Abstract
Objectives
Oncologists estimate patients’ prognosis to guide care. Evidence suggests oncologists tend to overestimate life expectancy, which can lead to care with questionable benefits. Information obtained from geriatric assessment may improve prognostication for older adults. In this study, we created a geriatric assessment-based prognostic model for older adults with advanced cancer and compared its performance to alternative models.
Materials and Methods:
We conducted a secondary analysis of a trial (URCC 13070; PI: Mohile) capturing geriatric assessment and vital status up to one year for adults age ≥70 years with advanced cancer. Oncologists estimated life expectancy as 0-6 months, 7-12 months, and >1 year. Three statistical models were developed: (1) a model including age, sex, cancer type, and stage (basic model), (2) basic model + Karnofsky Performance Status (≤50, 60-70, and 80+) (KPS model), and (3) basic model + 16 binary indicators of geriatric assessment impairments (GA model). Cox regression was used to model one-year survival; c-indices and time-dependent c-statistics assessed model discrimination and stratified survival curves assessed model calibration.
Results:
We included 484 participants; mean age was 75; 48% had gastrointestinal or lung cancer. Overall, 43% of patients died within one year. Oncologists classified prognosis accurately for 55% of patients, overestimated for 35%, and underestimated for 10%. C-indices were 0.61 (basic model), 0.62 (KPS model), and 0.63 (GA model). The GA model was well-calibrated.
Conclusions:
The GA model showed moderate discrimination for survival, similar to alternative models, but calibration was improved. Further research is needed to optimize geriatric assessment-based prognostic models for use in older adults with advanced cancer.
Keywords: geriatric assessment, advanced cancer, prediction modeling
Introduction
Oncologists must use information available to them to estimate their patients’ life expectancy to optimize decisions about cancer treatment and supportive care. Accurate estimation of life expectancy for older adults with cancer can be challenging due to heterogeneity in the presence of comorbidities, functional and cognitive impairments, and geriatric syndromes.1–3 In the advanced cancer setting, several studies show that oncologists tend to overestimate their patients’ life expectancy.4–7 When oncologists overestimate life expectancy, they may miss opportunities to discuss transitions in goals of care and thus help patients prepare for end-of-life decisions.
As such, there is a need to improve oncologists’ prediction of life expectancy among older adults with advanced cancers. One way to achieve this goal is by developing robust prognostic models that can (1) effectively distinguish between patients who are likely to die earlier versus later (i.e., discrimination) and (2) accurately capture observed survival across the range of predicted life expectancy estimates (i.e., calibration).8 Researchers have attempted to use traditional oncology assessments like the Karnofsky Performance Status9 (KPS) and Eastern Cooperative Oncology Group Performance Status for prognostication in patients with advanced cancer, but they have resulted in only moderate discrimination.10 Other models have tried to incorporate symptoms11,12 or intake and consciousness level,11 but these efforts have resulted in little improvement in model discrimination. Thus, new approaches for improving prognostication among older adults with advanced cancers are needed.
Geriatric assessment,13,14 a set of validated, patient-reported and objective measures to assess comorbidity, functional status, physical performance, cognitive status, psychological status, nutritional status, polypharmacy, and social support, has been shown to uncover age-related problems (e.g., cognitive impairment) not captured in standard oncology assessments.15,16 Recent studies show that poor physical function and nutritional status captured via the geriatric assessment are associated with worse survival in older adults with advanced cancers.17,18 While these studies highlight important prognostic factors in older adults with advanced cancers, they have not explicitly focused on evaluating the performance of prognostic models using the geriatric assessment. In this study, we create a geriatric assessment-based prognostic model and compare its performance (discrimination – overall and at specific time points - as well as calibration) to alternative models and oncologist-estimated life expectancy.
Materials and Methods
Data source and study population
We conducted a secondary analysis of a community-based, cluster-randomized trial.19 The trial was conducted within the University of Rochester Cancer Center (URCC) National Cancer Institute Community Oncology Research Program (NCORP) and enrolled patients who were aged 70 years and above, had a diagnosis of an incurable stage III/IV solid tumor or lymphoma, had at least one (out of 8) impaired geriatric assessment domains other than polypharmacy, and were considering or receiving any kind of cancer treatment.20,21 The treating oncologist was also enrolled. Individuals who had planned surgery or decided to forgo cancer treatment were excluded. For this study, participants who did not have a completed survival form (n= 57) were excluded, resulting in a study population of 484 patients and 121 oncologists. A comparison of patient characteristics from the included and excluded populations is provided in Supplemental Table 1.
Oncologist-reported measures
Two oncologist-reported measures were evaluated at study enrollment. The first was the treating oncologist’s estimate of patient life expectancy assessed as follows: “Considering the patient’s health, and underlying medical conditions, what would you estimate the patient’s overall life expectancy to be?” This question was adapted from a previous study of seriously ill older patients (including those with cancer),22 and this estimate was intended to include the current diagnosis of advanced cancer. Responses included 0-6 months, 7-12 months, 1 to 2 years, 2 to 5 years, >5 years. As active follow-up was only conducted for one year, we collapsed the responses for our analysis into the following categories: 0-6 months, 7-12 months, and >1 year.
