Abstract
Background:
Optic neuritis (ON) is the most common manifestation of myelin oligodendrocyte glycoprotein antibody associated disorder (MOGAD) and multiple sclerosis (MS). Acute ON in MOGAD is thought to be associated with more severe optic disc edema than in other demyelinating diseases, but this has not been quantitatively confirmed. The goal of this study was to determine whether optical coherence tomography (OCT) can distinguish acute optic neuritis (ON) in myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) from multiple sclerosis (MS), and establish the sensitivity of OCT as a confirmatory biomarker of ON in these entities.
Methods:
This was a multicenter cross-sectional study of MOGAD and MS patients with peripapillary retinal nerve fiber layer (pRNFL) thickness measured with OCT within two weeks of acute ON symptom. Cirrus HD-OCT (Carl Zeiss Meditec, Inc. Dublin, CA, USA) was used to measure the pRNFL during acute ON. Eyes with prior optic neuritis or disc pallor were excluded. A receiver operating characteristic (ROC) curve analysis was performed to assess the ability of pRNFL thickness to distinguish MOGAD from MS.
Results:
Sixty-four MOGAD and 50 MS patients met study inclusion criteria. Median age was 46.5 years (interquartile range [IQR]: 34.3–57.0) for the MOGAD group and 30.4 years (IQR: 25.7–38.4) for the MS group (p<0.001). Thirty-nine (61%) of MOGAD patients were female compared to 42 (84%) for MS (p=0.007). The median pRNFL thickness was 164μm (IQR: 116–212) in 96 acute MOGAD ON eyes compared to 103μm (IQR: 93–113) in 51 acute MS ON eyes (p<0.001). The ROC area under the curve for pRNFL thickness was 0.81 (95% confidence interval 0.74–0.88) to discriminate MOGAD from MS. The pRNFL cutoff that maximized Youden’s index was 118μm, which provided a sensitivity of 74% and specificity of 82% for MOGAD. Among 31 MOGAD and 48 MS eyes with an unaffected contralateral eye or a prior baseline, the symptomatic eye had a median estimated pRNFL thickening of 45μm (IQR: 17–105) and 7.5μm (IQR: 1–18), respectively (p<0.001). All MOGAD affected eyes had a ≥5μm pRNFL thickening, whereas 26 (54%) MS affected eyes had a ≥5μm thickening.
Conclusion:
OCT-derived pRNFL thickness in acute ON can help differentiate MOGAD from MS. This can aid with early diagnosis and guide disease-specific therapy in the acute setting before antibody testing returns, and help differentiate borderline cases. In addition, pRNFL thickening is a sensitive biomarker for confirming acute ON in MOGAD, which is clinically helpful and could be used for adjudication of attacks in future MOGAD clinical trials.
Keywords: Myelin oligodendrocyte glycoprotein (MOG); MOG antibody-associated disease (MOGAD); optic neuritis, optical coherence tomography (OCT); peripapillary retinal nerve fiber layer (pRNFL)
Introduction
Myelin oligodendrocyte glycoprotein (MOG) antibody associated disease (MOGAD) is a recently described central nervous system (CNS) demyelinating disease, which is distinct from multiple sclerosis (MS) and aquaporin-4 (AQP4) antibody-positive neuromyelitis optica spectrum disorder (NMOSD).(1–5) While MOGAD can present with various manifestations, optic neuritis (ON) is the most common feature.(3, 6–10) It is important to distinguish MOGAD from MS and other demyelinating disorders because both acute and chronic treatments are different.(11) While MOG antibody testing can help differentiate these entities, MOG antibody testing often takes weeks to return and is not available in some countries. In addition, while MOG antibody testing is highly specific,(12) false-positives can still occur (especially at low-titers), owing to the fact that the prevalence of MOGAD is relatively low, even in patients with demyelinating syndromes, including ON.(13–16) Thus, it would be helpful to have other methods of more quickly identifying patients with ON that may be from MOGAD and help differentiate MOGAD from other demyelinating disorders, especially in cases of low-positive MOG antibody titers.
It has been well documented that ON from MOGAD is associated with both more frequent and extensive optic disc edema at onset compared to other demyelinating disorders, with optic disc edema present in up to 86% of cases of MOGAD ON.(10, 17–19). Optical coherence tomography (OCT) has the ability to quantify optic disc swelling by measuring the peripapillary retinal nerve fiber layer thickness (pRNFL) and might serve as a way to confirm ON and differentiate MOGAD from other demyelinating disorders, such as MS. Therefore, the goal of this study was to determine whether the degree of pRNFL thickening during the acute phase of ON helps discriminate a MOGAD from MS etiology, and to establish the sensitivity of OCT in confirming ON in these entities.
