Abstract
Purpose:
Older adults with head and neck cancer have increased postoperative complications, longer hospital stays, and higher rates of mortality. Geriatric assessment (GA) provides a measure of overall health status and is preferable to using age alone for assessing fitness for surgery. We sought to determine whether a patient’s frailty as determined by a novel electronic GA is associated with outcomes after head and neck cancer (HNC) surgery.
Methods:
We conducted a retrospective review of 159 patients aged 75 and older referred to the Geriatrics Service at Memorial Sloan Kettering Cancer Center for preoperative evaluation prior to undergoing HNC surgery. All patients completed the electronic Rapid Fitness Assessment (eRFA) within 60 days prior to surgery. The accumulated geriatric deficit (AGD) score includes twelve domains from the eRFA with a point assigned for each domain in which there is a deficit and a final point related to comorbidities. Three other metrics were individually assessed: age, Karnofsky Performance Scale (KPS), and number of comorbidities. We utilized multivariable linear regression and T-tests to determine whether frailty is associated with longer length of hospital stay, 30-day intensive care unit (ICU) admission, and 30-day and 90-day postoperative mortality.
Results:
Patients with a higher AGD score spent more time in the hospital postoperatively (1.0 day increase per unit increase in AGD; 95% CI: 0.21– 1.9; p = 0.015). Lower KPS was also associated with statistically significant longer length of stay (−2.70 day change per increasing index KPS; 95% CI: −4.30 – −1.00; days; p=0.002), while age and comorbidity were not found to be statistically associated with length of stay. Higher AGD score remained significantly associated with longer length of stay on multivariable analysis (0.93 day increase per unit increase in AGD; 95% CI 0.15– 1.71; p=0.019). AGD was the only metric associated with increased risk of ICU admission (6.6 vs 5.0 geriatric deficits for those admitted vs not admitted to ICU; p=0.024).
Conclusions:
Frailty is associated with increased length of hospital stay and ICU admission in older adults with HNC undergoing surgery. GA can be used to counsel patients on the expected postoperative course.
Keywords: Frailty, Head and Neck Cancer, Surgery, Geriatric Assessment
INTRODUCTION
Older adults receiving surgery for head and neck cancers experience increased postoperative complications, longer hospital stays, greater dependence on tracheostomy and feeding tubes, and higher rates of mortality.1–3 Additionally, poor performance status and greater burden of comorbidity have also been associated with worse outcomes in older adults following head and neck surgery4–6. However, many older adults can tolerate head and neck surgeries with careful selection criteria and collaborative management with geriatricians7,8. Thus, treatment decisions among patients with head and neck cancer should be guided by evaluations of patients’ overall health status rather than age alone.9
Frailty refers to the decline in multiple physiologic systems resulting in decreased functional reserves and increased risk of adverse events. In older adults, frailty results not only from damage to physiologic systems, but also from decreased physical activity, poor nutrition, and stressor events such as falls, hospitalizations, and increased dependence on others for care.10 Frailty, as measured by brief inventories, has been associated with hospital length of stay, morbidity, and mortality in older adult head and neck cancer patients.11–14
The gold standard for assessing frailty is geriatric assessment (GA), which obtains information on functional status, cognitive status, social support, nutritional status, polypharmacy, and comorbidities.15 GA has been recommended in the care of older adults with cancer since impairments in the GA are associated with adverse outcomes.16–21 However, GA is not commonly utilized in clinical practice, due to time and logistical barriers.22,23 To overcome barriers to GA, a novel electronic and patient-centered GA termed the electronic Rapid Fitness Assessment (eRFA) was developed.22,23 The eRFA is fast, comprehensive, and requires minimal effort from clinical staff.22,23
The aim of this study is to assess the relationship between geriatric assessment and postoperative outcomes for older adults with head and neck cancer. Additionally, we separately assessed the association between postoperative outcomes and age, performance status, and comorbidity, which are other common metrics used as a surrogate for frailty in the absence of GA. We hypothesized that frailty, as determined by a geriatric assessment, is associated with postoperative morbidity and mortality. Understanding which older adults are more likely to experience worse outcomes would allow for opportunities to better counsel patients on the risks of undergoing surgery as well as to prevent or manage toxicities that may arise from surgery.