The second measure was oncologist rated Karnofsky Performance Status (KPS),9 reported only at patient enrollment as a range from 0% (dead) to 100% (normal, no evidence of disease). For analysis, we grouped KPS into three groups: 50% and lower, 60-70%, and 80% and higher.
Survival
Vital status and date of death or end of follow-up were ascertained using study forms and verified at each study site by the study coordinator. As part of the trial protocols, survival was assessed only up to one year following study enrollment.
Geriatric assessment
Geriatric assessment was performed at study enrollment, including 16 individual tests19 covering the following health domains: physical performance, functional status, comorbidity, cognition, nutrition, social support, polypharmacy, and psychological health. For our analysis, we created a binary variable for each of these 16 tests to indicate whether a deficit was present or not, as done previously in the COACH trial (see detailed content published elsewhere20,21).
Other patient characteristics
The trial also recorded demographic information including self-reported age, gender, and race/ethnicity. Cancer type and stage were assessed at enrollment. Additional self-reported information regarding marital status, household income, and educational attainment were also collected at enrollment.
Statistical analysis
Characteristics of the study population were summarized. Kaplan-Meier methods were used to describe survival at one year. Among those with complete data (n=429), we compared the oncologists’ estimates with observed survival and measured agreement based on the three categories (0-6 months, 7-12 months, and >1 year) using the weighted kappa.23
We constructed three statistical models to predict survival. The basic model included age, sex, cancer type, and stage, representing information routinely available to oncologists. The KPS model included the basic model plus the addition of KPS categories (50% and lower, 60-70%, and 80% and higher), allowing exploration of whether and how subjective KPS assessment improves prognostication. Third, the GA model included the basic model plus the 16 binary indicators of geriatric assessment-defined deficits. We did not include KPS in the GA model as we wanted to describe the performance of a model that used objective measures of geriatric health instead of subjective assessments for prognostication. Finally, we also evaluated the performance of oncologists’ life expectancy estimates in predicting survival, which likely includes both objective assessment of the health status and tumor characteristics, but also subjective assessments that may reflect the oncologists’ clinical impression of fitness or frailty. However, we do not refer to this as a model, as it only represents a single variable.
Cox proportional hazards regression was used to model one-year survival as a function of the specified model predictors. Model discrimination measures the predictive ability of a model to accurately rank individuals according to their survival time. We evaluated model discrimination via ten-fold cross-validation24 using Harrell’s concordance statistic (or c-index),25 as well as the time-dependent area under the receiver operating curve (AUC) or c-statistic evaluated at 30, 90, 180, and 365 days. Concordance statistics range from 0 to 1 with a value of 0.5 representing prediction no better than chance. Concordance statistics ranging from 0.5-0.6 are often considered as poor, 0.6-0.7 as moderate, 0.7-0.8 as good, 0.8-0.9 as very good, and 0.9 and above as excellent.26 Calibration, or the agreement between the model-predicted and observed survival estimates,27 was visually inspected. We plotted stratified observed versus model-predicted survival curves by approximating the baseline survival function within four prognostic groups, defined at the 18th, 50th, and 84th percentiles, using methods described by Royston et al.28 Observed and model-predicted curves that largely overlap suggest well-calibrated models. As the oncologist model was a simple stratification of observed survival across three prognostic groups, we plotted observed survival only.
The University of North Carolina at Chapel Hill Institutional Review Board (IRB) determined this research to be exempt from IRB review.
Results
Patient characteristics and overall survival
Table 1 summarizes the characteristics of the study population. In total, 484 patients were included in the study with a median age of 75 years and almost half were diagnosed with a gastrointestinal or lung cancer. The median time from advanced cancer diagnosis to study enrollment was 227 days. Among the 16 individual geriatric assessment tests, the most common deficit identified was polypharmacy (84%) followed by deficits in physical performance defined by the Short Physical Performance Battery (81%) and the OARS Physical Health (75%).
Table 1.
