Abstract
Objectives:
To evaluate whether PROMIS pediatric patient-reported outcome measures serve as valid endpoints in a clinical trial of a chronic pediatric illness.
Study design:
We evaluated the responsiveness of PROMIS Pediatric measures collected through the Clinical Outcomes of Methotrexate Binary Therapy in Practice (COMBINE) trial, a multicenter, randomized, double-blind, placebo-controlled, pragmatic clinical trial in pediatric patients with CD. We examined the relationships between changes in PROMIS pediatric measures and changes in disease activity by evaluating patient-reported outcome (PRO) score changes among patients who did or did not experience improvement in disease activity.
Results:
Participants included 266 children and adolescents with CD from a total of 35 institutions. Over the course of follow-up, participants showed improvement in most PRO domains, with the largest effect sizes observed for the clinically improved group. Patients who maintained steroid-free remission showed significantly lower PRO scores for Pain Interference, Fatigue and IBD Symptom and higher Positive Affect scores.
Conclusions:
This study demonstrates the responsiveness of the Pediatric PROMIS measures of Fatigue and Pain Interference as study endpoints in a large, multi-center pragmatic trial in pediatric CD, extending a growing body of research supporting the use of PROMIS Pediatric measures as reliable PRO endpoints for clinical trials.
Keywords: patient-reported outcomes, inflammatory bowel disease, Crohn’s disease, PROMIS, child
Patient reported outcome (PRO) measures reflect physical, mental and social health of patients, and serve as valuable endpoints in clinical monitoring. [1,2] In pediatric chronic disease, PRO measures are particularly important in understanding the relapsing-remitting or progressive nature of a disease and its effect on symptom burden, physiological comorbidities and disruptions in daily life. [3] It is critical to be able to reliably and validly measure the effects of chronic disease on children and adolescents using PROs in order to evaluate diagnostic and therapeutic approaches that affect health-related quality of life.
The Patient-Reported Outcomes Measurement Information System (PROMIS) is a National Institutes of Health (NIH) funded system of PRO measures developed to measure physical, mental, and social health in chronic disease populations. PROMIS Pediatric measures are specifically designed to measure these domains in child respondents and include self-report measures available for children ages 8–17. [4,5] The PROMIS measures have been shown to discriminate between levels of disease activity in a range of pediatric chronic conditions[6–10] and assess health-related quality of life (HRQOL) longitudinally [11]. Specifically, in pediatric Crohn’s disease, content validity has been established for the PROMIS measures pertaining to pain interference and fatigue [12]. Additionally, in a direct-to-patient internet cohort, these domains have been shown to be responsive to changes in disease status and HRQOL. [11,13,14] Thus, PROMIS Pediatric measures appear well suited as clinical trial endpoints. Yet, limited studies have used these measures in the clinical setting and/or as clinical trial endpoints. [15,16] It is critical that clinical trials begin to include PRO measures as trial endpoints, as their inclusion promotes a comprehensive evaluation of the clinical effectiveness of an intervention, in the context of a child’s physical, emotional, and social health, and based on the child’s lived experiences of their disease and treatments. Prior to broad adoption of PROMIS Pediatric measures as trial endpoints, proof-of-concept data are needed to demonstrate responsiveness.
In this study we evaluated the performance of PROMIS Pediatric measures, as well as demonstrate responsiveness of these measures obtained longitudinally in the context of a multi-center pragmatic trial. We hypothesized that the measures would reflect positive and negative changes in clinical status and that the measures would remain stable once the disease was in remission.
Methods:
The Clinical Outcomes of Methotrexate Binary Therapy in Practice (COMBINE, NCT02772965) study is a multicenter, randomized, double-blind, placebo-controlled, pragmatic clinical trial to compare the effectiveness of anti-TNF monotherapy versus anti-Tumor Necrosis Factor (TNF) combination therapy with low dose oral methotrexate in the induction and maintenance of steroid free remission in children and young adults with Crohn’s disease. In this study, we evaluated the performance of PROMIS Pediatric measures and are not reporting on the clinical outcomes of the trial. General eligibility criteria include a diagnosis of Crohn’s disease, age <21 years, initiation of infliximab or adalimumab at the discretion of the treating physician in the six weeks prior to randomization, and no contraindication to methotrexate. Although enrollment in the COMBINE trial has closed, study follow-up is ongoing.
In this analysis, we defined study baseline (Time 1) as the month of each participant’s first PRO completion. Randomization to methotrexate or placebo typically occurred shortly after baseline, but for 22% of participants there was an interval of 1–3 months between baseline and randomization. Over a follow-up period of up to 3 years, standardized clinical information (eg, disease characteristics including the short Pediatric Crohn’s Disease Activity Index[17] and Physician Global Assessment [18], laboratory data, adverse events, medication use, etc.) was collected during study visits. In addition, children ages 8–20 years completed PROMIS Pediatric measures at enrollment and at the time of (or close to) follow up visits scheduled approximately 3, 6, 12, 24, and, in some cases, 36 months following randomization. If a patient missed an appointment, they were able to complete the PRO survey at the following visit. Most questionnaires (94% of total) were self-administered using paper and pen. Study staff manually entered responses in two separate spreadsheets to ensure reliability, and discrepancies were resolved by checking the original records. During the COVID-19 pandemic, questionnaires were also self-administered online (4%) and administered by study coordinators by telephone (2%).
