Abstract
Objective
To compare key seizure and outcome characteristics between neonates with and without cardiopulmonary disease (CPD).
Study design
The Neonatal Seizure Registry (NSR-1) is a multicenter, prospectively acquired cohort of neonates with clinical or EEG-confirmed seizures. CPD was defined as congenital heart disease, congenital diaphragmatic hernia, and exposure to extracorporeal membrane oxygenation. We assessed continuous electroencephalographic monitoring (cEEG) strategy, seizure characteristics, seizure management, and outcomes for neonates with and without CPD.
Results
We evaluated 83 neonates with CPD and 271 neonates without CPD. Neonates with CPD were more likely to have EEG-only seizures (40% vs. 21%, P < .001) and experience their first seizure later than those without CPD (174 vs. 21 hours of age, p<0.001), but they had similar seizure exposure (many-recurrent electrographic seizures 39% vs. 43%, p=0.27). Phenobarbital was the primary initial antiseizure medication (ASM) for both groups (90%), and both groups had similarly high rates of incomplete response to initial ASM administration (66% vs. 68%, p=0.75). Neonates with CPD were discharged from the hospital later (hazard ratio 0.34, 95%CI 0.25–0.45, p<0.001), although rates of in-hospital mortality were similar between the groups (hazard ratio 1.13, 95%CI 0.66–1.94, p=0.64).
Conclusion
Neonates with and without CPD had a similarly high seizure exposure, but neonates with CPD were more likely to experience EEG-only seizures and had seizure onset later in the clinical course. Phenobarbital was the most common seizure treatment, but seizures were often refractory to initial ASM. These data support guidelines recommending cEEG in neonates with CPD and indicate a need for optimized therapeutic strategies.
Seizures are a well-documented manifestation of neonatal brain dysfunction and injury, often resulting from hypoxic-ischemic injury (HIE), ischemic stroke, intracranial hemorrhage, and central nervous system infection.(1–3) Current management practices are based mostly upon data collected in neonates with acute symptomatic seizures due to these well-characterized primary neurologic disorders. Seizures also occur in neonates with cardiopulmonary diseases (CPD), defined herein as congenital heart disease (CHD), congenital diaphragmatic hernia (CDH), and exposure to extracorporeal membrane oxygenation (ECMO). Neonates with CPD may have abnormal brain development, abnormal brain function due to cardiopulmonary pathophysiology, and complications associated with required life-sustaining technology.(4–9) These conditions may result in acute acquired brain injuries that manifest as seizures. Single-center studies of neonates with CPD have reported clinical and electrographic seizure incidence ranging from 1–20%.(10–17) Thus, consensus-based guidelines recommend performing a minimum of 24 hours of continuous electroencephalography (cEEG) monitoring for neonates with CPD.(18) However, few data are available regarding distinct seizure presentation or treatment challenges in neonates with CPD. If seizure characteristics differ between neonates with and without CPD, then more specific cEEG and seizure management strategies may be necessary to optimize outcomes.
In this study, we aimed to compare the clinical presentation, seizure characteristics, cEEG strategies, seizure management, and outcomes in neonates with and without CPD. We hypothesized that neonates with CPD, compared with neonates without CPD, would experience a comparably high incidence of electrographic seizures due to acute brain injury and that seizures would more often be refractory to first-line anti-seizure medications (ASM).
Methods
The Neonatal Seizure Registry’s first cohort (NSR-I) was a prospective cohort of consecutive neonates (less than 44 weeks postmenstrual age at the time of seizure onset) with clinical or EEG-confirmed seizures enrolled at seven tertiary care pediatric hospitals from January 2013 to April 2015.(1, 19–22) This cohort included neonates enrolled at six of the NSR sites. These institutions adhere to the American Clinical Neurophysiology Society’s (ACNS) guideline for cEEG in neonates(18, 23), which recommends cEEG for differential diagnosis of suspicious clinical events and screening for electrographic seizures for a minimum of 24 hours in high-risk neonatal populations. High-risk neonatal populations include those with acute etiologies such as encephalopathy, intracranial infection, and intracranial hemorrhage, and neonates with underlying CPDs such as CHD requiring surgical repair with cardiopulmonary bypass (CPB) or undergoing ECMO. Participating sites received Institutional Review Board approval for the study, and a waiver of informed consent was granted.
Clinical and EEG data were abstracted from electronic medical records into a secure REDCap database.(24) Clinical data included: (1) demographics; (2) cEEG characteristics (indications, age at onset, location); (3) seizure characteristics (exposure, semiology, timing); (4) seizure treatment (ASM, dosing, therapeutic drug levels, seizure responsiveness); and (5) disposition (mortality, discharge on ASM). EEG data were acquired clinically according to ACNS guidelines(18, 23) and analyzed by electroencephalographers at each site. The clinical EEG reports were abstracted into the study database. All neonates had at least one EEG recorded. If multiple EEG studies were acquired during the study period, then data from subsequent EEG studies were also included in the database (EEG start and stop date/time, presence of electrographic seizures). EEG monitoring indication was recorded only for the initial EEG performed on all neonates. Electrographic seizures were defined as a sudden and abnormal EEG event defined by a repetitive and evolving pattern with a minimum peak-to-peak voltage of two microvolts and duration of at least ten seconds with (electroclinical) or without (EEG-only) concurrent clinical signs.(23) Electrographic seizure exposure was categorized as none, low (<7 seizures), many-recurrent (≥7 seizures), or status epilepticus (summed duration of seizures comprising ≥50% of any one-hour epoch). EEG-only seizures were defined as seizures evident on EEG but without any identifiable clinical manifestation. The exact date and time of the initial clinical and/or electrographic seizure were recorded. The initial ASM loading dose was defined as the amount of medication administered in mg/kg in the first bolus of the initial ASM, and the total loading dose was defined as the total amount of medication administered in mg/kg in the initial 72 hours of treatment with an ASM. Incomplete response to initial ASM was defined as the occurrence of ≥1 electrographic seizure occurring >30 minutes after the initial loading dose of at least 20mg/kg of phenobarbital, 15mg/kg of phenytoin or fosphenytoin, or 40mg/kg of levetiracetam.
Neonates were categorized as having or not having CPD. Neonates with CPD were defined as those with CHD, CDH, or ECMO exposure. Neonates with CPD were analyzed as those with CHD-only, CDH-only, ECMO-only, and multiple diagnoses (≥2 diagnoses of CHD, CDH, and/or ECMO). Primary underlying seizure etiologies were not limited for neonates with CPD to maximize the number of neonates included in the cohort, and included acute symptomatic etiologies such as HIE, ischemic stroke, intracranial hemorrhage, and intracranial infection. Neonates without CPD were included if they had a primary seizure etiology of HIE, ischemic stroke, intracranial hemorrhage, or intracranial infection without a comorbid CPD.
Statistical analyses were performed using Stata (StataCorp LLC) version 15.1. Standard descriptive statistics included frequencies and percentages to summarize categorical variables and median and interquartile ranges (IQR) to summarize continuous variables due to skewness in distribution. To compare CPD and non-CPD cohorts or within the CPD cohort across diagnosis subgroups, the Pearson chi-squared or Fisher exact test (for variables with ≤5 subjects per group) was used as appropriate to assess binary and nominal categorical variables, and the Kruskal Wallis test was used to assess continuous variables. For certain categorical variables (such as seizure etiology), individual tests of comparison were run between the CPD and non-CPD groups for each response option. Cox proportional hazards model was used to assess the difference in time to death and time to hospital discharge between the two groups, with the estimated hazard ratio (HR) reported.