Characteristics of 484 Participants Included in the Study Population
| Patient characteristics* | n | % |
|---|---|---|
| Age, median years (IQR) | 75 (72, 80) | |
| Sex | ||
| Female | 238 | 49 |
| Male | 245 | 51 |
| Time from advanced cancer diagnosis to enrollment, median days (IQR) | 227 (65, 660) | |
| Cancer type | ||
| Gastrointestinal | 116 | 24 |
| Lung | 117 | 24 |
| Breast | 66 | 14 |
| Genitourinary | 67 | 14 |
| Other | 117 | 24 |
| Stage | ||
| III | 43 | 9 |
| IV | 427 | 88 |
| Other | 13 | 3 |
| Domain: Polypharmacy | ||
| Polypharmacy (5+ medications) | 408 | 84 |
| Domain: Cognition | ||
| BLESSED Orientation-Memory-Concentration | 11 | 2 |
| Mini Cog (based on word recall and clock drawing) | 166 | 34 |
| Domain: Nutrition | ||
| Weight loss (>10% change from 6 months ago) | 71 | 15 |
| Body mass index <21 (low weight) | 60 | 12 |
| Mini Nutrition Assessment (≤ 11 points) | 278 | 57 |
| Domain: Physical Performance | ||
| Timed “Up and Go” (≥ 13.5 seconds) | 200 | 41 |
| Short Physical Performance Battery (≤ 9 points) | 392 | 81 |
| Falls (any history of falls in the prior 6 months) | 125 | 26 |
| OARS Physical Health (any limitation defined as “a lot”) | 364 | 75 |
| Domain: Functional Status | ||
| Activities of Daily Living (any deficit identified) | 138 | 29 |
| Instrumental Activities of Daily Living (requiring help or unable to do) | 275 | 57 |
| Domain: Comorbidity | ||
| OARS Comorbidity (3 illnesses or 1 that interferes a great deal) | 308 | 64 |
| Domain: Psychological Health | ||
| Generalized Anxiety Disorder-7 (≥ 10 points) | 39 | 8 |
| Geriatric Depression Scale (≥ 5 points) | 106 | 22 |
| Domain: Social Support | ||
| OARS Medical Social Support (as “some”, “a little”, or “none of the time”) | 143 | 30 |
| Estimated life expectancy | ||
| 0-6 months | 29 | 6 |
| 7-12 months | 106 | 22 |
| 1+years | 340 | 70 |
| Missing | 9 | 2 |
Overall, 43% of the population died within one year. Among patients with known survival times and oncologist generated life expectancy estimates (n=429, Table 2), oncologists estimated that 23 patients (5%) would survive 0-6 months, 93 patients (22%) would survive 7-12 months, and 313 patients (73%) would survive >1 year. This is contrasted with actual survival where 105 patients (24%) died within 0-6 months, 84 patients (20%) died within 7-12 months, and only 240 patients (56%) survived to >1 year. Overall, oncologists’ accurately estimated life expectancy in 55% of patients, but overestimated life expectancy in 35% of patients and underestimated it in 10% of patients (weighted kappa=0.21).
Table 2.
Cross-Classification of Oncologist-Estimated Life Expectancy and Observed Survival in the Trial Participants with Complete Data.
| Oncologist estimate | Actual survival |
|||
|---|---|---|---|---|
| 0-6 months | 7-12 months | 1+ years | Total | |
| 0-6 months | 10 (2.3%) | 8 (1.9%) | 5 (1.2%) | 23 (5.4%) |
| 7-12 months | 43 (10.0%) | 20 (4.7%) | 30 (7.0%) | 93 (21.7%) |
| 1+ years | 52 (12.1%) | 56 (13.1%) | 205 (47.8%) | 313 (73.0%) |
|
| ||||
| Total | 105 (24.5%) | 84 (19.6%) | 240 (55.9%) | 429 (100%) |
Note: The analysis is limited to patients with complete information on oncologist-estimated life expectancy and non-censored survival times (i.e., complete follow-up through one year from enrollment). The classification table displays agreement and disagreement between oncologists’ estimates of life expectancy (rows) and observed survival (columns). Oncologists’ overestimation of survival is noted in light grey, while underestimation is noted in dark grey. All percentages represent cell percentages (i.e., a proportion of the total number of study participants with complete information, n=429).
Prognostic model performance
Discrimination
Table 3 reports Harrell’s c-index indicating the discrimination between predicted and observed survival times based on each model, as well as the c-statistic at each specific time point. Overall, the GA model resulted in the highest c-index of 0.63 (0.56, 0.69), but was similar to the c-indices for the basic model of 0.61 (0.59, 0.65), the KPS model of 0.62 (0.55, 0.68), and oncologists’ life expectancy estimates of 0.61 (0.50, 0.71). The time-dependent c-statistic for the KPS model and oncologists’ life expectancy estimates were highest for the 30-day time point and decreased slightly over time, whereas the time-dependent c-statistic was relatively stable across time for the basic and GA models. Parameter estimates from all models are included in Supplemental Tables 2–5.
Table 3.
C-Indices and Time-Dependent Areas Under the Receiver Operating Curves for the Three Multivariable Models and for Oncologist-Reported Life Expectancy Estimates
| Measure | Basic model | KPS model | GA model | |
|---|---|---|---|---|
| Age, sex, cancer type, and stage | Basic model + KPS | Basic model + 16 GA impairments | Oncologist-estimated life expectancy categories | |
| C-index (95% CI) | 0.59 (0.54, 0.65) | 0.62 (0.55, 0.68) | 0.63 (0.56, 0.69) | 0.61 (0.5, 0.71) |
| Time-dependent AUC (95% CI) | ||||
| 30 days | 0.61 (0.21, 1.00) | 0.73 (0.44, 1.00) | 0.65 (0.39, 0.91) | 0.77 (0.33, 1.00) |
| 90 days | 0.62 (0.54, 0.70) | 0.67 (0.54, 0.80) | 0.68 (0.57, 0.79) | 0.67 (0.48, 0.87) |
| 180 days | 0.61 (0.53, 0.69) | 0.64 (0.55, 0.73) | 0.66 (0.60, 0.72) | 0.65 (0.45, 0.84) |
| 365 days | 0.61 (0.52, 0.69) | 0.63 (0.54, 0.73) | 0.65 (0.56, 0.74) | 0.64 (0.51, 0.76) |
Abbreviations: area under the receiver operating curve=AUC, Karnofsky Performance Status=KPS, geriatric assessment=GA, confidence interval=CI
Calibration
Figure 1A–C shows the predicted and observed survival curves for the four statistical models stratified by four prognostic groups identified by the 18th, 50th, and 84th percentiles of survival times. The dashed lines represent the predicted survival times and the solid lines represent the observed survival times. Figure 1D displays observed survival across the three oncologist-estimated life expectancy categories. The basic model showed good calibration with observed and predicted curves largely overlapping, with slight underestimation of survival in the highest quartile group. The KPS model had the worst calibration, with underestimation of survival in the lowest survival quartile but overestimation in the second to lowest survival quartile. Finally, the GA model was generally well-calibrated with curves overlapping, with slight overestimation of survival in the highest quartile group. In general, the spread between the four lines was most pronounced in the GA model, with improved distinction of the lowest survival group.