PROMIS Measures
We administered PROMIS Pediatric Pain Interference[19] and Fatigue[20] measures as 8-item short forms and Positive Affect [21] as a 4-item short form. We also evaluated IBD symptoms using a 4-item measure. (Schuchard 2021) The PRO questions were the same for all ages, and children were instructed to complete the measures on their own, without assistance from parents. The scores for these measures are predictions of individuals’ level of health based on the item properties and the individual’s pattern of responses to the items, as opposed to a simple sum or average of the responses. We used Mplus 8 [22] and the published item parameters to produce item response theory (IRT) scores. Scores were converted to the PROMIS T-scale (T-score = 10 × score + 50). The measures are scored in the direction of their concept name, such that higher Positive Affect corresponds to better outcomes, whereas higher IBD Symptoms, Pain Interference, or Fatigue correspond to worse outcomes.
Clinical Anchors
We measured disease severity using the short Pediatric Crohn’s Disease Activity Index (sPCDAI)[17] and Physician Global Assessment (PGA)[18]. The sPCDAI includes items specific to Crohn’s disease, including abdominal pain, diarrheal stools and general well-being. The sPCDAI is scored from 0–90 with items that assess abdominal pain, stools, weight, extra-intestinal manifestations, and well-being. Disease activity was classified as inactive (sPCDAI < 15), mild (15 to <30), or moderate-severe (≥ 30). The Physician Global Assessment (PGA) is a physician rating of disease activity using a Likert scale of 1 (inactive) to 4 (severe)[18]. Physician assessment of disease severity was performed independently of patient survey.
Additionally, the COMBINE trial protocol identifies a set of outcomes that indicate treatment failure at various points in the three-year follow-up phase of the study. Pre-specified outcomes considered treatment failure included inability to achieve remission by week 26, failure to taper off steroids by week 16, hospitalization or surgery after week 25, sPCDAI ≥ 15 at two or more consecutive visits beyond week 26, use of steroids for a period of over 10 weeks cumulatively beyond week 16, and discontinuation of anti-TNF therapy due to lack of effectiveness or toxicity
Statistical Analyses
We examined the relationships between changes in PROs and changes in the clinical anchors by evaluating PRO score changes among patients who did or did not experience improvement in disease activity. Then we examined the trajectories of PRO scores for patients that did or did not experience treatment failures during the course of the study. Analyses were conducted in R Version 4.0.0.[23] We initially focused on changes that occurred between baseline and the first follow up because this interval most closely followed initiation of therapy and showed the largest changes in patients’ clinical outcomes. First follow-up visit PROs included PROs completed 2–5 months post-baseline (n = 235). Participants were divided into three subgroups based on their sPCDAI scores: (1) inactive disease at both baseline and first follow-up, (2) improved between baseline and first follow-up by a decrease of 15 or more on sPCDAI, or (3) active disease at baseline and not improved at first follow-up. For each subgroup, we used dependent-samples t-tests and Cohen d effect sizes to examine change on each PRO. These procedures were repeated after dividing participants into three subgroups based on their PGA scores: (1) inactive at Time 1 and Time 2, (2) improved between Time 1 and Time 2 by a decrease on PGA of 1 or more, or (3) active at Time 1 and not improved at Time 2. Only disease activity indices collected the same month as a patient’s PRO completion were included in the analyses. A Cohen d statistic was used to evaluate effect size (moderate is 0.5 to 0.79, large ≥0.8).[24]
To examine post-treatment PRO score trajectories for participants that did and did not experience treatment failure, we used multilevel regression models to regress sPCDAI and PRO scores on time with by-participant random intercepts and slopes using the lmerTest package in R. [25] These models were used to examine post-baseline change on each measure, controlling for the baseline scores on the measure by including them as a covariate. Time was coded as years, with time zero equal to the participant’s first follow-up PRO. To test whether post-baseline scores on average differed by treatment outcome, we included a predictor in each model indicating whether patients did or did not experience a treatment failure during the course of the study. Patient sex and mean-centered age at baseline were included as covariates in each model because these variables often show significant associations with PROs.
Results:
Study Sample
The evaluation of response rate included all enrolled participants (n=299). All other analyses included all eligible participants that completed baseline and at least one follow-up PRO measure before March 2021 (n=266), which included children and adolescents with Crohn’s disease from a total of 35 institutions. The ages ranged between 8 and 20 years, 64% were male and 82% White. Baseline scores showed that 67% of participants had either mild or moderate-severe disease based on the PGA and 50% of patients had either mild or moderate-severe disease based on the sPCDAI at baseline. Additional demographic and clinical characteristics of study participants are shown in Table 1. Because PROs were collected as part of visits that occurred during routine practice, the number and timing of follow-up PROs varied across patients. The number of follow-up PROs per participant ranged from 1 to 8 (median = 4). The time interval between baseline and the participant’s first follow-up PRO ranged from 2 to 25 months (interquartile range = 3–5 months). The time interval between baseline and the participant’s last follow-up PRO ranged from 2 to 39 months (interquartile range = 10–25 months).