Results
Demographic and Clinical Characteristics
The cohort of 354 neonates included 83 neonates (23%) with CPD and 271 neonates (77%) without CPD. The CPD cohort included 73 neonates (88%) with CHD, 5 neonates (6%) with CDH, and 26 neonates (31%) who received ECMO. Multiple CPD diagnoses occurred in 20 neonates (24%), including 16 neonates (80%) with CHD and ECMO, 2 neonates (10%) with CDH and ECMO, 1 neonate (5%) with CHD and CDH, and 1 neonate (5%) with CHD, CDH, and ECMO. Table I provides clinical characteristics of the CPD cohort.
Table 1:
Clinical Characteristics of the Cardiopulmonary Disease Cohort. Data are presented as n (%) or median [interquartile range].
| Congenital Heart Disease Cohort (n= 73) | |
|
| |
| Prenatal diagnosis | 36 (50%) |
|
| |
| CHD defect | |
| Hypoplastic left heart syndrome | 16 (22%) |
| Transposition of great arteries | 7 (10%) |
| Other* | 52 (71%) |
|
| |
| Single ventricle pathology | 30 (41%) |
|
| |
| Surgical intervention completed | 48 (66%) |
|
| |
| Age at surgery (hours of age) | 81 [35, 167] |
|
| |
| CPB utilized | 29 (60%) |
|
| |
| CPB duration (minutes) | 95 [76, 154] |
|
| |
| DHCA utilized | 12 (25%) |
|
| |
| DHCA duration (minutes) | 38 [35, 50] |
|
| |
| Congenital Diaphragmatic Hernia Cohort (N= 5) | |
|
| |
| Prenatal diagnosis | 5 (100%) |
|
| |
| Surgical intervention Completed^ | 3 (60%) |
|
| |
| Age at surgery (hours of age) | 58 [27, 94] |
|
| |
| Patch repair | 2 (67%) |
|
| |
| ECMO utilized | 3 (60%) |
|
| |
| Extracorporeal Membrane Oxygenation Cohort (N=26) | |
|
| |
| ECMO indications | |
| CHD | 8 (31%) |
| CDH | 3 (12%) |
| Intractable metabolic acidosis | 2 (8%) |
| Inability to wean CPB | 2 (8%) |
| PPHN | 3 (12%) |
| Pulmonary hypoplasia | 2 (8%) |
| Sepsis | 2 (8%) |
| Other+ | 15 (58%) |
|
| |
| ECMO Type | |
| Veno-arterial | 24 (92%) |
| Veno-venous | 1 (4%) |
| Unknown | 1 (4%) |
|
| |
| Cannulation Site | |
| Chest | 11 (42%) |
| Neck | 14 (54%) |
| Unknown | 1 (4%) |
|
| |
| Age at surgery (hours of age) | 176 [21, 507] |
Abbreviations: CDH, congenital diaphragmatic hernia; CHD, congenital heart disease; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; ECMO, extracorporeal membrane oxygenation; PPHN, persistent pulmonary hypertension of the newborn.
Individual neonates could have more than one diagnosis. Other CHD diagnoses included atrial septal defect, bicuspid aortic valve, cardiac rhabdomyomas, coarctation of the aorta with and without arch hypoplasia, congenital pulmonary airway malformation, double inlet left ventricle, Ebstein’s anomaly of the tricuspid valve, heterotaxy syndrome with a single ventricle, hypertrophic cardiomyopathy, levocardia, patent ductus arteriosus, patent foramen ovale, pulmonary atresia, pulmonary stenosis, small aortic and mitral anulus, tetralogy of Fallot, total anomalous pulmonary venous return, transposition of the great arteries, truncus arteriosus, unbalanced atrioventricular septal defect, and ventricular septal defect.
Two neonates with CDH died before undergoing repair of the defect.
Patients could have multiple indications. ECMO indications affecting only 1 patient per category are listed as ‘Other’ and included cardiac arrest, intractable shock, low cardiac output, lung mass, pneumothorax, respiratory failure, and meconium aspiration syndrome.
Surgical intervention occurred in 48/73 neonates (66%) with CHD at a median of 81 hours of age (IQR 35, 167). Among 48 neonates undergoing surgical repair, CPB was utilized in 29 neonates (60%) for a median duration of 95 minutes (IQR 76, 154), and deep hypothermic circulatory arrest was utilized in 12 neonates (25%) for a median duration of 38 minutes (IQR 35, 50). Among 5 neonates with CDH, 3 neonates (60%) underwent surgical intervention at a median of 58 hours of age (IQR 27, 94). Three neonates (60%) with CDH underwent ECMO, only 1 of whom underwent surgery. Among 26 neonates who received ECMO, 24 (92%) had veno-arterial (VA) ECMO, 1 (4%) had veno-venous (VV) ECMO, and 1 (4%) had unknown ECMO type. ECMO cannulation was in the neck for 14/26 neonates (54%), chest for 11/26 neonates (42%), and unknown for 1/26 neonate (4%). ECMO cannulation occurred at a median of 176 hours of age (IQR 21, 507). The most common indication for ECMO use was CHD.
The cohort of 271 neonates without CPD included 144 neonates (53%) with HIE, 55 neonates (20%) with ischemic stroke, 44 neonates (16%) with intracranial hemorrhage, and 28 neonates (10%) with intracranial infection. Table II compares the CPD and non-CPD cohorts. Neonates with CPD had a lower birthweight (3.1 vs. 3.3 kilograms, p=0.06), lower gestational age (38.6 vs. 39.3 weeks, p=0.001), and higher Apgar scores at 1 minute (7 vs 2, p<0.001) and 5 minutes (8 vs. 6, p=0.002) compared with neonates without CPD.
