Abstract
Humoral alloimmunity of organ transplant recipient to donor can lead to antibody-mediated rejection (ABMR), causing thousands of organ transplants to fail each year worldwide. However, the mechanisms of adaptive immune cell responses at the basis of humoral alloimmunity have not been entirely understood. In this review, we discuss how recent investigations have allowed to uncover the key contributions of T follicular helper and B cells, and their coordinated actions in driving donor-specific antibody generation and the immune progression towards ABMR. We show how recognition of the role of T follicular helper-B cell interactions may allow to elaborate improved clinical strategies for immune monitoring and to identify novel therapeutic targets to tackle ABMR that will ultimately allow to improve organ transplant survival.
Keywords: Alloimmunity, humoral response, T follicular helper cells, B cells, organ transplantation
Antibody-mediated rejection: an ongoing threat for organ transplant survival
Solid-organ transplantation (see Glossary) has emerged as the gold standard therapy for millions of individuals with end-stage organ failure worldwide from a clinical and an economic standpoint. However, thousands of transplanted organs fail each year, for which the first cause identified is represented by antibody-mediated organ rejection (ABMR) in kidney transplantation but also in heart, lung and liver [1]. ABMR is observed in organ recipients transplanted with an allograft from a genetically incompatible donors, and results from deleterious IgG donor-specific antibody (DSA) interactions with the allogeneic endothelium that triggers C1q-dependant complement activation, and recruitment of cytotoxic NK and monocytes cells that ultimately lead to severe inflammation and injury in both microvascular and macrovascular compartments of the organ allograft [2,3]. There are strong associations between the degree of human leukocyte antigen (HLA) mismatches within donor-recipient pairs and ABMR, and anti-HLA DSA are present and used for diagnosis, consistent with a pathogenic upstream involvement of adaptive cellular immune responses represented by alloantigen-specific T and B cells [4,5].
Yet, it is only recently that detailed mechanistic studies investigating the underlying contribution of T follicular helper (TFH) and B cells to the generation of DSAs and development of ABMR have become available in animal models and in human [6–9]. Additionally, progresses have been made in understanding why patients mounting DSAs do progress to ABMR and some do not, and what cellular and molecular states of TFH and B cells underlie the early onset of ABMR versus a late de novo occurrence of ABMR after organ transplantation. These recent discoveries have the potential to benefit in the future the transplant community for the diagnosis, risk stratification and therapeutic interventions of patients undergoing ABMR.
TFH cell contribution to humoral alloimmunity in organ transplantation
TFH cell and germinal center responses at the basis of DSA generation
TFH cells are a defined subset of “B helper” CD4 T cells that reside in germinal centers (GC) and actively recirculate in blood, and are pivotal in orchestrating the elaboration of antibody responses against protein antigens including HLA molecules [10,11]. Although the role of TFH cells has long sparked the interest of the transplant immunologists, clear recognition of their contribution to humoral alloimmunity has been documented only through the last five years [12–34] (Table 1). A growing number of studies in humans have shown that circulating TFH (cTFH), detected as CD4+ CXCR5+ cells, are expanded in frequencies or numbers in blood of patients mounting DSA post-transplant as compared to those who do not develop DSAs. These cTFH cells can be either in central (CCR7+/CD62L+) or effector (CCR7−/CD62L−) memory differentiation states, display GC TFH-like activation features (ICOS+PD-1+) and Th1 or Th17 polarized phenotypes (CXCR3+ or CCR6+). Importantly, cTFH cells are enriched in CD40L+ donor-specific cells and respond to donor-antigen by producing IL-21, which can be detected in pre-transplant (indicating pre-existing memory to donor), or post-transplant at time of de novo generation DSA emergence [21] [24]. While overall functional potency or direct alloimmune responses of TFH cells can be easily assessed in vitro (e.g (i) TFH and B cells co-cultured with SEB [17], (ii) TFH and B cells co-cultured with irradiated donor PBMCs [35] or (iii) recipient PBMCs co-cultured with irradiated donor PBMCs [36]), indirect alloimmune responses are more difficult to detect in vitro [37] (e.g recipient PBMCs pulsed with donor cell lysate [24,38]). Longitudinal studies have shown that expansion of alloreactive ICOS+PD-1+ cTFH cells in blood tracks with GC reactivity in allograft-draining lymph nodes and precede DSA formation [19].
Table 1.
TFH cell responses in humoral alloimmunity in transplantation
| Model | location | TFH cell immune state and function | References |
|---|---|---|---|
| rat kidney transplant | GC TFH | proliferating Ki67+ GC TFH, increased IL-21+ TFH in splenic follicles and in serum from rats with ABMR (model using low dose cyclosporine) | [12] |
| mouse skin transplant | GC TFH | increased splenic ICOS+ PD-1+ TFH in skin rejection, which can be inhibited by anti-IL-21R Ab | [13] |
| human kidney transplant recipients | cTFH | increased IL-21+ and CFSElow donor-reactive blood TH (CD3+ CD8-) cells in patients with de novo DSA | [14] |
| mouse kidney transplant | GC TFH | increased CXCR5+ ICOS+ GC TFH from the draining lymph nodes of the transplanted kidney in mice with ABMR | [15] |
| mouse skin transplant | GC TFH | expansion of CXCR5+ PD-1hi GC TFH in response to a skin graft, which could be diminished by selective anti-CD28 treatment. The selective CD28 blockade inhibition of TFH-B cell interactions was CTLA-4-dependent and TFH-specific | [16] |
| human kidney transplant recipients | cTFH | increased of proliferating Ki67+ ICOS+ TFH, CCR7+CD127+ TFH and IL-21+ donor-specific TFH in DSA+ABMR+ versus DSA+ABMR-patients | [17] |
| human kidney transplant recipients | cTFH | ICOS+PD-1+CD38+CXCR5+ TFH detected in highly sensitized patients, which were decreased after desensitization by belatacept and proteasome inhibitor | [18] |
| mouse skin transplant | cTFH | ICOS+ PD-1+ TFH are enriched for donor-specific cells, ICOS+ PD-1 TFH expansion precedes DSA formation | [19] |
| human kidney transplant recipients | cTFH | decreased CXCR5+ICOS+PD-1+ TFH precedes de novo DSA formation | [20] |
| human kidney transplant recipients | cTFH | increased IL-21+ donor-specific T cells pre-transplant and post-tranplant predict allograft rejection | [21] |
| human kidney transplant recipients | cTFH | increased CD25+ CCR6+ TFH after in vitro restimulation correlates with DSA generation | [22] |
| mouse heart transplant | GC TFH | increased number of secondary follicles and TFH cells in spleen at day 50 post-transplant from mice showing heart ABMR | [23] |
| human kidney transplant recipients | cTFH | increased CD62L-CXCR3+ TFH and IL-21+ donor-specific TFH are associated with early DSA generation post-transplant | [24] |
| mouse heart | GC TFH | increased TFH numbers, GC size, high serum IL-21 in alemtuzumab-induced | [25] |
| transplant | chronic ABMR model, which could be reduced by anti-LFA-1 treatment | ||
