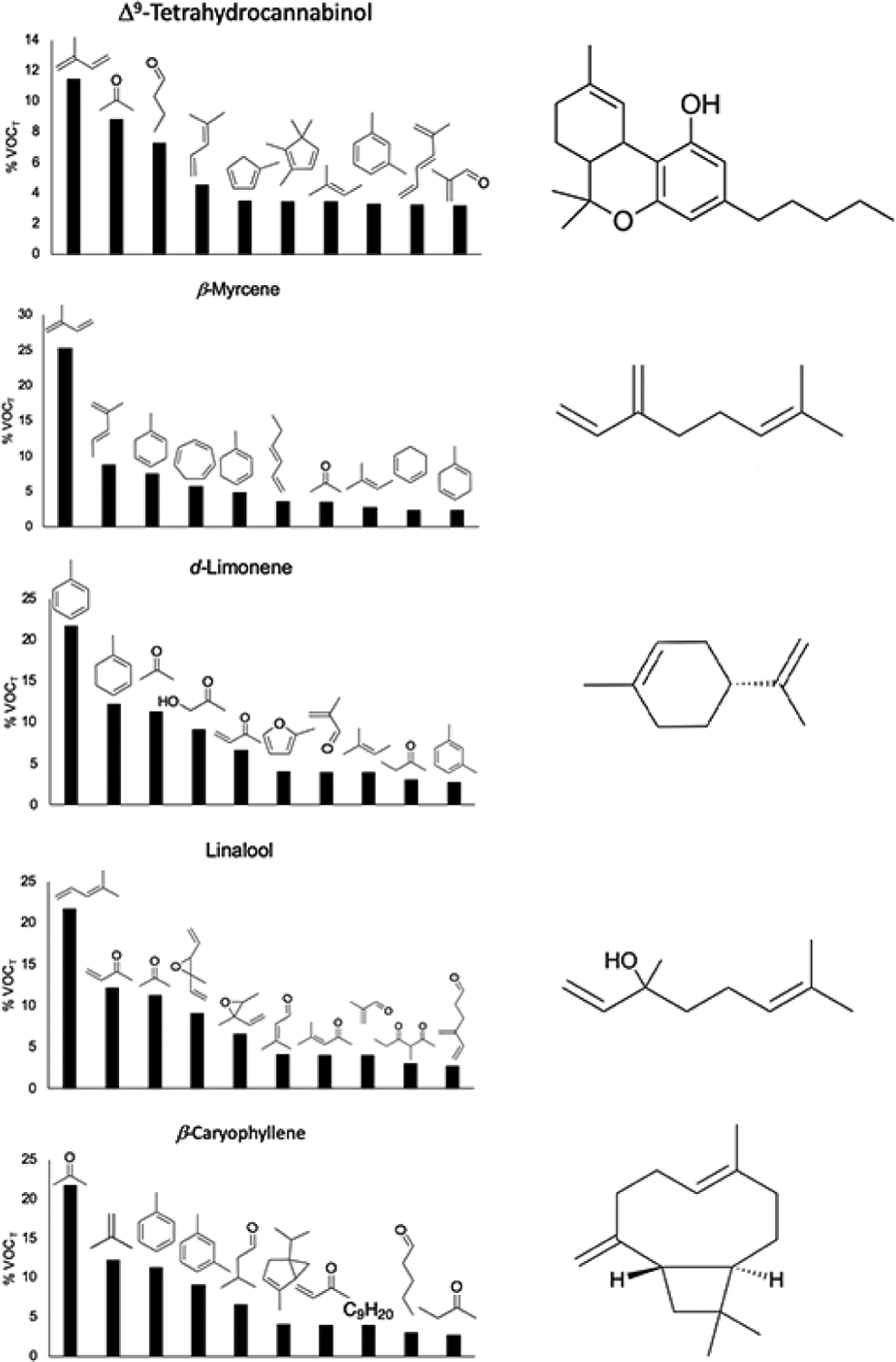
Relative levels of VOCs emitted by Δ9-THC and four high abundance terpene flavorants present in many CECs. Data derived from Meehan-Atrash (2021).33
Heavy metals as contaminants in CECs and other cannabis products are also cause for concern, as at least one case report exists wherein a patient suffered from giant cell interstitial pneumonia that was attributed to cobalt exposure from a CEC.103 An analytical method for determining levels of heavy metals in such aerosols has been recently published, and spike/recovery experiments also demonstrated that heavy metal can transfer to the aerosol in CECs and standard cannabis smoking.104 Generation of heavy metals especially during vaping due to devices can not be rules out.