Table 2:
Comparison of Demographic, EEG Monitoring, Seizure Characteristics, Seizure Treatment, and Outcomes Between the Cardiopulmonary Disease and Non-Cardiopulmonary Disease Cohorts. Data are presented as N (%) or median [interquartile range]
| Variable | Cardiopulmonary Disease Cohort (N=83) | Non-Cardiopulmonary Disease Cohort (N=271) | p-value |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Sex, female | 36 (43%) | 122 (45%) | 0.79 |
|
| |||
| Gestational age (weeks) | 38.6 [37, 40] | 39.3 [38, 40] | 0.001 |
|
| |||
| Birth weight (kilograms) | 3.1 [2.6, 3.5] | 3.3 [2.8, 3.6] | 0.06 |
|
| |||
| Apgar, 1 minute | 7 [3, 8] | 2 [1, 7] | <0.001 |
|
| |||
| Apgar, 5 minutes | 8 [6, 9] | 6 [3, 9] | 0.002 |
|
| |||
| EEG Monitoring Characteristics | |||
|
| |||
| EEG Location | <0.001 | ||
| Neonatal ICU | 39 (47%) | 265 (98%) | |
| Pediatric ICU | 2 (2%) | 5 (2%) | |
| Cardiac ICU | 42 (50%) | 0 (0%) | |
| Other | 0 (0%) | 1 (0.4%) | |
|
| |||
| EEG Indication | <0.001 | ||
| Clinical events | 40 (48%) | 142 (52%) | |
| Encephalopathy | 13 (16%) | 72 (27%) | |
| Clinical events and encephalopathy | 8 (10%) | 53 (20%) | |
| Paralysis | 2 (2%) | 0 (0.0%) | |
| Other | 20 (24%) | 4 (2%) | |
|
| |||
| Age at start of EEG (hours) | 141 [53, 305] | 29 [10, 68] | <0.001 |
|
| |||
| Seizure Characteristics | |||
|
| |||
| Primary Seizure Etiology | <0.001 | ||
| HIE | 30 (36%) | 144 (53%) | |
| Ischemic stroke | 22 (26%) | 55 (20%) | |
| Hemorrhage | 12 (14%) | 44 (16%) | |
| Infection | 1 (1%) | 28 (10%) | |
| Other*** | 18 (22%) | 0 (0%) | |
|
| |||
| Clinical Seizure Prior to EEG Monitoring | 47 (57%) | 188 (69%) | 0.03 |
|
| |||
| Age at first seizure [clinical or EEG] (hours) | 173.7 [45.7, 339.1] | 21.3 [9.7, 56.7] | <0.001 |
|
| |||
| EEG-only seizures present | 33 (40%) | 56 (21%) | <0.001 |
|
| |||
| EEG seizure exposure | 0.27 | ||
| None | 11 (13%) | 40 (15%) | |
| Rare (<7) | 15 (18%) | 64 (24%) | |
| Many-recurrent (≥7) | 32 (39%) | 116 (43%) | |
| Status epilepticus | 24 (29%) | 48 (18%) | |
| Unknown | 1 (1%) | 3 (1%) | |
|
| |||
| Treatment Characteristics | |||
|
| |||
| Initial ASM | 0.03 | ||
| Phenobarbital | 69 (85%) | 247 (92%) | |
| Phenytoin / fosphenytoin | 2 (2%) | 1 (0.4%) | |
| Levetiracetam | 7 (9%) | 7 (3%) | |
| Other | 1 (1%) | 1 (0.4%) | |
| N/A – no load given | 2 (2%) | 14 (5%) | |
|
| |||
| ASM(s) administered+ | |||
| Benzodiazepine intermittent dose | 10 (12%) | 55 (20%) | 0.09 |
| Benzodiazepine infusion | 14 (17%) | 26 (10%) | 0.07 |
| Phenobarbital | 76 (92%) | 255 (94%) | 0.41 |
| Phenytoin / fosphenytoin | 28 (34%) | 97 (36%) | 0.73 |
| Levetiracetam | 39 (47%) | 81 (30%) | 0.004 |
| Topiramate | 3 (4%) | 7 (3%) | 0.62 |
| Vitamins# | 6 (7%) | 7 (3%) | 0.08 |
| Other ASM^ | 4 (5%) | 3 (1%) | 0.03 |
|
| |||
| Phenobarbital prior to EEG | 20 (24%) | 137 (51%) | <0.001 |
|
| |||
| Phenobarbital total loading dose (mg/kg) | 40 [20, 50] | 30 [20, 40] | 0.02 |
|
| |||
| Highest phenobarbital level | 45 [31, 59] | 45 [35, 53] | 0.74 |
|
| |||
| Phenytoin / fosphenytoin total loading dose (mg/kg) | 20 [20, 30] | 20 [20, 22] | 0.09 |
|
| |||
| Levetiracetam total loading dose (mg/kg) | 40 [20, 60] | 40 [30, 60] | 0.48 |
|
| |||
| Incomplete response to initial ASM loading dose | N=77 | N=261 | 0.75 |
| 51 (66%) | 178 (68%) | ||
|
| |||
| Disposition | |||
|
| |||
| Discharge or Death on ASM | 68 (82%) | 183 (68%) | 0.01 |
|
| |||
| Disposition | 0.01 | ||
| Death | 25 (30%) | 37 (14%) | |
| Home | 53 (64%) | 193 (71%) | |
| Transfer | 3 (4%) | 25 (9%) | |
| Hospice | 2 (2%) | 12 (4%) | |
| Long-term care facility | 0 (0%) | 4 (2%) | |
|
| |||
| Age at death (days) | 23 [12, 50] | 5 [3, 18] | 0.001 |
|
| |||
| Age at discharge (days) | 37 [20, 63] | 12 [9, 22] | <0.001 |
Abbreviations: HIE, hypoxic ischemic encephalopathy; ASM, antiseizure medication; ICU, intensive care unit.
Other primary seizure etiologies include brain malformation (6 CHD, 1 CDH), inborn errors of metabolism (1 CHD), neonatal epilepsy (3 CHD), and other (5 CHD, 2 multi-diagnoses).
Multiple Antiseizure medications could be administered throughout the hospital course.
Vitamins include pyridoxine, folinic acid, and pyridoxal-5-phosphate.
Other medications include acetazolamide, bumetanide, carbamazepine, lacosamide, lidocaine, oxcarbazepine, valproic acid, and other vitamins (calcitriol, calcium, magnesium, thiamine).
EEG Monitoring Characteristics
All neonates in both cohorts underwent cEEG monitoring because they were considered high-risk populations. Compared with neonates without CPD, neonates with CPD more often underwent cEEG in the cardiac intensive care unit than the neonatal intensive care unit (CICU: 50% vs. 0%; NICU 47% vs. 98%; p<0.001) and had cEEG initiated later in their clinical course (141 vs. 29 hours of age, p<0.001). The cEEG indication varied between neonates with and without CPD. Neonates with CPD were less likely to be monitored for encephalopathy and/or suspicious clinical events, and they more likely to be monitored for “other” indications such as institutional cEEG protocols (clinical events with or without encephalopathy: 73% vs. 98%; other: 24% vs. 2%; p<0.001). All neonates with and without CPD had one EEG recorded. Of neonates with CPD, 34 (41%) had a second EEG and 11 (13%) had a third EEG. Of neonates without CPD, 71 (26%) had a second EEG and 20 (7%) had a third EEG.
Seizure Characteristics
The primary seizure etiology varied between the groups. Although HIE was the most common primary seizure etiology for neonates with and without CPD, neonates with CPD were more likely to have other etiologies as the primary seizure etiology (22% vs. 0%, p<0.001), including neonatal epilepsies (n=3), brain malformations (n=7), and metabolic disorders (n=1). Neonates with CPD were less likely to have clinical seizures prior to cEEG initiation (57% vs. 69%, p=0.03) and more likely to experience EEG-only seizures (40% vs. 21%, p<0.001). Further, neonates with CPD experienced their first seizure, either clinical or electrographic seizures, later in their course (173.7 vs. 21.3 hours of age, p<0.001). Among neonates who experienced electrographic seizures, they were most often identified on the initial cEEG, including 64/83 (77%) of neonates with CPD and 220/271 (81%) of neonates without CPD. The yield of seizure detection on the second EEG was 32% (11/34) for neonates with CPD and 20% (14/71) for neonates without CPD. The yield of seizure detection on the third EEG was 27% (3/11) for neonates with CPD and 5% (1/20) for neonates without CPD. Electrographic seizure exposure did not vary significantly by the presence of CPD, as neonates with and without CPD had similarly high rates of many-recurrent electrographic seizures (39% vs. 43%) and status epilepticus (29% vs. 18%, p=0.27). Notably, electrographic seizure exposure varied within the CPD cohort (p=0.05); neonates with ECMO and multiple CPD diagnoses experienced higher rates of status epilepticus (43% and 45%, respectively). Neonates with CHD-only experienced many-recurrent seizures (42%) and the lone neonate with CDH-only did not experience any electrographic seizures (0%). Table III provides within-group differences between the subgroups of neonates with CPD (CHD, CDH, ECMO, and multiple diagnoses).