| mouse skin transplant | GC TFH | expansion of CXCR5+ PD-1hi Bcl6+ donor-specific TFH that upregulated CTLA4 in response to a skin graft. Anti-CD28 treatment led to superior inhibition of donor-specific TFH and DSA formation compared to CTLA4-Ig. | [26] |
| human kidney transplant recipients | cTFH | CXCR5+ CCR7lo PD-1hi TFH correlated with de novo HLA sensitization post-transplant | [27] |
| human kidney transplant recipients | cTFH | increased CD40L+PD-1+ TFH in patients with de novo DSA at 1-year post-transplant | [28] |
| human kidney transplant recipients | cTFH | increased CXCR5+ CCR7+ TFH in ABMR patients with class II DSA compared to those with class I DSA | [29] |
| human kidney transplant recipients | cTFH | increased of both CXCR3-CCR6-TFH and CXCR3-CCR6+ TFH in patients with ABMR | [30] |
| non human primate kidney transplant | GC TFH | increased ICOS+PD-1hi GC TFH and size of GC in sensitized primates with ABMR after T cell–depleting induction | [31] |
| human kidney transplant recipients | cTFH | increased numbers of TFH cells in patients with pre-existent DSA | [32] |
| non human primate kidney transplant | GC TFH | Increased PD-1hi GC TFH and number of GCs in ABMR which could be decreased by anti-CD40 and belatacept treatment | [33] |
| mouse heart transplant | GC TFH | differentiation of transferred TCR-transgenic CD4 T cells (mimicking indirect pathway) into TFH phenotype that allow GC formation after heart transplantation of T cell-deficient mice | [34] |
Distinct TFH cell phenotypic and molecular states underlie DSA pathogenicity, ABMR severity and timing
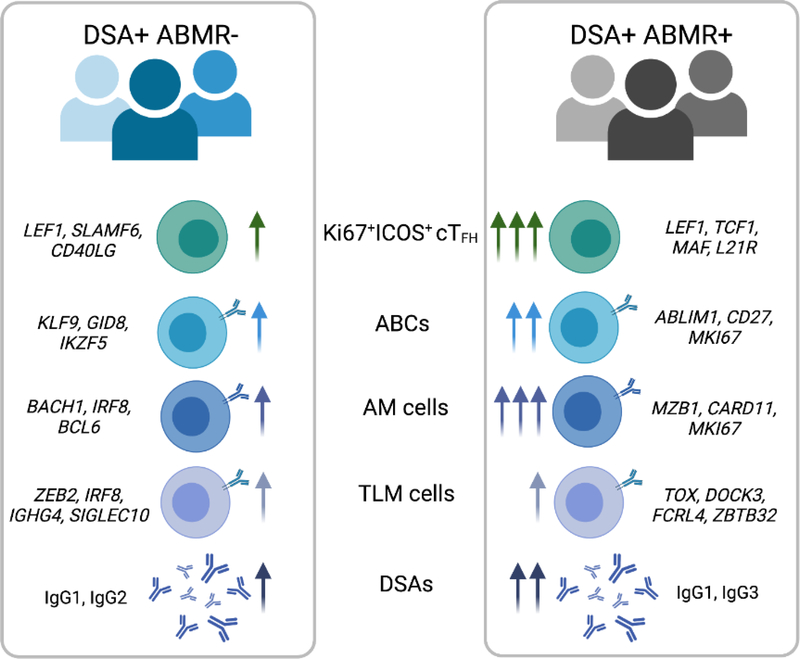
One main indicator of DSA pathogenic potential is the evidence for histological antibody-related injury lesions in the allograft (and thus ABMR diagnosis), which is associated with DSAs of predominant IgG1/IgG3 isotypes with the most potential to activate complement and recruit cytotoxic FcγR+ innate cells [39,40]. Although the abovementioned studies have linked TFH response to DSA formation, their involvement in promoting DSA pathogenicity and ABMR was yet unclear. Recently, Louis et al. have designed a study to specifically compare patients mounting post-transplant DSA and undergoing ABMR (DSA+ABMR+) to those mounting DSA but with no evidence of ABMR throughout two years of clinical follow-up (DSA+ABMR−) [17]. Louis et al. found in DSA+ABMR+ patients increased frequencies of cTFH cells with unique enrichment in cTFH clusters comprising proliferating Ki67+ICOS+ and early memory precursor CCR7+CD127+ cells. The emergence of these Ki67+ICOS+ cTFH clusters coincided with concomitant expansion of blood Ki67+ activated CD20+CD38lo B cells and CD20+CD38hi plasma-blasts, correlated with plasma-released CXCL13 and levels of DSA generated, suggesting a more robust, GC reaction in DSA+ABMR+ compared to the DSA+ABMR− condition. In addition to their increased cell numbers in DSA+ABMR+ patients, these Ki67+ICOS+ cTFH were specifically polarized with dominant Th1 and Th17 features, which paralleled the detection of IgG1 and IgG3-switched DSAs in patients’ sera. This is consistent with the known roles of INF-g, IL-17 and IL-21 in isotype switching toward IgG3 [41]. Also, IgG1 and IgG3 are most likely to be the first IgG subclasses to be generated as dictated by their genomic order, and thus, detected during acute ABMR occurring post-transplant, whereas IgG4 DSAs are usually detected in late and chronic forms of ABMR [39,42]. The transcriptional analyses of these DSA+ABMR+ associated cTFH clusters supported an enrichment in precursors (LEF1, TCF1), effectors (CD28, MAF, IL-21R) and Th1-polarized gene signatures. This distinct transcriptional programing of cTFH cells during ABMR resulted consistently in their potent functional capacity in vitro to provide help to cognate B cells that differentiated into plasma cells in response to vigorous cTFH cell help and generated DSAs enriched in IgG1 and IgG3 isotypes. In DSA+ABMR- patients, there was a lower amplitude of Ki67+ICOS+ cTFH with differing transcriptional programming (LEF1, SLAMF6, CD40LG), lower CXCL13 and thus most likely extrafollicular responses resulting in less pathogenic DSAs generated [17] (Figure 1).
Figure 1. Differing cellular and molecular states of TFH and B cells underlying progression to ABMR.
Common circulating TFH (cTFH) and B cell subsets can be found elevated in blood of patients developing DSA but who do not progress to ABMR (DSA+ABMR−) and patients developing DSA who progress to ABMR (DSA+ABMR+). These proliferating donor-specific cTFH, activated B cells (ABCs), activated memory (AM) and tissue-like memory (TLM) cell subsets were elevated at different magnitudes in blood, indicated by the arrows, and displayed distinct transcriptional programming, indicated by gene names in italics. These changes in cTFH and B cell subsets are linked to distinct magnitude and IgG subclass composition of donor-specific antibodies (DSAs) generated in the two types of patients. This figure was created with BioRender.
Analyzing frequencies and phenotypes of cTFH clusters with histological and clinical profiles of patients, Louis et al. found that the magnitude of the expansion of the Ki67+ICOS+ and CCR7+CD127+ cTFH cells was mostly associated with late onset and a more severe phenotype of ABMR, which was linked to significant decrease in kidney allograft survival [17]. These more severe forms of ABMR were characterized by more microvascular inflammation but also extensive arteritis and interstitial inflammation, that are both also features of T-cell–mediated rejection, suggesting an allograft-infiltrating TFH cell component which has been demonstrated by confocal microscopy by other groups [43,44].
These allograft-infiltrating TFH cells likely arise from cTFH cells that would have migrated from blood to target tissues as they display a CXCR5+PD-1+ phenotype. Also, intriguingly, these local TFH cells can cluster to form of ectopic/tertiary lymphoid structures defined by dense cell aggregates of TFH-B cells reminiscent of B cell follicles/GC structures of secondary lymphoid organs [43,45]. Recent data have also found that TFH cells to be located within allografts of patients showing chronic ABMR [7]. Interestingly, concomitant with the presence of CXCR5+ PD-1+ cells, a substantial number of these allograft-infiltrating CD4 T cells in ABMR were CXCR5−PD-1+, indicative of a peripheral helper phenotype as previously described in autoimmunity [46,47].
These findings in patients are consistent with preclinical animal models demonstrating the crucial implication of TFH cells in promoting DSA responses towards ABMR, as deletion of TFH cells at the time of transplant resulted in significantly less severe allograft ABMR in mice [15]. Using several other animal models, including rats and non-human primates, as well as other models of transplantation such as heart and skin transplant, studies have consistently shown that TFH cells by their capacity to respond to donor antigens by producing IL-21, providing potent B cell help, and promoting GC hypertrophy and reactivity, are important in the generation of isotype-switched IgG DSAs that leads to ABMR and premature allograft failure (Table 1). Along with integration of routine clinical and virologic parameters, characterization of cTFH and B cell phenotypic and functional profiles have clinical potential as biomarkers for patient management to predict DSA generation before its onset and distinguish the less robust phenotype (DSA+ABMR−) from pathogenic (DSA+ABMR+) humoral responses after organ transplantation.