Etiology and causes of EVALI
Prior to outbreak of EVALI, cannabis vaping-associated acute lung injuries (ALIs) were reported several times in the literature. McMahon et al. (2016) described a severe acute pneumonitis in a 19 year old male from dabbing butane hash oil (BHO, a common cannabis extract).84 BAL revealed elevated levels of lymphocytes and eosinophils, but lacked any evidence of bacterial, fungal, or viral infection.84 It was suggested that some impurity may have caused a chemical injury leading to acute hypersensitivity pneumonitis.84 He et al. (2017) reported a 54 year old male regular consumer of cannabis e-cigarettes that developed dyspnea and low O2 saturation.105 Computed tomography (CT) showed extensive airspace opacification and bronchoscopy indicated alveolar hemorrhage with leukocyte infiltration.105 Though the patient reported vaping supercritical CO2-extracted cannabis oil in an e-cigarette, the authors suspected the cannabis oil may have been contaminated with the nicotine e-cigarette solvents GL and PG.105 Anderson and Zechar (2019) detailed a case of severe pneumonitis in an 18 year old female associated with dabbing BHO.106 BAL was not performed, but lung CT showed bilateral patchy infiltrates, and blood workup showed leukocytosis, with no evidence of microbiological infection.106 The acute lung injury was attributed to harmful degradation products (or chemical interactions upon vaping) that form due to high heat during the dabbing process.106
The EVALI outbreak began as a gradual increase in patients reporting severe respiratory distress associated with vaping starting approximately in June of 2019, and cases quickly increased through August, peaked in mid-September, after which they gradually decreased until February 2020 when the CDC discontinued monitoring.7 EVALI patients reported a rapid onset of acute and severe respiratory distress after using e-cigarette products, with 82% of patients reporting the use of e-cigarettes containing Δ9-THC.7 Patients present with an airway-centered acute lung injury with severe bronchiolitis accompanied by mucosal edema and desquamation of bronchial epithelium.107 LLM, unlike foamy macrophages commonly caused by tobacco smoking, were a common feature which led to the suspicion that EVALI was a type of lipoid pneumonia induced by lipophilic ingredients in CEC.107 However, other histological markers of lipoid pneumonia were lacking, which led Butt et al. (2020) to conclude the acute lung injury was a type chemical pneumonitis induced by some unknown toxic ingredient or a degradation product thereof.107 More recent research showed that LLM are a common feature in healthy adult smokers and nicotine e-cigarette users, and do not serve as a specific marker for EVALI.108
Toxicological explanations for VEA pulmonary toxicity are conflicting, and a clear indication to this compound’s proinflammatory action on bronchial epithelial cells and pulmonary macrophages is not clear.11, 109 VEA has been shown to generate ketene, a highly toxic gas, when vaped, but due to the instability of this compound in the presence of water it is not clear if this molecule would persist in the aerosol long enough to cause a toxic response, as it may rapidly decompose to acetic acid in the humid respiratory tract before contacting tissue.110 It has also been suggested that VEA can disrupt the natural respiratory compression/decompression cycle of the alveoli by increasing the surface viscosity of pulmonary surfactant, potentially impairing oxygen diffusion leading to tissue hypoxia and inflammation.111 Inflammation may be further exacerbated by CB1R-dependent phospholipid/sphingolipid peroxidation79 that may synergistically add to lipid peroxidation caused reactive oxygen species generated by macrophage respiratory bursts,112 and CB2R-dependent attenuation of epithelial cell chemotaxis may prevent clearance of the inhaled toxicants leading to accumulation of redox cycling molecules such as terpenes in an inflammatory feedback loop. Continued lipid peroxidation can further impact alveolar compression/decompression, and also lead to tissue permeability that may result in pneumonia.
Conclusion
Despite the recent deadly outbreak of EVALI, cannabis vaping has continued to proliferate and novel products are introduced at a daunting rate. Compounds already with little safety data at low concentrations are now present at concentrations that, in some cases, are orders of magnitude higher than any previously reported literature. It must be stressed that novel cannabis inhalation formats share so little in common with cannabis smoking that the pool of relevant literature with which consumers and regulators can use to assess their safety is lacking in appropriately relevant data. In order to address these deficits, research on CEC chronic and acute respiratory toxicity/acute lung injuries along with biomarkers of EVALI must use realistic devices and cannabis oil compositions that simplify any internal and external variables. In order to work around academia’s inability to access state-level legal CECs, researchers must self-manufacture products that match their experimental requirements. In any case, dedicated attention to cannabis industry trends and user habits is necessary to design meaningful and relevant studies.
Acknowledgments:
The National Institutes of Health (NIH) 1R01HL135613 and Toxicology Training Grant 5T32ES007026-43 supported this study.