Table 3.
EEG Monitoring, Seizure Characteristics, Seizure Treatment, and Outcomes of the Cardiopulmonary Disease Cohort. Data are presented as n (%) or median [interquartile range].
| CHD (N=55) | CDH (N=1) | ECMO (N=7) | Multiple Diagnoses! (N=20) | |
|---|---|---|---|---|
|
| ||||
| Demographics | ||||
|
| ||||
| Sex, female | 24 (44%) | 1 (100%) | 5 (71%) | 6 (30%) |
|
| ||||
| Gestational age (weeks) | 39 [38, 40] | 39 [n/a] | 36.7 [27, 40] | 38.2 [37, 39] |
|
| ||||
| Birth weight (kilograms) | 3.1 [2.6, 3.4] | 2.62 [n/a] | 2.7 [0.7, 3.2] | 3.3 [2.9, 3.7] |
|
| ||||
| Apgar score, 1 minute | 7 [4, 8] | 1 [n/a] | 2 [1, 8] | 6 [4, 8] |
|
| ||||
| Apgar score, 5 minutes | 8 [7, 9] | 5 [n/a] | 5 [3, 9] | 8 [6, 8] |
|
| ||||
| EEG Monitoring Characteristics | ||||
|
| ||||
| EEG Location | ||||
| Cardiac ICU | 27 (49%) | 0 (0%) | 1 (14%) | 14 (70%) |
| Neonatal ICU | 27 (49%) | 1 (100%) | 5 (71%) | 6 (30%) |
| Pediatric ICU | 1 (2%) | 0 (0%) | 1 (14%) | 0 (0%) |
|
| ||||
| EEG Indication | ||||
| Clinical events | 33 (60%) | 1 (100%) | 0 (0%) | 6 (30%) |
| Encephalopathy | 5 (9%) | 0 (0%) | 2 (29%) | 6 (30%) |
| Clinical events and encephalopathy | 6 (11%) | 0 (0%) | 2 (29%) | 0 (0%) |
| Paralysis | 7 (0%) | 0 (0%) | 1 (14%) | 1 (5%) |
| Other | 11 (20%) | 0 (0%) | 2 (29%) | 7 (35%) |
|
| ||||
| Age at EEG onset (hours) | 118 [52, 221] | 260 [n/a] | 87 [6, 2887] | 209 [81, 376] |
|
| ||||
| Seizure Characteristics | ||||
|
| ||||
| Primary Seizure Etiology* | ||||
| HIE | 15 (27%) | 0 (0%) | 6 (86%) | 9 (45%) |
| Ischemic stroke | 16 (29%) | 0 (0%) | 0 (0%) | 6 (30%) |
| Hemorrhage | 8 (15%) | 0 (0%) | 1 (14%) | 3 (15%) |
| Infection | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other | 15 (27%) | 1 (100%) | 0 (0%) | 2 (10%) |
|
| ||||
| Clinical seizure prior to EEG | 36 (65%) | 1 (100%) | 3 (43%) | 7 (35%) |
|
| ||||
| Age at first seizure [clinical or EEG] (hours) | 120 [29, 240] | 240 [n/a] | 97 [6, 2887] | 289 [208, 491] |
|
| ||||
| EEG-only seizures present | 19 (35%) | 0 (0%) | 4 (57%) | 10 (50%) |
|
| ||||
| EEG seizure Exposure | ||||
| None | 9 (16%) | 1 (100%) | 0 (0%) | 1 (5%) |
| Rare (<7) | 11 (20%) | 0 (0%) | 2 (29%) | 2 (10%) |
| Many-recurrent (≥7) | 23 (42%) | 0 (0%) | 1 (14%) | 8 (40%) |
| Status epilepticus | 12 (22%) | 0 (0%) | 3 (43%) | 9 (45%) |
| Unknown | 0 (0%) | 0 (0%) | 1 (14%) | 0 (0%) |
|
| ||||
| Treatment Characteristics | ||||
|
| ||||
| Initial loading ASM | ||||
| Phenobarbital | 45 (82%) | 1 (100%) | 5 (71%) | 18 (90%) |
| Phenytoin / fosphenytoin | 1 (2%) | 0 (0%) | 0 (0%) | 1 (5%) |
| Levetiracetam | 6 (11%) | 0 (0%) | 1 (14%) | 0 (0%) |
| Other | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| N/a – no load | 2 (4%) | 0 (0%) | 1 (14%) | 1 (5%) |
|
| ||||
| ASM(s) administered* | ||||
| Benzodiazepine intermittent dose | 6 (11%) | 0 (0%) | 0 (0%) | 4 (20%) |
| Benzodiazepine infusion | 7 (13%) | 0 (0%) | 1 (14%) | 6 (30%) |
| Phenobarbital | 50 (91%) | 1 (100%) | 6 (86%) | 19 (95%) |
| Phenytoin / fosphenytoin | 16 (29%) | 0 (0%) | 2 (29%) | 10 (50%) |
| Levetiracetam | 25 (45%) | 0 (0%) | 4 (57%) | 10 (50%) |
| Topamax | 3 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Vitamins# | 6 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other ASM% | 2 (4%) | 0 (0%) | 1 (14%) | 1 (5%) |
|
| ||||
| Phenobarbital prior to EEG | 13 (24%) | 1 (100%) | 1 (14%) | 5 (25%) |
|
| ||||
| Phenobarbital total loading dose (mg/kg)^ | 40 [20, 50] | 20 [n/a] | 35 [20, 50] | 40 [35, 60] |
|
| ||||
| Highest phenobarbital level | 51 [32, 64] | 29 [n/a] | 43 [42, 51] | 31 [22, 58] |
|
| ||||
| Phenytoin / fosphenytoin total loading dose (mg/kg or mg PE /kg) | 20 [20, 30] | n/a | 29 [27, 30] | 20 [20, 34] |
|
| ||||
| Levetiracetam total loading dose (mg/kg) | 25 [20, 50] | n/a | 40 [30, 60] | 60 [40, 60] |
|
| ||||
| Incomplete response to initial ASM loading dose | 31 (61%) | 0 (0%) | 4 (67%) | 16 (84%) |
|
| ||||
| Disposition | ||||
|
| ||||
| Discharge or death on ASM | 48 (87%) | 1 (100%) | 3 (43%) | 16 (80%) |
|
| ||||
| Disposition | ||||
| Death | 7 (13%) | 0 (0%) | 5 (71%) | 13 (65%) |
| Home | 45 (82%) | 1 (100%) | 2 (29%) | 5 (25%) |
| Transfer | 1 (2%) | 0 (0%) | 0 (0%) | 2 (10%) |
| Hospice | 2 (7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Long term care facility | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
|
| ||||
| Age at death (days) | 7 [5, 60] | n/a | 60 [21, 125] | 23 [13, 35] |
|
| ||||
| Age at discharge (days) | 33 [19, 57] | 107 [n/a] | 62 [40, 84] | 61 [37, 63] |
Abbreviations - HIE, hypoxic ischemic encephalopathy; ASM, antiseizure medication; ICU, intensive care unit.
Multiple diagnoses include 16 CHD + ECMO, 2 CDH + ECMO, 1 CHD + CDH, 1 CHD + CDH + ECMO.