Effector B cell contribution to humoral alloimmunity in organ transplantation
Memory B cells as effectors for DSA generation
For obvious reasons, efforts in understanding of the immune pathogenesis of humoral alloimmunity and ABMR have focused on the characterization of the B cell response and its alloreactive potential [48–58] (Table 2). Most studies have identified important changes in the B cell compartment during an ongoing alloimmune humoral response; with increase in frequencies or number of antigen-experienced (i.e. memory) B cells in patients developing DSA in post-transplant. These memory B cells (MBCs) were generally isotype-switched (IgD−) and expressing the key costimulatory receptor CD27, which is acquired through GC experience [59]. Importantly, MBCs are highly enriched in donor HLA-specific cells and strongly respond to polyclonal stimulation comprised of TLR9 agonists and cytokine cocktails leading to DSA production in vitro [54,60]. These cells can also be detected pre-transplant (indicating pre-existing MBCs to donor-HLA antigens), or post-transplant by donor-HLA-specific ELISPOT at time of de novo DSA emergence [57]. Longitudinal studies have further shown that donor-HLA-specific MBC expansion in blood tracked with increased in GC size containing GL-7+CD95+ GC B cells, was proportional to DSA titers, and not surprisingly preceded of several weeks DSA emergence in circulation [53,57]. The phenotype of these cells was described as Ki67+CD19+CD27+IgD− in both animal models as well as in transplant patients manifesting DSAs, reminiscent of the activated B cells previously reported after flu vaccination [31,61]. Based on these previous data, Louis et al. inquired whether these proliferating B cells can distinguish asymptomatic DSA+ABMR− patients from the DSA+ABMR+ status [17]. Louis et al. found that Ki67+CD19+CD27+IgD− cells could be further separated into CD20+CD38lo activated B cells and CD20-CD38hi antibody-secreting cells. While CD20−CD38hi cells were consistently elevated in all DSA+ situations, CD20+CD38lo were highest in DSA+ABMR+ patients [17]. The emergence of both B cell subsets in blood coincided with increase in Ki67+ICOS+ cTFH cells, release of CXCL13 and the peak of DSA levels in circulation, overall indicative of GC-dependent TFH-B cell interactions during DSA responses that are exacerbated during ABMR [17].
Table 2.
Effector B cell responses in humoral alloimmunity in transplantation
| Model | location | Effector B cell immune state and function | References |
|---|---|---|---|
| human kidney transplant recipients | circulating B cells | increased T-bet+CD27+CD21+ memory B cells transcriptionally and functionally poised for plasma cell differentiation in ABMR | [48] |
| human kidney transplant recipients | intragraft B cells | intragraft B cells have transcriptional resemblance with mouse innate B cells with capacity to differentiate into plasma cells expressing self-reactive antibodies, driven by local intragraft antigens | [49] |
| rat kidney transplant | GC B cells | CD45R+CD27+ memory B cells in splenic follicles from rats with ABMR, which can be diminished by high cyclosporine treatment | [12] |
| mouse heart transplant | GC B cells | Alloreactive B cells contribute to transplantation tolerance by foregoing germinal center responses while retaining their ability to function as antigen-presenting cells and by actively suppressing de novo alloreactive B cell responses | [50] |
| mouse skin transplant | GC B cells | increased GL-7+CD95+ B cells correlate with ICOS+ PD-1 cTfh expansion and precedes DSA formation, which can be diminished by belatacept and anti-CD28 treatment | [19] |
| rat kidney transplant | GC B cells | presence of B cell follicles and AID mRNA in spleen of rats in ABMR model (low cyclosporine-induced) which can be diminished by anti-BAFF treatment | [51] |
| human kidney transplant recipients | circulating B cells | expansion of Ki67+CD20hiCD38loCD27+IgD- activated B cells in DSA+ABMR+ versus DSA+ABMR- patients | [17] |
| human kidney transplant recipients | circulating B cells | CD27-IgD+ anive and CD27+IgD− memory B cells detected in highly sensitized patients, which were decreased after desensitization by belatacept and proteasome inhibitor | [52] |
| human kidney transplant recipients | circulating B cells | increased IgD-CD27+CD38- memory B cells at time of DSA detection before onset of ABMR | [53] |
| human kidney transplant recipients | circulating B cells | high frequencies of donor-specific memory B cells in patients with acute and chronic ABMR | [54] |
| human kidney transplant recipients | circulating B cells | donor-specific memory B cells are detected by ELISPOT before transplantation in sensitized patients | [55] |
| non human primate kidney transplant | circulating B cells | increased blood Ki67+CD20+CD27+IgD− memory B cells in sensitized primated with ABMR after T cell–depleting induction | [31] |
| human kidney transplant recipients | circulating B cells | expansion of CD19+CD27-IgD- and IL-21R+ B cells in patients developping anti-HLA antibodies | [56] |
| human kidney transplant recipients | circulating B cells | high frequencies of donor-specific memory B cells are found at ABMR diagnosis and before transplantation, regardless of circulating DSA | [57] |
| non human primate kidney transplant | circulating B cells | presence of CD20+CD27+ B cells in ABMR which could be decreased by anti-CD40 and belatacept treatment | [33] |
| human kidney transplant recipients | circulating B cells | increased IgD-CD27+ switched memory and decreased IgD+ CD27- naive B cells during the first month post-transplant predicted de novo DSA development | [58] |
Memory B cell subsets derived from distinct inflammatory signals are involved during ABMR
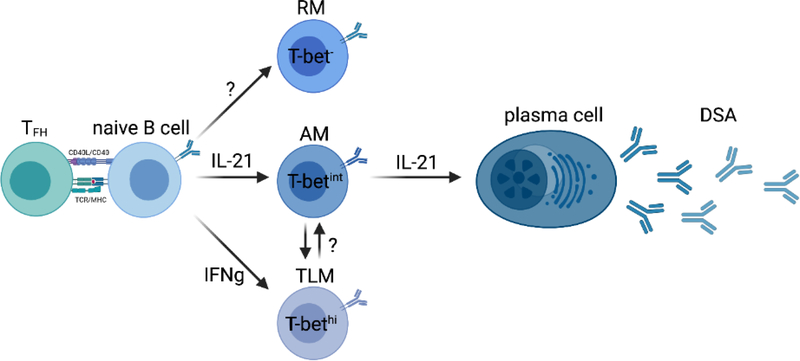
Organ transplantation is characterized by a sustained antigenic exposure and an IL-21 and IFN-g biased chronic inflammatory environment [62], both of which are known to deeply affect B cell differentiation and function resulting in the generation of unusual MBCs lacking CD21 and expressing the transcription factor T-bet [63,64]. A recent study have identified the expansion of unusual MBC clusters lacking CD21 and expressing T-bet, which largely distinguished patients without DSA from all DSA+ patients [48]. According to CD27 expression, Louis et al. further delineated CD27+CD21− activated memory (AM) from CD27−CD21− tissue-like memory (TLM) cells, while their resting memory (RM) counterparts were identified as CD27+CD21+ cells similar to other studies [65]. Although increased frequencies of both AM and TLM cells were an immune feature of all DSA+ patients, the AM cell expansion was much more pronounced in the DSA+ABMR+ group. T-bet was not expressed in RM cells, while expressed at intermediate levels in AM cells and most highly expressed in TLM cells, consistent with previously published data outside of the field of transplantation (Figure 2) [66].
Figure 2. Alloreactive B cell differentiation according to cytokine instructions.
Fate of naive B cells dictated by IFN-g and TFH cell-derived IL-21 and according to T-bet expression: resting memory (RM) cells represent the self-renewal reservoir of B cell memory, activated memory (AM) cells are poised for differentiation into donor-specific antibody-producing plasma cells driven by IL-21, and tissue-like memory (TLM) cells in an inhibitory state driven by IFN-g signals. Plasticity between AM and TLM cell fate may exist. This figure was created with BioRender. Abbreviations: CD, cluster of differentiation; MHC, major histocompatibility complex; TCR, T cell receptor.