We thank Thivanka Muthumalage for useful discussion
List of Abbreviations
- Δ9-THC
Δ9-tetrahydrocannabinol
- Δ8-tetrahydrocannabinol
- Δ10-THC
Δ10-tetrahydrocannabinol
- CBD
cannabidiol
- ENDS
electronic nicotine delivery systems
- CEC
cannabis e-cigarette
- EVALI
e-cigarette or vaping product use-associated lung injury
- HHC
hexahydrocannabinol
- CBC
cannabichromene
- CBL
cannabicylol
- CBT
cannabicitran
- MCT
medium chain triglyceride
- PG
propylene glycol
- GL
glycerol
- VEA
vitamin E acetate
- BAL
bronchoalveolar lavage
- AM
alveolar macrophage
- LPS
lipopolysaccharide
- LLM
lipid-laden macrophage
- VOC
volatile organic compound
- ALI
acute lung injury
- CT
computed tomography
- BHO
butane hash oil
Biography
Jiries Meehan-Atrash short biography
Dr. Jiries Meehan-Atrash graduated from the State University of New York at New Paltz with a Bachelor of Science in Chemistry in May 2014 and in June 2021 received his PhD in Chemistry from Portland State University with a dissertation titled Chemical Characterization of Toxicologically Relevant Molecules in Cannabis Concentrates and Vaporizer Aerosols under the mentorship of Dr. Robert M. Strongin. Jiries is currently as postdoctoral fellow at the University of Rochester Medical Center in the laboratory of Dr. Irfan Rahman.
Dr. Irfan Rahman short biography
Irfan Rahman, PhD is a Dean’s Professor of Environmental Medicine, Medicine (Pulmonary), and Public Health Sciences at the University of Rochester Medical Center, NY and Director of Flavoring Inhalation Toxicology Center. His research interests include oxidative stress, inflammation, molecular clock, mitochondrial dysfunction, epigenetics, and cellular senescence by tobacco smoke/tobacco products (cigarette smoke, e-cigarettes, waterpipe/hookah, and cigars). Dr. Rahman is an author of over three hundred (300) publications in peer-reviewed journals with an ‘H impact factor of 105. He is the author/editor of a book on ‘Inflammation, Aging, Diet and Nutrition’, and awarded as Highly Cited Researchers by Thomson Reuters.
Footnotes
Declarations: The authors have declared that no competing interests exist.
References
- (1).Wollscheid KA, and Kremzner ME (2009) Electronic cigarettes: safety concerns and regulatory issues. Am J Health Syst Pharm 66, 1740–1742. [DOI] [PubMed] [Google Scholar]
- (2).Etter JF, and Bullen C (2011) Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction 106, 2017–2028. [DOI] [PubMed] [Google Scholar]
- (3).Earleywine M, and Barnwell SS (2007) Decreased respiratory symptoms in cannabis users who vaporize. Harm Reduct J 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, and Benowitz NL (2007) Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther 82, 572–578. [DOI] [PubMed] [Google Scholar]
- (5).Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, and Donaghe H (2013) Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 14, 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).CDC. (2020) Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products, cdc.gov.
- (7).Krishnasamy VP, Hallowell BD, Ko JY, Board A, Hartnett KP, Salvatore PP, Danielson M, Kite-Powell A, Twentyman E, Kim L, Cyrus A, Wallace M, Melstrom P, Haag B, King BA, Briss P, Jones CM, Pollack LA, and Ellington S (2020) Update: Characteristics of a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury - United States, August 2019-January 2020. MMWR Morb Mortal Wkly Rep 69, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(2020) Inhalable Cannabinoid Product Processor Requirements, In OAR 845-025-3265 (Commission, O. L. C., Ed.), Oregon State Archives, Salem, OR. [Google Scholar]
- (9).Inslee J (2019) Addressing the Vaping Use Public Health Crisis, In Executive Order 19–03 (Governor, O. o. t., Ed.), Olympia, WA. [Google Scholar]
- (10).(2021) Ingredients Prohibited, In CCR 212–3 (Marijuana Enforcement Division, C. D. o. R., Ed.), Colorado Department of State, Denver, CO. [Google Scholar]
- (11).Muthumalage T, Lucas JH, Wang Q, Lamb T, McGraw MD, and Rahman I (2020) Pulmonary Toxicity and Inflammatory Response of E-Cigarette Vape Cartridges Containing Medium-Chain Triglycerides Oil and Vitamin E Acetate: Implications in the Pathogenesis of EVALI. Toxics 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Schwotzer D, Gigliotti A, Irshad H, Dye W, and McDonald J (2021) Phytol, not propylene glycol, causes severe pulmonary injury after inhalation dosing in Sprague-Dawley rats. Inhal Toxicol, 1–8. [DOI] [PubMed] [Google Scholar]
- (13).Jiang H, Ahmed CMS, Martin TJ, Canchola A, Oswald IWH, Garcia JA, Chen JY, Koby KA, Buchanan AJ, Zhao Z, Zhang H, Chen K, and Lin YH (2020) Chemical and Toxicological Characterization of Vaping Emission Products from Commonly Used Vape Juice Diluents. Chem Res Toxicol 33, 2157–2163. [DOI] [PubMed] [Google Scholar]