Other primary seizure etiologies include brain malformation (6 CHD, 1 CDH), inborn errors of metabolism (1 CHD), neonatal epilepsy (3 CHD), and other (5 CHD, 2 multi-diagnoses).
Multiple Antiseizure medications could be administered throughout the hospital course.
Total loading dose is the total amount of medication administered in mg/kg in initial 72 hours of treatment.
Vitamins include pyridoxine, folinic acid, and pyridoxal-5-phosphate.
Other Medications/Vitamins include acetazolamide, bumetanide, carbamazepine, lacosamide, lidocaine, oxcarbazepine, valproic Acid, and other vitamins (calcitriol, calcium, magnesium, thiamine).
Seizure Treatment
Although phenobarbital was the most prescribed ASM in both cohorts, neonates with CPD were more likely to receive levetiracetam as both the initial loading ASM (9% vs. 3%, p=0.03) and as an administered ASM during the hospital course (47% vs. 30%, p=0.004) compared with neonates without CPD. Compared with neonates without CPD, neonates with CPD were less likely to receive phenobarbital prior to cEEG initiation (24% vs. 51%, p<0.001). Although neonates with CPD ultimately received a higher total loading dose of phenobarbital (40 vs. 30 mg/kg, p=0.02), the two groups achieved comparable therapeutic drug levels (highest phenobarbital level of 45 mcg/mL for both groups, p=0.74). Levetiracetam and (fos)phenytoin dosing was similar for both groups. Treatment response was similar for both groups, including high rates of incomplete response to initial loading ASM in neonates with and without CPD (66% vs. 68%, p=0.75).
Short-Term Outcomes
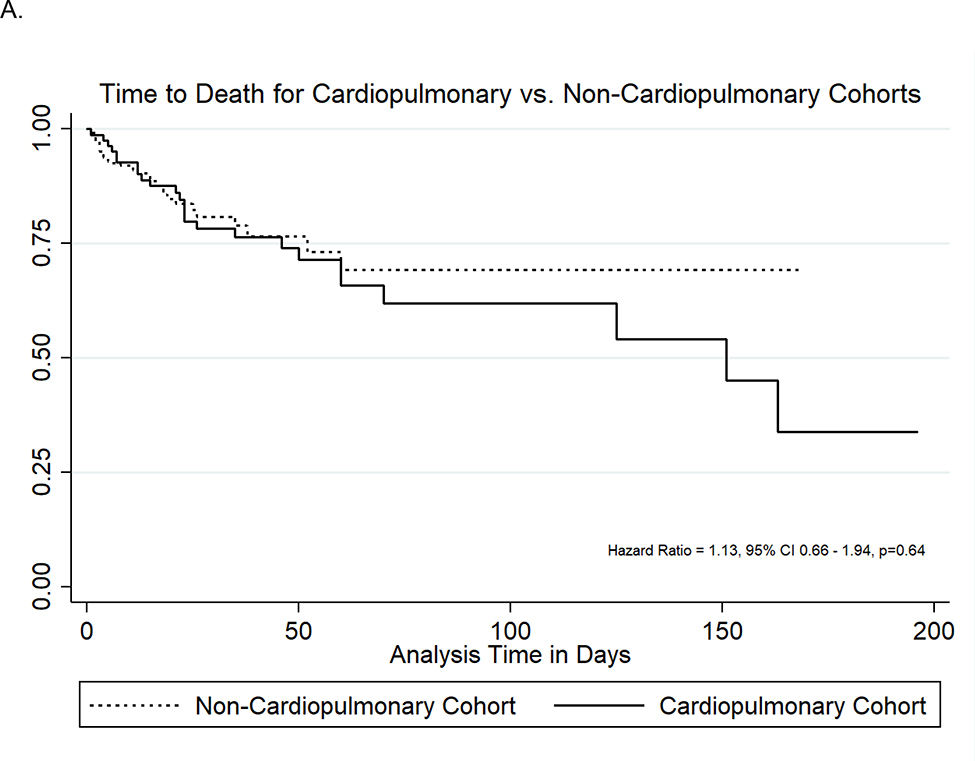
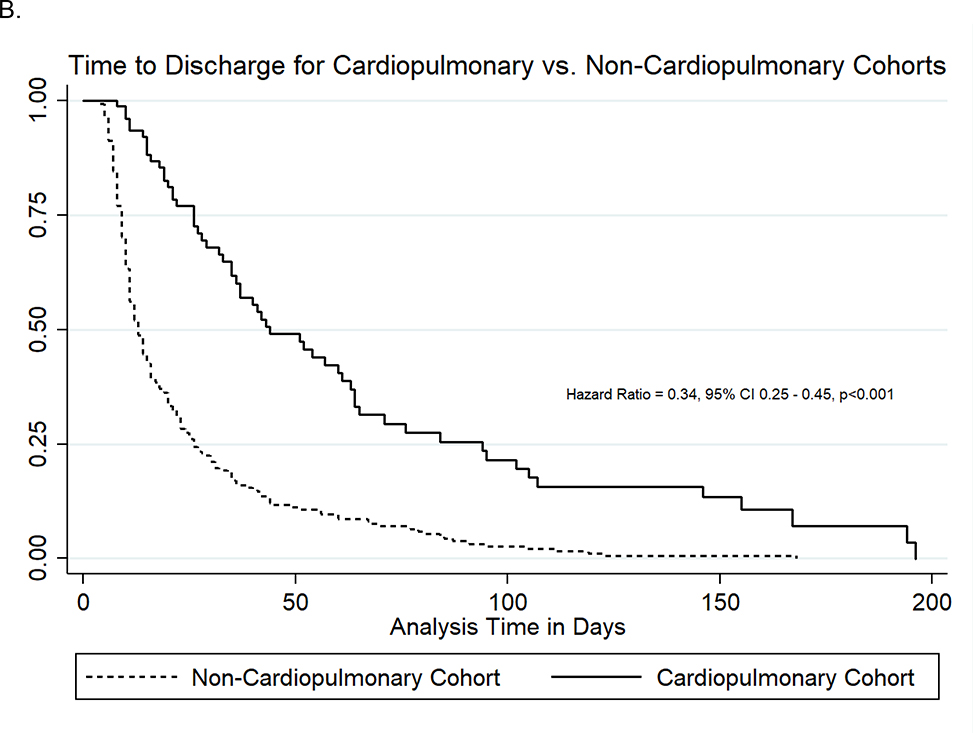
Although the raw rates of in-hospital mortality varied between the cohorts (30% in CPD cohort vs. 14% in non-CPD cohort), neonates with and without CPD had similar rates of in-hospital death per time when accounting for their differential hospitalization durations (HR 1.13, 95%CI 0.66–1.94, p=0.64). Within the CPD cohort, the high rates of in-hospital mortality were primarily driven by neonates who required ECMO (71% mortality) or had multiple CPD diagnoses (65% mortality) (Table III). Overall, 13% of neonates with CHD died and no neonates with a sole diagnosis of CDH died. The rate of hospital discharge was about 70% lower for neonates with CPD compared to neonates without CPD (HR 0.34, 95%CI 0.25–0.45, p<0.001). The Figure demonstrates the time-to-event analysis for in-hospital mortality and hospital discharge for both cohorts. Neonates with CPD were more likely to be discharged or die while maintained on an ASM (82% vs. 68%, p=0.01).
Figure 1:
Time-to-Death (A) and Time-to-Discharge (B) Curves for Cardiopulmonary Disease and Non-Cardiopulmonary Disease Cohorts.