RM cells, that do not express T-bet, represented the great majority of MBCs in healthy individuals and in patients who do not develop DSA [48]. Their transcriptional profile is consistent with a resting and central memory state with self-renewal capabilities. Conversely, AM and TLM cells shared the features of expression of T-bet and downregulation of CD21, and displayed several fundamental differences in their phenotype, differentiation, transcriptomic states and effector functions. AM cells comprised both proliferating isotype-switched (Ki67+IgD−) and unswitched (Ki67+IgD+) B cells which were predominantly found elevated in early ABMR (memory response) and late (de novo response) ABMR, respectively. Transcriptionally, AM cells appeared poised for plasma cell differentiation with high expression levels of the transcription factors IRF4 and Blimp1, as well as the genes MZB1 and XBP1, that are known to modulate plasma cell differentiation [67,68]. Specifically, unlike those from the DSA+ABMR− group, AM cells of DSA+ABMR+ patients were selectively enriched for IGHV germ line genes that have been previously documented to predominate during organ rejection, including IGHV3–7, IGHV3–15, IGHV3–23 and IGHV3–74 [69–71]. Consistently, AM cells could be detected within dense infiltrating B cell clusters from kidney allografts with diagnosis of acute ABMR. Their pathogenic IGHV3–7, IGHV3–15, IGHV3–23 and IGHV3–74 transcripts could also be detected within the global transcriptome of kidney allograft showing ABMR [48]. This restricted IGHV usage reflects clonal selection likely due to a common pool of antigens that may drive organ rejection, although direct evidence that the expansion of these clones is alloimmune requires additional in vitro and animal studies. Interestingly, Grover et al. demonstrated that antibodies encoded by IGHV3–23 gene were recognizing components of bacteria [72]. Whether antibodies encoded by these dominant IGHV genes recognize HLA molecules or are crossreactive to HLA due to molecular mimicry remains to be investigated.
In Louis et al. study, AM cells could be generated in vitro after priming of naive B cells and their stimulation with IL-21. Because of their CD27 expression, AM cells likely represent GC emigrants and not surprisingly were more responsive to cognate TFH cell help. They expressed high levels of IL-21R and CD40 at baseline and produced DSAs when co-cultured with TFH cells in an IL-21 dependent manner [48]. Conversely, and consistent with previous findings [73,74], TLM cells lacked CD27 and CD40, phenotypically resembled exhausted cells, were enriched with for the inhibitory receptors CD72, CD32B and FcRL5, and therefore were hyporesponsive to TFH cell help and did not produced DSA in vitro. Compared to DSA+ABMR−, TLM cells from DSA+ABMR+ patients displayed a more pronounced exhaustion-like transcriptional profile with increased in TOX, DOCK3 and FCRL4 (Figure 1). Their immune repertoire was different than that of AM cells and lacked enrichment in the pathogenic IGHV associated with ABMR. TLM cells could be generated in vitro from naive B cell priming and stimulation with IFN-g but not IL-21 [48]. Consistent with their T-bethi phenotype, they have been recently shown to localize outside of GCs, to be virtually absent from the lymphatic circulation [75]. Also, it has been shown that these cells were mainly generated through the extrafollicular pathway, had poor affinity maturation, generated short-lived plasma cells in response to TLR stimulation and IFN-g, signals mainly found outside GCs [76,77]. As both IL-21 and IFN-g coexist during ABMR, both T-betint AM cells and T-bethi TLM cells are generated and expanded in patients. It is like that TLM cells can counterbalance the hyperactivation state of AM cells in the context of chronic activation. Indeed, TLM cells from HIV infected patients were reported to be responsive to the binding of circulating IgG3 and C1q (also present in excess during ABMR) onto their surface IgM, conveying a strong B cell receptor-mediated inhibitory signal to these cells [78].
Coordinated TFH/B cell responses determines the progression towards ABMR
Identification of novel TFH and B cell subsets that shape humoral alloimmunity
The contribution of TFH and B cells to the immune pathogenesis of DSA generation and progression to ABMR is now increasingly documented. However, only few studies have concomitantly profiled TFH and B cell compartments and investigated the importance of TFH-B cell interactions in response to common alloantigenic stimulations and proinflammatory instructions. Identification of coordinated adaptive TFH/B cell immune signatures can be linked to specific trajectories of disease severity and have clinical implications for the design of more targeted and personalized therapeutics in ABMR. The emergence of novel high-dimensional and integrative technologies such as high-dimensional flow cytometry, next generation RNA-sequencing and multiplex immunoassays, can be used for deep profiling of patient clinical phenotypes. These methodologies have been applied in multiple discipline of medicine including autoimmunity, infectious disease and cancer, and are now ready for prime time in the field of transplantation.
Multidimensional approaches have been used to concomitantly profile several TFH and B cell immune signatures in kidney transplant recipients undergoing active humoral alloimmune responses. Recent works have identified highly coordinated responses of TFH and activated B cells at phenotypic, transcriptional and functional levels in the asymptomatic DSA+ABMR- versus the pathogenic DSA+ABMR+ clinical trajectory. Common cell subsets were identified in both DSA+ABMR− and DSA+ABMR+ patients when compared to no DSA situation, including Ki67+ICOS+ cTFH, Ki67+CD27+IgD− CD20+CD38lo activated B cells (ABCs), AM and TLM B cell subsets [17,48]. However, patients in the two cohorts, while well matched for clinical parameters (including the lack of ongoing viral infections) and analyzed at similar time points post-transplant and with a similar clinical follow-up of 2 years, differed in cTFH and B cell subsets: (i) in absolute numbers and frequencies, (ii) phenotype, and (iii) transcriptional programming (Figure 1). More importantly, these quantitative and qualitative cellular differences correlated with the amount and quality (class-switching) of DSAs, as well as with levels of CXCL13 produced as an indicator of ongoing GC reaction in DSA+ABMR+ versus DSA+ABMR− patients. Specifically, cTFH and B cells from DSA+ABMR+ patients are both activated and proliferating, more poised for cTFH-B cell interaction and GC activation, for elevated DSA production and capacity to class-switch towards the pathogenic IgG1 and IgG3 as compared to cTFH and B cells in DSA+ABMR- patients. Thus, differences between DSA+ABMR− and DSA+ABMR+ patients are most likely driven by distinct cTFH and B cell profiles and may be used as surrogate markers for predictive, diagnostic or therapeutic purposes in the clinic. However, other immune triggers (e.g active infection, inflammation) may induce phenotypic and functional changes in cTFH and B cells, which may participate to modulate their capacity to promote DSA formation [79], and these remain to be determined in future studies. In combination with histological and long-term clinical follow-up data, recent data further identified that DSA+ABMR+ patients with highest frequencies of both Ki67+ICOS+ cTFH and ABCs displayed more allograft arteritis and interstitial inflammation, and progressed to premature kidney allograft loss, compared to those with lower frequencies of the two cell subsets [17]. Similar conclusions were reached for high frequencies of AM B cells in the same cohort of deeply phenotyped kidney transplant patients. Comparable blood cTFH and activated B cells subsets were independently identified by several other groups, which were also predictive of poor prognosis in patients mounting DSA with or without ABMR [14,53] [80–82]. These findings highlight the importance of simultaneous immune monitoring of cTFH and activated B cells for improving patient’s risk stratification and clinical management (see Clinician’s Corner).
TFH and B cells as concomitant therapeutic targets in ABMR
There is an urgent need for innovative treatments to assist clinicians in the management of ABMR. Although patients with acute ABMR have an acceptable response to standard-of-care treatment (i.e. corticosteroids, plasmapheresis and intravenous immunoglobulins), the risk of relapse upon treatment withdrawal is high (>40%) [83]. In addition, the use of the B cell- and plasma cell-depleting agents Rituximab and Bortezomib was not associated with significant improvement of long-term outcome in ABMR [84,85]. For chronic forms of ABMR, no efficient immunosuppressive treatment exists and the poor response to the standard-of-care treatment is linked to allograft loss [86]. Moreover, it is difficult to predict clinical outcome at diagnosis, upon ABMR treatment and before complete treatment withdrawal. For these reasons, more specific targeted therapies aiming TFH-B cell interactions may represent a timely option as the abovementioned ABMR therapy depleting DSAs and plasma cells are insufficient. One successful use of biotherapy in organ transplantation has been illustrated by the use of the costimulatory blockade molecule belatacept (CTLA4-Ig) that received FDA approval for prophylaxis of humoral organ rejection in kidney transplantation, which significantly diminished the incidence of de novo DSA and improved long-term allograft function, when compared to cyclosporine [87]. However, the use of belatacept has not been tested so far for ABMR treatment.