Methods
This was a multicenter retrospective case series of patients with ON from MOGAD and MS who underwent OCT scanning at the time of the acute ON attack. Inclusion criteria were: 1) ON in an eye or eyes that was not previously affected and 2) Availability of OCT within 2 weeks of the onset of ON. All MOGAD patients included in the study had positive serum MOG antibodies by a live or fixed cell based assay (CBA) with full length MOG in its native conformational form and met the diagnostic criteria for MOGAD.(12, 20, 21) MS patients met the 2017 revised McDonald criteria.(22)
The Mayo Clinic Institutional Review Board approved this retrospective study. For cases contributed by other medical centers, the pertinent institutional review boards approved the study with a waiver of informed consent due to the retrospective nature of the study. Data were shared in a de-identified manner with the lead site.
Optic neuritis was diagnosed based on a combination of at least three of the following clinical findings: decreased visual acuity, pain with eye movement, visual field defect, a relative afferent pupillary defect, changes in color vision, fundus examination findings, and/or consistent magnetic resonance imaging (MRI) findings.(14) To minimize the effect of patients with prior subclinical ON that might affect pRNFL thickness, patients were excluded if they had pallor at the time of the ON attack in the affected eye.
All imaging was performed using Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA). Patients with high refractive error (+/− 6 diopters) and images with artifact, segmentation errors or signal strength < 6 were excluded to adhere to the APOSTEL recommendations and OSCAR-IB consensus criteria for quality assessment of OCT images.(23, 24) The pRNFL thickness measurements were obtained using the optic disc cube 200 × 200 Cirrus protocol that covered the 6 × 6 mm2 area centered on the optic disc. The macular cube 512 × 128 Cirrus protocol centered on the fovea was used for the GCIPL segmentation and measurements.
The OCT-derived pRNFL thickness during acute ON from MOGAD and MS were compared. In addition, the pRNFL thickening in affected eyes was calculated using a prior baseline of the affected eye if an OCT scan was available prior to the onset of ON, or an estimated pRNFL thickening in affected eyes was calculated by using the unaffected contralateral eye as a surrogate of the affected eye’s baseline pRNFL thickness if a prior baseline OCT was not available.
Statistics
Demographics were compared between groups with two-sample t-test and chi-squared test. OCT parameters were compared between groups using linear generalized estimating equations (GEE) models to account for within-subject inter-eye correlation (for cases with both eyes included). Analyses were performed unadjusted and adjusted for age, sex, and race (categorized as White and non-White). Pearson correlation coefficients are reported for the association of age and OCT parameters. The performance of pRNFL thickness for discriminating between the MOGAD and MS groups was assessed using receiver operating characteristic (ROC) curves and summarized using the area under the curve (AUC). The “optimal” cutoffs were evaluated based on maximizing Youden’s index.(25) Statistical analyses were performed using R Version 4.0.4 (https://www.r-project.org/).
Data availability
Anonymized data not published within this article are available, from the corresponding author on reasonable request from any qualified investigator.
Results
The study included 64 MOGAD patients (96 eyes) and 50 MS patients (51 eyes) with OCT measurements within two weeks of onset of symptoms in eye(s) without prior ON. Median age at time of ON was 46.5 years (interquartile range [IQR]: 34.3–57.0) for the MOGAD and 30.4 years (IQR: 25.7–38.4) for the MS group (p<0.001) (Table 1). There were nine pediatric MOGAD patients and one pediatric MS patient that were younger than 18. Thirty-nine (61%) MOGAD patients were female compared to 42 (84%) for MS (p=0.007). Twenty-nine (45%) MOGAD patients had bilateral simultaneous ON while only one (2%) MS patient had bilateral simultaneous ON.
Table 1:
Comparison of acute optic neuritis from MOG antibody associated disease and multiple sclerosis
| MOGAD N=64 subjects (96 eyes) |
Multiple sclerosis N=50 subjects (51 eyes) |
p-value | |
|---|---|---|---|
| Age at onset (years), median (IQR) | 46.5 (34.3–57) | 30.4 (25.7–38.4) | <0.001 |
| Female sex, n (%) | 39 (61%) | 42 (84%) | 0.01 |
| Ethnicity, n (%) | White, 37 (58%) African American, 5 (8%) Asian, 17 (27%) Hispanic, 4 (6%) Other/unknown, 1 (2%) |
White, 27 (54%) African American, 16 (32%) Asian, 2 (4%) Hispanic, 0 (0%) Other/unknown, 5 (10%) |
<0.001 |
| Affected eye pRNFL, μm, median (IQR) | 164 (116–212) | 103 (93–113) | <0.001 |
| pRNFL thickening1,2, μm, median (IQR) | 45.5 (17–105) | 7.5 (1–18) | <0.001 |
| GCIPL thickness, μm, median (IQR) | 79 (76–83.5) | 79 (75–84) | 0.72 |
Abbreviations: MOG (myelin oligodendrocyte glycoprotein), pRNFL (peripapillary retinal nerve fiber layer), GCIPL (ganglion cell inner plexiform layer).