Table 1.
Baseline Participant Characteristics
| n (%) | |
|---|---|
| Total study sample | 266 (100%) |
| Age at baseline | |
| 8–12 | 98 (37%) |
| 13–17 | 157 (59%) |
| 18–20 | 11 (4%) |
| Sex | |
| Male | 170 (64%) |
| Female | 96 (36%) |
| Race/Ethnicity a | |
| Hispanic/Latino | 7 (3%) |
| Black or African American | 27 (10%) |
| White | 217 (82%) |
| Other | 13 (5%) |
| Physician Global Assessment b | |
| Inactive | 62 (33%) |
| Mild | 83 (44%) |
| Moderate-Severe | 43 (23%) |
| Short Pediatric Crohn’s Disease Activity Index c | |
| Inactive (<15) | 92 (50%) |
| Mild (15–<30) | 58 (32%) |
| Moderate-Severe (≥30) | 33 (18%) |
| Perianal disease * d | |
| Yes | 22 (22%) |
| No | 79 (78%) |
| Disease locations * e | |
| Ileum | 150 (82%) |
| Colon | 140 (77%) |
| Upper tract | 114 (62%) |
| Other Measurements f | Mean (SD) |
| Weight (kg) | 49 (16) |
| Height (cm) | 156 (15) |
| Disease duration (years) | 0.7 (1.4) |
| C-reactive protein (mg/L) | 3.7 (8.8) |
| Erythrocyte sedimentation rate (mm/h) | 17 (15) |
| Albumin (g/dL) | 4.0 (0.5) |
| Hematocrit (%) | 38 (4) |
Note. Missing = not assessed the same calendar month as the baseline PRO questionnaire.
Missing n = 2
Missing n = 78
Missing n = 83
Missing n = 165
Missing n = 83
Missing n = 73–145
Categories are not mutually exclusive.
PRO Response Rate
We evaluated the attainability of administering and collecting PROs during clinic visits and found collection of PRO measures were initially above 90% for the first 3 visits, with only a modest decline over time. Despite data collection ongoing during the beginning of the COVID-19 pandemic, only by visit 10 and beyond did we see rates of PRO completion below 80%. Importantly, very few questionnaires (0.1%) were missing entire PROMIS domains, and 97% of questionnaires were completed with zero missing items. (Table II; available at www.jpeds.com)
Table 2:
Feasibility/Missing Data Summary
| Study Visit | PROs collected, N (%)* | ||
|---|---|---|---|
| Baseline | 294 (98) | ||
| Visit 3 | 239 (90)_ | ||
| Visit 4 | 206 (85) | ||
| Visit 6 | 163 (80) | ||
| Visit 10 | 121 (76) | ||
| Missing Items | |||
| Total number of PRO questionnaires collected: 1090 | |||
| Number of questionnaires with 0 missing items: 1061 | |||
| Number of missing items per questionnaire (out of 26 items) | Range: 0–14 | Mean: 0.1 | |
Percentages based on number of PROs collected divided by the total number of outpatient visits that occurred for each time point.
Changes in PROs and Disease Activity
We first grouped participants by whether they had active disease at baseline and improved at first follow-up, active disease at baseline and did not improve, or whether they had inactive disease at baseline and remained inactive. Although participants in all 3 groups showed improvement in most PRO domains, as expected, the largest effect sizes were observed for the clinically improved group. Participants in this group showed significant improvement on IBD symptoms, pain interference, and fatigue. The largest effect sizes were seen in the improved group for the Pain Interference domain (d = −0.93) and IBD symptoms (d = −0.85). (Table 3) Of note, IBD symptoms, Pain Interference, and Fatigue also improved significantly in the group that was initially determined to be inactive and stayed inactive at follow up. The improvement in positive affect was not statistically significant for any group. Although the main analysis group participants by sPCDAI, a second analysis group participants based on PGA and also showed significant improvement on IBD symptoms, Pain interference, and Fatigue but not positive affect. (Table 4; available at www.jpeds.com)
Table 3.