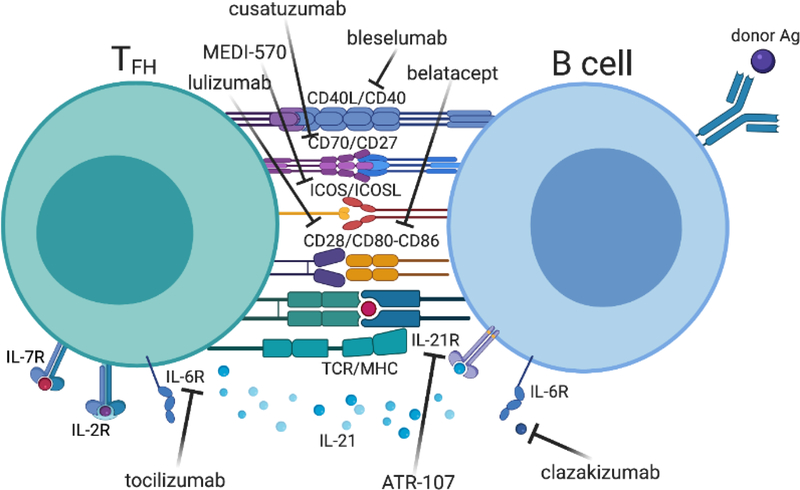
Targeting costimulatory molecules during ABMR
Because several costimulatory receptor pairs (CD28/CD80-CD86, CD40/CD40L, ICOS/ICOSL and CD70/CD27 axes) are at the basis of optimal control of TFH cell help to B cells, other costimulatory molecules antagonists are currently evaluated in transplantation (Figure 3, Key Figure). Donor-specific GC TFH cells, which upregulate CTLA4, and CD95+ GL7+ GC B cells can be significantly inhibited by anti-CD28 domain antibody at a greater extent than by CTLA4-Ig [26]. Of note, the superior inhibition of GC TFH-B cell interactions with selective CD28 blockade was CTLA-4 dependent and TFH cell specific [16]. Additionally, the new anti-CD28 molecule (lulizumab), because it preserves the regulatory CTLA4-mediated signals and controls the proliferative response of pre-existing TFH cells better than belatacept, has raised high interest [88,89] and is currently tested in clinical trials. An open-label single-arm Phase I clinical trial (NCT04066114 I) is currently recruiting patients to evaluate the safety of lulizumab in the context of combined immunosuppressive regimen (steroids, belatacept, tocilizumab and everolimus) in kidney transplantation. Another open-label randomized Phase II clinical trial (NCT04903054 II) was launched to evaluate the safety and efficacy of lulizumab compared to tacrolimus as the primary immunosuppressant in first-time renal transplant recipients. While interventional clinical trials using anti-CD40L agents were halted because of severe thromboembolic complications [90], anti-CD40 is now developed as a safer and promising alternative. Anti-CD40 effectively disrupt GCs by reducing GC TFH-cell numbers and their IL-21 production resulting in attenuated DSA production in animal models. Preliminary trials using bleselumab have shown its acceptable efficacy and safety for preventing acute rejection in kidney transplantation [91] and further clinical trials are ongoing. Although efficacy of ICOS-Ig was unsuccessful at preventing allograft rejection [92], anti-ICOS efficiently inhibited DSA generation in islet xenografts recipients, and when combined with anti-CD40 led to decrease in DSA titers and improved histology as compared with anti-CD40 alone in a cardiac model of chronic ABMR [93]. A double-blind randomized Phase I clinical trial (NCT01127321 III) is evaluating the safety and tolerability of the anti-ICOS MEDI-570 in patients with lupus and another open-label single-arm Phase I clinical trial (NCT02520791 IV) was launched to determine the safety and maximum tolerated dose of MEDI-570 in patients with T-cell Lymphoma. Thus, future clinical trials evaluating the potential use of anti-ICOS in transplantation are warranted. CD70/CD27 are mutually expressed on TFH and B cells; CD70 stimulates cytokine production in TFH cells [94] while it synergizes with the CD40 pathway to activate B cells and antibody production, and CD27, the ligand of CD70, is expressed on activated/memory TFH and B cells as well as plasma cells [95]. In transplant animals, anti-CD70 administration diminished prolonged corneal or heart allograft survival [96,97]. While there are ongoing clinical trials with the anti-CD70 cusatuzumab in acute myeloid leukemia [98], studies in transplant recipients are lacking and warranted.
Figure 3. Key therapeutic targets in TFH cell crosstalk with cognate B cell (key figure).
The major costimulatory and cytokine receptors involved in cognate donor-antigen specific TFH-B cell interaction are depicted. Biotherapies currently evaluated in clinical trials and their specific targets are represented. This figure was created with BioRender. Abbreviations: CD, cluster of differentiation; ICOS, inducible T cell costimulator; IL, interleukin; MHC, major histocompatibility complex; TCR, T cell receptor.
Targeting cytokines to treat ABMR
Biotherapeutic targeting of cytokines axes mediating TFH-B cell interactions may result in complex immune modulation beyond TFH and B cell responses. However, recent data encourage further cytokine inhibition-based biotherapeutic development in ABMR. IL-6 has known direct effects on B cell maturation and differentiation towards plasma cells, and also controls plasma cell homeostasis [99]. Additionally, IL-6 plays a major role in TFH-cell differentiation and function, and B cells and plasmablasts produce large amounts of IL-6, which in turn, stimulate TFH cells via IL-6R [100]. The IL-6R antagonist tocilizumab administration resulted in reduction in TFH cells in experimental models [101], and leaded to stabilization of kidney allograft function and significant decreased in DSA MFI levels in patients with chronic ABMR [102]. A recent double-blind randomized phase II clinical trial (NCT03444103 V) has evaluated the safety, tolerability and efficacy of clazakizumab in kidney transplant recipients with late ABMR. Ten patients received placebo and 10 patients received clazakizumab for 12 weeks, and in the second part, all 20 patients were treated for 40 weeks with clazakizumab. 5/20 patients under active treatment developed serious infectious events and 2/20 patients developed diverticular disease complications, leading to trial withdrawal. The mean renal function decline during the first part of the trial was slower with clazakizumab compared to placebo. Importantly, clazakizumab was associated with significantly decreased DSA levels, resolution of ABMR activity, and diminished ABMR-related gene-expression patterns [103]. While IL-21 is a master regulator of optimal TFH–B cell cross talk [104], its therapeutic blockade was found to have double-edge sword effects in clinical settings and should be used with caution. Donor-reactive cTFH cells produce IL-21 [17], which is detected at high levels in sera in experimental models [25]. Anti-IL-21R administration has been tested in vitro resulting in diminished plasma cells differentiation and IgG production in a coculture model of cTFH-B cells stimulated with donor antigen [35], and prolonged allograft survival in vivo in mice models [105]. Safety and efficacy data are accumulating in clinical trials in lupus and rheumatoid arthritis for anti-IL-21 [106] and in healthy subjects for anti-IL-21R [107]. Thus, blocking IL-21/IL-21R axis should be tested in future clinical trials in transplantation.
Recent in vitro data exposing cTFH cells to IL-2 induced their reprogramming with increased T-bet expression and IFN-g production leading to reduced IgG production by cognate B cells [108]. On the B cell side, an excessive expression of T-bet was also associated with diminished proliferation capacity, response to TFH cell signals and programming towards a B cell inhibitory state [109]. Overall, data support that a biased Th1 polarization on both TFH and B cells may diminish their effector function and lead, if sustained exposure, to cell state resembling exhaustion. Signals that may diminished effector function while favoring hyporesponsiveness of TFH and B cells, like blocking IL-21 and administration of low-dose IL-2 and IFN-g warrant further investigations. However, while cytokine manipulation appears promising, this should also be done with caution, and account for the complexity of interplay between the cognate signals involved in TFH-B cell cross talk and for the pleiotropic effects of cytokines beyond TFH and B cells.