METHODS
Sample
The study sample consists of 159 consecutive patients aged 75 and older with cancer who were referred to the Geriatrics Service at Memorial Sloan Kettering Cancer Center (MSKCC) for preoperative evaluation prior to undergoing any head and neck cancer surgery between 2015 and 2019. At MSKCC, all patients aged 75 and older are evaluated preoperatively either by their primary care physician, a cardiologist, or a geriatrician. Whether patients are referred to a geriatrician at MSKCC depends on patient and surgeon preference. Since 2015, when the eRFA was introduced, all patients referred to the Geriatrics Service completed GA within 60 days of surgery. All patients in this study were comanaged by geriatricians during their hospital course and received interventions to manage their geriatric deficits. Patient and tumor characteristics were extracted from the electronic medical record including tumor staging (AJCC 7th edition), cancer type, operating room time, and body mass index (BMI). For patients with a missing BMI (n=4) or marital status (n=1), we imputed the median or mode, respectively.
Geriatric Assessment
The GA utilized in this study is the eRFA, which was developed by the Geriatrics Service at MSKCC.22 The eRFA can be completed by the patient alone or with assistance from a caregiver either in a clinic via an electronic tablet or at home via the internet. Two components of the eRFA, Mini-Cog test and Timed Up and Go, are completed by nursing while the patient is in the clinic.
The eRFA collects information from 12 domains using validated assessments to determine frailty by computing an accumulated geriatric deficits (AGD) score. The domains include patient-rated Karnofsky Performance Scale (KPS), activities of daily living, instrumental activities of daily living, history of fall(s) in the past year, Timed Up and Go, Mini-Cog, major distress, depression, social activity limitation, poor social support, number of medications, and weight loss in the past six months.22 The final component of the AGD score is a measure of comorbidity. Patients receive one point for each domain in which there is a deficit and a point for having four or more of the pre-defined comorbidities; the total sum corresponding to the AGD score.24 The median AGD score in this cohort was 5, consistent with prior work on eRFA.24
Postoperative outcomes were also assessed based on age, performance status, and comorbidity, which were available from eRFA. Comorbidity was determined by ICD9 and ICD10 codes prior to surgery and included 13 conditions: heart disease, arthritis, cerebrovascular disease, renal impairment, lipid disorder, liver disease, peptic ulcer disease, diabetes, hypertension, peripheral vascular disease, chronic obstructive pulmonary disease, cognitive impairment, or thyroid disorders. Patients receive one point for each comorbidity, and this final count was used as a continuous variable. Patient-reported Karnofsky Performance Scale (KPS) ranges from 0–100, with values at increments of 10 corresponding to a patient’s ability to perform ordinary tasks was also collected.
Outcomes
Outcomes of this study are the length of postoperative hospital stay, 30-day intensive care unit (ICU) admission, 30-day hospital readmission, and 30-day and 90-day mortality. The Length of postoperative stay was defined as the number of days from admission to discharge from the hospital. ICU admission was defined as admission to the ICU within 30 days of surgery. 30-day readmission was defined as readmission to the hospital within 30 days of the surgery date. The reasons for ICU admission and 30-day readmission were determined through chart review. Seven patients had less than a 30 day follow-up time and were excluded from ICU analysis and readmission analysis. Three additional patients who had a planned readmission for a second operation were also excluded from the readmission analysis. Any death within 30 or 90 days of surgery was defined as 30-day and 90-day mortality, respectively. Overall survival was defined as death from any cause. Overall survival time was measured from the date of surgery to death from any cause.
Statistical Analysis
Univariable and multivariable linear regression was used to assess the association between patient and tumor characteristics and length of hospital stay. Age, BMI, and operating room time were continuous variables while marital status and prior recurrence were binary. All variables assessed are known predictors in oncology that are associated with treatment outcomes, which we were interested in evaluating in this specific cohort. Statistically significant variables (p < 0.05) in the univariable analysis were included in the multivariable models. Age was included in the multivariable models, regardless of statistical significance on univariable analysis. T-tests were used to test associations between patient characteristics and ICU admission or 30-day readmission. All statistical analysis was performed in R software (version 4.0).
RESULTS
The mean patient age was 81.5 years and most patients were male (59%), white (87%), and married (59%). The mean time to complete the eRFA was 11.9 minutes. Geriatric deficits were common in this cohort and 45% of patients had 6 or more deficits (Table 1). The more common subsites included oral cavity squamous cell carcinoma (SCC), skin cancers (including SCC, melanoma, and Merkel cell carcinoma), thyroid cancers (including papillary and anaplastic), and salivary gland cancers. In our cohort, the more common procedures during surgery were a radical or modified neck dissection (95%), partial or complete parotidectomy (21%), and a tissue graft or flap (21%). More than a quarter of patients (29%) had undergone prior head and neck surgery and 30.8% presented with a recurrence of a prior head and neck cancer at the time of GA (Table 2).