Figure 1A-D. Stratified survival curves to assess calibration for three prediction models and oncologists’ prognostic estimates.
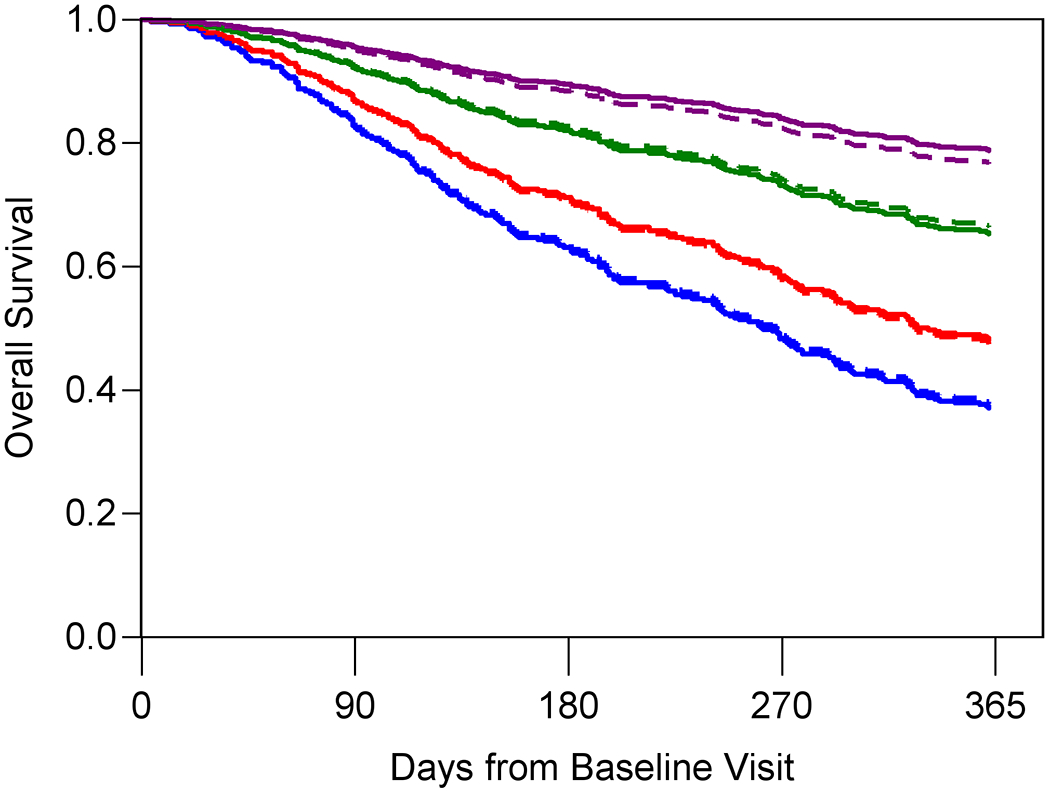
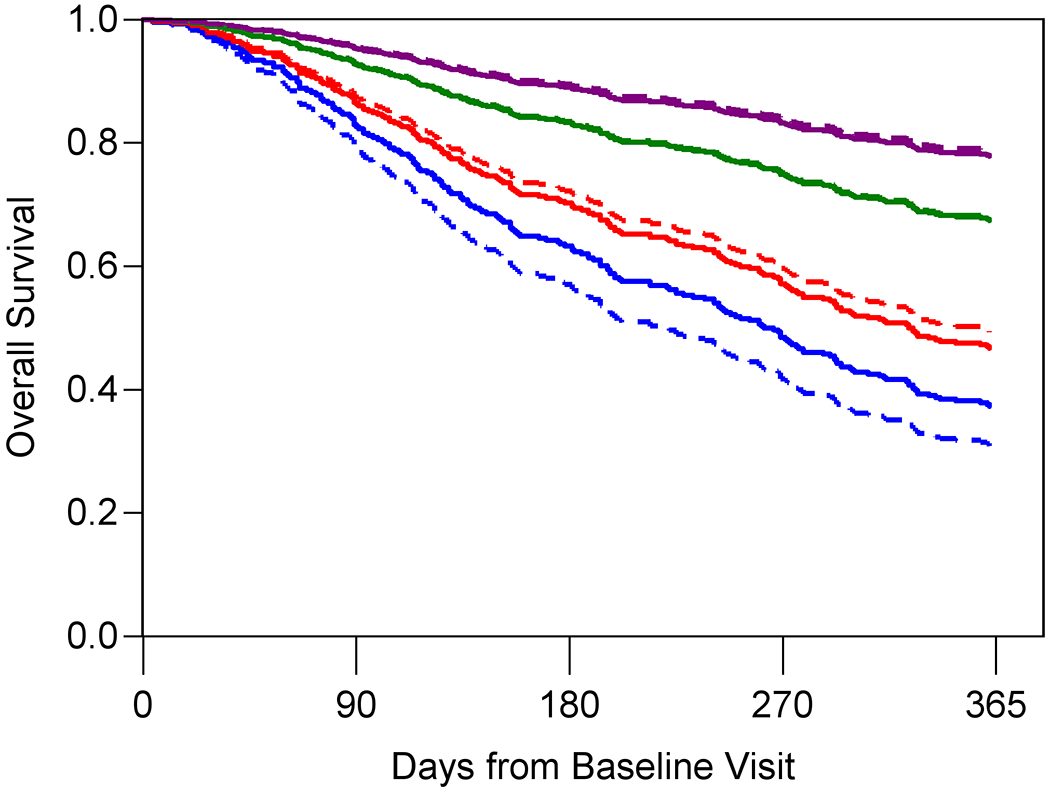
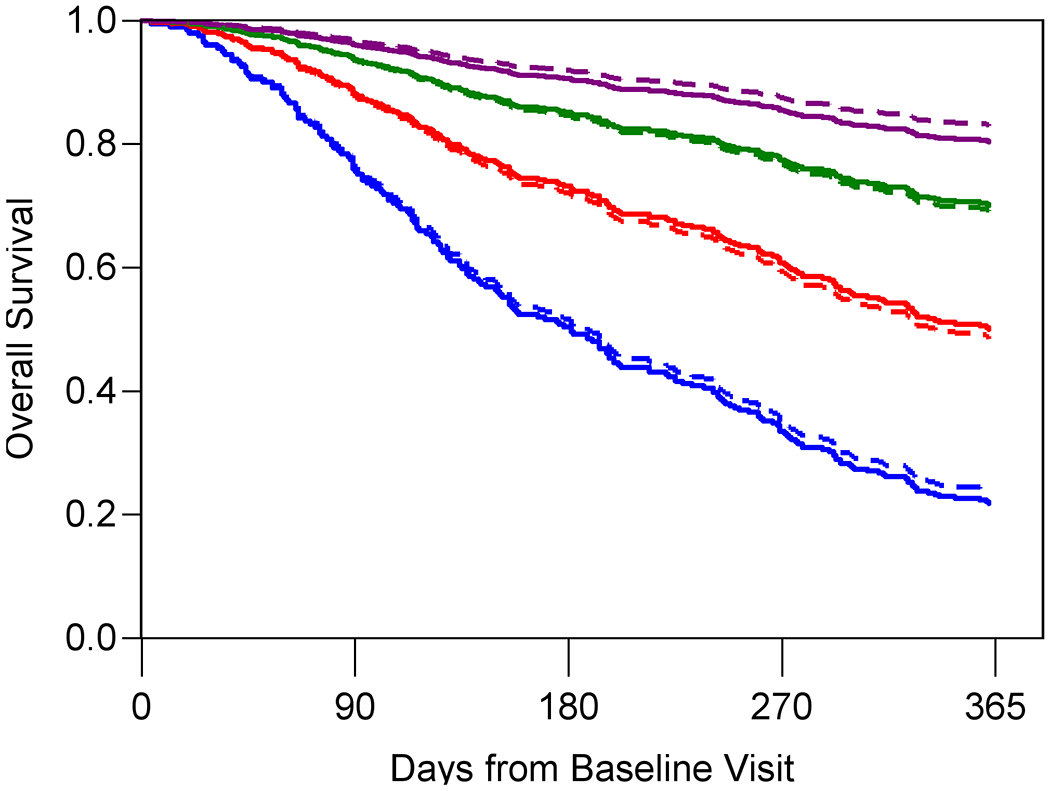
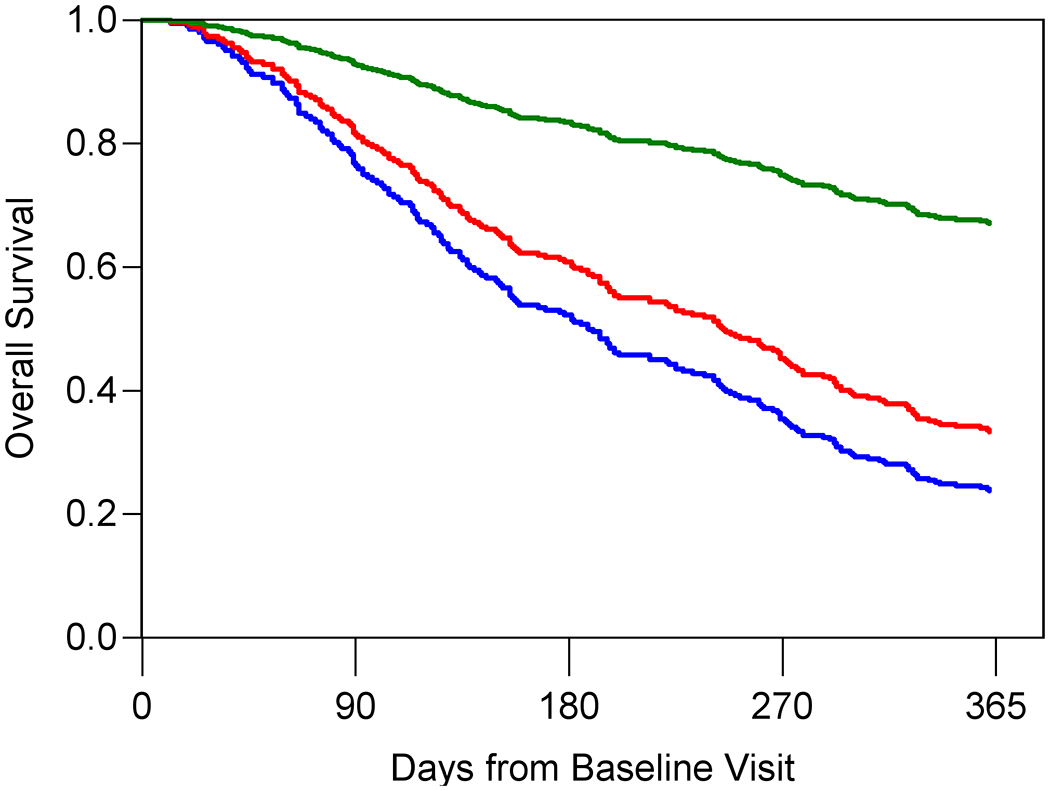
The blue, red, green, and purple lines represent the model-predicted survival times for patients in the <18th percentile, between the 18th and <50th percentile, between the 50th and <84th percentile and >84th percentile, respectively. The dashed lines represent the predicted survival times and the solid lines represent the observed survival times. Panel D only include three observed lines as the Oncologist model is a simple stratification of observed survival based on three prognostic categories: 0-6 months (blue), 7-12 months (red), and 1+ year (green). Observed and model-predicted curves that largely overlap suggest well-calibrated models.
Discussion
In this study enrolling older adults with advanced cancer, we found that prognostic models using commonly available clinical information or augmented by additional data from GA had moderate discrimination of survival. Discrimination using these models was similar to oncologists’ life expectancy estimates alone. However, our results suggest that information from GA can enhance model calibration, an important but often overlooked aspect of predictive performance.29 Poorly calibrated models that, for example, overestimate life expectancy can lead to overly aggressive treatment decisions and delayed referrals to palliative care or hospice, while model that underestimate life expectancy may lead to withholding potentially beneficial therapy.