Concluding remarks
Understanding of the pivotal role of adaptive immune cell responses in directing DSA generation leading to ABMR has been substantially improved by the profiling of cellular and molecular cues concomitantly occurring in TFH and B cells. The coordinated TFH-B cells responses in ABMR are now recognized to be underlied by common allo-antigeneic and dominant inflammatory triggers, and to involve several key cognate costimulatory receptors and cytokine signaling pathways. As depleting plasma cells and DSAs is certainly not efficient enough to tackle ABMR, targeting the upstream TFH-B cell interaction represents a novel attractive option for future biotherapeutic strategies in ABMR. Future investigations need to address whether therapeutic manipulation of TFH/B cell fate for promoting exhausted/tolerogenic profiles versus effector function is safe, and whether combined targeting of TFH-B cell interaction, their plasma cell progeny and DSAs will result in prolonged allograft survival in ABMR (see Outstanding Questions).
Outstanding questions.
Are both TFH and B cell clonally expanded during ABMR? Are there dominant TFH and B cell clones that drive DSA generation and ABMR? Are these exclusively allospecific, or also comprise of autoreactive or crossreactive expanded clones?
What is the role of regulatory T and B cells in the control of humoral alloimmunity? Are regulatory T and B cell responses also coordinated?
Can effector TFH-B cell be reprogrammed into regulatory or exhausted TFH-B cells and vice-versa?
Do TFH and B cell responses resolve after standard-of-care treatment of ABMR? Can monitoring TFH and B cell identify patients in need for second-line therapy in ABMR?
Can the use of IL-21/IL-21R blockers or low-dose IL-2 promote anti-inflammatory/tolerogenic environment to counteract pro-inflammatory signals in ABMR?
Is personalized targeting of cytokines and TFH-B cell interactions enough to tackle ABMR or should it be implemented to conventional antibody removal and plasma cell depletion?
Clinician’s corner
Immune monitoring of organ transplant recipients in the clinic is largely based on the measurement of anti-HLA DSAs. Implementation of additional non-invasive immune monitoring with assessment of concomitant blood cTFH and B cells can help clinician to improve precision diagnosis of ABMR, to identify specific immunophenotypes of patients, to identify ongoing DSA generation and predict future ABMR occurrence for improved patient management.
Unified assays for measuring cTFH and B cells and their donor-reactive potential, as preformed memory or de novo responses in both pre-transplant and post-transplant settings, are currently under thorough investigation by several independent laboratories in order to render them readily usable in clinical practice.
Because patients with the highest frequencies of activated cTFH and B cells at ABMR diagnosis have increased risk of allograft loss long-term, measuring these cells at diagnosis may help risk-stratify patients at high risk based their TFH and B cell immunophenotypes. In addition, measuring activated cTFH and B cells may help predict non-responder patients to treatment of ABMR and predict relapse after treatment discontinuation.
Development of novel biotherapeutic tools is growing exponentially, with the already successful use of belatacept which remarkable efficacy in preventing de novo DSA responses is largely due its intrinsic effect on optimal control of TFH-B cell interactions. Rituximab is part of the armamentarium of biotherapy used in ABMR, in addition to standard-of-care treatment (corticosteroids, plasmapheresis and intravenous immunoglobulins). However, its immunological impact in terms of successful GC B cell and regulatory B cell depletion (without targeting TFH cells), DSA reduction, and its clinical benefits in terms of allograft survival are yet to be demonstrated in larger clinical studies. As for Bortezomib, while depleting the direct source of DSAs, it certainly triggers paradoxical effect upstream plasma cells such as TFH and B cell proliferation compensating the plasma cell loss, resulting in the absence of significant decreased in DSA and lack of clinical effects in ABMR, when used alone.
Other promising biotherapeutic agents including tocilizumab (which targets TFH-B cell interaction) have yielded promising results on diminishing TFH cells and DSA levels, and stabilizing allograft function during ABMR with low rate of adverse events. This paves the way for further testing of the clinical efficacy and safety of cytokine manipulation to temper TFH and B cell responses.
Highlights.
The effector mechanisms by which donor-specific antibodies injure organ transplants leading to antibody-mediated rejection are now well recognized, but the pivotal role of the adaptive immune cell responses upstream of such humoral alloimmunity has been much less appreciated.
Knowledge of the contributions of both TFH and B cells to pathogenic humoral alloimmunity may improve outcomes for patients. Of particular interest is how the coordinated TFH and B cell responses can shape DSA pathogenicity and lead to ABMR.
This allows to understand how patients would benefit from concomitant monitoring both TFH and B cell responses to improve patient management and why targeting their interactions is key to tackle ABMR therapeutically.
Acknowledgments
This work was supported by the following grants: R21-AI116746 (DM), R01-AI130010 (DM) and the Human Immunology Program at the Starzl Transplantation Institute.
Glossary
- Allograft
a tissue or an organ removed from an individual and implanted to another individual from the same species
- Alloimmunity
an immune response that attacks cells or tissues from another individual of the same species
- Costimulatory receptor
a class of receptors expressed by immune cells that regulates the activation and generation of effector or regulatory cell responses
- Cytokine
substances such as interferons, interleukins or growth factors, that are secreted by certain cells of the immune system and have effects on other cells
- Humoral
relating to the body fluids especially with regard to immune responses involving antibodies
- Human leukocyte antigen (HLA)
complex of genes in humans which encodes cell surface proteins responsible for the regulation of the immune system including immunity against allograft
- Memory
immunity mediated by certain immune cell types that have previously encountered a given antigen and that on reexposure to the same antigen strongly and rapidly initiates a recall immune response
- Organ rejection
process in which the immune system of an organ transplant recipient attacks the transplanted organ or tissue
- Transplantation
the process of taking a living organ or tissue and implanting it in another part of the body or in another body
Footnotes
Disclaimer Statement
The authors declare no conflicts of interest.
Resources
I - https://clinicaltrials.gov/ct2/show/NCT04066114
II - https://clinicaltrials.gov/ct2/show/NCT04903054
III - https://clinicaltrials.gov/ct2/show/NCT01127321
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loupy A and Lefaucheur C (2018) Antibody-Mediated Rejection of Solid-Organ Allografts. N. Engl. J. Med. 379, 1150–1160 [DOI] [PubMed] [Google Scholar]
- 2.Valenzuela NM and Reed EF (2017) Antibody-mediated rejection across solid organ transplants: manifestations, mechanisms, and therapies. J. Clin. Invest. 127, 2492–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas KA et al. (2015) The perfect storm: HLA antibodies, complement, FcγRs, and endothelium in transplant rejection. Trends Mol Med 21, 319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery RA et al. (2018) HLA in transplantation. Nat Rev Nephrol 14, 558–70 [DOI] [PubMed] [Google Scholar]
- 5.Tambur AR et al. (2020) Sensitization in transplantation: Assessment of risk (STAR) 2019 Working Group Meeting Report. Am J Transplant 20, 2652–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walters GD and Vinuesa CG (2016) T Follicular Helper Cells in Transplantation. Transplantation 100, 1650–1655 [DOI] [PubMed] [Google Scholar]