Table 1:
Patient Demographics and Components of eRFA with Cut-offs defining Geriatric Deficits
| N=159 | |
|---|---|
| Gender: | |
| Female | 65 (41%) |
| Male | 94 (59%) |
| Age (standard deviation) | 81.5 (5.1) |
| eRFA Completion Time in Minutes (standard deviation) | 11.9 (6.8) |
| KPS: | |
| ≥90 | 102 (64%) |
| ≤80 | 56(35%) |
| Missing | 1 |
| Activities of Daily Living (Higher is better): | |
| ADL Score ≥ 14 | 89 (56%) |
| ADL Score ≤13 | 69 (44%) |
| Missing | 1 |
| Instrumental Activities of Daily Living Score (Higher is better): | |
| iADL Score ≥ 16 | 93 (59%) |
| iADL Score ≤ 15 | 65 (41%) |
| Missing | 1 |
| Fall in the Past Year: | |
| No | 111 (72%) |
| Yes | 43 (23%) |
| Missing | 5 |
| TUG Score (Lower is better): | |
| TUG Score < 10 seconds | 105 (71%) |
| TUG Score ≥ 10 Seconds | 44 (29%) |
| Missing | 11 |
| Mini-Cog Score (Higher is better): | |
| ≥ 3 | 121 (82%) |
| ≤ 2 | 26 (18%) |
| Missing | 13 |
| Social Support Score (Higher is better): | |
| ≥17 | 97 (61%) |
| ≤16 | 61 (39%) |
| Missing | 1 |
| Social Activity Limitation Score (Lower is better): | |
| ≤ 7 | 74 (47%) |
| ≥ 8 | 84 (53%) |
| Missing | 1 |
| Weight Loss of ≥10 pounds: | |
| No | 127 (84%) |
| Yes | 24 (16%) |
| Missing | 8 |
| Distress Thermometer Score (Lower is better): | |
| ≤ 3 | 62 (39%) |
| ≥4 | 97 (61%) |
| Depression based on Geriatric Depression Score (Lower is better): | |
| No | 64 (41%) |
| Yes | 93 (59%) |
| Missing | 2 |
| Number of Medications: | |
| ≤ 4 | 78 (57%) |
| ≥ 5 | 60 (43%) |
| Mean Accumulated Geriatric Deficit (standard deviation) | 5.12 (3.0) |
| Accumulated Geriatric Deficit Score (Lower Is better): | |
| Score ≤ 5 | 88 (55%) |
| Score > 5 | 71 (45%) |
eRFA: electronic Rapid Fitness Assessment; KPS: Karnofsky Performance Status; TUG: Timed Up and Go.
Table 2:
Tumor and Surgical Characteristics
| N=159 | |
|---|---|
| Prior History of Cancer | |
| No | 63 (40%) |
| Yes | 96 (60%) |
| Prior History of Head and Neck Cancer | |
| No | 87 (55%) |
| Yes | 72 (45%) |
| Prior History of Head and Neck Radiation | |
| No | 132 (83%) |
| Yes | 27 (17%) |
| Prior History of Head and Neck Surgery | |
| No | 113 (71%) |
| Yes | 46 (29%) |
| Head and Neck Cancer Type | |
| Thyroid | 23 (15%) |
| Skin | 34 (21%) |
| Oral Cavity | 60 (38%) |
| Oropharynx | 5 (3.1%) |
| Hypopharynx | 5 (3.1%) |
| Larynx | 7 (4.4%) |
| Salivary Gland | 12 (7.6%) |
| SCC of Mandible | 2 (1.3%) |
| SCC of Maxilla | 3 (1.9%) |
| SCC of Unknown Primary | 3 (1.9%) |
| Miscellaneous | 5 (3.1%) |
| T Stage (AJCC 7) | |
| 0 | 6 (3.7%) |
| Tis | 1 (0.6%) |
| 1 | 26 (17%) |
| 2 | 28 (18%) |
| 3 | 19 (12%) |
| 4 | 23 (15%) |
| Recurrent | 51 (32%) |
| Metastatic | 4(2.5%) |
| N Stage (AJCC 7) | |
| 0 | 50 (31%) |
| 1 | 32 (21%) |
| 2 | 17 (11%) |
| 3 | 4 (2.5%) |
| Recurrent | 51 (32%) |
| Metastatic | 4 (2.5%) |
| M Stage (AJCC 7) | |
| 0 | 103 (65%) |
| 1 | 4 (2.5%) |
| Recurrent | 51 (32%) |
| Surgical Procedures | |
| Radical or Modified Neck Dissection | 151 (95%) |
| Mandibulectomy* | 26 (16%) |
| Parotidectomy* | 33 (21%) |
| Thyroidectomy* | 21 (13%) |
| Maxillectomy* | 17 (11%) |
| Glossectomy* | 22 (14%) |
| Laryngectomy* | 14 (9%) |
| Resection of the Buccal Mucosa | 15 (9.4%) |
| Pharyngectomy | 4 (2.5%) |
| Wide Local Excision | 24 (15%) |
| Auriculectomy | 5 (3.1%) |
| Tissue Graft or Flap^ | 34 (21%) |
| Orbitectomy | 2 (0.1%) |
| Resection of Retromolar Trigone | 1 (0.1%) |
| Ethmoidectomy | 1 (0.1%) |
| Resection of Lip | 1 (0.1%) |
| Esophagectomy | 1(0.1%) |
SCC: Squamous Cell Carcinoma.