We also reported variation in discriminative performance of the four models over time. Interestingly, the time-dependent c-statistic at 30-days for the oncologists’ life expectancy estimates and KPS model were 0.77 and 0.73, respectively, while only 0.61 for the basic and 0.65 for the GA models. Despite the imprecision of these estimates due the occurrence of few early events, this finding may reflect oncologists’ ability to subjectively perceive when a patient is imminently dying, which other objective tools cannot detect. Taken together, our evaluation of time-dependent discrimination suggests that the selection of a specific prognostic model to inform decision-making will depend, to some extent, on the relevant time horizon for the decision.
Prognostic models of life expectancy in general populations of older adults, such as the Schonberg, Lee, and Lund-Lewis models, have reported c-statistics or c-indices in the range of 0.75-0.83.30–34 These models incorporate similar domains of health as the geriatric assessment, including comorbidities and activities of daily living. So, why are these models so much more successful in discriminating survival than the models evaluated in our study? In a general pool of older adults, there is greater variation in health status and prognosis than in a restricted cohort of older adults with advanced cancer, where prognosis is generally poor overall. This restriction in prognostic profiles makes it more challenging to separate those who are likely to die early from those who are likely to die later. So, how can we surmount this challenge and improve discriminative performance? One potential approach for future work is to incorporate more diverse sources of information for determining prognosis by including subjective assessments from patients, caregivers, and oncologists alongside objective assessments of cancer features and measures of geriatric health. A recent study of older adults with prostate cancer suggests that adding patient-reported outcomes to other claims-based indicators of health may improve prognostic discrimination.35 Another approach would be to utilize the full-extent of detail contained within the geriatric assessment for model prediction. Our current analysis included only binary indicators of geriatric assessment results using pre-selected cutpoints,19 which results in the loss of predictive information. Instead of using cut-points, future research should examine use of the entire range of assessment scores for prognostication. Finally, instead of focusing on improving discriminative model performance, prognostic tools could instead focus on embracing some uncertainty and transparently communicating that uncertainty to patients. There is ongoing research investigating the use of best case, worst case, and typical scenarios in prognostic communication to patients with advanced cancer,36–38 indicating this is largely an acceptable communication strategy.