n=31 MOGAD and 48 MS eyes
Estimated using the contralateral unaffected eye as a surrogate for the baseline pRNFL thickness or a baseline optical coherence tomography scan prior to ON onset (if available), n=31 MOGAD and 48 MS subjects/eyes
MOGAD optic neuritis has more severe peripapillary RNFL thickening than MS optic neuritis.
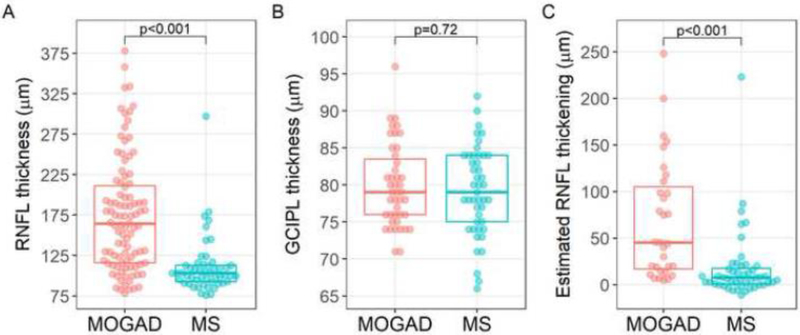
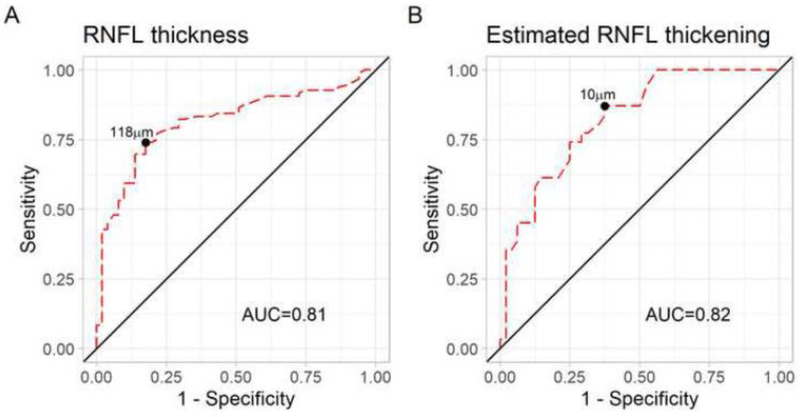
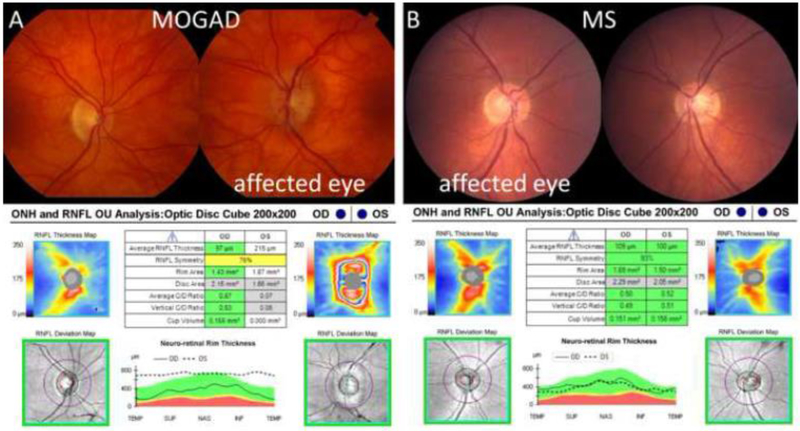
The median pRNFL thickness was 164μm (IQR: 116–212) in 96 acute MOGAD ON eyes compared to 103μm (IQR: 93–113) in 51 acute MS ON eyes (p<0.001) (Table 1, Figure 1A). There was no difference in GCIPL thickness in MOGAD ON eyes compared to MS ON eyes at the time of the acute ON (Table 1, Figure 1B). The AUC for pRNFL thickness was 0.81 (95% confidence interval (CI) 0.74 – 0.88) to discriminate MOGAD vs MS (Figure 2A). The pRNFL cutoff that maximized Youden’s index was 118μm, which provided a sensitivity of 74% and specificity of 82% for distinguishing MOGAD from MS. Only 2 (4%) MS eyes had a pRNFL thickness ≥175μm compared to 43 (45%) of MOGAD eyes, and therefore a pRNFL cutoff of 175μm provided a sensitivity of 45% and specificity of 96% for distinguishing MOGAD from MS. After excluding the nine pediatric MOGAD patients (13 eyes) and one MS pediatric patient (one eye), the AUC for pRNFL thickness was similar at 0.79 (95% CI 0.71 – 0.87) to discriminate MOGAD vs MS. Representative OCT scans and fundus photos during ON from a MOGAD and MS patient are shown in Figure 3.