PROs by Clinical Groups Defined by sPCDAI Change
| Active - Improved | Active - Not Improved | Inactive - Inactive | |
|---|---|---|---|
| n | 39 | 32 | 60 |
| IBD Symptoms | |||
| Baseline mean (SD) | 57 (±7) | 56 (±5) | 53 (±7) |
| First follow-up mean (SD) | 51 (±6) | 55 (±6) | 50 (±6) |
| Effect size (Cohen’s d) | −0.85 | −0.10 | −0.55 |
| p-value | <0.001 | 0.59 | <0.001 |
| Pain Interference | |||
| Baseline mean (SD) | 53 (±14) | 56 (±11) | 41 (±13) |
| First follow-up mean (SD) | 40 (±12) | 49 (±11) | 36 (±11) |
| Effect size (Cohen’s d) | −0.93 | −0.61 | −0.37 |
| p-value | <0.001 | <0.01 | <0.01 |
| Fatigue | |||
| Baseline mean (SD) | 50 (±17) | 55 (±13) | 44 (±14) |
| First follow-up mean (SD) | 41 (±16) | 50 (±15) | 38 (±13) |
| Effect size (Cohen’s d) | −0.58 | −0.51 | −0.35 |
| p-value | <0.001 | <0.01 | <0.01 |
| Positive Affect | |||
| Baseline mean (SD) | 47 (±10) | 45 (±7) | 50 (±9) |
| First follow-up mean (SD) | 49 (±10) | 45 (±8) | 52 (±8) |
| Effect size (Cohen’s d) | 0.27 | 0.00 | 0.18 |
| p-value | 0.10 | 0.98 | 0.17 |
Table 4:
PROs by Clinical Groups Defined by PGA Change
| Active - Improved | Active - Not Improved | Inactive - Inactive | |
|---|---|---|---|
| n | 88 | 13 | 40 |
| IBD Symptoms | |||
| Time 1 mean (SD) | 56 (7) | 56 (7) | 52 (6) |
| Time 2 mean (SD) | 51 (6) | 57 (7) | 49 (5) |
| Effect size (Cohen’s d) | −0.65 | 0.10 | −0.29 |
| p-value | <0.001 | 0.73 | 0.08 |
| Pain Interference | |||
| Time 1 mean (SD) | 49 (14) | 53 (14) | 40 (13) |
| Time 2 mean (SD) | 41 (12) | 51 (12) | 37 (11) |
| Effect size (Cohen’s d) | −0.64 | −0.21 | −0.26 |
| p-value | <0.001 | 0.46 | 0.11 |
| Fatigue | |||
| Time 1 mean (SD) | 49 (17) | 54 (14) | 42 (13) |
| Time 2 mean (SD) | 42 (15) | 50 (22) | 38 (13) |
| Effect size (Cohen’s d) | −0.45 | −0.32 | −0.28 |
| p-value | <0.001 | 0.30 | 0.08 |
| Positive Affect | |||
| Time 1 mean (SD) | 47 (8) | 45 (10) | 51 (9) |
| Time 2 mean (SD) | 49 (9) | 43 (6) | 53 (8) |
| Effect size (Cohen’s d) | 0.17 | −0.32 | 0.15 |
| p-value | 0.11 | 0.30 | 0.35 |
Post-baseline Trajectories
During the post-baseline period of the study, participants that remained in the study and completed PROs for at least one year post-baseline (n = 187; 70% of total) showed small but statistically significant improvement across time on sPCDAI and Pain Interference (Table 5). There was no significant change on IBD Symptoms, Fatigue, or Positive Affect.
Table 5.
Post-baseline PRO Score Trajectories
| Model Estimate | Standard Error | p-value | |
|---|---|---|---|
| sPCDAI | |||
| Time (annual change in score) | −1.8 | 0.4 | <0.001 |
| Treatment failure (yes vs. no) | 4.7 | 1.2 | <0.001 |
| Sex (female vs. male) | 1.9 | 1.2 | 0.10 |
| Baseline age (years) | 0.2 | 0.2 | 0.31 |
| IBD Symptoms | |||
| Time (annual change in score) | −0.4 | 0.3 | 0.14 |
| Treatment failure (yes vs. no) | 3.9 | 0.7 | <0.001 |
| Sex (female vs. male) | 1.2 | 0.7 | 0.09 |
| Baseline age (years) | 0.2 | 0.1 | 0.18 |
| Pain Interference | |||
| Time (annual change in score) | −1.7 | 0.5 | <0.01 |
| Treatment failure (yes vs. no) | 5.1 | 1.4 | <0.001 |
| Sex (female vs. male) | 1.2 | 1.3 | 0.37 |
| Baseline age (years) | 0.0 | 0.2 | 0.95 |
| Fatigue | |||
| Time (annual change in score) | −0.9 | 0.6 | 0.11 |
| Treatment failure (yes vs. no) | 4.1 | 1.6 | <0.01 |
| Sex (female vs. male) | 3.1 | 1.6 | 0.048 |
| Baseline age (years) | 0.3 | 0.3 | 0.25 |
| Positive Affect | |||
| Time (annual change in score) | 0.0 | 0.4 | 0.97 |
| Treatment failure (yes vs. no) | −2.7 | 1.0 | <0.01 |
| Sex (female vs. male) | −1.7 | 0.9 | 0.06 |
| Baseline age (years) | −0.3 | 0.2 | 0.07 |
Note. Each model controlled for baseline scores by including them as a covariate. Model estimates may be interpreted as the difference in sPCDAI score or PRO T-score associated with each of the following: an increase in time of one year; experiencing a treatment failure at any point in the study versus no treatment failure; female versus male; one year older in age at baseline. The models for sPCDAI and Fatigue included by-subject random intercepts but not random slopes because the full models with random slopes did not converge.