Results from this study should be viewed considering several points. First, vital status was assessed through active follow-up; therefore, survival times were unknown for those without a returned form. However, characteristics of patients missing a survival time were similar to those with an observed survival time, and thus selection bias is unlikely. Second, in the trial, oncologists’ life expectancy estimates were categorized into windows of time (i.e., 0-6 months, 7-12 months, >1 year), which leads to a loss of information. In turn, this categorization can incorrectly classify an oncologist’s estimate as inaccurate even when it is largely accurate (e.g., an oncologist’s estimates life expectancy at 7 months – categorized as 7-12 months – but the patient dies in month 6 (categorized as 0-6 months observed survival). Because of this categorization of oncologists’ life expectancy estimates in this secondary analysis of trial data, we could not directly compare our findings with previous studies that reported oncologist-estimated life expectancy by weeks or months.37 Third, we did not have access to information on cancer treatment history or specific tumor markers, which could potentially improve survival prediction. Ultimately, for this information to be useful for real-time clinical prognostication, it has to be collected in a consistent, standardized, and structured format in all patient records. Fourth, we internally validated our models using established cross-validation methods;24 however, external validation is a critical step for establishing the value of all prognostic models.39 Finally, recent trials19,40–44 have demonstrated clear benefits of geriatric assessment as a supportive care intervention for older adults with advanced cancers to identify vulnerabilities, reduce chemotherapy toxicity, improve communication, satisfaction with care, and quality of life. Therefore, regardless of the findings of the present study, geriatric assessment is a useful tool for improving patient-centered outcomes and is now recommended by the American Society for Clinical Oncology.1,2
In summary, we found that a prognostic model combining common clinical data with geriatric assessment showed moderate discrimination of one-year survival and improved calibration over other approaches to prognostic estimation. Accurate prognostication in this population is critical, as it plays a central role in individualized treatment decision-making. Efforts to further improve prognostic model performance through integration of information from patients, caregivers, and oncologists and more flexible analytic approaches are warranted.
Supplementary Material
Acknowledgements
We wish to acknowledge Susan Rosenthal, MD for her editorial assistance and funding from the University of Rochester Cancer Center (URCC) National Cancer Institute Community Oncology Research Program (NCORP) Research Base (UG1CA189961). We also wish to acknowledge the contributions of Margaret Sedenquist, a co-leader of SCOREBoard, who provided input as a patient advocate on this manuscript, and who recently passed away from COVID19.
Grant support
The work was supported by the Patient-Centered Outcomes Research Institute (PCORI) Program contract (4634 to SGM), the National Cancer Institute at the National Institute of Health (UG1 CA189961 to Morrow and Mustian; K99CA237744 to KPL), the National Institute on Aging at the National Institute of Health (K24 AG056589 to SGM; R33 AG059206 to SGM; KL2 TR001999 to NG), and the Wilmot Research Fellowship Award (grant number is not applicable; to KPL). This work was made possible by the generous donors to the Wilmot Cancer Institute (WCI) geriatric oncology philanthropy fund.
Footnotes
Conflicts of Interest
Dr. Lund’s spouse is a paid employee of GlaxoSmithKline and owns stock in the company. Dr. Loh reports being paid as a consultant to Pfizer and Seattle Genetics.
Publisher's Disclaimer: Disclaimer
All statements in this report, including its findings and conclusions, are solely those of the authors, do not necessarily represent the official views of the funding agencies, and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
References
- 1.Mohile SG, Dale W, Somerfield MR, et al. : Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology Summary. J Oncol Pract 14:442–446, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohile SG, Dale W, Somerfield MR, et al. : Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 36:2326–2347, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurria A, Wildes T, Blair SL, et al. : Senior adult oncology, version 2.2014: clinical practice guidelines in oncology. J Natl Compr Canc Netw 12:82–126, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Glare P, Virik K, Jones M, et al. : A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 327:195–8, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christakis NA, Lamont EB: Extent and determinants of error in physicians’ prognoses in terminally ill patients: prospective cohort study. West J Med 172:310–3, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gramling R, Gajary-Coots E, Cimino J, et al. : Palliative Care Clinician Overestimation of Survival in Advanced Cancer: Disparities and Association With End-of-Life Care. J Pain Symptom Manage 57:233–240, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Thai V, Ghosh S, Tarumi Y, et al. : Clinical prediction survival of advanced cancer patients by palliative care: a multi-site study. Int J Palliat Nurs 22:380–7, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Steyerberg EW, Vickers AJ, Cook NR, et al. : Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 21:128–38, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesko CR, Buchanan AL, Westreich D, et al. : Generalizing Study Results: A Potential Outcomes Perspective. Epidemiology 28:553–561, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang RW, Caraiscos VB, Swami N, et al. : Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract 10:e335–41, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Chow E, Abdolell M, Panzarella T, et al. : Predictive model for survival in patients with advanced cancer. J Clin Oncol 26:5863–9, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Anderson F, Downing GM, Hill J, et al. : Palliative performance scale (PPS): a new tool. J Palliat Care 12:5–11, 1996 [PubMed] [Google Scholar]