Figure 1: Optical coherence tomography measures in acute optic neuritis in myelin oligodendrocyte glycoprotein antibody associated disease and multiple sclerosis.
Plots demonstrating the distribution of peripapillary retinal nerve fiber layer (pRNFL) thickness (A), ganglion cell-inner plexiform layer (GCIPL) thickness (B) and estimated pRNFL thickening (C) by group. The boxplot lines correspond to the 25th, 50th and 75th percentiles. Comparisons between groups were performed using linear generalized estimating equations (GEE) models, adjusted for age, sex and race.
Figure 2: Receiver operating characteristic (ROC) curves for peripapillary retinal nerve fiber layer (pRNFL) thickness and estimated pRNFL thickening during acute optic neuritis (ON) for discriminating myelin oligodendrocyte glycoprotein antibody associated disease (MOGAD) from multiple sclerosis (MS).
ROC curves demonstrating the performance of pRNFL thickness (A) an estimated pRNFL thickening (B) during acute ON for discriminating MOGAD vs MS.
Figure 3: Optical coherence tomography (OCT) scans and fundus photographs during acute optic neuritis (ON) in myelin oligodendrocyte glycoprotein antibody associated disease (MOGAD) and multiple sclerosis (MS).
(A) The upper images show the fundus photographs of a 69-year-old female who presented with unilateral acute left ON from MOGAD. The bottom image shows the OCT demonstrating a peripapillary retinal nerve fiber layer (pRNFL) thickness of 215μm in the affected left eye and 97μm in the unaffected right eye. (B) The upper images show the fundus photographs of a 39-year-old female who presented with unilateral acute right ON from MS. The bottom image shows a pRNFL thickness of 109μm in the affected right eye and 100μm in the unaffected left eye.
OCT is very sensitive in detecting acute optic neuritis in MOGAD patients
In addition to assessing the pRNFL values from affected ON eyes, we performed analyses of the estimated pRNFL thickening in affected eyes by using the unaffected contralateral eye as a surrogate of the affected eye’s baseline pRNFL thickness (or a prior baseline of the affected eye if an OCT scan was available prior to onset of ON). Among 31 MOGAD and 48 MS patients with an unaffected contralateral eye (26 MOGAD eyes and 41 MS eyes) or a prior baseline (5 MOGAD eyes and 7 MS eyes), the median estimated pRNFL thickening beyond the unaffected eye or prior baseline was 45μm (IQR, 17–105) and 7.5μm (IQR, 1–18), respectively (p<0.001) (Table 1, Figure 1C). All MOGAD affected eyes had a ≥ 5μm thickening and 27 (87%) had a ≥10μm thickening, whereas 26 (54%) MS affected eyes had a ≥ 5μm thickening and only 18 (38%) had a ≥10μm thickening.
The AUC for estimated pRNFL thickening was 0.82 (95% CI 0.73 – 0.91) to discriminate MOGAD from MS (Figure 2B). The estimated pRNFL thickening cutoff that maximized Youden’s index was 10μm, which provided a sensitivity of 63% and specificity of 87% for discriminating MOGAD from MS.
Demographic Factors and pRNFL thickening
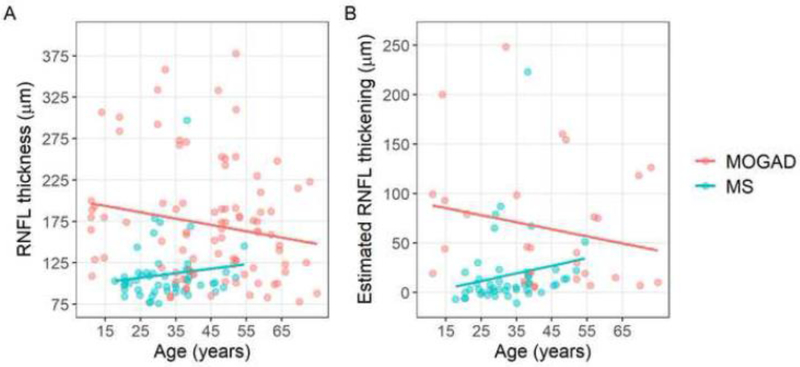
Analyses with multi-variable models including age, sex and race, revealed similar findings when comparing pRNFL thickness and estimated pRNFL thickening between MOGAD and MS acute ON eyes (p<0.001 for all). Interestingly, younger age appeared to be associated with increased severity of pRNFL swelling in MOGAD (pRNFL thickness: r=−0.24, p=0.02; estimated RNFL thickening: r=−0.29, p=0.07) but not MS (pRNFL thickness: r=0.14, p=0.30; estimated pRNFL thickening: r=0.19, p=0.20). These relationships are shown in Figure 4. Race and sex were not associated independently of age with pRNFL thickness or estimated pRNFL thickening in MOGAD or MS eyes (data not shown).