Complete or partial
Includes free flap reconstruction procedures
The mean operating room time in all patients was 393 minutes. Operating room time was associated with cancer type, with thyroid cancers having the shortest mean operating room time (303 minutes) and cancers of the oropharynx, hypopharynx, and larynx having the longest operating room time (502 minutes). Post-hoc analysis showed that AGD, KPS, and comorbidity were not significantly associated with operating room time, however, age was (11 minutes decrease per additional year of age; 95% CI: −17.0– −4.1; p=0.002).
Length of Stay
Patients with higher AGD had a longer postoperative length of stay (1.0 day increase per unit increase in AGD; 95% CI: 0.21– 1.9; p = 0.015) (Table 3). The association remained on multivariable regression controlling for age, operating room time, and BMI (0.93 day increase per unit increase in AGD; 95% CI: 0.15– 1.71; p=0.019) (Table 3). In the multivariable regression, operation room time and BMI remained associated with length of stay (p<0.001 and p=0.012, respectively). Lower KPS was also associated with statistically significant longer length of stay in both univariable (−2.70 day change per increasing index in KPS; 95% CI: −.4.30– −1.00 days; p=0.002) and multivariable analysis (−2.60 day change per increasing index in KPS; 95% CI: −4.10– −1.10; p=0.001). Age and comorbidity were not associated with length of stay in either the univariable or multivariable analysis.
Table 3:
Univariable and Multivariable Analysis of Length of Stay
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Characteristic | Coefficient | 95% CI | p | Coefficient | 95% CI | p |
| Accumulated Geriatric Deficit | 1.0 | 0.21 – 1.9 | 0.015 | 0.93 | 0.15 – 1.71 | 0.019 |
| Karnofsky Performance Status | −2.70 | −4.30 – −1.00 | 0.002 | |||
| Age | 0.05 | −0.48 – 0.58 | 0.9 | 0.24 | −0.26 – 0.73 | 0.3 |
| Comorbidity | −0.46 | −1.7 – 0.82 | 0.5 | |||
| Body Mass Index | −0.55 | −1.1 – 0.00 | 0.049 | −0.64 | −1.13 – −0.14 | 0.012 |
| Operating Room Time (minutes) | 0.03 | 0.02 – 0.04 | <0.001 | 0.03 | 0.02 – 0.04 | <0.001 |
| Previous Recurrence (ref: No Recurrence) | −0.5 | −5.9 – 4.9 | 0.9 | |||
| Married (ref: Not Married) | −1.0 | −6.2 – 4.1 | 0.7 | |||
ICU Admission and 30-Day Readmission
A total of 13 patients were admitted into the ICU within 30 days of their surgery and 11 patients were readmitted within 30 days of their hospitalization. Of the 13 patients admitted to the ICU, 7 patients were admitted for respiratory failure due to aspiration, laryngeal edema, or other cause, 3 for altered mental status or delirium, 1 for alcohol withdrawal, 1 for myocardial infarction, and 1 for hemodialysis (Supplementary Table 1). In univariable analysis, AGD was statistically higher in patients who were admitted to the ICU (6.6 vs 5.0 impairments; p = 0.024). Age, KPS, and comorbidities did not differ between patients who were and were not admitted to the ICU (Table 4). The reasons for readmission within 30 days included cellulitis (3 patients), acute kidney injury, deep vein thrombosis, influenza and hyponatremia, multiple falls, pulmonary embolism, pharyngeal-cutaneous fistula, and wound dehiscence (Supplementary Table 2). Patients who were readmitted within 30 days had no statistically significant difference in AGD score, KPS score, age, or comorbidities as patients who were not readmitted (Table 4).