Steroid-Free Remission Versus Treatment Failure
We compared post-baseline PRO data between the 54 patients who met any of the components of the primary study endpoint indicating treatment failure and the 133 participants who were able to maintain steroid-free remission for the duration of the study. When compared with patients who experienced a treatment failure, patients who maintained steroid-free remission showed lower PRO scores in the pain interference, fatigue and IBD symptom domains and higher positive affect scores during the follow-up period (Figure). Treatment failure during follow-up was associated with scoring on average 4 points worse on IBD Symptoms (P < .001), 5 points worse on Pain Interference (p < 0.001), 4 points worse on Fatigue (p = 0.01), and 3 points worse on Positive Affect (p < 0.01) (Table 5).
Figure 1.
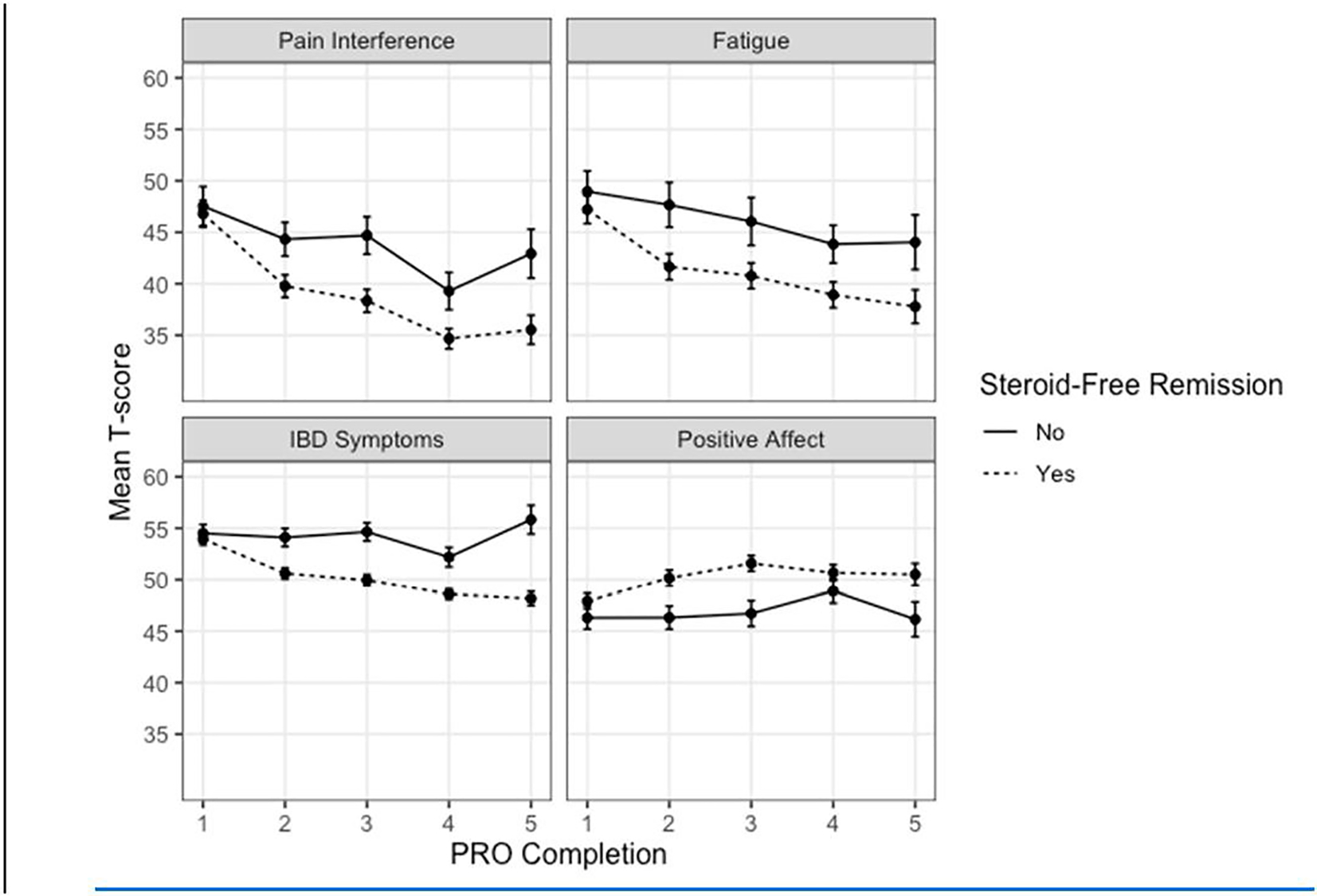
Mean T-scores for 187 participants that remained in the study and completed PROs for at least one year post-baseline. Means were calculated by the order of PRO completion (1 = baseline, 2 = participants’ first follow-up PRO, etc.) up to participants’ fourth follow-up PRO. Higher scores indicate worse outcomes on Pain Interference, Fatigue, and IBD symptoms and better outcomes on Positive Affect. Bars represent the standard error of the mean.