- (14).Meehan-Atrash J, Luo W, McWhirter KJ, Dennis DG, Sarlah D, Jensen RP, Afreh I, Jiang J, Barsanti KC, Ortiz A, and Strongin RM (2021) The influence of terpenes on the release of volatile organic compounds and active ingredients to cannabis vaping aerosols. RSC Advances 11, 11714–11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Meehan-Atrash J, Luo W, McWhirter KJ, and Strongin RM (2019) Aerosol Gas-Phase Components from Cannabis E-Cigarettes and Dabbing: Mechanistic Insight and Quantitative Risk Analysis. ACS Omega 4, 16111–16120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Miech RA, Patrick ME, O’Malley PM, Johnston LD, and Bachman JG (2020) Trends in Reported Marijuana Vaping Among US Adolescents, 2017–2019. JAMA 323, 475–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Braymiller JL, Barrington-Trimis JL, Leventhal AM, Islam T, Kechter A, Krueger EA, Cho J, Lanza I, Unger JB, and McConnell R (2020) Assessment of Nicotine and Cannabis Vaping and Respiratory Symptoms in Young Adults. JAMA Netw Open 3, e2030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Schauer GL, Dilley JA, Roehler DR, Sheehy TJ, Filley JR, Broschart SC, Holland KM, Baldwin GT, Holmes-Chavez AK, and Hoots BE (2021) Cannabis sales increases during COVID-19: Findings from Alaska, Colorado, Oregon, and Washington. Int J Drug Policy 98, 103384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Gieringer DH (2001) Cannabis “Vaporization”: A Promising Strategy for Smoke Harm Reduction. J. Cannabis Ther 1, 153–170. [Google Scholar]
- (20).Lik H (2013) Electronic cigarette, (Organization, U. S. P. a. T., Ed.), Ruyan Investment (Holdings) Limited, United States. [Google Scholar]
- (21).Gieringer DH, Laurent J, and Goodrich S (2004) Cannabis Vaporizer Combines Efficient Delivery of THC with Effective Suppression of Pyrolytic Compounds. J. Cannabis Ther 4, 7–27. [Google Scholar]
- (22).Hazekamp A, Ruhaak R, Zuurman L, van Gerven J, and Verpoorte R (2006) Evaluation of a vaporizing device (Volcano) for the pulmonary administration of tetrahydrocannabinol. J Pharm Sci 95, 1308–1317. [DOI] [PubMed] [Google Scholar]
- (23).Fataar F, and Hammond D (2019) The Prevalence of Vaping and Smoking as Modes of Delivery for Nicotine and Cannabis among Youth in Canada, England and the United States. Int J Environ Res Public Health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Loflin M, and Earleywine M (2014) A new method of cannabis ingestion: the dangers of dabs? Addict Behav 39, 1430–1433. [DOI] [PubMed] [Google Scholar]
- (25).Meehan-Atrash J, Luo W, and Strongin RM (2017) Toxicant Formation in Dabbing: The Terpene Story. ACS Omega 2, 6112–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Stogner JM, and Miller BL (2015) The Dabbing Dilemma: A Call for Research on Butane Hash Oil and Other Alternate Forms of Cannabis Use. Subst Abus 36, 393–395. [DOI] [PubMed] [Google Scholar]
- (27).Stogner JM, and Miller BL (2015) Assessing the Dangers of “Dabbing”: Mere Marijuana or Harmful New Trend? Pediatrics 136, 1–3. [DOI] [PubMed] [Google Scholar]
- (28).Etter JF (2015) Electronic cigarettes and cannabis: an exploratory study. Eur Addict Res 21, 124–130. [DOI] [PubMed] [Google Scholar]
- (29).Jones CB, Hill ML, Pardini DA, and Meier MH (2016) Prevalence and correlates of vaping cannabis in a sample of young adults. Psychol Addict Behav 30, 915–921. [DOI] [PubMed] [Google Scholar]
- (30).Popova L, McDonald EA, Sidhu S, Barry R, Maruyama Richers TA, Sheon NM, and Ling PM (2017) Perceived harms and benefits of tobacco, marijuana, and electronic vaporizers among young adults in Colorado: implications for health education and research. Addiction 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Sirikantaramas S, Taura F, T. Y, Ishikawa Y, Morimoto S, and Shoyama Y (2005) Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of the glandular trichomes. Plant Cell Physiol. 46, 1578–1582. [DOI] [PubMed] [Google Scholar]
- (32).Perrotin-Brunel H, van Roosmalen MJE, van Spronsen J, Verpoorte R, Peters CJ, and Witkamp GJ (2010) Supercritical fluid extraction of cannabis: experiments and modelling of the process design. ISASF-Graz, 1–6. [Google Scholar]
- (33).Meehan-Atrash J (2021) Chemical Characterization of Toxicologically Relevant Molecules in Cannabis Concentrates and Vaporizer Aerosols, In Chemistry, Portland State University, Portland, OR. [Google Scholar]
- (34).Duell AK, Pankow JF, Gillette SM, and Peyton DH (2018) Boiling points of the propylene glycol + glycerol system at 1 atmosphere pressure: 188.6–292 degrees C without and with added water or nicotine. Chem Eng Commun 205, 1691–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lovestead TM B. TJ (2017) Determination of Cannabinoid Vapor Pressures to Aid in Vapor Phase Detection of Intoxication. Forensic Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lynch J, Lorenz L, Brueggemeyer JL, Lanzarotta A, Falconer TM, and Wilson RA (2021) Simultaneous Temperature Measurements and Aerosol Collection During Vaping for the Analysis of Delta(9)-Tetrahydrocannabinol and Vitamin E Acetate Mixtures in Ceramic Coil Style Cartridges. Front Chem 9, 734793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Flockhart I, Wheatley G, Willian Dring, S., and Archer L (2004) Methods of preparing cannabinoids from plant material, (Organization, W. I. P., Ed.).