Figure 4: Relationship of age with peripapillary retinal nerve fiber layer (pRNFL) thickness and estimated pRNFL thickening in acute optic neuritis (ON) in myelin oligodendrocyte glycoprotein antibody associated disease (MOGAD) and multiple sclerosis (MS).
Scatterplots demonstrating the relationship of age with pRNFL thickness (A) and estimated pRNFL thickening (B) in acute ON in MOGAD (red) and MS (blue).
Discussion
Our retrospective multicenter study demonstrated that MOGAD ON is associated with a larger increase in pRNFL thickness than MS, which may be useful to help differentiate MOGAD from MS in eyes with a first episode of acute ON. A pRNFL cutoff of 118μm provided a sensitivity of 74% and specificity of 82%; while a RNFL cutoff of 175μm provided excellent specificity of 96%, albeit a lower sensitivity of 45% for diagnosing MOGAD. Furthermore, the increased pRNFL thickening seen in acute ON from MOGAD, as compared to MS, raises the possibility that the pathophysiologic underpinnings of optic nerve damage, and/or their severity, may differ between these conditions, consistent with the discrepancy observed in OCT outcomes after ON.(15, 26) In MOGAD, the ON often involves the orbital portion of the optic nerve and is longitudinally extensive,(10, 27) which likely contributes to the greater pRNFL thickening, relative to MS ON.
The finding that OCT-derived pRNFL thickness in acute ON can aid in differentiating MOGAD from MS is important since antibody testing can take weeks to return, so there is an unmet need for a biomarker at the time of presentation that can aid with early diagnosis and guide therapy in the acute setting. In addition, pRNFL thickness can be used to help differentiate these entities in the areas where MOG antibody testing is unavailable or cost prohibitive. Many clinicians do not offer acute steroid treatment for typical ON from MS based on the results of the Optic Neuritis Treatment Trial (ONTT) that showed that intravenous methylprednisolone improves the speed of recovery, but not the ultimate visual outcome.(28) However, the ONTT had very few MOGAD patients,(29) and therefore the results from the landmark trial better applies to typical ON. MOGAD ON is often steroid responsive and a recent study suggested that earlier treatment with steroids led to better visual outcomes.(30–33) While MOG antibody testing is considered by many to be sine qua non to diagnose MOGAD, the results often take two weeks to return, which is outside the one week window that early steroids were thought to improve outcomes.(32) Based on our study, OCT showing significant pRNFL thickening at onset of ON suggests MOGAD and could support early treatment with steroids with a prolonged oral prednisone taper until antibody testing returns.
The finding that OCT can help differentiate ON from MOGAD and MS is also important in light of the recent finding that MOG antibody testing has suboptimal diagnostic performance at low titers.(13) While the specificity of MOG antibodies is about 98% for MOGAD, a recent study evaluating MOG antibody testing at a single tertiary center found an overall positive predictive value of only 72% and was near 50% at lower titers for.(13) Many of the false positive MOG antibodies were in patients with MS. With ON being the most common manifestation of both MOGAD and MS, OCT can help differentiate MOGAD and MS in borderline cases, which is important because chronic treatment is different for these diseases.(18, 34, 35)
While there were some differences in age, sex, and race between the two cohorts, the difference in pRNFL thickening remained significantly different after accounting for these variables. OCT pRNFL thickness declines with age,(36–38) potentially biasing against detection of elevation in the older MOGAD cohort; however, the MOGAD cohort exhibited significant pRNFL thickening compared to the younger MS cohort. Additionally, the effects of age on pRNFL thickness are very small compared to the severity of pRNFL thickening and discrepancy between MOGAD and MS that we observed in acute ON. Even after removing the pediatric MOGAD and MS patients from the analysis, pRNFL thickness remained higher in the MOGAD patients than MS, and the AUC was not significantly different. There was a similar proportion of Whites in the two cohorts, but the MOGAD cohort did have more Asians and African Americans. This could have partially contributed to the thicker pRNFL in the MOGAD cohort since Asians and African Americans have been found to have a thicker pRNFL than Whites,(37, 39) but the difference between MOGAD and MS remained significantly different after accounting for race.