Discussion
The use of reliable PROs can improve a clinician’s ability to monitor meaningful changes over the course of a patient’s disease and is important in clinical research in order to develop treatment and monitoring plans that consider and optimize a child’s health-related quality of life. Additionally, the incorporation of PROs as clinical trial endpoints will help evaluate whether clinical interventions improve symptoms and physical and mental functioning as reported directly by the patient, consistent guidance from the Food and Drug Administration. [26] Although PROMIS measures have been shown to be responsive to changes in disease status in pediatric CD and other chronic pediatric diseases[3,6–11,14], however limited data exists regarding the use of PROMIS measures as clinical trial endpoints. [15,16] This study is the first to use PROMIS measures as endpoints in a clinical effectiveness trial in pediatric IBD, demonstrating both attainability and responsiveness of these measures obtained longitudinally in the context of a multi-center pragmatic trial. This study is further evidence that PROMIS scores are responsive to clinical changes in pediatric CD, track with other markers of clinical improvement, and can be reliably used in a clinical trial setting. Given that only very few studies have used PROMIS Pediatric measures as clinical trial endpoints[15,16], this study adds to a growing body of literature supporting adoption of PROMIS Pediatric measures as trial endpoints measures in pediatric chronic disease research.
Our study also evaluated PROMIS over an extended follow-up period of up to 3 years. Throughout the trial, high rates of completion of the PROs were maintained and only fell below 80% by the tenth follow up visit. The high rates of completion demonstrate the attainability of including PROs in pragmatic trials and prospective observational research. Ultimately, including PROs routinely in clinical trials will enhance our ability to assess a pediatric patient’s health status over time.
As expected, patients who showed meaningful clinical improvement on sPCDAI also showed significant improvement on IBD Symptoms, Pain Interference, and Fatigue. Score changes on these three PRO measures constituted medium to large effect sizes, exceeding estimates of a Minimally Clinically Important Difference (MCID) for PRO measures [27–30]. These findings suggest that inclusion of these measures can be useful as clinical trial endpoints. It is important to note that significant improvements on PROs were also observed among patients who did not show improvement on disease activity indices, indicating that children classified as having inactive disease often self-report some degree of IBD-related symptoms, and PROs may detect changes in these symptoms that are not captured by other clinical metrics. There was little or no change on study outcomes after the first follow-up visit, with improvement of < 2 points per year on average for sPCDAI and Pain Interference and < 1 point for the other PROs. The results suggest that clinically meaningful improvements on IBD Symptoms, Pain Interference, and Fatigue are most likely to be observed within the first few months after initiating anti-TNF therapy, and these improvements may be retained over the following 1–2 years.
In contrast, we did not see significant improvement on Positive Affect between study baseline and the first follow-up visit, making this PRO less useful as a clinical trial endpoint for pediatric IBD than Fatigue and Pain Interference. However, patients who experienced a treatment failure had lower Positive Affect on average than those who did not during the follow-up period of the study, suggesting an association between disease activity and affect over the long term.
An important strength of this study is the methodological rigor of the parent clinical trial, including the robust sample size, double-blind, randomized design, and close attention to data quality and follow-up, as well as the pragmatic nature of the trial without mandated procedures and processes, replicating real life experiences of care. The high rates of PRO completion over an extended follow up period allowed us to evaluate a large cohort of pediatric CD patients during induction of therapy, long-term follow-up and, in some instances, treatment failure. In contrast to many PRO studies, this extended follow up period allowed for monitoring PRO responsiveness with disease evolution.
Limitations in our study include homogeneity of treatment, as the COMBINE study protocol required all patients initiate anti-TNF therapy. Therefore, we were unable to conclude how PROMIS measures may perform overtime in patients not on anti-TNF medications. Additionally, generalizability is somewhat limited, as our cohort was primarily white and had chosen to enroll in the COMBINE study, reflecting high degree of engagement and possibly reflective of high degree of PRO completion. It is critical that future studies of PROs in children prioritize diversity of race/ethnicity, socioeconomic background, and therapy options in order to reflect the broader population of children with CD.
Acknowledgments
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (U19AR069525 [to C,F.]) and grants from the Patient Centered Outcomes Research Institute (to M.K.) and the Helmsley Charitable Trust (to M.K.).
This study demonstrates the responsiveness of the Pediatric PROMIS measures of Fatigue and Pain Interference in the context of a large, multi-center pragmatic trial in Pediatric Crohn’s disease, thus extending a growing body of research supporting the validity of these measures in clinical practice and observational research. Taken together, there is now robust evidence that PROMIS Pediatric measures can serve as reliable PRO endpoints for clinical trials and may complement more traditional endpoints including disease severity indices and mucosal healing.
Abbreviations:
- IBD
Inflammatory bowel disease
- CD
Crohn’s Disease
- HRQOL
Health-related quality of life
- PROMIS
Patient-Reported Outcomes Measurement Information System
- MCID
Minimal clinically important difference
- PROMIS
Patient Reported Outcomes Measurement Information System
- sPCDAI
short Pediatric Crohn’s Disease Activity Index
Additional members of the COMBINE Study Group
These contributors participated in data collection and reviewed the manuscript.