Discussion
In this large, multicenter cohort of children with neonatal seizures with and without CPD, we found high rates of seizures, particularly EEG-only seizures, which were often resistant to initial ASM administration. These findings provide additional evidence for neonatal guidelines that recommend cEEG monitoring of high risk neonatal populations,(18) such as those with CPD, and they also highlight the need for more effective ASM management strategies.
Seizures have been well-characterized for neonates with acute brain injuries such as perinatal asphyxia, ischemic stroke, intracranial hemorrhage, and central nervous system infection.(1–3, 25, 26) Less is known about neonates with seizures in the setting of underlying CPD (including CHD, CDH, or ECMO) because prior studies of neonates with CPD are limited to single-center cohorts.(10, 11, 13, 15, 16) Thus, this study provides multi-center data regarding seizure incidence, characteristics, and outcomes in a contemporary cohort of neonates with multiple types of CPD and guideline-directed use of cEEG. Our data indicate there are several important differences in seizure presentation between neonates with and without CPD. Neonates with CPD are less likely to present with clinically-evident seizures, are more likely to experience EEG-only (subclinical) seizures which would not be detected by clinical observation alone, ( are more often well-appearing at the time of birth (higher Apgar scores) and then experience seizures later in their clinical course, have a comparably high seizure exposure, and ( experience an equally low response rate to ASMs in comparison to neonates without CPD. Overall, these findings indicate that neonates with CPD are a high-risk group for EEG-only seizures near the time of CPD reparative procedures and that seizures are often refractory to initial treatment.
It is established that neonates with CPDs often have abnormal, immature brain structure pre-operatively.(4–7) However, in our cohort, they tended to appear well at birth and subsequently experienced decompensation with seizures detected later in their clinical course, presumably in the post-operative period because the median clinical and EEG seizure onset in CPD group was after the median surgical time. Additionally, neonates with CPDs were often sedated and paralyzed in the post-operative period, limiting the utility of neurologic examination to assess encephalopathy and the ability to observe for clinical events concerning for seizure. Without cEEG monitoring in the high-risk, post-operative period, electrographic seizures would not be detected and seizure burden would not be accurately assessed. Thus, protocol-driven cEEG in this period is necessary to accurately detect and manage seizures in neonates with CPD.(18)
Our data also demonstrate that phenobarbital is the most frequently administered first-line ASM for neonates with and without CPD, but neonates with CPD are more likely than neonates without CPD to receive levetiracetam during their hospital course. Clinicians may be concerned about potential systemic side effects in neonates with CPD and thus administer levetiracetam in place of phenobarbital in some patients.(27–30) However, data from the NEOLEV2 trial, a randomized controlled trial assessing the efficacy of phenobarbital vs. levetiracetam as first-line treatment for neonatal seizures indicated that phenobarbital was significantly more efficacious than levetiracetam for seizure cessation in a heterogenous group of neonates.(31) The optimal balance between safety and seizure efficacy in neonates with CPD requires further evaluation.
In our study, although neonates with and without CPD received similar initial ASMs to treat seizures, neonates with CPD received higher doses of phenobarbital compared with neonates without CPD, although both groups ultimately achieved similar therapeutic ASM levels. This finding suggests that neonates with CPD may require larger ASM doses compared with neonates without CPD to achieve expected ASM levels, potentially related to higher volumes of distribution and blood loss related to surgical procedures,(32, 33) and that seizures are often refractory even with appropriate ASM levels, as demonstrated in prior studies.(34) We did not collect data regarding ASM administration and serum level timing, thereby limiting our ability to evaluate the role of ASM administration timeliness on seizure responsiveness. More detailed data regarding ASM management would be beneficial in future studies of seizures in neonates with CPD.
Although the majority (78%) of neonates with CPD experienced seizures in the context of acute brain injury (HIE, stroke, hemorrhage, or intracranial infection), 22% of neonates with CPD had early-onset epilepsies due to genetic syndromes, brain malformations, or inborn errors of metabolism. Given that many cardiac anomalies occur in the context of genetic conditions and/or concurrent brain malformations, it may be challenging to assess whether neonatal seizures in this population arise from an acute symptomatic cause, an endogenous congenital cause that predisposes to epilepsy, or both.(21, 35) An additional important consideration in this discussion of etiology is the role of the maternal-fetal-placental environment and its interplay with underlying genetic predispositions.(36–38) Ongoing research has unveiled how time-dependent destructive processes in the fetal environment can predict acute and chronic outcomes following birth, as well as elucidate the time-course of clinical symptoms in neonates and infants presenting with seizures or other neurologic impairments.(39, 40) For example, prenatal insults sustained in the first two trimesters may present with delayed neurologic complications, similar to the time-course noted in our patients with CPD. NSR-I did not collect prenatal data such as placental pathology and markers of maternal health to assess the impact of the maternal-fetal-placental environment on our patients. We cannot assess brain lesions which may include anomalous brain development, destructive brain lesions, or both. Patients with CPDs may be at risk for both forms of brain lesions, and the underlying brain lesion characteristics determine epileptogenic network development that ultimately informs acute and chronic seizure responsiveness to treatment. Neuroimaging data are not available to delineate further this information in our cohort, but understanding these differences may help clinicians tailor seizure management, as the duration of ASM treatment may differ by etiology. Early ASM discontinuation may be appropriate with acute symptomatic etiologies,(41, 42) and prolonged ASM treatment may be appropriate with epilepsy. In our cohort, neonates with CPD were more likely to die or be discharged home on an ASM than neonates without CPD. Discharge on an ASM, especially despite the longer hospital course for neonates with CPD, may reflect that clinicians were concerned about a higher risk for future epilepsy and/or concerned about the potential risk of recurrent convulsive seizures in these medically complex patients. Further, early diagnosis of genetic syndromes or structural brain malformations may impact epilepsy management over the lifespan with the introduction of precision based therapies for genetic epilepsies and more widespread use of epilepsy surgery for lesional epilepsy.(43)
We defined CPDs as the presence of CHD, CDH, or the need for ECMO. Among neonates with CPD, seizures have been best characterized in those with CHD. Among neonates with CHD, the reported seizure incidence has varied over time as earlier studies described clinical seizures without EEG assessment. Single-center studies of neonates with CHD undergoing cEEG demonstrated an incidence of 0–6% for clinical seizures and 1–20% for electrographic seizures.(11, 13, 14, 16) Neonates with CHD have seizure onset at a median of 21–22 hours after surgery,(14) have a high seizure burden,(10, 16, 44) and have a high incidence of EEG-only seizures.(11)
The incidence of seizures in neonates who require ECMO has also been previously described in single-center studies. In a single-center study of 99 neonatal and pediatric patients on ECMO, the incidence of electrographic seizures was 9% in the NICU cohort, 27% in the CICU cohort, and 8% in the PICU cohort, with pre-ECMO low cardiac output as the only significant risk factor for seizure occurrence (p=0.03).(15) There was also a trend toward higher seizure occurrence in patients with VA ECMO, persistent pulmonary hypertension, an abnormal EEG background, and a history of CHD surgery. A separate single-center study evaluated 70 neonatal and pediatric patients who underwent cEEG for a minimum of 24 hours after ECMO initiation.(17) Electrographic seizures occurred in 23% of the cohort, with status epilepticus in 7%. Subclinical seizures were present in 56% of patients with seizures, and seizures began within 24 hours of ECMO initiation in 50% of patients. Patients with electrographic seizures were younger (0.6 months vs. 6.7 months, p=0.03). A more recent single-center study of seizures in pediatric patients exposed to ECMO evaluated 201 patients, including 92 neonates.(45) Seventeen neonates (18%) experienced ES, occurring at a median of 2.8 hours after cEEG monitoring was initiated. Neonates experienced a median of 21 seizures in the first 24 hours of cEEG monitoring, and the presence of electrographic seizures was associated with ipsilateral brain injury.