In addition to differentiating MOGAD and MS, this study demonstrated that OCT has the potential to be used to confirm a diagnosis of ON in previously unaffected eyes, especially in MOGAD patients. MOGAD ON was associated with a median pRNFL increase of 45.5μm with 87% having at least a 10μm increase and all attacks associated with at least a 5μm increase. The test-retest pRNFL variability for Cirrus OCT is about 4μm,(40) and therefore pRNFL thickening is a sensitive marker for detecting acute ON attacks from MOGAD in eyes that have not been previously affected by ON. In contrast, MS ON was associated with only a median pRNFL increase of 7μm with about half having at least a 5μm increase, and only a third of attacks having an increase of 10μm. Having objective evidence of a relapse is important for clinical practice and this information will be important for planning clinical trials and adjudication of ON attacks. While ON in equivocal cases is typically confirmed by contrast-enhanced MRI of the orbits, preliminary results from the inebilizumab trial on NMOSD patients (which included some patients with MOGAD) found that 51% of MRI scans showed asymptomatic enhancement during an inter-attack surveillance MRI’s,(41) and therefore it would be helpful to have other means of confirming equivocal cases. The observation that pRNFL thickening occurs in the vast majority of MOGAD ON indicates OCT can add a readily available, cost effective and rapid confirmatory biomarker for ON in MOGAD patients. For MS patients, pRNFL thickening is less sensitive, but can still be helpful in confirming in about half of ON attacks.
Limitations to this study include its retrospective nature. Being drawn mostly from tertiary care centers, the cohort may have been biased toward more atypical or severe disease. This study only included Cirrus OCT’s to allow the data to be compared in a uniform fashion, and therefore these specific cutoffs may not be applicable to other OCT devices. In addition, this study only examined eyes that were not affected by prior ON. An atrophic optic nerve from prior ON may not have as much optic disc edema or pRNFL thickening and therefore these findings can only be applied to eyes without prior ON. Lastly, OCT alone would not be helpful in differentiating other causes of disc edema, such as nonarteritic anterior ischemic optic neuropathy or papillitis.
Future studies comparing AQP4 antibody seropositive NMOSD and MOGAD will be important because of their overlapping presentation but differing prognosis and treatment.(42, 43) Because AQP4 antibody seropositive NMOSD is associated with more posterior optic nerve involvement and less optic disc edema,(17, 44) OCT may also help differentiate acute ON in MOGAD from NMOSD in a similar fashion to MS, but future studies are required to confirm this.
In conclusion, OCT is helpful in distinguishing acute ON from MOGAD and MS at the time of presentation. Therefore, OCT may provide guidance on treatment of acute ON before the results of the antibody testing return, and can facilitate the differentiation of MOGAD from MS in cases with overlapping clinical features or in the setting of low MOG antibody titer. In addition, the thickened pRNFL that typically accompanies acute ON can be used as objective evidence of an ON attack, especially for MOGAD, which can be used to help confirm ON and to adjudicate attacks in future clinical trials.
Highlights.
Acute optic neuritis in myelin oligodendrocyte glycoprotein antibody associated disease (MOGAD) was associated with more severe peripapillary retinal nerve fiber layer thickening (pRNFL) on optical coherence tomography (OCT) compared to multiple sclerosis.
The ROC area under the curve for pRNFL thickness was 0.81 to discriminate acute optic neuritis from MOGAD vs multiple sclerosis.
All MOGAD affected eyes had at least a 5μm pRNFL thickening, while only around half of affected eyes for multiple sclerosis had at least a 5μm pRNFL thickening.
pRNFL thickening is a sensitive biomarker for confirming acute optic neuritis in MOGAD, which is clinically helpful and could be used for adjudication of attacks in future MOGAD clinical trials.
Acknowledgments
Funding/Support: This work was supported by the Department Laboratory Medicine and Pathology and the Center for MS and Autoimmune Neurology, Mayo Clinic, Rochester, MN, the Leonard and Mary Lou Hoeft Career Development Award in Ophthalmology Research, and an RO1 from the National Institute of Neurological Disorders and Stroke (R01NS113828 to E. Flanagan). H. E. Moss was supported by grants from the NIH (K23 EY 024345, P30 EY 026877) and Research to Prevent Blindness (unrestricted grant to Stanford University Department of Ophthalmology). This work was also supported by the NIH/NINDS (K23NS117883 to E.S. Sotirchos; R01NS082347 to P.A. Calabresi), National MS Society (RG-1606-08768 to S. Saidha), and the Caring Friends NMO Research Fund.