- (38).Perrotin-Brunel H, Kroon MC, van Roosmalen MJE, van Spronsen J, Peters CJ, and Witkamp GJ (2010) Solubility of non-psychoactive cannabinoids in supercritical carbon dioxide andcomparison with psychoactive cannabinoids. J Supercrit Fluids 55, 603–608. [Google Scholar]
- (39).White J, Poovendran D, and Kershaw S (2017) Cannabidiol (CBD) Pre- Review Report. Agenda Item 5.2, In Expert Committee on Drug Dependence Thirty-ninth Meeting, World Health Organization, Geneva. [Google Scholar]
- (40).Myrcene [Liquid] Chemical Data Sheet.
- (41).Stull DR (1947) Vapor Pressure of Pure Substances. Organic and Inorganic Compounds. Ind. Eng. Chem 39, 517–540. [Google Scholar]
- (42).d-Limonene Chemical Data Sheet.
- (43).Clará RA, Marigliano ACG, and Sólimo HN (2009) Density, Viscosity, and Refractive Index in the Range (283.15 to 353.15) K and Vapor Pressure of α-Pinene, d-Limonene, (±)-Linalool, and Citral Over the Pressure Range 1.0 kPa Atmospheric Pressure. Journal of Chemical & Engineering Data 54, 1087–1090. [Google Scholar]
- (44).Lecat M (1929) Azeotropismus in binären Systemen, bestehend aus einem Alkohol, gemischt mit einem Amin, einem Nitroderivative, einem Äther (Oxyd), oder Wasser. Zeitschrift für anorganische und allgemeine Chemie 186, 119–140. [Google Scholar]
- (45).Winstein S, Morse BK, Grunwald E, Jones HW, Corse J, Trifan D, and Marshall H (2002) Neighboring Carbon and Hydrogen. VII. Reactivity of Some Alicyclic and Bicyclic Derivatives1,2,3. Journal of the American Chemical Society 74, 1127–1132. [Google Scholar]
- (46).(2021) 2020 National Drug Threat Assessment, (Justice, D. o., Ed.), Drug Enforcement Agency. [Google Scholar]
- (47).Abernethy A (2019) Hemp Production and the 2018 Farm Bill, In Congressional Testimony before the Senate Committee on Agriculture, Nutrition, and Forestry, Food and Drug Administration. [Google Scholar]
- (48).(2020) Implementation of the Agriculture Improvement Act of 2018, In 21 CFR §1308 and §1312 (Drug Enforcement Administration (DEA), D. o. J., Ed.), US Government Publishing Office. [Google Scholar]
- (49).Hanus LO, Meyer SM, Munoz E, Taglialatela-Scafati O, and Appendino G (2016) Phytocannabinoids: a unified critical inventory. Nat Prod Rep 33, 1357–1392. [DOI] [PubMed] [Google Scholar]
- (50).Hollister LE, and Gillespie HK (1973) Delta-8- and delta-9-tetrahydrocannabinol comparison in man by oral and intravenous administration. Clin Pharmacol Ther 14, 353–357. [DOI] [PubMed] [Google Scholar]
- (51).(2021) The Unregulated Distribution And Sale Of Consumer Products Marketed As Delta-8 THC US Cannabis Council, uscannabiscouncil.org.
- (52).Duffy B, Li L, Lu S, Durocher L, Dittmar M, Delaney-Baldwin E, Panawennage D, LeMaster D, Navarette K, and Spink D (2020) Analysis of Cannabinoid-Containing Fluids in Illicit Vaping Cartridges Recovered from Pulmonary Injury Patients: Identification of Vitamin E Acetate as a Major Diluent. Toxics 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Adams R, Pease DC, Cain CK, Baker BR, Clark JH, Wolff H, and Wearn RB (1940) Conversion of Cannabidiol to a Product with Marihuana Activity. A Type Reaction for Synthesis of Analogous Substances. Conversion of Cannabidiol to Cannabinol. Journal of the American Chemical Society 62, 2245–2246. [Google Scholar]
- (54).Webster GR, Sarna L, and Mechoulam R (2002) Conversion of cbd to delta8-thc and delta9-thc United States.