Table 4:
Association of Accumulated Geriatrics Deficit, Karnofsky Performance Status, Comorbidity and age with 30-day Readmission and Intensive Care Unit Admission.
| AGD Score (SD) | p | ||
|---|---|---|---|
| 30-day Readmission | Yes | 5.6 (3.3) | 0.6 |
| No | 5.1 (3.0) | ||
| ICU Admission | Yes | 6.6 (2.2) | 0.024 |
| No | 5.00 (3.0) | ||
| KPS (SD) | |||
| 30-day Readmission | Yes | 85 (14) | 0.9 |
| No | 86 (14) | ||
| ICU Admission | Yes | 82 (9.3) | 0.3 |
| No | 85 (15) | ||
| Age (SD) | |||
| 30-day Readmission | Yes | 82 (3.3) | 0.8 |
| No | 81 (4.8) | ||
| ICU Admission | Yes | 81 (6.9) | 0.9 |
| No | 82 (4.6) | ||
| Comorbidity (SD) | |||
| 30-day Readmission | Yes | 3.3 (2.4) | 0.5 |
| No | 3.8 (2.0) | ||
| ICU Admission | Yes | 3.5 (2.2) | 0.7 |
| No | 3.8 (2.0) |
SD: Standard Deviation.
PostOperative Mortality
Among our cohort, there were a total of 44 deaths, with median follow-up of 25 months among survivors. There were no deaths within 30 days of surgery and only one death within 90 days of surgery. Kaplan-Meier estimated overall survival at 1 year was 86% (95% CI 81% - 92%) and at 2 years was 74% (95% CI 66% - 82%) (Figure 1).
Figure 1:
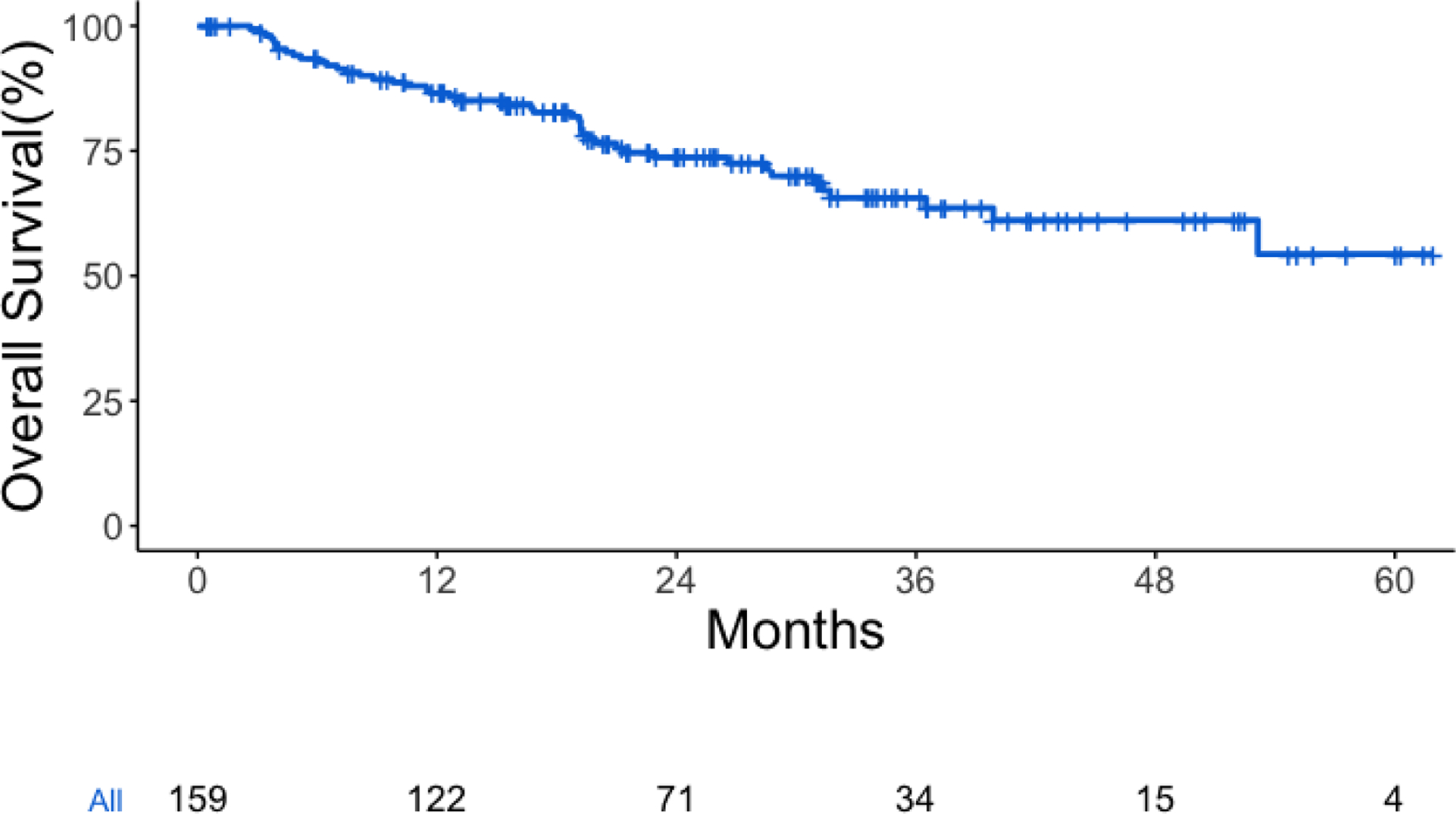
Overall survival for all patients
DISCUSSION
In our sample of older adults with head and neck cancer who underwent surgery, we found that frailty, as defined by increasing geriatric deficits, was associated with a longer length of hospital stay and a higher risk of ICU admission. Performance status was only associated with a longer length of stay while age and comorbidity were not associated with any postoperative outcomes. Since there was only one death within 90 days of surgery, associations between frailty and postoperative mortality could not be assessed in our dataset.
Overall, the outcomes for older adults undergoing head and neck cancer surgery in our study were very favorable. The 1- and 2-year overall survival rates of 86% and 74% are high considering our sample included patients with a mean age of 81.5 years and over 5 geriatric deficits. Additionally, more than a third of patients had either recurrent or metastatic disease. The rates of ICU admission of 8.2% and 30-day readmission of 9.0% were also acceptably low.
The patients in our study received geriatric assessment and co-management, which likely contributed to the favorable outcomes observed. Geriatric co-management involves patient-specific interventions to address geriatric deficits, which may have weakened the associations between frailty and outcomes in our study. However, AGD was still associated with the length of stay and ICU admission. Frail patients likely received more supportive care services and were more often admitted to the ICU, which may have both contributed to the longer length of hospital stay.