CDH can occur in isolation or as a part of a genetic syndrome with associated brain malformations that can increase the risk of neonatal-onset epilepsy. As a result of CDH with or without an underlying genetic association, patients often have an impact on lung size and function and cardiac output, with common symptoms requiring ECMO support. As previously noted, ECMO use is associated with a risk of brain injury which commonly manifests as seizure in neonates. The incidence of acute seizures in the CDH population with and without ECMO exposure has not been well characterized, but seizures are a documented complication.(46, 47)
Our work has several limitations. First, specific pathways or protocols for seizure management were not implemented across the NSR centers and care was determined by treating clinicians, thereby yielding variability in how and when seizures were managed. However, this variability across clinicians and hospitals inherently adds to the generalizability of the conclusions. Similarly, paralytic and sedative use were not standardized across NSR sites, but varying use of these medications in the post-operative period could affect seizure identification and seizure exposure. Second, our cohort includes a small number of neonates with CDH, thereby reducing the generalizability of our results to this population, especially given that we are only evaluating neonates with CDH who experienced seizures and some had additional CPD diagnoses. However, as noted above, only a few prior studies have evaluated neonatal seizures in CDH, and thus our data help to better understand the seizure profiles for these patients. Lastly, the data available for review for the cohort were limited by the scope of the initial NSR goals. Although we report ASM dosing and serum ASM levels, data were not collected regarding the timing of these doses and levels. This precludes conclusions regarding ASM efficacy and pharmacokinetics which may differ among neonates with CPD given their concurrent use of technology which impacts fluid status, volume of distribution, and hepatic metabolism.(33) Additionally, the NSR did not collect data regarding ASM adverse events. Given that some commonly used ASMs may cause bradycardia, hypotension, and respiratory depression, the underlying pathophysiology of the CPDs may impact the acceptability of these adverse effects. In our cohort, neonates with CPD were more likely to receive levetiracetam compared with neonates without CPD. We hypothesize that the side effect profile of levetiracetam may have been preferred for neonates with CPD, leading to increased use in this group, although providers were not questioned about the rationale for their ASM choices. Future studies are needed to evaluate the adverse events associated with ASM administration in neonates with CPD to ensure our efforts to treat seizures are not causing harm. Further, NSR did not collect genetic, prenatal/fetal, or placental data, limiting our ability to assess the potential impact of specific genetic variations and prenatal exposures on our neonates with CPD, and perhaps limiting our explanation of some of the key differences noted between neonates with and without CPD. Finally, this cohort does not include neuroimaging data or long-term outcome data, limiting our ability to correlate seizure exposure with neuroimaging assessments of brain injury severity or long-term patient outcomes. Clinicians must consider that neonates with CPD are certainly at risk for developmental as well as acquired brain lesions, both of which may be small and below the resolution threshold of clinically-available neuroimaging technology, but undoubtedly influence epileptogenesis, response to antiseizure treatment, and neurodevelopmental outcomes.
Our data reinforce that neonates with CPD are at high risk for seizures from brain injury following surgical repairs, co-existing neonatal epilepsies, or a combination of both, and therefore support guidelines that recommend cEEG in neonates with CPD, particularly following surgical intervention. Consideration of seizure etiology is important, as some neonates with CPD experience seizures due to a non-acute etiology, and this may inform decisions regarding ASM administration duration. Given the occurrence and persistence of seizures in this population, future studies are needed regarding ASM efficacy and adverse events in neonates with CPD to develop optimized seizure management approaches. Future work should also be directed at exploring the impact of the maternal-fetal-placental environment and genetics on the neurologic presentations and long-term outcomes of neonates with cardiopulmonary diseases.
Acknowledgments
Supported by the Pediatric Epilepsy Research Foundation (multi-center Neonatal Seizure Registry / A120625); the National Institutes of Health (NINDS K02 102598 [to C.W.]). The study sponsor had no role in (1) study design (2) the collection, analysis, and interpretation of data (3) the writing of the report and (4) the decision to submit the paper for publication. R.S. serves as Associate Editor for Neurology; is a consultant for the Epilepsy Study Consortium; and receives royalties from UpToDate for authorship of topics related to neonatal seizures. The other authors declare no conflicts of interest.
Abbreviations:
- CDH
Congenital Diaphragmatic Hernia
- cEEG
Continuous Electroencephalography
- CHD
Congenital Heart Disease
- ECMO
Extracorporeal Membrane Oxygenation
- HIE
Hypoxic Ischemic Encephalopathy
- NSR-1
Neonatal Seizure Registry-1
Footnotes
Data statement
De-identified individual participant data (including data dictionaries) and statistical analysis are available for review upon request from the journal editor or reviewers.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glass HC, Shellhaas RA, Wusthoff CJ, Chang T, Abend NS, Chu CJ, et al. Contemporary Profile of Seizures in Neonates: A Prospective Cohort Study. J Pediatr. 2016;174:98–103 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass HCW, Y.W. Epidemiology of Neonatal Seizures. Journal of Pediatric Neurology. 2009;7:13–7. [Google Scholar]
- 3.Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med. 2013;18:185–91. [DOI] [PubMed] [Google Scholar]
- 4.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–36; discussion 36–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McQuillen PS, Goff DA, Licht DJ. Effects of congenital heart disease on brain development. Prog Pediatr Cardiol. 2010;29:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–38. [DOI] [PubMed] [Google Scholar]
- 7.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–41. [DOI] [PubMed] [Google Scholar]
- 8.Peyvandi S, Kim H, Lau J, Barkovich AJ, Campbell A, Miller S, et al. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J Thorac Cardiovasc Surg. 2018;155:291–300 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danzer E, Zarnow D, Gerdes M, D’Agostino JA, Siegle J, Bebbington MW, et al. Abnormal brain development and maturation on magnetic resonance imaging in survivors of severe congenital diaphragmatic hernia. J Pediatr Surg. 2012;47:453–61. [DOI] [PubMed] [Google Scholar]
- 10.Helmers SL, Wypij D, Constantinou JE, Newburger JW, Hickey PR, Carrazana EJ, et al. Perioperative electroencephalographic seizures in infants undergoing repair of complex congenital cardiac defects. Electroencephalogr Clin Neurophysiol. 1997;102:27–36. [DOI] [PubMed] [Google Scholar]