Footnotes
Declaration of Competing Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jitprapaikulsan J, Chen JJ, Flanagan EP, Tobin WO, Fryer JP, Weinshenker BG, et al. Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein Autoantibody Status Predict Outcome of Recurrent Optic Neuritis. Ophthalmology. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Jarius S, Ruprecht K, Stellmann JP, Huss A, Ayzenberg I, Willing A, et al. MOG-IgG in primary and secondary chronic progressive multiple sclerosis: a multicenter study of 200 patients and review of the literature. J Neuroinflammation. 2018;15(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140(12):3128–38. [DOI] [PubMed] [Google Scholar]
- 4.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: The MOGADOR study. Neurology. 2018;90(21):e1858–e69. [DOI] [PubMed] [Google Scholar]
- 6.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation. 2016;13(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber MS, Derfuss T, Metz I, Bruck W. Defining distinct features of anti-MOG antibody associated central nervous system demyelination. Ther Adv Neurol Disord.2018;11:1756286418762083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reindl M, Jarius S, Rostasy K, Berger T. Myelin oligodendrocyte glycoprotein antibodies: How clinically useful are they? Curr Opin Neurol. 2017;30(3):295–301. [DOI] [PubMed] [Google Scholar]
- 10.Chen JJ, Flanagan EP, Jitprapaikulsan J, Chiriboga A, Fryer JP, Leavitt JA, et al. Myelin Oligodendrocyte Glycoprotein Antibody–Positive Optic Neuritis: Clinical Characteristics, Radiologic Clues, and Outcome. Am J Ophthalmol. 2018;195:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tajfirouz DA, Bhatti MT, Chen JJ. Clinical Characteristics and Treatment of MOG-IgG-Associated Optic Neuritis. Curr Neurol Neurosci Rep. 2019;19(12):100. [DOI] [PubMed] [Google Scholar]
- 12.Reindl M, Schanda K, Woodhall M, Tea F, Ramanathan S, Sagen J, et al. International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm. 2020;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sechi E, Buciuc M, Pittock SJ, Chen JJ, Fryer JP, Jenkins SM, et al. Positive Predictive Value of Myelin Oligodendrocyte Glycoprotein Autoantibody Testing. JAMA Neurol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan MB, Stern C, Flanagan EP, Pittock SJ, Kunchok A, Foster RC, et al. Population-Based Incidence of Optic Neuritis in the Era of Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein Antibodies. Am J Ophthalmol. 2020;220:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filippatou AG, Mukharesh L, Saidha S, Calabresi PA, Sotirchos ES. AQP4-IgG and MOG-IgG Related Optic Neuritis-Prevalence, Optical Coherence Tomography Findings, and Visual Outcomes: A Systematic Review and Meta-Analysis. Front Neurol. 2020;11:540156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soelberg K, Jarius S, Skejoe H, Engberg H, Mehlsen JJ, Nilsson AC, et al. A population-based prospective study of optic neuritis. Mult Scler. 2017;23(14):1893–901. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Tan S, Chan TCY, Xu Q, Zhao J, Teng D, et al. Clinical features of demyelinating optic neuritis with seropositive myelin oligodendrocyte glycoprotein antibody in Chinese patients. Br J Ophthalmol. 2018;102(10):1372–7. [DOI] [PubMed] [Google Scholar]
- 18.Chen JJ, Bhatti MT. Clinical phenotype, radiological features, and treatment of myelin oligodendrocyte glycoprotein-immunoglobulin G (MOG-IgG) optic neuritis. Curr Opin Neurol. 2020;33(1):47–54. [DOI] [PubMed] [Google Scholar]
- 19.Biotti D, Bonneville F, Tournaire E, Ayrignac X, Dalliere CC, Mahieu L, et al. Optic neuritis in patients with anti-MOG antibodies spectrum disorder: MRI and clinical features from a large multicentric cohort in France. J Neurol. 2017;264(10):2173–5. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Chiriboga AS, Majed M, Fryer J, Dubey D, McKeon A, Flanagan EP, et al. Association of MOG-IgG Serostatus With Relapse After Acute Disseminated Encephalomyelitis and Proposed Diagnostic Criteria for MOG-IgG-Associated Disorders. JAMA Neurol. 2018;75(11):1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waters PJ, Komorowski L, Woodhall M, Lederer S, Majed M, Fryer J, et al. A multicenter comparison of MOG-IgG cell-based assays. Neurology. 2019;92(11):e1250–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–73. [DOI] [PubMed] [Google Scholar]