Jeremy Adler, MD, MSc
University of Michigan
Ann Arbor, MI
Rana F. Ammoury, MD
Children’s Hospital of The King’s Daughters
Norfolk, VA
Dorsey Bass, MD
Lucile Packard Children’s Hospital, Stanford
Palo Alto, CA
Julie Bass, DO
Children’s Mercy
Kansas City, MO
Keith Benkov, MD
Mount Sinai Kravis Children’s Hospital
New York, NY
Athos Bousvaros, MD, MPH
Boston Children’s Hospital
Boston, MA
Brendan Boyle, MD, MPH
Nationwide Children’s Hospital
Columbus, OH
José M. Cabrera, MD
Children’s Wisconsin
Milwaukee, WI
Richard Colletti, MD
University of Vermont
Burlington, VM
Jill M. Dorsey, MD
University of Florida
Jacksonville, FL
Dawn R. Ebach, MD
University of Iowa
Iowa City, IA
Ann M. Firestine, MD
Nationwide Children’s Hospital
Columbus, OH
Ajay Gulati, MD
University of North Carolina
Chapel Hill, NC
Edward J. Hoffenberg, MD
Children’s Hospital Colorado
Aurora, CO
Traci W. Jester, MD
Children’s of Alabama
Birmingham, AL
Jess L. Kaplan, MD
Mass General for Children
Boston, MA
Subra Kugathasan, MD
Emory University
Atlanta, GA
Mark E. Kusek MD
Children’s Hospital and Medical Center Omaha
Omaha, NE
Ian Leibowitz, MD
Children’s National
Washington, DC
Tiffany M. Linville, MD
Levine Children’s Specialty Center
Charlotte, NC
Peter Margolis, MD, PhD
Cincinnati Children’s Hospital
Cincinnati, OH
Phillip Minar, MD, MS
Cincinnati Children’s Hospital
Cincinnati, OH
Zarela Molle Rios, MD
Nemours Children’s Health
Wilmington, DE
Jonathan Moses, MD
UH Rainbow Babies and Children’s Hospital
Cleveland, OH
Pablo J. Palomo, MD
Nemours Children’s Hospital
Orlando, FL
Helen Pappa, MD, MPH
Saint Louis University
Saint Louis, MO
Dinesh S. Pashankar, MD
Yale New Haven Hospital
New Haven, CT
Shehzad A. Saeed, MD
Dayton Children’s
Dayton, OH
Charles M. Samson, MD
Washington University
Saint Louis, MO
Kelly C. Sandberg, MD
Dayton Children’s
Dayton, OH
Steven J. Steiner, MD
Indiana University
Carmel, IN
Jeffrey B. Brown, MD
Lurie Children’s Hospital of Chicago
Chicago, IL
Jillian S. Sullivan, MD, MSc
University of Vermont
Burlington, VM
Jeanne Tung, MD
University of Oklahoma
Oklahoma, City
Prateek Wali.
State University of New York Upstate
Syracuse, NY
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Broderick J, DeWit EM, Rothrock N, Crane P, Forrest CB. Advances in Patient Reported Outcomes: The NIH PROMIS Measures. EGEMs (Generating Evidence & Methods to Improve Patient Outcomes) 2013;1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jensen RE, Bjorner JB. Applying PRO Reference Values to Communicate Clinically Relevant Information at the Point-of-care. Medical Care, vol. 57, Lippincott Williams and Wilkins; 2019, p. S24–30. [DOI] [PubMed] [Google Scholar]
- [3].Brenner EJ, Long MD, Mann CM, Lin L, Chen W, Reyes C, et al. Validity and Responsiveness of the Patient-reported Outcomes Measurement Information System in Children With Ulcerative Colitis. Journal of Pediatric Gastroenterology & Nutrition 2021;73:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH roadmap cooperative group during its first two years. Medical Care 2007;45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Forrest CB, Bevans KB, Tucker C, Riley AW, Ravens-Sieberer U, Gardner W, et al. Commentary: The patient-reported outcome measurement information system (PROMIS®) for children and youth: Application to pediatric psychology. Journal of Pediatric Psychology 212;37:614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].DeWalt DA, Gross HE, Gipson DS, Selewski DT, DeWitt EM, Dampier CD, et al. PROMIS® pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Quality of Life Research 2015;24:2195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dampier C, Barry V, Gross HE, Lui Y, Thornburg CD, Dewalt DA, et al. Initial Evaluation of the Pediatric PROMIS® Health Domains in Children and Adolescents With Sickle Cell Disease. Pediatric Blood and Cancer 2016;63:1031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hinds PS, Nuss SL, Ruccione KS, Withycombe JS, Jacobs S, Deluca H, et al. PROMIS pediatric measures in pediatric oncology: Valid and clinically feasible indicators of patient-reported outcomes. Pediatric Blood and Cancer. 2013. [DOI] [PubMed] [Google Scholar]