- (55).Holler JM S. ML; Paul SN; Past MR; Paul BD (2008) Isomerization of delta-9-THC to delta-8-THC when tested as trifluoroacetyl-, pentafluoropropionyl-, or heptafluorobutyryl- derivatives. J. Mass Specrom 43, 674–679. [DOI] [PubMed] [Google Scholar]
- (56).Golombek P, Muller M, Barthlott I, Sproll C, and Lachenmeier DW (2020) Conversion of Cannabidiol (CBD) into Psychotropic Cannabinoids Including Tetrahydrocannabinol (THC): A Controversy in the Scientific Literature. Toxics 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Marzullo P, Foschi F, Coppini DA, Fanchini F, Magnani L, Rusconi S, Luzzani M, and Passarella D (2020) Cannabidiol as the Substrate in Acid-Catalyzed Intramolecular Cyclization. Journal of Natural Products 83, 2894–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Kiselak TD, Koerber R, and Verbeck GF (2020) Synthetic route sourcing of illicit at home cannabidiol (CBD) isomerization to psychoactive cannabinoids using ion mobility-coupled-LC-MS/MS. Forensic Sci Int 308, 110173. [DOI] [PubMed] [Google Scholar]
- (59).Cooke J (2021) How To Make Delta 8 THC: CBD to Δ8 THC Step-By-Step, DailyCBD, dailycbd.com.
- (60).Erickson BE (2021) Delta-8-THC craze concerns chemists, In Chemical & Engineering News, American Chemical Society, Washington DC. [Google Scholar]
- (61).Sullivan K (2021) Delta-8 THC is legal in many states, but some want to ban it National Broadcasting Company, nbc.com.
- (62).Johnson-Arbor K, and Smolinske S (2021) The current state of delta-8 THC. Am J Emerg Med. [DOI] [PubMed] [Google Scholar]
- (63).Effex D (2021) You’ve heard about delta-8. Now, meet delta-10., Leafly Holdings, Inc, Leafly.com.
- (64).Srebnik M, Lander N, Breuer A, and Mechoulam R (1984) Base-catalysed double-bond isomerizations of cannabinoids: structural and stereochemical aspects. Journal of the Chemical Society, Perkin Transactions 1 12, 2881–2886. [Google Scholar]
- (65).HHC - Hexahydrocannabinol Products - Bearly Legal Hemp, Bearly Legal Hemp, bearlylegalhemp.com.
- (66).Pray IW, Atti SK, Tomasallo C, and Meiman JG (2020) E-cigarette, or Vaping, Product Use-Associated Lung Injury Among Clusters of Patients Reporting Shared Product Use - Wisconsin, 2019. MMWR Morb Mortal Wkly Rep 69, 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Muthumalage T, Friedman MR, McGraw MD, Ginsberg G, Friedman AE, and Rahman I (2020) Chemical Constituents Involved in E-Cigarette, or Vaping Product Use-Associated Lung Injury (EVALI). Toxics 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, Braselton M, Brosius CR, Caron KT, Chambers D, Corstvet J, Cowan E, De Jesus VR, Espinosa P, Fernandez C, Holder C, Kuklenyik Z, Kusovschi JD, Newman C, Reis GB, Rees J, Reese C, Silva L, Seyler T, Song MA, Sosnoff C, Spitzer CR, Tevis D, Wang L, Watson C, Wewers MD, Xia B, Heitkemper DT, Ghinai I, Layden J, Briss P, King BA, Delaney LJ, Jones CM, Baldwin GT, Patel A, Meaney-Delman D, Rose D, Krishnasamy V, Barr JR, Thomas J, Pirkle JL, and Lung Injury Response Laboratory Working, G. (2020) Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N Engl J Med 382, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).(October 29, 2020) Non-Cannabis Additives in Inhalable Cannabinoid Products: Rationale for Rulemaking, (Commission, O. L. C., Ed.), Salem, OR. [Google Scholar]
- (70).Jensen RP, Strongin RM, and Peyton DH (2017) Solvent Chemistry in the Electronic Cigarette Reaction Vessel. Sci Rep 7, 42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Peace MR, Butler KE, Wolf CE, Poklis JL, and Poklis A (2016) Evaluation of Two Commercially Available Cannabidiol Formulations for Use in Electronic Cigarettes. Front Pharmacol 7, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Grotenhermen F (2003) Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin Pharmacokinet 42, 327–360. [DOI] [PubMed] [Google Scholar]
- (73).Huestis MA, S. ML, (2007) Human Cannabinoid Pharmacokinetics and Interpretation of Cannabinoid Concentrations in Biological Fluids and Tissues, In Marijuana and the Cannabinoids (ElSohly M, Ed.), Humana Press, Totowa, New Jersey. [Google Scholar]
- (74).Anderson GD, and Chan LN (2016) Pharmacokinetic Drug Interactions with Tobacco, Cannabinoids and Smoking Cessation Products. Clin Pharmacokinet 55, 1353–1368. [DOI] [PubMed] [Google Scholar]
- (75).Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, and Casellas P (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232, 54–61. [DOI] [PubMed] [Google Scholar]