Our findings align with the results of prior studies that show frailty’s association with a longer length of postoperative stay.25 However, most prior studies used brief surveys or tools to measure frailty, whereas, our study measured frailty using a novel electronic GA that patients can largely complete at home. The eRFA reduces barriers to the implementation of GA in clinical practice and can aid in identifying opportunities to better manage geriatric syndromes. Co-management of frail older adults with cancer by geriatricians is associated with better outcomes postoperatively.26–28 GA can also help oncologists make treatment recommendations and improve the tolerability of chemotherapies.29,30 Impairments in instrumental activities of daily living can be treated by physical therapists31 and psychiatric interventions can help patients who are depressed or distressed.18,32
Strengths of this study include the use of a novel electronic GA in the preoperative evaluation of older adults with cancers of the head and neck. The eRFA contains detailed information on 13 domains of geriatric impairment as well as demographic and clinical data. We were also able to compare frailty as measured by eRFA to other metrics including age, performance status, and the number of comorbidities. This study has several limitations, including the single-institution retrospective design. Although our cohort is limited to head and neck cancer, there remains heterogeneity in the subtypes of head and neck cancer and their respective adjuvant therapies and prognoses, which could confound the study results. For example, 10-year survival rates can approach 85% among older adults patients with papillary thyroid cancer, while cutaneous SCC with nodal metastases has been associated with poor survival following surgery and postoperative radiation.33,34 Additionally, all patients in this study were comanaged by geriatricians who directed interventions to address geriatric deficits, which may have diminished the associations between frailty and outcomes and also contributed to the low rates of ICU and 30-day readmissions. Finally, older and more frail patients with head and neck cancer were potentially more likely to receive a referral to the Geriatrics Service for comanagement, so our cohort of patients may not be representative of an unselected group of older adults with head and neck cancer.
In conclusion, we found that frailty, as determined by a novel electronic GA, was associated with a longer length of stay and a higher risk of ICU admission among older adults with cancers of the head and neck. GA can guide clinicians in their management of older adults with cancer receiving head and neck surgery and can inform patients of their post-surgical outcomes. Future studies should assess the association between frailty and the use of inpatient support services and whether the electronic GA can be utilized to improve post-surgical outcomes with less involvement from the geriatrics service.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI statement:
The authors have no relevant financial conflicts of interest.