- 13.Hurria A, Cirrincione CT, Muss HB, et al. : Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol 29:1290–6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurria A, Gupta S, Zauderer M, et al. : Developing a cancer-specific geriatric assessment: a feasibility study. Cancer 104:1998–2005, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Mohile SG, Magnuson A, Pandya C, et al. : Community Oncologists’ Decision-Making for Treatment of Older Patients With Cancer. J Natl Compr Canc Netw 16:301–309, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolly TA, Deal AM, Nyrop KA, et al. : Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist 20:379–85, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruijnen CP, van Harten-Krouwel DG, Koldenhof JJ, et al. : Predictive value of each geriatric assessment domain for older patients with cancer: A systematic review. J Geriatr Oncol 10:859–873, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Kenis C, Baitar A, Decoster L, et al. : The added value of geriatric screening and assessment for predicting overall survival in older patients with cancer. Cancer 124:3753–3763, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Mohile SG, Epstein RM, Hurria A, et al. : Communication With Older Patients With Cancer Using Geriatric Assessment: A Cluster-Randomized Clinical Trial From the National Cancer Institute Community Oncology Research Program. JAMA Oncol 6:196–204, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loh KP, Mohile SG, Epstein RM, et al. : Willingness to bear adversity and beliefs about the curability of advanced cancer in older adults. Cancer 125:2506–2513, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loh KP, Mohile SG, Lund JL, et al. : Beliefs About Advanced Cancer Curability in Older Patients, Their Caregivers, and Oncologists. Oncologist 24:e292–e302, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fried TR, Bradley EH, O’Leary J: Changes in prognostic awareness among seriously ill older persons and their caregivers. J Palliat Med 9:61–9, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Cohen J A Coefficient of Agreement for Nominal Scales. Educational and Psychological Measurement. 1960: 20(1): 37–46, [Google Scholar]
- 24.Hastie T, Tibshirani R, and Friedman J. The Elements of Statistical Learning. New York, NY, USA: Springer New York Inc., 2009, p. 241–247. , [Google Scholar]
- 25.Harrell FE Jr., Califf RM, Pryor DB, et al. : Evaluating the yield of medical tests. JAMA 247:2543–6, 1982 [PubMed] [Google Scholar]
- 26.ePrognosis. Available at https://eprognosis.ucsf.edu/index.php, accessed on February 25, 2021,
- 27.Harrell FE Jr. Regression Modeling Strategies. 2nd ed. New York, NY: Springer-Verlag; 2015, [Google Scholar]
- 28.Royston P, Altman DG: External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol 13:33, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Calster B, McLernon DJ, van Smeden M, et al. : Calibration: the Achilles heel of predictive analytics. BMC Med 17:230, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gagne JJ, Glynn RJ, Avorn J, et al. : A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 64:749–59, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund JL, Kuo TM, Brookhart MA, et al. : Development and validation of a 5-year mortality prediction model using regularized regression and Medicare data. Pharmacoepidemiol Drug Saf 28:584–592, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SJ, Lindquist K, Segal MR, et al. : Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 295:801–8, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Schonberg MA, Davis RB, McCarthy EP, et al. : Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med 24:1115–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross RK, Kuo TM, Webster-Clark M, et al. : Validation of a 5-Year Mortality Prediction Model among U.S. Medicare Beneficiaries. J Am Geriatr Soc, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan HJ, Zhou X, Spratte BN, et al. : Patient-Reported vs. Claims-Based Measures of Health for Modeling Life Expectancy in Men with Prostate Cancer. J Urol:101097JU0000000000001355, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiely BE, McCaughan G, Christodoulou S, et al. : Using scenarios to explain life expectancy in advanced cancer: attitudes of people with a cancer experience. Support Care Cancer 21:369–76, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Moth EB, Blinman P, Stefanic N, et al. : Estimating survival time in older adults receiving chemotherapy for advanced cancer. J Geriatr Oncol 11:617–625, 2020 [DOI] [PubMed] [Google Scholar]
- 38.Kiely BE, Tattersall MH, Stockler MR: Certain death in uncertain time: informing hope by quantifying a best case scenario. J Clin Oncol 28:2802–4, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Collins GS, Reitsma JB, Altman DG, et al. : Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 162:55–63, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Kleckner AS, Wells M, Kehoe LA, et al. : Using Geriatric Assessment to Guide Conversations Regarding Comorbidities Among Older Patients With Advanced Cancer. JCO Oncol Pract:OP2100196, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohile SG, Mohamed MR, Culakova E et al. A geriatric assessment (GA) intervention to reduce treatment toxicity in older patients with advanced cancer: A University of Rochester Cancer Center NCI community oncology research program cluster randomized clinical trial (CRCT). J Clin Oncol 2020;38:12009a, [Google Scholar]
- 42.Li D, Sun C-L, Kim H. Geriatric assessment-driven intervention (GAIN) on chemotherapy toxicity in older adults with cancer: A randomized controlled trial. J Clin Oncol. 2020;38(15_suppl):12010, [Google Scholar]
- 43.Soo WK, King M, Pope A et al. Integrated geriatric assessment and treatment (INTEGERATE) in older people with cancer planned for systemic anticancer therapy. J Clin Oncol 2020;38:12011a, [Google Scholar]
- 44.Qian CL, Knight HP, Ferrone CR et al. Randomized trial of a perioperative geriatric intervention for older adults with cancer. J Clin Oncol 2020;38(15 suppl):12012a, [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