- (76).Staiano RI, Loffredo S, Borriello F, Iannotti FA, Piscitelli F, Orlando P, Secondo A, Granata F, Lepore MT, Fiorelli A, Varricchi G, Santini M, Triggiani M, Di Marzo V, and Marone G (2016) Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J Leukoc Biol 99, 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Sarafian T, Montes C, Harui A, Beedanagari SR, Kiertscher S, Stripecke R, Hossepian D, Kitchen C, Kern R, Belperio J, and Roth MD (2008) Clarifying CB2 receptor-dependent and independent effects of THC on human lung epithelial cells. Toxicol Appl Pharmacol 231, 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Sarafian T K. S; Khoshaghideh F; Tashkin DP; Roth MD. (2003) delta-9-Tetrahydrocannabinol dusrupts mitochondrial function and cell energetics. Am J Physiol Lung Cell Mol Physiol 284, L298–L306. [DOI] [PubMed] [Google Scholar]
- (79).Zawatsky CN, Abdalla J, and Cinar R (2020) Synthetic cannabinoids induce acute lung inflammation via cannabinoid receptor 1 activation. ERJ Open Res 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Coffey RG, Snella E, Johnson K, and Pross S (1996) Inhibition of macrophage nitric oxide production by tetrahydrocannabinol in vivo and in vitro. International Journal of Immunopharmacology 18, 749–752. [DOI] [PubMed] [Google Scholar]
- (81).Zheng Z-M, and Specter SC (1996) Delta-9-tetrahydrocannabinol suppresses tumor necrosis factor α maturation and secretion but not its transcription in mouse macrophages. International Journal of Immunopharmacology 18, 53–68. [DOI] [PubMed] [Google Scholar]
- (82).Jie-Liu T, Lancz G, Specter S, and Bullock H (1992) Marijuana and immunity: Tetrahydrocannabinol-mediated inhibition of growth and phagocytic activity of the murine macrophage cell line, P388D1. International Journal of Immunopharmacology 14, 253–262. [DOI] [PubMed] [Google Scholar]
- (83).Baldwin GC, Tashkin DP, Buckley DM, Park AN, Dubinett SM, and Roth MD (1997) Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am J Respir Crit Care Med 156, 1606–1613. [DOI] [PubMed] [Google Scholar]
- (84).McMahon MJ, Bhatt NA, Stahlmann CG, and Philip AI (2016) Severe Pneumonitis after Inhalation of Butane Hash Oil. Ann Am Thorac Soc 13, 991–992. [DOI] [PubMed] [Google Scholar]
- (85).Turcotte C, Blanchet MR, Laviolette M, and Flamand N (2016) Impact of Cannabis, Cannabinoids, and Endocannabinoids in the Lungs. Front Pharmacol 7, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Ribeiro A, F.-d.-P. V, Pinheiro ML, Vitoretto LB, Mariano-Souze DP, Quinteiro-Filho WM, Akamine AT, Almeida VI, Quevedo J, Dal-Pizzol F, Hallak JE, Zuardi AW. (2012) Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A2A receptor. European Journal of Pharmacology 678, 78–85. [DOI] [PubMed] [Google Scholar]
- (87).Muthumalage T, and Rahman I (2019) Cannabidiol differentially regulates basal and LPS-induced inflammatory responses in macrophages, lung epithelial cells, and fibroblasts. Toxicol Appl Pharmacol 382, 114713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Leigh NJ, and Goniewicz ML (2020) Effect of aerosolized nicotine on human bronchial epithelial cells is amplified after co-administration with cannabidiol (CBD): a pilot in vitro study. BMC Pharmacol Toxicol 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Leigh NJ, and Goniewicz ML (2020) Acute Effect of Electronic Cigarette- Generated Aerosol From Flavored CBD-Containing Refill Solutions on Human Bronchial Epithelial Cells. Front Physiol 11, 592321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Van der Kooy F, Pomahacova B, and Verpoorte R (2008) Cannabis smoke condensate I: the effect of different preparation methods on tetrahydrocannabinol levels. Inhal Toxicol 20, 801–804. [DOI] [PubMed] [Google Scholar]
- (91).Bechtold J, Simpson T, White HR, and Pardini D (2015) Chronic adolescent marijuana use as a risk factor for physical and mental health problems in young adult men. Psychol Addict Behav 29, 552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Arterberry BJ, Treloar Padovano H, Foster KT, Zucker RA, and Hicks BM (2019) Higher average potency across the United States is associated with progression to first cannabis use disorder symptom. Drug Alcohol Depend 195, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Rohr AC (2013) The health significance of gas- and particle-phase terpene oxidation products: a review. Environ Int 60, 145–162. [DOI] [PubMed] [Google Scholar]