References
- 1.Al-Qurayshi Z, Sullivan CB, Schwalje A, et al. Presentation and Outcomes of Elderly Patients Undergoing Head and Neck Surgeries: A National Perspective. Otolaryngol Head Neck Surg. 2020;163(2):335–343. doi: 10.1177/0194599820911727 [DOI] [PubMed] [Google Scholar]
- 2.Cramer JD, Patel UA, Samant S, Smith SS. Postoperative Complications in Elderly Patients Undergoing Head and Neck Surgery: Opportunities for Quality Improvement. Otolaryngol Head Neck Surg. 2016;154(3):518–526. doi: 10.1177/0194599815618204 [DOI] [PubMed] [Google Scholar]
- 3.Subramaniam N, Balasubramanian D, Rka P, et al. Peri-operative outcomes following major surgery for head and neck cancer in the elderly: institutional audit and case-control study. J Laryngol Otol. 2018;132(8):742–747. doi: 10.1017/S0022215118001135 [DOI] [PubMed] [Google Scholar]
- 4.Fancy T, Huang AT, Kass JI, et al. Complications, Mortality, and Functional Decline in Patients 80 Years or Older Undergoing Major Head and Neck Ablation and Reconstruction. JAMA Otolaryngol Head Neck Surg. Published online October 10, 2019. doi: 10.1001/jamaoto.2019.2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.L’Esperance HE, Kallogjeri D, Yousaf S, Piccirillo JF, Rich JT. Prediction of mortality and morbidity in head and neck cancer patients 80 years of age and older undergoing surgery. Laryngoscope. 2018;128(4):871–877. doi: 10.1002/lary.26858 [DOI] [PubMed] [Google Scholar]
- 6.Adjei Boakye E, Johnston KJ, Moulin TA, et al. Factors Associated With Head and Neck Cancer Hospitalization Cost and Length of Stay-A National Study. Am J Clin Oncol. 2019;42(2):172–178. doi: 10.1097/COC.0000000000000487 [DOI] [PubMed] [Google Scholar]
- 7.Shuman AG, Korc-Grodzicki B, Shklar V, Palmer F, Shah JP, Patel SG. A new care paradigm in geriatric head and neck surgical oncology. J Surg Oncol. 2013;108(3):187–191. doi: 10.1002/jso.23370 [DOI] [PubMed] [Google Scholar]
- 8.Shuman AG, Patel SG, Shah JP, Korc-Grodzicki B. Optimizing perioperative management of geriatric patients with head and neck cancer. Head Neck. 2014;36(5):743–749. doi: 10.1002/hed.23347 [DOI] [PubMed] [Google Scholar]
- 9.Porceddu SV, Haddad RI. Management of elderly patients with locoregionally confined head and neck cancer. Lancet Oncol. 2017;18(5):e274–e283. doi: 10.1016/S1470-2045(17)30229-2 [DOI] [PubMed] [Google Scholar]
- 10.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams P, Ghanem T, Stachler R, Hall F, Velanovich V, Rubinfeld I. Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg. 2013;139(8):783–789. doi: 10.1001/jamaoto.2013.3969 [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DP, Sklar MC, de Almeida JR, et al. Frailty as a predictor of outcomes in patients undergoing head and neck cancer surgery. Laryngoscope. 2020;130(5):E340–E345. doi: 10.1002/lary.28222 [DOI] [PubMed] [Google Scholar]
- 13.Noor A, Gibb C, Boase S, Hodge J-C, Krishnan S, Foreman A. Frailty in geriatric head and neck cancer: A contemporary review. Laryngoscope. 2018;128(12):E416–E424. doi: 10.1002/lary.27339 [DOI] [PubMed] [Google Scholar]
- 14.Fu TS, Sklar M, Cohen M, et al. Is Frailty Associated With Worse Outcomes After Head and Neck Surgery? A Narrative Review. Laryngoscope. 2020;130(6):1436–1442. doi: 10.1002/lary.28307 [DOI] [PubMed] [Google Scholar]
- 15.Korc-Grodzicki B, Downey RJ, Shahrokni A, Kingham TP, Patel SG, Audisio RA. Surgical considerations in older adults with cancer. J Clin Oncol. 2014;32(24):2647–2653. doi: 10.1200/JCO.2014.55.0962 [DOI] [PubMed] [Google Scholar]
- 16.Williams GR, Dunham L, Chang Y, et al. Geriatric Assessment Predicts Hospitalization Frequency and Long-Term Care Use in Older Adult Cancer Survivors. JOP. 2019;15(5):e399–e409. doi: 10.1200/JOP.18.00368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badgwell B, Stanley J, Chang GJ, et al. Comprehensive geriatric assessment of risk factors associated with adverse outcomes and resource utilization in cancer patients undergoing abdominal surgery. J Surg Oncol. 2013;108(3):182–186. doi: 10.1002/jso.23369 [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Yamasaki M, Sugimoto K, et al. Risk Evaluation of Postoperative Delirium Using Comprehensive Geriatric Assessment in Elderly Patients with Esophageal Cancer. World J Surg. 2016;40(11):2705–2712. doi: 10.1007/s00268-016-3602-2 [DOI] [PubMed] [Google Scholar]