- [9].Selewski DT, Collier DN, MacHardy J, Gross HE, Pickens EM, Cooper AW, et al. Promising insights into the health related quality of life for children with severe obesity. Health and Quality of Life Outcomes 2013;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Selewski DT, Massengill SF, Troost JP, Wickman L, Messer KL, Herreshoff E, et al. Gaining the Patient Reported Outcomes Measurement Information System (PROMIS) perspective in chronic kidney disease: a Midwest Pediatric Nephrology Consortium study. Pediatric Nephrology 2014;29:2347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brenner EJ, Long MD, Mann CM, Chen W, Reyes C, Lin L, et al. Responsiveness of the Patient-reported Outcomes Measurement Information System (PROMIS) Pediatric Measures to Changes in Disease Status and Quality of Life among Children and Adolescents with Crohn’s Disease. Inflammatory Bowel Diseases 2021;27:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Forrest CB, Forrest KD, Clegg JL, de la Motte A, Amaral S, Grossman AB, et al. Establishing the content validity of PROMIS Pediatric pain interference, fatigue, sleep disturbance, and sleep-related impairment measures in children with chronic kidney disease and Crohn’s disease. Journal of Patient-Reported Outcomes 2020;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arvanitis M, DeWalt DA, Martin CF, Long MD, Chen W, Jaeger B, et al. Patient-Reported Outcomes Measurement Information System in Children with Crohn’s Disease. Journal of Pediatrics 2016;174:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brenner EJ, Long MD, Mann CM, Chen W, Reyes C, Lin L, et al. Responsiveness of the Patient-reported Outcomes Measurement Information System (PROMIS) Pediatric Measures to Changes in Disease Status and Quality of Life Among Children and Adolescents With Crohn’s Disease. Inflammatory Bowel Diseases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Padidela R, Whyte MP, Glorieux FH, Munns CF, Ward LM, Nilsson O, et al. Patient-Reported Outcomes from a Randomized, Active-Controlled, Open-Label, Phase 3 Trial of Burosumab Versus Conventional Therapy in Children with X-Linked Hypophosphatemia. Calcified Tissue International 2021;108:622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kimura Y, Schanberg LE, Tomlinson GA, Riordan ME, Dennos AC, del Gaizo V, et al. The Childhood Arthritis & Rheumatology Research Alliance Start Time Optimization of Biologics in Polyarticular Juvenile Idiopathic Arthritis Study (STOP-JIA): A Comparative Effectiveness Study of CARRA Consensus Treatment Plans for Untreated Polyarticular. 2021. [DOI] [PMC free article] [PubMed]
- [17].Kappelman MD, Crandall W v., Colletti RB, Goudie A, Leibowitz IH, Duffy L, et al. Short pediatric Crohn’s disease activity index for quality improvement and observational research. Inflammatory Bowel Diseases 2011;17:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Colletti RB, Baldassano RN, Milov DE, Margolis PA, Bousvaros A, Crandall W v., et al. Variation in care in pediatric crohn disease. Journal of Pediatric Gastroenterology and Nutrition 2009;49:297–303. [DOI] [PubMed] [Google Scholar]
- [19].Varni JW, Stucky BD, Thissen D, Dewitt EM, Irwin DE, Lai JS, et al. PROMIS pediatric pain interference scale: An item response theory analysis of the pediatric pain item bank. Journal of Pain 2010;11:1109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lai JS, Stucky BD, Thissen D, Varni JW, DeWitt EM, Irwin DE, et al. Development and psychometric properties of the PROMIS® pediatric fatigue item banks. Quality of Life Research 2013;22:2417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Forrest CB, Devine J, Bevans KB, Becker BD, Carle AC, Teneralli RE, et al. Development and psychometric evaluation of the PROMIS Pediatric Life Satisfaction item banks, child-report, and parent-proxy editions. Quality of Life Research 2018;27:217–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Muthén LK, Muthén BO. Statistical Analysis With Latent Variables User’s Guide. 1998.
- [23].R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- [24].Cohen J Statistical Power Analysis for the Behavioral Sciences (2nd ed.). New York: Routledge Academic; 1988. [Google Scholar]
- [25].Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software 2017;82:1–26. [Google Scholar]
- [26].Food and Drug Administration. Public Workshop on Patient-Focused Drug Development: Guidance 4 – Incorporating Clinical Outcome Assessments into Endpoints for Regulatory Decision Making. 2019. [Google Scholar]
- [27].Wyrwich KW, Bullinger M, Aaronson N, Hays RD, Patrick DL, Symonds T. Estimating clinically significant differences in quality of life outcomes. Quality of Life Research, vol. 14, Qual Life Res; 2005, p. 285–95. [DOI] [PubMed] [Google Scholar]
- [28].Copay AG, Subach BR, Glassman SD, Polly DW, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine Journal 2007;7:541–6. [DOI] [PubMed] [Google Scholar]
- [29].Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10:407–15. [DOI] [PubMed] [Google Scholar]
- [30].McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Review of Pharmacoeconomics and Outcomes Research 2011;11:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