- (94).Bitzer ZT, Goel R, Reilly SM, Elias RJ, Silakov A, Foulds J, Muscat J, and Richie JP Jr. (2018) Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic Biol Med 120, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Calandra MJ, Wang Y, Impellizzeri J, Frank S, de Saint Laumer J-Y, Leocata S, and Chaintreau A (2016) Terpene hydroperoxide chemistry in citrus oils; reaction with endogenous aldehydes to form peroxyhemiacetals. Flavour and Fragrance Journal 31, 241–249. [Google Scholar]
- (96).Bezard M, Gimenez-Arnau E, Meurer B, Grossi L, and Lepoittevin JP (2005) Identification of carbon-centred radicals derived from linalyl hydroperoxide, a strong skin sensitizer: a possible route for protein modifications. Bioorg Med Chem 13, 3977–3986. [DOI] [PubMed] [Google Scholar]
- (97).Kao D, Chaintreau A, Lepoittevin JP, and Gimenez-Arnau E (2011) Synthesis of allylic hydroperoxides and EPR spin-trapping studies on the formation of radicals in iron systems as potential initiators of the sensitizing pathway. J Org Chem 76, 6188–6200. [DOI] [PubMed] [Google Scholar]
- (98).Wolkoff P, Clausen PA, Larsen K, Hammer M, Larsen ST, and Nielsen GD (2008) Acute airway effects of ozone-initiated d-limonene chemistry: importance of gaseous products. Toxicol Lett 181, 171–176. [DOI] [PubMed] [Google Scholar]
- (99).Doyle M, Sexton KG, Jeffries H, Bridge K, and Jaspers I (2004) Effects of 1,3-butadiene, isoprene, and their photochemical degradation products on human lung cells. Environ Health Perspect 112, 1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Nilsson AM, Bergstrom MA, Luthman K, Nilsson JLG, and Karlberg AT (2005) A conjugated diene identified as a prohapten: Contact allergenic activity and chemical reactivity of proposed epoxide metabolites. Chemical Research in Toxicology 18, 308–316. [DOI] [PubMed] [Google Scholar]
- (101).Bezard M, Karlberg AT, Montelius J, and Lepoittevin JP (1997) Skin sensitization to linalyl hydroperoxide: support for radical intermediates. Chem Res Toxicol 10, 987–993. [DOI] [PubMed] [Google Scholar]
- (102).Karlberg AT, Magnusson K, and Nilsson U (1992) Air oxidation of d-limonene (the citrus solvent) creates potent allergens. Contact Dermatitis 26, 332–340. [DOI] [PubMed] [Google Scholar]
- (103).Fels Elliott DR, Shah R, Hess CA, Elicker B, Henry TS, Rule AM, Chen R, Golozar M, and Jones KD (2019) Giant cell interstitial pneumonia secondary to cobalt exposure from e-cigarette use. Eur Respir J 54. [DOI] [PubMed] [Google Scholar]
- (104).Mallampati SR, McDaniel C, and Wise AR (2021) Strategies for Nonpolar Aerosol Collection and Heavy Metals Analysis of Inhaled Cannabis Products. ACS Omega 6, 17126–17135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).He T, Oks M, Esposito M, Steinberg H, and Makaryus M (2017) “Tree-in-Bloom”: Severe Acute Lung Injury Induced by Vaping Cannabis Oil. Ann Am Thorac Soc 14, 468–470. [DOI] [PubMed] [Google Scholar]
- (106).Anderson RP, and Zechar K (2019) Lung injury from inhaling butane hash oil mimics pneumonia. Respir Med Case Rep 26, 171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Butt YM, Smith ML, Tazelaar HD, Laslo TV, Swanson KL, Cecchini MJ, Boland JM, Bois MC, Boyum JH, Froemming AT, Khoor A, Mira-Avendano I, Patel A, and Larsen BT (2019) Pathology of Vaping-Associated Lung Injury. NEJM 381. [DOI] [PubMed] [Google Scholar]
- (108).Shields PG, Song MA, Freudenheim JL, Brasky TM, McElroy JP, Reisinger SA, Weng DY, Ren R, Eissenberg T, Wewers MD, and Shilo K (2020) Lipid laden macrophages and electronic cigarettes in healthy adults. EBioMedicine 60, 102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Feldman R, Stanton M, and Suelzer EM (2021) Compiling Evidence for EVALI: A Scoping Review of In Vivo Pulmonary Effects After Inhaling Vitamin E or Vitamin E Acetate. J Med Toxicol 17, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Wu D, and O’Shea DF (2020) Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proc Natl Acad Sci U S A 117, 6349–6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Lee H (2020) Vitamin E acetate as linactant in the pathophysiology of EVALI. Med Hypotheses 144, 110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Rahman I, and Adcock IM (2006) Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 28, 219–242. [DOI] [PubMed] [Google Scholar]