- 11.Clancy RR, Sharif U, Ichord R, Spray TL, Nicolson S, Tabbutt S, et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia. 2005;46:84–90. [DOI] [PubMed] [Google Scholar]
- 12.Clancy RR, McGaurn SA, Wernovsky G, Gaynor JW, Spray TL, Norwood WI, et al. Risk of seizures in survivors of newborn heart surgery using deep hypothermic circulatory arrest. Pediatrics. 2003;111:592–601. [DOI] [PubMed] [Google Scholar]
- 13.Andropoulos DB, Mizrahi EM, Hrachovy RA, Stayer SA, Stark AR, Heinle JS, et al. Electroencephalographic seizures after neonatal cardiac surgery with high-flow cardiopulmonary bypass. Anesth Analg. 2010;110:1680–5. [DOI] [PubMed] [Google Scholar]
- 14.Naim MY, Gaynor JW, Chen J, Nicolson SC, Fuller S, Spray TL, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:169–78; discussion 78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JJ, Banwell BL, Berg RA, Dlugos DJ, Ichord RN, Kilbaugh TJ, et al. Electrographic Seizures in Children and Neonates Undergoing Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med. 2017;18:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban KC, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–64. [DOI] [PubMed] [Google Scholar]
- 17.Okochi S, Shakoor A, Barton S, Zenilman AR, Street C, Streltsova S, et al. Prevalence of Seizures in Pediatric Extracorporeal Membrane Oxygenation Patients as Measured by Continuous Electroencephalography. Pediatr Crit Care Med. 2018;19:1162–7. [DOI] [PubMed] [Google Scholar]
- 18.Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. 2011;28:611–7. [DOI] [PubMed] [Google Scholar]
- 19.Glass HC, Soul JS, Chu CJ, Massey SL, Wusthoff CJ, Chang T, et al. Response to antiseizure medications in neonates with acute symptomatic seizures. Epilepsia. 2019;60:e20–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shellhaas RA, Chang T, Wusthoff CJ, Soul JS, Massey SL, Chu CJ, et al. Treatment Duration After Acute Symptomatic Seizures in Neonates: A Multicenter Cohort Study. J Pediatr. 2017;181:298–301 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shellhaas RA, Wusthoff CJ, Tsuchida TN, Glass HC, Chu CJ, Massey SL, et al. Profile of neonatal epilepsies: Characteristics of a prospective US cohort. Neurology. 2017;89:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass HC, Shellhaas RA, Tsuchida TN, Chang T, Wusthoff CJ, Chu CJ, et al. Seizures in Preterm Neonates: A Multicenter Observational Cohort Study. Pediatr Neurol. 2017;72:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchida TN, Wusthoff CJ, Shellhaas RA, Abend NS, Hahn CD, Sullivan JE, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol. 2013;30:161–73. [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80. [DOI] [PubMed] [Google Scholar]
- 26.Sheth RD, Hobbs GR, Mullett M. Neonatal seizures: incidence, onset, and etiology by gestational age. J Perinatol. 1999;19:40–3. [DOI] [PubMed] [Google Scholar]
- 27.Sharpe CM, Capparelli EV, Mower A, Farrell MJ, Soldin SJ, Haas RH. A seven-day study of the pharmacokinetics of intravenous levetiracetam in neonates: marked changes in pharmacokinetics occur during the first week of life. Pediatr Res. 2012;72:43–9. [DOI] [PubMed] [Google Scholar]
- 28.Khan O, Chang E, Cipriani C, Wright C, Crisp E, Kirmani B. Use of intravenous levetiracetam for management of acute seizures in neonates. Pediatr Neurol. 2011;44:265–9. [DOI] [PubMed] [Google Scholar]
- 29.Rakshasbhuvankar A, Rao S, Kohan R, Simmer K, Nagarajan L. Intravenous levetiracetam for treatment of neonatal seizures. J Clin Neurosci. 2013;20(8):1165–7. [DOI] [PubMed] [Google Scholar]
- 30.Thibault C, Naim MY, Abend NS, Licht DJ, Gaynor JW, Xiao R, et al. A retrospective comparison of phenobarbital and levetiracetam for the treatment of seizures following cardiac surgery in neonates. Epilepsia. 2020;61:627–35. [DOI] [PubMed] [Google Scholar]
- 31.Sharpe C, Reiner GE, Davis SL, Nespeca M, Gold JJ, Rasmussen M, et al. Levetiracetam Versus Phenobarbital for Neonatal Seizures: A Randomized Controlled Trial. Pediatrics. 2020;145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thibault C, Massey SL, Abend NS, Naim MY, Zoraian A, Zuppa AF. Population Pharmacokinetics of Phenobarbital in Neonates and Infants on Extracorporeal Membrane Oxygenation and the Influence of Concomitant Renal Replacement Therapy. J Clin Pharmacol. 2021;61:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thibault C, Massey SL, Naim MY, Abend NS, Zuppa AF. Population Pharmacokinetics of IV Phenobarbital in Neonates After Congenital Heart Surgery. Pediatr Crit Care Med. 2020;21:e557–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341:485–9. [DOI] [PubMed] [Google Scholar]
- 35.Numis AL, da Gente G, Sherr EH, Glass HC. Whole-exome sequencing with targeted analysis and epilepsy after acute symptomatic neonatal seizures. Pediatr Res. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scher MS. “The First Thousand Days” Define a Fetal/Neonatal Neurology Program. Front Pediatr. 2021;9:683138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brosens I, Puttemans P, Benagiano G. Placental bed research: I. The placental bed: from spiral arteries remodeling to the great obstetrical syndromes. Am J Obstet Gynecol. 2019;221:437–56. [DOI] [PubMed] [Google Scholar]
- 38.Harris LK, Benagiano M, D’Elios MM, Brosens I, Benagiano G. Placental bed research: II. Functional and immunological investigations of the placental bed. Am J Obstet Gynecol. 2019;221:457–69. [DOI] [PubMed] [Google Scholar]
- 39.Penn AA, Wintermark P, Chalak LF, Armstrong J, Redline R, Scher MS, et al. Placental contribution to neonatal encephalopathy. Semin Fetal Neonatal Med. 2021:101276. [DOI] [PubMed] [Google Scholar]
- 40.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22:633–5. [DOI] [PubMed] [Google Scholar]
- 41.Glass HC, Soul JS, Chang T, Wusthoff CJ, Chu CJ, Massey SL, et al. Safety of Early Discontinuation of Antiseizure Medication After Acute Symptomatic Neonatal Seizures. JAMA Neurol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fitzgerald MP, Kessler SK, Abend NS. Early discontinuation of antiseizure medications in neonates with hypoxic-ischemic encephalopathy. Epilepsia. 2017;58:1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blumcke I, Coras R, Busch RM, Morita-Sherman M, Lal D, Prayson R, et al. Toward a better definition of focal cortical dysplasia: An iterative histopathological and genetic agreement trial. Epilepsia. 2021;62:1416–28. [DOI] [PubMed] [Google Scholar]
- 44.Rappaport LA, Wypij D, Bellinger DC, Helmers SL, Holmes GL, Barnes PD, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation. 1998;97:773–9. [DOI] [PubMed] [Google Scholar]
- 45.Sansevere AJ, DiBacco ML, Akhondi-Asl A, LaRovere K, Loddenkemper T, Rivkin MJ, et al. EEG features of brain injury during extracorporeal membrane oxygenation in children. Neurology. 2020;95:e1372–e80. [DOI] [PubMed] [Google Scholar]
- 46.Lott IT, McPherson D, Towne B, Johnson D, Starr A. Long-term neurophysiologic outcome after neonatal extracorporeal membrane oxygenation. J Pediatr. 1990;116:343–9. [DOI] [PubMed] [Google Scholar]
- 47.Bernbaum J, Schwartz IP, Gerdes M, D’Agostino JA, Coburn CE, Polin RA. Survivors of extracorporeal membrane oxygenation at 1 year of age: the relationship of primary diagnosis with health and neurodevelopmental sequelae. Pediatrics. 1995;96:907–13. [PubMed] [Google Scholar]