- 7.Louis K et al. (2021) Targeting T Follicular Helper Cells To Control Humoral Allogeneic Immunity. Transplantation 105, e168–e180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Besouw NM et al. (2019) The role of follicular T helper cells in the humoral alloimmune response after clinical organ transplantation. HLA 94, 407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong AS et al. (2021) Regulation of Alloantibody Responses. Front Cell Dev Biol 9, 706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt N et al. (2014) Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 35, 436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crotty S (2019) T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 50, 1132–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steines L et al. (2021) Disruption of Tfh:B Cell Interactions Prevents Antibody-Mediated Rejection in a Kidney Transplant Model in Rats: Impact of Calcineurin Inhibitor Dose. Front Immunol 12, 657894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nian Y et al. (2021) IL-21 Receptor Blockade Shifts the Follicular T Cell Balance and Reduces De Novo Donor-Specific Antibody Generation. Front Immunol 12, 661580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subburayalu J et al. (2021) Characterization of follicular T helper cells and donor-specific T helper cells in renal transplant patients with de novo donor-specific HLA-antibodies. Clin Immunol 226, 108698. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed MT et al. (2021) Follicular T cells mediate donor-specific antibody and rejection after solid organ transplantation. Am J Transplant 21, 1893–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Muraglia GM et al. (2021) Superior inhibition of alloantibody responses with selective CD28 blockade is CTLA-4 dependent and T follicular helper cell specific. Am J Transplant 21, 73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis K et al. (2020) Coordinated Circulating T Follicular Helper and Activated B Cell Responses Underlie the Onset of Antibody-Mediated Rejection in Kidney Transplantation. J Am Soc Nephrol 31, 2457–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alishetti S et al. (2020) Desensitizing highly sensitized heart transplant candidates with the combination of belatacept and proteasome inhibition. Am J Transplant 20, 3620–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Muraglia GM et al. (2020) Circulating T follicular helper cells are a biomarker of humoral alloreactivity and predict donor-specific antibody formation after transplantation. Am J Transplant 20, 75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danger R et al. (2019) CXCR5+PD1+ICOS+ Circulating T Follicular Helpers Are Associated With de novo Donor-Specific Antibodies After Renal Transplantation. Front Immunol 10, 2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Besouw NM et al. (2019) The Number of Donor-Specific IL-21 Producing Cells Before and After Transplantation Predicts Kidney Graft Rejection. Front Immunol 10, 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahdal S et al. (2018) Residual Activatability of Circulating Tfh17 Predicts Humoral Response to Thymodependent Antigens in Patients on Therapeutic Immunosuppression. Front Immunol 9, 3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chhabra M et al. (2018) Germinal Center Alloantibody Responses Mediate Progression of Chronic Allograft Injury. Front Immunol 9, 3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macedo C et al. (2019) Impact of Induction Therapy on Circulating T Follicular Helper Cells and Subsequent Donor-Specific Antibody Formation After Kidney Transplant. Kidney Int Rep 4, 455–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwun J et al. (2018) IL-21 Biased Alemtuzumab Induced Chronic Antibody-Mediated Rejection Is Reversed by LFA-1 Costimulation Blockade. Front Immunol 9, 2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badell IR et al. (2018) Selective CD28 Blockade Results in Superior Inhibition of Donor-Specific T Follicular Helper Cell and Antibody Responses Relative to CTLA4-Ig. Am J Transplant 18, 89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cano-Romero FL et al. (2019) Longitudinal profile of circulating T follicular helper lymphocytes parallels anti-HLA sensitization in renal transplant recipients. Am. J. Transplant. 19, 89–97 [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki K et al. (2018) Increased CD40L+PD-1+ follicular helper T cells (Tfh) as a biomarker for predicting calcineurin inhibitor sensitivity against Tfh-mediated B-cell activation/antibody production after kidney transplantation. Int Immunol 30, 345–355 [DOI] [PubMed] [Google Scholar]
- 29.Désy O et al. (2018) Allogeneic dendritic cells stimulated with antibodies against HLA class II polarize naive T cells in a follicular helper phenotype. Sci Rep 8, 4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W et al. (2017) Low proportion of follicular regulatory T cell in renal transplant patients with chronic antibody-mediated rejection. Sci Rep 7, 1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burghuber CK et al. (2016) Antibody-Mediated Rejection in Sensitized Nonhuman Primates: Modeling Human Biology. Am. J. Transplant. 16, 1726–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Graav GN et al. (2015) Follicular T helper cells and humoral reactivity in kidney transplant patients. Clin. Exp. Immunol. 180, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim EJ et al. (2014) Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am. J. Transplant. 14, 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conlon TM et al. (2012) Germinal center alloantibody responses are mediated exclusively by indirect-pathway CD4 T follicular helper cells. J. Immunol. 188, 2643–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Leur K et al. (2017) IL-21 Receptor Antagonist Inhibits Differentiation of B Cells toward Plasmablasts upon Alloantigen Stimulation. Front Immunol 8, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Besouw NM et al. (2019) The Number of Donor-Specific IL-21 Producing Cells Before and After Transplantation Predicts Kidney Graft Rejection. Front Immunol 10, 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waanders MM et al. (2008) Monitoring of indirect allorecognition: wishful thinking or solid data? Tissue Antigens 71, 1–15 [DOI] [PubMed] [Google Scholar]
- 38.Korin YD et al. (2005) A novel flow assay for the detection of cytokine secreting alloreactive T cells: application to immune monitoring. Hum. Immunol. 66, 1110–1124 [DOI] [PubMed] [Google Scholar]
- 39.Lefaucheur C et al. (2016) IgG Donor-Specific Anti-Human HLA Antibody Subclasses and Kidney Allograft Antibody-Mediated Injury. J. Am. Soc. Nephrol. 27, 293–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lefaucheur C et al. (2018) Complement-Activating Anti-HLA Antibodies in Kidney Transplantation: Allograft Gene Expression Profiling and Response to Treatment. J. Am. Soc. Nephrol. 29, 620–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pène J et al. (2004) Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J. Immunol. 172, 5154–5157 [DOI] [PubMed] [Google Scholar]
- 42.Vidarsson G et al. (2014) IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Leur K et al. (2018) Characterization of ectopic lymphoid structures in different types of acute renal allograft rejection. Clin Exp Immunol 192, 224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liarski VM et al. (2014) Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci Transl Med 6, 230ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thaunat O et al. (2010) Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J. Immunol. 185, 717–728 [DOI] [PubMed] [Google Scholar]
- 46.Rao DA et al. (2017) Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bocharnikov AV et al. (2019) PD-1hiCXCR5− T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight 4, e130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louis K et al. (2021) T-bet+CD27+CD21− B cells poised for plasma cell differentiation during antibody-mediated rejection of kidney transplants. JCI Insight 6, 148881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asano Y et al. (2021) Innate-like self-reactive B cells infiltrate human renal allografts during transplant rejection. Nat Commun 12, 4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khiew SH et al. (2020) Transplantation tolerance modifies donor-specific B cell fate to suppress de novo alloreactive B cells. J Clin Invest 130, 3453–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steines L et al. (2020) Anti-BAFF Treatment Interferes With Humoral Responses in a Model of Renal Transplantation in Rats. Transplantation 104, e16–e22 [DOI] [PubMed] [Google Scholar]
- 52.Alishetti S et al. (2020) Desensitizing highly sensitized heart transplant candidates with the combination of belatacept and proteasome inhibition. Am J Transplant 20, 3620–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischman C et al. (2019) Circulating B Cells With Memory and Antibody-Secreting Phenotypes Are Detectable in Pediatric Kidney Transplant Recipients Before the Development of Antibody-Mediated Rejection. Transplant Direct 5, e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luque S et al. (2019) Value of monitoring circulating donor-reactive memory B cells to characterize antibody-mediated rejection after kidney transplantation. Am J Transplant 19, 368–380 [DOI] [PubMed] [Google Scholar]
- 55.Karahan GE et al. (2017) A Memory B Cell Crossmatch Assay for Quantification of Donor-Specific Memory B Cells in the Peripheral Blood of HLA-Immunized Individuals. Am J Transplant 17, 2617–2626 [DOI] [PubMed] [Google Scholar]
- 56.Santilli V et al. (2016) Cellular immune profile of kidney transplant patients developing anti-HLA antibodies during childhood. Pediatr Nephrol 31, 1001–1010 [DOI] [PubMed] [Google Scholar]
- 57.Lúcia M et al. (2015) Preformed circulating HLA-specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int. 88, 874–887 [DOI] [PubMed] [Google Scholar]
- 58.Todeschini M et al. (2013) In kidney transplant patients, alemtuzumab but not basiliximab/low-dose rabbit anti-thymocyte globulin induces B cell depletion and regeneration, which associates with a high incidence of de novo donor-specific anti-HLA antibody development. J Immunol 191, 2818–2828 [DOI] [PubMed] [Google Scholar]
- 59.Jung J et al. (2000) Regulation of CD27 expression in the course of germinal center B cell differentiation: the pivotal role of IL-10. Eur. J. Immunol. 30, 2437–2443 [DOI] [PubMed] [Google Scholar]