- 19.Hurria A, Wildes T, Blair SL, et al. Senior adult oncology, version 2.2014: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12(1):82–126. doi: 10.6004/jnccn.2014.0009 [DOI] [PubMed] [Google Scholar]
- 20.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603. doi: 10.1200/JCO.2013.54.8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012;215(4):453–466. doi: 10.1016/j.jamcollsurg.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 22.Shahrokni A, Tin A, Downey RJ, et al. Electronic Rapid Fitness Assessment: A Novel Tool for Preoperative Evaluation of the Geriatric Oncology Patient. J Natl Compr Canc Netw. 2017;15(2):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahrokni A, Vishnevsky BM, Jang B, et al. Geriatric Assessment, Not ASA Physical Status, Is Associated With 6-Month Postoperative Survival in Patients With Cancer Aged ≥75 Years. Journal of the National Comprehensive Cancer Network. 2019;17(6):687–694. doi: 10.6004/jnccn.2018.7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahrokni A, Sarraf S, Alexander K, et al. Geriatric comanagement and surgical outcomes of older cancer patients. JCO. 2018;36(15_suppl):10035–10035. doi: 10.1200/JCO.2018.36.15_suppl.10035 [DOI] [Google Scholar]
- 25.Lin H-S, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatrics. 2016;16(1):157. doi: 10.1186/s12877-016-0329-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahrokni A, Tin AL, Sarraf S, et al. Association of Geriatric Comanagement and 90-Day Postoperative Mortality Among Patients Aged 75Years and Older With Cancer. JAMA Netw Open. 2020;3(8):e209265. doi: 10.1001/jamanetworkopen.2020.9265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filippova OT, Chi DS, Long Roche K, et al. Geriatric co-management leads to safely performed cytoreductive surgery in older women with advanced stage ovarian cancer treated at a tertiary care cancer center. Gynecologic Oncology. 2019;154(1):77–82. doi: 10.1016/j.ygyno.2019.04.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saur NM, Montroni I. Geriatric Comanagement: A Secret Ingredient of the Elusive Recipe. JAMA Netw Open. 2020;3(8):e209460. doi: 10.1001/jamanetworkopen.2020.9460 [DOI] [PubMed] [Google Scholar]
- 29.Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36(22):2326–2347. doi: 10.1200/JCO.2018.78.8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalsi T, Babic-Illman G, Ross PJ, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. British Journal of Cancer. 2015;112(9):1435–1444. doi: 10.1038/bjc.2015.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farley E, McCarthy L, Pergolotti M. Rehabilitation Strategies in Older Adult Oncology Patients: a Focus on Occupational and Physical Therapy. Curr Geri Rep. 2017;6(4):255–263. doi: 10.1007/s13670-017-0228-7 [DOI] [Google Scholar]
- 32.Canoui-Poitrine F, Reinald N, Laurent M, et al. Geriatric assessment findings independently associated with clinical depression in 1092 older patients with cancer: the ELCAPA Cohort Study. Psycho-Oncology. 2016;25(1):104–111. doi: 10.1002/pon.3886 [DOI] [PubMed] [Google Scholar]
- 33.Varra V, Woody NM, Reddy C, et al. Suboptimal Outcomes in Cutaneous Squamous Cell Cancer of the Head and Neck with Nodal Metastases. Anticancer Res. 2018;38(10):5825–5830. doi: 10.21873/anticanres.12923 [DOI] [PubMed] [Google Scholar]
- 34.Uruno T, Miyauchi A, Shimizu K, et al. Favorable surgical results in 433 elderly patients with papillary thyroid cancer. World J Surg. 2005;29(11):1497–1501; discussion 1502–1503. doi: 10.1007/s00268-005-7953-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