- 23.Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, Saidha S, Martinez-Lapiscina EH, Lagreze WA, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5. [DOI] [PubMed] [Google Scholar]
- 26.Sotirchos ES, Filippatou A, Fitzgerald KC, Salama S, Pardo S, Wang J, et al. Aquaporin-4 IgG seropositivity is associated with worse visual outcomes after optic neuritis than MOG-IgG seropositivity and multiple sclerosis, independent of macular ganglion cell layer thinning. Mult Scler. 2020;26(11):1360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson AP, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22(4):470–82. [DOI] [PubMed] [Google Scholar]
- 28.Beck RW, Cleary PA, Anderson MM Jr., Keltner JL, Shults WT, Kaufman DI, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992;326(9):581–8. [DOI] [PubMed] [Google Scholar]
- 29.Chen JJ, Tobin WO, Majed M, Jitprapaikulsan J, Fryer JP, Leavitt JA, et al. Prevalence of Myelin Oligodendrocyte Glycoprotein and Aquaporin-4-IgG in Patients in the Optic Neuritis Treatment Trial. JAMA Ophthalmol. 2018;136(4):419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramanathan S, Mohammad S, Tantsis E, Nguyen TK, Merheb V, Fung VSC, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89(2):127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etemadifar M, Abbasi M, Salari M, Etemadifar F, Tavakoli H. Comparing myelin oligodendrocyte glycoprotein antibody (MOG-Ab) and non MOG-Ab associated optic neuritis: Clinical course and treatment outcome. Mult Scler Relat Disord. 2019;27:127–30. [DOI] [PubMed] [Google Scholar]
- 32.Stiebel-Kalish H, Hellmann MA, Mimouni M, Paul F, Bialer O, Bach M, et al. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol Neuroimmunol Neuroinflamm. 2019;6(4):e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kezuka T, Ishikawa H. Diagnosis and treatment of anti-myelin oligodendrocyte glycoprotein antibody positive optic neuritis. Jpn J Ophthalmol. 2018;62(2):101–8. [DOI] [PubMed] [Google Scholar]
- 34.Chen JJ, Flanagan EP, Bhatti MT, Jitprapaikulsan J, Dubey D, Lopez Chiriboga ASS, et al. Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder. Neurology. 2020;95(2):e111–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whittam DH, Cobo-Calvo A, Lopez-Chiriboga AS, Pardo S, Gornall M, Cicconi S, et al. Treatment of MOG-IgG-associated disorder with rituximab: An international study of 121 patients. Mult Scler Relat Disord. 2020;44:102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung CK, Yu M, Weinreb RN, Ye C, Liu S, Lai G, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012;119(4):731–7. [DOI] [PubMed] [Google Scholar]
- 37.Alasil T, Wang K, Keane PA, Lee H, Baniasadi N, de Boer JF, et al. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma. 2013;22(7):532–41. [DOI] [PubMed] [Google Scholar]
- 38.Sotirchos ES, Gonzalez Caldito N, Filippatou A, Fitzgerald KC, Murphy OC, Lambe J, et al. Progressive Multiple Sclerosis Is Associated with Faster and Specific Retinal Layer Atrophy. Ann Neurol. 2020;87(6):885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caldito NG, Saidha S, Sotirchos ES, Dewey BE, Cowley NJ, Glaister J, et al. Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: a longitudinal study. Brain. 2018;141(11):3115–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mwanza JC, Chang RT, Budenz DL, Durbin MK, Gendy MG, Shi W, et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010;51(11):5724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paul F, Wingerchuk D, Weinshenker B, Bennett J, Kim H, Pittock S, et al. Quiescent MRI activity in neuromyelitis optica spectrum disorder: results from the N-MOmentum randomized placebo-controlled trial. Abstract ECTRIMS - ACTRIMS. 2020. [Google Scholar]
- 42.Prasad S, Chen J. What You Need to Know About AQP4, MOG, and NMOSD. Semin Neurol. 2019;39(6):718–31. [DOI] [PubMed] [Google Scholar]
- 43.Hacohen Y, Palace J. Time to separate MOG-Ab-associated disease from AQP4-Ab-positive neuromyelitis optica spectrum disorder. Neurology. 2018;90(21):947–8. [DOI] [PubMed] [Google Scholar]
- 44.Chen JJ, Pittock SJ, Flanagan EP, Lennon VA, Bhatti MT. Optic neuritis in the era of biomarkers. Surv Ophthalmol. 2020;65(1):12–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article are available, from the corresponding author on reasonable request from any qualified investigator.