- 60.Wehmeier C et al. (2020) Donor-specific B Cell Memory in Alloimmunized Kidney Transplant Recipients: First Clinical Application of a Novel Method. Transplantation 104, 1026–1032 [DOI] [PubMed] [Google Scholar]
- 61.Ellebedy AH et al. (2016) Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol. 17, 1226–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walsh PT et al. (2004) Routes to transplant tolerance versus rejection; the role of cytokines. Immunity 20, 121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knox JJ et al. (2019) T-bet+ memory B cells: Generation, function, and fate. Immunol. Rev. 288, 149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Myles A et al. (2019) T-bet+ B cells: A common denominator in protective and autoreactive antibody responses? Curr. Opin. Immunol. 57, 40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koutsakos M et al. (2018) Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci Transl Med 10, [DOI] [PubMed] [Google Scholar]
- 66.Knox JJ et al. (2017) T-bet+ B cells are induced by human viral infections and dominate the HIV gp140 response. JCI Insight 2, e92943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lau D et al. (2017) Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci Immunol 2, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nutt SL et al. (2015) The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160–171 [DOI] [PubMed] [Google Scholar]
- 69.Cheng J et al. (2011) Ectopic B-cell clusters that infiltrate transplanted human kidneys are clonal. Proc. Natl Acad. Sci. USA 108, 5560–5565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pineda S et al. (2019) Characterizing pre-transplant and post-transplant kidney rejection risk by B cell immune repertoire sequencing. Nature Commun 10, 1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weinberger J et al. (2015) Immune Repertoire Profiling Reveals that Clonally Expanded B and T Cells Infiltrating Diseased Human Kidneys Can Also Be Tracked in Blood. PloS One 10, e0143125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grover RK et al. (2012) The costimulatory immunogen LPS induces the B-Cell clones that infiltrate transplanted human kidneys. Proc. Natl Acad. Sci. USA 109, 6036–6041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Portugal S et al. (2015) Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife 4, e07218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moir S et al. (2008) Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205, 1797–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson JL et al. (2020) The Transcription Factor T-bet Resolves Memory B Cell Subsets with Distinct Tissue Distributions and Antibody Specificities in Mice and Humans. Immunity 52, 842–855.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jenks SA et al. (2018) Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 49, 725–739.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Austin JW et al. (2019) Overexpression of T-bet in HIV infection is associated with accumulation of B cells outside germinal centers and poor affinity maturation. Sci Transl Med 11, eaax0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kardava L et al. (2018) IgG3 regulates tissue-like memory B cells in HIV-infected individuals. Nat. Immunol. 19, 1001–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suàrez-Fernández P et al. (2021) Circulatory follicular helper T lymphocytes associate with lower incidence of CMV infection in kidney transplant recipients. Am J Transplant 21, 3946–3957 [DOI] [PubMed] [Google Scholar]
- 80.Laguna-Goya R et al. (2020) Imbalance favoring follicular helper T cells over IL10+ regulatory B cells is detrimental for the kidney allograft. Kidney Int 98, 732–743 [DOI] [PubMed] [Google Scholar]
- 81.Bestard O and Grinyó J (2019) Refinement of humoral rejection effector mechanisms to identify specific pathogenic histological lesions with different graft outcomes. Am. J. Transplant. 19, 952–953 [DOI] [PubMed] [Google Scholar]
- 82.Chenouard A et al. (2017) Renal Operational Tolerance Is Associated With a Defect of Blood Tfh Cells That Exhibit Impaired B Cell Help. Am. J. Transplant. 17, 1490–1501 [DOI] [PubMed] [Google Scholar]
- 83.Viglietti D et al. (2018) Complement-binding anti-HLA antibodies are independent predictors of response to treatment in kidney recipients with antibody-mediated rejection. Kidney Int 94, 773–787 [DOI] [PubMed] [Google Scholar]
- 84.Sautenet B et al. (2016) One-year Results of the Effects of Rituximab on Acute Antibody-Mediated Rejection in Renal Transplantation: RITUX ERAH, a Multicenter Double-blind Randomized Placebo-controlled Trial. Transplantation 100, 391–399 [DOI] [PubMed] [Google Scholar]
- 85.Eskandary F et al. (2018) A Randomized Trial of Bortezomib in Late Antibody-Mediated Kidney Transplant Rejection. J Am Soc Nephrol 29, 591–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Viglietti D et al. (2018) Dynamic Prognostic Score to Predict Kidney Allograft Survival in Patients with Antibody-Mediated Rejection. J. Am. Soc. Nephrol. 29, 606–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vincenti F et al. (2016) Belatacept and Long-Term Outcomes in Kidney Transplantation. N. Engl. J. Med. 374, 333–343 [DOI] [PubMed] [Google Scholar]
- 88.Poirier N et al. (2010) Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transl Med 2, 17ra10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ville S et al. (2016) Anti-CD28 Antibody and Belatacept Exert Differential Effects on Mechanisms of Renal Allograft Rejection. J. Am. Soc. Nephrol. 27, 3577–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kawai, null et al. (2000) Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat. Med. 6, 114. [DOI] [PubMed] [Google Scholar]
- 91.Harland RC et al. (2020) Efficacy and safety of bleselumab in kidney transplant recipients: A phase 2, randomized, open-label, noninferiority study. Am J Transplant 20, 159–171 [DOI] [PubMed] [Google Scholar]
- 92.Lo DJ et al. (2015) A pilot trial targeting the ICOS-ICOS-L pathway in nonhuman primate kidney transplantation. Am. J. Transplant. 15, 984–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guillonneau C et al. (2005) Inhibition of chronic rejection and development of tolerogenic T cells after ICOS-ICOSL and CD40-CD40L co-stimulation blockade. Transplantation 80, 546–554 [PubMed] [Google Scholar]
- 94.García P et al. (2004) Signalling via CD70, a member of the TNF family, regulates T cell functions. J. Leukoc. Biol. 76, 263–270 [DOI] [PubMed] [Google Scholar]
- 95.Jacquot S et al. (1997) CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J. Immunol. 159, 2652–2657 [PubMed] [Google Scholar]
- 96.Narimatsu A et al. (2020) Blockade of costimulatory CD27/CD70 pathway promotes corneal allograft survival. Exp Eye Res 199, 108190. [DOI] [PubMed] [Google Scholar]
- 97.Dai H et al. (2011) Blockade of CD27/CD70 pathway to reduce the generation of memory T cells and markedly prolong the survival of heart allografts in presensitized mice. Transpl. Immunol. 24, 195–202 [DOI] [PubMed] [Google Scholar]
- 98.Riether C et al. (2020) Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat Med 26, 1459–1467 [DOI] [PubMed] [Google Scholar]
- 99.Jordan SC et al. (2017) Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation 101, 32–44 [DOI] [PubMed] [Google Scholar]
- 100.Chavele K-M et al. (2015) Cutting edge: circulating plasmablasts induce the differentiation of human T follicular helper cells via IL-6 production. J. Immunol. 194, 2482–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim I et al. (2014) Anti-interleukin 6 receptor antibodies attenuate antibody recall responses in a mouse model of allosensitization. Transplantation 98, 1262–1270 [DOI] [PubMed] [Google Scholar]
- 102.Choi J et al. (2017) Assessment of Tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a Potential Treatment for Chronic Antibody-Mediated Rejection and Transplant Glomerulopathy in HLA-Sensitized Renal Allograft Recipients. Am. J. Transplant. 17, 2381–2389. [DOI] [PubMed] [Google Scholar]
- 103.Doberer K et al. (2021) A Randomized Clinical Trial of Anti-IL-6 Antibody Clazakizumab in Late Antibody-Mediated Kidney Transplant Rejection. J Am Soc Nephrol 32, 708–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ettinger R et al. (2005) IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 175, 7867–7879 [DOI] [PubMed] [Google Scholar]
- 105.de Leur K et al. (2019) The Effects of an IL-21 Receptor Antagonist on the Alloimmune Response in a Humanized Mouse Skin Transplant Model. Transplantation 103, 2065–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ignatenko S et al. (2016) Safety, PK, and PD of recombinant anti-interleukin-21 monoclonal antibody in a first-in-human trial. Int J Clin Pharmacol Ther 54, 243–252 [DOI] [PubMed] [Google Scholar]
- 107.Hua F et al. (2014) Anti-IL21 receptor monoclonal antibody (ATR-107): Safety, pharmacokinetics, and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study. J Clin Pharmacol 54, 14–22 [DOI] [PubMed] [Google Scholar]
- 108.Cubas R et al. (2015) Reversible Reprogramming of Circulating Memory T Follicular Helper Cell Function during Chronic HIV Infection. J. Immunol. 195, 5625–5636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Portugal S et al. (2017) Atypical memory B cells in human chronic infectious diseases: An interim report. Cell. Immunol. 321, 18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]